Abstract
Resistance of human immunodeficiency virus type 1 (HIV-1) to antiretroviral agents results from target gene mutation within the pol gene, which encodes the viral protease, reverse transcriptase (RT), and integrase. We speculated that mutations in genes other that the drug target could lead to drug resistance. For this purpose, the p1-p6gag-p6pol region of HIV-1, placed immediately upstream of pol, was analyzed. This region has the potential to alter Pol through frameshift regulation (p1), through improved packaging of viral enzymes (p6Gag), or by changes in activation of the viral protease (p6Pol). Duplication of the proline-rich p6Gag PTAP motif, necessary for late viral cycle activities, was identified in plasma virus from 47 of 222 (21.2%) patients treated with nucleoside analog RT inhibitor (NRTI) antiretroviral therapy but was identified very rarely from drug-naïve individuals. Molecular clones carrying a 3-amino-acid duplication, APPAPP (transframe duplication SPTSPT in p6Pol), displayed a delay in protein maturation; however, they packaged a 34% excess of RT and exhibited a marked competitive growth advantage in the presence of NRTIs. This phenotype is reminiscent of the inoculum effect described in bacteriology, where a larger input, or a greater infectivity of an organism with a wild-type antimicrobial target, leads to escape from drug pressure and a higher MIC in vitro. Though the mechanism by which the PTAP region participates in viral maturation is not known, duplication of this proline-rich motif could improve assembly and packaging at membrane locations, resulting in the observed phenotype of increased infectivity and drug resistance.
Currently available combination antiretroviral therapy fails to achieve optimal suppression of viral replication in 20 to 45% of patients (21). A leading factor for failure is the development of resistance by mutation within the pol gene, encoding the viral reverse transcriptase (RT) and protease, which are the targets of currently used antiretroviral agents. In general, initial or primary mutations modify the active sites of these viral enzymes, followed by stepwise accumulation of secondary or compensatory mutations leading to restored enzyme functionality (5, 13).
Given the extreme plasticity of the human immunodeficiency virus type 1 (HIV-1) genome, we speculated that genetic changes at a distance could contribute to the process of drug resistance. For this purpose, we analyzed the p1-p6gag-p6pol region localized immediately upstream of pol. The p1 region carries structures regulating gag-pol frameshift activities. The p6gag region encodes a protein involved in the late viral cycle—Pol packaging, particle size determination, and budding (8, 11, 30, 31). The transframe protein encoded by the p6pol region acts as a regulator of protease activation (16, 18, 20). In addition, the p7-p1 and p1-p6 cleavage sites adapt to facilitate processing by a mutant protease (2, 9, 34). Thus, the p1-p6gag-p6pol region has the potential, by various mechanisms—greater Pol production through frameshift regulation, enhanced packaging of viral enzymes (p6Gag), or control of activation of the viral protease (p6Pol)—to induce downstream changes leading to resistance to antiretroviral agents through a mechanism of gene or protein dosing or titration. In the present study, we show that changes in the p6 region lead to a complex viral phenotype that includes increased infectivity and resistance to nucleoside analog RT inhibitors (NRTIs), that we attribute to changes in the p6Gag frame. This is counterbalanced by a delay in protein maturation and diminished viral release, a phenotype that we attribute to the corresponding transframe modification in p6Pol.
MATERIALS AND METHODS
Analysis of p1-p6gag-p6pol sequences.
RNA from plasma virions from HIV-1 infected patients (n = 296) was isolated, reverse transcribed, amplified via nested PCR, and sequenced as previously described (5). Samples were collected in Switzerland from patients undergoing genotypic analysis of resistance. Only one sequence per patient was included in the analysis.
Site-directed mutagenesis, viral production, and resistance testing.
A 9-nucleotide insertion was introduced by site-directed mutagenesis (Quickchange; Stratagene, Basel, Switzerland) in the NL4-3 laboratory viral strain. This insertion codes for Ala-Pro-Pro in the Gag frame and for Ser-Pro-Thr in the transframe Pol. The resulting construct is hereafter described as APP/SPT recombinant. Viruses were obtained by HeLa cell transfection (GenePORTER transfection reagent; Axon labs, Baden, Switzerland). Constructs were confirmed by sequencing. Analysis of the susceptibility phenotype to RT inhibitors used a standardized recombinant virus susceptibility assay (12).
Particle release.
Subconfluent COS-7 cells were transfected with 2 μg of the pNL4-3 or APP/SPT clones in six-well plates. Efficiency of particle release was determined by measurement of HIV-1 p24 antigen enzyme-linked immunosorbent assay (ELISA) (Abbott, North Chicago, Ill.) in supernatant at 7, 9, 19, 24, 30, 41, and 45 h posttransfection.
One-cycle infectivity assay.
GHOST cells (stable transduced with chemokine receptor CXCR4 and with the green fluorescence protein under the control of the HIV-1 long terminal repeat; provided by D. Littman and V. K. Ramani, AIDS Research and Reference Reagent Program) were seeded in 48-well plates (3 × 104 cells/well). Infection was done in triplicate (inoculum of 3,000 pg of p24 antigen) in 300 μl of Dulbecco's modified Eagle's medium supplemented with Glutamax (2 mM; GIBCO Life Technologies, Basel, Switzerland), gentamicin (50 μg/ml), and 10% (vol/vol) fetal calf serum (FCS) (GIBCO) by a spinoculation technique: 3 h of centrifugation with 1,500 × g at 22°C, in the presence of Polybrene (20 μg/ml) (1). The experiment was performed with concentrations of zidovudine ranging from 500 to 0 nM, including a preincubation time of 2 h in the presence of drug. Cells were trypsinized 24 h postinfection, harvested in 3 ml of phosphate-buffered saline–5% FCS–2 mM EDTA, and resuspended in 250 μl of cellFIX solution (Becton Dickinson, Erembodegem, Belgium). The infectious titer was determined by fluorescence-activated cell sorting analysis as the proportion of green fluorescence protein-positive cells.
Competitive replication assay.
Fitness determination was performed by growth competition experiments as previously described (33).Viral stocks were titrated by endpoint dilution in MT-2 cells to calculate 50% tissue culture infective doses per ml. Cultures between viruses APP/SPT and NL4-3 were carried out at an initial proportion of 1:1 during four passages in two replicas. MT-2 cells (104) were infected at a multiplicity of infection of 0.1, both in the absence and in presence of 0.1 μM zidovudine. For the next passage, fresh MT-2 cells were infected with 10 μl of supernatant of the preceding passage. After isolation of viral RNA, reverse transcription and amplification were performed using 118MIGU (5′AGACAGGCTAATTTTTTAGGGAA) and 117MIGD (5′CCCCAGACCTGAAGCTCTCT); PCR products, differing in size, were resolved in 20% acrylamide gels; and the proportion of each virus in the competition was estimated by densitometry of specifics bands. Fitness lines were obtained from the representation of the ratio of the competing virus APP/SPT to the NL4-3 in each passage, referred to as the initial proportion (Rn/Ro). The fitness lines were obtained by linear regression and the slope represents the fitness of the APP/SPT virus in relation to NL4-3.
RT activity.
The RT activity assay was carried out according to the manufacturer's protocol (Lenti RT activity assay; Cavidi Tech, Uppsala, Sweden). Briefly, 50 μl of filtered culture supernatant was serially diluted (1/5, 1/25, 1/125) and added to a 96-well plate, coated with poly(rA) (enzyme template), with 150 μl of reaction solution containing BrdUTP as the enzyme substrate. Polymerization was allowed to proceed for 3 h at 33°C. Immunological product detection with alkaline phosphatase (AP)-conjugated anti-BrdU antibody was carried out at 33°C, during 90 min. Bound antibody was determined colorimetrically with an AP substrate, para-nitrophenyl phosphate, in a standard microtiter plate reader (405 nm), at 0.5, 1, 2, 4, and 6 h after addition of the AP substrate.
Protein analysis.
At 20 h posttransfection, COS-7 cells were metabolically labeled with 2 ml of Dulbecco's modified Eagle's medium (methionine-cysteine-free, 10% dialyzed FCS) containing 50 μCi of [35S]methionine-[35S]cysteine/ml (35S : Easy Tag EXPRESS; NEN, Life Science Products, Boston, Mass.). Samples were collected at 23, 26, 29, 32, 35, 38, 41, 44, and 57 h posttransfection. To analyze cell-associated viral proteins, transfected cells were washed with phosphate-buffered saline, lysed in 500 μl of radioimmunoprecipitation assay (RIPA) buffer (1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS], 2 mM EDTA, 150 mM NaCl, 50 mM Tris-HCl, pH 8) supplemented with complete protease inhibitor cocktail (Roche Diagnostics), and centrifuged 30 min at 21,000 × g at 4°C. Viral proteins were immunoprecipitated from lysates using anti-HIV human immunoglobulin G (NIH AIDS Research and Reference Reagent Program) and protein A-Sepharose CL-4B beads (Amersham Pharmacia Biotech) and separated by SDS-polyacrylamide gel electrophoresis (PAGE) (5 to 15% gradient gel). To analyze particle-associated proteins, virions in the culture supernatant were concentrated through a 20% sucrose cushion by ultracentrifugation (90 min with a centrifugal force of 100,000 × g, at 4°C) and lysed in 200 μl of RIPA buffer. RT content was determined by quantification of bands counts with Instant Imager (Electronic Autoradiography; Packard Instrument Company, Meriden, Conn.). RT content was normalized to p24 antigen content and expressed as picograms of RT per nanogram of p24.
For pulse-chase analysis, COS-7 cells were metabolically labeled 48 h posttransfection for 1 h (pulse). Chase samples were collected 1, 2, 3, and 6 h postpulse.
Protease autoprocessing.
Autocleavage efficiency was assessed by expression of an nucleocapsid-transframe-p6Pol-protease (NC-TF-p6-PR) polyprotein in a transcription and translation TNT T7 rabbit reticulocyte lysate (Promega, Madison, Wis.), following a published protocol (35). Inserts were cloned into BamHI and EcoRI sites of pET3 vector (Novagen, Madison, Wis.). Samples collected after 30, 45, 60, and 90 min of reaction were resolved by SDS-PAGE, and processing efficiency was evaluated by Instant Imager.
Electron microscopy.
Transfected cells grown on glass coverslips were fixed with 2% glutaraldehyde in 0.1 M Sörensen phosphate buffer, pH 7.4, for 60 min at 4°C. Cells were postfixed in 2% osmium tetroxide in Sörensen buffer at room temperature for 1 h, dehydrated in ethanol, and embedded in Epon. Embedded cells were separated from the coverslips after short treatment with liquid nitrogen and cut parallel to the substrate using a Leica Ultracut UCT microtome. The ultrathin sections were placed on Formvar-carbon-coated grids and stained with uranyl acetate and lead citrate. Grids were examined with a Philips CM10 electron microscope at 80 kV using a 30- to 50-μm-objective aperture. Size and maturation of viral particles were evaluated independently by two researchers blind to the nature of the viral clone used for transfection.
RESULTS
Polymorphism in p6.
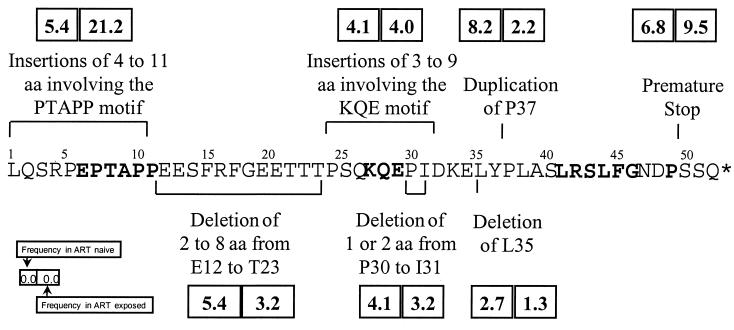
p1-p6gag-p6pol sequence analysis of plasma virus from 296 HIV-1-infected patients demonstrated more extensive polymorphism than previously reported (17, 29). Overall, 274 (93%) p6 sequences were unique and corresponded generally to HIV-1 subtype B (78%). Only seven (2%) sequences were classified as wild type (identical to reference NL4-3). There were numerous insertions and deletions (Fig. 1). Insertions were identified preferentially in the first 11 amino acids of p6Gag (with corresponding changes in the Pol frame), generally involving the polyproline motif Pro-Thr-Ala-Pro (PTAP). Extensive single-amino-acid polymorphism was observed throughout p6 (not shown) and exhibited no association with exposure to antiretroviral agents, the only exception being I31K,R,T,V (5.4% in naïve versus 14.4% in exposed; P = 0.043). Duplication of P37 was more frequently identified among treatment-naïve individuals, P = 0.042.
FIG. 1.
Polymorphism of p6Gag. Insertions and deletions in the p6Gag region in plasma viruses from HIV-1-infected individuals. Shown are the frequencies of different polymorphisms in plasma viruses from naïve (n = 74) versus antiretroviral-experienced patients (n = 222) (box and inset). The difference in frequency of insertions involving the PTAP motif reached statistical significance (P = 0.002). The LXSLF motif, involved in HIV-1 VPR packaging, was only rarely modified by single nucleotide polymorphism, and it was never a subject of insertion or deletion. Conserved p6Gag residues among HIV-1 M strains are shown in boldface type. Representative nucleotide sequences of p6 polymorphisms have been submitted to GenBank under accession no. AF282959 to AF282969.
Duplication of PTAP (most frequently PTAPPAPP) was identified in 44 of 222 (21.2%) patients, all of which had been exposed to NRTI prior to current treatment. Duplication of PTAP was identified in only 4 of 74 (5.4%) of treatment-naive individuals (P = 0.002) (Fig. 1). Furthermore, infection in two of the four treatment-naive patients carrying an APP/SPT duplication was suggestive of transmission of a drug-resistant or -exposed strain. This was determined in one patient by the identification of RT substitution T215D, characteristic of prior zidovudine exposure, and in the second patient by identification of the transmission source, exposed to NRTI, who carried a virus with the same p6 insertion.
Longitudinal analysis of stored plasma samples from three patients carrying viruses with a APP/SPT duplication was done to identify the pattern of selection of this particular insertion (Table 1). The duplication could be observed after 3 months of therapy, as the first mutation selected under NRTI pressure, or could emerge during the stepwise process of accumulation of resistance mutations leading to high-level NRTI resistance.
TABLE 1.
Longitudinal analysis of the selection of APP/SPT duplication in plasma virus from three patients receiving antiretroviral drug treatment
| Patient no. | No. of mo on ARTb | ARTa | Protein
|
Viremia (log RNA copies/ml) | ||
|---|---|---|---|---|---|---|
| p6 | PR | RT | ||||
| 51335 | Baseline | WT | L63P | WT | NAc | |
| 3 | DDI | APP/SPT | L63P | WT | NA | |
| 14 | D4T | APP/SPT | L63P | WT | NA | |
| 26 | D4T + 3TC + RTV | APP/SPT | L63P, V77I, V82A | M184V | 5.0 | |
| 38 | None | APP/SPT | L63P | WT | 4.5 | |
| 50418 | Baseline | WT | WT | WT | NA | |
| 14 | AZT | WT | WT | K70R | NA | |
| 33 | AZT + DDI | WT | WT | M41L, L210W, T215Y | NA | |
| 61 | AZT + DDI | APP/SPT | M36I | M41L | 4.7 | |
| 75 | AZT + 3TC | APP/SPT | M36I | M41L, D67N, M184V, T215Y, K219R | 4.2 | |
| 86 | D4T + RTV + SQV | APP/SPT | WT | M41L, D67N, M184V, T215Y, K219R | <2.6 | |
| 50220 | Baseline | WT | L63H | WT | NA | |
| 16 | AZT | WT | L63H | M41L, T215Y | NA | |
| 42 | DDI | WT | L63H | M41L, T215Y | NA | |
| 76 | AZT + 3TC | APP/SPT | L63H | M41L, M184V, T215Y | 4.5 | |
| 94 | AZT + 3TC | APP/SPT | L10I, L63H | M41L, D67N, M184V, T215Y | 5.3 | |
| 106 | D4T + RTV + SQV | WT | L63H | M41L, D67N, T215Y | 4.3 | |
ART, antiretroviral treatment; AZT, zidovudine; 3TC, lamivudine; DDI, didanosine; D4T, stavudine; RTV, ritonavir; SQV, saquinavir.
The first observation of APP/SPT selection is shown in boldface type.
NA, not available.
Early viral cycle phenotype of APP/SPT clones.
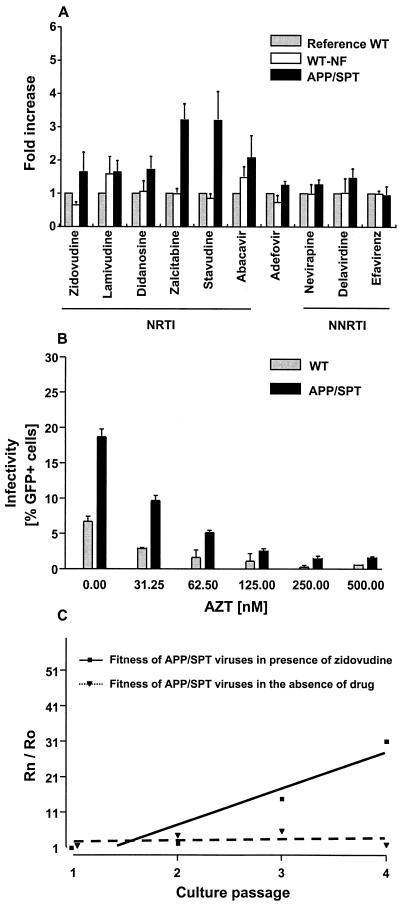
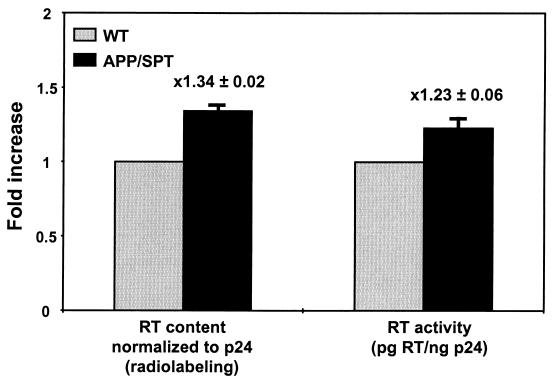
To better understand the role of PTAP motif insertions in the process of drug resistance, a duplication of amino acids APP/SPT was introduced by site-directed mutagenesis in the HIV-1 drug-susceptible laboratory strain NL4-3. Upon susceptibility testing, APP/SPT clones demonstrated an increase (mean ± standard error of the mean [SEM]) of (2.2 ± 0.3)-fold in resistance to NRTIs compared to NL4-3, without significant change in susceptibility to nonnucleoside RT inhibitors (mean ± SEM, 1.3 ± 0.1) (Fig. 2A). Thereafter, APP/SPT clones were tested in a one-cycle infectivity assay in the presence of zidovudine (Fig. 2B). After p24 antigen-normalized input, there was greater infectivity of GHOST cells (mean ± SEM, [1.9 ± 0.1]-fold increase) by APP/SPT virus in the presence or absence of zidovudine in comparison to the wild-type parental virus. Similar results were obtained with use of stavudine (data not shown). The modest increase in resistance to NRTIs led to APP/SPT clones effectively outgrowing the parental NL4-3 virus in the presence of subinhibitory concentrations of zidovudine (Fig. 2C). In this competitive kinetics assay performed over four culture passages in the presence or the absence of 100 nM of zidovudine, the slope of the regression line, representing the relative fitness of the APP clone, was 10.3 ± 2.5 in the presence of zidovudine and 0.34 ± 1.2 in the absence of zidovudine, compared to the fitness of the wild-type NL4-3. Analysis of protein content in virions by SDS-PAGE of radiolabeled particles revealed a mean 34% greater incorporation of RT in APP/SPT virions compared to parental NL4-3 (Fig. 3). This correlated with a 23% increase in reverse transcription (Fig. 3). Thus, the infectivity and growth advantage of APP/SPT clones observed in the presence of antiretroviral pressure correlates with the greater content and activity of RT in virions.
FIG. 2.
Evaluation of APP/SPT clones under antiretroviral selective pressure. (A) Analysis of the susceptibility phenotype to RT inhibitors used a standardized recombinant virus susceptibility assay. Shown are the mean changes in drug susceptibility for four independent clones carrying an APP/SPT duplication and three wild-type clones that were exposed to the site-directed mutagenesis process (WT-NF), in comparison to the parental NL4-3 strain (reference WT). Bars represent the SEM. (B) Clones carrying the APP/SPT duplication exhibited a greater infectivity of GHOST cells at any concentration of zidovudine (AZT) than the parental NL4-3 (WT). (C) A marked growth advantage of APP/SPT clones was observed when they were subjected to competition against the wild-type parental strain NL4-3 in the presence of 100 nM zidovudine. Shown is a representative competition from two independent experiments, performed over four culture passages. The slope of the regression line, representing the relative fitness of the APP/SPT clone was 10.3 ± 2.5 (in the presence of zidovudine; R2= 0.90) and 0.34 ± 1.2 (in the absence of zidovudine; R2= 0.04) compared to the fitness of the wild-type NL4-3. Rn, ratio at passage n; Ro, ratio at baseline.
FIG. 3.
Virion RT content and activity. RT content was determined by analysis of radiolabeled proteins from virions separated by SDS-PAGE. RT was quantified by radioimaging and expressed as a ratio of RT to p24. RT activity of viral particles was determined by analysis of reversion of an artificial RNA substrate, and the activity was expressed as picograms of RT per nanogram of p24 antigen. Shown are the means and SEMs (error bars) from triplicate APP/SPT clone data sets.
Late viral cycle phenotype of APP/SPT clones.
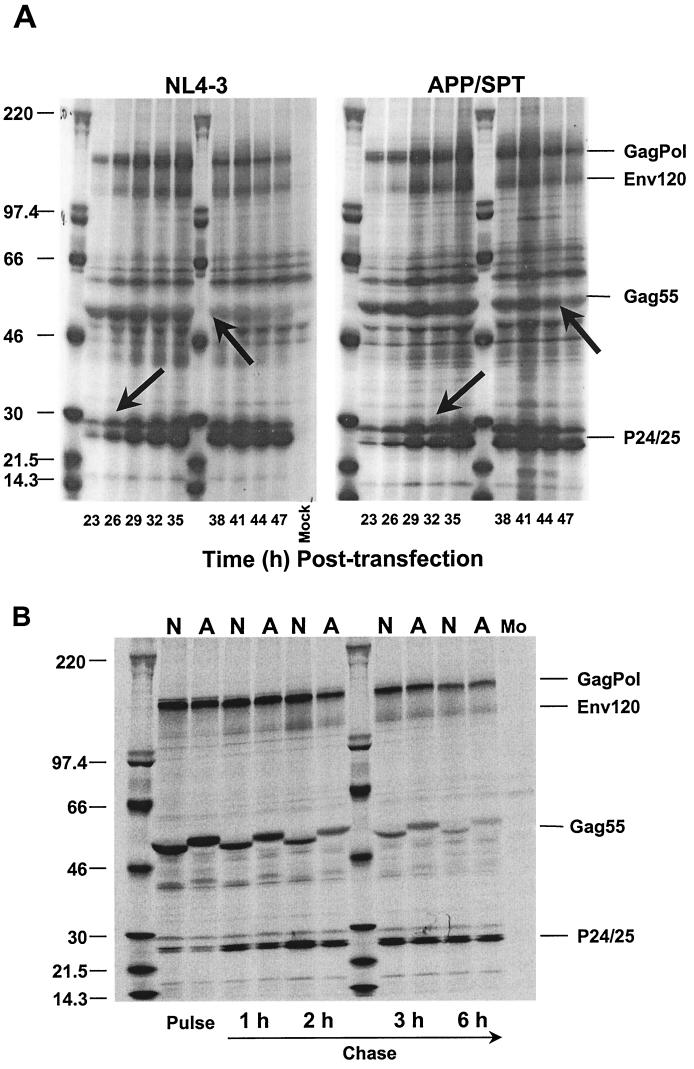
Analysis of protein maturation of APP/SPT clones identified a 3- to 6-h delay in the maturation process (Fig. 4A). However, the rate of Gag cleavage, as estimated by pulse-chase experiments, was comparable for the mutant and the parental NL4-3 virus (Fig. 4B). The observed delay could not be explained by a perturbation in autoprocessing of the protease, leading to a delay in initiation of cleavage (Fig. 4C). The rate of protease autocleavage, estimated by using an NC-TF-p6-PR expression vector in a reticulocyte lysate TNT system, indicates a similar rate of processing of the SPT precursor compared to wild-type precursor (19.5% versus 16% after 30 min, and 6.5% versus 5% of unprocessed precursor after a 45-min reaction).
FIG. 4.
Evaluation of late cycle phenotype of APP/SPT clones. (A) Protein maturation is delayed in APP/SPT clones by up to 6 h, as shown by radiolabeling experiments. The delay was estimated by the timing of completion of processing of the Gag55 precursor and of precursor p25 to mature p24 equimolarity (arrows). However, pulse-chase labeling experiments (B) indicated that once initiated, the rate of Gag cleavage was comparable for both APP/SPT (A) and wild-type (N) clones. The 3-amino-acid insertion in APP/SPT clones leads to a characteristic upward shift of Gag55. (C) The rate of protease autocleavage, estimated by using an NC-TF-p6-PR expression vector in a reticulocyte lysate transcription and translation (TNT) system, did not identify changes in the rate of processing of the SPT precursor (SPT is the 3-amino-acid duplication in the Pol frame corresponding to APP in the Gag frame) compared to wild-type precursor. Shown in panel C are results of TNT after 30 min of transcription-translation, including the unprocessed product of a D25E construct, generating an inactive protease (PR−).
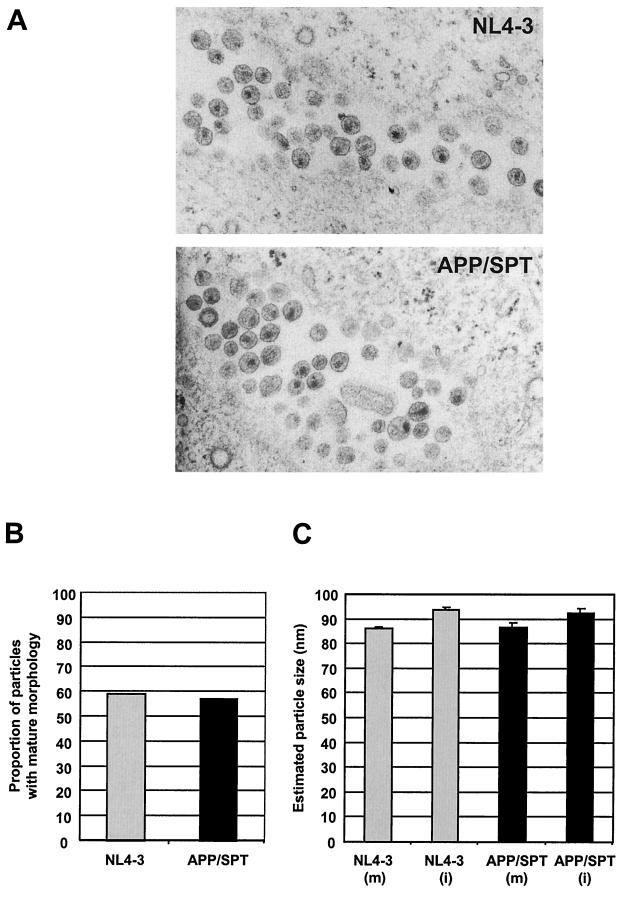
The observed modification was associated with a diminution in APP/SPT viral particle release, with a mean ± SEM at 45 h posttransfection of 17,007 ± 1,818 pg of p24/ml, versus 32,617 ± 3,520 pg of p24/ml for NL4-3 (Fig. 5A). Analysis of APP/SPT particles by SDS-PAGE (Fig. 5B) and by electron microscopy (Fig. 6A) revealed no apparent maturation, structural, or budding anomalies. Upon semiquantitative evaluation of NL4-3 and APP/SPT particles (n = 273), no differences were observed in the proportion of APP/SPT viral particles with mature morphology (57 versus 59% for NL4-3; P not significant [Fig. 6B]) or in diameter (mean ± SEM of 86.8 ± 1.7 and 92.6 ± 1.6 nm for mature and immature APP/SPT particles, respectively, compared to 86.2 ± 0.7 and 93.8 ± 1.0 nm, respectively, for NL4-3; P not significant [Fig. 6C]).
FIG. 5.
Particle release. (A) Analysis of the efficiency of particle release demonstrated a diminished production of viral particles of APP/SPT clones in the absence of drug pressure (shown are means and SEMs [error bars] of analyses in triplicate). (B) On SDS-PAGE, APP/SPT virions displayed a normal pattern of protein maturation. Shown are two independent clones (clones A and B) for NL4-3 and APP/SPT.
FIG. 6.
Electron microscopy. (A) Transfected COS-7 cells grown on glass coverslips were fixed and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate. No major abnormalities in APP/SPT particle morphology or in budding were observed. Upon semiquantitative evaluation of particles, no differences were observed in the proportion of virions with mature morphology, 57% for APP/SPT versus 59% for NL4-3 (B), or in diameter, mean ± SEM of 86.8 ± 1.7 and 92.6 ± 1.6 nm for mature (m) and immature (i) APP/SPT particles, compared to 86.2 ± 0.7 nm (m) and 93.8 ± 1.0 nm (i) for NL4-3 (C).
GenBank accession no.
Representative nucleotide sequences of p6 polymorphisms have been submitted to GenBank under accession no. AF282959 to AF282969.
DISCUSSION
Analysis of a large number of p1-p6gag-p6pol sequences from plasma virus from HIV-1-infected patients demonstrated more extensive polymorphism than previously reported (17,29), in particular due to the presence of numerous insertions and deletions. Insertions were identified preferentially in the first 11 amino acids of p6Gag (with corresponding changes in the Pol frame), involving the late assembly (L) domain of HIV-1, the motif PTAP. Deletion of PTAP results in the generation of noninfectious immature viral particles carrying a diminished content in Pol and retained by a tether to the cellular surface (8, 11, 30, 31).
Duplication of the initial 11 amino acids of p6 has been rarely reported in the genome databases or the literature. We only identified 15 sequences with this phenomenon among an estimated 5,800 HIV-1 Gag sequences submitted to the GenBank. Two entries correspond to HIV-1 proviral clones BRU and BH10 (GenBank accession no. K02013 and K02083), a third entry corresponds to a Nef-deficient attenuated HIV-1 strain (GenBank accession no. U37270), and nine entries correspond to isolates reported since 1990 (GenBank accession no. L11803, L03705, L11799, U46016, AF247522, L03705, AF067154, D86068, and AJ006287). Three sets of isolates were obtained from patients exposed to antiretrovirals, or from vertical transmission of the PTAP duplication from a mother exposed to zidovudine to her child (GenBank accession no. AF024003, AJ271445, and U53633). In contrast to the paucity of the PTAP duplication phenomenon in the databases, the present study identified this event (most frequently PTAPPAPP) in plasma virus from one-fifth of patients exposed to antiretroviral therapy but very rarely from treatment-naïve individuals, except as result from transmission of a drug resistant strain. The p6 duplication could be the first mutation selected under NRTI pressure or emerge during the stepwise process of accumulation of resistance mutations, leading to high-level NRTI resistance. The strongest association was found for stavudine and didanosine therapy, where 6 of 16 patients (37.5%) failing bitherapy with these antiretroviral drugs carried viruses with the PTAP duplication. This is of particular interest given the limited understanding of the mechanisms of HIV-1 resistance to stavudine and didanosine (7, 22). We observed that the PTAP duplication may persist in plasma for extended periods after transmission or after treatment discontinuation, thus serving as marker of drug resistance by indicating previous exposure to NRTI agents.
When compared to the parental HIV-1 drug-susceptible laboratory strain NL4-3, molecular clones carrying a duplication of amino acids APP/SPT exhibited a modest decrease of susceptibility to NRTIs and improved early viral cycle activities (as reflected by an increased infectivity of GHOST cells). This phenotype is reminiscent of the inoculum effect described in bacteriology, where a larger input, or a greater infectivity of an organism with a wild-type antimicrobial target, leads to escape from drug pressure and a higher MIC in vitro (6). This allowed APP/SPT clones to effectively outgrow the parental NL4-3 virus in the presence of drug selective pressure. The observed resistance phenotype correlated with a greater incorporation of RT and an increased RT activity in APP/SPT particles compared to parental NL4-3.
APP/SPT clones exhibited a delay in initiation of the protein maturation process. We speculated that the duplication in p6Gag, which corresponds to a Ser-Pro-Thr duplication in p6Pol, could have delayed the release of the fully activated protease by disturbing autoprocessing of the nearby p1-p6Pol and p6Pol-protease scissile bonds (16, 18, 20). However, we did not identify a defect in autoprocessing of the protease by in vitro analysis of autocleavage efficiency. The observed modification in protein maturation led to production of lower amounts of viral particles. Thus, we conclude that in the absence of antiretroviral pressure, the perturbation of late viral cycle activities by an unclear mechanism offsets the early cycle benefit of APP/SPT viral particles. This would account for the rarity of wild-type viruses carrying an insertion at the PTAP motif in nature.
The phenomenon of viral escape from selective pressure described herein could also correspond to the general mechanism of drug target gene dosing associated with antimicrobial drug resistance in bacteria, parasites, and fungi. Well-known examples are the resistance of Mycobacterium tuberculosis to isoniazid through up-promoter mutations in inhA that increase the enoyl reductase drug target, resistance in the parasite Leishmania donovani to methotrexate through dihydrofolate reductase target amplification, and resistance in Candida glabrata through overexpression of the 14α-demethylase (4, 15, 27). Gene amplification has been described for vaccinia virus, where duplication of the virus-encoded small subunit of the ribonucleotide reductase gene, M2, allows escape from selective pressure of hydroxyurea (25). Otherwise, resistance to antiviral drugs has been almost exclusively due to mutation in target viral genes or in genes involved in intracellular activation of the drug. Examples include resistance to acyclovir, ganciclovir, or foscavir in herpesvirus; resistance to lamivudine in hepatitis B virus through mutation in the viral DNA polymerase; and resistance to acyclovir or ganciclovir through mutations in the herpes simplex- or varicella zoster virus-encoded thymidine kinase or in the cytomegalovirus-encoded phosphotransferase (3). However, the explanation of the observed phenotype by invoking a mechanism of gene or protein dosing can be challenged: titration of the enzyme (RT), already in apparent excess in the virion (approximately 100 molecules per particle), will not change the ratio of chain terminator (e.g., zidovudine) to deoxynucleoside triphosphate.
Thus, identification of improved packaging of Pol may be an epiphenomenon of more-relevant changes in the maturation, budding, or function of the APP/SPT viruses leading to greater infectivity and to drug resistance. Interference with function of the L domain or blocking of budding with proteasome inhibitors is associated with release of tightly linked multimers of particles (19, 32). Thus, an L domain mutant might release clusters of particles that have a higher-than-normal specific infectivity because they contain multiple copies of the viral genome. In the case of Sindbis virus, mutants have been identified that package large numbers of nucleocapsids and are highly infectious even though particle release is decreased nearly 10-fold (10). However, by using electron microscopy, we failed to observe changes in particle release or in nucleocapsid content that would support this hypothesis.
Despite the modest benefit conferred by p6 insertions in vitro, its high prevalence in vivo underscores its fitness value. This is in keeping with the behavior of most single RT and protease mutations selected under drug pressure (e.g., Thr215Tyr in the RT) that confer modest increases of 50% inhibitory concentrations in in vitro resistance testing and, however, are central to viral escape in vivo and to the multistep process of selection of high level resistant mutants (23). Since the selective advantage of PTAP duplication results in a highly prevalent mechanism of viral escape in the patient population, this could also suggest that the effective intracellular levels achieved by NRTIs are only marginally above the level necessary to control viral replication.
The mechanism by which the PTAP region participates in Pol packaging has not been defined. Proline-rich sequences are commonly found in situations requiring rapid recruitment of proteins, such as during initiation of transcription, signaling cascades, and cytoskeletal rearrangements (14). Recent work has underscored the role of PTAP in ubiquitination of HIV-1 Gag (26, 28). Duplication of polyproline motifs could improve cellular protein recruitment at membrane locations, modulating viral assembly and enhancing Pol incorporation into the budding virion. The description of a p6 antisense oligomer that successfully blocks HIV-1 replication and the ongoing drug discovery efforts based on polyproline peptidomimetics (14, 24) highlight the interest in expanding knowledge on this incompletely explored region of the HIV-1 genome.
ACKNOWLEDGMENTS
This work was supported by grants from the Swiss National Science Foundation and the Santos Suarez Foundation (to A.T.) and the FIS (to C.L.-G.).
We thank D. Richman and H. Göttlinger for comments, G. Greub and D. Bugnon for statistical analysis, V. Soriano for clinical material, and T. Klimkait for pNL-NF.
ADDENDUM IN PROOF
Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, has been recently described as binding the PTAP domain of HIV-1 p6 (L. Verplank, F. Bouamr, T. J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C. A. Carter, Proc. Natl. Acad. Sci. USA 98:7724–7729, 2001). In this paper, the p6 region from pBH10, a HIV-1 clone that carries two PTAP motifs, was used in a yeast two-hybrid assay. Deletion of the first motif reduced Tsg101 binding by 50%, deletion of the second PTAP motif reduced binding by 25%, and deletion of both eliminated binding. These results underscore the biological relevance of PTAP motif duplication.
REFERENCES
- 1.Bahnson A. Centrifugal enhancement of retroviral mediated gene transfer. J Virol Methods. 1995;54:131–134. doi: 10.1016/0166-0934(95)00035-s. [DOI] [PubMed] [Google Scholar]
- 2.Bally F, Martinez R, Peters S, Sudre P, Telenti A. Polymorphism of HIV-1 Gag p7/p1 and p1/p6 cleavage sites. Clinical significance and implications for resistance to protease inhibitors. AIDS Res Hum Retrovir. 2000;16:1209–1213. doi: 10.1089/08892220050116970. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini J, Naesens L, De Clercq E. New antivirals: mechanism of action and resistance development. Curr Opin Microbiol. 1998;1:535–546. doi: 10.1016/s1369-5274(98)80086-6. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um S K, Wilson T, Collins D, De Lisle G, Jacobs W R., Jr inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 5.Bleiber G, Munoz M, Ciuffi A, Meylan P, Telenti A. Individual contribution of protease and reverse transcriptase to infectivity, replication and protein maturation of antiretroviral drug-resistant human immunodeficiency virus type 1. J Virol. 2001;75:3291–3300. doi: 10.1128/JVI.75.7.3291-3300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook I. Inoculum effect. Rev Infect Dis. 1989;11:361–368. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- 7.Coakley E P, Gillis J M, Hammer S M. Phenotypic and genotypic resistance patterns of HIV-1 isolates derived from individuals treated with didanosine and stavudine. AIDS. 2000;14:F9–F15. doi: 10.1097/00002030-200001280-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dettenhoffer M, Yu X-F. Proline residues in human immunodeficiency virus type 1 p6Gag exert a cell type-dependent effect on viral replication and virion incorporation of Pol proteins. J Virol. 1999;73:4696–4704. doi: 10.1128/jvi.73.6.4696-4704.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyon L, Croteau G, Thibeault D, Poulin F, Pilote L, Lamarre D. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J Virol. 1996;70:3763–3769. doi: 10.1128/jvi.70.6.3763-3769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaedigk-Nitschko K, Schlesinger M J. Site-directed mutations in Sindbis virus E2 glycoprotein's cytoplasmic domain and the 6K protein lead to similar defects in virus assembly and budding. Virology. 1991;183:206–214. doi: 10.1016/0042-6822(91)90133-v. [DOI] [PubMed] [Google Scholar]
- 11.Göttlinger H G, Dorfman T, Sodroski J G, Haseltine W A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci USA. 1991;88:3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hertogs K, de Bethune M P, Ivens T, Schel P, Van Cauwenberge A, Van den Eynde C, Van Derwen V, Azijn H, Van Houtte M, Peeters F, Staszewski S, Conant M, Bloor S, Kemp S, Larder B, Pauwels R. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother. 1998;42:269–276. doi: 10.1128/aac.42.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch M S, Brun-Vezinet F, D'Aquila R T, Hammer S M, Johnson V A, Kuritzkes D R, Loveday C, Mellors J W, Clotet B, Conway B, Demeter L M, Vella S, Jacobsen D M, Richman D D. Antiretroviral drug resistance testing in adult HIV-1 infection: recommendations of an International AIDS Society-USA panel. JAMA. 2000;283:2417–2426. doi: 10.1001/jama.283.18.2417. [DOI] [PubMed] [Google Scholar]
- 14.Kay B K, Williamson M P, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- 15.Kündig C, Leblanc E, Papdopoulou B, Ouellette M. Role of the locus and of the resistance gene on gene amplification frequency in tethotrexate resistant Leishmania tarentolae. Nucleic Acids Res. 1999;27:3653–3659. doi: 10.1093/nar/27.18.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis J M, Clore G M, Gronenborn A M. Autoprocessing of HIV-1 protease is tightly coupled to protein folding. Nat Struct Biol. 1999;6:868–875. doi: 10.1038/12327. [DOI] [PubMed] [Google Scholar]
- 17.Louwagie J, McCutchan F E, Peeters M. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993;7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Partin K, Zybarth G, Ehrlich L, DeCrombrugghe M, Wimmer E, Carter C. Deletion of sequences upstream of the proteinase improves the proteolytic processing of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1991;88:4776–4780. doi: 10.1073/pnas.88.11.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patnaik A, Chau V, Wills J W. Ubiquitin is part of the retrovirus budding machinery. Proc Natl Acad Sci USA. 2000;97:13069–13074. doi: 10.1073/pnas.97.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulus C, Hellebrand S, Tessmer U, Wolf H, Kräusslich H-G, Wagner R. Competitive inhibition of human immunodeficiency virus type-1 protease by the Gag-Pol transframe protein. J Biol Chem. 1999;274:21539–21543. doi: 10.1074/jbc.274.31.21539. [DOI] [PubMed] [Google Scholar]
- 21.Perrin L, Telenti A. HIV treatment failure: testing for HIV resistance in clinical practice. Science. 1998;280:1871–1873. doi: 10.1126/science.280.5371.1871. [DOI] [PubMed] [Google Scholar]
- 22.Petropoulos C J, Parkin N T, Limoli K L, Lie Y S, Wrin T, Huang W, Tian H, Smith D, Winslow G A, Capon D J, Whitcomb J M. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000;44:920–928. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schinazi R F, Larder B, Mellors J W. Mutation in retroviral genes associated with drug resistance: 2000–2001 update. Int Antivir News. 2000;8:65–91. [Google Scholar]
- 24.Sei S, Yang Q E, O'Neill D, Yoshimura K, Nagashima K, Mitsuya H. Identification of a key target sequence to block human immunodeficiency virus type 1 replication within the gag-pol transframe domain. J Virol. 2000;74:4621–4633. doi: 10.1128/jvi.74.10.4621-4633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slabaugh M B, Roseman N A, Mathews C K. Amplification of the ribonucleotide reductase small subunit gene: analysis of novel joints and the mechanism of gene duplication in vaccinia virus. Nucleic Acids Res. 1989;17:7073–7088. doi: 10.1093/nar/17.17.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strack B, Calistri A, Accola M A, Palu G, Göttlinger H G. A role for ubiquitin ligase recruitment in retrovirus release. Proc Natl Acad Sci USA. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanden Bossche H, Marichal H P, Odds F, Le Jeune L, Coene M-C. Characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother. 1992;36:2602–2610. doi: 10.1128/aac.36.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogt V M. Ubiquitin in retrovirus assembly: actor or bystander? Proc Natl Acad Sci USA. 2000;97:12945–12947. doi: 10.1073/pnas.97.24.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura F K, Diem K, Learn G H, Riddell S, Corey L. Intrapatient sequence variation of the gag gene of human immunodeficiency virus type 1 plasma virions. J Virol. 1996;70:8879–8887. doi: 10.1128/jvi.70.12.8879-8887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu X-F, Dawson L, Tian C-J, Flexner C, Dettenhofer M. Mutations of the human immunodeficiency virus type 1 p6Gag domain results in reduced retention of Pol proteins during virus assembly. J Virol. 1998;72:3412–3417. doi: 10.1128/jvi.72.4.3412-3417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X-F, Matsuda Z, Yu Q-C, Lee T-H, Essex M. Role of the C terminus Gag protein in human immunodeficiency virus type 1 virion assembly and maturation. J Gen Virol. 1995;76:3171–3179. doi: 10.1099/0022-1317-76-12-3171. [DOI] [PubMed] [Google Scholar]
- 32.Yuan B, Campbell S, Bacharach E, Rein A, Goff S P. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J Virol. 2000;74:7250–7260. doi: 10.1128/jvi.74.16.7250-7260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuste E, Sanchez-Palomino S, Casado C, Domingo E, Lopez-Galindez C. Drastic fitness loss in human immunodeficiency virus type 1 upon serial bottleneck events. J Virol. 1999;73:2745–2751. doi: 10.1128/jvi.73.4.2745-2751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y-M, Imamichi H, Imamichi T, Lane H C, Falloon J, Vasudevachari M B, Salzman N P. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J Virol. 1997;71:662–6670. doi: 10.1128/jvi.71.9.6662-6670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zybarth G, Carter C. Domains upstream of the protease (PR) in human immunodeficiency virus type 1 Gag-Pol influence PR autoprocessing. J Virol. 1995;69:3878–3884. doi: 10.1128/jvi.69.6.3878-3884.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]