Abstract
Recombinant Norwalk virus-like particles (rNV VLPs) were administered to BALB/c mice by the intranasal (i.n.) route to evaluate the induction of mucosal antibody responses. The results were compared to systemic and mucosal responses observed in new and previous studies (J. M. Ball, M. E. Hardy, R. L. Atmar, M. E. Connor, and M. K. Estes, J. Virol. 72:1345–1353, 1998) after oral administration of rNV VLPs. Immunizations were given in the presence or absence of a mucosal adjuvant, mutant Escherichia coli heat-labile toxin LT(R192G). rNV-specific immunoglobulin G (IgG) and fecal IgA were evaluated by enzyme-linked immunosorbent assay. The i.n. delivery of rNV VLPs was more effective than the oral route at inducing serum IgG and fecal IgA responses to low doses of rNV particles. Vaginal responses of female mice given VLPs by the i.n. and oral routes were also examined. All mice that received two immunizations with low doses i.n. (10 or 25 μg) of rNV VLPs and the majority of mice that received two high doses orally (200 μg) in the absence of adjuvant had rNV-specific serum IgG, fecal, and vaginal responses. Additional experiments evaluated whether rNV VLPs can function as a mucosal adjuvant by evaluating the immune responses to two soluble proteins, keyhole limpet hemocyanin and chicken egg albumin. Under the conditions tested, rNV VLPs did not enhance the serum IgG or fecal IgA response to these soluble proteins when coadministered by the i.n. or oral route. Low doses of nonreplicating rNV VLPs are immunogenic when administered i.n. in the absence of adjuvant, and addition of adjuvant enhanced the magnitude and duration of these responses. Recombinant NV VLPs represent a candidate mucosal vaccine for NV infections in humans.
Norwalk virus (NV) is a frequent cause of acute gastroenteritis in developed and developing countries. The Centers for Disease Control and Prevention attributed 42% of outbreaks of acute nonbacterial gastroenteritis in the United States from 1976 to 1980 to NV (25). Recent estimates obtained by using new and improved diagnostic assays developed over the past decade for the detection of NV infections indicate that greater than 90% of outbreaks of acute nonbacterial gastroenteritis are caused by NV or Norwalk-like agents (17, 36). Outbreaks frequently occur in day care centers, schools, nursing homes, hospitals, and the military. The increasing clinical significance of these infections suggests that an effective vaccine could be useful (16).
NV is classified as a human calicivirus based on sequencing and characteristics of the viral genome (positive-sense, single-stranded, nonenveloped RNA viruses with a single capsid protein) (8, 22, 26). NV and NV-like agents are difficult to study because they cannot be cultivated in cell culture systems, and no animal model is available. In spite of these difficulties, the cloning and expression of the single capsid protein resulted in the assembly of empty virus-like particles (VLPs) that are similar to native Norwalk virions in size and appearance (23). Our laboratory is examining the usefulness of these VLPs as a candidate for a mucosal vaccine because of the following useful properties. First, the VLPs are stable at low pH, so they can be administered orally. Second, they can be lyophilized and stored at 4°C in water or phosphate-buffered saline (PBS) for at least 3 years without degradation. Third, the VLPs are easily made by using the baculovirus expression system; yields of more than 22 mg per 9 × 108 cells are obtained in sufficient purity for vaccine evaluation and successful crystallization (33). Fourth, the unique structure of the single protein that folds to make a VLP suggests these particles can be modified to be an antigen delivery system (33). Finally, the recombinant NV (rNV) VLPs are immunogenic when tested in inbred and outbred mice and in volunteers following oral administration, even in the absence of a mucosal adjuvant (2, 3).
Most nonreplicating proteins administered alone by mucosal routes induce poor if measurable immune responses. Only a few natural antigens, including bacterial toxins such as cholera toxin (CT) or Escherichia coli labile toxin (LT), consistently stimulate strong mucosal responses (18). These antigens are also useful as mucosal adjuvants to stimulate mucosal responses to unrelated coadministered antigens. Intranasal (i.n.) immunization with a variety of antigens has induced significant increases in specific immunoglobulin A (IgA) responses at intestinal, pulmonary, and other mucosal surfaces, such as the vagina (1, 4, 5, 11, 13, 24, 28, 29, 32). In this study, we tested the potential of rNV VLPs as an i.n. immunogen and determined if this route of immunization stimulates mucosal (fecal and vaginal) antibodies. We also evaluated if VLPs can function as a mucosal adjuvant when given with soluble proteins, such as keyhole limpet hemocyanin (KLH) or chicken egg albumin (OVA).
MATERIALS AND METHODS
Mice.
Inbred 6- to 8-week-old female BALB/c mice (Charles River Laboratories, Portage, Mich.) were used for all immunizations. Mice were housed in microisolator cages.
Animal inoculations and sample collection to evaluate the response to rNV VLPs administered orally or i.n.
BALB/c mice (six to seven mice per group) were immunized orally or i.n. with rNV VLPs (DynCorp, Rockville, Md.) at 0 and 21 days postinoculation (dpi). The rNV VLPs were administered in the presence or absence of 10 μg of a mutant Escherichia coli labile toxin, LT(R192G) (12). Control mice received PBS (pH 7.4) or PBS with LT(R192G). rNV VLPs were administered orally by gavage with a stainless steel intubation needle (Popper and Sons, Inc., New Hyde Park, N.Y.). The concentrations of rNV VLPs administered orally were 200 μg in the absence or presence of LT(R192G) and 10 μg in the presence of LT(R192G). The i.n. immunization was performed with 10 or 25 μg of rNV VLPs administered in the absence of adjuvant and with 10 μg of rNV VLPs in the presence of 10 μg of of LT(R192G). Prior to i.n. immunization, mice were anesthesized with 30 to 40 μl of a mixture of ketamine (37.5 mg/ml), xylazine (1.9 mg/ml), and acepromazine (0.37 mg/ml) delivered intraperitoneally (i.p.). The i.n. immunization was administered with a 10-μl Eppendorf pipette tip, alternating drops through both nares. The drops were placed gently at the tip of the nares, with a maximum volume of 7 μl given per administration and with up to two repeat administrations given during a 15-min period (maximum volume, 21 μl).
Fecal and serum samples were collected at 0, 21, 36, and 417 dpi. Fecal samples were collected from individual mice with a fecal collection cage as previously described (3). Fecal samples were extracted by making a 5 to 10% (wt/vol) fecal suspension in PBS containing 0.1% Tween 20, soybean trypsin inhibitor (0.1 mg/ml), and Merthiolate (Lilly; 0.1 mg/ml). Each fecal suspension was vortexed, sonicated for 10 min, and centrifuged for 10 min in a microcentrifuge (3). The fecal supernatant was collected and stored at −80°C. Blood samples were collected by tail bleed; after clotting and centrifugation, serum samples were collected and stored at −20°C until tested.
Vaginal samples were collected on 40, 125, 221, and 365 dpi from the groups of mice that received rNV VLPs in the presence of LT(R192G) to determine the longevity of antibody response induced by the VLPs. Vaginal samples were collected on different days from the serum and fecal samples to minimize stress to the mice from the vaginal sampling. Vaginal samples were collected, processed, and stored as described previously (20) with minor modifications. Briefly, mice received i.p. anesthesia before vaginal sample collection with vaginal wicks (2 by 25 mm; Polyfiltronics, Inc., Rockland, Mass.). To facilitate introduction and removal of wicks, 10 μl of PBS was instilled into the vagina with a 10-μl pipette to tip followed by insertion of the wick. Protease inhibitors were added to the vaginal washes as described above for fecal sample processing.
Animal inoculations and sample collection to evaluate if rNV VLPs can function as a mucosal adjuvant.
BALB/c mice (six to seven per group) were immunized orally with 2.5 mg of OVA (Calbiochem-Novabiochem, La Jolla, Calif.) or i.n. with either 500 μg of OVA (10) or 100 μg of KLH (Calbiochem-Novabiochem) administered in the presence or absence of rNV VLPs at 0 and 14 dpi or 0 and 21 dpi. OVA was suspended in sterile MilliQ water at an initial concentration of 53 mg/ml. The protein concentration was determined by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, Ill.) with bovine serum albumin as the standard. The VLPs were prepared in Spodoptera frugiperda (Sf9) insect cells as described previously (3). The preparation was examined by negative-stain electron microscopy to ensure that the VLPs were intact. Bacteriologic cultures in Lennox L and thioglycolate broth incubated for a minimum of 2 weeks at 37°C were done to ensure sterility of the preparation. Endotoxin levels were measured with the Limulus amebocyte lysate assay (Association of Cape Cod, Woods Hole, Mass.). Positive control groups received OVA in the presence of 10 μg of LT(R192G). Negative controls received PBS and rNV VLPs. In a follow-up experiment, BALB/c mice were given two i.n. immunizations consisting of OVA administered in the presence or absence of rNV VLPs.
Serum and fecal samples were collected at 0, 14, and 28 dpi and processed as described above. In a follow-up experiment, serum and fecal samples were collected at 0, 21, and 35 dpi.
Antibody ELISAs. (i) Preparation of rNV VLP antigen-coated microtiter plates.
For enzyme-linked immunosorbent assays (ELISAs), 96-well polyvinyl chloride plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated with rNV antigen in selected columns by adding 100 μl of rNV particles per well (0.35 μg/ml, based on the BCA assay) and incubating the plates for 4 h at room temperature. To block nonspecific protein binding, the plates were incubated overnight at 4°C with 5% (wt/vol) dry milk in PBS (5% BLOTTO) for serum IgG assays or 10% BLOTTO for rNV-specific fecal IgA assays.
(ii) Serum IgG ELISA.
Individual serum samples were tested for rNV-specific IgG on VLP antigen-coated plates as previously described (19). Background binding was also analyzed by adding individual serum samples to wells lacking antigen. Absorbance measurements were done at 450 nm with a Titertek Multiskan Plus automatic plate reader (ICN Flow, Costa Mesa, Calif.). End point titer values were determined as the reciprocal of the highest dilution that had an absorbance value greater than or equal to 0.1 above the background (absorbance of the well lacking antigen)
(iii) Fecal IgA ELISA.
Two separate ELISA protocols were done for each stool specimen to determine rNV-specific and total fecal IgA by protocols previously described (3). The level of rNV-specific IgA was calculated from a standard curve that was determined by the absorbance values of the mouse IgA standard (Southern Biotechnology Assoc., Birmingham, Ala.) added to each plate. The level of rNV-specific fecal IgA was calculated from the linear portion of a standard curve that was determined by the absorbance values of the IgA standard added to each plate. Total fecal IgA was determined by capturing all fecal extract IgA molecules with goat anti-mouse IgA (Southern Biotech Assoc.). The rNV-specific IgA level was expressed in nanograms per milliliter, and each corresponding total IgA level was expressed in micrograms per milliliter. Individual fecal IgA responses were expressed as a ratio of rNV-specific IgA (nanograms per milliliter) to total IgA (micrograms per milliliter) (nanograms of rNV-specific IgA per microgram of total IgA). This ratio was used to determine the fecal response due to the daily variation in IgA concentration in fecal samples.
(iv) Vaginal rNV-specific IgA ELISAs.
Ninety-six-well polyvinyl chloride plates were coated with rNV in selected columns as described above. After an overnight blocking at 4°C with 5% BLOTTO, 75 μl of an undiluted vaginal sample per well or a 1:5 dilution of the sample was added, and the sample was serially diluted twofold down the plate and incubated for 2 h at 37°C. The protocol was completed as described above for the rNV-specific fecal IgA ELISA or the serum IgG ELISA.
(v) Serum OVA-specific ELISA and serum KLH-specific ELISA.
Polyvinyl chloride 96-well plates were coated with OVA or KLH by placing 100 μl of OVA or KLH/well (50 μg/ml, based on the BCA assay). The plates were incubated for 4 h at room temperature. Nonspecific protein binding was blocked overnight at 4°C with 5% BLOTTO. Individual serum samples were prepared the following day in 5% BLOTTO and serially diluted twofold down the plate. Individual serum samples (75 μl/well) were also analyzed in wells lacking antigen to determine background binding. Control mouse serum samples (75 μl/well) were added to each plate with pooled final blood samples (dpi 28 or 35) from the groups that received OVA with LT or KLH with LT. Plates were then incubated for 2 h at 37°C to permit antibody binding. Plates were washed six times with 0.05% Tween 20 in PBS (PBS-T) and incubated for 1 h at room temperature with 75 μl of horseradish peroxidase-conjugated goat anti-mouse IgG per well (Sigma Chemical Co.) diluted 1:7,500 in 2.5% BLOTTO. Reactions were developed with 100 μl of 4% 3, 3′, 5, 5′ tetramethylbenzidine (TMB) peroxidase liquid substrate system containing 0.02% hydrogen peroxide (Kirkegaard and Perry Laboratories Gaithersburg, Md.) per well for 8 min. Color development was stopped by adding 100 μl of 1 M phosphoric acid. Absorbance measurements were made at 450 nm. End point titer values are the reciprocal of the highest dilution that had an absorbance value greater than or equal to 0.1 above the background (absorbance of well without antigen).
(vi) ELISAs for fecal OVA-specific IgA, fecal KLH-specific IgA, and total fecal IgA.
Plates were coated as described above, except they were blocked overnight at 4°C with 10% BLOTTO. Individual stool suspensions were assayed for OVA- or KLH-specific fecal IgA. Purified mouse IgA standard (Sigma Chemical Company, St. Louis, Mo.) was diluted in 1% BLOTTO–0.5% fetal bovine serum (FBS), added at an initial concentration of 0.5 μg/ml, and serially diluted twofold down the plate. The plates were incubated at room temperature for 4 h and then blocked with 10% BLOTTO overnight at 4°C. Stool extracts were diluted 1:1 with 2% BLOTTO–1% FBS and serially diluted twofold down the plate containing 1% BLOTTO–0.5% FBS. The plates were incubated for 2 h at 37°C. After six washes with PBS-T, 75 μl of horesradish peroxidase-conjugated goat anti-mouse IgA (Sigma, St. Louis, Mo.) diluted 1:10,000 in 2.5% BLOTTO–0.5% FBS was added to each well. The conjugated antibody was incubated at 37°C for 1 h. The reaction was developed with 100 μl of TMB substrate per well. Color development was stopped by addition of 100 μl of 1 M phosphoric acid per well. Absorbance measurements were made at 450 nm. The level of OVA-specific or KLH-specific IgA was calculated from the linear portion of a standard curve that was determined by the absorbance value of the IgA standard. Total fecal IgA was determined as previously described (3), and each fecal response was expressed as a ratio of nanograms of specific IgA per microgram of total IgA as described above.
(vii) Data and statistical analysis.
Geometric mean titers (GMTs) were determined for every group of mice. All nonresponders were included in the computation of the GMT. The lowest serum dilution tested (1:10) was divided by 2 and used as the titer for the negative samples (i.e., negative samples were assigned a titer of 5). Standard errors were calculated for the log-transformed titers. The mean ratio of specific to total fecal IgA was calculated for each group. The stool samples in which rNV- or OVA-specific IgA levels were below detection were included in the calculation of the mean and assigned a value of one-half the minimum detectable IgA level (31.25 ng). Calculations of the mouse IgA standard curve were done with CA-Crickett Graph III (Computer Associates International, Inc., Islandia, N.Y.). NV-specific vaginal IgA data were expressed in nanograms per milliliter.
Statistical analyses were performed with SPSS version 7.0 for Windows (SPSS, Inc., Chicago, Ill.). Antibody titers or levels of antibodies between groups were compared by using the Kruskal-Wallis test followed by the Mann-Whitney U rank sum test.
RESULTS
i.n. administration of rNV VLPs induces a systemic immune response.
Previous studies showed that high doses (200 μg) of rNV VLPs administered four times orally to mice over a 3-week interval are immunogenic in the absence of adjuvant (3). The present study tested the effectiveness of i.n. administration of low doses of rNV VLPs as immunogens and used as a positive control a simplified regimen of giving two oral doses of 200 μg of VLPs at a 3-week interval. Low doses of VLPs were administered i.n. in the presence or absence of LT(R192G). Serum rNV-specific IgG was lacking (titer, <10) in all preimmune samples taken prior to initial immunization (data not shown). Postimmune (dpi 36) samples from control mice that received PBS (Fig. 1A) or PBS with LT(R192G) also lacked rNV-specific serum IgG (data not shown).
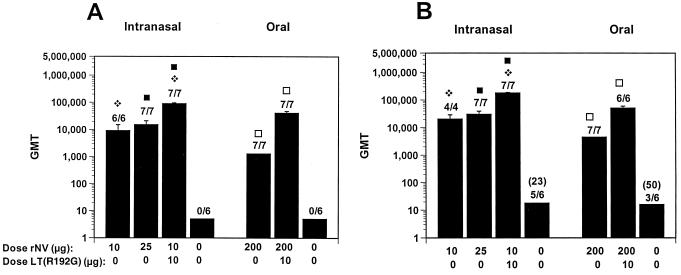
FIG. 1.
Serum IgG responses after in and oral administration to BALB/c mice of rNV VLPs in the presence or absence of LT(R192G). (A and B) Serum IgG responses at 36 dpi (A) and 417 dpi (B). The x axis shows the dose of VLPs administered on days 1 and 21, as well as the presence or absence of adjuvant. The GMT was calculated, including responders and nonresponders. The error bars show the standard error of the mean. Above each column is the number of responders over the total number of mice tested, and the GMT of only the responders is shown in parentheses. Statistical analysis was done with the Mann-Whitney U test. For each panel, identical symbols above two different columns indicate that these two groups were significantly different.
Following the i.n. administration of 10 or 25 μg of rNV VLPs without LT (R192R), 100% of the mice had an rNV-specific serum IgG response, with GMTs of 9,123 and 15,216, respectively (Fig. 1A). Titers varied from 2,560 to 40,280. Coadministration of 10 μg of rNV VLPs with LT(R192G) significantly enhanced (P < 0.05, Mann-Whitney U test) the serum IgG response (GMT of 90,447). Titers varied between 20,480 and 327,680.
The responses to the VLPs administered orally in the presence or absence of LT(R192G) were similar to those seen previously when the VLPs were administered with CT (3). The oral administration of 200 μg of rNV VLPs in the absence of adjuvant induced rNV-specific serum IgG responses (GMT = 1,280; range, 160 to 10,240) in all (100%, n = 7) mice, and antibody titers were enhanced (P = 0.002) when the VLPs were administered with adjuvant (Fig. 1A). There were no responders in the mice that received 10 μg of VLPs orally with LT(R192G) (data not shown). Postimmune (dpi 36) samples from control mice that received PBS with LT(R192G) also lacked rNV-specific serum IgG. The induced antibody responses were long-lived, because rNV-specific serum IgG responses were also detected more than 1 year (417 dpi) after immunization (Fig. 1B).
i.n. administration of rNV VLPs induces mucosal (fecal and vaginal) immune responses.
Individual stool samples were assayed for rNV-specific and total IgA by ELISA. Following the i.n. administration of 10 or 25 μg of rNV VLPs in the absence of adjuvant, 100% of the mice had rNV-specific fecal IgA responses (Fig. 2A). Coadministration of 10 μg of rNV VLPs with LT(R192G) significantly enhanced (P < 0.05) the fecal IgA response to the VLPs.
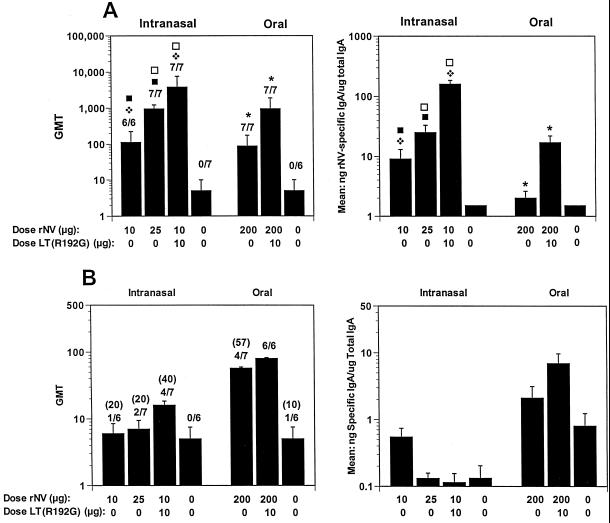
FIG. 2.
Fecal antibody responses after i.n. and oral administration to BALB/c mice of rNV VLPs in the presence or absence of LT(R192G). (A and B) Fecal IgA responses at 36 dpi (A) and 417 dpi (B). The x axis shows the dose of VLPs administered on days 1 and 21, as well as the presence or absence of adjuvant. The left panel y axis shows the GMT of antibody for each group of mice, and the right panel y axis represents the response expressed as mean of the ratio of NV-specific IgA in nanograms per milliliter to total IgA in micrograms per milliliter. The GMT of only the responders is shown in parentheses. Statistical analysis was done with the Mann-Whitney U test. For each panel, identical symbols above different columns indicate that these two groups were significantly different. There were no statistical differences between the groups in panel B.
Specific fecal IgA response was induced in 100% (7/7) of mice after the oral administration of 200 μg of VLPs in the absence of adjuvant (Fig. 2A), a result that was greater than the 83% (10 of 12) of responders of BALB/c mice following immunization with the same dose given in four immunizations (3). The magnitude of the response was significantly higher (P = 0.039) in the group that received 200 μg of VLPs orally in the presence of LT(R192G). No specific fecal IgA was detected in mice that received low doses of VLPs (10 μg) orally in the presence of LT(R192G) (data not shown). The fecal antibody responses induced by i.n. administration of 10 μg of VLPs with adjuvant or oral administration of 200 μg of VLPs with or without adjuvant were still positive at 417 dpi (Fig. 2B). A slightly higher background was obtained in the ELISA at this late time point, because a titer of 10 was obtained for the serum from one of the control mice administered PBS without adjuvant. It is unknown if the slightly higher background occurs spontaneously in some older mice, but animals were kept in microisolaters throughout these experiments and were not exposed to aerosolized VLPs.
Although NV is only known to infect the gastrointestinal tract, rNV VLPs may be used as an antigen delivery system for mucosal immunization. For this purpose, it would be useful to know if immunization with these VLPs can induce antibody in other mucosal sites, such as the vagina. Individual vaginal samples were assayed for rNV-specific and total IgA by ELISA. Following the i.n. administration of 10 μg of rNV VLPs, four of six mice responded, whereas administration of 25 μg of VLPs in the absence of adjuvant or coadministration of 10 μg of rNV VLPs with LT(R192G) resulted in 100% of the mice having rNV-specific vaginal IgA responses (Fig. 3A). There was no significant difference (P > 0.068) in the responses between the groups that received 10 or 25 μg of VLPs in the absence of adjuvant and the group that received 10 μg of VLPs in the presence of LT(R192G).
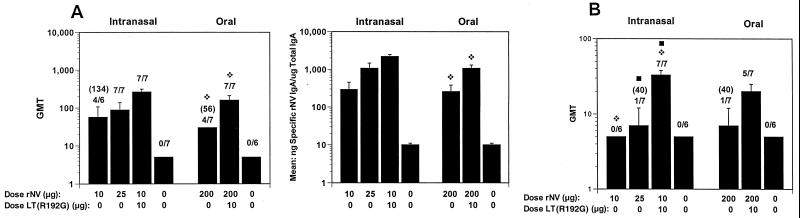
FIG. 3.
Vaginal antibody responses after i.n. and oral administration to BALB/c mice of rNV VLPs in the presence or absence of LT(R192G). (A and B) Vaginal responses at 40 dpi (A) and 365 dpi (B). The GMT was calculated, including responders and nonresponders. The error bars show the standard error of the mean. Above each column is the number of responders over the total number of mice tested, and the GMT of only the responders is shown in parentheses. The y axis on the left panel shows the GMT of antibody for each group of mice, and the y axis on the right panel represents the response expressed as mean of the ratio of NV-specific IgA in nanograms per milliliter to total IgA in micrograms per milliliter. Statistical analysis was done with the Mann-Whitney U test. Identical symbols above two different columns indicate that these two groups were significantly different.
Specific vaginal IgA responses also were induced in the majority of mice (57% [four of seven]) after the oral administration of 200 μg of VLPs in the absence of adjuvant (Fig. 3A). The magnitude of the response was significantly higher (P = 0.015) in the group that received 200 μg of VLPs orally in the presence of LT(R192G). The longevity of vaginal IgA induced by oral or i.n. administration of rNV VLPs was determined. Specific vaginal IgA wanted by 125 dpi in the groups immunized i.n. with 10 μg of VLPs without adjuvant or orally with 200 μg without adjuvant and by 221 dpi in the group immunized i.n. with 25 μg of VLPs without adjuvant (data not shown). Specific vaginal antibody responses were still present 365 dpi for animals immunized with 10 μg of VLPs and adjuvant nasally or 200 μg of VLPs orally with adjuvant (Fig. 3B).
Can orally administered rNV VLPs function as a mucosal adjuvant?
OVA is a soluble protein that is a poor immunogen when given orally. To evaluate if rNV VLPs possess adjuvant properties, OVA was administered orally in the presence or absence of VLPs (Fig. 4). Serum OVA-specific IgG antibody responses were evaluated initially (Fig. 4A). No OVA-specific serum IgG (titer, ≤20) was detected in samples taken prior to initial immunization (data not shown). The group that received OVA with LT(R192G) served as a positive control for evaluation of the adjuvant properties of the VLPs. Postimmune samples from control mice that received PBS with LT or PBS with rNV VLPs lacked OVA-specific serum IgG and fecal IgA (data not shown). After oral administration with 2.5 mg of OVA alone, 33% of the mice (two of six) had an OVA-specific serum IgG response, with a GMT of 40 (Fig. 4A). Coadministration of OVA with 20 or 200 μg of rNV VLPs did not enhance the serologic immune response. Likewise, coadministration of OVA with 20 μg of LT(R192G) did not significantly enhance the specific serum IgG response to OVA, with titers ranging from 80 to 81,920 and a GMT of 2,031. The majority of mice (83%) had a detectable serum response to OVA after coadministration of LT(R192G).
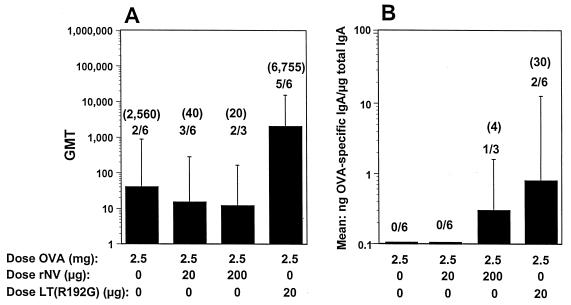
FIG. 4.
BALB/c mouse antibody responses to the oral administration of OVA in the presence of rNV VLPs or LT(R192G). (A) Serum IgG responses. (B) Fecal IgA responses. The x axis shows the dose of OVA, rNV VLPs, or LT(R192G) administered by the oral route. For panel A, the y axis represents the GMT of antibody for each group of mice. For panel B, the y axis represents the response expressed as the mean of the ratio of OVA-specific IgA in nanograms per milliliter to total IgA in micrograms per milliliter. The GMT of only the responders is shown in parentheses. Statistical analysis was done with the Mann-Whitney U test. There were no statistically significant differences between any of the groups.
Assays also were done to detect fecal OVA-specific IgA and total IgA (Fig. 4B). There was no detectable specific fecal IgA in the group that received orally administered OVA (2.5 mg). Coadministration of 20 μg of rNV VLPs did not enhance the immune response. Coadministration of 200 μg of rNV VLPs induced a low detectable response in one mouse of the group. Coadministration of 10 μg of LT(R192G) with OVA resulted in a detectable fecal response in one-third of the mice. These data suggest that rNV VLPs at doses between 20 and 200 μg are not effective as an adjuvant when given orally with OVA at a 2-week interval.
Can i.n. administered rNV VLPs function as a mucosal adjuvant?
rNV VLPs were highly immunogenic when administered i.n. at low doses in the absence of adjuvant (see above). We next tested the responses to OVA administered i.n. in the presence or absence of rNV VLPs (Fig. 5) to evaluate the potential of VLPs as a mucosal adjuvant.
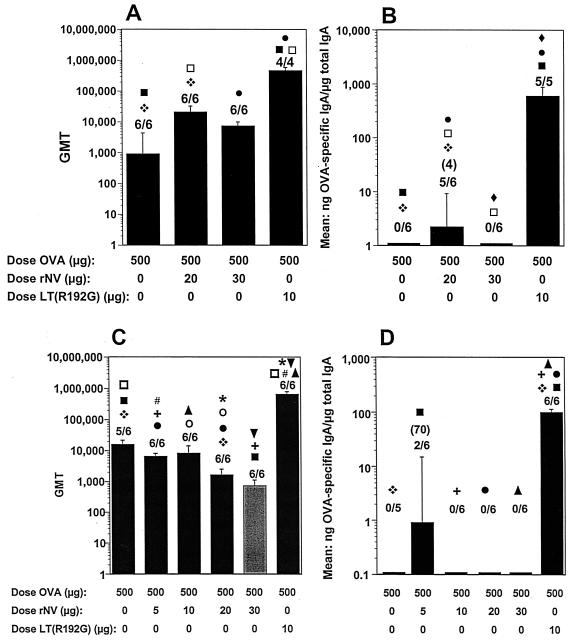
FIG. 5.
BALB/c mouse antibody responses after i.n. administration with OVA in the presence of rNV VLPs or LT(R192G). (A and B) Serum IgG responses (A) and fecal IgA responses (B) 28 dpi for mice immunized with a two week interval. (C and D) Serum IgG responses (C) and fecal IgA responses (D) 35 dpi for mice immunized with a 3-week interval. The x axis shows the dose of OVA, rNV VLPs, or LT(R192G) administered by the i.n. route on days 1 and 14 (A and B) or on days 1 and 21 (C and D). The lightly shaded column received OVA with 30 μg of rNV VLPs on days 1 and 14. For panels A and C, the y axis represents the GMT of antibody for each group of mice. For panels B and D, the y axis represents the response expressed as the mean of the ratio of OVA-specific IgA in nanograms per milliliter to total IgA in micrograms per milliliter. The GMT of only the responders is shown in parentheses. Statistical analysis was done with the Mann-Whitney U test. For each panel, identical symbols above two different columns indicate that these two groups were significantly different.
Postimmune samples from control mice that received PBS with LT(R192G) or PBS with rNV VLPs did not induce OVA-specific serum IgG or fecal IgA (data not shown). Following the i.n. administration of 500 μg of OVA, six of six (100%) of the mice had an OVA-specific serum IgG response, with a GMT of 905 (Fig. 5A). Titers varied between 40 and 20,480. Coadministration of OVA with 20 μg of rNV VLPs significantly enhanced the serum IgG response, with a GMT of 20,480 (P = 0.033). Specific serum IgG was induced in 100% of the mice, and titers varied between 10,240 and 81,920. Coadministration of OVA with 30 μg of VLPs did not significantly enhance the immune response to OVA, while coadministration of 10 μg of LT (R192G) with OVA significantly enhanced the serum IgG response to OVA (GMT of 463,409, P = 0.009). Titers varied between 163,840 and 655,360. OVA-specific serum IgG responses were also significantly higher after coadministration of LT(R192G) when compared to coadministration of OVA and 20 μg of VLPs (P < 0.05).
There was no detectable specific fecal IgA in mice that received i.n. administration of 500 μg of OVA (Fig. 5B). Coadministration of 20 μg of rNV VLPs induced a detectable IgA response, with a mean ratio of 2.2 (P = 0.035); detectable specific fecal IgA was induced in 83% (five of six) of the mice. Coadministration of OVA with 30 μg of VLPs did not induce detectable specific fecal IgA. Coadministration of 10 μg of LT(R192G) enhanced the immune response with a mean ratio of 594 (P = 0.006), with detectable specific IgA in 100% of the mice. These results suggested that rNV VLPs might have some adjuvant activity when 20 μg is coadministered i.n. with antigen.
A follow-up experiment evaluated the response to KLH or OVA administered i.n. in the presence or absence of VLPs on a different immunization schedule from that used previously (3-instead of 2-week intervals between doses) (Fig. 5C and D and 6, respectively). One group (0.5 mg of OVA with 30 μg of rNV VLPs) received two immunizations at a 2-week interval (Fig. 5C and D). Positive control groups received KLH in presence of mutant LT (10 μg) or OVA in presence of mutant LT (10 μg).
Following the i.n. administration of 500 μg of OVA in the absence of adjuvant at a 3-week interval, five of six mice had OVA-specific serum IgG responses, with a GMT of 15,520 (Fig. 5C). Coadministration of 5, 10, or 20 μg of VLPs did not enhance the serum immune response. The GMT of 718 following coadministration of OVA with 30 μg of VLPs at a 2-week interval was statistically lower (P = 0.021) than the GMT of 7,240 following the identical immunization schedule in the previous experiment. The coadministration of OVA with LT enhanced the serum IgG responses, with a GMT of 655,360 (P = < 0.005). Titers in the latter group varied from 327,680 to 1,310,720.
There was no detectable OVA-specific fecal IgA following the i.n. administration of 500 μg of OVA in the absence of adjuvant (Fig. 5D). The coadministration of OVA with 5 μg of rNV VLPs resulted in a weak response in 33% of the mice, with a mean ratio of 0.5, but the VLPs did not significantly enhance the response to OVA (P = 0.58). There was no detectable OVA-specific fecal IgA after the i.n. coadministration of 10 or 20 μg of VLPs or after after coadministration of 30 μg of VLPs at a 2-week interval. Coadministration of 10 μg of LT enhanced the response, with a mean ratio of 99 (P = 0.005), and 100% of the mice responded.
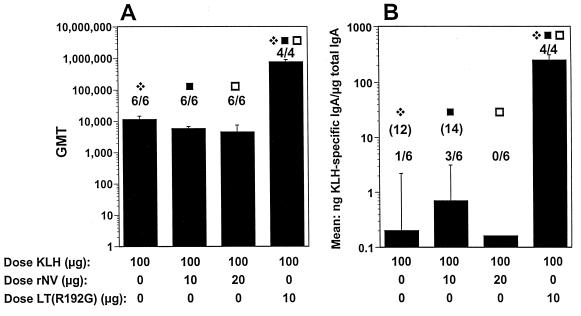
Following the i.n. administration of 100 μg of KLH, 100% (six of six) of the mice had KLH-specific serum IgG responses on dpi 35, with a GMT of 11,494 (Fig. 6A). Coadministration of 10 or 20 μg of VLPs did not enhance the KLH-specific serum IgG response, although LT did significantly enhance these responses (GMT of 1,555,718, P < 0.01). Following the i.n. administration of 100 μg of KLH in the absence of adjuvant, 16% (one of six) of the mice had a KLH-specific fecal IgA response, with a mean ratio of 0.25 (Fig. 6B). The coadministration of 10 or 20 μg of rNV VLPS did not enhance the fecal response. The coadministration of LT enhanced the response to KLH (P = 0.011), with a mean ratio of 250, and 100% of the mice had KLH-specific fecal IgA responses.
FIG. 6.
BALB/c mouse antibody responses after i.n. administration with KLH. (A and B) Serum IgG responses (A) and fecal IgA responses (B) 35 dpi for mice immunized with a 3-week interval. The x axis shows the dose of KLH, rNV VLPs, or LT administered on days 1 and 21. For panel A, the y axis shows the GMT of antibody for each group of mice. The coadministration of rNV VLPs at different doses did not enhance the response, but the response was enhanced with coadministration of 10 μg of LT (P = 0.009; Mann-Whitney test). For panel B, the y axis represents the response expressed as mean of the ratio of KLH-specific IgA in nanograms per milliliter to total IgA in micrograms per milliliter. The coadministration of rNV VLPs did not enhance the response, but the coadministration of LT enhanced the response to KLH (P = 0.011). The GMT of only the responders is shown in parentheses. Statistical analysis was done with the Mann-Whitney U test. For each panel, identical symbols above two different columns indicate that these two groups were significantly different.
Although the initial data (Fig. 5A and B) raised the possibility that 20 μg of VLPs might have some weak mucosal adjuvant activity when coadministered i.n., the repeat experiments with OVA (although with a slightly different immunization schedule) failed to confirm the initial results. Although the serum responses to both KLH and OVA alone were good with the 3-week immunization schedule, LT(R192G) significantly enhanced both the serum and fecal responses in contrast to the VLPs.
DISCUSSION
Our laboratory is interested in the immunogenicity of recombinant gastrointestinal virus vaccines, including VLPs from both NV and rotavirus (2, 3, 7, 29, 30). The route of administration of an immunogen and other variables, such as frequency, dose and timing, are important factors that influence the immune response (21). We are particularly interested in determining if nonreplicating VLPs are immunogenic when delivered by mucosal routes. The particulate nature of the VLPs facilitates mucosal immunization. Although oral immunization has advantages over parenteral immunization, especially for enteric pathogens, vaccination by other mucosal routes is also of interest. This report describes studies that confirm that rNV VLPs administered orally to mice are immunogenic, and use of a simplified protocol with only two oral immunizations with rNV VLPs given in the absence of adjuvant can establish systemic and mucosal immune responses in the majority of mice. Although oral immunization is effective with rNV VLPs, we also showed that lower doses of VLPs can be effective by i.n. immunization.
The oral administration of many antigens, especially nonreplicating antigens, generally does not induce an immune response, but may produce tolerance after repeated exposure. Oral tolerance is not induced by repeated (two or four) oral immunizations with rNV VLPs to mice (2, 3; this study). Our new data show that the i.n. route is more effective than the oral route at inducing specific IgG and fecal IgA responses with 10-fold or lower doses of rNV particles. Only two i.n. immunizations with rNV VLPs in the absence of adjuvant are needed to establish systemic and mucosal immune responses. The i.n. route induced strong serum IgG, fecal IgA, and vaginal IgA responses. Although rNV VLPs are stable at the acidic pH of the stomach, the large doses of VLPs needed to maintain oral immunogenicity suggest that some degradation of the VLPs may occur as these particles traverse the gastrointestinal tract. In contrast, the VLPs given i.n. are not exposed to proteolytic enzymes compared to antigen given by the gastrointestinal tract.
The mechanisms of induction of the antibody responses following i.n. immunization probably relate to interactions between VLPs and aggregates of lymphoid tissue, the nasal associated lymphoid tissue (NALT), found in rodents at the nasopharyngeal opening (31). However, after i.n. immunization in mice, it is uncertain whether NALT is the only or major site of antigen uptake (31, 38). Part of the antigen dose may be swallowed or inhaled after i.n. immunization, resulting in the development of a more general immune response instead of a pure local response (27). Antigen swallowing seems unlikely in our studies, because oral administration of low doses of VLPs was not as effective as i.n. administration of similar doses of VLPs. In anesthesized animals, i.n. dosing has been shown to deliver antigen to the lungs rather than the stomach, at least with human papillomavirus VLPs (4). However, we expect the strong humoral and mucosal antibody responses observed by the i.n. route likely resulted from local processing of antigen, as has been demonstrated with i.n. immunization with human immunodeficiency virus reverse transcriptase (32). rNV VLPs administered i.n. may be taken up by the microfold or membranous epithelial (M) cells present in the NALT and adjacent systemic lymph node compartments (38) or the bronchial associated lymphoid tissue (BALT). Alternatively, the rNV VLPs may interact with a specific cellular receptor, be taken up, and then presented to immune cells. While an interaction with a specific receptor seems unlikely because NV is thought to have a restricted tropism only for humans, this possibility cannot be ruled out, since radioactive rNV VLPs can bind to cultured intestinal cells from both humans and animals, and low levels of internalization into such cells have been detected (37). Future studies will address the mechanism of uptake of VLPs delivered by the different routes.
In our initial study, i.n. immunizations were given in the presence or absence of a mucosal adjuvant. Several mucosal adjuvants have been developed recently in an attempt to enhance the immunogenicity of nonreplicating antigens. While currently no effective adjuvants are approved for oral use in humans, CT and LT are adjuvants tested widely in preclinical studies (9, 35), and mutant toxins are being developed (6, 12, 14, 15, 18). We evaluated one mutant LT, LT(R192G), that has a single amino acid substitution in position 192 that decreases toxicity while still retaining the adjuvant properties. Phase I studies have shown oral administration of LT(R192G) is devoid of significant toxicity (M. J. Oplinger, S. Baqar, A. F. Trofa, J. D. Clements, P. Gibbs, G. Pazzaglia, A. L. Bourgeois, and D. A. Scott, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-10, p. 193, 1997). LT(R192G) was effective as an adjuvant by the i.n. route. Although the presence of adjuvant enhanced the response and longevity of the mucosal responses to rNV, the VLPs administered i.n. without adjuvant elicited strong systemic responses in all of the mice and at least short-term strong mucosal immune responses in the majority of mice.
The inherent immunogenicity of rNV VLPs raised the question of whether the VLPs are capable of functioning as an adjuvant by the i.n. or oral route. The preliminary results in this study are not encouraging, although further work is warranted. In the present study, oral coadministration of low (20 μg) or high (200 μg) doses of rNV VLPs did not increase the serum antibody response to high doses of OVA. High oral doses (200 μg) of rNV VLPs produced some detectable fecal IgA antibody response to OVA, so it might be of interest to determine if greater responses would be induced with higher doses of coadministered VLPs. One dose (20 μg) of the rNV VLPs coadministered i.n. with OVA at a 2-week interval increased both serum and fecal OVA-specific responses, although LT was more effective. It will be worthwhile to test lower doses of OVA and KLH. Also, since KLH and OVA have the tendency to induce oral tolerance, additional studies with tetanus toxoid, a biologically relevant and less toleragenic protein, might more clearly show VLPs can function as an adjuvant.
Phase I human trials have shown rNV VLPs to be safe and immunogenic by the oral route (2). Future studies will need to test if rNV VLPs induce protective immunity when given by the oral or i.n. route to humans. The i.n. route has the advantage that it elicits stronger antibody responses at lower doses of VLPs. These responses are consistently found in mucosal and systemic immune compartments (1). Our data show i.n. administration of rNV VLPs similarily induces both systemic and mucosal (fecal and vaginal) responses in mice. The i.n. route may be an acceptable alternative to oral immunization in humans because topical and nebulized drugs have been used safely for several years in humans. This strategy may target human lungs, which do not have an organized BALT, but have an enormous surface area and an extremely thin tissue lining, which can increase the speed of absorption of many drugs (34) and potential vaccines. Other advantages of the i.n. route of immunization include exposure to a reduced number of proteases in the lung that degrade proteins and peptides. Recent studies have shown that i.n. vaccination in humans with recombinant CT B subunit can elicit specific vaginal IgA and IgG antibodies and antibody-secreting cells (5, 24). It remains unclear if i.n. administration of all VLPs will result in similar responses, but similar data have been obtained in mice with i.n. administration of human papillomavirus VLPs (4).
An important question is whether the preclinical responses detected in mice will induce clinical immunity following NV challenge in volunteers. Currently, this cannot be predicted, because the relationship between host immunity and resistance to infection remains poorly understood for NV. One might assume that mucosal immunity, mainly IgA, will play a key role in protection, since IgA is the predominant antibody at mucosal surfaces and NV infections are localized to the gastrointestinal tract. However, previous volunteer studies did not find a correlation between the presence of IgA (or any antibody) detected by ELISA and protection from challenge with NV (reviewed in reference 16). Antibody may still be protective against infection and disease if neutralizing antibody could be measured. This will require cultivation of NV or development of a suitable animal model, which is not yet available. It is possible that some protection is mediated by innate or NV-specific cellular immune responses, but this remains to be determined. Immunization and challenge experiments with volunteers that measure NV-specific humoral and cellular responses will be needed to answer these questions. Previous studies with volunteers did demonstrate induction of short-term immunity, but these studies did not test optimized vaccination or challenge schedules. It is possible that optimal immunization with VLPs will be more effective (discussed in reference 16). Currently, it is clear that rNV VLPs are immunogenic when administered without adjuvant by mucosal routes to mice and they represent a candidate vaccine for humans. These VLPs can also be considered as a carrier for protective epitopes of other pathogens with enteric, respiratory, or vaginal routes of entry, since the NV capsid is composed of a single protein, the structure of which has recently been determined (33), and these VLPs induce strong immunity in various mucosal sites.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI 42646, AI 65299, and T32-DK07664 and by Advanced Technology Program grant 004949-003 from the Texas Higher Education Coordinating Board.
We thank Robert Atmar, Max Ciarlet, and Sue Crawford for helpful discussions.
REFERENCES
- 1.Almeida A J, Alpar H O. Nasal delivery of vaccines. J Drug Target. 1996;3:455–467. doi: 10.3109/10611869609015965. [DOI] [PubMed] [Google Scholar]
- 2.Ball J M, Graham D Y, Opekun A R, Gilger M A, Guerrero R A, Estes M K. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999;117:40–48. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 3.Ball J M, Hardy M E, Atmar R L, Conner M E, Estes M K. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998;72:1345–1353. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist C, Johansson E-L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong C, Friberg M, Clements J D. LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine. 1998;16:732–740. doi: 10.1016/s0264-410x(97)00255-7. [DOI] [PubMed] [Google Scholar]
- 7.Ciarlet M, Crawford S E, Barone C, Bertolotti-Ciarlet A, Ramig R F, Estes M K, Conner M E. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J Virol. 1998;72:9233–9246. doi: 10.1128/jvi.72.11.9233-9246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke I N, Lambden P R. The molecular biology of caliciviruses. J Gen Virol. 1997;78:291–301. doi: 10.1099/0022-1317-78-2-291. [DOI] [PubMed] [Google Scholar]
- 9.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 10.de Geus B, Dol-Bosman M, Scholten J W, Stok W, Bianchi A. A comparison of natural and recombinant cholera toxin B subunit as stimulatory factors in intranasal immunization. Vaccine. 1997;15:1110–1113. doi: 10.1016/s0264-410x(97)00007-8. [DOI] [PubMed] [Google Scholar]
- 11.de Haan A, Renegar K B, Small P A J, Wilschut J. Induction of a secretory IgA response in the murine female urogenital tract by immunization of the lungs with liposome-supplemented viral subunit antigen. Vaccine. 1995;13:613–616. doi: 10.1016/0264-410x(94)00062-r. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douce G, Fontana M, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2828. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estes M K, Ball J M, Guerrero R A, Opekun A R, Gilger M A, Pacheco S S, Graham D Y. Norwalk virus vaccines: challenges and progress. J Infect Dis. 2000;181:S367–S373. doi: 10.1086/315579. [DOI] [PubMed] [Google Scholar]
- 17.Fankhauser R L, Noel J S, Monroe S S, Ando T, Glass R I. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998;178:1571–1578. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- 18.Freytag L C, Clements J D. Bacterial toxins as mucosal adjuvants. Curr Top Microbiol Immunol. 1999;236:215–236. doi: 10.1007/978-3-642-59951-4_11. [DOI] [PubMed] [Google Scholar]
- 19.Graham D Y, Jiang X, Tanaka T, Opekun A R, Madore H P, Estes M K. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 20.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A-M. Mucosal immunity: implications for vaccine development. Immunobiology. 1992;184:157–179. doi: 10.1016/S0171-2985(11)80473-0. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Graham D Y, Wang K N, Estes M K. Norwalk virus genome cloning and characterization. Science. 1990;250:1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Wang M, Graham D Y, Estes M K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson E-L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan J E, Gary G W, Baron R C, Singh N, Schonberger L B, Feldman R, Greenberg H B. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann Intern Med. 1982;96:756–761. doi: 10.7326/0003-4819-96-6-756. [DOI] [PubMed] [Google Scholar]
- 26.Lambden P R, Caul E O, Ashley C R, Clarke I N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 27.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–273. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 28.Morris C B, Cheng E, Thanawastien A, Cardenas-Freytag L, Clements J D. Effectiveness of intranasal immunization with HIV-gp160 and an HIV-1 env CTL epitope peptide (E7) in combination with the mucosal adjuvant LT(R192G) Vaccine. 2000;18:1944–1951. doi: 10.1016/s0264-410x(99)00447-8. [DOI] [PubMed] [Google Scholar]
- 29.O'Neal C M, Clements J D, Estes M K, Conner M E. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J Virol. 1998;72:3390–3393. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pabst R, Tschernig T. Current view of NALT, LALT, and BALT in humans. Mucosal Immunol Update. 1997;5:1–3. (Abstract.) [Google Scholar]
- 32.Pacheco S E, Gibbs R A, Ansari-Lari A, Rogers P. Intranasal immunization with HIV reverse transcriptase: effect of dose in the induction of helper T cell type 1 and 2 immunity. AIDS Res Hum Retrovir. 2000;16:2009–2017. doi: 10.1089/088922200750054747. [DOI] [PubMed] [Google Scholar]
- 33.Prasad B V V, Hardy M E, Dokland T, Bella J, Rossmann M G, Estes M K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 34.Service R F. Drug delivery takes a deep breath. Science. 1997;277:1199–1200. doi: 10.1126/science.277.5330.1199. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 36.Vinje J, Altena S A, Koopmans M. The incidence and genetic variability of small round-structured viruses in outbreaks of gastroenteritis in The Netherlands. J Infect Dis. 1997;176:1374–1378. doi: 10.1086/517325. [DOI] [PubMed] [Google Scholar]
- 37.White L J, Ball J M, Hardy M E, Tanaka T N, Kitamoto N, Estes M K. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J Virol. 1996;70:6589–6597. doi: 10.1128/jvi.70.10.6589-6597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H Y, Russell M W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]