Abstract
The protein NS3 of Dengue virus type 2 (DEN-2) is the second largest nonstructural protein specified by the virus and is known to possess multiple enzymatic activities, including a serine proteinase located in the N-terminal region and an NTPase-helicase in the remaining 70% of the protein. The latter region has seven conserved helicase motifs found in all members of the family Flaviviridae. DEN-2 NS3 lacking the proteinase region was synthesized as a fusion protein with glutathione S-transferase in Escherichia coli. The effects of 10 mutations on ATPase and RNA helicase activity were examined. Residues at four sites within enzyme motifs I, II, and VI were substituted, and six sites outside motifs were altered by clustered charged-to-alanine mutagenesis. The mutations were also tested for their effects on virus replication by incorporation into genomic-length cDNA. Two mutations, both in motif I (G198A and K199A) abolished both ATPase and helicase activity. Two further mutations, one in motif VI (R457A,R458A) and the other a clustered charged-to-alanine substitution at R376KNGK380, abolished helicase activity only. No virus was detected for any mutation which prevented helicase activity, demonstrating the requirement of this enzyme for virus replication. The remaining six mutations resulted in various levels of enzyme activities, and four permitted virus replication. For the two nonreplicating viruses encoding clustered changes at R184KR186 and D436GEE439, we propose that the substituted residues are surface located and that the viruses are defective through altered interaction of NS3 with other components of the viral replication complex. Two of the replicating viruses displayed a temperature-sensitive phenotype. One contained a clustered mutation at D334EE336 and grew too poorly for further characterization. However, virus with an M283F substitution in motif II was examined in a temperature shift experiment (33 to 37°C) and showed reduced RNA synthesis at the higher temperature.
The four serotypes of Dengue virus (types 1 to 4) belong to the family Flaviviridae, which consists of the genera Flavivirus, Pestivirus, and Hepacivirus (52). The dengue virus genome is positive-sense RNA of 11 kb and encodes the proteins C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5 in a single open reading frame. Co- and posttranslational polyprotein processing by host and viral proteinases generates three structural proteins, namely, C (capsid), M (membrane associated) and E (envelope), and seven nonstructural (NS) proteins, NS1 through NS5 (reviewed in reference 45). Biochemical functions have been demonstrated for some nonstructural proteins. NS5 possesses RNA-dependent RNA polymerase activity (49). A complex of NS2B and NS3 acts as a chymotrypsin-like serine proteinase; the N-terminal 30% of NS3 is sufficient for this activity (15, 42). The C-terminal 70% of NS3 has seven motifs characteristic of RNA helicases of the DExH subfamily. Recombinant proteins containing the C-terminal helicase region of dengue virus NS3 possess nucleoside triphosphatase (NTPase) (10, 33) and RNA helicase activities (33).
RNA helicases catalyze the unidirectional unwinding of duplex RNAs (containing a single-stranded RNA region of at least 3 nucleotides [nt]) in the presence of a divalent cation and require the hydrolysis of the β-γ bond of a suitable deoxynucleoside triphosphate or nucleoside triphosphate (NTP) (usually ATP) as an energy source (32, 40). Known and putative RNA helicases of viral origin possess conserved amino acid sequence motifs enabling their classification into three distinct superfamilies. Superfamilies 1 and 2 have seven conserved motifs, while superfamily 3 has only three (30). The helicase of the flavivirus Dengue virus type 2 (DEN-2) is a member of superfamily 2, which includes the helicases of the pestivirus Bovine viral diarrhea virus (BVDV) and the hepacivirus Hepatitis C virus (HCV). Helicases can be further classified into DEAD, DExH, and DExx subfamilies based on the sequence of motif II (35, 46). The multifunctional flavivirus NS3 helicase protein is believed to be a component of the viral RNA replication complex with the RNA-dependent RNA polymerase NS5 protein (26). There is evidence that NS3 interacts with both NS5 and stem-loop structures in the genomic 3′ untranslated region, possibly playing an important role in the initiation of negative-strand RNA synthesis (8, 26).
Several X-ray crystal structures of the HCV NS3 helicase domain have been determined (9, 29, 58) and together with site-directed mutagenesis have helped to define the function of some helicase motifs. The first reported mutagenesis studies of HCV and other positive-strand viruses targeted the helicase motifs I, II, III, and VI. Motif I (GxGKT), conserved in all three superfamilies, is involved in binding the β and γ phosphate groups of NTPs. Motif II (DExH), also present in all three superfamilies, is predicted to bind Mg2+, making a complex with the terminal phosphates of the NTP. Several residues and motifs have been implicated in the coupling of NTP hydrolysis with RNA unwinding; they are the histidine residue of motif II, motif III (TAT box), and the glutamine and arginine residues of motif VI ([Q/x]RxGRxxR) (16, 18, 21, 28, 41, 51, 53, 58). More recently, the roles of residues outside motifs were examined using site-directed mutagenesis and a crystal structure of the HCV NS3 helicase-(dU)8 complex (29, 34, 41). Several conserved HCV helicase residues which contact the oligonucleotide were shown to be involved in RNA binding, duplex unwinding, and polynucleotide-stimulated ATPase activity.
This study investigated the importance of selected residues in the DEN-2 NS3 helicase region for enzyme activity and viral replication. Two types of mutations were introduced. The first type was the substitution of residues within motifs, I, II, and VI, and the second type was the replacement with alanine of amino acids in clusters of charged amino acids outside motifs. Mutant proteins were synthesized as N-terminally truncated fusion proteins in Escherichia coli, purified, and assayed for ATPase and RNA helicase activities. Mutations were also incorporated into genomic-length DEN-2 cDNA to investigate the effects of changes on viral yield. This work is the first report of extensive mutagenesis of a flavivirus helicase, examining both enzyme activity and viral replication.
MATERIALS AND METHODS
Cell lines, virus, and antiserum.
BHK-21 and Aedes albopictus C6/36 cells were grown and maintained as described previously (43). Stocks of DEN-2 viruses were prepared, and titers were determined by plaque assay in C6/36 cells at 28°C (22). Concentrated stocks of some viruses were produced by precipitation with polyethylene glycol (12). The preparation of rabbit polyclonal antiserum directed against DEN-2 NS3 (residues 355 to 593) has been described (50).
Constructs encoding NS3 fusion proteins.
For the following cloning strategies, the locations of restriction enzyme sites cleaving in DEN-2 cDNA (25) are shown in superscript, and sites present in plasmid vectors are not numbered. To obtain a plasmid encoding the C-terminal region of the DEN-2 NS3 protein, an NdeI5002-SpeI cDNA fragment (containing nt 5002 to 6375 of the DEN-2 New Guinea C [NGC] genome) was excised from the vector pSV.NS3, which encodes full-length NS3 and contains a stop codon at the 3′ end of the NS3 gene (50), and cloned into the SmaI site of pGEX-3X (Pharmacia Biotech). The resulting plasmid pGX74%NS3 encodes the glutathione S-transferase (GST) fused to residues 161 to 618 of DEN-2 NS3 (Fig. 1). The plasmid pGX74%NS3 was used as a template for mutagenesis by overlap extension PCR (OE-PCR) (24). Sequences of oligonucleotides used in mutagenesis are shown in Table 1. Six mutant constructs (Fig. 1) derived from pGX74%NS3 were prepared by replacing the BamHI-XhoI5426 fragment (BamHI is located in the pGEX-3X multiple cloning site immediately upstream of SmaI) with a mutagenized fragment prepared by OE-PCR; the constructs were pGX74%NS3169–173, pGX74%NS3179–181, pGX74%NS3184–186, pGX74%NS3G198A, pGX74%NS3K199A and pGX74%NS3M283F. The plasmid pGX74%NS3R457A,R458A was constructed by removing the mutagenized XhoI5426-SnaBI fragment from the plasmid pSV.NS2B/3457,458 (50) and ligating this into XhoI5426-BsaAI-digested pGX74%NS3. The remaining three mutant constructs were derived from pGX74%NS3 by replacing the XhoI5426-Ppu MI5852 fragment with mutagenized fragments prepared by OE-PCR; they were pGX74%NS3334–336, pGX74%NS3376–380, pGX74%NS3436–439. The PCR-derived regions of all clones were sequenced.
FIG. 1.
Constructs used to synthesize NS3 fusion proteins and prepare mutant viruses. The seven helicase motifs are shaded (29); residue numbers within NS3 are given. On the left are the full (pGX) and abbreviated (G) designations of the truncated NS3 gene constructs in pGEX-3X. The G designations are also used for the corresponding encoded mutant fusion proteins where appropriate. On the right are the names of the plasmids containing genomic-length cDNA (pDVWS) and derived virus (V). Antiserum raised in rabbits against a bacterial fusion protein (50) was directed against a segment of NS3 (vertical stripes). For clustered charged-to-alanine mutants, the underlined residues were mutated to alanine.
TABLE 1.
Oligonucleotides used in OE-PCR
| Mutant | Primer no. | Primer designationa | Sequenceb (5′ to 3′) |
|---|---|---|---|
| E169KSIE173c | 2618 | a | 4980ATCGAAGGTCGTGGGATCCCCCTATG5005 |
| 4095 | b | 5020CAGACTGcAgcAAGTATTGcAGACAATCCAGAGAT5055 | |
| 4096 | c | 5045TTGTCTgCAATACTTgcTgCAGTGTGGGCTATAGCA5010 | |
| 2619 | d | 5568CTCATGTCCAGAACTCCACGACG5545 | |
| E179DD181 | 2618 | a | 4980ATCGAAGGTCGTGGGATCCCCCTATG5005 |
| 7538 | b | 5050GAGATCGcAGcTGcTATTTTTCGAAAGAG5078 | |
| 3640 | c | 5082TTTTCTCTTTCGAAAAATGgCAgCTgCGATCTCTGGATTGTC5041 | |
| 2619 | d | 5568CTCATGTCCAGAACTCCACGACG5545 | |
| R184KR186 | 2618 | a | 4980ATCGAAGGTCGTGGGATCCCCCTATG5005 |
| 4097 | b | 5065TATTTTTgcAgcGgcAAAATTGACCATCATGGACC5099 | |
| 4098 | c | 5085CAATTTTgcCgcTgcAAAAATATCATCTTCGATCTCTGG5047 | |
| 2619 | d | 5568CTCATGTCCAGAACTCCACGACG5545 | |
| G198A | 2618 | a | 4980ATCGAAGGTCGTGGGATCCCCCTATG5005 |
| 2620 | b | 5107GGAGCGGctAAGACGAAGAGATACCTTCCG5136 | |
| 2621 | c | 5126CTCTTCGTCTTagCCGCTCCTGGGTGGAGG5097 | |
| 2619 | d | 5126CTCTTCGTCTTagCCGCTCCTGGGTGGAGG5097 | |
| K199A | 2618 | a | 5126ATCGAAGGTCGTGGGATCCCCCTATG5005 |
| 2624 | b | 5107GGAGCGGGAgcGACGAAGAGATACCTTCCG5136 | |
| 2625 | c | 5124CTTCGTCgcTCCCGCTCCTGGGTGGAGGTC5095 | |
| 2619 | d | 5568CTCATGTCCAGAACTCCACGACG5545 | |
| M283F | 2618 | a | 4980ATCGAAGGTCGTGGGATCCCCCTATG5005 |
| 2627 | b | 5364CATCtTcGACGAAGCCCATTTCACAGACCC5393 | |
| 2626 | c | 5374CGTCgAaGATGATCAGGTTGTAATTTGGC5346 | |
| 2619 | d | 5568CTCATGTCCAGAACTCCACGACG5545 | |
| D334EE336 | 3638 | a | 4792GAAGAAGCCGCGGTCTTGGCATTGGAGCCTG4822 |
| 4099 | b | 5517CATGGcTGcAGcAAGAGAAATCCCTGAACG5546 | |
| 4100 | c | 5533CTCTTgCTgCAgCCATGATTGGTGCAT5507 | |
| 4318 | d | 6123CACAAAGGTTTTCCTTGCTTCTCCTCTCAAGCGG6090 | |
| R376KNGK380 | 3638 | a | 4792GAAGAAGCCGCGGTCTTGGCATTGGAGCCTG4822 |
| 4104 | b | 5641TGCCTGgcAgcAAATGGAgcGAAAGTGATACAACTC5676 | |
| 4105 | c | 5667CACTTTCgcTCCATTTgcTgcCAGGCAAGCTGCTATATC5629 | |
| 4318 | d | 6123CACAAAGGTTTTCCTTGCTTCTCCTCTCAAGCGG6090 | |
| D436GEE439 | 3638 | a | 4792GAAGAAGCCGCGGTCTTGGCATTGGAGCCTG4822 |
| 4106 | b | 5821CTAACAGcTGGTGcAGcGCGGGTGATCCTGGCA5853 | |
| 4107 | c | 5842CCCGCgCTgCACCAgCTGTTAGTATAACTGG5812 | |
| 4318 | d | 6123CACAAAGGTTTTCCTTGCTTCTCCTCTCAAGCGG6090 |
Primers designated a and d are flanking primers; primers designated c and b are mutagenic primers.
All sequences are listed 5′ to 3′, and the substituted nucleotides are in lowercase type. Nucleotide numbering refers to the NGC DEN-2 sequence of Irie et al. (25).
For clustered charged-to-alanine mutants, the underlined residues were mutated to alanine. Amino acid numbering refers to the position of the residue in NS3.
Synthesis and purification of NS3 proteins.
The recombinant proteins containing an N-terminal GST tag were expressed in E. coli DH5α cells grown at 37°C in Luria-Bertani medium containing ampicillin (100 μg/ml). Synthesis of the recombinant proteins was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside. Two hours later, the cells were collected by centrifugation, resuspended in ice-cold phosphate-buffered saline containing lysozyme (33 μg/ml), and held on ice for 10 min. Triton X-100, at a final concentration of 0.1%, was added to the cells prior to their sonication on ice for 2 min. The cell lysate was clarified by centrifugation at 12,000 × g for 10 min. The soluble fraction was mixed with glutathione-Sepharose 4B beads (Pharmacia Biotech) and gently mixed at 4°C for 30 min. The beads were washed three times with phosphate-buffered saline, and bound protein was eluted twice in elution buffer (10 mM glutathione and 50 mM Tris-HCl [pH 8.0]) at 25°C for 15 min. All protein preparations were adjusted to 10% glycerol and stored at −70°C. Protein concentrations were estimated by densitometer scanning of Coomassie blue-stained acrylamide gels and Bradford assay (Bio-Rad).
ATPase assay.
The ATPase assay was a modified procedure of Warrener et al. (54). Briefly, the final volume of the standard assay used to test mutated proteins was 10 μl, containing 50 mM Tris-HCl (pH 8.0), 10 mM NaCl, 2.5 mM MgCl2, 1 μCi of [α-32P]ATP (800 Ci/mmol; DuPont) and 0.4 pmol of protein sample. Reaction mixes were incubated for 1 h at 24°C, and the reactions were terminated by the addition of EDTA to a final concentration of 20 mM. A 0.5-μl sample of the reaction mixture was spotted onto plastic-backed polyethyleneimine-cellulose sheets, and 32P-labeled ATP and ADP were separated by ascending chromatography in 0.375 M potassium phosphate (pH 3.5). The sheets were dried and exposed to X-ray film. The percentage of conversion of ATP to ADP was estimated by measuring the radioactivity in separated nucleotides by liquid scintillation counting.
The values of Km and kcat were calculated from a Lineweaver-Burk plot of ATP hydrolysis activity over a range of ATP concentrations from 1 to 5 mM. The concentration of poly(A), if present, was 0.17 μg/μl (0.5 mM measured as mononucleotide equivalents).
Helicase assay.
A partial double-stranded RNA (dsRNA) substrate was prepared using a modified pGEM-4Z (Promega) plasmid. A 24-bp region of the polylinker was removed by digestion with EcoRI and HindIII, filling in of recessed ends with Klenow DNA polymerase, and blunt-end ligation to generate the plasmid pGEM-4ZΔ24.
NdeI-digested plasmid pGEM-4ZΔ24 was transcribed with T7 RNA polymerase to produce a 259-nt strand. BanI-digested plasmid pGEM-4ZΔ24 was transcribed with SP6 RNA polymerase in the presence of [α-32P]ATP to produce a radiolabeled 144-nt strand. Reaction mixes were treated with RQI RNase-Free DNase (Promega) and extracted with phenol-chloroform, and the RNA was precipitated with ethanol. Transcripts were combined in annealing buffer containing 10 mM Tris-HCl (pH 7.5), 0.1 mM EDTA, and 200 mM NaCl. The mixture was heated for 5 min at 95°C and 1 h at 65°C and then was allowed to cool to room temperature over 3 h. RNA sample buffer (5×; 25 mM EDTA, 0.25% bromophenol blue, 50% glycerol, 0.5% sodium dodecyl sulfate [SDS]) was added to the hybridization mixture, which was then electrophoresed through a 6% polyacrylamide gel (acrylamide-bisacrylamide [30:0.8], 0.5× TBE [90 mM Tris borate, pH 7.5; 2 mM EDTA], 0.1% SDS). The region of the gel containing the RNA duplex was localized by autoradiography, excised from the gel, and pulverized, and RNA was eluted overnight at 37°C with a solution containing 500 mM ammonium acetate, 10 mM magnesium acetate, 1 mM EDTA, and 0.1% SDS. The eluted RNA was precipitated with ethanol and resuspended in water.
The RNA helicase assay was carried out in a total volume of 20 μl containing radiolabeled substrate, 25 mM MOPS (morpholinepropanesulfonic acid)-KOH (pH 6.5), 5 mM ATP, 3 mM MnCl2, 2 mM dithiothreitol, 100 μg/ml bovine serum albumin, 5 U of RNasin (Promega), and 1 pmol of protein. Reaction mixtures were incubated for 30 min at 37°C and terminated by the addition of 5× RNA sample buffer. The reaction mixture was analyzed by electrophoresis through a 6% polyacrylamide gel. Gels were dried and exposed to the storage phosphor screen (Molecular Dynamics). The phosphor screen was analyzed using a STORM PhosphorImager system, and ImageQuant image analysis software (Molecular Dynamics) was used to estimate the percentage of 32P-labeled fragment unwound.
Insertion of mutations into genomic-length DEN-2 cDNA.
The plasmid pDVWS501, which contains genomic-length DEN-2 cDNA, was described in detail (22). For these experiments, the mutated NS3 helicase fusion proteins were examined for ATPase and RNA helicase activity, and then the mutations were inserted into genomic-length DEN-2 cDNA to study their effects on virus replication (except the G198A mutant) (Fig. 1).
The plasmids pDVWS501NS3334–336, pDVWS501NS3376–380, and pDVWS501NS3436–439 were prepared by replacing the BstBI5069-BstBI6046 fragment of pDVWS501 with a mutagenized fragment prepared by OE-PCR. Sequences of oligonucleotides used in mutagenesis are shown in Table 1. The plasmids pDVWS501NS3K199A, pDVWS501NS3M283F, and pDVWS501NS3R457A,R458A were prepared by removing the mutagenized BstBI5069-BstBI6046 fragments from the corresponding pGX74%NS3 plasmid and ligation into BstBI-digested pDVWS501 (Fig. 1).
The other three mutations were initially constructed in the plasmid pDVSO8298 (pSPORT 1 containing nt 4494 to 8744 of DEN-2 NGC) prior to ligation into pDVWS501. A cDNA fragment encoding the mutation EDD (residues 179 to 181) (underlined residues changed to alanine) was cloned into pDVSO8298 by removing the mutagenized NsiI4700-Ppu MI5854 fragment from the corresponding pSV.NS2B/3 plasmid and ligation into NsiI-PpuMI-digested pDVSO8298. The two remaining charged-to-alanine mutations, EKSIE (169–173) and RKR (184–186), were introduced into NsiI-PpuMI-digested pDVSO8298 as OE-PCR fragments. For all three mutants, NsiI4700-StuI7874 mutagenized fragments were then removed from the appropriate pDVSO8298 plasmid and ligated into NsiI-StuI-digested pDVWS501. PCR-derived regions were sequenced.
Production of virus from genomic-length cDNA.
Procedures for transcription of RNA, electroporation, and immunofluorescence of BHK-21 cells and passaging of virus in C6/36 cells have been described (22). Briefly, capped transcripts were produced from plasmids containing genomic-length DEN-2 cDNA using the Promega RiboMAX kit. Approximately 7 to 10 μg of transcript RNA and 50 μg of carrier tRNA were electroporated into BHK-21 cells, which were then incubated at 33 or 37°C. The cells were examined for immunofluorescence 4 to 6 days later using anti-E monoclonal antibodies (20). At 7 days the culture medium was used to infect C6/36 cells. Four to five days later, the culture medium from the C6/36 cells was used to initiate a second passage in C6/36 cells. When approximately 50% of the cells exhibited cytopathic effects, or 4 days later if no cytopathic effects were visible, these second passage virus stocks were collected, and titers were determined by plaque assay in C6/36 cells.
To confirm that each mutation was present after electroporation and passaging, total RNA was extracted from infected C6/36 cells or supernatant, and reverse transcription (RT)-PCR of viral RNA was performed (22, 43). The complete NS2B and NS3 genes were sequenced to confirm the presence of the introduced mutation and the absence of any other changes that may have been introduced during virus passaging.
Temperature shift experiments.
RNA extracts of infected cells were prepared using RNeasy columns (Qiagen) for analysis of viral RNA content by dot blot hybridization. A 32P-labeled DNA probe spanning nt 5364 to 6123 of the DEN-2 genome was produced by random primed labeling using [α-32P]dATP (3,000 Ci/mmol), labeling mix-dCTP and pd(N)6 (Pharmacia Biotech). RNA samples were diluted in RNA dilution buffer (diethyl pyrocarbonate-treated H2O-20× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]-formaldehyde [5:3:2]) and held at 65°C for 15 min to remove RNA secondary structure. The samples were applied to a Hybond-N+ nylon membrane (Amersham), presoaked in 10× SSC, using an SRC 96 D Minifold I dot blotter (Schleicher and Schuell). The membrane was allowed to dry at room temperature, cross-linked with UV light, and prehybridized at 68°C for 2 h in hybridization solution (5× SSC, 1% SDS, 1% bovine serum albumin). Labeled DNA probe at 107 cpm/ml was heated at 95°C and added to fresh hybridization solution, and the membrane was incubated for a further 16 h in the presence of the probe. The membrane was then washed twice at room temperature in 2× SSC and 0.1% SDS, and this was followed by two washes at 68°C in 0.1× SSC and 0.1% SDS. Bound radioactivity was detected using the STORM PhosphorImager system (Molecular Dynamics).
To assess the effect of temperature shift on viral protein synthesis, infected BHK-21 cells were radiolabeled with trans-[35S]methionine (1,150 Ci/mmol) for 2 h. The methods for labeling procedures, cell lysis, radioimmunoprecipitation (RIP), gel electrophoresis, and fluorography have been described (44, 50).
RESULTS
Mutagenesis of the helicase region of NS3.
In order to test the importance of residues in the helicase region of DEN-2 NS3 for enzyme activity and virus replication, two types of mutations were introduced. First, changes were made in motifs I, II, and VI. These were single alanine substitutions G198A and K199A (motif I) and a double change at R457A,R458A (motif VI). In motif II, the substitution was M283F; phenylalanine is the second most common residue (after methionine) at this position in positive-strand viruses (30). Based on previous mutational studies and X-ray crystallography data of related viral RNA helicases (16, 19, 21, 28, 41, 51, 53), these motifs are known to be involved in the binding and hydrolysis of ATP and/or the coupling of helicase and ATPase activities. Thus, we hypothesized that the first three mutations would reduce enzyme activity and virus replication, although there were no previous studies on the replication of flaviviruses carrying these types of mutations. The possible effect of the substitution M283F was unknown.
The second type of mutation was the replacement with alanine of three amino acids in clusters of charged amino acids. Charged amino acids are likely to occupy exposed positions in the tertiary structure and therefore interact with other proteins (1, 13). Several studies have demonstrated an association between the flavivirus NS3 protein and other viral nonstructural proteins, including NS2B and NS5, both in vitro and during viral replication (5, 6, 8, 14, 15, 26, 57). The central region of NS3, spanning amino acids 161 through 463, was scanned for clusters of five residues which contained at least three charged amino acids (2, 55). Six such clusters outside helicase motifs (30) were chosen for mutagenesis, and the charged residues were changed to alanine (Fig. 1). These were as follows: E169A, K170A, and E173A; E179A, D180A, and D181A; R184A, K185A, and R186A; D334A, E335A, and E336A; R376A, K377A, and K380A; and lastly, D436A, E438A, and E439A. Alanine was chosen as the replacement amino acid since it removes the side chain beyond the beta carbon and also minimizes any steric effects within the polypeptide caused by the replacement (11). It was of interest to determine whether these changes in hydrophilicity outside helicase motifs modified the enzyme activity of NS3 in the absence of any other viral protein or whether any effects of the changes could be detected only by examining virus replication, when not only helicase activity but also interactions between NS3 and other viral or host proteins may be required.
Synthesis of truncated parental and mutant NS3 in E. coli.
To provide a source of flavivirus NS3 protein for biochemical studies, truncated (amino acids 161 to 618) parental and mutant polypeptides were synthesized as GST fusion proteins in E. coli DH5α cells. Proteins were purified from the cell lysate by affinity chromatography, and purified parental GST:74% NS3 fusion protein (G2) with a molecular mass of 78 kDa was detected following SDS-polyacrylamide gel electrophoresis (Fig. 2A, lane 2). In addition to the G2 protein, several proteins of lower molecular mass were also detected. These were possibly generated by either proteolytic degradation or premature translational termination, as they were recognized by anti-GST and anti-NS3 antibodies (Fig. 2B and C, lanes 1). GST (26 kDa) was also synthesized in E. coli to use as a negative control for the in vitro enzyme assays (Fig. 2A, lane 3; Fig. 2B, lane 2). Preparations of all mutant fusion proteins used for enzyme assays are shown in Fig. 2D.
FIG. 2.
Analysis on 10% polyacrylamide gels of partially purified parental and mutant GST:74%NS3 fusion proteins. (A) Coomassie blue staining. (B) Immunoblot using anti-GST antibodies (Pharmacia). (C) Immunoblot using anti-NS3 antiserum. (D) Coomassie blue staining of the GST:74%NS3 mutant fusion proteins used for enzyme assays. Size markers are shown on the left.
NS3-mediated ATPase activity.
The ATPase activity of increasing amounts of the parental NS3 fusion protein G2 was first measured over 45 min in the presence of 5 mM ATP (Fig. 3A). The rate of hydrolysis was directly proportional to the amount of enzyme. Using 1 pmol of enzyme and 5 mM ATP, the rate of hydrolysis was linear from 15 to 90 min (not shown). To determine the Km of G2, the ATPase activity of 1 pmol of G2 was measured at ATP concentrations from 1 to 5 mM for 60 min in the presence or absence of poly(A). The Lineweaver-Burk plots were linear in this range (Fig. 3B). The Km values for the parental protein were 3.0 or 2.6 mM in the absence or presence of poly(A), respectively. Corresponding kcat values were 1.2 and 1.5 s−1. The measure of catalytic efficiency, kcat/Km, increased from 4.0 × 102 (mol/liter)−1s−1 in the absence of poly(A) to 5.8 × 102 (mol/liter)−1s−1 in the presence of poly(A). Thus, the stimulation of ATPase activity (1.45-fold) by poly(A) was low.
FIG. 3.
(A) ATPase activity of increasing amounts of parental GST:74%NS3 fusion protein measured by the production of [α32P]ADP. (B) Lineweaver-Burk plots of ATPase activity in the presence or absence of 0.5 mM poly(A). A reaction volume of 10 μl contained 1 pmol of enzyme.
The results of testing the ATPase activity of the mutant NS3 fusion proteins are shown in Fig. 4. The chromatographic analyses of a typical experiment are displayed in Fig. 4A, the means of three experiments are shown in Fig. 4B, and an overall summary is presented in Table 2. As expected, mutation of the highly conserved G198 and K199 residues within ATP binding motif I abolished ATPase activity (Fig. 4A, lanes 7 and 8). Mutation of the less-conserved residues in motifs II and VI also reduced ATPase activity (Fig. 4A, lanes 9 and 13), although to a lesser extent than the motif I mutants. The clustered charged-to-alanine mutants located outside helicase motifs demonstrated a range of ATPase activities, from levels lower (Fig. 4A, lanes 10 and 11) to levels higher (Fig. 4A, lanes 4, 5, 6, and 12) than that of the parental protein. It is interesting that mutated clusters located within the linker region (residues 161 to 188) between the final proteinase box 4 (residues 145 to 155) (3) and upstream of the first helicase motif I (residues 188 to 205) stimulated ATPase activity.
FIG. 4.
(A) ATPase activities of parental and mutant 74%NS3 fusion proteins. Radioactive ADP and ATP were separated by thin-layer chromatography. (B) Percent hydrolysis of ATP to ADP. Means (columns) and range of values (error bars) from three independent experiments are shown.
TABLE 2.
Yields of mutant viruses following electroporation of RNA into BHK-21 cells and two passages of virus in C6/36 cells
| Site(s) mutated | Virus
|
IFa | Virus titerb (PFU/ml) | Approx plaque size (mm) | RT-PCRc | ATPase activityf | Helicase activityf | |
|---|---|---|---|---|---|---|---|---|
| Mutant | Temp (°C)d | |||||||
| V2 | 33 | ++++ | (1.1 ± 0.1) × 106 | 4 | Yes | + | + | |
| V2 | 37 | ++++ | (7.3 ± 0.8) × 106 | 4 | Yes | |||
| E169KSIE173 | V169–173 | 33e | ++++ | (2.7 ± 0.2) × 106 | 3 | Yes | ↑ | ↑ |
| V169–173 | 37 | ++++ | (2.2 ± 0.5) × 106 | 3 | Yes | |||
| E179DD181 | V179–181 | 33e | +++ | (4.9 ± 1.0) × 105 | 1 | Yes | ↑ | ↑ |
| V179–181 | 37 | +++ | (1.4 ± 0.2) × 106 | 1 | Yes | |||
| R184KR186 | V184–186 | 33 | − | None detected | No | ↑ | ↑ | |
| V184–186 | 37 | − | None detected | No | ||||
| K199A | VK199A | 33 | − | None detected | No | − | − | |
| VK199A | 37 | − | None detected | No | ||||
| M283F | VM283F | 33 | +++ | (4.7 ± 1.0) × 105 | 2 | Yes | ↓ | ↑ |
| VM283F | 37 | − | None detected | No | ||||
| D334EE336 | V334–336 | 33 | ++++ | (7.3 ± 1.1) × 102 | 1 | Yes | ↓ | ↓ |
| V334–336 | 37 | − | None detected | No | ||||
| R376KNGK380 | V376–380 | 33 | − | None detected | No | ↓ | − | |
| V376–380 | 37 | − | None detected | No | ||||
| D436GEE439 | V436–439 | 33 | − | None detected | No | ↑ | ↑ | |
| V436–439 | 37 | − | None detected | No | ||||
| R457A,R458A | VR457A,R458A | 33 | − | None detected | No | ↓ | − | |
| VR457A,R458A | 37 | − | None detected | No | ||||
Immunofluorescence (IF) in BHK-21 cells at 5 to 6 days postelectroporation. IF was scored as follows: −, no positive cells; +, 1 to 25% positive cells; ++, 25 to 50% positive cells; +++, 50 to 75% positive cells; ++++, 75 to 100% positive cells.
Plaque titers after passaging in C6/36 are expressed as means ± one standard deviation. Each virus was derived at least twice from RNA transcripts; therefore, the result shown for each virus is the average of two or more experiments.
Detection of product after RT-PCR. All positive samples retained the required mutation and had no other changes in the NS2B/3 genes.
Temperature at which BHK-21 cells were incubated immediately after electroporation.
Data for this virus are published in reference 37.
RNA helicase activity of NS3 mutants.
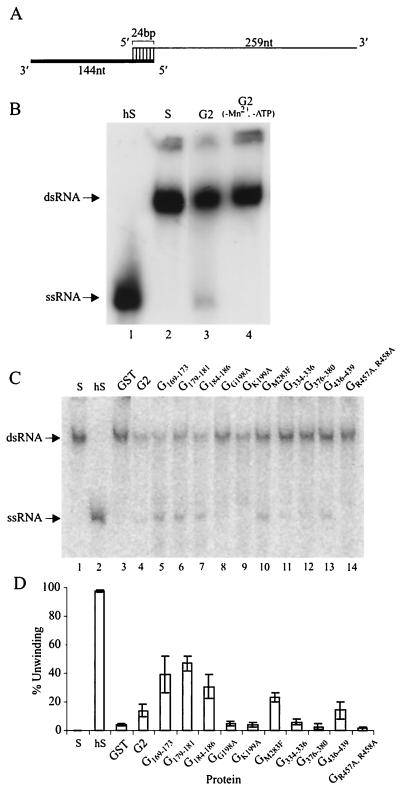
Helicase activity was tested by using an RNA substrate which consisted of a 259-nt RNA strand hybridized to a 144-nt radiolabeled RNA strand to produce a partially duplex RNA substrate containing 3′ single-stranded regions with a 24-bp duplex region (Fig. 5A). The G2 protein had RNA helicase activity in the presence of Mn2+ and ATP as shown by the release of the radiolabeled strand (Fig. 5B, lane 3). In the absence of the G2 protein (lane 2), or Mn2+ and ATP (lane 4), no activity was detected.
FIG. 5.
RNA helicase assay of 74%NS3 fusion proteins. (A) Structure of the 3′-tailed dsRNA substrate; the lower strand of RNA was labeled with [α-32P]ATP. (B) RNA helicase activity of the parental G2 protein. Lane 1, heated RNA substrate (hS); lane 2, untreated RNA substrate (S); lane 3, RNA helicase activity of 1 pmol of purified G2 protein; lane 4, same as lane 3 but omitting ATP and Mn2+. (C) RNA helicase activity of mutant fusion proteins. (D) Percent unwinding of dsRNA to single-stranded RNA (ssRNA). Means (columns) and range of values (error bars) from three independent experiments are shown.
Next, the mutant NS3 proteins were examined in the RNA helicase assay (Fig. 5C) (summary shown in Table 2). As expected, mutation of the ATP binding motif I abolished RNA helicase activity (lanes 8 and 9); the extent of unwinding was the same as for GST only (lane 3), confirming that no RNA helicase activity was detected in the absence of ATPase activity. Interestingly, mutation M283F in motif II demonstrated reduced ATPase activity (Fig. 4A, lane 9) and increased helicase activity (Fig. 5C, lane 10) compared to the parental G2 protein (Fig. 4A, lane 3, and Fig. 5C, lane 4). Also, the protein with changes R457A and R458A in motif VI which retained ATPase activity (Fig. 4A, lane 13) showed no detectable RNA unwinding (Fig. 5C, lane 14). These results demonstrate that the NTPase and RNA helicase activities of the DEN-2 NS3 protein can be functionally uncoupled by mutations within motifs.
Mutation of the clustered charged regions external to the helicase motifs had variable effects on RNA unwinding. The three mutants G169–173, G179–181, and G184–186, upstream of motif I, demonstrated a large increase in RNA unwinding (Fig. 5C, lanes 5 to 7) compared with parental NS3, which corresponded to their increased ATPase activity (Fig. 4). However, the G436–439 mutant, which also exhibited enhanced ATP hydrolysis, showed only a slight increase in helicase activity over parental G2 (Fig. 5C, lane 13). The remaining two mutants, G334–336 and G376–380, each demonstrated ATPase activity but only low or no RNA unwinding (Fig. 5C, lanes 11 and 12). These results show uncoupling of ATPase and helicase activity by mutagenesis outside enzyme motifs.
Analysis of virus replication.
To test the effects of the mutations described above on virus replication, all mutants except G198A were incorporated into genomic-length cDNA. We considered it unnecessary to test both the motif I mutants, G198A and K199A.
Virus was produced from genomic-length cDNA by established procedures (22). RNA was transcribed and electroporated into BHK-21 cells, and the cells were incubated at 33 or 37°C. BHK-21 cells were tested for immunofluorescence with anti-E antibodies. Medium from the transfected BHK-21 cells was passaged twice in C6/36 cells at 28°C, and the virus titer was determined after the second passage by plaque assay in C6/36 cells. Viral RNA was then amplified by RT-PCR, and the entire NS2B and NS3 genes were sequenced to check that the mutation was retained during the passaging and that no other changes were present within this region. These procedures were completed at least twice for each construct, and the results are summarized in Table 2.
The parental virus V2 and mutant viruses V169–173, and V179–181 grew to comparable titers of 105 to 106 PFU/ml following initial electroporation at 33 or 37°C (Table 2), although both mutant viruses showed a small-plaque phenotype. The corresponding NS3 fusion proteins G2 and mutants G169–173, and G179–181 all possessed in vitro ATPase and RNA helicase activities. For viruses VM283F and V334–336, virus was detected following electroporation at 33°C only, at reduced titers (4.7 × 105 and 7.3 × 102 PFU/ml, respectively), and with a small-plaque phenotype (Table 2). These results suggested that VM283F and V334–336 were restricted in replication and possibly heat sensitive. The GM283F and G334–336 fusion proteins both had reduced ATPase activity in vitro (Fig. 4B) and helicase activity that was either increased or reduced, respectively (Fig. 5D).
No virus was detected for five constructs. The lack of virus from the three constructs containing mutation K199A (motif I) or R457A,R458A (motif VI) or clustered charged-to-alanine changes R376KNGK380 corresponded to the lack of helicase activity detected for the corresponding fusion proteins. However, clustered changes at R184KR186 and D436GEE439 did not reduce helicase activity of the fusion proteins, and yet no virus was recovered. We hypothesize that these residues are required for other NS3 functions, such as the interaction with proteins in the viral replication complex.
Growth of viruses V2, V169–173, V179–181, and VM283F in BHK cells.
To examine further the properties of the viruses, more concentrated stocks were prepared by polyethylene glycol precipitation of all viruses except V334–336. Virus V334–336 did not replicate adequately to obtain sufficient titers for further experiments.
BHK-21 cells were infected at a multiplicity of infection (MOI) of 1, and cells were incubated at 33 or 37°C. Experiments maintaining the BHK-21 cells at 39°C were unsuccessful because of poor cell survival. The culture medium was sampled at 72 h after infection, and virus titers were determined by plaque assay in C6/36 cells (Fig. 6). Of the four viruses, only VM283F showed significant temperature sensitivity. At 72 h after infection, supernatant from cells infected with virus VM283F and maintained at 33°C contained (3.0 ± 0.4) × 104 PFU/ml, whereas cells maintained at 37°C contained (5.7 ± 0.4) × 102 PFU/ml. The presence of each mutation in recovered virus was reconfirmed by RT-PCR and sequencing.
FIG. 6.
Replication of selected mutant viruses in BHK-21 cells at 33 and 37°C (MOI of 1.0). The cell culture medium was sampled at 72 h postinfection, and the virus titers were determined by plaque assay on C6/36 cells (28°C). Error bars show one standard deviation of the plaque titer.
Temperature shift experiments with virus VM283F.
To assess the effect of temperature shift on viral RNA synthesis and replication of VM283F, duplicate BHK-21 cell monolayers were infected with the parental V2 or mutant VM283F viruses at an MOI of 10 and incubated for 48 h at 33°C. At this time monolayers were maintained at 33°C or shifted to 37°C.
At 24 h after the shift (72 h after infection) the cell culture medium was assayed for virus yield (Fig. 7A). The titers for the mutant and parental viruses were both higher at 33°C than at 37°C. However, the shift to 37°C clearly had a greater effect on mutant VM283F than on V2. The reductions in titer (log10) were 0.7 and 1.9, respectively. At 0, 16, and 24 h after the shift (48, 64, and 72 h after infection) RNA extracts of infected cells were prepared for analysis of accumulated viral RNA content by dot blot hybridization. Overall, the V2-infected cells contained more viral RNA than those infected with the mutant VM283F (Fig. 7B), consistent with the higher yield of virus from the former (Fig. 7A). Following the shift, V2-infected cells showed similar viral RNA content at the two temperatures at 64 and 72 h, whereas for the mutant VM283F, the cells maintained at 33°C clearly had more viral RNA than at 37°C. Both positive- and negative-strand viral RNA were detected by the dsDNA probe.
FIG. 7.
(A) Replication of parental V2 and VM283F viruses in BHK-21 cells shifted from 33 to 37°C at 48 h postinfection (h p.i.). Virus titers in cell culture fluid at 72 h p.i. were determined by plaque assay on C6/36 cells (28°C). Error bars show one standard deviation of the plaque titer. (B) Dot blot hybridizations of total infected cell RNA using a 32P-labeled dsDNA viral probe. Cells were harvested at 48, 64, and 72 h p.i. (C) Analysis by gel electrophoresis of 35S-labeled protein immunoprecipitated by anti-NS3 antiserum (50). Mock-infected cells (lanes 2, 5, and 6) and size markers (lane 1) are shown. The bottom panel shows amounts of total cell protein in cell lysates before immunoprecipitation. RIPs were prepared using anti-NS3 antiserum. The samples in lanes 7, 8, 9, and 10 are from independent duplicate experiments.
To assess the effect of the temperature shift on viral protein synthesis, the accumulations of labeled proteins at the permissive (33°C) and nonpermissive (37°C) temperatures were compared. The timing of the experiment was similar to that described above for the dot blot assay. BHK-21 cell monolayers were infected with the V2 or mutant VM283F viruses at an MOI of 10 and incubated for 48 h at 33°C. At 48 h after infection monolayers were maintained at 33°C or shifted to 37°C. At 0 and 24 h postshift, media were replaced with fresh medium lacking methionine for 2 h. Proteins were then radiolabeled with [35S]methionine for a further 2 h. As a measure of overall viral protein synthesis, the amount of NS3 protein was assessed by RIP of cell lysates (Fig. 7C). As for viral RNA, less NS3 was detected in the cells infected by the mutant virus VM283F than by V2. In addition, cells infected by the mutant contained less NS3 at the higher temperature (Fig. 7C, lanes 7 and 8 compared with lanes 9 and 10), whereas V2-infected cells (Fig. 7C, lanes 11 and 12) were more similar in NS3 content.
The bottom panel of Fig. 7C shows that the amount of protein in cell lysates before immunoprecipitation varied little. This demonstrated that the differences in levels of NS3 seen following RIP were probably due to the availability of template viral RNA for protein synthesis and not to variation in the number of cells in the monolayer or efficiency of lysis. RIPs were also performed with anti-E monoclonal antibodies, and identical results were obtained with respect to the relative amounts of viral proteins (data not shown). Therefore, the mutant virus VM283F was temperature (heat) sensitive in RNA synthesis, protein synthesis, and virus yield.
DISCUSSION
Ten sites distributed through the helicase region of DEN-2 NS3 were mutagenized in these experiments. Four were located in enzyme motifs, and a further six that were rich in charged amino acids were altered by charged-to-alanine mutagenesis of three residues (Fig. 1).
Ten mutant proteins were synthesized in E. coli and tested in vitro for their effects on ATPase and RNA helicase activities. The ATPase activity of the parental protein G2 was stimulated only modestly by poly(A) in these experiments. The increase in the kcat/Km ratio was 1.45-fold, corresponding to an increase in Vmax of 1.25-fold. This was in contrast to the results obtained by Li et al. (33) who showed a 9.7-fold increase in Vmax for a DEN-2 NS3 in the presence of poly(A), using a protein with a similar N-terminal truncation but containing a C-terminal His tag rather than the much larger N-terminal GST tag of our experiments. Other significant differences in NTPase activities among NS3 proteins in the presence of polynucleotides have also been described (4, 31, 47, 48, 54). The reasons for the differences have not been identified, but they probably reflect variation in the types, sizes, and locations of fused peptides; the degree of truncation of the enzymes; the methods of expression (e.g., in bacteria, insect, or mammalian cells); the purification procedures; and the assay conditions.
Patterns of activity.
In our experiments we examined both ATPase and helicase activity in vitro. Five patterns of activity were observed, and they are discussed in turn below: (i) no ATPase and no helicase, (ii) enhanced ATPase and enhanced helicase, (iii) reduced ATPase and no helicase, (iv) reduced ATPase and reduced helicase, and (v) reduced ATPase and enhanced helicase.
(i) No ATPase and no helicase.
Only two of the ten mutant fusion proteins assayed for enzymatic activity in this study lacked both in vitro ATPase and RNA helicase activities. They contained a substitution of the invariant G198 or K199 residues in NTP-binding motif I. Substitution of the residue corresponding to K199 in BVDV and HCV was previously shown to greatly reduce ATPase and RNA helicase activities (21, 23, 28, 38, 53).
(ii) Enhanced ATPase and enhanced helicase.
All six proteins with charged-to-alanine mutations had ATPase activity. Of these, four proteins—G169–173, G179–181, G184–186, and G436–439—were more active than parental G2 (Fig. 4). Increased ATPase corresponded to increased helicase activity (Fig. 5; Table 2). Previous studies have also shown enhanced NTPase activity for some flavivirus and poxvirus enzyme mutants. Li et al. (33) generated a DEN-2 NS3 mutant Q184NGN187, comparable to our R184KRK187, and demonstrated that it had a twofold increase in ATPase activity in the absence of poly(A). Substitution of the conserved histidine residue of motif II with alanine in NS3 of HCV and Japanese encephalitis virus, and in the NTP phosphohydrolase II (NPH-II) of Vaccinia virus also caused an increase in NTPase activity in the absence of poly(A) compared with parental protein (17, 23, 51).
(iii) Reduced ATPase and no helicase.
In this study, the motif VI mutant GR457A,R458A and the charged-to-alanine mutant G376–380 had reduced ATPase activity and no helicase activity, identifying two regions required for coupling of the two activities. The role of the arginine residues in motif VI has been examined in Vaccinia virus NPH-II and HCV NS3 helicases. Mutation of the first arginine (corresponding to R457 in DEN-2) in NPH-II and of the second arginine (corresponding to R458 in DEN-2) in HCV decreased RNA binding (7, 18, 28). Thus, by comparison with these viruses, the lack of detectable helicase activity of the DEN-2 GR457A,R458A double mutant protein was possibly due to inhibition of RNA binding. In contrast to GR457A,R458A, mutagenesis of the region corresponding to G376–380 has not been reported, and analysis of the HCV structure at this location provides no understanding of the role of these residues in enzyme activity. However, the substitution of basic residues by alanine may also have an adverse effect on RNA binding. Recent structure-based mutagenesis of HCV NS3 helicase demonstrated that substitution with alanine of several residues (external to helicase motifs) proposed to interact with RNA also uncoupled the two enzyme activities (34).
(iv) Reduced ATPase and reduced helicase.
The remaining charged-to-alanine mutant G334–336 showed reduced levels of both ATPase and RNA helicase activities.
(v) Reduced ATPase and enhanced helicase.
The only protein which demonstrated reduced ATPase activity and increased helicase activity with respect to parental G2 was the motif II mutant GM283F. The mutation in this protein was of particular interest because the residue at this position (methionine in DEN-2 motif II L280IIMDEAH287) (30) has not been previously mutagenized for any virus. Phenylalanine commonly occurs at this position in positive-strand viruses (30). Analysis of the HCV NS3 crystal structure indicates that the adjoining aspartic and glutamic acid residues potentially interact with the bound ATP γ-phosphate and amino acid residues in motif I via Mg2+ binding (29), and both residues are required for NTPase and helicase activities (51, 53). However, the reason for the increase in helicase activity observed in this study is unknown. Analysis of the HCV helicase structure demonstrates that the residue equivalent to DEN-2 M283 is buried within the secondary structure, suggesting that it is not directly involved in ATPase or helicase activity (J. C. Whisstock, personal communication).
With the availability of genomic-length cDNA, we were able to test the effect on virus replication of nine mutations modifying ATPase and helicase activity in vitro. As described above, the mutations produced five patterns of enzyme activity in vitro, and their effects on replication were of considerable interest. The introduction of helicase mutations into viral genomes has been reported for only one positive-strand RNA virus, BVDV (21). For BVDV, three mutations, two point mutations in motifs I and II and a deletion mutant in motif VI, abolished helicase activity and virus replication (21). Likewise for DEN-2, the mutations in motif I (K199A) and motif VI (R457A, R458A) that abolished helicase activity also prevented virus replication. In addition, the charged-to-alanine mutation at R376KNGK380 in DEN-2 NS3 also stopped helicase activity and virus replication. Thus, for both these members of the family Flaviviridae, lack of helicase in vitro correlated directly with no detectable virus replication.
On the other hand, mutations permitting helicase activity in vitro did not necessarily permit virus replication. There were four mutations that allowed replication, i.e., E169KSIE173, E179DD181, M283F, and D334EE336 (although with smaller plaque phenotype and in two instances temperature sensitivity), and two that did not, i.e., R184KR186 and D436GEE439. With the exception of M283F (motif II mutant), these mutations were all of the charged-to-alanine type and therefore likely to be located on the surface of NS3 and involved in protein-protein or RNA-protein interactions (1, 13). NS3 has been identified in NS3-NS5 complexes (8, 26) and associated with the nonstructural proteins NS1, NS2A, NS4A, and NS5 in replication complexes (27, 36, 57). It is therefore likely that subtle or substantial changes in these interactions led to the observed range of phenotypes. In particular, the charged residues at R184KR186, D334EE336, and D436GEE439 are worthy of further investigation. Less severe mutagenesis of these sites would establish the relative contribution of individual residues to enzyme activity and virus replication. Additional experiments which may assist in determining the roles of residues are binding assays in vitro for analysis of protein-protein and RNA-protein interactions (10). Since the hydrophilicity profiles of proteins specified by viruses within the genus Flavivirus are highly conserved (56), the results obtained using DEN-2 would be potentially applicable across the genus.
We had limited success in producing temperature-sensitive mutants by charged-to-alanine mutagenesis. Here, one potential mutant (V334–336) was obtained after mutagenesis of six different sites. Only one temperature-sensitive mutant produced by this technique has been reported for the flaviviruses, in NS1 of Yellow fever virus (39). However, we were successful in generating a temperature-sensitive mutant, VM283F, with the M283F substitution in motif II. As noted above for the hydrophilicity profiles, the conservation of the sequence MDEAH in motif II suggests the results with VM283F may hold for other flaviviruses. The data shown in Fig. 7B demonstrated that cells infected with this virus were defective in RNA synthesis at the nonpermissive temperature. Further experiments are in progress to determine the basis of this defect.
In summary, we have identified residues in the NS3 helicase region of DEN-2 within and outside motifs that modify enzyme activities in vitro and alter virus phenotype. Mutations that abolished helicase but not ATPase activity were identified. In some instances enhancement of enzyme activities was observed. Absence of helicase activity in vitro correlated directly with lack of virus replication. Our results are likely to be applicable to other flaviviruses and are of importance in the development of antiviral strategies directed at the inhibition of NTP and RNA binding, the coupling of NTPase and RNA helicase activities, and protein-protein interactions within the replication complex.
ACKNOWLEDGMENTS
This work was supported by grants from the National Health and Medical Research Council of Australia and the World Health Organization Global Program for Vaccines and Immunization.
REFERENCES
- 1.Alber T. Mutational effects on protein stability. Annu Rev Biochem. 1989;58:765–798. doi: 10.1146/annurev.bi.58.070189.004001. [DOI] [PubMed] [Google Scholar]
- 2.Bass S H, Mulkerrin M G, Wells J A. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci USA. 1991;88:4498–4502. doi: 10.1073/pnas.88.10.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazan J F, Fletterick R J. Detection of a trypsin-like serine protease domain in flaviviruses and pestiviruses. Virology. 1989;171:637–639. doi: 10.1016/0042-6822(89)90639-9. [DOI] [PubMed] [Google Scholar]
- 4.Borowski P, Niebuhr A, Mueller O, Bretner M, Felczak K, Kulikowski T, Schmitz H. Purification and characterization of West Nile virus nucleoside triphosphatase (NTPase)/helicase: evidence for dissociation of the NTPase and helicase activities of the enzyme. J Virol. 2001;75:3220–3229. doi: 10.1128/JVI.75.7.3220-3229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers T J, Grakoui A, Rice C M. Processing of the yellow fever virus nonstructural polyprotein: a catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J Virol. 1991;65:6042–6050. doi: 10.1128/jvi.65.11.6042-6050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers T J, Nestorowicz A, Amberg S M, Rice C M. Mutagenesis of the yellow fever virus NS2B protein: effects on proteolytic processing, NS2B-NS3 complex formation, and viral replication. J Virol. 1993;67:6797–6807. doi: 10.1128/jvi.67.11.6797-6807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S C, Cheng J C, Kou Y H, Kao C H, Chiu C H, Wu H Y, Chang M F. Roles of the AX4GKS and arginine-rich motifs of hepatitis C virus RNA helicase in ATP- and viral RNA-binding activity. J Virol. 2000;74:9732–9737. doi: 10.1128/jvi.74.20.9732-9737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C J, Kuo M D, Chien L J, Hsu S L, Wang Y M, Lin J H. RNA-protein interactions: involvement of NS3, NS5, and 3′ noncoding regions of Japanese encephalitis virus genomic RNA. J Virol. 1997;71:3466–3473. doi: 10.1128/jvi.71.5.3466-3473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho H S, Ha N C, Kang L W, Chung K M, Back S H, Jang S K, Oh B H. Crystal structure of RNA helicase from genotype 1b hepatitis C virus. A feasible mechanism of unwinding duplex RNA. J Biol Chem. 1998;273:15045–15052. doi: 10.1074/jbc.273.24.15045. [DOI] [PubMed] [Google Scholar]
- 10.Cui T, Sugrue R J, Xu Q, Lee A K, Chan Y C, Fu J. Recombinant dengue virus type 1 NS3 protein exhibits specific viral RNA binding and NTPase activity regulated by the NS5 protein. Virology. 1998;246:409–417. doi: 10.1006/viro.1998.9213. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham B C, Wells J A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 12.Della P A, Westaway E G. Rapid preparation of hemagglutinins of togaviruses from infected cell culture fluids. Appl Microbiol. 1972;23:158–160. doi: 10.1128/am.23.1.158-160.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond S E, Kirkegaard K. Clustered charged-to-alanine mutagenesis of poliovirus RNA-dependent RNA polymerase yields multiple temperature-sensitive mutants defective in RNA synthesis. J Virol. 1994;68:863–876. doi: 10.1128/jvi.68.2.863-876.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falgout B, Miller R H, Lai C J. Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of a domain required for NS2B-NS3 protease activity. J Virol. 1993;67:2034–2042. doi: 10.1128/jvi.67.4.2034-2042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falgout B, Pethel M, Zhang Y M, Lai C J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol. 1991;65:2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassmann C W, Isken O, Behrens S E. Assignment of the multifunctional NS3 protein of bovine viral diarrhea virus during RNA replication: an in vivo and in vitro study. J Virol. 1999;73:9196–9205. doi: 10.1128/jvi.73.11.9196-9205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross C H, Shuman S. Mutational analysis of vaccinia virus nucleoside triphosphate phosphohydrolase II, a DExH box RNA helicase. J Virol. 1995;69:4727–4736. doi: 10.1128/jvi.69.8.4727-4736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross C H, Shuman S. The QRxGRxGRxxxG motif of the vaccinia virus DExH box RNA helicase NPH-II is required for ATP hydrolysis and RNA unwinding but not for RNA binding. J Virol. 1996;70:1706–1713. doi: 10.1128/jvi.70.3.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross C H, Shuman S. Vaccinia virus RNA helicase: nucleic acid specificity in duplex unwinding. J Virol. 1996;70:2615–2619. doi: 10.1128/jvi.70.4.2615-2619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruenberg A, Wright P J. Processing of dengue virus type 2 structural proteins containing deletions in hydrophobic domains. Arch Virol. 1992;122:77–94. doi: 10.1007/BF01321119. [DOI] [PubMed] [Google Scholar]
- 21.Gu B, Liu C, Lin-Goerke J, Maley D R, Gutshall L L, Feltenberger C A, Del Vecchio A M. The RNA helicase and nucleotide triphosphatase activities of the bovine viral diarrhea virus NS3 protein are essential for viral replication. J Virol. 2000;74:1794–1800. doi: 10.1128/jvi.74.4.1794-1800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gualano R C, Pryor M J, Cauchi M R, Wright P J, Davidson A D. Identification of a major determinant of mouse neurovirulence of dengue virus type 2 using stably cloned genomic-length cDNA. J Gen Virol. 1998;79:437–446. doi: 10.1099/0022-1317-79-3-437. [DOI] [PubMed] [Google Scholar]
- 23.Heilek G M, Peterson M G. A point mutation abolishes the helicase but not the nucleoside triphosphatase activity of hepatitis C virus NS3 protein. J Virol. 1997;71:6264–6266. doi: 10.1128/jvi.71.8.6264-6266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 25.Irie K, Mohan P M, Sasaguri Y, Putnak R, Padmanabhan R. Sequence analysis of cloned dengue virus type 2 genome (New Guinea-C strain) Gene. 1989;75:197–211. doi: 10.1016/0378-1119(89)90266-7. [DOI] [PubMed] [Google Scholar]
- 26.Kapoor M, Zhang L W, Ramachandra M, Kusukawa J, Ebner K E, Padmanabhan R. Association between NS3 and NS5 proteins of dengue virus type 2 in the putative RNA replicase is linked to differential phosphorylation of NS5. J Biol Chem. 1995;270:19100–19106. doi: 10.1074/jbc.270.32.19100. [DOI] [PubMed] [Google Scholar]
- 27.Khromykh A A, Sedlak P L, Westaway E G. trans-complementation analysis of the flavivirus Kunjin NS5 gene reveals an essential role for translation of its N-terminal half in RNA replication. J Virol. 1999;73:9247–9255. doi: 10.1128/jvi.73.11.9247-9255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D W, Kim J, Gwack Y, Han J H, Choe J. Mutational analysis of the hepatitis C virus RNA helicase. J Virol. 1997;71:9400–9409. doi: 10.1128/jvi.71.12.9400-9409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J L, Morgenstern K A, Griffith J P, Dwyer M D, Thomson J A, Murcko M A, Lin C, Caron P R. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 30.Koonin E V, Dolja V V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- 31.Kuo M D, Chin C, Hsu S L, Shiao J Y, Wang T M, Lin J H. Characterization of the NTPase activity of Japanese encephalitis virus NS3 protein. J Gen Virol. 1996;77:2077–2084. doi: 10.1099/0022-1317-77-9-2077. [DOI] [PubMed] [Google Scholar]
- 32.Lain S, Riechmann J L, Garcia J A. RNA helicase: a novel activity associated with a protein encoded by a positive strand RNA virus. Nucleic Acids Res. 1990;18:7003–7006. doi: 10.1093/nar/18.23.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H T, Clum S, You S H, Ebner K E, Padmanabhan R. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J Virol. 1999;73:3108–3116. doi: 10.1128/jvi.73.4.3108-3116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C, Kim J L. Structure-based mutagenesis study of hepatitis C virus NS3 helicase. J Virol. 1999;73:8798–8807. doi: 10.1128/jvi.73.10.8798-8807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luking A, Stahl U, Schmidt U. The protein family of RNA helicases. Crit Rev Biochem Mol Biol. 1998;33:259–296. doi: 10.1080/10409239891204233. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie J M, Khromykh A A, Jones M K, Westaway E G. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 37.Matusan A E, Kelley P G, Pryor M J, Whisstock J C, Davidson A D, Wright P J. Mutagenesis of the dengue virus type 2 NS3 proteinase and the production of growth-restricted virus. J Gen Virol. 2001;82:1647–1656. doi: 10.1099/0022-1317-82-7-1647. [DOI] [PubMed] [Google Scholar]
- 38.Min K H, Sung Y C, Choi S Y, Ahn B Y. Functional interactions between conserved motifs of the hepatitis C virus RNA helicase protein NS3. Virus Genes. 1999;19:33–43. doi: 10.1023/a:1008184522153. [DOI] [PubMed] [Google Scholar]
- 39.Muylaert I R, Galler R, Rice C M. Genetic analysis of the yellow fever virus NS1 protein: identification of a temperature-sensitive mutation which blocks RNA accumulation. J Virol. 1997;71:291–298. doi: 10.1128/jvi.71.1.291-298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paolini C, De Francesco R, Gallinari P. Enzymatic properties of hepatitis C virus NS3-associated helicase. J Gen Virol. 2000;81:1335–1345. doi: 10.1099/0022-1317-81-5-1335. [DOI] [PubMed] [Google Scholar]
- 41.Paolini C, Lahm A, De Francesco R, Gallinari P. Mutational analysis of hepatitis C virus NS3-associated helicase. J Gen Virol. 2000;81:1649–1658. doi: 10.1099/0022-1317-81-7-1649. [DOI] [PubMed] [Google Scholar]
- 42.Preugschat F, Yao C W, Strauss J H. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J Virol. 1990;64:4364–4374. doi: 10.1128/jvi.64.9.4364-4374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pryor M J, Gualano R C, Lin B, Davidson A D, Wright P J. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J Gen Virol. 1998;79:2631–2639. doi: 10.1099/0022-1317-79-11-2631. [DOI] [PubMed] [Google Scholar]
- 44.Pryor M J, Wright P J. The effects of site-directed mutagenesis on the dimerization and secretion of the NS1 protein specified by dengue virus. Virology. 1993;194:769–780. doi: 10.1006/viro.1993.1318. [DOI] [PubMed] [Google Scholar]
- 45.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 931–959. [Google Scholar]
- 46.Schmid S R, Linder P. D-E-A-D protein family of putative RNA helicases. Mol Microbiol. 1992;6:283–291. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 47.Suzich J A, Tamura J K, Palmer-Hill F, Warrener P, Grakoui A, Rice C M, Feinstone S M, Collett M S. Hepatitis C virus NS3 protein polynucleotide-stimulated nucleoside triphosphatase and comparison with the related pestivirus and flavivirus enzymes. J Virol. 1993;67:6152–6158. doi: 10.1128/jvi.67.10.6152-6158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura J K, Warrener P, Collett M S. RNA-stimulated NTPase activity associated with the p80 protein of the pestivirus bovine viral diarrhea virus. Virology. 1993;193:1–10. doi: 10.1006/viro.1993.1097. [DOI] [PubMed] [Google Scholar]
- 49.Tan B H, Fu J, Sugrue R J, Yap E H, Chan Y C, Tan Y H. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology. 1996;216:317–325. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- 50.Teo K F, Wright P J. Internal proteolysis of the NS3 protein specified by dengue virus 2. J Gen Virol. 1997;78:337–341. doi: 10.1099/0022-1317-78-2-337. [DOI] [PubMed] [Google Scholar]
- 51.Utama A, Shimizu H, Hasebe F, Morita K, Igarashi A, Shoji I, Matsuura Y, Hatsu M, Takamizawa K, Hagiwara A, Miyamura T. Role of the DExH motif of the Japanese encephalitis virus and hepatitis C virus NS3 proteins in the ATPase and RNA helicase activities. Virology. 2000;273:316–324. doi: 10.1006/viro.2000.0417. [DOI] [PubMed] [Google Scholar]
- 52.van Regenmortel M H V, Fauqet C M, Bishop D H L, Arstens C E B, Estes M K, Lemon S M, Maniloff J, Mayo M A, McGeoch D J, Pringle C R, Wickner R B. Virus taxonomy. San Diego, Calif: Academic Press; 2000. [Google Scholar]
- 53.Wardell A D, Errington W, Ciaramella G, Merson J, McGarvey M J. Characterization and mutational analysis of the helicase and NTPase activities of hepatitis C virus full-length NS3 protein. J Gen Virol. 1999;80:701–709. doi: 10.1099/0022-1317-80-3-701. [DOI] [PubMed] [Google Scholar]
- 54.Warrener P, Tamura J K, Collett M S. RNA-stimulated NTPase activity associated with yellow fever virus NS3 protein expressed in bacteria. J Virol. 1993;67:989–996. doi: 10.1128/jvi.67.2.989-996.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wertman K F, Drubin D G, Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westaway E G, Blok J. Taxonomy and evolutionary relationships of flaviviruses. In: Gubler D J, Kuno G, editors. Dengue and dengue hemorrhagic fever. Wallingford, Conn: CAB International; 1997. pp. 147–173. [Google Scholar]
- 57.Westaway E G, Mackenzie J M, Kenney M T, Jones M K, Khromykh A A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao N, Hesson T, Cable M, Hong Z, Kwong A D, Le H V, Weber P C. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol. 1997;4:463–467. doi: 10.1038/nsb0697-463. [DOI] [PubMed] [Google Scholar]