Abstract
Previous studies utilizing Gag polyprotein budding assays with transfected cells reveal that the equine infectious anemia virus (EIAV) Gag p9 protein provides a late assembly function mediated by a critical Y23P24D25L26 motif (L-domain) to release viral particles from the plasma membrane. To elucidate further the role of EIAV p9 in virus assembly and replication, we have examined the replication properties of a defined series of p9 truncation and site-directed mutations in the context of a reference infectious molecular proviral clone, EIAVuk. Characterization of these p9 proviral mutants revealed new functional properties of p9 in EIAV replication, not previously elucidated by Gag polyprotein budding assays. The results of these studies demonstrated that only the N-terminal 31 amino acids of a total of 51 residues in the complete p9 protein were required to maintain replication competence in transfected equine cells; proviral mutants with p9 C-terminal truncations of 20 or fewer amino acids remained replication competent, while mutants with truncations of 21 or more residues were completely replication defective. The inability of the defective p9 proviral mutations to produce infectious virus could not be attributed to defects in Gag polyprotein expression or processing, in virion RT activity, or in virus budding. While proviral replication competence appeared to be associated with the presence of a K30K31 motif and potential ubiquitination of the EIAV p9 protein, mutations of these lysine residues to methionines produced variant proviruses that replicated as well as the parental EIAVuk in transfected ED cells. Thus, these observations reveal for the first time that EIAV p9 is not absolutely required for virus budding in the context of proviral gene expression, suggesting that other EIAV proteins can at least in part mediate late budding functions previously associated with the p9 protein. In addition, the data define a function for EIAV p9 in the infectivity of virus particles, indicating a previously unrecognized role for this Gag protein in EIAV replication.
Equine infectious anemia virus (EIAV) is a member of the lentivirus subfamily of retroviruses. EIAV is genetically the simplest lentivirus in that it contains only three accessory genes (rev, tat, and S2) in addition to the canonical gag, pol, and env genes. This genetic simplicity offers distinct advantages for using EIAV as a model system to study the basic aspects of lentiviral replication, including mechanisms involved in virion budding. As with all retroviruses, EIAV proviral expression in infected cells produces three predominant polyprotein precursors, Gag, Gag-Pol, and Env, that form the structural and enzymatic proteins of the viral particle (18). The Gag polyprotein of EIAV is about 55 kDa in size and comprises in order the matrix (MA p15), capsid (CA p26), nucleocapsid (NC p11), and p9 core proteins. Despite extensive studies into the molecular mechanisms of retrovirus budding over the past 20 years, the viral and cellular components and processes that mediate virion assembly and budding remain poorly defined.
To focus on the role of Gag proteins in retrovirus assembly and budding, numerous studies have employed assays of virion budding from cells transfected with plasmids expressing Gag polyproteins with or without the viral protease. The results of these studies have in general demonstrated several characteristic Gag functions, including the role of N-terminal myristylation of the MA protein in targeting the Gag polyprotein to the plasma membrane (M-domain) (2, 6, 22, 34, 35), the role of CA domains in multimerization of Gag proteins (I-domain) (7, 17), the role of NC motifs in genomic RNA incorporation, and the role of various Gag late assembly domains (L-domain) in releasing budding virions from the plasma membrane (2, 42, 50).
The retrovirus L-domain is of particular interest, since it is representative of highly specific virus-cell interactions that must occur to direct host cellular processes to achieve virus assembly and budding. Studies to date have demonstrated that different retroviruses may utilize different viral proteins and structural motifs to accomplish the same late budding function. For example, human immunodeficiency virus type 1 (HIV-1) (a lentivirus) (21, 27), Rous sarcoma virus (a type C oncornavirus) (39), human T-cell leukemia virus type 1 (a type C oncornavirus), and Mason-Pfizer monkey virus (a type D retrovirus) (51) all contain proline-rich L-domains consisting of PPXY and/or P(T/S)AP in various Gag protein locations. Interestingly, proline-rich motifs have also been identified in other enveloped viruses, such as vesicular stomatitis virus (a rhabdovirus) (14) and NS3 of bluetongue virus (47), suggesting a common role in viral budding. It has been demonstrated that proline-rich L-domains can in vitro bind specifically with WW-domains of cellular proteins, although there has been no direct demonstration of this putative interaction in virus-infected cells.
In contrast to the proline-rich L-domains frequently found in other viruses, we have used Gag budding assays to define a YPDL L-domain in the Gag p9 protein of EIAV (42) and demonstrated further that this L-domain specifically recruits the cellular adapter protein AP-2 to the sites of viral budding in infected equine cells (43). In addition, the involvement of YXXL domains has recently been demonstrated in tyrosine-based signal transduction and internalization (23, 33, 40) and in the budding and infectivity of other envelope viruses, including HIV-1 (16), bovine leukemia virus (28, 49), and Epstein-Barr virus (4). While the EIAV and HIV-1 L-domains are distinct, we have shown that the YPDL and PTAP motifs are functionally interchangeable and positionally independent in Gag budding assays (39), perhaps indicating different entry points into a common cellular process that facilitates viral budding. Further studies are required to elucidate the virus-cell interactions that so efficiently achieve the assembly and release of viral particles at the plasma membrane.
Although Gag budding assays are useful for determining the function of these proteins in virus assembly and budding, it has been well established that Gag proteins also perform critical functions during virus infection of target cells, indicating the multifunctional nature of these relatively small proteins. Thus, it is likely that the late assembly functions identified with HIV-1 p6 and EIAV p9 reflect only a single aspect of a multifunctional Gag protein. In support of this hypothesis, recent studies have indicated that HIV-1 p6 is also involved in the incorporation of Vpr (3, 29, 31), envelope (36), and polymerase (52) proteins into viral particles, in ubiquitination of Gag polyproteins (41, 45, 47, 48), and in the determination of particle size (19, 20, 27). Thus, it is important that studies of Gag functional properties be performed in the context of virus replication to reveal interactions with other viral and cellular proteins necessary for viral infectivity.
To examine further the function of EIAV p9 in viral replication we have now performed a comprehensive characterization of the replication properties of a series of truncation and site-directed mutants constructed in a reference pathogenic proviral clone, EIAVuk (12). The results of the studies described herein reveal new important insights into the fundamental mechanisms of EIAV assembly and budding that were not evident in previously reported Gag polyprotein budding assays and also indicate a critical role of p9 sequences distinct from the L-domain in viral infectivity. Thus, these data demonstrate the multifunctional roles of EIAV p9 in replication and suggest complex interactions with other viral proteins and with cellular proteins in viral exit and entry.
MATERIALS AND METHODS
DNA mutagenesis.
Two versions, EIAVuk and cmvEIAVuk, of proviral DNA plasmids were used for this report. EIAVuk is an in vivo pathogenic proviral DNA cloned in a low-copy-number plasmid, pLG338-30 (12, 15). When transfected into fetal equine kidney (FEK) and equine dermal (ED) cells, EIAVuk is replication competent and produces viruses that are also infectious to FEK and ED cells. The p9 mutants in the context of the EIAVuk backbone were used to test their replication competencies in both FEK and ED cells. The cmvEIAVuk version is fundamentally the same as EIAVuk except that a 650-bp-long DNA fragment containing the cytomegalovirus promoter was inserted between the U3 and R regions of the 5′ long terminal repeat (LTR) to obtain a higher level of protein expression in transfected Cos-1 cells. Therefore, the cmvEIAVuk constructs were used when large amounts of viruses were needed for biochemical analysis.
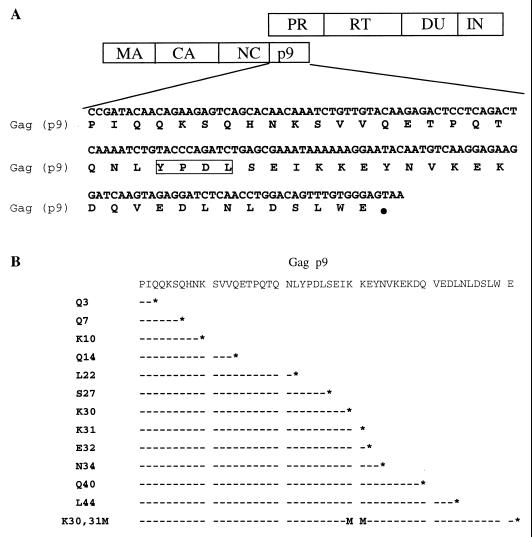
The p9 mutants (Fig. 1) were generated using two methods: ligation-mediated PCR or two rounds of PCR using an overlap extension procedure (9, 25). A series of p9 truncations was constructed by introducing stop codons at different positions of the p9 coding sequence. Site-directed mutation of K30K31 to M30M31 was constructed similarly using the overlapping PCR method. All mutants were sequenced to confirm the specified mutations. Because the pol reading frame overlaps with gag at the p9 region, certain mutations resulted in concomitant alterations in the preprotease sequence, but they were found not to affect mature protease activity.
FIG. 1.
Summary of p9 truncations engineered into the reference EIAVuk proviral clone. (A) Diagram of the Gag and Pol genome and amino acid sequences of EIAV p9. The boxed YPDL is the L-domain for EIAV budding and release. (B) Schematic summary of p9 truncations. Each p9 mutant is denoted by a single-letter amino acid followed by a number to represent the residue of p9 that is replaced with a stop codon. For example, the K31 mutant designation indicates that the lysine residue at the 31st position of p9 is replaced with a stop codon (asterisk).
Cell transfection.
FEK, ED, and African green monkey kidney Cos-1 cells were cultured in minimal essential medium. All media were supplemented with 2 mM l-glutamine, 10% (vol/vol) fetal bovine serum, 100 U of penicillin, and 100 μg of streptomycin per ml. The cell culture products were obtained from Life Technologies Inc. (Gibco BRL). All plasmid DNA preparations used for transfection were isolated using the Midiprep kit (Qiagen). Transfections were carried out using GenePorter 2 reagents (Gene Therapy Systems) according to the manufacturer's recommendations. Typically, 60 mm petri dishes were used in this study unless otherwise noted. Briefly, a total of 6 μg of purified plasmid DNA was mixed with DNA diluent and GenePorter 2 lipid film and then added to cells that were split the day before transfection and at 50 to 70% confluence. One day after transfection, media were replaced with fresh minimal essential medium.
Reverse transcriptase (RT) assays.
Approximately 10 to 15 μl of cell culture supernatant was used for each RT activity assay as described previously (30). In certain cases, supernatant virus was first pelleted by ultracentrifugation, and the viral pellet was resuspended for the RT assay. Briefly, the supernatant was mixed with 50 μl of reaction buffer containing 100 mM Tris-HCl (pH 8.0), 12 mM KCl, 10 mM MgCl2, 10 mM dithiothreitol, 0.1% NP-40, 10 U of poly(rA) · poly(dT)12-13 (Amersham Pharmacia Biotech), and 0.25 μl of [3H]TTP (TRK424; Amersham). After 1.0 to 1.5 h of incubation at 37°C, the mixtures were transferred to a DEAE membrane (catalog no. 1205-405; Wallac Inc.) and dried. The membrane was then washed three times in 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate) for 10 min each time. The membrane was quickly rinsed with 95% ethanol to facilitate drying. Finally, the membrane was assayed for radioactivity with a 96-well reader in a Wallac Betaplate liquid scintillation counter.
Protein analysis.
Clarified transfection supernatants were centrifuged to pellet viral particles through a 20% glycerol–phosphate-buffered saline cushion in an SW55 rotor at 34,800 rpm at 4°C for 50 min. Then the viral pellets were resuspended in phosphate-buffered saline solution for protein analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. To examine the intracellular expression of viral proteins, transfected Cos-1 cells grown on 60-mm-diameter dishes were lysed with 500 μl of lysis buffer (25 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% deoxycholic acid, 1% Triton 100, protease inhibitor cocktail, 0.1% SDS). Immunoblot analysis was performed as previously described (11). SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, Ill.) was used to visualize the bands after blotting with a monoclonal antibody against p26 (10) or a reference horse (Lady) immune serum (32). Horse peroxidase-conjugated goat anti-horse immunoglobulin G (IgG) F(ab′)2 was purchased from Jackson ImmunoResearch, and anti-mouse immunoglobulin G peroxidase conjugate was purchased from Sigma (catalog no. A4416).
RESULTS
Characterization of EIAV p9 sequences required for replication competence.
We have previously reported that the deletion of EIAV p9 or site-directed mutagenesis of the p9 Y23P24D25L26 L-domain produces a defect in the release of budding virus particles from Cos-1 cells transfected with Gag expression plasmids (42). To extend the p9 function observations made in the Gag budding assay, we sought to examine the role of EIAV p9 in viral replication in equine cells. Towards this goal, we generated a series of p9 truncation mutants in our reference infectious proviral clone (12), EIAVuk, by introducing termination codons at various positions in the p9 sequence, as summarized in Fig. 1. Although these mutations also altered the preprotease sequences in the overlapping pol reading frame, the coding sequences of mature viral protease were not affected and the protease activity was retained (see below). EIAV p9 truncation mutations were designated according to the site of the engineered termination codon. For example, K31 substitutes a termination codon at the position of lysine-31, such that the truncated protein product should contain only the N-terminal 30 amino acids of p9. A total of 12 different C-terminal truncations were constructed to express protein products containing the N-terminal segments of the parental (51 amino acids) p9 protein ranging in length from 2 to 44 amino acids.
The replication competence of each EIAV p9 truncation mutant was then evaluated in parallel in transfected ED cells by measurements of extracellular RT activity at various time points posttransfection. As summarized in Fig. 2, the results of these transfection experiments revealed a striking discrimination between replication-competent and defective p9 truncation mutants. Transfection of ED cells with proviruses expressing truncated p9 proteins containing 30 or fewer of the N-terminal amino acids of the parental p9 protein yielded only background levels of extracellular RT activity over the 35-day observation period, indicating that these mutant p9 proviral clones were replication defective. The mutant p9 proviruses that were replication defective in transfected equine cells included K10, S27, and K31 (as shown in Fig. 2), as well as Q3, Q7, Q14, L22, and K30 (data not shown). In contrast to these replication-defective truncation mutants, proviruses expressing 31 or more of the N-terminal amino acids of EIAV p9 were replication competent in transfected ED cells, as evidenced by the production of increasing levels of extracellular RT over the 35-day observation period. Replication-competent p9 truncation mutants included L44, N34, and E32 (Fig. 2), as well as Q40 (data not shown). These transfection experiments conclusively demonstrated that only the N-terminal 31 amino acids of p9 were required for replication competence.
FIG. 2.
Replication properties of EIAV proviral p9 mutants in transfected ED cells as monitored by extracellular RT activity. ED cells were transfected in parallel with the various proviral mutant DNA samples, and supernatants from transfected cells were collected at weekly intervals posttransfection for measurements of RT activity, as described in Materials and Methods. Proviral p9 mutants are under the EIAVuk backbone as described in Fig. 1.
Interestingly, the transition from replication competence to the replication-defective phenotype was absolute, with a truncation of only one additional amino acid. Thus, the E32 p9 truncation was replication competent, but the K31 truncation was completely replication defective, as were all larger truncations. While the proviral clones expressing at least 31 amino acids of p9 were replication competent in transfected ED cells, these p9 proviral mutants characteristically displayed a lag in virus replication kinetics compared to the parental EIAVuk (Fig. 2), despite standardization of the transfection procedures in repeated experiments. This lag in replication could not be attributed to a slow reversion of the termination codons introduced into the various p9 truncation mutants, since sequencing of virus produced by replication-competent p9 truncation proviruses revealed a conservation of the engineered termination codons. It is important to also note that transfections of cultured primary FEK cells revealed a similar pattern of replication competence among the p9 truncations, indicating that the proviral p9 mutant replication phenotypes were not cell dependent.
Effects of p9 truncations on extracellular virion production
While the preceding experiments define the minimum p9 sequences required for EIAV replication, they do not elucidate the specific defect in p9 truncations that fail to support viral replication. In the light of previous studies indicating the role of EIAV p9 in virus budding, one possible explanation is that replication-defective p9 truncation mutants failed to produce virus particles from transfected cells. To examine this hypothesis, we next assayed the level of extracellular virion production by both replication-competent and -defective p9 truncation mutants compared to the parental EIAVuk provirus. For these assays we utilized a cmvEIAVuk proviral plasmid in which viral gene expression is driven by a cytomegalovirus promoter inserted into the viral LTR to increase levels of viral gene expression in transfected Cos-1 cells. The p9 truncations shown in Fig. 1 were subcloned into cmvEIAVuk expression plasmids, and the resulting constructs were then used to transfect Cos-1 cells. Cos-1 cells are not susceptible to infection by EIAVuk and thus were chosen as transfection targets to prevent subsequent rounds of infection by progeny virions produced from the transfected proviral clones.
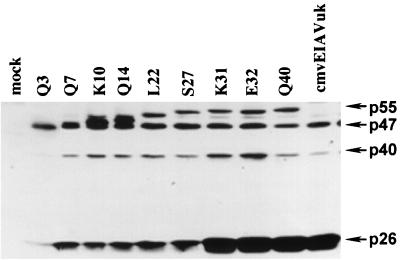
The cultured Cos-1 cells were transfected in parallel with equal amounts of DNA containing the respective p9 truncations in cmvEIAVuk expression plasmids. Three days after transfection, the medium was collected from the respective cell cultures, and EIAV virions were pelleted from equal volumes of supernatant medium by ultracentrifugation. The quantity of extracellular viral particles produced was then analyzed by SDS-PAGE and immunoblotting with a high-titer reference equine immune serum to identify virion Gag proteins. The data in Fig. 3 demonstrate that the p9 truncations produced viral particles at slightly different levels. The p9 truncations containing 30 or more N-terminal amino acids, including K31, E32, and Q40, produced levels of extracellular viral particles similar to those of the parental cmvEIAVuk provirus. The p9 truncations containing only 26 or fewer N-terminal amino acids of p9 produced about 2- to 3-fold fewer extracellular virions than the parental cmvEIAVuk provirus.
FIG. 3.
Effects of p9 truncations on production of extracellular virions. Cos-1 cells were transfected in parallel with equal amounts of cmvEIAVuk plasmid DNA preparations from the indicated p9 truncation mutants. After 3 days, equal volumes of supernatant medium from each culture were collected and subjected to ultracentrifugation to pellet virus particles. The quantities of virions from different p9 truncations were then analyzed by SDS-PAGE and immunoblotting with a high-titer reference equine immune serum to blot virion Gag proteins. Full-length Gag precursor p55, the intermediates p47 (MA-CA-NC) and p40 (MA-CA), and the mature capsid protein p26 are indicated by arrows. The data presented in this figure are representative of at least three independent transfections. Estimations of relative levels of extracellular virion Gag protein production by each proviral mutant were made using densitometer scans of Gag protein bands in the immunoblot.
The fact that virion production was reduced with p9 truncation mutants missing the p9 L-domain (Y23P24D25L26) was in agreement with the previously demonstrated role of this motif in facilitating viral budding. However, the observed reduction in extracellular virion production alone could not be correlated with the absolute nature of the replication phenotypes. This lack of correlation is best exemplified by the observation that the extracellular virion production was virtually identical between the replication-defective K31 mutant and the replication-competent E32 mutant (Fig. 3).
Taken together, these data definitively demonstrated that the lack of replication competence observed with p9 truncation mutants likely cannot be attributed to a defect in extracellular virion production, assuming that the transfected Cos-1 cells utilized for Fig. 3 accurately reflect viral budding in the ED cells used for Fig. 2. The data also indicated for the first time that the EIAV p9 protein is not absolutely required for extracellular particle production in the context of proviral gene expression, suggesting that viral proteins other than p9 may mediate viral budding, albeit at somewhat reduced levels. This observation is in distinct contrast to previous studies using EIAV Gag budding assays that indicate a requirement for the p9 protein and its L-domain for efficient budding from Cos-1 cells transfected with Gag expression plasmids (42).
Effects of p9 truncations on virion RT incorporation and activity.
Another possible explanation for the replication-defective phenotype of the p9 truncation mutants is that the p9 protein may perform a role in Pol incorporation into viral particles, as previously described for HIV-1 p6 (52). Alternatively, truncations in p9 may cause defects in Gag-Pol processing, preventing the activation of protease and the maturation of RT in viral particles. To test directly the influence of p9 truncations on virion RT incorporation and activity, we analyzed the levels of RT activity in viral particles produced by replication-competent and -defective p9 truncations, normalized to total Gag protein content. After 3 days, virus particles produced from the Cos-1 cells transfected with each p9 truncation mutant were pelleted from the culture medium by ultracentrifugation and resuspended to achieve a standard concentration of EIAV Gag proteins. Then equal amounts of virus were assayed for virion RT activity (Fig. 4). The results of these assays revealed similar amounts of RT activity per unit of Gag protein, regardless of the portion of p9 contained in the Gag polyprotein. For example, virions produced from a truncation mutant (Q3) containing only the N-terminal two amino acids of p9 displayed about 90% of the RT activity observed in the same amount of viral particles produced by the parental EIAVuk provirus containing a full-length p9 protein. Similarly, the replication-competent E32 and replication-defective K31 viral particles displayed virtually identical normalized RT values. These data indicated that the EIAV p9 protein does not influence incorporation of Pol into viral particles or the cleavage processing of Gag-Pol precursor proteins to active RT in the virion. Thus, the replication-competent and replication-defective phenotypes of p9 truncation mutants evidently cannot be correlated with RT incorporation or processing. The cleavage and activation of the RT enzyme from the Gag-Pol polyprotein also support the conclusion that the various p9 mutations did not affect viral protease activity, despite modifications in the preprotease sequences in certain mutants.
FIG. 4.
Effect of p9 truncations on incorporation of RT activity into progeny virions. Cos-1 cells were transfected in parallel with equal quantities of the indicated cmvEIAVuk proviral plasmid DNAs using standard transfection conditions. After 3 days, virus particles were pelleted by centrifugation of equal volumes of cell supernatant medium, and the quantity of EIAV Gag p26 was determined by Western blotting with a reference murine monoclonal antibody, as described in Materials and Methods. Based on p26 content, equal amounts of EIAV virions were then assayed for RT activity. The presented data represent the averages of results from at least three experiments.
Effects of p9 truncations on Gag protein expression and processing.
The preceding studies indicated similar levels of virion production and RT activity in viral particles produced by replication-competent and -defective p9 truncation proviral mutants but did not address the issue of Gag polyprotein expression in transfected cells and maturation cleavages in budding virions. Thus, we next compared the levels of Gag polyprotein expression and processing in Cos cells transfected with selected replication-competent and replication-defective p9 mutant proviruses. As described above, we once again utilized our cmvEIAVuk expression plasmids to achieve high levels of proviral gene expression in transfected Cos-1 cells. After 3 days, the supernatants were harvested from the transfected cells and analyzed for virus production, while the transfected cells were lysed, and the cellular extracts were analyzed for EIAV Gag protein expression and processing.
Figure 5A presents the patterns of Gag protein expression in the Cos-1 cells transfected with various p9 truncation proviral mutants, as revealed by Western blotting of cellular extracts with a reference equine immune serum. As demonstrated in the Western blot for the parental cmvEIAVuk transfection, the immunoblot detects the intracellular expression of full-length Gag polyprotein (p55 containing p15-p26-p11-p9), various cleavage intermediates, such as p47 (p15-p26-p11) and p40 (p15-p26), and the mature capsid protein p26. The decreasing apparent molecular weight of the intracellular “p55” Gag polyproteins produced by the various p9 mutants clearly reflects the smaller size of these polyprotein species due to the p9 truncations.
FIG. 5.
Effects of p9 truncations on Gag protein expression and proteolytic processing. Equal amounts of plasmid DNA samples containing the respective p9 truncation mutations were used to transfect Cos-1 cells. After 3 days, the transfected cells and supernatant medium were collected for analysis of intracellular and virion-associated EIAV Gag proteins, respectively. (A) Transfected cells were lysed, and intracellular EIAV Gag protein expression was analyzed by SDS-PAGE and Western blotting using the reference (Lady) equine immune serum. (B) Supernatant media from the transfected cells were subjected to ultracentifugation to pellet virus particles that were resuspended and quantified for p26 content using a murine monoclonal antibody. Based on virion p26 content, equal amounts of pelleted virus were then analyzed by SDS-PAGE and immunoblotting with Lady serum for Gag protein profiles. The various p9 mutants are listed on the top of the gel. The full-length Gag precursor p55 is indicated with an arrow. The p9 truncation mutations generated a series of Gag precursors with decreasing molecular weights from right to left, as expected. The major processing intermediates, p47 (MA-CA-NC) and p40 (MA-CA), and mature capsid protein p26 are also indicated by arrows.
The data shown in Fig. 5A indicate similar levels of intracellular Gag protein expression and processing in cells transfected with the parental cmvEIAVuk and the selected replication-competent and -defective proviral clones containing various p9 truncations. From these data it could be concluded that the observed differences in replication phenotypes could not be absolutely correlated with differences in Gag polyprotein expression levels or in the efficacy of intracellular processing of the Gag polyprotein. The similarity in Gag polyprotein expression levels and processing between a replication-competent and -defective p9 proviral mutant is made readily apparent by a comparison of the adjacent lanes analyzing the replication-competent E32 proviral expression and the replication-defective K31 proviral expression.
Figure 5B summarizes the Gag protein profiles of extracellular virions isolated from the Cos-1 cells transfected with the various truncated p9 proviral expression plasmids. For this analysis, virus particles were pelleted from cell supernatants by ultracentrifugation, and the resuspended viral pellet was analyzed for EIAV Gag protein concentration by Western blotting with the reference equine immune serum. Equal amounts of pelleted Gag protein from each proviral DNA transfection were then analyzed by SDS-PAGE and immunoblotting to provide a comparison of the extent of protein processing in a standardized amount of virion-associated Gag. The virion protein profiles in Fig. 5B revealed a difference in the extent of Gag polyprotein processing in the viral particles that was not evident intracellularly (cf. Fig. 5A). As observed in the cmvEIAVuk particles, Gag polyprotein processing to mature capsid p26 appeared to proceed equally efficiently in the parental cmvEIAVuk particles compared to the p9 truncation mutants Q40, E32, and K31. However, uncleaved Gag polyprotein became more evident in the viral particles produced by the S27 and L22 p9 truncations, and the levels of uncleaved Gag polyprotein became predominant in the viral particles produced by the p9 truncations Q14, K10, Q7, and Q3. These observations suggest that the EIAV p9 protein can influence the efficacy of proteolytic processing of the Gag polyprotein during viral budding and maturation, either by altering the Gag polyprotein structure or influencing viral protease activity. These defects in Gag polyprotein proteolytic processing could contribute to the replication-defective nature of viral particles produced by the more extensive p9 truncations but are not significant in virions containing the shorter p9 truncations.
However, the defect in Gag polyprotein processing did not correlate consistently with the replication-competent and replication-defective phenotypes among p9 truncation mutants. For example, the replication-competent E32 mutant and the replication-defective K31 mutant displayed apparently identical Gag polyprotein processing efficiency, despite the absolute differences in the infectivities of the respective virions. Therefore, these observations demonstrated that the Gag polyprotein processing defects were not responsible for the inability of the K31 mutant viral particles to successfully infect cells.
Role of the K30K31 sequences of p9 in viral replication.
The preceding studies together focused attention on the K30K31 segment of EIAV p9 as being critical for the maintenance of replication competence. In this regard, recent studies have revealed that 2 to 5% of HIV-1 p6 found in virions is monoubiquitinated at lysine residues, perhaps suggesting an involvement of ubiquitination in HIV-1 assembly or infectivity (38, 45). We next examined the potential role of this p9 segment in viral replication by mutating the K30K31 doublet to M30M31 in the context of the EIAVuk proviral clone. Methionine substitutions were selected to maintain the general size properties of the lysine residues, while changing the charge and abolishing the potential for ubiquitination at this site. The replication properties of the KK/MM p9 mutant were then compared in parallel to those of the parental EIAVuk in transfected ED cells, as described for the p9 truncation mutants (cf. Fig. 2). The data in Fig. 6 demonstrated similar replication kinetics and levels for the KK/MM p9 mutant and the parental EIAVuk provirus in transfected ED cells. Thus, these data indicated that the K30K31 sequence of p9 could be functionally replaced by an MM sequence, evidently suggesting that the charged residues and their potential ubiquitination were not required for viral budding and infectivity of EIAV. These observations are consistent with recent studies indicating that the two lysine residues in HIV-1 p6 that are targets for ubiquitination can be mutated with any effect on virus replication (37).
FIG. 6.
Role of the K30K31 sequences of EIAV p9 in viral replication. The EIAVuk proviral clone was specifically mutated to substitute methionine residues for both the K31 and K32 residues. The replication properties of the KK/MM mutant were then analyzed by transfection of ED cells and compared to a parallel transfection with an identical amount of the parental EIAVuk proviral plasmid. Viral replication was monitored by extracellular RT activity, as described in the legend for Fig. 2.
DISCUSSION
We have previously reported experiments using Gag polyprotein budding assays that indicated a critical role for the EIAV p9 protein in the release of budding virions from transfected Cos-1 cells, similar to the assembly role defined for HIV-1 p6 (8, 42). Based on selected mutagenesis of the EIAV p9 protein, we further defined a YPDL motif in p9 that provided the late budding function mediated by PTAP-based L-domains in HIV-1 Gag polyprotein budding, and we demonstrated a specific interaction of EIAV p9 with the adapter protein AP-2 in virus-infected equine cells (43). The results of these studies suggest that the EIAV p9 protein, and the YPDL L-domain in particular, may specifically recruit components of the cellular endocytosis machinery for viral budding from plasma membranes of infected cells.
Despite the informative nature of the Gag polyprotein budding assays for assessing p9 function, we remained concerned about two limitations of these functional assays. First, the functions of p9 are defined in the absence of the expression of other non-Gag viral proteins that may also contribute to viral assembly and budding. For example, interactions between Gag and Env proteins influence polarity of virus budding and release (13, 26, 36). In addition, certain accessory gene proteins, such as Vif, appear to influence virus assembly in HIV-1 (5) and caprine arthritis encephalitis virus (24), and Vpu can enhance virus particle release through an L-domain-independent mechanism (46). Second, the Gag polyprotein budding system monitors p9 functions related to virus assembly but does not monitor the potential functions of the p9 protein in virus infection of target cells. The latter limitation has become increasingly relevant as there has been accumulating evidence that retroviral Gag proteins perform critical functions during both viral infection and budding. Therefore, we initiated the present studies to examine the functional roles of EIAV p9 in the context of an infectious proviral clone and a full complement of viral protein expression in infected or transfected cells.
The results of the present studies indeed reveal for the first time novel aspects of p9 function in viral replication that were not evident from Gag polyprotein budding assays. The analysis of a series of p9 truncation mutants in the context of a reference proviral construct clearly demonstrated that only the first 31 amino acids of the p9 protein are required for replication competence, suggesting that the C-terminal 20 amino acids are not essential for viral budding or infection. However, it should be noted that replication-competent p9 proviral mutations did display delayed replication kinetics and lower replication levels than the parental EIAVuk proviral clone, indicating an optimization of p9 functional interactions by the complete protein. It is noteworthy that all of the replication-competent p9 truncation mutants retained the YPDL L-domain of p9 and that replication-defective truncation mutants lacking the YPDL sequences displayed threefold-lower levels of virus budding than parental EIAVuk. These observations are in general in agreement with the previously defined role of p9 in virus budding from the plasma membrane. However, several of the replication-defective p9 truncation mutants also contained the YPDL L-domain, indicating the presence in p9 of other functional domains necessary for viral replication.
In this regard, a remarkable observation was the absolute demarcation between replication-competent and replication-defective phenotypes in the p9 C-terminal truncation series with the progression from the E32 to K31 mutants that differed by only one amino acid. These p9 variants displayed similar levels of expression and processing of Gag polyproteins, production of extracellular virions, and incorporation of virion-associated RT activity. Despite these similarities in viral gene expression, processing, and viral budding, the virions produced by the E32 mutant were replication competent and the K31 virions were completely replication defective. These observations indicate for the first time a critical function of the p9 protein in viral infectivity, in addition to its previously defined role in viral budding. Based on the hypothesis that the EIAV p9 protein recruits cellular endocytosis machinery to facilitate viral budding, it is possible that the p9 protein may interact with cellular endocytic molecules during viral penetration and uncoating in target cells. This model then implies that the N-terminal 30-amino-acid sequence of p9 is functionally budding competent and infectivity defective, while the addition of a single amino acid restores the infectivity function.
The replication phenotypes associated with the series of C-terminal p9 proviral truncation mutants clearly highlighted the highly charged K31E32 sequences as a critical functional borderline in terms of viral infectivity and thus replication competence. In light of recent reports indicating the importance of the ubiquitination of HIV-1 p6 lysine residues for viral assembly, it seemed plausible that the functional properties of EIAV p9 may depend on ubiquitination of the K30K31 residues at the functional border. To test this hypothesis directly, we engineered and evaluated the replication properties in transfected ED cells of a proviral mutant in which the K30K31 residues of p9 were mutated to M30M31. The results of these transfection experiments revealed that the KK/MM p9 proviral mutant displayed replication kinetics and levels similar to those of the parental EIAVuk provirus in transfected cells. These observations demonstrated conclusively that the K30K31 residues are not required for EIAV budding or infectivity, and thus ubiquitin modification of these particular lysines cannot be an important factor in viral replication, although it does not exclude the possibility of the ubiquitination of other lysine residues in p9. Thus, these EIAV studies suggest that additional experiments are required to assess further the importance of ubiquitination as a common factor in the budding and infectivity of retroviruses.
In addition to revealing a functional role of EIAV p9 in viral infectivity, the comparative study of the series of p9 truncation proviral mutations also elucidated new aspects of the role of p9 in viral assembly and budding that were not evident from earlier Gag budding assays (42). For example, Gag budding assays indicated an absolute requirement for the YPDL L-domain for virion budding from transfected Cos-1 cells; Gag mutants lacking the p9 protein or with a YPDL deletion or specific L-domain point mutations in the Gag polyprotein failed to produce detectable extracellular particles from transfected cells (cf. Fig. 5 and reference 42). In contrast, the present experiments using p9 mutations in the context of a complete provirus genome clearly demonstrated that virion budding was reduced by only about threefold in truncation variants lacking the YPDL L-domain. These marked differences in the influence of the p9 mutation and its L-domain on virion budding efficiency in the Gag polyprotein and proviral budding assays cannot be attributed to the differences in experimental conditions. Both assays utilized transfection of Cos-1 cells with expression plasmids that produced similar levels of viral protein. Thus, we interpret these observations to imply that other non-Gag proteins may also contribute to viral budding, perhaps by providing a redundant and somewhat less efficient L-domain budding function. These results also emphasize the importance of assessing viral protein functions in the context of replicating virus and natural target cells.
The present studies also demonstrated for the first time new information about the role of EIAV p9 and other viral proteins during assembly. Several published reports have indicated the importance of HIV-1 p6 in the incorporation of viral RT in virions (52), but this function does not appear to be associated with the EIAV p9 protein. Both replication-competent and -defective p9 truncation proviral mutants contained similar levels of RT activity when normalized to virion Gag protein content. Thus, the infectivity defect observed in proviruses containing less than the N-terminal 31 amino acids of p9 could not be attributed to a lack of Pol protein incorporation or processing to active RT. We interpret the EIAV data to indicate that the primary function of EIAV Gag p9 is interaction with cellular proteins rather than other viral proteins. In this regard, it is of interest that localization experiments indicate a lack of association of p9 with other viral core proteins (44). Also, the fact that Gag p6 is absent from HIV-1 core preparations (1) is consistent with our interpretation.
Taken together, our data are consistent with a general model in which EIAV p9 performs at least two functions during viral replication, one related to viral budding and the other related to viral infectivity. The viral budding function has previously been correlated at least in part with a specific recruitment by p9 of the cellular adapter protein AP-2 at the site of viral budding. It is possible that the infectivity function of p9 may be mediated by a similar interaction, since the cellular adapter proteins are part of the endocytic machinery of the cell and localized to the plasma membrane where virus penetration occurs. Alternatively, p9 may interact with different components of the endocytosis and exocytosis machinery of the cell during viral budding and infection. These possible mechanisms are currently under investigation. Regardless of the exact functional mechanism of EIAV p9, the present studies support the concept that the “structural” proteins of retroviruses likely perform multiple functions in adapting cellular processes to efficiently accomplish viral assembly and budding from cells and effective penetration and uncoating during infection of target cells. A better understanding of these Gag-related functions can lead to the identification of new virus-specific functions that can be targeted by novel antiviral drugs.
ACKNOWLEDGMENTS
Chaoping Chen and Feng Li contributed equally to this study.
This work was supported by National Institutes of Health grant 5RO1 CA49296 from the National Cancer Institute.
We acknowledge the assistance of the DNA Sequencing Facility of the Biomedical Research Support Facilities at the University of Pittsburgh.
REFERENCES
- 1.Accola M A, Ohagen A, Gottlinger H G. Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6(gag) J Virol. 2000;74:6198–6202. doi: 10.1128/jvi.74.13.6198-6202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accola M A, Strack B, Gottlinger H G. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J Virol. 2000;74:5395–5402. doi: 10.1128/jvi.74.12.5395-5402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachand F, Yao X J, Hrimech M, Rougeau N, Cohen E A. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 gag precursor. J Biol Chem. 1999;274:9083–9091. doi: 10.1074/jbc.274.13.9083. [DOI] [PubMed] [Google Scholar]
- 4.Beaufils P, Choquet D, Mamoun R Z, Malissen B. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 1993;12:5105–5112. doi: 10.1002/j.1460-2075.1993.tb06205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borman A M, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burniston M T, Cimarelli A, Colgan J, Curtis S P, Luban J. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J Virol. 1999;73:8527–8540. doi: 10.1128/jvi.73.10.8527-8540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell S, Rein A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J Virol. 1999;73:2270–2279. doi: 10.1128/jvi.73.3.2270-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Sheng S, Shao Z, Guo P. A dimer as a building block in assembling RNA. A hexamer that gears bacterial virus phi29 DNA-translocating machinery. J Biol Chem. 2000;275:17510–17516. doi: 10.1074/jbc.M909662199. [DOI] [PubMed] [Google Scholar]
- 10.Chong Y H, Payne S L, Issel C J, Montelaro R C, Rushlow K E. Characterization of the antigenic domains of the major core protein (p26) of equine infectious anemia virus. J Virol. 1991;65:1007–1012. doi: 10.1128/jvi.65.2.1007-1012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook R F, Berger S L, Rushlow K E, McManus J M, Cook S J, Harrold S, Raabe M L, Montelaro R C, Issel C J. Enhanced sensitivity to neutralizing antibodies in a variant of equine infectious anemia virus is linked to amino acid substitutions in the surface unit envelope glycoprotein. J Virol. 1995;69:1493–1499. doi: 10.1128/jvi.69.3.1493-1499.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook R F, Leroux C, Cook S J, Berger S L, Lichtenstein D L, Ghabrial N N, Montelaro R C, Issel C J. Development and characterization of an in vivo pathogenic molecular clone of equine infectious anemia virus. J Virol. 1998;72:1383–1393. doi: 10.1128/jvi.72.2.1383-1393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosson P. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 14.Craven R C, Harty R N, Paragas J, Palese P, Wills J W. Late domain function identified in the vesicular stomatitis virus M protein by use of rhabdovirus-retrovirus chimeras. J Virol. 1999;73:3359–3365. doi: 10.1128/jvi.73.4.3359-3365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham T P, Montelaro R C, Rushlow K E. Lentivirus envelope sequences and proviral genomes are stabilized in Escherichia coli when cloned in low-copy-number plasmid vectors. Gene. 1993;124:93–98. doi: 10.1016/0378-1119(93)90766-v. [DOI] [PubMed] [Google Scholar]
- 16.Deschambeault J, Lalonde J P, Cervantes-Acosta G, Lodge R, Cohen E A, Lemay G. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J Virol. 1999;73:5010–5017. doi: 10.1128/jvi.73.6.5010-5017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franke E K, Yuan H E, Bossolt K L, Goff S P, Luban J. Specificity and sequence requirements for interactions between various retroviral Gag proteins. J Virol. 1994;68:5300–5305. doi: 10.1128/jvi.68.8.5300-5305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed E O. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 19.Garnier L, Parent L J, Rovinski B, Cao S X, Wills J W. Identification of retroviral late domains as determinants of particle size. J Virol. 1999;73:2309–2320. doi: 10.1128/jvi.73.3.2309-2320.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnier L, Ratner L, Rovinski B, Cao S X, Wills J W. Particle size determinants in the human immunodeficiency virus type 1 Gag protein. J Virol. 1998;72:4667–4677. doi: 10.1128/jvi.72.6.4667-4677.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlinger H G, Dorfman T, Sodroski J G, Haseltine W A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci USA. 1991;88:3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guthmann M D, Tal M, Pecht I. A secretion inhibitory signal transduction molecule on mast cells is another C-type lectin. Proc Natl Acad Sci USA. 1995;92:9397–9401. doi: 10.1073/pnas.92.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmache A, Bouyac M, Audoly G, Hieblot C, Peveri P, Vigne R, Suzan M. The vif gene is essential for efficient replication of caprine arthritis encephalitis virus in goat synovial membrane cells and affects the late steps of the virus replication cycle. J Virol. 1995;69:3247–3257. doi: 10.1128/jvi.69.6.3247-3257.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 26.Hourioux C, Brand D, Sizaret P Y, Lemiale F, Lebigot S, Barin F, Roingeard P. Identification of the glycoprotein 41(TM) cytoplasmic tail domains of human immunodeficiency virus type 1 that interact with Pr55Gag particles. AIDS Res Hum Retrovir. 2000;16:1141–1147. doi: 10.1089/088922200414983. [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Orenstein J M, Martin M A, Freed E O. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J Virol. 1995;69:6810–6818. doi: 10.1128/jvi.69.11.6810-6818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inabe K, Nishizawa M, Tajima S, Ikuta K, Aida Y. The YXXL sequences of a transmembrane protein of bovine leukemia virus are required for viral entry and incorporation of viral envelope protein into virions. J Virol. 1999;73:1293–1301. doi: 10.1128/jvi.73.2.1293-1301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo E, Mammano F, Cohen E A, Gottlinger H G. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J Virol. 1995;69:2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein D L, Rushlow K E, Cook R F, Raabe M L, Swardson C J, Kociba G J, Issel C J, Montelaro R C. Replication in vitro and in vivo of an equine infectious anemia virus mutant deficient in dUTPase activity. J Virol. 1995;69:2881–2888. doi: 10.1128/jvi.69.5.2881-2888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y L, Bennett R P, Wills J W, Gorelick R, Ratner L. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J Virol. 1995;69:6873–6879. doi: 10.1128/jvi.69.11.6873-6879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montelaro R C, Parekh B, Orrego A, Issel C J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984;259:10539–10544. [PubMed] [Google Scholar]
- 33.Nesterov A, Carter R E, Sorkina T, Gill G N, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono A, Freed E O. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J Virol. 1999;73:4136–4144. doi: 10.1128/jvi.73.5.4136-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono A, Orenstein J M, Freed E O. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J Virol. 2000;74:2855–2866. doi: 10.1128/jvi.74.6.2855-2866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott D E, Chertova E N, Busch L K, Coren L V, Gagliardi T D, Johnson D G. Mutational analysis of the hydrophobic tail of the human immunodeficiency virus type 1 p6(Gag) protein produces a mutant that fails to package its envelope protein. J Virol. 1999;73:19–28. doi: 10.1128/jvi.73.1.19-28.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ott D E, Coren L V, Chertova E N, Gagliardi T D, Schubert U. Ubiquitination of HIV-1 and MuLV Gag. Virology. 2000;278:111–121. doi: 10.1006/viro.2000.0648. [DOI] [PubMed] [Google Scholar]
- 38.Ott D E, Coren L V, Copeland T D, Kane B P, Johnson D G, Sowder R C, Yoshinaka Y, Oroszlan S, Arthur L O, Henderson L E. Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus. J Virol. 1998;72:2962–2968. doi: 10.1128/jvi.72.4.2962-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parent L J, Bennett R P, Craven R C, Nelle T D, Krishna N K, Bowzard J B, Wilson C B, Puffer B A, Montelaro R C, Wills J W. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1995;69:5455–5460. doi: 10.1128/jvi.69.9.5455-5460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J G, Schreiber A D. Determinants of the phagocytic signal mediated by the type IIIA Fc gamma receptor, Fc gamma RIIIA: sequence requirements and interaction with protein-tyrosine kinases. Proc Natl Acad Sci USA. 1995;92:7381–7385. doi: 10.1073/pnas.92.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patnaik A, Chau V, Wills J W. Ubiquitin is part of the retrovirus budding machinery. Proc Natl Acad Sci USA. 2000;97:13069–13074. doi: 10.1073/pnas.97.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puffer B A, Parent L J, Wills J W, Montelaro R C. Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J Virol. 1997;71:6541–6546. doi: 10.1128/jvi.71.9.6541-6546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puffer B A, Watkins S C, Montelaro R C. Equine infectious anemia virus Gag polyprotein late domain specifically recruits cellular AP-2 adapter protein complexes during virion assembly. J Virol. 1998;72:10218–10221. doi: 10.1128/jvi.72.12.10218-10221.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts M M, Oroszlan S. The preparation and biochemical characterization of intact capsids of equine infectious anemia virus. Biochem Biophys Res Commun. 1989;160:486–494. doi: 10.1016/0006-291x(89)92459-5. [DOI] [PubMed] [Google Scholar]
- 45.Schubert U, Ott D E, Chertova E N, Welker R, Tessmer U, Princiotta M F, Bennink J R, Krausslich H G, Yewdell J W. Proteasome inhibition interferes with Gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci USA. 2000;97:13057–13062. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz M D, Geraghty R J, Panganiban A T. HIV-1 particle release mediated by Vpu is distinct from that mediated by p6. Virology. 1996;224:302–309. doi: 10.1006/viro.1996.0532. [DOI] [PubMed] [Google Scholar]
- 47.Strack B, Calistri A, Accola M A, Palu G, Gottlinger H G. A role for ubiquitin ligase recruitment in retrovirus release. Proc Natl Acad Sci USA. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogt V M. Ubiquitin in retrovirus assembly: actor or bystander? Proc Natl Acad Sci USA. 2000;97:12945–12947. doi: 10.1073/pnas.97.24.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willems L, Gatot J S, Mammerickx M, Portetelle D, Burny A, Kerkhofs P, Kettmann R. The YXXL signalling motifs of the bovine leukemia virus transmembrane protein are required for in vivo infection and maintenance of high viral loads. J Virol. 1995;69:4137–4141. doi: 10.1128/jvi.69.7.4137-4141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang Y, Cameron C E, Wills J W, Leis J. Fine mapping and characterization of the Rous sarcoma virus Pr76gag late assembly domain. J Virol. 1996;70:5695–5700. doi: 10.1128/jvi.70.8.5695-5700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasuda J, Hunter E. A proline-rich motif (PPPY) in the Gag polyprotein of Mason-Pfizer monkey virus plays a maturation-independent role in virion release. J Virol. 1998;72:4095–4103. doi: 10.1128/jvi.72.5.4095-4103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu X F, Dawson L, Tian C J, Flexner C, Dettenhofer M. Mutations of the human immunodeficiency virus type 1 p6Gag domain result in reduced retention of Pol proteins during virus assembly. J Virol. 1998;72:3412–3417. doi: 10.1128/jvi.72.4.3412-3417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]