Abstract
Recombinant adenoviruses that express high levels of the simian virus 40 (SV40) small-t (ST) antigen have been used to study the requirement for ST to drive cell cycle proliferation of confluent human diploid fibroblasts. This occurs when either large-T (LT) antigen or serum is added to provide a second signal. While cells readily completed S phase in these experiments, they were found to accumulate with 4N DNA content. Cellular and nuclear morphology, as well as the biochemical status of cyclin B complexes, showed that these cells entered mitosis but were blocked prior to mitotic metaphase. The defect appears to reflect an inability of cells overexpressing ST to form organized centrosomes that duplicate and separate normally during the cell cycle and, therefore, the absence of a mitotic spindle. The ability of ST to bind protein phosphatase 2A was required for this pattern, suggesting that altered phosphorylation of key centrosomal components may occur when ST is overexpressed. Although the possible significance of ST effects on the centrosome cycle is not fully understood, these findings suggest that ST could influence chromosomal instability patterns that are a hallmark of SV40-transformed cells and LT expression.
The early region of simian virus 40 (SV40) encodes three proteins that can be detected in nonpermissive, transforming infections (44, 50). The best understood of these is the large-T (LT) antigen, which binds tumor suppressor proteins p53 and pRb and has DNA binding, helicase, and transactivation capabilities (reviewed in references 12, 22, and 32). An amino-terminal dnaJ domain is also required for viral DNA replication and transformation (40, 49). In LT, the dnaJ domain modulates the protein stability of p130 and p107, members of the pRb family. (41). A key function of the small-t (ST) antigen is its binding to protein phosphatase 2A (PP2A). ST mimics cellular regulatory B subunits (28, 29, 48) of this trimeric enzyme and, presumably, modifies the substrate specificity and intracellular localization of PP2A (36, 39). ST expression in primary cells results in activation of key cellular kinases and growth regulators, such as mitogen-activated protein kinase, its kinase MEK, and the ion transporter, the Na-H antiporter (19, 38). These enzymes are all more highly phosphorylated in the presence of ST, consistent with an inhibition of phosphatase activity against these target molecules.
ST enhances the efficiency of virus transformation and tumor formation in animal model systems. A role for ST in hamsters (2) or transgenic mice is particularly apparent in nondividing tissues (5), consistent with the general concept that ST enhances cell cycle progression. Considerable evidence to support this concept came from early tissue culture studies (18, 23), one of which showed that a few rounds of cell division could bypass the ST requirement in a hamster cell system (23).
ST is not required for the transformation of all cell types in cultures. However, whenever ST is required, its ability to interact with PP2A has proven to be essential for transformation (25, 33). Human diploid fibroblasts (HDFs) are particularly dependent upon ST in SV40-mediated transformation (3, 7, 33). Neither focus formation nor anchorage-independent growth occurred when human cells were transfected with constructs that express LT but no ST. When both ST and LT were introduced, transformation resulted with good efficiency.
One of the earliest steps leading to cell transformation by SV40 is the induction of cell cycle progression. When defective recombinant adenoviruses (Ads) that independently express LT or ST were used to study cell cycle reentry of confluent, density-arrested HDFs, neither LT nor ST expression alone was sufficient to drive confluent HDFs back into the cell cycle. Coinfection with Ad-LT and Ad-ST, however, allowed the majority of the culture to progress through G1 and S phases of the cell cycle (34). The joint requirement for these SV40 proteins reflected the ability of LT to decrease levels of the cyclin kinase inhibitor p21 in HDFs, while ST expression led to decreased levels of p27. Interestingly, fresh serum addition also decreased p21 levels, leading to the prediction that Ad-ST-infected cells would induce cell cycle progression in the presence of fresh serum, a prediction that was confirmed experimentally.
In the course of studies with Ad-ST, there appeared to be a block in the progression of cells through G2/M, despite efficient cell cycle reentry. The present report extends studies of the cell cycle in this system, with particular emphasis on the failure of cells to complete mitosis. This correlated with an altered centrosome cycle as a consequence of the inhibition of PP2A by ST.
MATERIALS AND METHODS
Cell culture, synchronization, and infection.
HDFs were isolated from infant foreskins and grown for not more than nine passages at a split ratio of 1:10. Ad-EIA/B-transformed human embyronic kidney (293) cells were used to grow recombinant Ads. All cells were maintained in Dulbecco's modified Eagle medium (DME) containing 10% fetal calf serum (FCS), penicillin-streptomycin, and l-glutamine at 37°C in 6% CO2.
The construction of the recombinant Ads has been previously described (33). The viruses express wild-type (WT) ST (Ad-ST) or mutant forms of ST. One virus, Ad-43/45, expresses ST with a double mutation (P43L K45N) in the dnaJ domain. A second recombinant virus, Ad-103, expresses ST with a mutant PP2A interaction domain (C103S). Ad-CMV is a control virus which contains the cytomegalovirus (CMV) immediate-early promoter found in all other constructs but no ST coding sequences.
HDFs arrest naturally at confluence with a G0/G1 2N DNA content. Cell cycle analysis by flow cytometry (see below) showed that >90% of the cells had a 2N DNA content. Subconfluent HDFs were arrested by two methods. First, cells were plated in the presence of 2.5 mM hydroxyurea (HU; Sigma) and then infected. HU was added to noninfected cells at the time of plating and again at the end of the 1-h infection period. Cells were released from the HU block by being washed twice with phosphate-buffered saline (PBS) and addition of fresh DME and FCS to the cells. Such cells entered S phase almost immediately. In a few experiments, subconfluent HDFs were arrested in G1 by incubation for 36 to 48 h in DME containing 0.5% FCS. Following readdition of serum, such cells reached S phase after about 16 to 20 h. As for confluent cultures, cells arrested either by HU or by low serum showed predominantly a 2N DNA content.
Cell cycle analysis by flow cytometry.
Analysis of HDFs by flow cytometry was described previously (34). Briefly, nuclei were prepared from trypsinized cells using Triton X-100 and stained with propidium iodide in the presence of RNase before analysis on a Becton Dickinson flow cytometer using Cell-Quest software.
Detection of BrdU incorporation.
Subconfluent HDFs on coverslips were transfected with 2 μg of a plasmid expressing ST antigen plus 0.5 μg of a plasmid expressing green fluorescent protein (GFP) using Lipofectamine. The plasmids used were derivatives of pCMVt, a plasmid that encodes a cDNA version of ST under the control of the CMV promoter. On the morning after transfection, residual reagents were removed by aspiration of the transfection medium and fresh medium containing 0.5% FCS was added to the cells. Two days later (3 days posttransfection), bromodeoxyuridine (BrdU) was added, the mixture was incubated for 8 h, and then cells were fixed in 10% formaldehyde to preserve GFP staining and permeabilized by washing in PBS containing 0.5% NP-40. Fixed cells were treated for 20 min with 2 N HCl to denature double-stranded DNA, thus exposing BrdU residues for antibody recognition. The timing of this step was quite critical because prolonged exposure to acid eliminated the GFP signal. Nuclei containing incorporated BrdU were detected by using antibodies to BrdU (Boehringer) and rhodamine-conjugated secondary antibodies.
Western blotting.
Cells were washed twice with ice-cold PBS and scraped in a 1-ml volume of PBS. Cells were pelleted by centrifugation at 5,000 rpm in a Beckman J2-HS centrifuge for 5 min at 4°C. The supernatant was removed, and the cells were lysed with cold lysis buffer (50 mM Tris [pH 7.4], 200 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 2% glycerol, 0.5% NP-40) supplemented with protease and phosphatase inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 10 μg each of leupeptin, pepstatin, and aprotinin per ml; 1 mM NaF, and 1 mM sodium orthovanadate). Extracts were incubated on ice for 15 min with periodic vigorous vortexing. Insoluble material was then removed by centrifugation at 14,000 rpm in a Beckman J2-HS centrifuge for 10 min at 4°C and transfer of the supernatant to a clean microcentrifuge tube. Total protein concentrations were determined by using the Bio-Rad Protein Assay system with bovine serum albumin as the calibration standard. Equal amounts of total protein were resolved by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and transferred to Immobilon membranes (Millipore). For cyclin B Western assays, the membranes were incubated with anti-cyclin B primary antibody (1:1,000 in PBS–0.1% Tween 20–5% milk; Santa Cruz Biotechnology), followed by a horseradish peroxidase-conjugated secondary antibody. Monoclonal antibody PAB419 (17) was used at a dilution of 1:50 for detection of ST antigen. Proteins were visualized with enhanced-chemiluminescence reagents (Pierce Chemical).
Cyclin B immunoprecipitation kinase assays.
Cells were extracted in cold lysis buffer as described above. Equal amounts of protein (typically, 50 to 100 μg) were used in all experiments and were incubated with 1 μg of anti-cyclin B antibody. Extracts and antibody were incubated for 2 h at 4°C. Immunoprecipitates were collected by using protein A-agarose beads (Santa Cruz Biotechnology). The beads were washed three times in lysis buffer and twice in kinase buffer (50 mM HEPES [pH 7.4], 10 mM MgCl2, 1 mM MnCl2, 1 mM dithiothreitol). Immunoprecipitates were resuspended in 40 μl of kinase buffer containing 25 μg of histone H1 (Gibco BRL) per ml and 15 μCi of [γ-32P]ATP (3,000 mCi/mmol; Amersham) and then incubated at 37°C for 30 min. Reactions were stopped by the addition of sodium dodecyl sulfate sample buffer and then boiled for 5 min.
Immunofluorescence microscopy.
For visualization of mitotic antigens by the antimitotic protein monoclonal 2 antibody (MPM-2; Upstate Biotechnology Inc.) and DNA by 4′,6′-diamidino-2-phenylindole (DAPI; Sigma) staining, cells grown on coverslips were fixed at room temperature for 20 min with 3.7% formaldehyde in PBS containing Ca2+ and Mg2+ and permeabilized for 5 min with 0.3% NP-40 in PBS. For MPM-2 staining, fixed cells were incubated with MPM-2 antibody (1:100 in PBS) for 1 h at 37°C, washed extensively, and then incubated with a fluorescein-conjugated secondary antibody (4 μg/ml, 37°C for 1 h). To detect chromosomal DNA morphology, DAPI was used at a concentration of 2 μg/ml. For γ-tubulin staining, formalin-fixed cells on coverslips were permeabilized for 6 min in cold methanol and then first incubated with mouse anti-γ-tubulin (Sigma; 1:8,000 in PBS containing 25% FCS). Centrin was visualized by using monoclonal antibody 20H5, a kind gift of Jeffrey Salisbury (Mayo Clinic, Rochester, Minn.) (30, 35). For anticentrin antibody staining, cells were fixed and permeabilized in cold methanol-acetone (1:1) at −20°C for 10 min and the primary antibody was used at a dilution of 1:500 in PBS containing 25% fetal bovine serum. Secondary anti-mouse antibody for both anti-γ-tubulin and anti-centrin staining was fluorescein tagged and was used at 1:1,000 (PBS containing 40% fetal bovine serum). All stained coverslips were mounted onto slides by using Gelvatol (Sigma) containing 1,4-diazabicyclo[2,2,2]octane (0.1 mg/ml; Sigma).
RESULTS
Cell cycle induction by Ad-ST and serum requires the PP2A binding function.
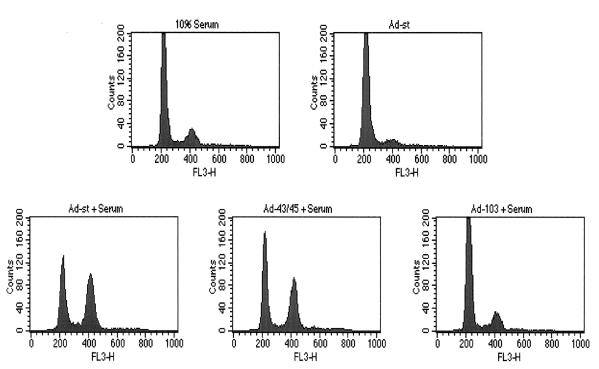
Previous studies showed that confluent (density-arrested) HDFs require two signals to reenter the cell cycle, one provided by SV40 LT or fresh serum and the other provided by ST. Cell cycle induction in this system depends on the ability of ST antigen to bind and inhibit PP2A. This was shown by using Ad-103, a recombinant virus that encodes the C103S mutant form of ST (Fig. 1). Cells infected with this mutant form of Ad-ST did not show significant S or G2/M populations, even in the presence of 10% serum. In contrast, a virus that expressed mutant ST with an altered dnaJ domain (Ad-43/45) was able to drive cell cycle progression in the presence of serum. Although not shown here, comparable levels of ST protein were found in cells infected with the various Ads if the multiplicity of Ad-103 was increased two- to threefold. However, no significant cell cycling was found, even with multiplicities as high as 50 PFU/cell. In contrast, even 5 PFU of WT Ad-ST per cell led to cell cycle reentry.
FIG. 1.
Cell cycle induction by Ad-ST in the presence of serum. Confluent HDFs were mock infected or infected at 20 PFU/cell with Ad-ST or the mutant Ad-43/45 or Ad-103. In profiles labeled + Serum, cells were incubated after infection in DME containing 10% FCS. For the Ad-ST control (upper right panel), cells were incubated in DME containing only 0.5% FCS after infection. At 32 h postinfection, cells were trypsinized and processed for flow cytometry.
Cell cycle progression of transfected HDF.
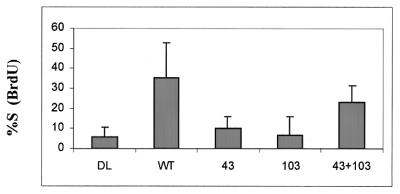
Ad-ST experiments have the advantage that the entire population of cells can be infected, making it possible to detect alterations in levels of proteins such as the cyclin kinase inhibitors p21 and p27. However, the levels of ST expressed by the recombinant viruses are very high, beyond even those expressed by cells that are productively infected with SV40. To examine ST effects on cell cycle progression in a different system, we turned to transfections of subconfluent HDFs. When cells are mock transfected and then placed in low concentrations of serum the next day, they become increasingly quiescent over the next 2 days. By 60 to 72 h posttransfection, there is little ongoing cell cycling, as measured by BrdU incorporation (Fig. 2). In contrast, 25 to 30% of cells transfected with plasmids that express ST continue to incorporate BrdU under these conditions, indicative of continued S phase progression. As for the Ad-ST experiments, the PP2A-binding mutant form of ST (mutant 103) was particularly defective in allowing continued DNA synthesis. In contrast to experiments with recombinant Ads, the dnaJ mutant (mutant 43/45) also had a greatly reduced ability to support cell cycling in transfection experiments. This suggests that the dnaJ domain of ST may contribute to the stimulation of cell growth but that the need for this domain may be overcome when high levels of ST are expressed in cells. Interestingly, cotransfections with the 103 and 43/45 mutant plasmids showed at least partial complementation between these two mutant forms of ST antigen.
FIG. 2.
BrdU incorporation (S phase progression) by transfected cells. HDFs plated on coverslips (30 to 40% confluent) were transfected with 0.5 μg of pEGFP and 2 μg of a pCMVt-derived construct that expressed WT ST, the P43L K45N mutant (column 43), C103S (column 103), or a mixture of the two mutant constructs (column 43+103). These plasmids all encode cDNA versions of ST. The DL control plasmid is not a cDNA but carries a deletion of the ST splice donor and makes no ST protein or truncated fragment. On the day after infection, the medium was changed to DME–0.5% serum. At 48 h later, BrdU was added, the mixture was incubated for 8 h, and then coverslips were fixed for immunofluorescence assay using anti-BrdU antibodies and a rhodamine-tagged secondary antibody. Data are expressed as the percentage of GFP-positive cells showing positive anti-BrdU staining.
Overexpression of ST affects G2/M progression.
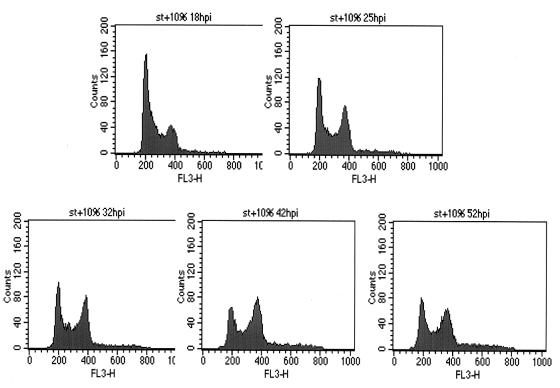
During the course of the Ad-ST experiments described above, we noticed an accumulation of cells in G2/M in the fluorescence-activated cell sorter (FACS) profiles (Fig. 1; note the Ad-ST plus serum panel, in particular). As shown in Fig. 3, Ad-ST-infected cells showed significant G2/M accumulation by 1 day postinfection, reaching a maximum of 40% of the cells in G2/M. The accumulation in G2/M persisted for even as long as 52 h postinfection, when only a small fraction of the cells appeared to reenter G1, reducing the G2/M peak. There was no evidence in these experiments of a cycling tetraploid population, which would have been evident if peaks with greater DNA contents had appeared at later times.
FIG. 3.
Kinetics of cell cycle induction by ST in the presence of serum. Confluent HDFs were infected with Ad-ST at 20 PFU/cell, and then medium containing 10% serum was added back at the end of the infection period. Cells were collected and processed for flow cytometry at 18, 25, 32, 42, and 52 h postinfection (hpi).
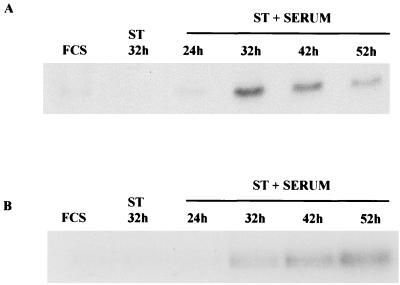
The expression of cyclin B is necessary as cells transit G2 and enter mitosis (20). To determine whether ST was affecting the expression of cyclin B and therefore inhibiting the progression of the cells through G2, Western blot analyses were performed to ascertain the cyclin B protein levels in cells induced by ST plus serum. As shown in Fig. 4A, cells infected with Ad-ST in the presence of serum showed an increase in the amount of cyclin B expressed by 24 h postinfection and levels increased further by 32 h postinfection. High levels of cyclin B persisted for at least another day, additional evidence that cells cannot complete mitosis where cyclin B destruction occurs normally in the cell cycle (20).
FIG. 4.
Cyclin B and cyclin B-associated kinase levels of arrested cells. (A) Confluent HDFs were infected with Ad-ST at 20 PFU/cell and then maintained in 10% FCS after infection. Extracts were prepared at 24, 32, 42, and 52 h postinfection, and levels of cyclin B were compared to those found in controls (Ad-ST alone, FCS alone) by Western blot analysis. (B) The same extracts were used for immunoprecipitation-kinase assays in which histone H1 was the substrate for cyclin B1-associated kinase activity.
Accumulated cyclin B is typical of both G2 and M phases of the cell cycle. Activation of the cyclin B-dependent kinase cdc2 does not occur in G2 but is typical of the prophase stage of mitosis. To measure cdc2 activity, cyclin B-cdc2 complexes were immunoprecipitated by using an anti-cyclin B monoclonal antibody and then assaying immune complexes for the ability to phosphorylate H1 histone in the presence of [γ-32P]ATP. Cells infected with Ad-ST in the presence of serum showed high levels of cyclin B kinase, which persisted for more than 20 h with only a slight decline (Fig. 4B). These data suggest that the cells have indeed entered mitosis and are either unable to degrade their cyclin B, which is required for exit from mitosis, or have not reached anaphase, the stage at which cyclin B would normally be degraded.
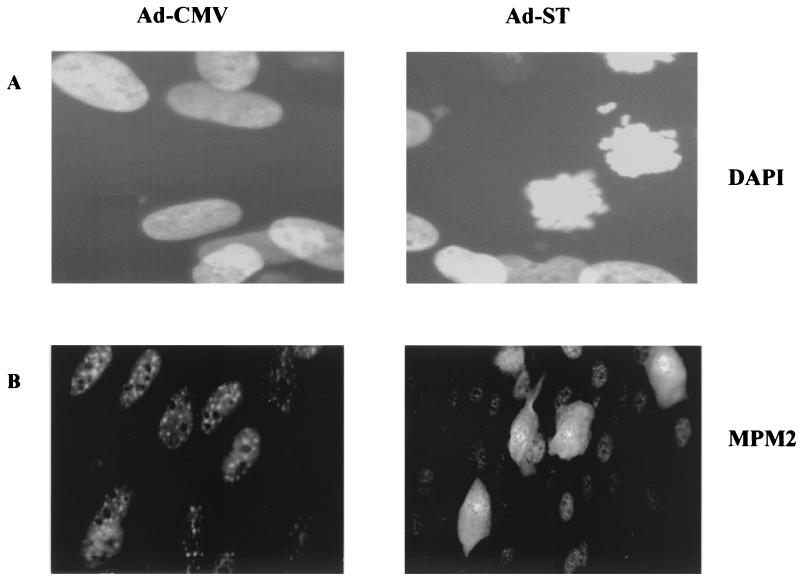
Immunohistochemistry provided additional evidence that Ad-ST-infected cells actually enter mitosis. Cells were stained with antibodies to MPM-2, a phosphorylated epitope shared by a set of proteins phosphorylated at the onset of mitosis (6, 9). As seen in Fig. 5B, many cells in Ad-ST-infected cultures (right panel) showed increased MPM-2 staining. These cells were rounder and showed stain in both their nuclear and cytoplasmic portions. This staining pattern would be expected for normal cells because the initiation of mitosis is associated with the breakdown of the nuclear envelope. In addition, dense, dark areas in the nuclear areas of cells stained with anti-MPM2 antibody appeared like a ring of condensed chromosomes. This suggested that cells had undergone chromosome condensation and entered mitosis. Cells stained with DAPI (Fig. 5A) confirmed the presence of condensed chromosomes that were not aligned on a metaphase plate. Thus, these cells have a prometaphase profile in which cells have undergone chromosome condensation and breakdown of the nuclear membrane and have high levels of cyclin B-cdc2 kinase activity.
FIG. 5.
MDM-2 staining and chromosomal morphology in arrested cells. HDFs were plated on coverslips and then infected with Ad-ST in the presence of 10% serum. At 32 h postinfection, cells were fixed with formalin and permeabilized with NP-40 as described in Materials and Methods. The cells in panels A were stained with DAPI, and those in panels B were stained with anti-MPM-2 antibody. Ad-ST-infected cells are shown on the right side of both sets, while control Ad-CMV-infected cells are on the left.
An analysis of the mitotic profiles of cells in one experiment is shown in Table 1. In this experiment, 63% of the cells had 4N DNA content and about half of these appeared to be in prophase (condensed chromatin not aligned on a metaphase plate). None of the cells showed metaphase or anaphase chromosomal patterns, suggesting that one block to cell cycle progression occurred in the progression to or through metaphase. Interestingly, this analysis also revealed an earlier premitotic block that had not been appreciated previously. Only half of the cells with 4N DNA content were actually in prophase, suggesting that the other half of these cells remained blocked in G2 and never entered mitosis. It is possible that confluent cells that overcome a G1 arrest now encounter a G2 checkpoint. ST may allow some, but not all, cells to bypass this checkpoint but then blocks their progression through M.
TABLE 1.
Distribution of mitotic figures in arrested, Ad-ST-infected cells
| Stage of cell cycle | No. of cells counted | % of totala |
|---|---|---|
| Interphase nuclei (noncondensed, envelope present) | 258 | 70 |
| Prophase nuclei (condensed DNA, nonaligned) | 113 | 30 |
| Metaphase nuclei (metaphase plate alignment) | 0 | 0 |
| Anaphase nuclei (separating chromosomes) | 0 | 0 |
FACS analysis showed that 63% of the cells had 4N DNA content.
PP2A binding is necessary for prometaphase block.
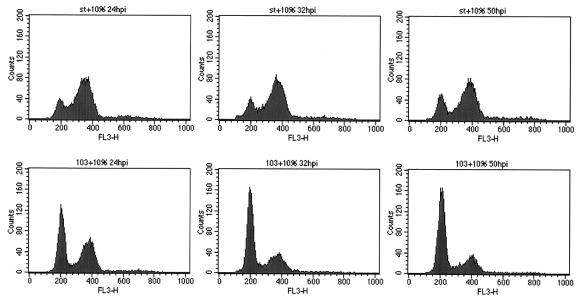
The dnaJ domain of ST was not required for the premetaphase block to cell cycle progression, because cells infected with Ad-43/45 showed the same MPM2 and DAPI staining patterns described above (data not shown). To analyze the role of the PP2A-binding domain, it was necessary to drive cell cycle progression in an ST-independent fashion, because cells infected with Ad-103 do not enter G1/S and progress to G2/M (Fig. 1). In contrast to confluent HDFs which cannot reenter the cell cycle in response to serum stimulation, serum-deprived subconfluent HDFs can proliferate in response to freshly added serum. Thus, HDFs were plated and grown to one-third confluence, deprived of serum to synchronize the cells in G0/G1, and then infected with Ad-ST and serum stimulated. As shown in Fig. 6, a majority of the cells infected with Ad-ST became blocked in G2/M and remained in this stage of the cell cycle for over 50 h. In contrast, cells infected with Ad-103 reached G2/M but failed to remain in mitosis and reentered G1 within 8 h. DAPI staining confirmed that Ad-103-infected cells decondensed their DNA and resumed interphase appearance at this time (data not shown). It is also worth noting that the experiments with the 103 mutant and similar experiments with Ad-LT (not shown) showed that the mitotic arrest was not an artifact related to Ad infection. Rather, mitotic arrest required ST antigen, specifically, its PP2A inhibition function. This suggests that sequestering of PP2A by ST may prevent critical dephosphorylation reactions required for the progression of mitosis.
FIG. 6.
The C103S mutation alters G2/M arrest patterns. Subconfluent cells were kept in DME–0.5% FCS for 38 h and then infected with Ad-ST (WT or C103S) at 20 PFU/cell. Medium containing 10% FCS was added after infection to allow the cells to reenter the cell cycle. Cells were collected at 24, 32, and 50 h postinfection (hpi) and processed for flow cytometry.
PP2A inhibition inhibits centrosome maturation and duplication.
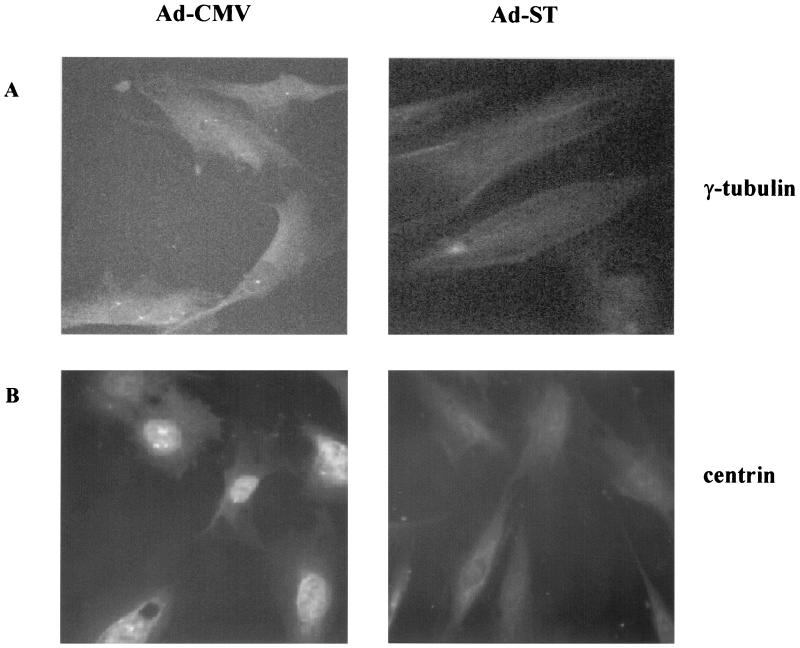
The prometaphase appearance of nuclei from Ad-ST-infected cells suggested that cells were unable to assemble a mitotic spindle. Indeed, staining of arrested cells with β-tubulin (data not shown) provided no evidence for a mitotic spindle, in agreement with the nonaligned appearance of the condensed chromosomes. A key component of the spindle apparatus is the centrosome, so we looked for centrosomal structures by staining for the centrosome-associated tubulin γ-tubulin. Centrosomes mature and thicken during G1 and S phases of the cell cycle, forming structures that are clearly detectable by γ-tubulin staining (27). In late S or early G2, centrosomes duplicate and then begin to move away from one another toward the poles of the cell. To determine how the normal centrosome cycle was affected in Ad-ST-infected cells, we compared the γ-tubulin staining patterns of uninfected and Ad-ST-infected subconfluent cells at defined times in the cell cycle. Cells were first plated in the presence of HU to arrest them at the G1/S border before infection. This procedure was used rather than serum deprivation because, following release of the HU block, cells transit S and proceed toward M in a more rapid and synchronized fashion. On the day after plating in HU, cells were infected with Ads and then kept in HU for 8 h to allow ST expression. Cells were then washed to remove HU and allowed to proceed through S and into G2. Centrosome patterns were studied at various times after release of the HU block. Figure 7A shows an example of the staining patterns of cells 10 h after the release of the HU block, and the data shown in Table 2 summarize the patterns from viewing many fields of cells. The 10-h time point was chosen because this was when the maximum numbers of duplicated and separated centrosomes were found in control cell populations. As shown in Table 2, centrosomes were clearly visible in nearly all Ad-CMV-infected cells, with 18% showing clearly separated centrosomes that were at or moving toward the poles of the cells. In contrast, none of the Ad-ST-infected cells showed duplicated centrosomes and only a few had distinct single centrosomes. The vast majority showed diffuse, disorganized staining with anti-γ-tubulin, suggesting that centrosomal maturation had not occurred and thickened centrosomes of detectable size and structure were not present in these cells. These findings suggest that the failure of ST-expressing cells to transit mitosis reflected the inability of their centrosomes to undergo a normal cycle to produce a normal spindle apparatus.
FIG. 7.
Anti-γ-tubulin and anticentrin immunofluorescence. HDFs were plated on coverslips in the presence of HU for 24 h and then mock infected or infected with Ad-ST at 20 PFU/cell for 8 h. Cell cycle progression was then initiated by washing out the HU and adding DME–10% FCS. At various times after HU release, cells were fixed and permeabilized and then stained for γ-tubulin. The cells shown were stained with anti-γ-tubulin (A) 10 h after HU release or with anticentrin antibody (B) 15 h after HU washout. Cells infected with Ad-ST are shown on the right side of each panel, and control Ad-CMV-infected cells are on the left.
TABLE 2.
Centrosomal patterns observed by immunofluorescence assay for γ-tubulin
| Pattern | % of cell infected with:
|
|
|---|---|---|
| Ad-CMV control | Ad-ST | |
| Duplicated centrosomes | 18 | 0 |
| Single centrosome | 75 | 21 |
| No apparent centrosomes | 7 | 74 |
Another indication that centrosomes were aberrant came from staining with an anticentrin antibody. Centrin is a structural component of the centrosome body but is also found in centrosome-free pools within the cell (30). As cells approached mitosis 15 h after release of the HU block, centrin was particularly enriched in the nuclei of Ad-CMV-infected cells (Fig. 7B). In contrast, centrin staining was much weaker in Ad-ST-infected cells and was not localized to the nuclear compartment. The disorganized, weak centrin staining in these cells further suggests a defect in normal centrosome dynamics when ST is overexpressed in HDFs.
DISCUSSION
During studies of the role of ST antigen in promoting cell cycle progression, a pronounced block to G2/M progression was noted in experiments using recombinant Ads which overexpress ST antigen. This was first apparent by an accumulation of cells with 4N DNA content in FACS analyses. Such cells showed the increased levels of cyclin B1 that would be expected in either G2 or early M, but the presence of active cyclin B-associated cdc2 activity suggested that at least some cells had moved into early mitosis. This was confirmed by immunofluorescence for the MPM-2 mitotic epitope (6, 9). In nonmitotic cells, MPM-2 staining is faint and localized to the nucleus. When cells enter mitosis, fluorescence intensity increases and staining is found throughout the cell because the integrity of the nuclear envelope is altered in mitotic cells. Of the cells infected with Ad-ST in the presence of serum, 30 to 50% showed this early mitotic staining pattern. DAPI staining showed that a similar fraction of cells contained highly condensed chromosomes that were not aligned in a metaphase arrangement. This pattern was observed for about half of the cells with 4N DNA content. The remaining half were arrested at an earlier point, in G2 but before M.
The interference with mitosis described here almost certainly reflects the overexpression of ST that occurs with the Ad vectors, but this finding raises the possibility that ST transiently interferes with mitotic progression, a possibility discussed later in greater detail. It is not surprising that low levels of ST do not cause the severe phenotypes found in the Ad-ST-infected cells because a complete block to mitotic completion would be incompatible with cell survival. Interestingly, a lethal mutation with phenotypes similar to those reported here was described in drosophila (37). In this case, P element insertion into the PP2A catalytic subunit inactivated its function. Homozygous mutant embryos totally deficient in PP2A showed overcondensed chromatin and an arrest between prophase and anaphase (37) at a stage in development where rapid mitotic cycles are required. Our studies of Ad-ST-infected cells confirm in a completely different system that PP2A activity is required for the assembly of a functional mitotic spindle and suggest that PP2A inhibition blocks an early step in centrosome assembly and maturation.
Consistent with the behavior of the drosophila mutant, mitotic arrest by ST depends on its binding to PP2A. This function of ST maps to its C-terminal half, in particular, to a CXXXPXC motif found at residues 97 to 103 (25). Studies with the C103S mutant indicated that it is the PP2A interaction function that accounts for the cell cycle arrest described in Ad-ST-infected HDFs. In contrast, mutation of the dnaJ domain of ST, a region involved in STs transactivation function, had no effect on the ability of ST to drive cell cycle progression and mitotic arrest (25).
A well-defined mitotic checkpoint responds to damage to the mitotic spindle. Proteins such as BUB1 and MAD1 (21) or the polo-like kinases are critical in the metaphase-anaphase transition and may well be sensitive to the state of PP2A in cells (8, 16). In yeast, there is strong genetic evidence that a specific PP2A B subunit is required for the spindle checkpoint response (47). However, there is a key difference here. Cells arrested by ST expression never form a mitotic spindle, so the block to mitotic progression occurs before this checkpoint is believed to function.
The absence of identifiable centrosomes in Ad-ST-infected cells suggests that arrest occurs not through activation of a checkpoint but because the spindle cannot form. The centrosome cycle is coupled to the cell cycle. However, the failure of Ad-ST-infected cells to assemble centrosomes does not correlate with an obvious difficulty in cell cycle transit because these cells clearly reach G2/M and many enter early mitosis. In addition, ST is known to promote cell cycle progression and to relieve p27-mediated inhibition of cell cycle kinases (34). One might expect that reduced p27 levels would result in overly active cyclin E-cdk2 and that this would lead to excessive centrosome amplification (10, 51). In contrast, the inability to detect any centrosomes in Ad-ST-infected cells suggests that an even earlier step in centrosome maturation or assembly, one that precedes centrosome duplication, is blocked.
It is possible that interference with some upstream activity indirectly affects the centrosome cycle, although this would have to be a function that is nonessential for cell cycle progression. Alternatively, inhibition of PP2A may directly alter a key centrosomal component (14), many of which are known to be regulated by phosphorylation. An interesting example is NPM/B23 (26), a protein associated with unduplicated centrosomes that dissociates from them after phosphorylation with cyclin E-2 cdk2. Other molecules are C-Nap1 (24), a protein that mediates centriole-centriole adhesion, and Nek2, a protein which influences centrosomal separation (15, 45). Some preliminary experiments have been performed for centrosomal proteins for which good reagents are available. While no differences in the kinetics or accumulation of Nek2 were found (unpublished observations), the reduced centrin staining found in Ad-ST-infected cells (Fig. 7B) lends further support to the idea that centrosomal components are directly affected. The expression and phosphorylation status of key centrosomal proteins like these should provide greater insights into whether effects of ST on the centrosome cycle are likely to be direct or indirect.
Finally, although the severe defects found in Ad-ST infections could not persist if cells were to survive, the possibility of transient interference with normal centrosome function evokes an interesting speculation on its possible contribution to chromosomal instability. This phenotype has been described for several tumor cell lines and results from a failure of the mitotic spindle checkpoint (1). When mitotic progression is blocked by drugs like colchinine and vinblastine, some tumor lines fail to arrest but rather decondense their chromosomes and reenter the cell cycle, leading to increased ploidy and subsequent chromosomal rearrangement. Defects in p53 activity, among other things, can contribute to bypass of mitotic arrest. In SV40 infections, in which LT binds and inhibits p53 activity, a transient delay to mitotic progression caused by altered activity of centrosomal proteins could lead to a similar scenario. It is very unlikely that the pronounced arrest described here ever occurs in a natural infection, where levels of viral proteins may be high immediately after infection but fall shortly thereafter. Lower levels are also present in stably transformed cells lines. However, even a temporary delay in the progression of cells through mitosis could promote aberrant cell cycle reentry and chromosomal decondensation.
There is increasing evidence that tumor viruses can interfere with normal mitotic progression, leading to altered ploidy and genetic instability. LT and the papillomavirus E6 protein serve as prototypes for proteins that alter p53-sensitive checkpoints (4, 31, 42, 43, 46), and mechanisms that influence the centrosome cycle have also been recently described in papillomavirus systems (11). LT expression leads to tetraploidization of permissive monkey cells (13) through unknown mechanisms. It will be interesting to formally test whether ST contributes to LT-driven genetic instability in systems where levels and duration of expression of the viral proteins can be limited and controlled.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant CA-21327 (K.R.). We acknowledge the support of the Lester Woods Foundation, through the Robert H. Lurie Comprehensive Cancer Center, in providing some of the equipment used in this study.
We appreciate the technical assistance of Marlena Wilson. We thank Jeffrey Salisbury (Mayo Clinic, Rochester, Minn.) for the kind gift of the anticentrin monoclonal antibody.
REFERENCES
- 1.Cahill D P, Lengauer C, Yu J, Riggins G J, Willson J K, Markowitz S D, Kinzler K W, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 2.Carbone M, Lewis A M, Matthews B J, Levine A S, Dixon K. Characterization of hamster tumors induced by simian virus 40 small t deletion mutants as true histiocytic lymphomas. Cancer Res. 1989;49:1565–1571. [PubMed] [Google Scholar]
- 3.Chang L-S, Pater M, Hutchinson N, di Mayorca G. Tranformation by purified early genes of simian virus 40. Virology. 1984;133:341–353. doi: 10.1016/0042-6822(84)90400-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang T H, Ray F A, Thompson D A, Schlegel R. Disregulation of mitotic checkpoints and regulatory proteins following acute expression of SV40 large T antigen in diploid human cells. Oncogene. 1997;14:2383–2393. doi: 10.1038/sj.onc.1201196. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y, Lee I, Ross S R. Requirement for the simian virus 40 small t antigen in tumorigenesis in transgenic mice. Mol Cell Biol. 1988;8:3382–3390. doi: 10.1128/mcb.8.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis F M, Tsao T Y, Fowler S K, Rao P N. Monoclonal antibodies to mitotic cells Proc. Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Ronde A, Sol C J A, van Strien A, ter Schegget J, van der Noordaa J. The SV40 small t antigen is essential for the morphological transformation of human fibroblasts. Virology. 1989;171:260–263. doi: 10.1016/0042-6822(89)90534-5. [DOI] [PubMed] [Google Scholar]
- 8.Descombes P, Nigg E A. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding M, Feng Y, Vandre D D. Partial characterization of the MPM-2 phosphoepitope. Exp Cell Res. 1997;231:3–13. doi: 10.1006/excr.1996.3439. [DOI] [PubMed] [Google Scholar]
- 10.Doxsey S J. The centrosome—a tiny organelle with big potential. Nat Genet. 1998;20:104–106. doi: 10.1038/2392. [DOI] [PubMed] [Google Scholar]
- 11.Duensing S, Lee L Y, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum C P, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci USA. 2000;97:10002–10007. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992;66:1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich T D, Laffin J, Lehman J M. Induction of tetraploid DNA content by simian virus 40 is dependent on T-antigen function in the G2 phase of the cell cycle. J Virol. 1994;68:4028–4030. doi: 10.1128/jvi.68.6.4028-4030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry A M, Mayor T, Nigg E A. Regulating centrosomes by protein phosphorylation. Curr Top Dev Biol. 2000;49:291–312. doi: 10.1016/s0070-2153(99)49014-3. [DOI] [PubMed] [Google Scholar]
- 15.Fry A M, Meraldi P, Nigg E A. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 1998;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glover D M, Hagan I M, Tavares A A. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- 17.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiscott J B, Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. II. In nonpermissive cells. J Virol. 1981;37:802–812. doi: 10.1128/jvi.37.2.802-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe A K, Gaillard S, Bennett J S, Rundell K. Cell cycle progression in monkey cells expressing simian virus 40 small t antigen from adenovirus vectors. J Virol. 1998;72:9637–9644. doi: 10.1128/jvi.72.12.9637-9644.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt T, Luca F C, Ruderman J V. The requirements for protein synthesis and degradation and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Gorbea C, Mahaffey D, Rechsteiner M, Benezra R. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc Natl Acad Sci USA. 1997;94:12431–12436. doi: 10.1073/pnas.94.23.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manfredi J J, Prives C. The transforming activity of simian virus 40 large tumor antigen. Biochim Biophys Acta. 1994;1198:65–83. doi: 10.1016/0304-419x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 23.Martin R, Setlow V, Edwards C, Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979;17:635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- 24.Mayor T, Stierhof Y D, Tanaka K, Fry A M, Nigg E A. The centrosomal protein C-Nap1 is required for cell cycle-regulated centrosome cohesion. J Cell Biol. 2000;151:837–846. doi: 10.1083/jcb.151.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mungre S, Enderle K, Turk B, Porras A, Wu Y-Q, Mumby M C, Rundell K. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J Virol. 1994;68:1675–1681. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda M, Horn H F, Tarapore P, Tokuyama Y, Smulian A G, Chan P-K, Knudsen E S, Hofmann I A, Snyder J D, Bove K E, Fukasawa K. Nucleophosmin/B23 is a target of cdk2/cyclin E in centrosome duplication. Cell. 2000;103:127–140. doi: 10.1016/s0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 27.Palazzo R E, Vogel J M, Schnackenberg B J, Hull D R, Wu X. Centrosome maturation. Curr Top Dev Biol. 2000;49:449–470. doi: 10.1016/s0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- 28.Pallas D C, Shahrik L K, Martin B L, Jaspers S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 29.Pallas D C, Weller W, Jaspers S, Miller T B, Lane W S, Roberts T M. The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J Virol. 1992;66:886–893. doi: 10.1128/jvi.66.2.886-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paoletti A, Moudjou M, Paintrand M, Salisbury J L, Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- 31.Passalaris T M, Benanti J A, Gewin L, Kiyono T, Galloway D A. The G2 checkpoint is maintained by redundant pathways. Mol Cell Biol. 1999;19:5872–5881. doi: 10.1128/mcb.19.9.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pipas J M. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porras A, Bennett J, Howe A, Tokos K, Bouck N, Henglein B, Sathyamangalam S, Thimmapaya B, Rundell K. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porras A, Gaillard S, Rundell K. The simian virus 40 small-t and large-T antigens jointly regulate cell cycle reentry in human fibroblasts. J Virol. 1999;73:3102–3107. doi: 10.1128/jvi.73.4.3102-3107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders M A, Salisbury J L. Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1994;124:795–805. doi: 10.1083/jcb.124.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shtrichman R, Sharf R, Kleinberger T. Adenovirus E4orf4 protein interacts with both Balpha and B′ subunits of protein phosphatase 2A, but E4orf4-induced apoptosis is mediated only by the interaction with Balpha. Oncogene. 2000;19:3757–3765. doi: 10.1038/sj.onc.1203705. [DOI] [PubMed] [Google Scholar]
- 37.Snaith H A, Armstrong C G, Guo Y, Kaiser K, Cohen P T. Deficiency of protein phosphatase 2A uncouples the nuclear and centrosome cycles and prevents attachment of microtubules to the kinetochore in Drosophila microtubule star (mts) embryos. J Cell Sci. 1996;109:3001–3012. doi: 10.1242/jcs.109.13.3001. [DOI] [PubMed] [Google Scholar]
- 38.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small t antigen with protein phosphatase 2A stimulates the Map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 39.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom G S, Mumby M C. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stubdal H, Zalvide J, Campbell K S, Schweitzer C, Roberts T M, DeCaprio J A. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas J T, Laimins L A. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J Virol. 1998;72:1131–1137. doi: 10.1128/jvi.72.2.1131-1137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson D A, Belinsky G, Chang T H, Jones D L, Schlegel R, Munger K. The human papillomavirus-16 E6 oncoprotein decreases the vigilance of mitotic checkpoints. Oncogene. 1997;15:3025–3035. doi: 10.1038/sj.onc.1201495. [DOI] [PubMed] [Google Scholar]
- 44.Tooze J. Molecular biology of the tumor viruses. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1981. [Google Scholar]
- 45.Uto K, Sagata N. Nek2B, a novel maternal form of Nek2 kinase, is essential for the assembly or maintenance of centrosomes in early Xenopus embryos. EMBO J. 2000;19:1816–1826. doi: 10.1093/emboj/19.8.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahl A F, Donaldson K L, Fairchild C, Lee F Y, Foster S A, Demers G W, Galloway D A. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med. 1996;2:72–79. doi: 10.1038/nm0196-72. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Burke D J. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:620–626. doi: 10.1128/mcb.17.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang S-I, Lickteig R L, Estes R, Rundell K, Walter G, Mumby M C. Control of protein phosphatase 2A by simian virus 40 small t antigen. Mol Cell Biol. 1991;11:1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zalvide J, Stubdal H, DeCaprio J A. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family members. Mol Cell Biol. 1998;18:1408–1415. doi: 10.1128/mcb.18.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zerrahn J, Knippschild U, Winkler T, Deppert W. Independent expression of the transforming amino-terminal domain of large T antigen from an alternatively spliced third SV40 early mRNA. EMBO J. 1993;12:4739–4746. doi: 10.1002/j.1460-2075.1993.tb06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou H, Kuang J, Zhong L, Kuo W L, Gray J W, Sahin A, Brinkley B R, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]