Abstract
Poliovirus (PV) replicates its genome in association with membranous vesicles in the cytoplasm of infected cells. To elucidate the origin and mode of formation of PV vesicles, immunofluorescence labeling with antibodies against the viral vesicle marker proteins 2B and 2BC, as well as cellular markers of the endoplasmic reticulum (ER), anterograde transport vesicles, and the Golgi complex, was performed in BT7-H cells. Optical sections obtained by confocal laser scanning microscopy were subjected to a deconvolution process to enhance resolution and signal-to-noise ratio and to allow for a three-dimensional representation of labeled membrane structures. The mode of formation of the PV vesicles was, on morphological grounds, similar to the formation of anterograde membrane traffic vesicles in uninfected cells. ER-resident membrane markers were excluded from both types of vesicles, and the COPII components Sec13 and Sec31 were both found to be colocalized on the vesicular surface, indicating the presence of a functional COPII coat. PV vesicle formation during early time points of infection did not involve the Golgi complex. The expression of PV protein 2BC or the entire P2 and P3 genomic region led to the production of vesicles carrying a COPII coat and showing the same mode of formation as vesicles produced after PV infection. These results indicate that PV vesicles are formed at the ER by the cellular COPII budding mechanism and thus are homologous to the vesicles of the anterograde membrane transport pathway.
Poliovirus (PV), the prototype member of the family Picornaviridae, contains an RNA genome of plus polarity with a single large open reading frame (ORF) coding for a polyprotein of 265 kDa. The translation product is cleaved by intrinsic viral proteases into about 20 proteins, of which intermediate as well as end products are functional (38, 83, 88; reviewed in reference 35). The proteins encoded by the P1 genomic region form the capsid, whereas most of the nonstructural proteins, encoded by the P2 and P3 genomic region, are involved in viral RNA replication (2, 27, 28, 37, 48, 55, 58, 82). The viral genome replicates asymmetrically with an excess of plus strands produced in multistranded replicative intermediates (2, 17, 31, 49). PV genome replication depends on the template RNA molecule carrying a cis-acting replicative element (see references 32 and 54), a primer (54), viral and cellular proteins and their proper interactions (2, 29, 30, 53), and the presence of membranes (10, 12, 14, 26, 74).
Genome replication of all plus-strand RNA viruses investigated so far takes place in membrane-bound replication complexes (see references 19 and 65 and references therein). However, the morphology of such replication complexes of different viruses is diverse, and different cellular compartments provide membranes for and are involved in viral replication (44, 56, 65). PV replication complexes are built up from individual membranous vesicles (PV vesicles) which are assembled into specific higher-order structures (11, 12, 15).
The PV replication complex is formed in cis, coupling translation of the same RNA, which is to be replicated in the complex, and vesicle formation (25). This suggests the involvement of translation-competent endoplasmic reticulum (ER) membranes in the formation of PV vesicles (25). This view is compatible with ultrastructural findings early in infection, when the appearance of seemingly ER-derived PV vesicles is observed (10). As infection progresses, formation of vesicular replication complexes changes the aspect and extent of vesiculation of the infected cell fundamentally (11, 21). Concomitantly, an increasing number of vesicles was reported to acquire characteristics of autophagic vacuoles (67, 73). At later stages, all cytoplasmic membranes, except the nuclear and plasma membranes and mitochondria, are no longer recognizable and have presumably contributed to PV vesicles (11, 21).
The exact role of membranes in PV RNA synthesis is still elusive. Likewise, not all of the different functions in RNA replication for the mostly multifunctional nonstructural proteins are known. The viral proteins 2BC and 2C, encoded in the P2 genomic region, are indispensable for viral RNA replication (9, 15, 57, 58, 81). They are exclusively associated with the membranes of the vesicles of the PV replication complex and can, therefore, be used as a histochemical marker for PV vesicles and the viral replication complex (10, 11, 24, 67). Several reports indicate that protein 2BC, in the absence of or in combination with other viral proteins, can induce vesicle formation (1, 13, 20, 73, 79, 80). The mechanism by which protein 2BC induces vesicles is not known, and thus it is not clear whether vesicle induction and formation are an intrinsic property of this viral protein or whether 2BC might interact with (i.e., activate or stimulate) cellular vesiculation processes. The second possibility seems quite conceivable, since several regulated viral activities, e.g., initiation, enhancement, and cessation of viral translation, depend on the interaction of viral and cellular molecules (2, 16, 29, 30, 53).
In uninfected eukaryotic cells, vesiculation processes are used for the bidirectional membrane and protein transport, i.e., the antero- and retrograde membrane pathway (for reviews, see references 39 and 72). The anterograde traffic starts with the production of transport vesicles at the ER. This process is mediated by the proteins of the COPII complex (7). The five COPII proteins Sar1, Sec23p and Sec24p, and Sec13 and Sec31 are sufficient to produce vesicles in an in vitro assay (45, 62). COPII-mediated vesicle formation was first extensively described in Saccharomyces cerevisiae. Mammalian homologues of COPII components have been cloned and functionally characterized more recently (40, 51, 52, 75, 78).
For the formation of COPII-coated vesicles at the ER, sequential binding of the COPII proteins has to take place. The GTPase Sar1, in the GTP-bound state, binds to the ER. The binding is mediated by the membrane protein Sec12p, a guanine exchange protein for Sar1 (8, 22, 40). Sec23p, a GTPase activating protein (87), complexed to Sec24p (36), then binds to Sar1-GTP. In the last step, the Sec13 complex consisting of Sec13 complexed to Sec31 is recruited to the ER membranes. This complex, referred to as Sec13-Sec31, polymerizes the COPII complex into a coat which brings about vesicle budding (4, 60, 61, 66, 76, 77).
Newly synthesized secretory proteins leave the ER in COPII-coated vesicles. After budding, COPII vesicles lose their coat and either fuse with one another to form the vesicular tubular clusters of the ER-Golgi intermediate compartment (6, 33, 69) or fuse with preexisting vesicular tubular clusters. The clusters then move to the center of the cell and deliver their cargo to the Golgi apparatus.
Recycling of selected proteins and lipids within the Golgi and back to the ER is effected by vesicles of the retrograde pathway. For vesicle formation, the cytosolic GTPase ARF1 binds, in the GTP-bound state, to membranes of the Golgi complex. ARF1 then recruits the COPI proteins, a cytosolic, preassembled complex of seven coat protein subunits (50), which induce budding of vesicles.
In the present study, we tested whether PV vesicles are derived from COPII vesicles and thus utilize a cellular mechanism of vesicle formation or whether viral proteins (i.e., protein 2BC) induce PV vesicles by a virus-specific mechanism. The well-characterized role of the Sec13-Sec31 complex prompted its use as an ER-to-Golgi transport marker of the anterograde pathway. ER membranes were delineated with antibodies (Ab) against several ER resident marker proteins. To define an involvement of the Golgi complex in PV vesicle formation, giantin was used as a marker for the cis-medial Golgi compartment (41, 42). To trace PV vesicles, monoclonal Ab (MAb) against viral P2-proteins were used. Colocalization of marker proteins was examined in BT7-H cells by double immunofluorescence (IF) labeling and confocal scanning laser microscopy. The highest possible resolution was obtained by applying deconvolution software during the digital image processing of serial optical sections through the entire cell.
Our results indicate that the vesicles produced early in PV infection originate from the ER and that vesicles emerging after PV infection as well as after expression of a viral nonstructural protein(s) are not induced and formed by the action of a specific viral protein(s) with the propensity to perform membrane vesiculation. Rather, they are formed on the ER using the COPII protein complex, and thus are, by origin and mode of formation, homologous to the vesicles of the anterograde membrane transport pathway. However, unlike vesicles of the anterograde membrane traffic, they do not fuse with (or mature to) the ER-Golgi intermediate compartment or the Golgi system but instead accumulate in the cytoplasm. Thus, PV effectively uses a cellular mechanism to prepare the structural support for its genome replication.
MATERIALS AND METHODS
Infection and transfection of BT7-H cells.
The monkey kidney cell line BT7-H, which stably expresses T7 RNA polymerase (86), was kindly provided by S. Lemon (University of Texas Medical Branch, Galveston). The cells were maintained in the presence of G-418 sulfate (Geneticin; Life Technologies, Gaithersburg, Md.). For infection or transfection, the antibiotic was omitted.
For infection with PV, the cells were grown as monolayer cultures on coverslips and were infected with PV Sabin type 1 strain LSC2ab, at a multiplicity of 200 PFU per cell. The virus was allowed to adsorb at 4°C for 45 min, and the infection was left to proceed at 36°C for 3.5 h.
Transfections were done in 3.5-cm-diameter plates with 2 μg of plasmid DNA and 10 μl of Lipofectin (Life Technologies) according to the manufacturer's procedure. The plasmids pE5PVΔP1 and pTM-PV2BC have been described (20, 79). A poly(A) stretch has been added to the pTM-PV2BC by N. Teterina, National Institutes of Health, Bethesda, Md. Expression time of plasmid-encoded proteins was for 6 or 8 h.
EM.
For electron microscopy (EM), cell cultures were trypsinized, fixed with 2.5% glutaraldehyde and 2% OsO4, and embedded in Epon 812 according to standard procedures. Sections were viewed in a Philips CM 100 electron microscope.
IF and Ab.
For IF, the adhering cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 as described previously (17). For simultaneous detection of two antigens, specimens were incubated with two primary Ab from different species followed by an incubation with the two corresponding antispecies Ab, each conjugated to a different fluorochrome.
For the simultaneous detection of giantin and viral 2B-containing proteins, only two MAb were available as primary Ab. To perform double IF, the anti-PV 2B MAb was fluorescein isothiocyanate (FITC) labeled and the specimens were incubated first with antigiantin MAb followed by the anti-mouse Ab coupled to Cy3. After washing, the preparations were incubated with 1% mouse serum for 20 min to block possible free paratopes on the anti-mouse Ab. Viral proteins were then detected by the FITC-labeled anti-PV 2B MAb. This signal was enhanced with a goat anti-FITC Ab coupled to Alexa 488 (Molecular Probes, Eugene, Oreg.).
As primary Ab against viral proteins the anti-PV 2B MAb (24) (dilution 1:4), which recognizes the viral 2B-containing proteins, and the same anti-PV 2B MAb coupled to FITC (dilution 1:200) were used. The FITC coupling was kindly done by S. Freigang and R. Zinkernagel (University of Zurich, Zurich, Switzerland). The ER-resident marker proteins were detected with an anti-p63 MAb in a dilution of 1:1,000, anti-p63 rabbit Ab in a dilution of 1:400 (68, 70) or anti-major ER glycoproteins (anti-MERG) rabbit Ab (MERG are Ab raised against solubilized ER membrane fraction and kindly provided by D. Meyer, University of California at Los Angeles) in a dilution of 1:600. Proteins of the COPII complex were identified with the MAb anti-Sec31 in a dilution of 1:50 or anti-Sec31 rabbit Ab in a dilution of 1:50 (77) and with anti-Sec13 rabbit Ab in a dilution of 1:50 (76). Membranes of the Golgi complex were visualized using an antigiantin MAb in a dilution of 1:800 (42).
The following fluorochrome-tagged secondary Ab were used: goat anti-rabbit Ab coupled to Cy2 (Jackson Immunoresearch Laboratories, West Grove, Pa.) diluted 1:400, goat anti-mouse Ab coupled to Cy3 (Jackson) diluted 1:600, and goat anti-FITC Ab coupled to Alexa 488 (Molecular Probes) diluted 1:400.
All IF specimens to be used in the confocal microscope were mounted in Mowiol 4-88 (Hoechst, Frankfurt, Germany) containing 1% N-propyl gallate (Sigma, Buchs, Switzerland) as antifading agent and left to set at 4°C overnight (43).
Microscopy and digital image processing.
Conventional microscopy was performed using an epifluorescence microscope (Nikon E800) equipped with suitable filters.
Digital optical sections were taken with a confocal laser scanning microscope (model TCS4D; Leica Lasertechnik, Heidelberg, Germany) equipped with a Leica 100×/nA 1.4 apochromatic objective lens and a Kr-Ar laser emitting the excitation wavelengths of 488, 568, and 647 nm.
Images were recorded in the simultaneous acquisition mode. The settings of the photomultipliers were adjusted to record the range of 1 to 255 intensity values. The image area had a size of 25 by 25 μm and contained 512 by 512 pixels, which resulted in a pixel size of approximately 50 by 50 nm. Image stacks of 44 successive horizontal optical sections were recorded. To fulfill the requirements of the Nyquist theorem (85), the thickness of the optical sections did not exceed 120 nm. The settings resulted in a slight overlap between successive optical sections and ensured that no signal was lost along the z axis.
Raw images were transferred to a Silicon Graphics O2 workstation and deconvolved by the Huygens deconvolution module (Scientific Volume Imaging BV, Hilversum, Holland) of the Imaris software packet (Bitplane AG, Zurich, Switzerland) operating in the maximum-likelihood estimation mode (84) and assuming Poisson distribution of background noise. Deconvolution substantially reduces background noise and compensates for aberrations of the optical system. For this process, a mathematical function (point spread function [34]) describing the differences between a defined object and its image obtained in a given confocal microscope is applied to the recorded image. The point spread function was generated by using spherical fluorescent latex beads (diameter, 0.22 μm) as a defined object (71, 84). When applied to images recorded with identical parameter settings as used for generating the point spread function, deconvolution allows the reconstruction of an image of the object that is almost free of background and shows enhanced contrast and resolution.
Visualization of data obtained by confocal microscopy.
To examine specimens after the deconvolution process, we used three different projections of the image stacks. Statistical single-pixel projections (histograms) show every pixel for its relative fluorescence intensity in both the red (x axis) and the green (y axis) channel. Maximal-intensity projections are superimpositions of all optical sections of a deconvolved image stack. Maximal-intensity projections thus are similar to the aspect of an image from a conventional fluorescence microscope, although at increased resolution. Pixels fulfilling the requirements of colocalization (see below) were highlighted yellow and added as a separate layer to the deconvolved image stack of both fluorescence channels. Isosurface pictures were generated using the ISOsurface module of the Imaris software package. They are three-dimensional surface renderings of fluorescent structures. To define the surface of a structure, maximal-intensity projections of the deconvolved image were inspected at high magnification. The threshold between structure and background was set where the signal intensity dropped by at least a factor of 3 within a distance of four pixels. To enhance the stereoscopic effect of the printed image, isosurface pictures were slightly tilted in the vertical plane.
Parameter settings for colocalization of two antigens.
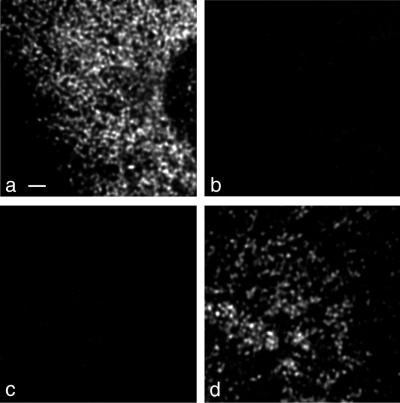
The significance of IF experiments demonstrating colocalization of two antigens depends on the extent of bleedthrough (cross talk), i.e., signal emitted by one fluorochrome which is detected in the channel of the other fluorochrome (18). The absence of visible cross talk was verified by using Ab yielding the strongest IF signals. Infected cells were stained with either Ab against ER marker p63 or PV protein 2B followed by Cy2- or Cy3-labeled antispecies Ab, respectively. Figure 1 shows black and white prints of maximal-intensity projections. No Cy2 signal (Fig. 1a) could be visualized in the Cy3 channel (Fig. 1b) and vice versa (Fig. 1c and d).
FIG. 1.
Signal cross talk between Cy2 and Cy3 detection channels. (a and b) Mock-infected cells were labeled with anti-p63 and Cy2-tagged antispecies Ab. No bleedthrough of signal was visible from the Cy2-detecting (a) into the Cy3-detecting (b) channel. (c and d) PV-infected cells were labeled with anti-2B and Cy3-tagged antispecies Ab. No bleedthrough from the Cy3-detecting (d) into the Cy2-detecting (c) channel was observed. Bar, 2 μm.
Background signal was determined by measuring the intensity of appropriate individual voxels on maximal-intensity projections. After deconvolution, background in both channels did not exceed 6%. Cross talk between channels, although not visible, was detectable in a similar way by measuring appropriate voxels on corresponding single optical sections. A maximum of 17% of the Cy3 signal (44 of 255 gray values [8 bit]) was picked up in the green channel, and 10% of the Cy2 signal (25 of 255) was picked up in the red channel. Cross talk increases linearly with signal intensity and intersects with the 6% background level at 35% intensity of the green and at 55% intensity of the red signal (Fig. 2d and e). Accordingly, colocalization of Cy2 and Cy3 signals was considered to be significant if above background or cross talk level, whichever was higher. For specimens with almost exclusive colocalization and little if any pure red or green signal, the colocalization threshold was set at background level (Fig. 2e).
FIG. 2.
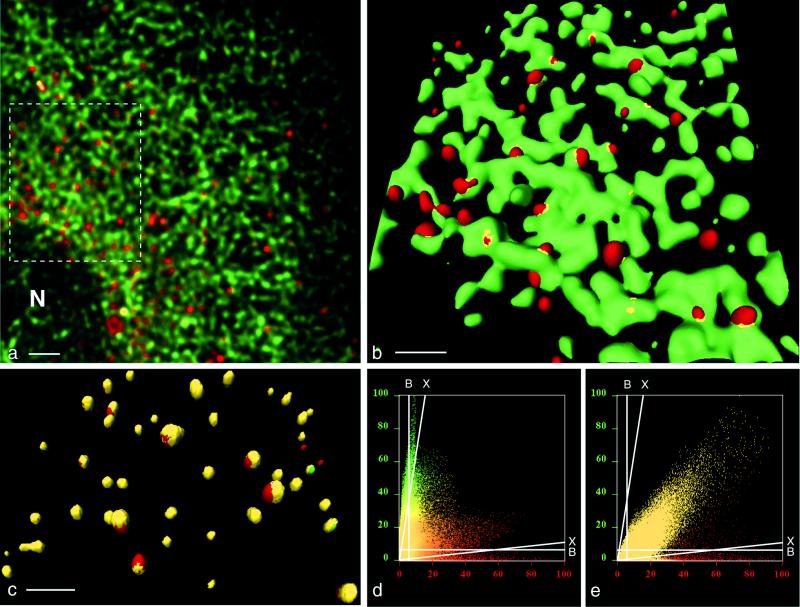
Formation of anterograde transport vesicles at the ER in uninfected BT7-H cells. (a and b) Detection of ER marker protein p63 (green) and COPII component Sec31 (red). (a) Maximal-intensity projection of an deconvolved image stack of 44 optical sections obtained by confocal microscopy. N, nucleus. Bar, 2 μm. (b) Three-dimensional view (isosurface) of the region outlined in panel a. Sec31-coated vesicles (red) are budding from the ER membranes (green). Colocalization (yellow) of p63 and Sec31 is restricted to collar-like transition sites between ER and vesicles. Bar, 1 μm (due to perspective, the bar size applies only to the foreground of the image). (c) Isosurface reconstruction of a part of a cell labeled for Sec13 (green) and Sec31 (red), showing a high degree of colocalization (yellow) of the two COPII components. Bar, 1 μm (in foreground). (d) Histogram of a stack of optical sections taken from a cell labeled for p63 (green) and Sec31 (red). Pixels carrying green or red signal are distributed according to their intensity in two distinct populations along their respective axes. (e) Histogram from a cell labeled for Sec13 and Sec31. Most pixels carry both signals. In panels d and e, background for either channel was 6% over the entire range of signal intensity (lines marked B). Cross talk increased with signal intensity from zero to a maximum of 17% in the green channel and 10% in the red channel (lines marked X) (see explanation in Material and Methods).
RESULTS
Visualization of vesicle formation in the anterograde membrane traffic in uninfected cells.
To disclose the process of vesiculation on the ER with high-resolution confocal scanning laser microscopy, we followed the formation of vesicles of the anterograde membrane pathway in uninfected BT7-H cells by simultaneous IF with Ab against the ER marker p63 and the COPII component Sec31. Anti-Sec31 is known to label selectively vesicles of the anterograde membrane pathway (77). Figure 2a shows a maximal-intensity projection from a part of a cell with p63-labeled green network, compatible with the aspect of ER, and several single red spots, representing Sec31-containing structures. The nuclear membrane is not labeled by the anti-p63 Ab, which is in agreement with earlier findings (70).
The three-dimensional view (Fig. 2b, isosurface picture) obtained from the area outlined in Fig. 2a shows a p63-containing network (green) representing ER and Sec31-labeled vesicles (red), some of which are budding off from the ER. p63 was not contained in the vesicular membranes, and colocalization (yellow) between p63 and Sec31 was restricted to a thin collar-like structure at the interphase between the vesicles and the ER. Exclusion of the ER-resident protein p63 from the vesicles of the anterograde membrane pathway is in accord with published data on the presence of p63 in the ER and absence in structures of the anterograde membrane traffic (70).
In parallel cell preparations, stained simultaneously with anti-Sec31 and anti-Sec13 Ab, a high degree of colocalization of the two COPII components in the vesicular structures could be observed (Fig. 2c). This finding confirms that the vesicle formation depicted in Fig. 2b was COPII mediated, since the Sec13-Sec31 complex is the last component of the COPII coat to be recruited onto ER membranes during vesicle formation (77).
The extent of colocalization was also monitored in histograms. Figure 2d shows a histogram corresponding to Fig. 2a, i.e., a cell stained for p63 and Sec31. The two signals colocalized only partially, and a high number of red or green pixels were distributed along their respective color axes. In contrast, in cells stained for Sec13 and Sec31 (Fig. 2e), the distribution of the majority of the pixels diagonally across the panel indicated an extensive colocalization of both antigens.
Visualization of PV-specific vesicle formation in PV-infected cells.
To visualize the process of the formation of PV vesicles, BT7-H cells were infected with PV, fixed at 3.5 h postinfection and stained with a MAb against PV protein 2B, i.e., specific for PV vesicles, and with anti-p63 Ab. Figure 3a shows that 2B-containing proteins associated with the ER by forming flat patches, in which viral vesicle marker and cellular ER marker proteins colocalized. The 2BC-carrying membrane sites seemed to undergo a budding process, whereby p63 was excluded from the vesicles. A collar-like ring (yellow), containing the viral and cellular marker proteins, was connecting vesicle and ER membranes. The corresponding histogram indicates that p63 and 2B-containing proteins only partially colocalize (not shown). However, the exclusion of ER-resident proteins from PV vesicles is not restricted to p63. In preparations stained with anti-2B MAb and anti-MERG Ab, recognizing a multitude of ER proteins, the ER proteins were similarly excluded from the PV vesicles (Fig. 3b).
FIG. 3.
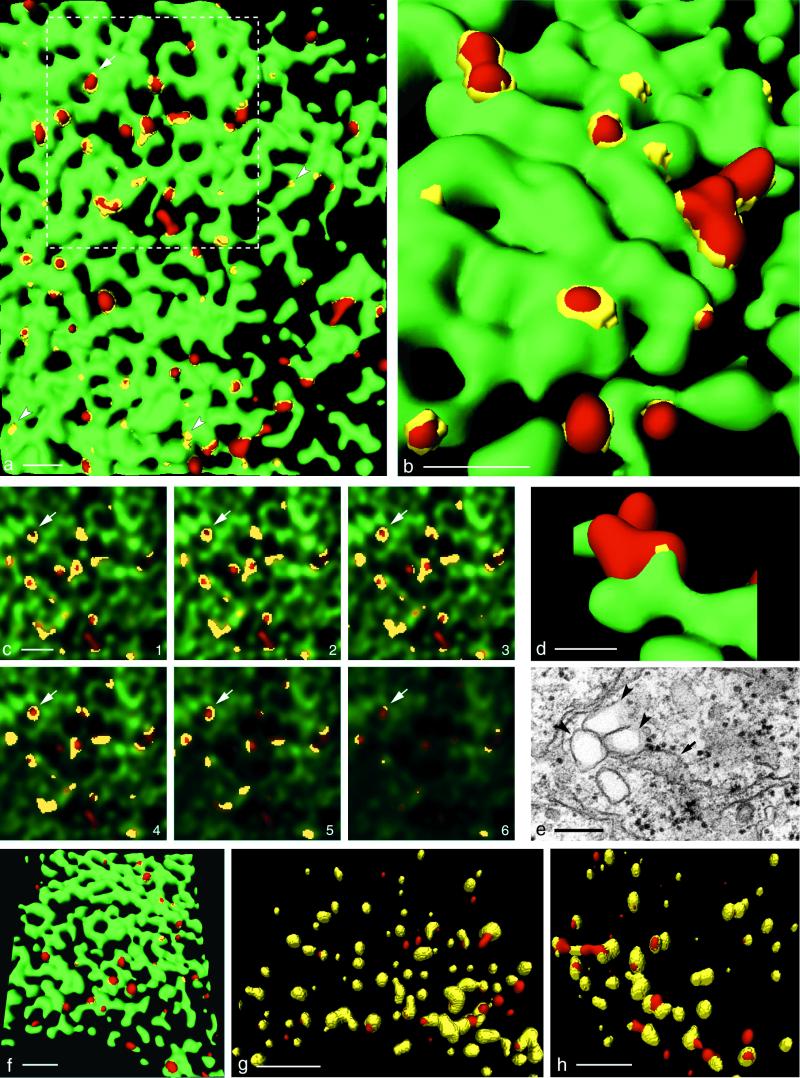
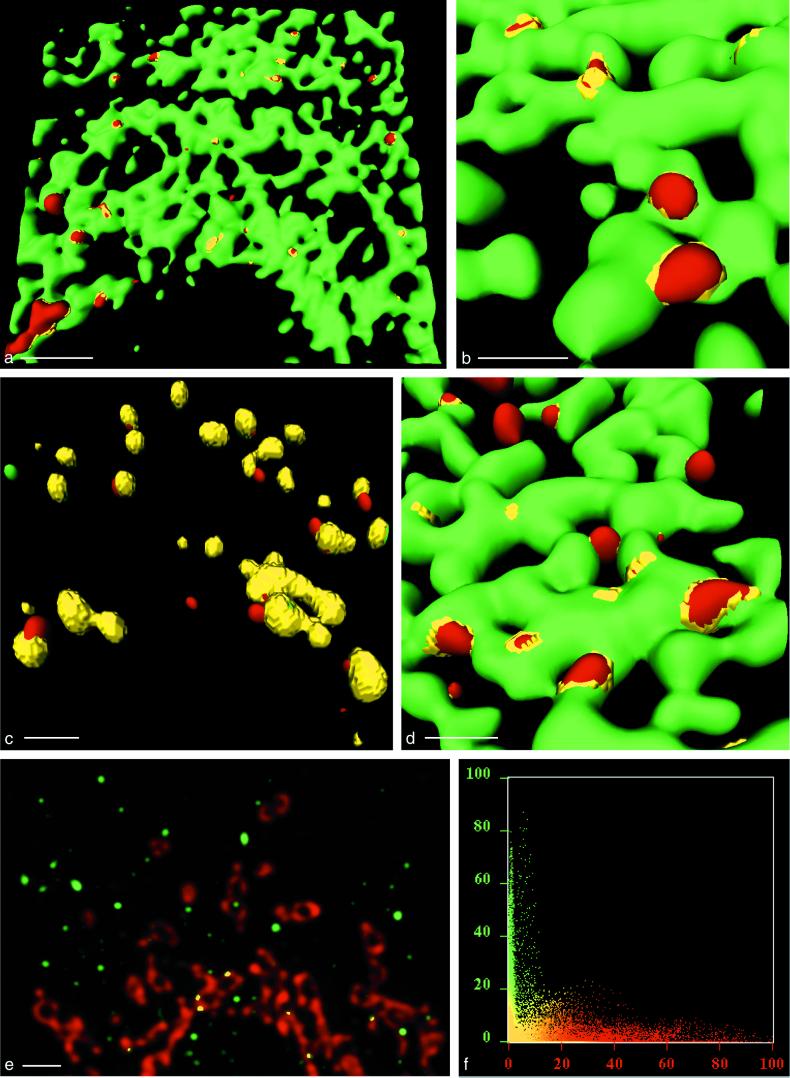
In PV-infected cells, formation of PV vesicles occurs at the ER. (a) Isosurface picture of a part of a cell labeled for p63 (green) and 2B (red). Colocalization of the markers is restricted to flat yellow patches (arrowheads), representing the sites of association of 2B marker protein with the ER and to collar-like transition sites between ER and vesicles (arrow; see also panel c). The dashed line confines the area shown in panel c. Bar, 1 μm. (b) Higher-magnification isosurface view showing part of a cell labeled for MERG, i.e., a series of ER-resident proteins (green) and 2B (red). Their colocalization (yellow) with 2B is in transition sites only, as for p63 and 2B in panel a. Bar, 0.5 μm (in foreground). (c) Consecutive optical sections through the zone of the cell outlined in Fig. 3a (dashed line). By following an emerging vesicle through the sections (arrows), it can be seen that the antigens p63 (green) and PV 2B (red) colocalize (yellow) to form a ring-like structure which evolves into a red vesicle. The thickness of an optical section is 70 nm. Bar, 1 μm. (d) A cluster of 2B-positive vesicles (red) is emerging from the ER labeled for p63 (green). Bar, 200 nm (in foreground). (e) EM picture of a section through a comparable cluster of vesicles (arrowheads) associated with the ER (arrow), partially carrying ribosomes. The vesicles are of similar size and arrangement as the vesicles found by confocal microscopy. Bar, 200 nm. (f) Isosurface picture showing anti-Sec31-labeled vesicles (red) which are budding from the ER (green) (p63) in a PV-infected cell. Bar, 2 μm (in foreground). (g and h) Vesicles were labeled with anti-2B Ab (red) and either with anti-Sec13 (g) or anti-Sec31 (h). Both COPII components largely colocalize (yellow) with 2B. Bars, 2 μm (in foreground).
Figure 3c is a gallery of six consecutive optical sections with the red and green channels superimposed. The sections correspond to the area outlined in Fig. 3a. In pursuing the yellow colocalization signal through such a stack of sections, colocalization between ER marker and viral vesicle marker is found to be a hollow, ring-like structure, which eventually evolves into a red vesicle. This finding confirms the isosurface representations in Fig. 3a and b.
In Fig. 3d, a larger, 2B-positive structure is shown, representing clustered vesicles attached to the ER. This aspect is compatible with ultrastructural findings (Fig. 3e). Such vesicular clusters, associated with the ER, have previously been found to represent an early stage of a PV replication complex (10). Figure 3d and e also show that the size of PV vesicles is comparable in the EM and the confocal microscope.
Formation of PV vesicles is homologous to the formation of vesicles of the anterograde membrane pathway.
The budding process of vesicles carrying the COPII complex was visualized in PV-infected BT7-H cells. Double IF for COPII and ER markers revealed that formation of COPII vesicles appears to be very similar in PV-infected cells (Fig. 3f) and in uninfected cells (Fig. 2b). In addition, the budding of COPII-coated vesicles during infection (Fig. 3f) resembles closely the emergence of PV vesicles at the ER (Fig. 3a). The apparent similarity in the formation of COPII and PV vesicles prompted us to test whether PV vesicles are coated with proteins of the COPII complex and thus would represent vesicles of the anterograde membrane traffic. To test for the presence of COPII coat proteins on PV vesicles, PV-infected BT7-H cells were fixed at 3.5 h postinfection and double stained with Ab against PV protein 2B and Sec13 or Sec31. Figure 3g and h show an extensive colocalization of each of the two COPII proteins with the viral 2B-containing proteins (yellow signal). All of the Sec13- and Sec31-positive vesicles carried the 2B marker and, therefore, are by definition PV vesicles. In contrast, few vesicles were positive for 2B-containing proteins only and not for the Sec13-Sec31 complex (red vesicles in Fig. 3g and h). The finding that all of the Sec13 and Sec31 proteins colocalized to 2B-containing structures was confirmed in corresponding histograms (not shown).
Comparing the location of COPII-positive vesicles within infected and noninfected cells indicates that COPII-positive PV vesicles tended to accumulate in the perinuclear region (Fig. 4a), whereas COPII vesicles of noninfected cells were distributed more evenly through the cytoplasm (Fig. 4b). In addition, PV vesicles tended to form larger units (Fig. 3b and d and 4a), increasing the average diameter of the observed structures (see Discussion).
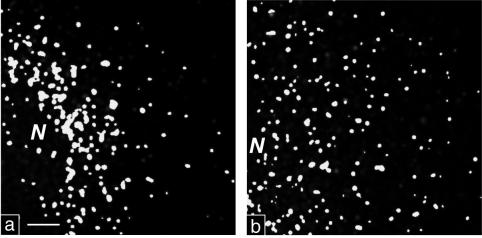
FIG. 4.
High-contrast black and white prints of maximal-intensity projections. (a) PV-infected cell stained for Sec13 and 2B. The irregularly sized colocalization signal is found concentrated in a perinuclear region. (b) Noninfected cell stained for Sec13 and Sec31. The uniform signal is rather evenly distributed through the cytoplasm. N, nucleus. Bar, 2 μm.
The finding that virtually no Sec13-Sec31 could be found on the ER or on vesicles without being colocalized to the viral marker proteins suggests that PV protein 2BC recruits the COPII complex to start vesicle formation. The observation that both COPII components, Sec13 and Sec31, are found on the PV vesicles suggests that the COPII complex is functional (3, 76, 77) and acts as the driving force in PV vesicle formation. Together, our data argue that PV vesicles are formed at the ER by a mechanism similar or, possibly, identical to that producing the vesicles of the anterograde pathway. The few vesicles which carry only 2B marker and no COPII proteins might either have lost the COPII coat or originate from a compartment different from the ER.
Vesicle formation after expression of PV protein 2BC alone and in the context of all nonstructural proteins.
Previous reports have indicated that the PV protein 2BC is the inducing protein for PV vesicle formation (1, 13, 20) and that the PV nonstructural protein 3A may contribute to the final morphology (73). Thus, it was of interest to determine whether vesicle formation initiated by protein 2BC would also be mediated by the COPII mechanism. This was investigated by expressing PV protein 2BC either in the absence of other viral proteins or, to assess a role of other nonstructural proteins in vesicle formation, within an ORF coding for all PV P2 and P3 proteins.
BT7-H cells stably expressing T7 RNA polymerase were transfected with plasmid DNA pTM-PV2BC and processed for IF. Figure 5a shows 2BC-carrying vesicles budding off from the ER, thereby excluding the ER marker p63. Details of the vesicle formation process at the ER (Fig. 5b) appear to be fully comparable to that in PV-infected cells (compare with Fig. 3b).
FIG. 5.
Vesicle formation in cells expressing PV protein 2BC alone or in the context of all nonstructural PV proteins. (a to c) Isosurface pictures of cells transfected with pTM-PV2BC and fixed 6 h posttransfection. (a and b) Cells were stained for p63 (green) and 2B (red). The emergence of the 2B-positive vesicles appears similar to that observed for vesicles after PV infection, and colocalization of p63 and PV 2BC is in collar-like structures only (compare with Fig. 3). Bars (in foreground), 2 μm (a) and 0.5 μm (b). (c) In pTM-PV2BC-transfected cells stained for Sec13 (green) and 2B (red), a high degree of colocalization (yellow) of the two markers is observed. Bar, 0.5 μm (in foreground). (d) Cells transfected with plasmid pE5PVΔP1 (coding for all proteins of the P2 and P3 genomic region) and stained for p63 (green) and 2B (red) at 8 h posttransfection. Vesicle formation is comparable to that during PV infection or expression of 2BC alone. Bar, 0.5 μm (in foreground). (e and f) PV-infected cells, stained with antigiantin MAb (red) and FITC-tagged anti-2B MAb, enhanced with a secondary anti-FITC-Alexa 488 Ab (green). (e) Maximal-intensity projection. The PV vesicles do not associate with the cis-medial Golgi compartment. Bar, 2 μm. (f) In the corresponding histogram, the two signals are largely separated.
Staining of parallel specimens with anti-2B and anti-COPII Ab showed that vesicle formation after expression of protein 2BC is also mechanistically comparable to that after PV infection. Similar to the findings in PV-infected cells, the COPII-positive vesicles in pTM-PV2BC-transfected cells carried the viral protein 2BC (Fig. 5c).
To determine whether the expression of additional nonstructural proteins influences vesicle formation or morphology, the ORF pE5PVΔP1 (79), which comprises the entire P2 and P3 regions of the PV genome, was expressed in BT7-H cells. The ER was visualized with anti-p63 Ab, and the vesicles were stained with anti-2B MAb (Fig. 5d). Vesicle formation in the presence of all nonstructural PV proteins proceeds comparably to that in PV-infected cells or in cells transfected with the construct coding for 2BC only.
The Golgi complex is not a primary target for the PV vesicle formation process.
The data reported above indicate that the PV vesicles originate from the ER and are homologous to vesicles of the anterograde membrane pathway. However, a few vesicles can be observed which do not carry the COPII proteins and, thus, could be from a different origin. Since there are controversial reports on the role of the Golgi apparatus in PV vesicle formation and on the extent to which the Golgi can possibly participate in this process (17, 46, 63), we tested whether the Golgi complex might be a second vesicle donor early in infection. We analyzed PV-infected cells stained with anti-2B MAb and with antigiantin Ab, which selectively labels the cis-medial Golgi membranes (41, 42).
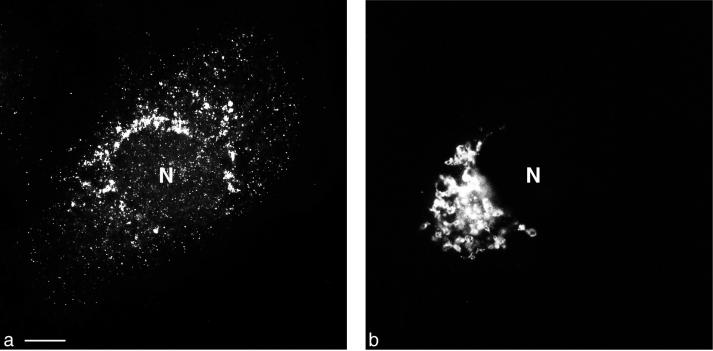
Conventional epifluorescence microscopy was used to show the overall distribution of 2B-containing PV proteins (Fig. 6a) and giantin in the same PV-infected cell (Fig. 6b). The two antigens form distinct patterns with no preferential association with each other. To assess any colocalization of PV vesicles and cis-medial Golgi membranes with the highest possible resolution, deconvolved optical sections from the confocal microscope were inspected. They indicated little if any colocalization of the two antigens (Fig. 5e), which was confirmed by a histogram analysis (Fig. 5f). Tracing occasional small colocalization signals through the stack of optical sections obtained from such cells indicated that, in contrast to the vesicles at the ER, no collar-like transition sites between the two types of membranes were built up. Rather, colocalization was restricted to small contact points between the cis-medial Golgi compartment and 2B-containing vesicles.
FIG. 6.
Conventional double fluorescence microscopy of a PV-infected cell. (a) Staining with FITC-tagged anti-2B MAb, enhanced with a secondary anti-FITC-Alexa 488 Ab. (b) Staining for giantin (cis-medial Golgi compartment). Distribution of the two signals in the cell is entirely different. Bar, 10 μm.
DISCUSSION
High-resolution confocal microscopy employing deconvolution software allowed us to visualize cellular structures at the limits of resolution. The primary output of the confocal microscope is optical sections, which are corrected (deconvolved) for optical aberrations and background noise and which can then be viewed singly or superimposed as stacks (maximal-intensity projection). Stacks of optical sections were used to generate histograms, which are helpful in analyzing image background and cross talk between channels and for judging the overall extent of colocalization of two target molecules (Fig. 2d and e). Three-dimensional views, which cover the entire depth of a cell, are derived from deconvolved maximal-intensity projections. They discriminate between structures below and above a predetermined level of fluorescence intensity. All structures with intensities above the threshold level are shown, irrespective of their actual fluorescence level, at the same brightness in a stereoscopic view.
The analysis of noninfected cells showed features expected from published experiments, i.e., vesicle budding on the ER and the exclusion of ER-resident proteins from vesicles of the anterograde membrane traffic (4, 70). The fact that we could clearly visualize the expected features made us confident that the high-resolution confocal microscopy used yields meaningful results and thus would produce reliable data also for PV-infected cells. The size of some COPII vesicles, whether budding or detached from the ER, exceeded that of published values for vesicles formed at ER membranes in vitro (7) but was slightly below those for vesicles or vesicular tubular clusters and transport containers found in vivo (5, 47, 59, 64). It should be kept in mind, however, that fluorescence microscopy does not necessarily reflect accurately the size of an object (64).
In PV-infected cells, vesicles in a broad range of apparent size (200 to 400 nm) were visualized. These structures are compatible in size and shape with PV vesicles already described in some detail in previous electron microscopic studies (10, 13–15, 21, 24). The observed clustering of vesicles (Fig. 3b and d and 4a) could well represent the formation of viral replication complexes (12) and is compatible with earlier data visualizing PV RNA synthesis by autoradiography (10). Thus, the three-dimensional (isosurface) reconstructions presented show the spatial distribution and colocalization of viral and cellular markers on the level of single vesicles and nascent replication complexes which, by this method, can be viewed in the context of the entire depth of and in a three-dimensional orientation within a cell.
Vesicular budding and the collar-like transition sites between ER and viral vesicle markers could be seen. Consequently, our findings directly demonstrate the exclusion of ER-resident marker proteins p63 and a series of other ER membrane proteins from the viral vesicular membranes during the budding process, similar to that observed for vesicles of the anterograde membrane traffic in uninfected cells (Fig. 2b). To prove the ER origin of PV vesicles and their possible homology with vesicles of the anterograde transport pathway, the possible presence of the COPII proteins Sec13 and Sec31 on PV vesicles was investigated. In uninfected cells, binding of Sec13 and Sec31 was shown to be dependent on the ordered prior binding of the upstream COPII components Sar1 and Sec23p-Sec24p (45) and Sar1 activation (3). The presence of the last heterodimeric protein in the COPII cascade, i.e., Sec13 and Sec31 on the 2B-carrying PV vesicles, thus implicates the association of an active complement of the COPII proteins. Our findings are interpreted to mean that PV vesicles are not formed by the action of one single PV protein (i.e., protein 2BC, or, as suggested in reference 73, a combination of proteins 2BC and 3A) but rather that PV vesicles—the key building blocks of the viral replication complex—are formed by utilizing the COPII machinery and thus can be considered homologous to the vesicles of the anterograde membrane traffic.
Protein 2BC triggers an extensive vesiculation of membranes in a cell, regardless of whether the protein is expressed alone from the plasmid pTM-PV2BC or in the context of all nonstructural proteins, i.e., translated either from ORF pE5PVΔP1 or from a replicating genome (13, 20, 79, 80). The following two observations suggest that the role of protein 2BC in this vesiculation process stems from the recruitment of the COPII proteins to the ER. Protein 2BC is strictly membrane bound (1, 10, 11, 20) and is found in flat patches on the ER even before vesicle budding becomes visible (Fig. 3a and 5a). The COPII proteins Sec13 and Sec31, however, are not found on membranes devoid of the viral protein 2BC (Fig. 3g and h), which indicates that the COPII cascade assembles on the ER membrane at the sites where protein 2BC is already present. Thus, protein 2BC might act as a priming protein for the COPII-mediated vesiculation, perhaps substituting cellular priming proteins, such as cargo or vSNARE proteins (reviewed in reference 72). Alternatively, 2BC might even replace Sar1 (or its function), as both exhibit NTPase activity (8, 58, 87).
Interaction of protein 2BC with the COPII mechanism could lead to a stimulation of vesicle production, resulting in the observed extensive vesiculation of infected cells. Alternatively, or additionally, protein 2BC could influence the extent of vesiculation of an infected cell by interfering with the intracellular fate of the PV-COPII vesicles. In uninfected cells, vesicles of the anterograde membrane traffic lose their COPII coat before fusing with vesicular tubular clusters of the intermediate compartment and being incorporated into the cis-Golgi complex (reviewed in reference 72). Since a majority of the PV vesicles carried the COPII components Sec13 and 31 on their surface (Fig. 3g and h and 4a), it might well be that the viral protein 2BC stabilizes the COPII coat on the vesicles, thus preventing a subsequent fusion process into the Golgi. This would, in turn, lead to the observed accumulation of the vesicles (23, 46).
Using high-resolution confocal microscopy to test whether 2B-positive vesicles show any association with the Golgi, we could not detect anything more than obviously fortuitous association of PV vesicles with the cis-medial Golgi compartment. In those few cases where association of PV vesicles and cis-medial Golgi structures scored as colocalization, no collar-like transition sites, but mere small contact points between vesicles and Golgi, were found.
The findings can be interpreted to mean that PV vesicles neither originate from nor fuse with the Golgi and that the Golgi is not a primary association site for PV nonstructural proteins. This is in contrast to earlier work (63); however, the present work used different host cells and a different virus strain and, most importantly, investigated early times of infection. A reduction in or lack of membrane supply to the Golgi may finally lead to its disintegration during later times of infection (17) and, consequently, to a secondary use of Golgi membranes by the PV replication machinery (67).
As a consequence of limited genome size, viruses have to economize the amount of proteins and, hence, functions that they encode. The use of cellular resources, i.e., using a preexisting membrane pathway to create structures indispensably necessary for viral replication, is an intriguing example of such an economizing strategy. How viral proteins interact with and influence the action of cellular COPII proteins deserves further investigation. Studying these interactions might well provide a means to elucidate more details of the underlying mechanisms of the cellular COPII-mediated vesicle budding process.
ACKNOWLEDGMENTS
We are grateful to D. Meyer for providing the MERG antibody and S. Lemon for providing BT7-H cells. We thank S. Freigang and R. Zinkernagel for FITC coupling of the 2B MAb and N. Teterina and E. Ehrenfeld for adding the poly(A) stretch to the plasmid pTM-PV2BC and for helpful discussions.
This work was supported by grants 31-055397.98/1 (K.B.) and 31-61455.00 (H.-P.H.) from the Swiss National Science Foundation; grant 10348 from INTAS, Brussels, Belgium (K.B.); and a grant from the Biomedical Research Council of Singapore (B.L.T., W.H.). R.C.R. was supported by the Gottlieb Daimler- und Karl Benz-Foundation, Ladenburg, Germany, and R.G. was supported by the Novartis Foundation and the Freiwillige Akademische Gesellschaft, Basel, Switzerland.
REFERENCES
- 1.Aldabe R, Carrasco L. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem Biophys Res Commun. 1995;206:64–76. doi: 10.1006/bbrc.1995.1010. [DOI] [PubMed] [Google Scholar]
- 2.Andino R, Rieckhof G E, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990;63:369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- 3.Aridor M, Balch W E. Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem. 2000;275:35673–35676. doi: 10.1074/jbc.C000449200. [DOI] [PubMed] [Google Scholar]
- 4.Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch W E. Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol. 1998;141:61–70. doi: 10.1083/jcb.141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannykh S I, Balch W E. Membrane dynamics at the endoplasmic reticulum Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannykh S I, Nishimura N, Balch W E. Getting into the Golgi. Trends Cell Biol. 1998;8:21–25. doi: 10.1016/s0962-8924(97)01184-7. [DOI] [PubMed] [Google Scholar]
- 7.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach M F, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 8.Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 9.Barton D J, Flanegan J B. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol. 1997;71:8482–8489. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;160:220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- 11.Bienz K, Egger D, Pfister T. Characteristics of the poliovirus replication complex. Arch Virol Suppl. 1994;9:147–157. doi: 10.1007/978-3-7091-9326-6_15. [DOI] [PubMed] [Google Scholar]
- 12.Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bienz K, Egger D, Rasser Y, Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983;131:39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- 14.Bienz K, Egger D, Rasser Y, Bossart W. Kinetics and location of poliovirus macromolecular synthesis in correlation to virus-induced cytopathology. Virology. 1980;100:390–399. doi: 10.1016/0042-6822(80)90530-9. [DOI] [PubMed] [Google Scholar]
- 15.Bienz K, Egger D, Troxler M, Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990;64:1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blyn L B, Towner J S, Semler B L, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolten R, Egger D, Gosert R, Schaub G, Landmann L, Bienz K. Intracellular localization of poliovirus plus- and minus-strand RNA visualized by strand-specific fluorescent in situ hybridization. J Virol. 1998;72:8578–8585. doi: 10.1128/jvi.72.11.8578-8585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brelje T C, Wessendorf M W, Sorenson R L. Multicolor laser scanning confocal immunofluorescence microscopy: practical application and limitations. Methods Cell Biol. 1993;38:97–181. doi: 10.1016/s0091-679x(08)61001-8. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Ahlquist P. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J Virol. 2000;74:4310–4318. doi: 10.1128/jvi.74.9.4310-4318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho M W, Teterina N, Egger D, Bienz K, Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994;202:129–145. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- 21.Dales S, Eggers H J, Tamm I, Palade G E. Electron microscopic study of the formation of poliovirus. Virology. 1965;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- 22.d'Enfert C, Wuestehube L J, Lila T, Schekman R. Sec12p-dependent membrane binding of the small GTP-binding protein Sar1p promotes formation of transport vesicles from the ER. J Cell Biol. 1991;114:663–670. doi: 10.1083/jcb.114.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doedens J R, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger D, Pasamontes L, Bolten R, Boyko V, Bienz K. Reversible dissociation of the poliovirus replication complex: functions and interactions of its components in viral RNA synthesis. J Virol. 1996;70:8675–8683. doi: 10.1128/jvi.70.12.8675-8683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger D, Teterina N, Ehrenfeld E, Bienz K. Formation of the poliovirus replication complex requires coupled viral translation, vesicle production, and viral RNA synthesis. J Virol. 2000;74:6570–6580. doi: 10.1128/jvi.74.14.6570-6580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etchison D, Ehrenfeld E. Comparison of replication complexes synthesizing poliovirus RNA. Virology. 1981;111:33–46. doi: 10.1016/0042-6822(81)90651-6. [DOI] [PubMed] [Google Scholar]
- 27.Flanegan J, Baltimore D. Poliovirus-specific primer dependent RNA polymerase able to copy poly(A) Proc Natl Acad Sci USA. 1977;74:3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamarnik A V, Andino R. Interactions of viral protein 3CD and poly(RC) binding protein with the 5′ untranslated region of the poliovirus genome. J Virol. 2000;74:2219–2226. doi: 10.1128/jvi.74.5.2219-2226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamarnik A V, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamarnik A V, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 31.Girard M. In vitro synthesis of poliovirus ribonucleic acid: role of the replicative intermediate. J Virol. 1969;3:376–384. doi: 10.1128/jvi.3.4.376-384.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodfellow I, Chaudhry Y, Richardson A, Meredith J, Almond J W, Barclay W, Evans D J. Identification of a cis-acting replication element within the poliovirus coding region. J Virol. 2000;74:4590–4600. doi: 10.1128/jvi.74.10.4590-4600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauri H P, Kappeler F, Andersson H, Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J Cell Sci. 2000;113:587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- 34.Hell S W, Stelzer E H K. Lens aberrations in confocal microscopy. In: Pawley J B, editor. Handbook of biological confocal microscopy. 2nd ed. New York, N.Y: Plenum; 1995. pp. 347–354. [Google Scholar]
- 35.Hellen C U, Krausslich H G, Wimmer E. Proteolytic processing of polyproteins in the replication of RNA viruses. Biochemistry. 1989;28:9881–9890. doi: 10.1021/bi00452a001. [DOI] [PubMed] [Google Scholar]
- 36.Hosobuchi M, Kreis T, Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- 37.Johnson K L, Sarnow P. Three poliovirus 2B mutants exhibit noncomplementable defects in viral RNA amplification and display dosage-dependent dominance over wild-type poliovirus. J Virol. 1991;65:4341–4349. doi: 10.1128/jvi.65.8.4341-4349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jore J, De Geus B, Jackson R J, Pouwels P H, Enger-Valk B E. Poliovirus protein 3CD is the active protease for processing of the precursor protein P1 in vitro. J Gen Virol. 1988;69:1627–1636. doi: 10.1099/0022-1317-69-7-1627. [DOI] [PubMed] [Google Scholar]
- 39.Klumperman J. Transport between ER and Golgi. Curr Opin Cell Biol. 2000;12:445–449. doi: 10.1016/s0955-0674(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 40.Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman J E, Balch W E. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linstedt A D, Foguet M, Renz M, Seelig H P, Glick B S, Hauri H P. A C-terminally-anchored Golgi protein is inserted into the endoplasmic reticulum and then transported to the Golgi apparatus. Proc Natl Acad Sci USA. 1995;92:5102–5105. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linstedt A D, Hauri H P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longin A, Souchier C, Ffrench M, Bryon P A. Comparison of anti-fading agents used in fluorescence microscopy: image analysis and laser confocal microscopy study. J Histochem Cytochem. 1993;41:1833–1840. doi: 10.1177/41.12.8245431. [DOI] [PubMed] [Google Scholar]
- 44.Mackenzie J M, Jones M K, Westaway E G. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J Virol. 1999;73:9555–9567. doi: 10.1128/jvi.73.11.9555-9567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuoka K, Orci L, Amherdt M, Bednarek S Y, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 46.Maynell L A, Kirkegaard K, Klymkowsky M W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992;66:1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mironov A A, Polishchuk R S, Luini A. Visualizing membrane traffic in vivo by combined video fluorescence and 3D electron microscopy. Trends Cell Biol. 2000;10:349–353. doi: 10.1016/s0962-8924(00)01787-6. [DOI] [PubMed] [Google Scholar]
- 48.Nomoto A, Detjen B, Pozzatti R, Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977;268:208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- 49.Novak J E, Kirkegaard K. Coupling between genome translation and replication in an RNA virus. Genes Dev. 1994;8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 50.Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner T H, Rothman J E. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 51.Paccaud J P, Reith W, Carpentier J L, Ravazzola M, Amherdt M, Schekman R, Orci L. Cloning and functional characterization of mammalian homologues of the COPII component Sec23. Mol Biol Cell. 1996;7:1535–1546. doi: 10.1091/mbc.7.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pagano A, Letourneur F, Garcia-Estefania D, Carpentier J L, Orci L, Paccaud J P. Sec24 proteins and sorting at the endoplasmic reticulum. J Biol Chem. 1999;274:7833–7840. doi: 10.1074/jbc.274.12.7833. [DOI] [PubMed] [Google Scholar]
- 53.Parsley T B, Towner J S, Blyn L B, Ehrenfeld E, Semler B L. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA. 1997;3:1124–1134. [PMC free article] [PubMed] [Google Scholar]
- 54.Paul A V, Rieder E, Kim D W, van Boom J H, Wimmer E. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J Virol. 2000;74:10359–10370. doi: 10.1128/jvi.74.22.10359-10370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul A V, van Boom J H, Filippov D, Wimmer E. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393:280–284. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen K W, van der Meer Y, Roos N, Snijder E J. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J Virol. 1999;73:2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfister T, Jones K W, Wimmer E. A cysteine-rich motif in poliovirus protein 2CATPase is involved in RNA replication and binds zinc in vitro. J Virol. 2000;74:334–343. doi: 10.1128/jvi.74.1.334-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfister T, Wimmer E. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J Biol Chem. 1999;274:6992–7001. doi: 10.1074/jbc.274.11.6992. [DOI] [PubMed] [Google Scholar]
- 59.Presley J F, Cole N B, Schroer T A, Hirschberg K, Zaal K J M, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 60.Pryer N K, Salama N R, Schekman R, Kaiser C A. Cytosolic Sec13p complex is required for vesicle formation from the endoplasmic reticulum in vitro. J Cell Biol. 1993;120:865–875. doi: 10.1083/jcb.120.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salama N R, Chuang J S, Schekman R W. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salama N R, Yeung T, Schekman R W. The Sec13p complex and reconstitution of vesicle budding from the ER with purified cytosolic proteins. EMBO J. 1993;12:4073–4082. doi: 10.1002/j.1460-2075.1993.tb06091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandoval I V, Carrasco L. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J Virol. 1997;71:4679–4693. doi: 10.1128/jvi.71.6.4679-4693.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scales S J, Pepperkok R, Kreis T E. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 65.Schaad M C, Jensen P E, Carrington J C. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 1997;16:4049–4059. doi: 10.1093/emboj/16.13.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 67.Schlegel A, Giddings T H, Jr, Ladinsky M S, Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J Virol. 1996;70:6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri H P. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J Cell Sci. 1993;104:671–683. doi: 10.1242/jcs.104.3.671. [DOI] [PubMed] [Google Scholar]
- 69.Schweizer A, Fransen J A, Matter K, Kreis T E, Ginsel L, Hauri H P. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990;53:185–196. [PubMed] [Google Scholar]
- 70.Schweizer A, Rohrer J, Slot J W, Geuze H J, Kornfeld S. Reassessment of the subcellular localization of p63. J Cell Sci. 1995;108:2477–2485. doi: 10.1242/jcs.108.6.2477. [DOI] [PubMed] [Google Scholar]
- 71.Shaw P J, Rawlins D J. The point-spread function of a confocal microscope: its measurement and use in deconvolution of 3-D data. J Microsc. 1991;163:151–165. [Google Scholar]
- 72.Springer S, Spang A, Schekman R. A primer on vesicle budding. Cell. 1999;97:145–148. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- 73.Suhy D A, Giddings T H, Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J Virol. 2000;74:8953–8965. doi: 10.1128/jvi.74.19.8953-8965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takeda N, Kuhn R J, Yang C F, Takegami T, Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in a membrane complex of infected HeLa cells. J Virol. 1986;60:43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang B L, Kausalya J, Low D Y, Lock M L, Hong W. A family of mammalian proteins homologous to yeast Sec24p. Biochem Biophys Res Commun. 1999;258:679–684. doi: 10.1006/bbrc.1999.0574. [DOI] [PubMed] [Google Scholar]
- 76.Tang B L, Peter F, Krijnse-Locker J, Low S H, Griffiths G, Hong W. The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol. 1997;17:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang B L, Zhang T, Low D Y, Wong E T, Horstmann H, Hong W. Mammalian homologues of yeast sec31p. An ubiquitously expressed form is localized to endoplasmic reticulum (ER) exit sites and is essential for ER-Golgi transport. J Biol Chem. 2000;275:13597–13604. doi: 10.1074/jbc.275.18.13597. [DOI] [PubMed] [Google Scholar]
- 78.Tani K, Oyama Y, Hatsuzawa K, Tagaya M. Hypothetical protein KIAA0079 is a mammalian homologue of yeast Sec24p. FEBS Lett. 1999;447:247–250. doi: 10.1016/s0014-5793(99)00303-8. [DOI] [PubMed] [Google Scholar]
- 79.Teterina N L, Egger D, Bienz K, Brown D M, Semler B L, Ehrenfeld E. Requirements for assembly of poliovirus replication complexes and negative-strand RNA synthesis. J Virol. 2001;75:3841–3850. doi: 10.1128/JVI.75.8.3841-3850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teterina N L, Gorbalenya A E, Egger D, Bienz K, Ehrenfeld E. Poliovirus 2C protein determinants of membrane binding and rearrangements in mammalian cells. J Virol. 1997;71:8962–8972. doi: 10.1128/jvi.71.12.8962-8972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teterina N L, Kean K M, Gorbalenya A E, Agol V I, Girard M. Analysis of the functional significance of amino acid residues in the putative NTP-binding pattern of the poliovirus 2C protein. J Gen Virol. 1992;73:1977–1986. doi: 10.1099/0022-1317-73-8-1977. [DOI] [PubMed] [Google Scholar]
- 82.Towner J S, Mazanet M M, Semler B L. Rescue of defective poliovirus RNA replication by 3AB-containing precursor polyproteins. J Virol. 1998;72:7191–7200. doi: 10.1128/jvi.72.9.7191-7200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toyoda H, Nicklin M J H, Murray M G, Anderson C W, Dunn J J, Sudier F W, Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986;45:761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- 84.van der Voort H T M, Strasters K C. Restoration of confocal images for quantitative image analysis. J Microsc. 1995;178:165–181. [Google Scholar]
- 85.Webb R H, Dorey C K. The pixelated image. In: Pawley J B, editor. Handbook of biological confocal microscopy. 2nd ed. New York, N.Y: Plenum; 1995. pp. 55–67. [Google Scholar]
- 86.Whetter L E, Day S P, Elroy-Stein O, Brown E A, Lemon S M. Low efficiency of the 5′ nontranslated region of hepatitis A virus RNA in directing cap-independent translation in permissive monkey kidney cells. J Virol. 1994;68:5253–5263. doi: 10.1128/jvi.68.8.5253-5263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- 88.Ypma-Wong M F, Dewalt P G, Johnson V H, Lamb J G, Semler B L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988;166:265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]