Abstract
Using vaccinia virus as a live vector, we show that the expression of human papillomavirus type 16 (HPV-16) E7 fused to a nonhemolytic portion of the Listeria monocytogenes virulence factor, listeriolysin O (LLO), induces an immune response that causes the regression of established HPV-16 immortalized tumors in C57BL/6 mice. The vaccinia virus construct expressing LLO fused to E7 (VacLLOE7) was compared with two previously described vaccinia virus constructs: one that expresses unmodified E7 (VacE7) and another that expresses E7 in a form designed to direct it to intracellular lysosomal compartments and improve major histocompatibility complex class II-restricted responses (VacSigE7LAMP-1). C57BL/6 mice bearing established HPV-16 immortalized tumors of 5 or 8 mm were treated with each of these vaccines. Fifty percent of the mice treated with VacLLOE7 remained tumor free 2 months after tumor inoculation, whereas 12 to 25% of the mice were tumor free after treatment with VacSigE7LAMP-1 (depending on the size of the tumor). No mice were tumor free in the group given VacE7. Compared to VacE7, VacSigE7LAMP-1 and VacLLOE7 resulted in increased numbers of H2-Db-specific tetramer-positive CD8+ T cells in mouse spleens that produced gamma interferon and tumor necrosis factor alpha upon stimulation with RAHYNIVTF peptide. In addition, the highest frequency of tetramer-positive T cells was seen in the tumor sites of mice treated with VacLLOE7. An increased efficiency of E7-specific lysis by splenocytes from mice immunized with VacLLOE7 was also observed. These results indicate that the fusion of E7 with LLO not only enhances antitumor therapy by improving the tumoricidal function of E7-specific CD8+ T cells but may also increase the number of antigen-specific CD8+ T cells in the tumor, the principle site of antigen expression.
Human papillomavirus (HPV) type 16 (HPV-16) infection in humans is associated with most cervical cancers (47), and expression of the early oncogenic proteins E6 and E7 is required to maintain the transformed state of the tumor cell. Therefore, E7 is an appropriate tumor-specific antigen and target for vaccine-based treatment of HPV-16-associated malignancies (9). Specific immunity against HPV-16 transformed tumors in murine models has been achieved by a number of vaccine protocols (reviewed in reference 38). These include administering E7 protein (14, 40), the CD8+ epitope in E7 specific for H-2Db (13), DNA that codes for E7 (8), or recombinant vaccinia virus vectors that express E7 (22, 23). An effective therapeutic response in most of these situations correlates with the induction of cytotoxic T lymphocytes (CTLs) specific for the E7 CD8+ epitope, RAHYNIVTF (13).
The role of CD8+ T cells in tumor immunity can be diverse. Not only are these cells able to lyse tumor targets that express tumor-specific antigen in the context of major histocompatibility complex (MHC) class I but also they secrete cellular mediators, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). Both IFN-γ and TNF-α have potent antitumor effects (27). The production of inducible nitric oxide synthase by macrophages requires both TNF-α and IFN-γ (12). The chemokines IP-10 (IFN-γ-inducible protein 10) and Mig (monokine induced by IFN-γ) are also produced by macrophages in response to IFN-γ. These chemoattractants mediate the infiltration of NK cells (37) and also inhibit angiogenesis (2, 32). TNF-α is also able to recruit NK cells to the tumor, providing a valuable mechanism by which tumor cells that have lost the expression of MHC class I molecules can be removed (16, 19). Possible direct effects of IFN-γ on tumor cells include the regulation of proteosome composition and hence antigen processing (45) and the upregulation of MHC class I expression (3) to enhance tumor immunogenicity.
Immunization with fusion products that consist of tumor antigen determinants and a nonantigenic determinant, either as naked DNA or purified protein, can significantly enhance tumor-specific immunity (1, 8, 14, 35). Previous work in our laboratory has shown that a recombinant Listeria monocytogenes construct that expresses a fusion of influenza virus nucleoprotein (NP) with listeriolysin O (LLO) at the N terminus is able to induce antigen-specific immunity that mediates the protection of mice against tumors expressing NP (26, 43). The hemolysin LLO is a secreted pore-forming protein that is essential for the escape of L. monocytogenes from the microbicidal environment of the macrophage phagolysosome (15). However, the form of LLO fused to NP used in these studies had been modified to remove the sequence that codes for the hemolytic portion of LLO (24). We recently described (G. Gunn et al., submitted for publication) a potent E7-based immunotherapeutic agent that could cause the regression of established HPV-16 immortalized transplantable mouse tumors and that also used L. monocytogenes as a vaccine vector and the E7 antigen fused to LLO. Interestingly, a similar vector that expressed E7 alone was quite ineffective in the same model tumor system. There are a number of genetic differences between these two recombinant listerial vectors, but a major difference is the form of the antigen expressed.
In order to address whether fusing E7 to LLO (LLOE7) enhances the immunogenicity of E7, in this study LLOE7 was delivered using a nonlisterial vector, vaccinia virus, and compared to two other forms of E7 that are expressed by totally isogenic vaccinia virus constructs and that are known to have different antitumor potencies. One construct expresses unmodified E7 (VacE7), and the other expresses E7 fused to lysosome-associated membrane protein 1 (LAMP-1) designed to improve MHC class II-restricted responses (VacSigE7LAMP-1) (22). We show that, compared to other vaccinia virus-based E7 vaccines, VacLLOE7 is a potent antitumor immunotherapeutic agent with important clinical potential for the treatment of cervical cancers.
MATERIALS AND METHODS
Tumor cell lines and maintenance.
The TC-1 cell line described previously (22) was obtained from the National Biological Resources Branch, National Cancer Institute, National Institutes of Health, Frederick, Md. EL4 and EL4-E7 (39) were kind gifts from Robert Tindle, Sir Albert Sakzewski Virus Research Center, Royal Children's Hospital, Brisbane, Queensland, Australia. Each line was maintained in RP-10, which consisted of RPMI 1640 (Cellgro) supplemented with 2 mM l-glutamine, 0.1 mM minimal essential medium with nonessential amino acids, 1 mM sodium pyruvate, 10 U of penicillin/ml, 10 μg of streptomycin/ml, 10% fetal bovine serum, and 10% NCTC medium (all from GIBCO BRL). TC-1 and EL4-E7 were maintained in RP-10 that also contained 400 μg of Geneticin/ml. All cells were grown in a 37°C incubator at 95% humidity and 10% CO2.
DNA constructs and generation of vaccinia virus recombinants.
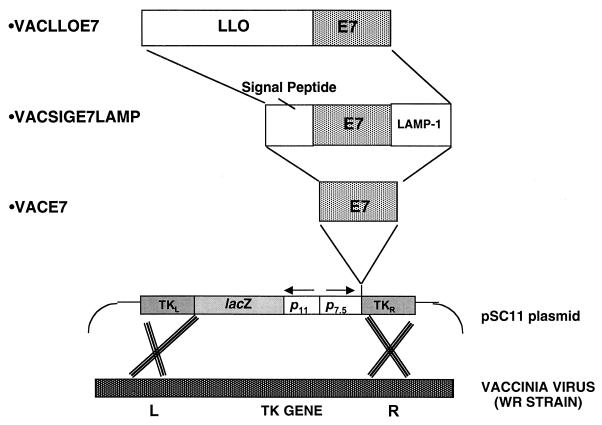
To ensure that the vaccines differed only by the form of E7 expressed, the vaccinia virus recombinants used were generated by transfection with pSC11-based plasmids. pSC11 is a plasmid that drives foreign gene expression with the vaccinia virus early-late promoter p7.5. Each construct differed only in the fusion product of E7 encoded. Figure 1 is a schematic of each plasmid that describes the different forms of E7 expressed by each recombinant.
FIG. 1.
Schematic of the different forms of E7 expressed by each vaccinia virus recombinant. In each form, the pSC11 vaccinia virus vector was used to insert the gene of interest into the thymidine kinase (TK) gene of the WR host strain of vaccinia virus.
The portion of LLO that is required for its sulfhydryl-dependent hemolytic activity was removed by using PCR to exclude amino acids 442 to 529, which contain the cysteine-dependent active site of LLO (24). An XhoI site was introduced into the 3′ end of the resultant truncated gene to allow ligation with the gene that codes for HPV-16 E7. The resultant fusion of LLO and E7 (LLOE7) was then cloned into the XbaI/NheI site of pAM401 upstream of the prfA gene for subsequent expression by Listeria (Gunn et al., submitted).
To allow expression by vaccinia virus, the LLOE7 sequence was cloned into pSC11. To do this, we first modified a portion of the LLOE7 sequence by PCR to alter a T5NT sequence at the 5′ end of the LLOE7 sequence to ensure its proper early transcription by vaccinia virus. We also introduced restriction sites to permit in-frame cloning of this fragment of LLO into a previously described pUC19 plasmid containing the full LLOE7 open reading frame (Gunn et al., submitted). The oligonucleotides synthesized for this modification were as follows: pGG55LO (3′GCATTTTCGCTTAAGC5′), where the underlined sequence represents an EcoRI site within the LLO sequence, and pGG55UPNXC (5′GGAATTCCATATGCCCGGGATGA7TAATGCTAGTCTTTATTACAC TTATATTAG3′), where the underlined sequence represents an NdeI/XbaI site. After PCR amplification, the resultant fragment was digested with EcoRI and NdeI and then ligated into similarly cut pUC19 containing the nonhemolytic sequence of LLO fused to E7. From here, the modified fusion protein was excised using XmaI and inserted into the XmaI site of pSC11. Successful introduction of these changes (without loss of the original sequence that codes for LLOE7) was confirmed by sequencing.
The recombinant virus was isolated by standard procedures (7). Briefly, the pSC11LLOE7 construct was used to transfect CV1 cells that had been infected with wild-type vaccinia virus strain WR (Western Reserve). Cell lysates obtained from this infection-transfection step contained vaccinia virus recombinants that were plaque purified three times on a thydimidine kinase-deficient cell line in the presence of bromodeoxyuridine and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining. Expression of the LLOE7 fusion product by plaque-purified vaccinia virus was verified by Western blotting using an antibody directed against the LLO protein sequence. VacSigE7LAMP-1 and VacE7 were made as described previously (44) using the same vaccinia virus vector (pSC11) and host strain of vaccinia virus (WR).
In vivo treatment of tumors.
Eight- to 10-week-old female C57BL/6 mice (Charles River Laboratories) were injected subcutaneously (s.c.) in the flank with 1 × 105 or 2 × 105 viable TC-1 cells in either phosphate-buffered saline (PBS) or Matrigel (BD Biosciences). TC-1 suspensions in Matrigel were prepared by adding 400 μl of Matrigel to 2 × 105 TC-1 cells in 100 μl of PBS. Upon the appearance of palpable tumors (7 days later), groups of eight mice were injected twice intraperitoneally (i.p.) on days 11 and 18 after tumor inoculation with 107 PFU of vaccinia virus. Tumor sizes were assessed every 2 to 3 days using calipers to determine the average diameter of each tumor. Mice were sacrificed when tumor sizes exceeded 25 mm.
In vitro CTL induction and activity.
Eight- to 10-week-old female C57BL/6 mice were injected twice i.p. with 107 PFU of vaccinia virus as described above. Thirteen days later, the spleens from two mice per vaccination group were pooled and homogenized in RP-10 using nylon mesh bags. Erythrocytes were lysed using Tris ammonium chloride solution and washed twice in RP-10. Primary cultures of 2 × 106 splenocytes/ml were incubated in upright T75 flasks with irradiated (30,000 rads) TC-1 tumor cells in 25 ml of RP-10 for 6 days. The ratio of splenocytes to TC-1 cells for each group was 100:1. Following this period, cells from each primary culture were prepared for a conventional 5-h chromium release assay at increasing effector/target (E:T) ratios using EL4, EL4 plus E7 peptide (RAHYNIVTF), or EL4-E7 as targets. The amount of 51Cr released into the culture supernatant of each well was determined by carefully transferring 50 μl of supernatant into 200 μl of Optiphase Supermix (Wallac, Perkin-Elmer). Samples were then read using a Microbeta scintillation counter (Wallac, Perkin-Elmer). The percent specific lysis was determined by using the following equation: [(test chromium release − spontaneous chromium release)/(total chromium release − spontaneous chromium release)] × 100. Spontaneous chromium release was determined by using 51Cr-pulsed targets in the absence of effector cells, and total chromium release was determined by adding an equal volume of 2% Triton X-100 to lyse pulsed targets. An average of three or four specific lysis values for each E:T ratio was then plotted.
Intracellular staining and analysis by flow cytometry.
Mice were injected twice i.p. on days 7 and 14 with 107 PFU of vaccinia virus. Splenocytes were harvested 7 days later and incubated with 1 μM peptide for 5 to 6 h in the presence of the Golgi transport inhibitor brefeldin A or monensin at a density of 107 cells/ml. Cells were washed twice and incubated in 50 μl of anti-mouse Fcγ receptor supernatant 2.4G2 (American Type Culture Collection) for 1 h or overnight at 4°C. Cells were stained for surface molecules, permeabilized, fixed using the permeabilization kit Golgi-Stop or Golgi-Plug (Pharmingen), and then stained for the cytokines IFN-γ and TNF-α. Typically, 400,000 events were acquired using the two-laser flow cytometer FacsCalibur and analyzed using Cellquest software (Becton Dickinson).
Proliferation assays.
Splenocytes from mice given two i.p. injections of vaccinia virus 7 days apart were pooled from two mice per vaccination group 10 days after the final injection. Following the removal of erythrocytes, the majority of B cells and macrophages were removed by passing the cells over a nylon wool column. Enriched T cells (2.5 × 105) were incubated for 66 h in triplicate with an equal number of syngeneic γ-irradiated splenocytes in a volume of 200 μl with increasing concentrations of recombinant E7 protein in flat-bottom 96-well plates. Cells were pulsed with 0.5 μCi of [3H]thymidine for the final 18 to 24 h of incubation and harvested onto filter mats using a Titertek harvester (Wallac, Perkin-Elmer). Samples in Microbetalux scintillant were then read using the Microbeta scintillation counter.
In vitro tumor analyses.
Tumors from mice were excised and cut into 2-mm pieces after removal of blood vessels and connective tissue by dissection. To isolate T cells, tumors were incubated for 30 min, with occasional shaking, in an enzyme mixture that consisted of 2 mg of collagenase P/ml, 1 mg of DNase I/ml,10 U of penicillin/ml, and 10 μg of streptomycin/ml in PBS at 37°C. The digested tissue was then passed through a nylon mesh bag, and the resultant cells were washed twice in RP-10 before being stained for flow cytometric analysis as described previously. Cells in Matrigel plugs were either isolated as described above or incubated in 300 μl of PBS overnight at 4°C before removal of the fibrous clot with forceps, passed through a nylon mesh bag, and then washed in PBS containing 5% fetal bovine serum and 0.05% azide.
RESULTS
VacLLOE7 is a better TC-1 tumor immunotherapeutic agent than VacE7 and VacSigE7LAMP-1.
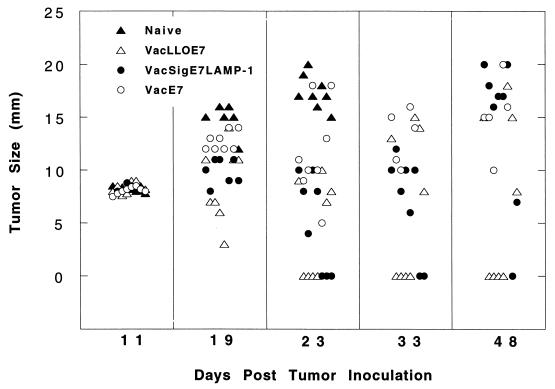
To assess the efficacy of VacLLOE7 for tumor therapy, we determined its ability to cause regression of established TC-1 tumors in C57BL/6 mice compared to the results for VacSigE7LAMP-1- and VacE7-vaccinated mice. A dose of 2 × 105 TC-1 cells were first injected s.c. into the flank of each mouse. Eleven days later, when the tumor was at least 8 to 9 mm in size, each mouse was treated with 107 PFU of vaccinia virus i.p. A boost of the same dose was given i.p. 7 days later. Tumor sizes were observed every 2 to 3 days. Five representative time points are shown in Fig. 2. At 23 days after tumor inoculation, none of the mice that had received VacE7 were tumor free, and by day 48, none had survived. In marked contrast to VacE7-treated mice, three of eight of the mice given VacsigE7LAMP-1 had resolved their tumors by day 23. However, two of eight of the regressed tumors grew out, so that at day 48, only one of the eight mice was tumor free. These results are in agreement with the observation made previously with these vaccinia virus constructs that VacSigE7LAMP-1 is far more effective than VacE7 at controlling the growth of TC-1 tumors in vivo (22). However, it should be noted that in that study (22), tumor challenges were approximately 10-fold lower than ours, so that at 7 days, when vaccination took place, only microscopic tumors were present. Because immunization took place before tumor growth could be measured, only tumor appearance after vaccination was monitored and plotted; 100% protection against tumor appearance by vaccination with VacSigE7LAMP-1 could be demonstrated (22). We show here that only 12% of animals can be cured of 8- to 9-mm macroscopic tumors using this vaccine. However, if immunizations are performed when the tumors are 3 to 5 mm in size, 25% of the mice can be cured of their tumors and remain tumor free (data not shown).
FIG. 2.
VacLLOE7 causes long-term regression of tumors established from 2 × 105 TC-1 cells injected s.c. into C57BL/6 mice. Mice were injected 11 and 18 days after tumor challenge with 107 PFU of VacLLOE7, VacSigE7LAMP-1, or VacE7/mouse i.p. or were left untreated (naive). Eight mice per treatment group were used, and the cross section for each tumor (average of two measurements) in each mouse is shown for the indicated days after tumor inoculation.
Effective eradication of TC-1 was also observed in mice that received VacLLOE7. Four of eight of these mice were tumor free 23 days after tumor inoculation. In contrast to the situation with VacSigE7LAMP-1, no tumors escaped in these mice, so that these mice were still tumor free at the end of the experiment, on day 48. The difference between the average sizes of tumors in VacSigE7LAMP-1-treated mice and VacLLOE7-treated mice on day 28 was statistically significant (P < 0.05, as determined by Student's t test), despite large deviations in tumor size between individual mice. We have performed this comparison between VacSigE7LAMP-1 and VacLLOE7 with tumors varying in size between 3 and 8 mm and have found that 5 of 24 mice remain tumor free in the group immunized with VacSigE7LAMP-1 compared to 12 of 24 mice remaining tumor free in the group immunized with VacLLOE7 (P < 0.05, as determined by the G2 likelihood ratio chi-square test). Thus, VacLLOE7 is able to slow the reappearance of TC-1 such that the mortality of mice is significantly improved over that seen following treatment with VacSigE7LAMP-1 or VacE7.
E7-specific CD8+ T-cell responses are enhanced in mice vaccinated with VacLLOE7 and VacSigE7LAMP-1 versus VacE7.
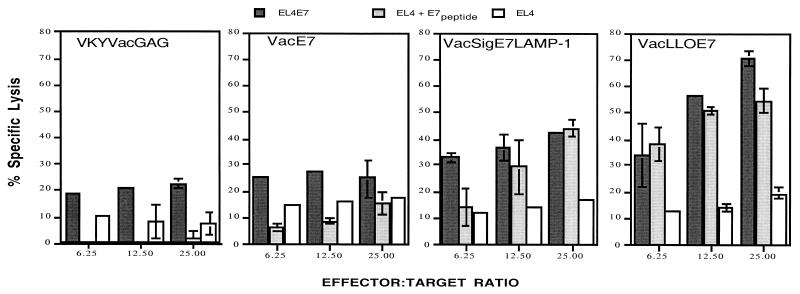
CTL responses to E6 and E7 have been identified elsewhere as correlating with effective immunotherapy of HPV immortalized tumors (9, 13, 24). Thus, we sought to determine whether the mechanism underlying the improved efficacy of VacLLOE7 involved enhanced lytic activity of cells isolated from mice treated with this construct. VacLLOE7 was again compared with VacSigE7LAMP-1 and VacE7. Vaccinia virus expressing human immunodeficiency virus (HIV) Gag (VacGag), which expresses an irrelevant antigen, was used as a negative control. Figure 3 shows that splenocytes from mice treated with VacE7 generated very little lysis above that seen with VacGag. In contrast, mice given VacSigE7LAMP-1 were able to generate CTLs that had significantly enhanced cytotoxic activity in the presence of E7. However, at an E:T ratio of 25:1, VacLLOE7-induced cells were capable of mediating at least 20% more lysis than VacSigE7LAMP-1-derived effectors.
FIG. 3.
CTL responses to E7 induced by VacLLOE7 surpass those seen with VacSigE7LAMP-1 and VacE7. Mice were injected twice i.p. 7 days apart with 107 PFU. Thirteen days after the final injection, spleen cells from two mice per treatment group were harvested and pooled as described in Materials and Methods. EL4 cells, EL4 cells pulsed with a peptide from HPV-16 E7 (RAHYNIVTF) (EL4 + E7 peptide), or EL4 cells stably transfected with full-length HPV-16 E7 (EL4E7) were compared with the parental EL4 cell line as targets for lysis. CTL activity is expressed as the average percent specific lysis for measurements at each E:T ratio as described in Materials and Methods. Error bars indicate standard deviations for measurements in triplicate or quadruplicate. Results shown are representative of three independent experiments.
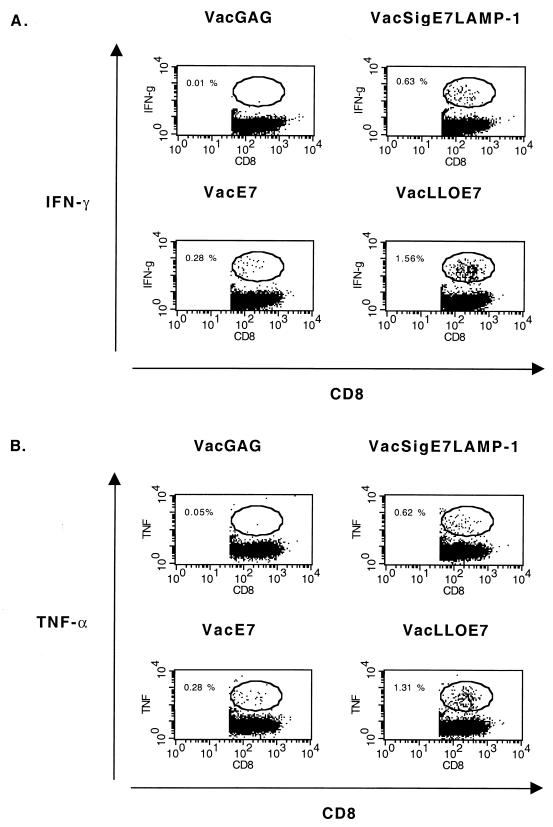
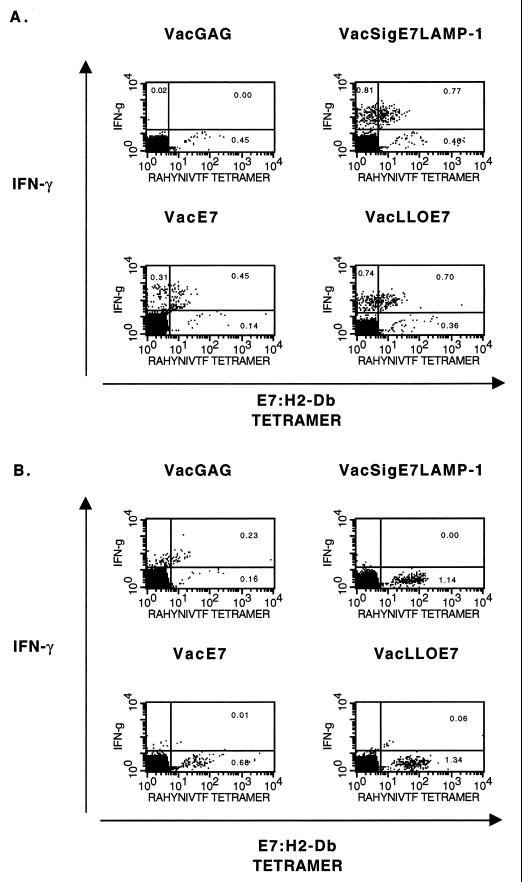
Since many of the tumoricidal effects of CD8+ T cells in vivo are mediated through the secretion of the inflammatory cytokines TNF-α and IFN-γ, we next examined the cytokine profiles of splenic E7-specific CD8+ T cells using an intracellular staining assay (25). Figure 4 contains representative dot plots obtained by flow cytometry that show the frequencies of E7-specific CD8+ T cells among activated (CD62Llo) CD8+ T cells in culture. The highest frequency of CD8+ T cells that produce IFN-γ and TNF-α in response to the E7 MHC class I epitope is obtained from mice vaccinated with VacLLOE7. The most striking observations are that the frequency of T cells producing IFN-γ and TNF-α in an antigen-specific manner is always highest in the spleens of mice treated with either VacSigE7LAMP-1 (0.63%) or VacLLOE7 (1.56% for IFN-γ and 1.31% for TNF-α), while IFN-γ and TNF-α production is lowest in the spleens of mice given VacE7 (0.28%).
FIG. 4.
Inflammatory cytokine production in response to RAHYNIVTF by CD8+ T cells is enhanced in the spleens of mice vaccinated with VacLLOE7. Following two i.p. injections 7 days apart with 107 PFU of VacGag, VacE7, VacSigE7LAMP-1, or VacLLOE7, spleen cells from two mice per treatment group were harvested 13 days after the second injection. Whole splenocytes were incubated with 1 μM HPV-16 E7 peptide RAHYNIVTF for 5 h in the presence of a Golgi transport inhibitor, and the levels of intracellular IFN-γ (A) and TNF-α (B) were determined by flow cytometry as described in Materials and Methods. Values shown are percentages of activated CD8+ T lymphocytes (gated on CD62L) that are IFN-γ positive. IFN-γ production in the presence of an irrelevant peptide (AMQMLKETI) from HIV Gag was no more than 10% the value shown for each vaccinia virus recombinant expressing E7. Responses shown are representative of three experiments.
The CD8+ T cells induced by these different vaccinia virus constructs were also quantified by determining the frequency of T cells in the spleen that are positive for fluorogenic E7–H2-Db tetramers. Peptide MHC tetramers have been used extensively to determine the numbers of bacterium- and virus-specific T cells in animal infection models (5, 6, 25). MHC tetramers have also been useful for identifying and characterizing T cells specific for tumor antigens in melanoma patients (21, 46) and in murine vaccination protocols with vaccinia virus (42). Thus, we wanted to determine if the number of IFN-γ-secreting T cells corresponds to the number of T cells that are positive for E7–H2-Db tetramers loaded with the dominant CTL epitope of E7, RAHYNIVTF.
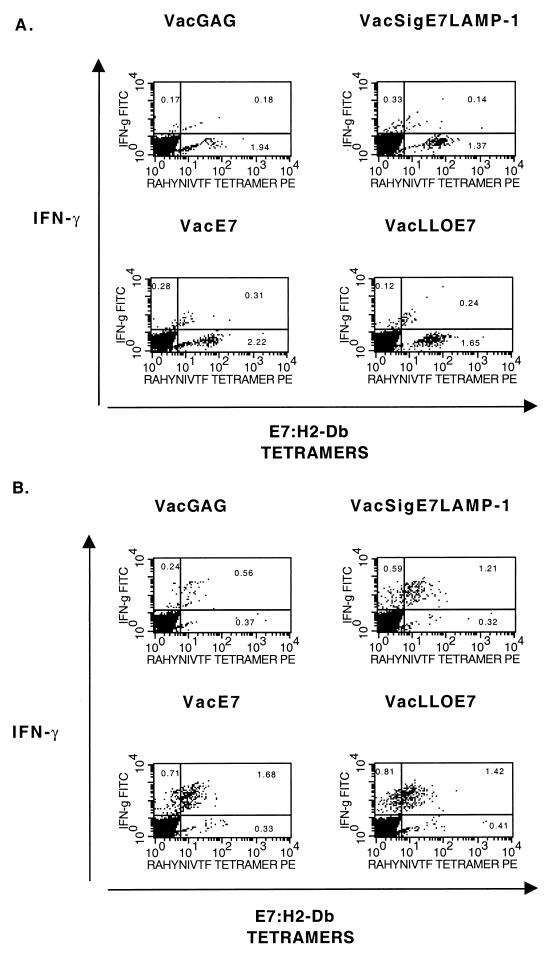
Interestingly, we found that only about half of the cells that produce IFN-γ when stimulated by RAHYNIVTF stain brightly with E7–H2-Db tetramers (Fig. 5A). We also found that unstimulated CD8+ T cells, i.e., that had been incubated with the H-2d-restricted control peptide from human immunodeficiency virus (HIV) Gag (AMQMLKETI), stained much more brightly with the tetramers such that almost twice as many cells were positive for E7–H-2Db (compare Fig. 5A and Fig. 5B). Of course, in Fig. 5B none of the cells show the production of IFN-γ because they are not Gag specific and, therefore, were not stimulated by the control peptide. Indeed, this population of cells represents the ex vivo pool of E7-specific CD8+ T cells induced by vaccination. Figure 5B shows that about twice as many tetramer-positive CD8+ T cells were present in the spleens of VacSigE7LAMP-1 (1.14%)- and VacLLOE7 (1.34%)-immunized mice as in those of VacE7 (0.68%)-immunized mice. We believe that the somewhat duller staining observed in Fig. 5A is due to the downregulation of the T-cell receptor (TCR) on activated T cells upon stimulation by antigen-presenting cells (APCs) presenting RAHYNIVTF. This is probably exacerbated by the presence of the Golgi inhibitor monensin, required for the detection of intracellular IFN-γ, which would block the export of newly synthesized TCR to the cell surface. The downregulation of TCR by engagement with peptide MHC is a well-known phenomenon (31, 41). Nevertheless, it is obvious from Fig. 5 that VacE7 is less able to generate RAHYNIVTF-specific CD8+ T cells than VacSigE7LAMP-1 and VacLLO-E7, which induce approximately equal numbers of IFN-γ-secreting T cells and tetramer-positive T cells on stimulation with the immunodominant epitope (Fig. 5A) or tetramer-positive T cells ex vivo (Fig. 5B).
FIG. 5.
VacLLOE7 and VacSigE7LAMP-1 induce similar numbers of tetramer-positive T cells that are specific for E7–H2-Db and that secrete IFN-γ in response to the HPV-16 peptide RAHYNIVTF. Spleen cells from mice vaccinated twice i.p. with each vaccinia virus construct were harvested 7 days after the last vaccination, incubated with 1 μM HPV-16 E7 (A) or HIV Gag (B) peptide, and stained for surface markers as described in Materials and Methods. Cells were gated on CD8+ CD62Llo, and IFN-γ production by E7–H2-Db tetramer-positive cells was determined. IFN-γ production in the presence of an irrelevant peptide (AMQMLKETI) from HIV Gag was less than 10% the value shown for each vaccinia virus recombinant expressing E7. Responses shown are representative of two independent experiments.
Therefore, the data described here indicate that the ability of each of these vaccinia virus constructs to eradicate TC-1 tumors correlates with their ability to induce E7-specific CTLs that recognize the RAHYNIVTF-H2b complex. The frequency of E7-H2b-specific cells that produce the inflammatory cytokines IFN-γ and TNF-α also supports the involvement of CD8+ effector cells in the treatment of established TC-1 tumors.
CD4+ T-cell-mediated responses to E7 are undetectable by proliferation measurements in mice vaccinated with VacLLOE7.
Previous studies have shown that modification of the E7 sequence to target it to peptide MHC class II loading compartments, via the Lamp-1 signal sequence and the N-terminal sequence, enhances the CD4+-restricted response to E7 over that seen with VacE7 (44). Not only were vaccinia virus constructs expressing SigE7LAMP-1 able to induce enhanced proliferative responses to the E7 CD4+ T-cell epitope, but these T cells were also required to mediate protection against TC-1 challenge (22).
The superior CTL activity and cytokine-secreting potential of CD8+ T cells from VacLLOE7 mice over those seen with mice treated with VacE7 therefore indicated that mice receiving this treatment may also have good CD4+ T-cell helper responses directed to E7. To test this hypothesis, we examined the proliferative activity of T cells from mice vaccinated with VacGag, VacE7, VacSigE7LAMP-1, and VacLLOE7.
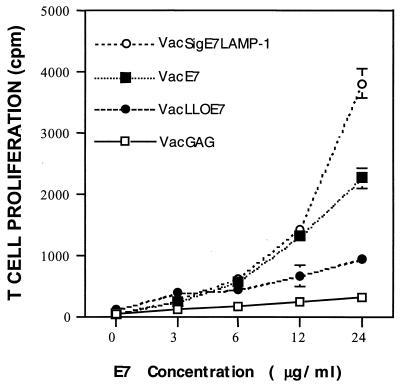
As expected, the proliferative response to E7 by T cells isolated from mice treated with VacSigE7LAMP-1 was higher than that seen with cells isolated from VacE7-treated mice (Fig. 6). However, to our surprise, very little proliferation specific for E7 was detected in cultures with T cells from VacLLOE7-vaccinated mice. In addition to this finding, we were unable to consistently detect CD4+ T cells that secrete IFN-γ in response to either the class II epitope contained within E7 (residues 31 to 62) or E7 protein (data not shown). Thus, in the absence of CD4+ T-cell help that is specific for E7, VacLLOE7 can induce CD8+ T cells that specifically target cells that express E7.
FIG. 6.
In vitro proliferative responses to exogenous E7 protein are significantly reduced in the spleens of mice vaccinated with VacLLOE7. Mice were given two i.p. injections of 107 PFU of VacGAG, VacE7, VacSigE7LAMP-1, or VacLLOE7. Ten days after the last injection, spleen cells from two mice per vaccination group were harvested and enriched for T cells over nylon wool columns. T cells from each vaccination group were incubated with equal numbers of syngeneic γ-irradiated APCs as described in Materials and Methods. T-cell proliferation is shown as the mean of triplicate measurements of [3H]thymidine incorporation at each concentration of E7. Error bars are standard deviations of the mean at each dose. Results shown are representative of two experiments.
CD8+ tetramer-positive T-cell numbers are enhanced in the tumors of mice treated with VacLLOE7.
Mice immunized with VacLLOE7 have poor proliferative responses to E7 protein compared to VacSigE7LAMP-1-immunized mice but better control the growth of TC-1 tumors in vivo. In addition, VacLLOE7 induces better CD8+ T-cell immunity in the spleen than VacSigE7LAMP-1, which is more effective than VacE7. We therefore examined whether these RAHYNIVTF-specific CD8+ T cells were present in the tumors of mice treated with each vaccinia virus construct. As described earlier for the tumor regression studies, 2 × 105 TC-1 cells were injected s.c. into C57BL/6 mice, and each treatment group was injected twice i.p. with the relevant vaccinia virus construct. Seven days after the final injection, spleens and tumors from mice in each group were removed and analyzed for E7-specific CD8+ T-cell contents. The spleens from two or three mice were pooled, as were the tumors.When the tumor had regressed completely, a biopsy of the tissue at the tumor site was performed. These cells were homogenized in the same way as the cells obtained from mice that still bore tumors at the site of implantation and resembled lymphocytes in size, as determined by their forward and side scatter fluorescence-activated cell sorting profiles (data not shown).
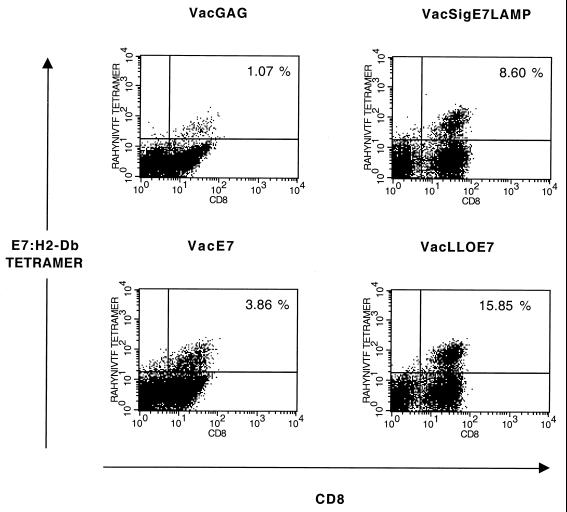
The cells remaining at the site of tumor inoculation were examined for T cells specific for E7–H2-Db. These were clearly visible without in vitro stimulation or gating for activated T cells (Fig. 7). The number of tetramer-positive cells in the tumors of mice given VacGAG constituted ∼1% of lymphocytes infiltrating the tumor. In mice that had been treated with VacE7, tetramer-positive T cells represented ∼4% of the total lymphocytes in the tumor, whereas ∼8% of the infiltrating lymphocytes in mice treated with VacSigE7LAMP-1 were tetramer positive. Most striking is that almost 16% of lymphocytes present at the site of tumor inoculation in VacLLOE7-treated mice were tetramer positive, indicating that the enhanced production of CTLs in the spleens of vaccinated mice (Fig. 3) translated into effector cells in the tumor.
FIG. 7.
Ex vivo frequencies of E7–H2-Db-specific tetramer-positive CD8+ T cells in the tumors of mice treated with VacLLOE7 are enhanced over those seen with VacSigE7LAMP-1 and VacE7. Tumors were homogenized in the presence of collagenase and DNase to aid in the isolation of T lymphocytes. Cells were gated for live lymphocytes based on their forward scatter and side scatter to exclude cellular debris, and CD8+ T cells reactive for E7–H2-Db were identified without in vivo stimulation or gating for activated T cells.
Because the clinical response to vaccination was heterogeneous for each vaccine, resulting in the necessity of harvesting tumors of various sizes for each group, tumor cells were mixed with Matrigel prior to s.c. inoculation. Matrigel is a reconstructed basement membrane in which solid tumors can grow and interact with infiltrating cells (18, 34) and as such is often used to study tumor invasion of basement membrane as well as angiogenesis and tumor infiltration. Matrigel plugs of the same size were harvested from each vaccine group 7 days after the last immunization. Half the plug from each mouse was treated with DNase and collagenase, while the other half was left untreated. The cells isolated from the tumors in each group were pooled as before. Table 1 summarizes our observations using Matrigel-embedded tumors in vivo. As observed in the absence of Matrigel, the number of tetramer-positive T cells specific for E7–H2-Db and located at the site of the tumor was markedly enhanced in mice treated with VacLLOE7 or VacSigE7LAMP-1. Again, mice treated with VacE7 had the lowest frequency. This trend was also observed for tumors that had been homogenized with enzyme compared with those that had not been incubated with DNase and collagenase. This result indicates that the enzymatic digestion of tumors does not have a great impact on cell surface receptors.
TABLE 1.
Enhanced accumulation of E7-specific CD8+ T cells in TC-1 tumors of mice treated with VacLLOE7 is not influenced by collagenase and DNase treatment of tumorsa
| Treatment | % of CD8+ tetramer-positive cells for the indicated sample in:
|
||||
|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
||||
| Spleen | Tumor with enzyme treatment | Spleen | Matrigel tumor with enzyme treatment | Matrigel tumor without enzyme treatment | |
| VacGag | 0.05 | 1.07 | 0.23 | 0.2 | 0.01 |
| VacE7 | 0.06 | 3.9 | 0.07 | 4.7 | 7.9 |
| VacSigE7LAMP | 0.04 | 8.6 | 0.19 | 15.6 | 18.3 |
| VacLLOE7 | 0.05 | 15.9 | 0.48 | 18.2 | 25.8 |
The percentages of RAHYNIVTF-specific cells in the spleens of treated mice are also shown for comparison. Values shown are percentages of cells that fall within the live lymphocyte gate.
We also used E7–H2-Db tetramers to measure the impact of TC-1 tumors on the number of E7-specific CD8+ T cells left in the spleen following treatment with each of the vaccinia virus constructs. In vitro, tetramer-positive spleen cells were discernible to very similar extents (0.14 to 0.31%) in all treatment groups, including those given VacGag (Fig. 8A). Stimulation of spleen cells with the RAHYNIVTF epitope, to determine the proportion of CD8+ tetramer-positive T cells capable of producing IFN-γ, demonstrated that IFN-γ production by cells from VacE7-immunized mice was as high (1.68%) as that seen with immune cells from mice treated with VacLLOE7 (1.42%) and VacSigE7LAMP-1 (1.21%). This finding is in contrast to the inferior IFN-γ production by T cells from VacE7-immunized mice compared to the other two vaccine constructs in the absence of TC-1 tumors (Fig. 4 and 5).
FIG. 8.
IFN-γ secretion by E7–H2-Db-specific tetramer-positive CD8+ cells in the spleens of tumor-bearing mice is indistinguishable between mice receiving different vaccinia virus constructs expressing E7. Spleen cells from vaccinated mice were harvested and stimulated as described in the legend to Fig. 5. Plots are of percentages of activated (CD62Llo) CD8+ cells that secreted IFN-γ in response to the HIV Gag control peptide (A) or RAHYNIVF (B).
Thus, the higher numbers of E7-specific T cells at the site of tumor inoculation in mice treated with VacLLOE7 suggest that the differences in efficacies of the vaccine constructs described here may be due not just to the levels of CD8+ T cells induced but also to the ability of these T cells to exit the periphery and home in on the tumor site.
DISCUSSION
In this report, we demonstrate an as-yet-undescribed immunity-enhancing function of nonhemolytic LLO expressed as a fusion protein with E7 by vaccinia virus.
In the past, a role for both CD4+ and CD8+ effectors specific for E7 in the eradication of tumors has been demonstrated (22, 40). However, others have shown that protection against E7 immortalized tumors is more dependent on CD8+ T-cell responses to E7 than on CD4+ T-cell responses (13). More pertinent to the work described in this report, Chen et al. (8) have found that the administration of DNA encoding a fusion of the gene for E7 with the gene for heat shock protein (HSP) 70 (HSP-70) results in CD8+ T-cell responses to E7 in the absence of CD4+ T-cell responses. As with the observations made in this study, such immune responses were able to cause the regression of TC-1 tumors in vivo. Therefore, the ability of VacLLOE7 to induce the regression of established TC-1 tumors in the absence of a proliferative CD4+ T-cell response to E7 is in agreement with previous work studying the treatment of HPV-16 immortalized tumors using immunity-enhancing therapy.
The reduced proliferative responses to exogenous E7 in vitro in the spleens of mice vaccinated with VacLLOE7 may be due to an ability of LLO to specifically enhance CD8+ T-cell responses. For example, other investigators have shown that fusion of HSP-70 to ovalbumin specifically enhances CD8+ T-cell responses to the ovalbumin peptide SIINFEKL (35) independently of CD4+ T-cell help (17). HSP fusions may achieve this result because of the ability of HSPs to transport misfolded proteins to the ubiquitination pathway and therefore bypass the MHC class II pathway (4) or because of their innate ability to potentiate the Th1 response (29) and enhance the expression of costimulatory molecules on professional APCs (10, 20, 30) such that CD4+ T-cell help is no longer required. Since the region of HSP-70 required for CTL enhancement does not include the peptide binding domain used for the transport of proteins (17), the latter explanation may be more likely. It is possible that LLOE7 uses similar mechanisms to achieve potent CTL activity in the absence of CD4+ T-cell responses to E7. We believe that LLOE7 more effectively targets the protein for rapid proteolysis in the cytosol due to the presence of a PEST-like sequence (28) near the amino terminus of LLO (11). This would enhance the presentation of E7 in the MHC class I pathway of antigen processing, leading to enhanced CD8+ T-cell responses. It would also decrease the access of E7 to the endocytic pathway and thus reduce E7 peptide loading of MHC class II molecules, with subsequent poor CD4+ T-cell responses to E7. In this scenario, CD4+ T-cell responses to antigens derived from the vaccinia virus vector may provide help during the priming of CD8+ T cells. We are currently investigating the possible role of LLO in the trafficking of its fusion partner to proteosomes.
We believe that boosting the number of preexisting CD8+ T cells specific for E7 mediates the resolution of TC-1 following treatment with VacLLOE7. This notion is supported by the enhanced CTL activity and E7 tetramer-positive CD8+ T cells in the spleens of mice treated with VacLLOE7. However, VacSigE7LAMP-1 is also capable of inducing a significant population of splenic antigen-specific CD8+ T cells, a finding which may explain the ability of this vaccine to eradicate tumors during the first 2 weeks of treatment. Long-term eradication of TC-1 tumors, which occurs only in mice treated with VacLLO-E7, is more likely to be affected by the migration of E7-specific CD8+ T cells to peripheral sites of antigen expression. The significantly enhanced numbers of tetramer-positive T cells specific for E7–H2-Db at the tumor site of mice treated with VacLLOE7 is an indicator of this scenario. The selective expression of a number of different adhesion molecules (e.g., CD44) and chemokine receptors (e.g., CCR5) may mediate the expedient exit of E7-specific CD8+ T cells from the spleen and draining lymph nodes to the tumor site (33, 36).
The findings in this paper therefore provide an additional option for the enhancement of immune responses to E7 and a possible immunotherapeutic agent for cervical cancers. The enhanced numbers of CD8+ T cells that are specific for E7 and that produce IFN-γ together with their ability to persist at the tumor site following treatment with a vaccinia virus recombinant that expresses LLOE7 may assist in overcoming the mechanisms used by tumors to evade host immunity in vivo.
ACKNOWLEDGMENTS
We thank our colleagues at the University of Pennsylvania, George Gunn, Gregory Beatty, Christian Peters, and Asha Abdool, for very helpful suggestions and technical assistance.
This work was supported by grants CA 72108 (to T.-C.W. and Y.P.), CA69632 (to Y.P.), and AI40957 (to S.N.I.)
REFERENCES
- 1.Acres B, Apostolopoulos V, Balloul J-M, Wreschner D, Xing P-X, Ali-Hadji D, Bizouarne N, Kieny M P, McKenzie I F C. MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines. Cancer Immunol Immunother. 2000;48:588–594. doi: 10.1007/PL00006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angiolillo A, Sgadari C, Taub D, Liao F, Farber J, Maheshwari S, Kleinman H, Reaman G, Tosato G. Human interferon-inducible protein is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Brodsky J L. Post-translational protein translation: not all HSC70s are created equal. Trends Biochem Sci. 1996;21:122–126. [PubMed] [Google Scholar]
- 5.Busch D H, Phillips I M, Vijh S, Pamer E G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 6.Callan M F C, Tan L, Annels N, Ogg G S, Wilson J D K, O'Callaghan C A, Steven N, McMichael A J, Rickenson A B. Direct visualization of antigen specific CD8+ T cells during the primary immune response to Epstein Barr virus. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinat virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C H, Wang T L, Hung C F, Yang Y, Young R A, Pardoll D M, Wu T C. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP-70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 9.Chen L P, Thomas E K, Hu S L, Hellstrom I, Hellstrom K E. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc Natl Acad Sci USA. 1991;88:110–114. doi: 10.1073/pnas.88.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- 11.Decatur A L, Portnoy D A. A PEST-like sequence in Listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–995. doi: 10.1126/science.290.5493.992. [DOI] [PubMed] [Google Scholar]
- 12.Drapier J C, Wietzerbin J, Hibbs J B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988;18:1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- 13.Feltkamp M C, Smits H L, Vierboom M P, Minnaar R P, de Jongh B M, Drijfhout J W, ter Schegget J, Melief C J, Kast W M. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 14.Fernando G, Murray B, Zhou J, Frazer I. Expression, purification and immunological characterization of the transforming protein E7, from cervical cancer-associated human papillomavirus type 16. Clin Exp Immunol. 1999;115:397–403. doi: 10.1046/j.1365-2249.1999.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gedde M, Higgins D, Tilney T, Portnoy D. Role of listeriolysin in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glas R, Franksson L, Une C, Eloranta M-L, Öhlen C, Örn A, Kärre K. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype: an adaptive component of NK cell-mediated responses. J Exp Med. 2000;191:129–138. doi: 10.1084/jem.191.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Q, Richmond J F, Suzue K, Eisen H N, Young R. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4+ T cell dependent. J Exp Med. 2000;191:403–408. doi: 10.1084/jem.191.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson B, Nannmark U. Ultrastructure of interactions between activated murine natural killer cells and melanoma cells in an extracellular matrix (Matrigel) environment. Nat Immun. 1996;15:98–106. [PubMed] [Google Scholar]
- 19.Kashii Y, Giorda R, Herberman R B, Whiteside T L, Vujanovic N L. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol. 1999;163:5358–5366. [PubMed] [Google Scholar]
- 20.Kol A T, Bourcier T, Lichtman A H, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophage. J Clin Investig. 1990;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 22.Lin K-Y, Guarnieri F, Staveley-O'Carroll K F, Levitsky H, August J T, Pardoll D, Wu T-C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 23.Meneguzzi G, Cerni C, Kieny M P, Lathe R. Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology. 1991;181:62–69. doi: 10.1016/0042-6822(91)90470-v. [DOI] [PubMed] [Google Scholar]
- 24.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:167–178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26.Pan Z-K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 27.Prevost-Blondel A, Roth E, Rosenthal F M, Pircher H. Crucial role of TNF-α in CD8 T cell-mediated elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J Immunol. 2000;164:3645–3651. doi: 10.4049/jimmunol.164.7.3645. [DOI] [PubMed] [Google Scholar]
- 28.Rechsteiner M, Rogers S W. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 29.Rico A, Angel S, Alonso C, Requena J. Immunostimulatory properties of the Leishmania infantum heat shock proteins HSP70 and HSP83. Mol Immunol. 1999;36:1131–1139. doi: 10.1016/s0161-5890(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 30.Ruedl C, Kopf M, Bachmann M F. CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo. J Exp Med. 1999;189:1875–1883. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SanJose E, Borroto A, Niedergang F, Alcover A, Alarcon B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–170. doi: 10.1016/s1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 32.Sgadari C, Farber J, Angiolillo A, Liao F, Teruya-Feldstein J, Burd P, Yao L, Gupta G, Kanegane C, Tosato G. Mig, the monokine induced by interferon gamma, promotes tumor necrosis in vivo. Blood. 1997;89:2635–2643. [PubMed] [Google Scholar]
- 33.Springer T. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multiple paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 34.Sterling-Levis K, White L, Trickett A, Gramacho C, Pittman S, Tobias V. Heterotransplantation of early B-lineage acute lymphoblastic leukemia using a solubilized attachment matrix (Matrigel) Cancer Res. 1993;53:1222–1225. [PubMed] [Google Scholar]
- 35.Suzue K, Zhou X, Eisen H, Young R. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taub D, Conlon K, Lloyd A, Oppenheim J, Kelvin D. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 37.Taub D D, Sayers T J, Carter C R, Ortaldo J R. α and β Chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 38.Tindle R. Human papillomavirus vaccines for cervical cancer. Curr Opin Immunol. 1996;8:643–650. doi: 10.1016/s0952-7915(96)80080-x. [DOI] [PubMed] [Google Scholar]
- 39.Tindle R W, Croft S, Herd K, Malcolm K, Geczy A F, Stewart T, Fernando G M. A vaccine conjugate of ‘ISCAR’ immunocarrier and peptide epitopes of the E7 cervical cancer-associated protein of human papillomavirus type 16 elicits specific Th1- and Th2-type responses in immunized mice in the absence of oil-based adjuvants. Clin Exp Immunol. 1995;101:265–271. doi: 10.1111/j.1365-2249.1995.tb08349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tindle R W, Fernando G J, Sterling J C, Frazer I H. A “public” T-helper epitope of E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc Natl Acad Sci USA. 1991;88:5887–5891. doi: 10.1073/pnas.88.13.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valitutti S, Muller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)-CD3-ζ complexes after antigenic stimulation. J Exp Med. 1997;185:1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valmori D, Levy F, Miconnet I, Zajac P, Spagnoli G C, Rimoldi D, Lienard D, Cerundolo V, Cerottini J C, Romero P. Induction of potent antitumor CTL responses by recombinant vaccinia encoding Melan-A peptide analogue. J Immunol. 2000;164:1125–1131. doi: 10.4049/jimmunol.164.2.1125. [DOI] [PubMed] [Google Scholar]
- 43.Weiskirch L M, Paterson Y. Listeria monocytogenes. A potent vaccine vector for neoplastic and infectious disease. Immunol Rev. 1997;158:159–169. doi: 10.1111/j.1600-065x.1997.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu T-C, Guarnieri F G, Staveley-O'Carroll K F, Viscidi R P, Levitsky H I, Hedrick L, Cho K R, August J T, Pardoll D M. Engineering an intracellular pathway for major histocompatibilty complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Waters J B, Fruh K, Peterson P A. Proteosomes are regulated by interferon γ: implications for antigen processing. Proc Natl Acad Sci USA. 1992;89:4928–4932. doi: 10.1073/pnas.89.11.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yee C, Savage P A, Lee P P, Davis M M, Greenberg P D. Isolation of high avidity melanoma-reactive CTL from heterogenous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 47.zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]