Abstract
Background
The relationship between postoperative cumulative systemic inflammation and cancer survival needs to be investigated. We developed an approach to the prognostication of postoperative esophageal cancer by establishing low and high cut-off values for the C-reactive protein (CRP) area under the curve (AUC) at 7 and 14 days after esophagectomy.
Methods
One hundred and twenty-five consecutive patients with biopsy-proven invasive esophageal squamous cell carcinoma (SCC) who underwent esophagectomies were evaluated. Postoperative CRP levels were analyzed for the first 14 days after surgery. The AUC on days 7 and 14 were calculated and compared with clinicopathological features and survival. The cut-off values for CRP at 7 days (CRP 7 d) and 14 days (CRP 14 d) were 599 mg/L and 1153 mg/L, respectively.
Results
The patients in the low CRP 7 d group had significantly better recurrence-free survival (RFS) and overall survival (OS), not that in the low CRP 14d group. The OS rates in the high CRP groups at PODs 1, 3, 10, and 14 were significantly lower than those in the low CRP groups. Postoperative complications were more common in the high CRP groups on PODs 3, 10, and 14. Univariate analyses revealed that pTNM stage, depth of tumor invasion, tumor location, lymph node involvement, and CRP 7 d were significant prognostic factors for both OS and RFS. The Cox proportional hazards model identified pTNM, tumor location, and CRP 7d as independent prognostic factors for the RFS and OS.
Conclusions
Early prediction of patients with postoperative complications, and adequate management will suppress the elevation of CRP 7 d and further suppress the CRP value in the late postoperative period, which may improve the prognosis of esophageal cancer patients after esophagectomy.
Keywords: Esophagectomy, Esophageal cancer, CRP, Prognosis, Complications
Background
Esophageal cancer is the tenth most common cancer, with 604,100 new cases worldwide. The fatality rate in 2020 was 544,076 cases [1]. Surgery is the standard treatment for patients with locoregional disease. Two-thirds of patients with esophageal cancer have advanced disease at diagnosis [2], and there is a high rate of recurrence, even after curative surgery [3–6]. Esophagectomy is a highly invasive procedure with several serious postoperative complications, including pneumonia, anastomotic leakage, and recurrent laryngeal nerve paralysis [7–9]. Previous studies showed that the postoperative complications worsen the prognosis in esophageal cancer patients [10–14]. C-reactive protein (CRP), which is synthesized in the liver, is a sensitive indicator of the systemic inflammatory response(SIR), which is stimulated by cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor-α (TNF-α) from tumor cells [15]. High concentrations of CRP are associated with high mortality, poor response to treatment, and recurrence of solid tumors [16]. Some studies have reported the association between postoperative CRP levels, complications, and prognosis [17–22]. However, these studies evaluated CRP level at one point on the postoperative day, and the timings of CRP evaluation were inconsistent, ranging from 1 PODs(Postoperative Day) to 2 months. If postoperative complications could be predicted by using CRP values on early postoperative period, it would be clinically very useful and would lead to treatment. Furthermore, indicators are needed to comprehensively evaluate the results of interventions for postoperative complications. Therefore, we thought of comprehensively evaluating the postoperative inflammatory response by graphing the postoperative CRP values and integrating the area under the curve (AUC). In the present study, to examine whether postoperative complications can be predicted by CRP values in early postoperative period, and we quantified CRP for 2 weeks after surgery, analyzed the relationships between cumulative CRP levels (early and late phase) and postoperative complications, and evaluated the clinical significance of postoperative CRP levels over time as a prognostic factor.
Methods
One hundred and seventy-three patients with esophageal cancer or cancer or esophagogastric junction aged 20–80 years old were enrolled in this study. They received surgery at Kawasaki Medical School Hospital between January 2010 and June 2021, and their patients' records were reviewed retrospectively. The patients were received esophagectomy with/or two or three field lymphadenectomies or total gastrectomy + lower esophagectomy was performed. Adenocarcinoma of the esophagus or esophagogastric junction, other types of cancer(small cell carcinoma), R2 resection, and combined other malignancy (advanced gastric cancer: two patients, head and neck cancer: seven patients) were excluded in this study. Total 125 patients were analyzed in this study (Fig. 1). Tumor staging was based on the UICC classification of malignant tumors (8th edition) [23]. Two or three cycles of neoadjuvant therapy consisting of 5-FU/cisplatin or 5FU/cisplatin/docetaxel were administered to nine cStage II/III cases after 2017, with esophagectomy conducted at 3–7 weeks after completion of chemotherapy. Transthoracic esophagectomy with two- or three-field lymph node dissections and esophageal reconstruction was performed using a gastric tube in the retrosternal or posterior mediastinal roots. A few patients underwent right side colon replacement in the retrosternal root. Thoracoscopic and laparoscopic-assisted approaches were also utilized. Trans-hiatal esophagectomy with middle, lower mediastinal, and abdominal lymphadenectomy was performed in cStage I cases. The details of these procedures were described in a previous report [24]. The present study was approved by the Institutional Review Board of Kawasaki Medical School (Authorization No: 5603–03). Data were collected from the medical records at Kawasaki Medical School Hospital, and details of this retrospective study were provided on the hospital’s home page. All patients were followed up regularly until November 2023 or death. Informed consent was obtained from all patients preoperatively. Routine laboratory measurements of preoperative CRP were performed at 1–4 weeks before surgery. In the nine patients who received neoadjuvant chemotherapy, the CRP measurements were performed at 3–5 days before surgery.
Fig. 1.
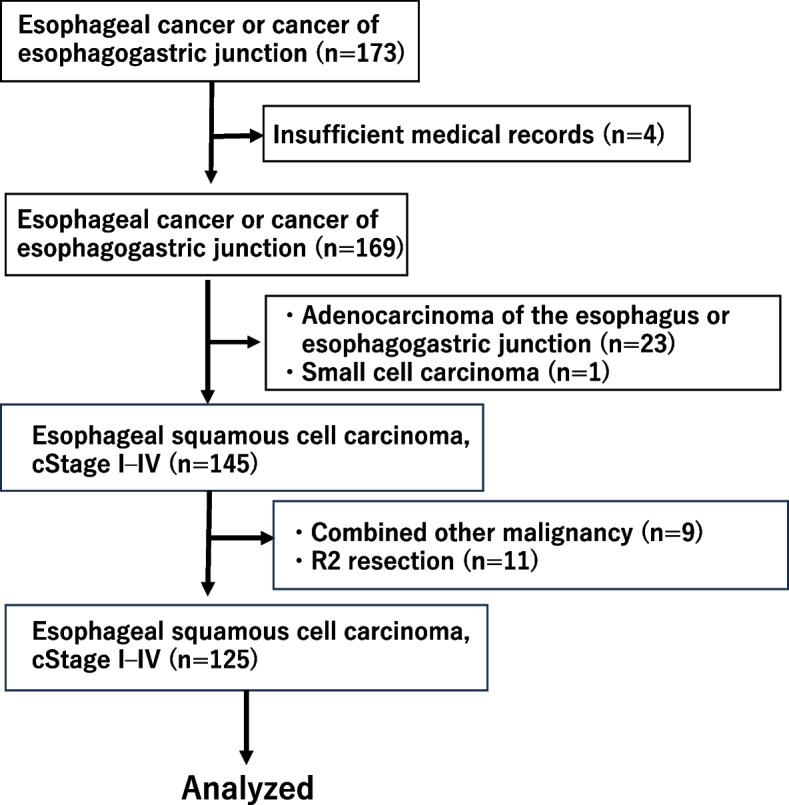
The patients flow diagram in this study
Evaluation of postoperative CRP
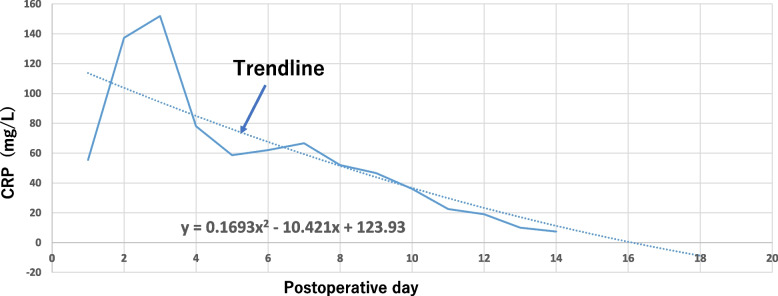
CRP was measured routinely on postoperative days (PODs) 1, 2, 3, 5, 7, 10, and 14. Additional measurements were performed according to each patient’s condition. CRP was measured using anti-human CRP mouse monoclonal antibody-sensitive latex (SEKISUI, Tokyo, Japan), with a normal cut-off value of 1.4 mg/L. The CRP values for 14 days after surgery were graphed using commercial software (Microsoft Excel 2019; Microsoft Inc., Redmond WA, USA) to create a curve trendline (Fig. 2). The area under the curve (AUC) trendline was calculated by definite integrals. CRP at 7 days (CRP 7 d) was defined as the cumulative CRP value for 7 days after surgery, and CRP at 14 days (CRP 14 d) was defined as the cumulative CRP value for 14 days after surgery. Four patients were excluded because of data unavailability for the CRP 14 d analysis. Also, CRPmax was evaluated in this study.
Fig. 2.
The CRP values (mg/L) on postoperative days. The trendline was created by Microsoft EXCEL (Dotted line). After then, area under the trendline as calculated by integral calculus
Postoperative complications
Significant postoperative complications were evaluated and classified according to the Clavien–Dindo classification as grade ≥ 2 [25]. In addition, we also investigated the patients with grade ≥ 3.
Statistical analysis
Overall survival (OS) was defined as the time between surgery and patient death or the availability of final information on vital status. Recurrence-free survival (RFS) was defined as the time between surgery and cancer recurrence or death. A time-dependent receiver operating characteristic curve was generated to evaluate the sensitivity and specificity of CRP 7 d, CRP 14 d, and CRP on PODs 1, 2, 3, 5, 7, 10, and 14 for predicting 3-year OS. Youden’s index was calculated to determine the optimal cut-off values for these parameters. The cut-off value of CRPmax was evaluated similarly. The chi-square test or Fisher’s exact test was used for categorical variables. The Mann–Whitney U-test was used for continuous variables. The Kaplan–Meier method was employed to estimate survival, and groups were compared using a two-sided log-rank test. Univariate and multivariate analyses for OS and RFS were performed using the Cox proportional hazards regression model, and the survival rate with 95% confidence interval (CI) was determined. Covariates for the Cox model were selected based on age and gender plus the following criteria: (i) the number of explanatory variables was approximately one-tenth the number of event occurrences; (ii) factors dependent on each other (collinearity) were not entered; (iii) useful independent prognostic factors were selected from previously published data, and factors with P < 0.2 in the univariate analyses were permitted. Statistical analyses were performed using commercial (JMP version 14; SAS, Tokyo, Japan) and open-source (R version 3.1.1; R Project for Statistical Computing, Vienna, Austria) software.
Results
The cut-off values for CRP 7 d and CRP 14 d were 599 mg/L (specificity: 0.409; sensitivity: 0.797; AUC: 0.56) and 1153 mg/L (specificity: 0.484; sensitivity: 0.702; AUC: 0.579), respectively. Based on these cut-off values, CRP 7 d and CRP 14 d were each divided into two groups (low and high CRP groups). The cut-off value of CRPmax was 16.17 mg/dL.
Patient characteristics
The mean follow-up time was 3.5 years. Table 1 summarizes the relationships between CRP 7 d and clinicopathological features in the patients. Preoperative CRP levels were significantly higher in the high CRP 7 d group (P < 0.001). Postoperative complications of grade ≥ 3 were more common in the high CRP7d group (P = 0.02), and anastomotic leakage was also more frequent in the high CRP7d group (P = 0.0334). Table 2 summarizes the relationships between CRP 14 d and clinicopathological features in the patients. Preoperative CRP levels were significantly higher in the high CRP 14 d group (P = 0.001). Postoperative complications with Grade ≥ 2 and 3 were more common in the high CRP 14 d group (P = 0.0008). Patients with anastomotic leakage, respiratory complications, and SSI were more likely to be in the high CRP14d group (P = 0.048, 0.0175, 0.0272, respectively).
Table 1.
Clinical characteristics of low- and high-CRP 7 d groups
| Covariates | Low CRP 7 d | High CRP 7 d | P |
|---|---|---|---|
| n | 39 | 86 | |
| Age, y:Median | |||
| < 67 | 22 | 42 | 0.448 |
| > 67 | 17 | 44 | |
| Sex | |||
| Male | 30 | 76 | 0.112 |
| Female | 9 | 10 | |
| Tumor location | |||
| Upper | 6 | 20 | 0.309 |
| Middle | 25 | 42 | |
| Lower | 8 | 24 | |
| Tumor length | |||
| < 50 mm | 20 | 40 | 0.7 |
| > 50 mm | 19 | 46 | |
| pTNM** | |||
| I | 19 | 27 | 0.182 |
| II | 3 | 15 | |
| III | 13 | 28 | |
| IV | 4 | 16 | |
| pT | |||
| T1 | 24 | 39 | 0.338 |
| T2 | 4 | 11 | |
| T3 | 10 | 28 | |
| T4 | 1 | 8 | |
| pN | |||
| pN0 | 19 | 35 | 0.868 |
| pN1 | 9 | 23 | |
| pN2 | 6 | 17 | |
| pN3 | 5 | 11 | |
| ****M-factor | |||
| M0 | 37 | 81 | 1 |
| M1 | 2 | 5 | |
| Procedure | |||
| Ivor Lewis | 4 | 15 | 0.63 |
| Thoracoscopic | 30 | 62 | |
| ***THE | 5 | 9 | |
| Blood loss | 150 g | 180 g | 0.207 |
| Operation time | 373 min | 372.5 min | 0.747 |
| preoperative CRP | 0.8 mg/L | 1.7 mg/L | *0.0001 |
| Neoadjuvant chemotherapy | 1 | ||
| (-) | 36 | 80 | |
| (+) | 3 | 6 | |
| Postoperative complications (Grade≥II) | 0.0816 | ||
| (-) | 24 | 37 | |
| (+) | 15 | 49 | |
| Anastomotic leakage | *0.0334 | ||
| (-) | 37 | 68 | |
| (+) | 2 | 18 | |
| Recurrent nerve palsy | 0.583 | ||
| (-) | 32 | 75 | |
| (+) | 7 | 11 | |
| Respiratory complications | 0.106 | ||
| (-) | 34 | 63 | |
| (+) | 5 | 23 | |
| SSI(Surgical Site Infection) | 0.106 | ||
| (-) | 34 | 63 | |
| (+) | 5 | 23 | |
| Postoperative complications(Grade≥III) | |||
| (-) | 28 | 42 | *0.02 |
| (+) | 11 | 44 | |
*Statistically significant
**pTNM: pathological TNM
***THE: Transhiatal esophagectomy
****M-factor: Distant metastasis
Table 2.
Clinical characteristics of low- and high-CRP 14 d groups
| Covariates | Low CRP 14d | High CRP 14d | p-value |
|---|---|---|---|
| n | 49 | 72 | |
| Age (y):Median | |||
| < 67 | 26 | 34 | 0.581 |
| > 67 | 23 | 38 | |
| Sex | |||
| Male | 37 | 65 | *0.041 |
| Female | 12 | 7 | |
| Tumor location | |||
| Upper | 7 | 18 | 0.195 |
| Middle | 31 | 34 | |
| Lower | 11 | 20 | |
| Tumor length | |||
| < 50 mm | 23 | 36 | 0.853 |
| > 50 mm | 26 | 36 | |
| **pTNM | |||
| I | 23 | 22 | 0.366 |
| II | 6 | 12 | |
| III | 14 | 26 | |
| IV | 6 | 12 | |
| pT | |||
| pT1 | 30 | 31 | 0.223 |
| pT2 | 4 | 11 | |
| pT3 | 12 | 26 | |
| pT4 | 3 | 4 | |
| pN | |||
| pN0 | 24 | 28 | 0.766 |
| pN1 | 11 | 20 | |
| pN2 | 8 | 14 | |
| pN3 | 6 | 10 | |
| ****M-factor | |||
| M0 | 47 | 67 | 0.7 |
| M1 | 2 | 5 | |
| Procedure | |||
| Ivor Lewis | 6 | 11 | 0.908 |
| Thoracoscopic | 37 | 53 | |
| ***THE | 6 | 8 | |
| Blood loss | 150 g | 200 g | 0.278 |
| Operation time | 375 min | 377 min | 0.804 |
| Preoperative CRP | 0.8 mg/L | 1.8 mg/L | *0.001 |
| Neoadjuvant chemotherapy | 1 | ||
| (-) | 45 | 67 | |
| (+) | 4 | 5 | |
| Postoperative complications(Grade≥2) | *0.0008 | ||
| (-) | 33 | 25 | |
| (+) | 16 | 47 | |
| Anastomotic leakage | *0.048 | ||
| (-) | 45 | 56 | |
| (+) | 4 | 16 | |
| Recurrent nerve palsy | 0.294 | ||
| (-) | 40 | 64 | |
| (+) | 9 | 8 | |
| Respiratory complications | |||
| (-) | 41 | 33 | *0.0175 |
| (+) | 6 | 18 | |
| SSI(Surgical Site Infection) | *0.0272 | ||
| (-) | 43 | 50 | |
| (+) | 6 | 22 | |
| Postoperative complications(Grade≥3) | *0.0001 | ||
| (-) | 38 | 28 | |
| (+) | 11 | 44 | |
*Statistically significant
**pTNM: pathological TNM
***THE: Transhiatal esophagectomy
****M-factor: Distant metastasis
Survival in the CRP 7 d groups
Overall, 59 patients had cancer recurrence and 49 patients died during the follow-up period. The 3- and 5-year RFS rates were 73.4% and 61.9% in the low CRP 7 d group and 49.6% and 40.9% in the high CRP 7 d group, respectively (P = 0.0117) (Fig. 3A). The 3- and 5-year OS rates were 81.8% and 74.6% in the low CRP 7 d group and 54.5% and 47.8% in the high CRP 7 d group, respectively (P = 0.0087) (Fig. 3B).
Fig. 3.
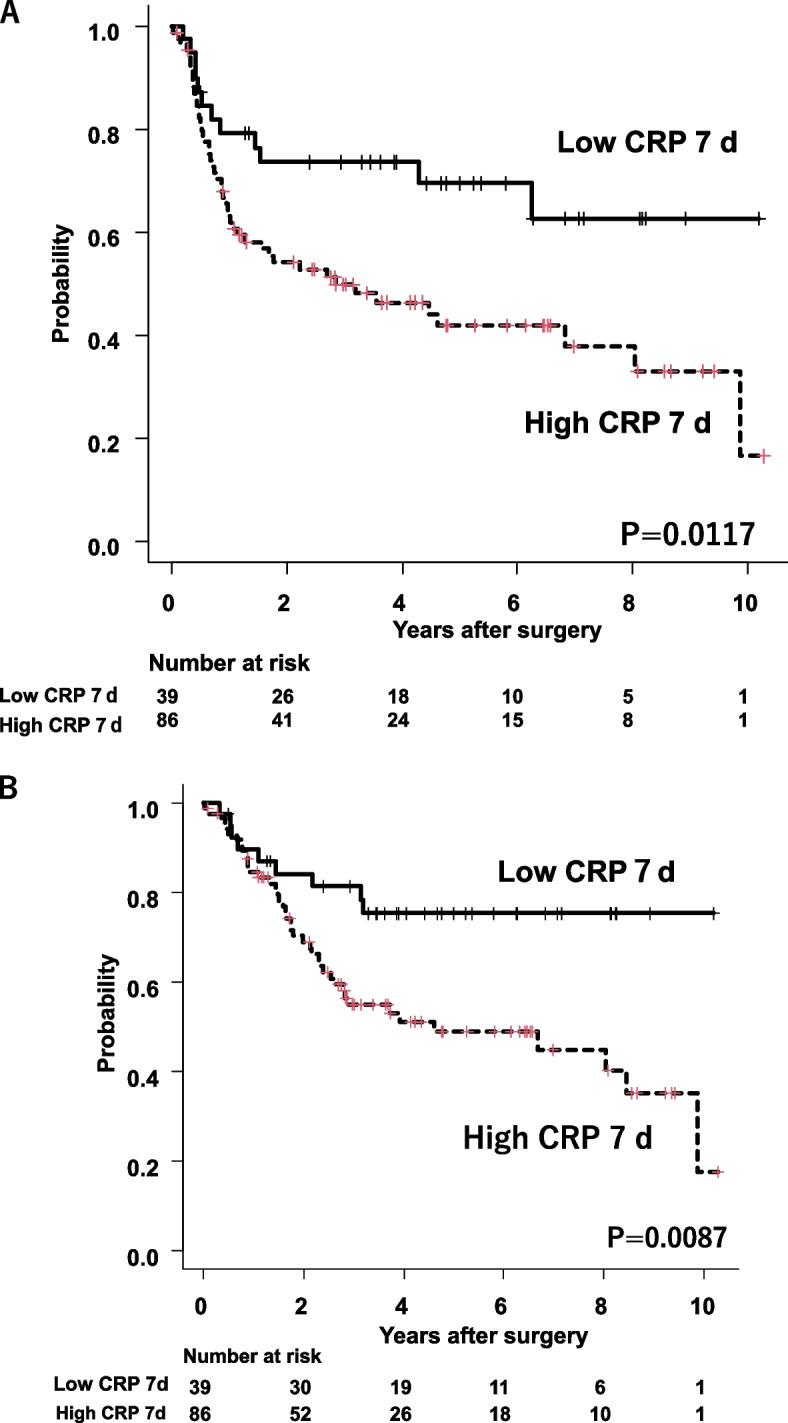
A Recurrence-free survival in the low and high CRP 7 d groups after esophagectomy. The low CRP 7 d group had significantly better survival than the high CRP 7 d group (P = 0.0117). B Overall survival between low and high CRP 7 d groups after esophagectomy. Low CRP 7 d group was significantly better survival than high CRP 7 d group (P = 0.0087)
Survival in the CRP 14 d groups
The 3- and 5-year RFS rates were 68% and 64.3% in the low CRP 14 d group and 50.7% and 40.5% in the high CRP 14 d group, respectively, with no significant difference (P = 0.132) (Fig. 4A). The 3- and 5-year OS rates also showed no significant difference (P = 0.111) (Fig. 4B).
Fig. 4.
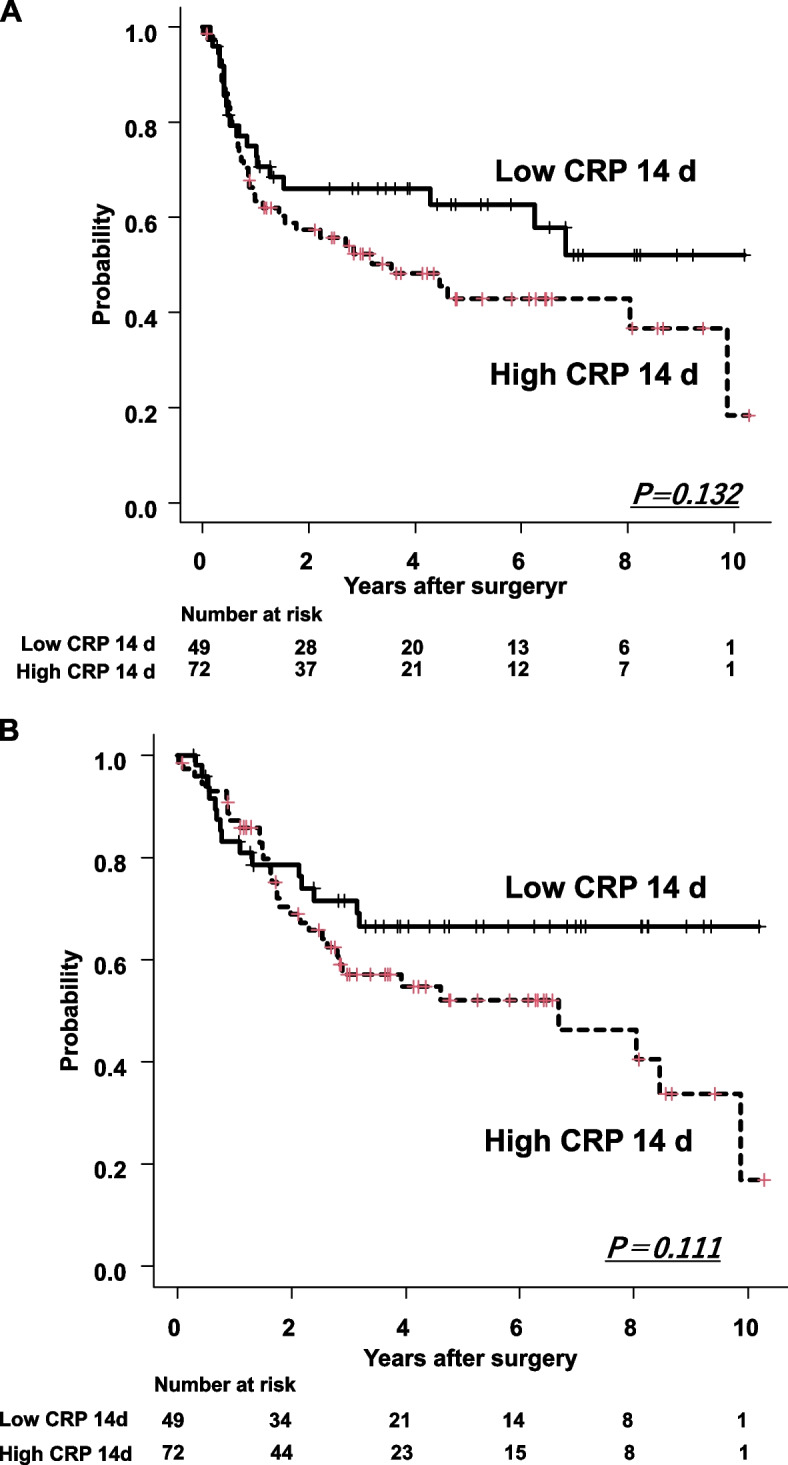
A Recurrence-free survival between low and high CRP 14 d groups after esophagectomy. Low CRP 14 d group was tendency to be better survival than high CRP 14 d group, but not significantly difference (P = 0.132). B Overall survival between low and high CRP 14 d groups after esophagectomy. Low CRP 14 d group tended to have better survival than the high CRP 14 d group, but not to a significant degree (P = 0.111)
Survival for patients with or without postoperative complications
Postoperative complications of grade ≥2 were noted in 66 of the 125 patients.
No significant differences were found in OS and RFS with or without postoperative complications (Fig. 5A,B).
Fig. 5.
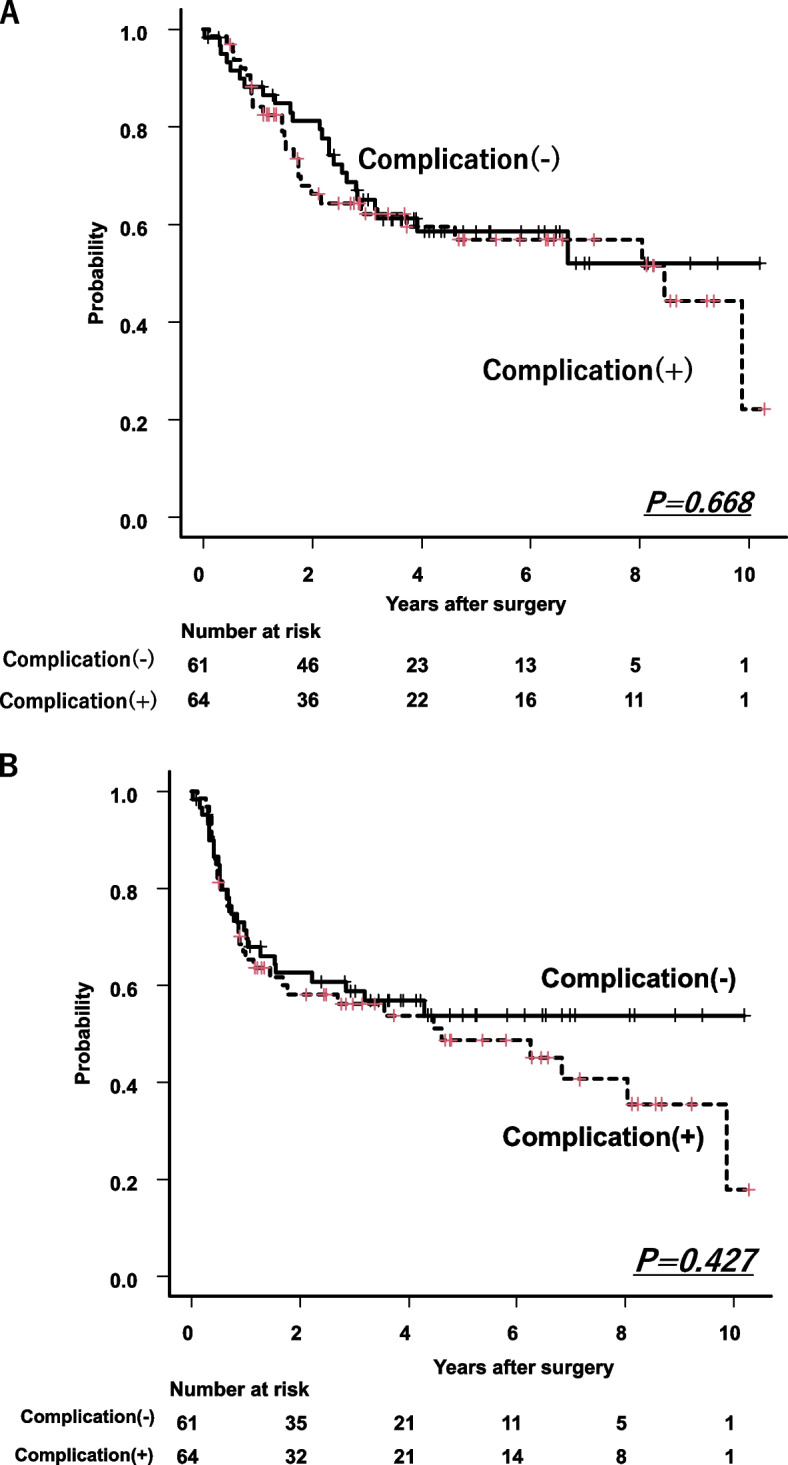
A Overall survival between with and without postoperative complications ≥ Grade 2. No significant differences between two groups (P = 0.668). B Recurrence free survival between with and without postoperative complications ≥ Grade2. No significant differences between two groups (P = 0.427)
Univariate analyses for patients with or without postoperative complications based on CRP 7 d and CRP 14 d
The low CRP 7 d group tended to be better RFS than the high CRP 7 d group with postoperative complications (P = 0.075, Table 3A). The low CRP 7 d group had significantly better OS than the high CRP 7 d group (P = 0.032, Table 3B).
Table 3.
Univariate analysis of OS and RFS in ESCC patients after esophagectomy with or without complications
| A Univariate analysis of OS and RFS in ESCC patients after esophagectomy with complications | ||||||
| RFS | OS | |||||
| Variables | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Postoperative CRP 7d | 2.371 | 0.913–6.157 | 0.075 | 1.848 | 0.633–5.398 | 0.261 |
| Low vs High | ||||||
| Postoperatve CRP 14d | 2.112 | 0.863–5.170 | 0.102 | 2.097 | 0.716–6.136 | 0.177 |
| Low vs High | ||||||
| B Univariate analysis of OS and RFS in ESCC patients after esophagectomy without complications | ||||||
| RFS | OS | |||||
| Variables | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Postoperative CRP 7d | 1.97 | 0.822–4.423 | 0.128 | 2.961 | 1.097–7.991 | *0.032 |
| Low vs High | ||||||
| Postoperatve CRP 14d | 1.073 | 0.480–2.397 | 0.864 | 1.259 | 0.545–2.909 | 0.59 |
| Low vs High | ||||||
*Statistically significant
Relationships between postoperative CRP values and survival or complications
The postoperative CRP values at PODs 1, 2, 3, 5, 7, 10, and 14 are shown in Fig. 6. The cut-off values for these parameters and the survival rates in the high and low CRP groups are shown in Table 4. The OS rates in the high CRP groups at PODs 1, 3, 10, and 14 were significantly lower than those in the low CRP groups (P = 0.0190, P = 0.019, P = 0.014, and P = 0.032, respectively). The RFS rates in the high CRP groups at PODs 1, 10, and 14 were significantly lower than those in the low CRP groups (P = 0.006, P = 0.012, and P = 0.004, respectively). The high CRP group of Day 3 tended to be worse survival compared to the low group (P = 0.063). The relationship between postoperative CRP levels and complications was analyzed. Postoperative complications were more common in the high CRP groups on PODs 3, 10, and 14 (P = 0.0068, P = 0.0066, and P = 0.00049, respectively).
Fig. 6.
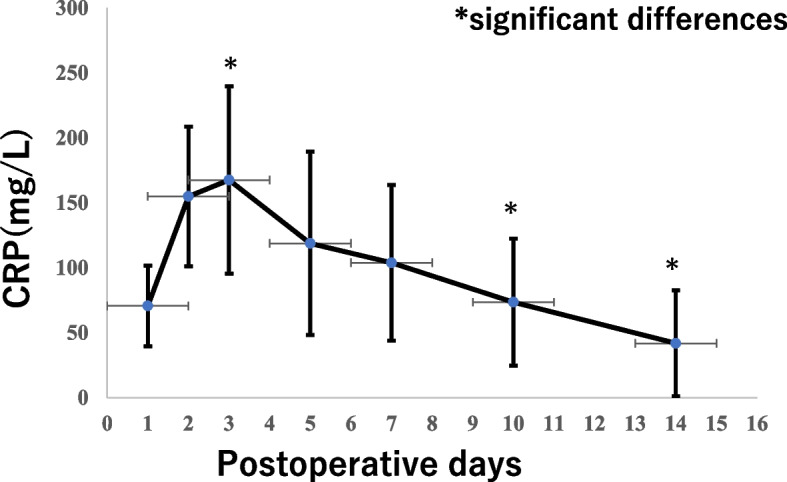
Postoperative CRP values for 14 days after surgery. The values are expressed as mean ± SD. Postoperative complications were more common in PODs3,10,14 (P = 0.0068, P = 0.0066, P = 0.00049)
Table 4.
The cut-off values of postoperative CRP for Overall Survival(OS),and OS or Recurrence-free survival(RFS) between postoperative high and low CRP values after esophagectomy patients
| CRP:cut-off values(mg/L) | Sensityvity/specificity | AUC | ||||
|---|---|---|---|---|---|---|
| PODs1 | 89 | 0.853/0.388 | 0.611 | |||
| PODs2 | 168.7 | 0.649/0.455 | 0.521 | |||
| PODs3 | 140.9 | 0.453/0.714 | 0.532 | |||
| PODs5 | 69 | 0.297/0.872 | 0.568 | |||
| PODs7 | 72.1 | 0.439/0.795 | 0.609 | |||
| PODs10 | 55.9 | 0.547/0.767 | 0.603 | |||
| PODs14 | 24.2 | 0.576/0.711 | 0.583 | |||
| For OS | For RFS | |||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| PODs1 | 2.017 | 1.122–3.625 | 0.019* | 2.135 | 1.244–3.664 | 0.0006* |
| PODs2 | 1.279 | 0.705^2.318 | 0.417 | 1.095 | 0.64–1.871 | 0.741 |
| PODs3 | 2.109 | 1.129–3.94 | 0.019* | 1.676 | 0.971–2.894 | 0.063 |
| PODs5 | 1.762 | 0.787–3.946 | 0.168 | 1.302 | 0.674–2.514 | 0.432 |
| PODs7 | 1.734 | 0.878–3.426 | 0.1132 | 1.416 | 0.789–2.54 | 0.244 |
| PODs10 | 2.305 | 1.181–4.499 | 0.014* | 2.13 | 1.184–3.833 | 0.012* |
| PODs14 | 2.109 | 1.062–4.186 | 0.032* | 2.456 | 1.333–4.525 | 0.004* |
*Statistically significant for OS
Univariate and multivariate Cox analyses
The univariate analyses revealed that pTNM stage, depth of tumor invasion, tumor location, extent of lymph node involvement, and CRP 7 d value were significant prognostic factors for both OS and RFS in patients with resectable esophageal cancer (Table 5). The Cox proportional hazards model for preoperative or postoperative clinicopathological factors and CRP 7 d identified pTNM, tumor location and CRP 7 d as independent prognostic factors for RFS and OS(Table 6).
Table 5.
Univariate analysis of overall survival (OS) and recurrence-free survival (RFS) in postoperative patients
| RFS | OS | |||||
|---|---|---|---|---|---|---|
| Variables | Ratio | 95% CI | p-value | Ratio | 95% CI | p-value |
| Age > 67 y | 1.031 | 0.621–1.714 | 0.904 | 1.078 | 0.621–1.871 | 0.789 |
| Gender (Male:Female) | 1.233 | 0.624–2.438 | 0.5471 | 1.156 | 0.542–2.464 | 0.7074 |
| pTNM Stages (I:II:III:IV) | 1.646 | 1.294–2.094 | 0.0001* | 1.724 | 1.32–2.251 | 0.0001* |
| Depth of tumor invasion | 1.529 | 1.212–1.929 | 0.0001* | 1.568 | 1.218–2.026 | 0.0005* |
| (T1:T2:T3:T4) | ||||||
| Lymph nodes metastasis | 1.636 | 1.292–2.073 | 0.0001* | 1.755 | 1.352–2.278 | 0.0001* |
| (N0:N1: N2:N3) | ||||||
| Distant metastasis | 1.014 | 0.364–2.825 | 0.979 | 1.037 | 0.369–2.908 | 0.9456 |
| (M0:M1) | ||||||
| Tumor size (≥ 50 mm) | 1.156 | 0.695–1.927 | 0.5735 | 1.202 | 0.692–2.089 | 0.5133 |
| Tumor location | 0.588 | 0.399–0.866 | 0.0072* | 0.592 | 0.392–0.894 | 0.0126* |
| (Upper:Middle,Lower) | ||||||
| Preoperative CRP (> 1.4 mg/L) | 1.479 | 0.874–2.505 | 0.145 | 1.634 | 0.917–2.912 | 0.096 |
| Postoperative CRP 7 d | 2.214 | 1.174–4.140 | 0.0140* | 2.542 | 1.235–5.233 | 0.0113* |
| (Low:High) | ||||||
| Postoperative CRP 14 d | 1.521 | 0.877–2.637 | 0.135 | 1.633 | 0.888–3.004 | 0.115 |
| (Low:High) | ||||||
| Postoperative complications(≥ Grade2) (Yes:No) | 1.23 | 0.737–2.052 | 0.428 | 1.129 | 0.649–1.963 | 0.668 |
| Anastomotic leakage | 1.297 | 0.682–2.465 | 0.428 | 1.043 | 0.502–2.191 | 0.9 |
| (Yes:No) | ||||||
| Respiratory complications | 1.495 | 0.850–2.626 | 0.163 | 1.462 | 0.775–2.756 | 0.2408 |
| (Yes:No) | ||||||
| SSI (Superficial and deep) | 1.087 | 0.603–1.961 | 0.781 | 0.882 | 0.448–1.734 | 0.716 |
| (Yes:No) | ||||||
| Postoperative complications (≥ Grade III) (Yes:No) | 1.464 | 0.880–2.436 | 0.143 | 1.398 | 0.804–2.431 | 0.236 |
| CRPmax | ||||||
| (Low:High) | 1.272 | 0.752–2.152 | 0.37 | 1.455 | 0.8111–2.61 | 0.208 |
*Statistically significant
Table 6.
Cox analysis of Recurrence-free Survival (RFS) and Overall Survival (OS) in ESCC patients after esophagectomy
| OS | RFS | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age > 67 y | 0.9 | 0.512–1.583 | 0.7148 | 0.837 | 0.4951–1.416 | 0.5073 |
| Gender (Male:Female) | 0.936 | 0.422–2.077 | 0.8714 | 1.09 | 0.529–2.245 | 0.8157 |
| pTNM (I,II/III,IV) | 4.147 | 2.139–8.04 | 0.00001* | 4.383 | 2.37–8.106 | 0.00001* |
| Location | 0.372 | 0.196–0.707 | 0.00254* | 0.304 | 0.163–0.569 | 0.000195* |
| (Upper/Middle,Lower) | ||||||
| CRP d7 (High vs Low) | 2.499 | 1.166–5.356 | 0.0185* | 2.011 | 1.036–3.906 | 0.03903* |
| Postoperative complications(≥ Grade II) | 0.703 | 0.390–1.266 | 0.2401 | 0.791 | 0.462–1.355 | 0.3933 |
*Statistically significant
*ESCC Esophageal squamous cell carcinoma
Discussion
This study attempted to evaluate postoperative CRP levels using an AUC method with definite integrals. CRP 7 d was identified as a significant prognostic factor in both univariate and Cox analysis. Furthermore, a detailed analysis of postoperative CRP levels revealed that CRP levels on PODs 1, 3, 10, and 14 were associated with prognosis, and postoperative complications were more common in the high CRP group on PODs 3, 10, and 14. In this study, we excluded esophageal and esophagogastric junction adenocarcinoma because surgical procedures for adenocarcinoma are less consistent than for squamous cell carcinoma, and because many adenocarcinoma patients are obese, which is estimated to lead to higher postoperative CRP levels.
There have been some reports on postoperative CRP levels in esophageal cancer patients [17–22, 26]. These studies evaluated the postoperative CRP using different methods to predict postoperative complications or prognosis. Matsuda et al. [18] reported that an intense postoperative inflammatory response, consisting of a delayed CRP peak (POD 3 or later) and persistent CRP elevation, was associated with a poor prognosis. However, their report did not assess the amount and sustained changes in CRP levels postoperatively. Other studies evaluated the postoperative CRP levels at a single point after esophagectomy, which was different from our report and that of Matsuda et.al. As a biomarker for predicting complications, it has been reported that postoperative complications were more likely to occur in the high CRP group on PODs 2–4 [17, 19, 21]. We similarly found that postoperative complications were more likely to occur in the high CRP group at PODs3, and it was noted that the patients with postoperative complications were more common in the high CRP group at PODs10.14. These findings suggested that early postoperative CRP levels(> 141 mg/L) can predict postoperative complications and insufficient treatment or delayed treatment effect for complications may have caused a delay in the inflammatory response. In the present study, we created a curve trendline for postoperative CRP levels (PODs 1–14) and evaluated the AUC values, suggesting that CRP 7d was an independent prognostic factor in both OS and RFS. We hypothesized that continuous exposure to cytokines after radical surgery for esophageal cancer might activate microscopic cancer cells and cause postoperative recurrence. This is the first attempt to comprehensively evaluate postoperative CRP levels after surgery. In breast cancer, post-surgery wound fluid contains large amounts of cytokines and growth factors and can promote the proliferation of breast cancer cells [27]. Hirai et al. [28]. reported that excessive surgical stress aggravated liver metastases in rat laparotomy and/or thoracotomy models. Studies on other tumors, including hepatocellular carcinoma and renal carcinoma, demonstrated that postoperative CRP levels affected the prognosis of these cancers [29, 30]. These findings imply that continuous exposure to inflammatory cytokines or excessive surgical stress contributes to cancer progression. Therefore, cumulative evaluation of CRP 7d and 14d is appropriate for postoperative CRP evaluation after esophagectomy.
Some papers have been published regarding postoperative CRP levels and prognosis [18–20, 22, 26]. Controversy has existed regarding the relationship between the timing of postoperative CRP evaluation and prognosis. Our data showed that OS was poor in the group with high CRP values PODs 1, 3, 10, and 14 days after surgery, but it is not possible to determine which period should be considered as important. Therefore, we believe that it is desirable to judge the postoperative CRP value comprehensively. In this study, CRP 7 d is a prognostic factor for esophageal cancer patients, while CRP 14 d is not. It is estimated that appropriate intervention may have been performed for patients who developed complications due to high CRP within one week after surgery, and as a result, CRP14 d decreased and did not become a prognostic factor. This may also be one of the reasons why the survival was not different between the presence or absence of postoperative complications. However, although many patients received appropriate treatment for postoperative complications and their CRP levels decreased, some cases were difficult to treat, and such cases may have insufficient CRP decreases and a worsened prognosis. Further examinations might be necessary.
The present study was limited by its retrospective design and the fact that postoperative CRP 7 d was an independent prognostic factor for esophageal cancer patients after esophagectomy both univariate and multivariate analysis. Furthermore, high CRP values at PODs 3 was associated with postoperative complications and poor prognosis. To prolong the survival of esophageal cancer patients, advances in surgical techniques and perioperative management are desired to prevent postoperative complications. Furthermore, it is necessary to pay attention to the CRP value on 3PODs in the early postoperative period, and take appropriate examinations to predict complications and interventions to decrease CRP 7d.
Conclusions
The cumulative postoperative CRP value for 1 week after surgery, designated CRP 7 d, was identified as an important prognostic factor after esophagectomy that may affect perioperative management. Attempts to minimalize postoperative complications, or minimalize postoperative CRP 7 d in the presence of complications, may improve the prognosis of esophageal cancer patients.
Acknowledgements
We would like to thank Miss Ayako Saki for her assistance in collecting data.
Abbreviations
- ESCC
Esophageal squamous cell carcinoma
- OS
Overall survival
- RFS
Recurrence free survival
- AUC
Area under the curve
- PODs
Postoperative days
Authors’ contributions
YF and SE: Statistical analysis and wrote Introduction, Methods, Results and Discussion. MH and HK: Assisted data collections and clinical practice. Figure and Table formation. KY: assisted statistical analysis and comprehensive check. TU: Final check of manuscript and suggested appropriate corrections. All authors have read and approved the manuscript for submission.
Funding
This study was not supported by any sponsor or funder.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because permission from our hospital and university was not given, but are available from the corresponding author on reasonable request.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions in our hospital or university.
Declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by The Research Ethics Committee(REC) of Kawasaki Medical School, approval number [5603–03]."
Informed consent: Informed consent was obtained from all individual participants included in the study, and it was obtained in writing prior to surgery.
Consent for publication
No consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed]
- 2.Tachimori Y, Ozawa S, Numasaki H, Ishihara R, Matsubara H, Muro K, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus : official journal of the Japan Esophageal Society. 2019;16(3):221–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dresner SM, Griffin SM. Pattern of recurrence following radical oesophagectomy with two-field lymphadenectomy. Br J Surg. 2000;87(10):1426–33. [DOI] [PubMed] [Google Scholar]
- 4.Hulscher JB, van Sandick JW, Tijssen JG, Obertop H, van Lanschot JJ. The recurrence pattern of esophageal carcinoma after transhiatal resection. J Am Coll Surg. 2000;191(2):143–8. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198(2):205–11. [DOI] [PubMed] [Google Scholar]
- 6.Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology. 1991;48(5):411–20. [DOI] [PubMed] [Google Scholar]
- 7.Kinugasa S, Tachibana M, Yoshimura H, Ueda S, Fujii T, Dhar DK, et al. Postoperative pulmonary complications are associated with worse short- and long-term outcomes after extended esophagectomy. J Surg Oncol. 2004;88(2):71–7. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260(2):259–66. [DOI] [PubMed] [Google Scholar]
- 9.Fujita H, Kakegawa T, Yamana H, Shima I, Toh Y, Tomita Y, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg. 1995;222(5):654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai T, Yamashita Y, Mukaida H, Kuwahara M, Inoue H, Toge T. Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today. 1998;28(6):576–9. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T, et al. Prognostic impact of postoperative morbidity after esophagectomy for esophageal cancer: Exploratory Analysis of JCOG9907. Ann Surg. 2017;265(6):1152–7. [DOI] [PubMed]
- 12.Rutegård M, Lagergren P, Rouvelas I, Mason R, Lagergren J. Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur J Surg Oncol. 2012;38(7):555–61. [DOI] [PubMed] [Google Scholar]
- 13.Booka E, Takeuchi H, Suda K, Fukuda K, Nakamura R, Wada N, et al. Meta-analysis of the impact of postoperative complications on survival after oesophagectomy for cancer. BJS Open. 2018;2(5):276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeki H, Tsutsumi S, Tajiri H, Yukaya T, Tsutsumi R, Nishimura S, et al. Prognostic Significance of Postoperative Complications After Curative Resection for Patients With Esophageal Squamous Cell Carcinoma. Ann Surg. 2017;265(3):527–33. [DOI] [PubMed]
- 15.Weinhold B, Rüther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997;327(( Pt 2)(Pt 2)):425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS ONE. 2015;10(12):e0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babic B, Tagkalos E, Gockel I, Corvinus F, Hadzijusufovic E, Hoppe-Lotichius M, et al. C-reactive Protein Levels After Esophagectomy Are Associated With Increased Surgical Trauma and Complications. Ann Thorac Surg. 2020;109(5):1574–83. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda S, Takeuchi H, Kawakubo H, Fukuda K, Nakamura R, Takahashi T, et al. Correlation Between Intense Postoperative Inflammatory Response and Survival of Esophageal Cancer Patients Who Underwent Transthoracic Esophagectomy. Ann Surg Oncol. 2015;22(13):4453–60. [DOI] [PubMed] [Google Scholar]
- 19.Harada K, Matsumoto C, Toihata T, Kosumi K, Iwatsuki M, Baba Y, et al. C-Reactive Protein Levels After Esophagectomy are Associated with Increased Surgical Complications and Poor Prognosis in Esophageal Squamous Cell Carcinoma Patients. Ann Surg Oncol. 2023;30(3):1554–63. [DOI] [PubMed] [Google Scholar]
- 20.Ibuki Y, Hamai Y, Hihara J, Emi M, Taomoto J, Furukawa T, et al. Role of Postoperative C-Reactive Protein Levels in Predicting Prognosis After Surgical Treatment of Esophageal Cancer. World J Surg. 2017;41(6):1558–65. [DOI] [PubMed] [Google Scholar]
- 21.Kano K, Aoyama T, Nakajima T, Maezawa Y, Hayashi T, Yamada T, et al. Prediction of postoperative inflammatory complications after esophageal cancer surgery based on early changes in the C-reactive protein level in patients who received perioperative steroid therapy and enhanced recovery after surgery care: a retrospective analysis. BMC Cancer. 2017;17(1):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsurahara K, Shiozaki A, Fujiwara H, Konishi H, Kudou M, Shoda K, et al. Relationship Between Postoperative CRP and Prognosis in Thoracic Esophageal Squamous Cell Carcinoma. Anticancer Res. 2018;38(11):6513–8. [DOI] [PubMed] [Google Scholar]
- 23.James D. Brierley MKGCW. UICC: TNM Classification of malignat tumors Eighth Edition: Wiley Blackwell, Kanehara,Tokyo, Japan; 2017.
- 24.Fujiwara Y, Endo S, Higashida M, Kubota H, Yoshimatsu K, Ueno T. The prognostic significance of preoperative nutritional/inflammatory markers and clinicopathological features in resectable esophagectomy patients: possibility of nutritional intervention. Esophagus. 2022. [DOI] [PubMed]
- 25.Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46(6):668–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kano K, Aoyama T, Maezawa Y, Hayashi T, Yamada T, Tamagawa H, et al. Postoperative Level of C-Reactive Protein Is a Prognosticator After Esophageal Cancer Surgery With Perioperative Steroid Therapy and Enhanced Recovery After Surgery Care. In vivo (Athens, Greece). 2019;33(2):587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segatto I, Berton S, Sonego M, Massarut S, Perin T, Piccoli E, et al. Surgery-induced wound response promotes stem-like and tumor-initiating features of breast cancer cells, via STAT3 signaling. Oncotarget. 2014;5(15):6267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirai T, Yoshimoto A, Iwata T, Yamashita Y, Kuwahara M, Toge T. Enhancing effect of thoraco-laparotomy on liver metastasis and the role played by active oxygens in its mechanism. Surg Today. 1997;27(11):1040–5. [DOI] [PubMed] [Google Scholar]
- 29.Ito K, Yoshii H, Sato A, Kuroda K, Asakuma J, Horiguchi A, et al. Impact of postoperative C-reactive protein level on recurrence and prognosis in patients with N0M0 clear cell renal cell carcinoma. J Urol. 2011;186(2):430–5. [DOI] [PubMed] [Google Scholar]
- 30.Shiba H, Furukawa K, Fujiwara Y, Futagawa Y, Haruki K, Wakiyama S, et al. Postoperative peak serum C-reactive protein predicts outcome of hepatic resection for hepatocellular carcinoma. Anticancer Res. 2013;33(2):705–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because permission from our hospital and university was not given, but are available from the corresponding author on reasonable request.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions in our hospital or university.