Abstract
Dynein is a minus-end-directed microtubule-associated motor protein involved in cargo transport in the cytoplasm. African swine fever virus (ASFV), a large DNA virus, hijacks the microtubule motor complex cellular transport machinery during virus infection of the cell through direct binding of virus protein p54 to the light chain of cytoplasmic dynein (LC8). Interaction of p54 and LC8 occurs both in vitro and in cells, and the two proteins colocalize at the microtubular organizing center during viral infection. p50/dynamitin, a dominant-negative inhibitor of dynein-dynactin function, impeded ASFV infection, suggesting an essential role for dynein during virus infection. A 13-amino-acid domain of p54 was sufficient for binding to LC8, an SQT motif within this domain being critical for this binding. Direct binding of a viral structural protein to LC8, a small molecule of the dynein motor complex, could constitute a molecular mechanism for microtubule-mediated virus transport.
Dynein is part of a large enzyme complex responsible for intracellular movement associated with microtubules (31, 32). Intracellular movement of membrane-bound organelles is linked to molecular motors; cytoplasmic dynein and kinesin are believed to be responsible for organelle movement in opposite directions along microtubules (11). Microtubules appear to project from a single perinuclear spot, the microtubular organizing center (MTOC), as the microtubule filaments radiate from this area. During mitosis it is called a centrosome and is responsible for the formation of the mitotic spindle. Cytoplasmic dynein mediates the return of vesicles to the microtubular organizing center and retains the vesicles at this cell location (23). The bidirectional nature of microtubule movement provides a shuttle transport to relocate organelles, endosomes, lysosomes, the Golgi apparatus (25), and also mRNA (15, 51). Cytoplasmic dyneins are part of a small family of minus-end-directed, microtubule-based motors implicated in organelle transport processes. These enzymes must contain motor, cargo-binding, and regulatory components (24, 30). The ATPase and microtubule motor domains are located within the large dynein heavy chains that form the globular heads and stems of the complex (59). Cargo-binding activity involves the intermediate chains and several classes of light chains that associate in a subcomplex at the base of the soluble dynein particle. Regulatory control involves phosphorylation of light-chain proteins associated with the heavy chains. LC8 cytoplasmic dynein exists in situ as a dimer (4, 49). The X-ray crystal structure of the LC8 dimer in complex with a 12-residue peptide from neuronal nitric oxide synthase (nNOS) has been solved, and the binding site is located in a pocket-forming concave surface (35).
A set of proteins have been described to bind LC8, although the sequence determinants needed for LC8 binding remain obscure (28, 35, 49). Virus entry into host cells requires targeting of their genome and accessory proteins across the plasma membrane and to the correct cellular compartment for viral replication to proceed. The involvement of microtubules in virus transport has been reported for a number of viruses (34, 52, 53, 62). Microtubule transport mediated by binding to microtubular motors has been described for adenovirus and herpes simplex virus. Adenovirus binds dynein intermediate chain through an adapter GTP-protein (34, 36). Herpes simplex virus protein UL34 binds dynein intermediate chain (62), and two lyssavirus proteins have been reported to bind the light chain of cytoplasmic dynein LC8 (26, 46).
Microtubule transport has also been described for vaccinia virus (41) and African swine fever virus (ASFV) (7, 10). ASFV is an enveloped icosahedral deoxyvirus which causes a devastating disease of swine (12, 56, 58). The virus genome is double-stranded DNA about 170 kb long and encodes about 150 open reading frames. ASFV is the only member of a new family of viruses called Asfarviridae. It replicates mainly in the cytoplasm in perinuclear factory areas, although some early DNA replication occurs in the nucleus (17). p30 and p54 are externally located virus proteins (20, 48) of 30 and 25 kDa, encoded by the virus genes CP204L and E183L, respectively (47, 61). Protein p54 is a late virus protein that is essential for virus replication, is incorporated into the external envelope of virions (47, 48), and participates in the first stages of virus infection (20). ASFV perinuclear location of the viral factory was related to microtubules, as it was inhibited by colchicine (7). In the present work we demonstrate that ASFV interacts with the dynein motor complex through the structural virus protein p54. The ASFV interaction with microtubular motor protein LC8 through p54 protein could represent one of a number of viral strategies to take advantage of cellular functions and ensure efficient virus transport and replication.
MATERIALS AND METHODS
Yeast two-hybrid and mutational analysis.
A fragment encoding the ASFV p54 gene downstream from the predicted transmembrane domain to the C terminus (residues 52 to 183) was cloned in pGBT9 (Clontech) and used to screen a pig macrophage cDNA library (37) using the yeast two-hybrid system (16). Clones encoding interacting proteins were selected on medium lacking histidine and by expression of β-galactosidase (β-gal). The sequence of inserts was determined. Smaller fragments of the p54 gene were cloned in pGBT9 and similarly tested for interaction with LC8 dynein. A library containing random amino acid substitutions in the p54 gene fragment (residues 52 to 183) between residues 149 and 161 was constructed. The mutants encoded either the wild-type (YTTVTTQNTASQT) or a mutant residue at each position. The fully mutated sequence was SSSGSSHSSGPHS. This was achieved by amplifying the COOH-terminal region of p54 (residues 139 to 183) by PCR using the degenerate PCR primer AAAGCGGCCGCGAGTGCTCATCCGACTGAGCCTT(AC)C(AT)CG(AT)CAG(GT)C(AT)CT(AT)CTCA(GC)A(AG)C(AT)CGT(GC)T(CT)CACA(AC)(AT)CAATGTCGGC and a 3′-end primer; digestion with NotI (NotI site shown in boldface), and ligation to the wild-type NH2-terminal fragment (residues 52 to 138), which was also digested with NotI and cloned in pGBT9. This library of mutants was transformed into yeast cells and tested for interaction with LC8 dynein. Individual point mutations were similarly constructed using the appropriate double-stranded oligonucleotide.
Interaction studies.
LC8 was expressed as a 6His-tagged protein (pET-LC8) and linked to a Ni2+-nitrilotriacetic acid (NTA)-agarose column (Qiagen). Insect cell extracts containing p54 overexpressed in a baculovirus vector were passed through the column (20). After extensive washing of columns with 30 mM Tris–100 mM NaCl (pH 7), retained and control proteins were eluted with 200 mM imidazole. Western blot analyses of eluted fractions were carried out with anti-p54 and anti-LC8 dynein antibodies (see below). Purified LC8 dynein cloned in plasmid pET-23a+ (Novogen) and expressed in Escherichia coli was incubated with the baculovirus-expressed p54 at 4°C for 2 h and then immunoprecipitated with monospecific affinity-purified pig antibodies against p54 or preimmune pig serum conjugated with protein A-Sepharose beads (Pharmacia). Immunocomplexes were analyzed by Western blot with specific anti-LC8 dynein serum.
Antibodies and immunofluorescence.
Vero cells were grown in chamber slides (Lab-Tek; Nunc), approximately 1.5 × 104 cells/chamber, allowed to attach, and then mock infected or infected with ASFV strain BA71V at a multiplicity of infection (MOI) of 1 to 10 and then fixed with acetone-methanol (1:1) for immunofluorescence analyses.
An affinity-purified rabbit antibody raised against LC8 dynein (R4058) was kindly supplied by S. King (31, 32) and used at a 1:200 dilution. Other antibodies used were a mouse monoclonal anti-c-Myc antibody (Clontech), actin phalloidin-fluorescein isothiocyanate (FITC) and anti-β-tubulin-indocarbocyanine (Cy3) conjugate (Sigma). Secondary antibodies used were Alexa 488-, rhodamine-, and Cy3-conjugated sheep anti-rabbit or goat anti-mouse immunoglobulin (Ig) antibodies (Sigma). A monospecific antiserum against p54 was raised in pigs using E. coli-expressed protein as the immunogen. Hyperimmune serum from a pig infected with the virus strain 1207 was used to detect ASFV. Both were visualized with 1:100 protein A-Alexa 488 fluor (Molecular Probes). Anti-p30 and anti-p72 antisera were obtained from pigs immunized against each recombinant protein produced in a baculovirus system. Specificity of labeling and absence of signal crossover were established by examination of singly labeled control samples. Conventional microscopy was carried out in a Leica photomicroscope with a digital camera, and digitized images were obtained with Qwin program (Leica). Confocal microscopy was carried out on an MRC1024 system (Bio-Rad) mounted on a Nikon Eclipse 300 microscope. Statistical analysis of colocalization was performed using Lasersharp Processing 3.2 program (Bio-Rad).
Infections, drug treatments, and transfections.
For virus titrations in the presence or absence of specific inhibitors, a recombinant ASFV expressing the β-galactosidase marker gene (BA71β-gal) (20) was used in Vero cells.
Nocodazole, a microtubule inhibitor, was used dissolved in dimethyl sulfoxide and added to the culture medium to a final concentration of 10 μM or 1 μM. Brefeldin A was used at 5 μg/ml in methanol as an inhibitor of the endoplasmic reticulum (ER)-Golgi and trans-Golgi network secretory pathway. Sodium orthovanadate (Na3VO4), a tyrosine phosphatase inhibitor (21, 60), was used at 100 μM or 10 μM in Dulbecco's modified Eagle's medium. Controls were simultaneously treated with solvents. Microtubule depolymerization was assessed with antitubulin-Cy3 staining of cells. Inhibitors did not affect cell viability, as tested by trypan blue staining. In a set of experiments, the inhibitors were added 2 h prior to infection at an MOI of 1. Then, virus was adsorbed to cells 4°C, and the cells were washed and incubated at 37°C for 48 h in the presence of each concentration of inhibitor. In a second set of experiments the inhibitor was added when infection had proceeded for 6 h. Cells and supernatants were collected after 48 h to determine the extracellular or cell-associated virus production as described (20). Early and late viral protein synthesis under different inhibitor concentrations was evaluated by Western blotting using antisera against early and late ASFV proteins.
For transient expression, full-length p54 was cloned into the expression vector pCMV (Clontech) and transfected into Vero cells using Lipofectamine 2000 transfection reagent (Gibco-BRL) according to the manufacturer's recommendations. To analyze the essential role of LC8 in ASFV infection, cells were transfected with a Myc-tagged p50/dynamitin expression construct (in plasmid pCMV) (13) and then infected 24 h later with the Ba71V ASFV, analyzing virus protein expression 24 h later (positive or negative cells). Cells were analyzed for expression of ASFV early or late proteins and p50/dynamitin by double immunofluorescence labeling. To carry out the experiment, specific pig antisera against the early p30 ASFV protein and late virus proteins p54 and p72 were used. Antibodies reacting with ASFV proteins were revealed with protein A-Alexa 488 fluor (green fluorescence; Molecular Probes). To detect p50/dynamitin–c-Myc expression, an anti-c-Myc monoclonal antibody with anti-mouse Ig-rhodamine was used. Cell samples were observed using confocal microscopy.
RESULTS
Interaction of ASFV p54 protein with LC8 of cytoplasmic dynein.
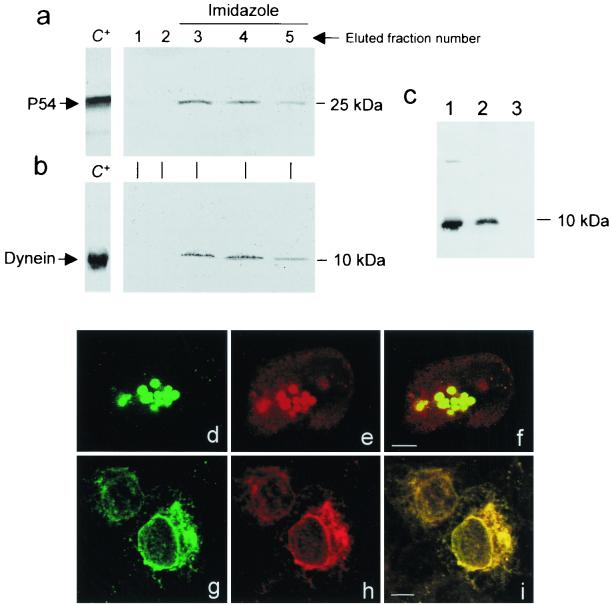
Previous results have shown that ASFV protein p54 is an essential virus protein involved in the early steps of viral infection (20, 48). p54 sequence resembles cytoskeletal proteins such as microtubule-associated proteins of 190 kDa (human and bovine) in containing a Pro-, Ala-, and Thr-rich domain with tandem repeats keeping the distance between prolines constant (1, 47). We identified cellular proteins that bind to p54 protein using the yeast two-hybrid system. A p54 gene fragment encoding the C-terminal part of the protein trimmed from the transmembrane domain was used to screen a porcine macrophage cDNA library. Four clones identical in size and composition were isolated encoding p54-interacting host proteins, which contained cDNAs encoding the light chain of cytoplasmic dynein, LC8. We confirmed the specific and direct interaction of p54 with LC8 in vitro by affinity chromatography. Recombinant p54 from baculovirus-infected cells interacted directly with hexahistidine-tagged LC8 bound to Ni-NTA-agarose resin, and the complex could subsequently be eluted with 200 mM imidazole. The eluted fractions were positive with anti-p54 (Fig. 1a) and anti-LC8 dynein antibodies (Fig. 1b). LC8 dynein-p54 complex was also efficiently pulled down by Sepharose beads linked to p54-specific antibodies (Fig. 1c), confirming the specific interaction between p54 and LC8.
FIG. 1.
Specificity of p54-LC8 dynein binding. Detection by Western blotting of protein p54 retained by His-tagged LC8 dynein linked to nickel-agarose columns. The fractions eluted from the affinity column were electrophoresed in 5 to 15% acrylamide gels, transferred to nitrocellulose filters, and probed with specific antisera. (a) Lanes 1 and 2 show the absence of p54 in eluted fractions after extensive washing of the column with washing buffer. Lanes 3 to 5 show detection of p54 protein eluted with 200 mM imidazole. (b) LC8 dynein detected by Western blotting in the same eluted fractions using an antidynein serum. Controls of purified recombinant LC8 dynein and baculovirus-expressed p54 are shown as a positive control (C+). (c) Coimmunoprecipitation of purified recombinant LC8 dynein and p54 with anti-p54 linked to Sepharose-protein A. Lane 1, Western blot of recombinant purified LC8 dynein. Lanes 2 and 3, coimmunoprecipitations of LC8 and p54 detected with anti-p54 antibodies and preimmune pig serum, respectively. LC8 dynein was detected using a specific polyclonal serum. (d and e) Subcellular localization of p54 protein (Alexa 488) in green (d) and LC8 dynein-rhodamine (e), in cells infected with ASFV at 24 h postinfection. (f) Colocalization of LC8 dynein and p54 in MTOC. Colocalization percentages were 99% for the viral protein and 75% for LC8 dynein. Magnification, ×100. (g and h) Localization of p54 (Alexa 488) in green (g) and LC8 dynein revealed with rhodamine (h) in Vero cells transfected with a plasmid expressing full-length p54. (i) Colocalization of both proteins in the merged image in yellow. Colocalization percentages were 99% for p54. Bar, 10 μm.
Subcellular localization of viral-cellular protein complex.
Confocal immunofluorescence microscopy was used to visualize the viral and cellular protein complex during ASFV infection of Vero cells using specific antibodies against LC8 and p54. p54 colocalized with light-chain dynein LC8 at rates over 98% probability, in a perinuclear location at the MTOC area (Fig. 1d to f). Sequential optical sections along the z axis showed a peripheral location for p54 with a central core of LC8 dynein (not shown).
Also, full-length p54 (pCMV-p54) transiently expressed in uninfected Vero cells colocalized with LC8 dynein (>98% probability) (Fig. 1g to i), demonstrating that p54 interacts with LC8 dynein in the absence of other viral proteins.
Mapping of LC8 binding region in p54.
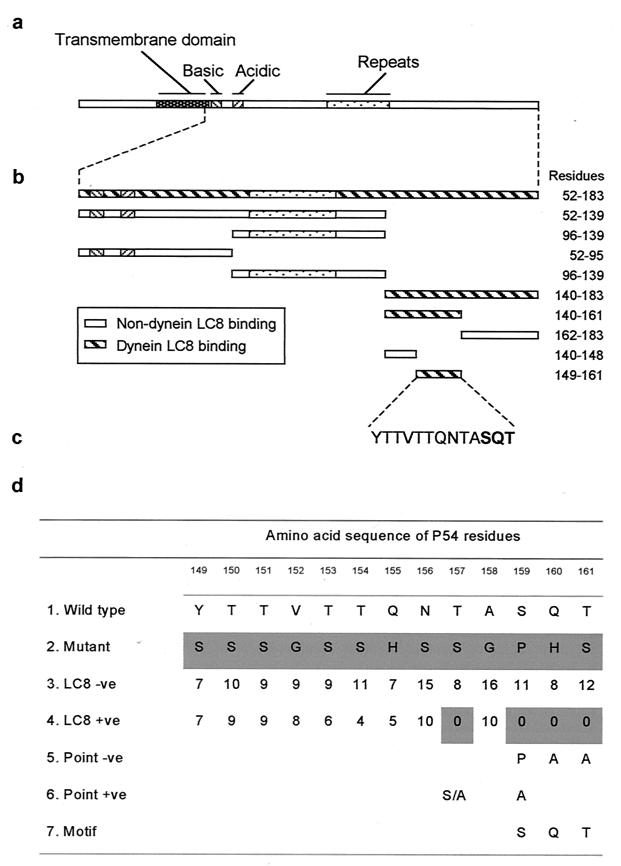
Interactions between different proteins with LC8 dynein have been characterized previously and are mediated by a variety of amino acid sequence motifs. To determine the residues of p54 required for LC8 binding, the yeast two-hybrid system was used. Expression of several truncated fragments of p54 as Gal4 binding domain (BD) fusions revealed that C-terminal amino acids Y149 to T161 were sufficient for binding to LC8 (Fig. 2a-c). Random mutations were introduced into this 13-amino-acid LC8-binding domain within the p54 gene fragment (residues 52 to 183). A library of mutants was constructed in which the p54 fragment could contain either a wild-type or mutant amino acid residue at positions 149 to 161 (Fig. 2d). Mutant p54 proteins which either bound or failed to bind LC8 in the yeast two-hybrid system were selected, and the encoded p54 genes were sequenced. In the p54 mutants which retained LC8 binding activity, no amino acid substitutions were observed in 4 residues (the T-SQT motif between residues 157 and 161), indicating that these were essential for binding function. Individual point mutations were introduced at each of these residues, and substitution of Q (160) or T (161) for A abolished binding, confirming that these were essential. No binding was observed when S (159) residue was replaced with P, but binding was observed when it was replaced with A. Replacing T (157) residue with S or A did not affect binding, suggesting that this is not an essential part of the binding motif. We conclude that the motif SQT (159 to 161) is critical for binding of p54 to LC8 but that some substitutions are possible at the S (159) residue, thus establishing the minimal sequences required for its biological function (Fig. 2). Using dodecapeptide libraries of various proteins known to bind to LC8 synthesized on an amino-derivatized cellulose membrane and by means of the pepscan technique (55), binding to this minimal stretch of p54 was also confirmed (not shown).
FIG. 2.
Mapping of the LC8 binding region in p54. (a) Domain structure of the p54 protein. The predicted transmembrane domain, basic and acidic regions, and variable region encoding amino acid repeats are indicated. (b) Deletion mutants of the p54 gene that were tested for interaction with LC8 in the yeast two-hybrid system. (c) Amino acid sequence of the minimal LC8 binding domain in p54 (residues 149 to 161) with critical residues in bold (SQT). (d) Summary of data from analysis of amino acid substitutions in the 149 to 161 LC8 binding domain of p54. Mutants contained either the wild-type or a mutant residue at each position between 149 and 161. Row 1 shows the wild-type amino acid sequence; row 2 shows the fully mutated sequence. Mutants were tested for binding to LC8 using the yeast two-hybrid system. Row 3 shows the number of times a substitution to the wild-type residue was observed at each position in non-LC8 binding mutants; row 4 shows the number of substitutions at each position in LC8-binding mutants; row 5 indicates point mutations which prevented LC8 binding; and row 6 indicates point mutations which did not prevent LC8 binding. Row 7 indicates the critical SQT motif needed for binding of p54 to LC8.
Relevance of microtubules and dynein motor complex in viral infection.
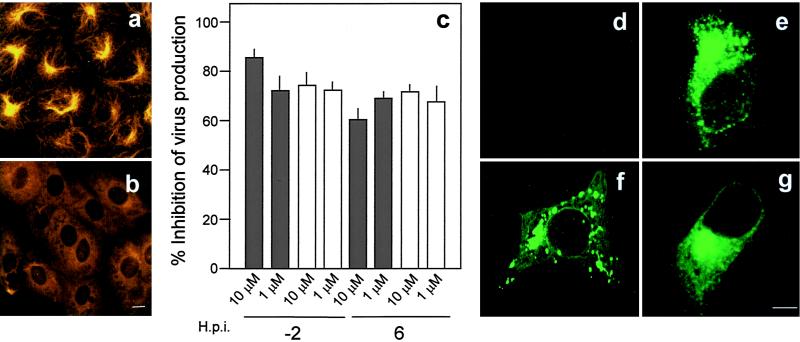
Microtubules have been suggested to be important in the molecular trafficking of ASFV to the perinuclear region during early stages of virus replication (7). Microtubule cytoskeleton is formed by tubulin filaments that appear to project from a single perinuclear spot, markedly stained with antitubulin antibodies (Fig. 3a), the microtubular organizing center. When microtubule depolymerization dismantled tubulin filaments after nocodazole treatment (Fig. 3b), the production of extracellular and intracellular virus at 48 h postinfection was significantly reduced (Fig. 3c). Similar inhibition percentages of virus production were observed by addition of nocodazole either 2 h before infection or after virus internalization (6 h postinfection, Fig. 3c). A greater than eightfold reduction in the amount of early (p30) and late (p54) ASFV protein accumulation in infected cells as detected by Western blotting was also observed in the presence of inhibitors (not shown). These results suggest an important role for microtubules in intracellular virus transport, since microtubule dismantling severely impairs virus production.
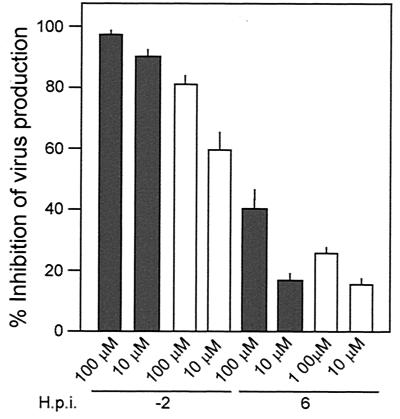
FIG. 3.
Microtubule depolymerization influence on virus production. (a and b) Microtubule cytoskeleton organized in filaments irradiating from the microtubular organizing center, shown as a perinuclear bright spot in untreated Vero cells (a) and Vero cells after microtubule depolymerization with nocodazole (b). Panels a and b were stained with a Cy3-labeled monoclonal anti-β-tubulin antiserum. (c) Effect of nocodazole on extracellular and intracellular ASFV production at 48 h postinfection. The inhibition of virus production from cells treated 2 h before infection or 6 h postinfection is presented as a percentage of the untreated infected control value. Solid and open bars represent extracellular and intracellular virus inhibition, respectively, as described in the text. This figure shows the mean inhibition values from three independent experiments ± standard error. (d) Control uninfected Vero cells. (e) Control cells infected with ASFV fixed at 24 h postinfection in the absence of inhibitors. (f) Depolymerization of microtubules with nocodazole caused dispersed cytoplasmic positive staining and loss of perinuclear localization. (g) Brefeldin A was added 6 h after infection, and perinuclear localization was maintained. Cells were stained with anti-p54 antiserum and protein A-Alexa 488. Bar, 10 μm.
To detect virus proteins by immunofluorescence of infected cells treated with different inhibitors, a hyperimmune serum against ASFV and antisera against early and late viral proteins were used (Fig. 3d to g). Instead of the characteristic accumulation of virus proteins in the perinuclear zone seen in untreated infected cells (Fig. 3e), in cells treated with nocodazole, virus proteins were detected in a dispersed pattern throughout the cytoplasm (Fig. 3d). Perinuclear localization of the viral proteins was not modified with brefeldin A, an inhibitor of ER-Golgi and trans-Golgi transport in the secretory pathway (Fig. 3g). Thus, alteration of virus localization with nocodazole was not due to an indirect effect because of the role of microtubules in maintaining localization of organelles such as the Golgi apparatus.
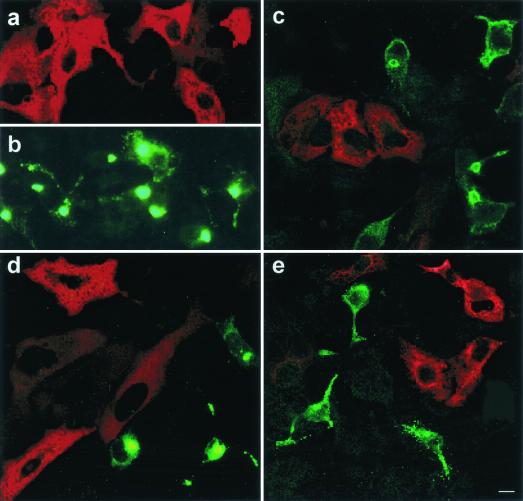
Microtubules seem to play a role in ASFV transport and localization of the viral proteins at the perinuclear area. To confirm that microtubular motor dynein is essential for initiation of ASFV infection, cells were transfected with a Myc-tagged pCMV plasmid expressing p50/dynamitin 24 h before infection. p50/dynamitin acts as a dominant-negative inhibitor of dynein-dynactin function (13). p50/dynamitin expression was found in transfected cells (Fig. 4a and c to e). No coexpression of both viral proteins and p50/dynamitin was found in the same cell. In control nontransfected infected cultures, virus infection was very efficient, and most cells were infected and expressed virus proteins (Fig. 4b). Nevertheless, cells transfected with an unrelated control plasmid (pCMV-βgal) supported infection and showed double staining for transfection and infection markers (not shown). These experiments suggested that inhibition of dynein function blocks the infection at a critical early step of ASFV infection.
FIG. 4.
Disruption of the dynein-dynactin complex results in inhibition of infection. (a, c, d, and e) A plasmid expressing Myc-tagged p50/dynamitin was transfected into Vero cells and detected using antibodies against the Myc epitope tag and secondary anti-mouse Ig-rhodamine (in red). (b, c, d, e) Cells were infected with ASFV and showed characteristic morphological changes. Virus proteins were detected with antibodies against p30 (c), anti-p54 (b and d), and anti-p72 primary antibodies (e) labeled with Alexa 488 (in green). Cells transfected with dynein dominant-negative mutant were not infected, and no simultaneous expression of both viral proteins and p50/dynamitin was found in the same cell. Percentages of infected cells were over 95% in control infected but nontransfected cultures, as shown in panel b. Bar, 10 μm.
Moreover, signaling pathways associated with minus-end cytosolic motility mediated by the dynein-dynactin motor complex (54) seemed to be relevant for viral internalization. An inhibitor of tyrosine phosphatase, sodium orthovanadate (Na3OV4), reduced intracellular and extracellular virus production by more than 60% at 48 h postinfection when added 2 h prior to infection (Fig. 5). In contrast, when the inhibitor was added at 6 h postinfection, when virus is already internalized, the drug had little effect on virus production compared to controls (Fig. 5) (20 to 40% inhibition). With immunofluorescence, virus internalization was not observed at 24 and 48 h postinfection in the cytoplasm of cells treated with Na3OV4 prior to infection (not shown), whereas when Na3OV4 inhibitor drug was added to the cultures after the early infection phase of internalization, viral proteins were found in the characteristic perinuclear location.
FIG. 5.
Influence of tyrosine phosphatase inhibitor Na3OV4 on virus production. Effect of Na3OV4 on extracellular and intracellular ASFV production found at 48 h postinfection. The inhibition of virus production from cells treated 2 h before infection or 6 h postinfection with Na3OV4 are presented as a percentage of the untreated infected cell control value. Solid and open bars represent extracellular and intracellular virus inhibition, respectively, as described in the text. This figure shows the mean inhibition values from three independent experiments ± standard error.
DISCUSSION
ASFV, a large DNA virus, hijacks the microtubule motor complex cellular transport machinery during virus infection of the cell through direct binding of virus protein p54 to the light chain of cytoplasmic dynein (LC8). LC8 dynein is part of a motor protein multicomplex that generates minus-end-directed movement by ATP hydrolysis along the microtubules (31, 32). Within the cell, proteins and vesicles are transported centripetally from the plasma membrane to the cell interior. Cell shuttling of both proteins and vesicles requires cytoskeletal filaments and molecular motor proteins (3, 33). Three motor protein superfamilies are involved in membrane transport: kinesin motors transport vesicles toward the microtubule plus end, cytoplasmic dynein transports cargo toward the microtubule minus end, and unconventional myosins convey cargo along actin filaments. Dynein is the most complex motor protein and involves multiple interactions. The dynein-associated adapter complex, dynactin, is required for cargo transport, and dynamitin overexpression releases this complex (13, 22). Centractin is a molecule linking the microtubular motor to the actin cytoskeleton by dynein binding (24).
Specific motor proteins are linked to particular cargoes. The nonmotor “tail” domains of motor polypeptides or associated subunits are thought to contain the information for cargo selection. On the organelle side, “receptor” proteins that interact with the motor tail domains are assumed to exist. Proteins that may dock molecular motors onto organelles with previously identified functions have recently been characterized (10, 39).
Sequence determinants of LC8 binding.
It is of great interest to define which molecules within dynein complexes directly contact the cargo. Five proteins with diverse cellular functions have been described to bind LC8, although the sequence determinants needed for LC8 binding remain obscure (28, 35, 49). Although a three-dimensional structure of LC8 bound to an nNOS peptide with the sequence KDTGIQVDR is currently available, this binding motif is absent in the other proteins known to interact with LC8 (35). Interestingly, the LC8 binding motif that we have identified in p54 differs from the motif defined for nNOS (28, 35, 49), IκBα (8), GKAP (guanylate kinase domain-associated protein), and myosin V (38), but it is similar to that of the apoptosis regulator Bim (42). The Bim/LC8 binding site, QDKSTQTPS, is close to the p54-LC8 binding site defined in this work (QNTASQTMS; Fig. 2). ASFV sequestering of LC8 cytoplasmic dynein transport might secondarily modify the binding of dynein to cellular targets and potentially alter cellular regulatory processes related to Bim, a member of the Bcl-2 family of apoptosis regulators. This might contribute to the apoptosis induced during ASFV infection (44), which is known to play an important role in virus pathogenesis (5, 6, 45)
Viral interaction with cytoplasmic dynein shuttle.
Viruses have evolved subtle strategies to ensure the efficient expression of their genes upon infection. This includes use of the cytoplasmic transport machinery during early steps of infection to enable the virus to reach the replication site (57). Once delivered into the cytosol, virions have to be transported to sites of replication, and some viruses make use of microtubules and microtubule-dependent motors to move from the cell periphery to the nucleus (29, 36, 62). This means that incoming virions expose on their surface not only targeting signals, but also signals for association with molecular motor complexes or their adapters (57).
We here provide evidence for an interaction between LC8 dynein and p54 that suggests a possible mechanism for transport of ASFV particles to the perinuclear factory area by a minus-end-directed movement along microtubules. Disruption of the dynactin complex by overexpression of one of its subunits, called p50/dynamitin, releases both dynactin and cytoplasmic dynein, blocking the dynein-dependent transport mechanism (13, 43), and this impedes ASFV infection. Adenovirus cytosolic minus- and plus-end-directed movements are supported by microtubules, and this migration is reduced by overexpression of dynamitin (53). It has been reported that a nonstructural adenovirus protein binds to an adapter GTPase, RagA, that in turn binds TCTEL1, a 14-kDa light-intermediate-chain dynein (34, 36). Association between the virus proteins and dynein intermediate chains has been also shown for herpes simplex virus type 1 (HSV-1) (62) and adenovirus (53), and light-chain dynein (LC8) binding was described for lyssavirus proteins (26, 46).
Understanding the molecular basis and functional consequences of these interactions would provide further insight in the molecular mechanisms linking motor proteins to viral infection. UL34 protein of HSV-1 interacts with the intermediate chain of cytoplasmic dynein IC-1a and UL31 protein (62), and the site of interaction has been mapped to the carboxyl-terminal 30 amino acids (63). A10L and L4R proteins of vaccinia virus accumulate in the vicinity of the MTOC in a dynein-dynactin complex-dependent fashion also demonstrated by expression of p50/dynamitin (41). In the present study, virus yields were also greatly affected at early infection steps by sodium orthovanadate (Na3OV4), an inhibitor drug previously reported to inhibit dynein-dependent movement (18). Na3OV4 is an inhibitor of tyrosine phosphatase activity and function (21), and it has been shown to inhibit minus-end-directed movement (54). Tyrosine phosphorylation regulates the structure and function of some cytoskeletal proteins (21, 60). When Na3OV4 was added prior to infection, virus production was strongly inhibited and there was no intracytoplasmic staining by immunofluorescence. Nevertheless, when the drug was added at 6 h postinfection, viral protein perinuclear localization was conserved and it had little effect on virus production. Therefore, inhibition of tyrosine phosphatases with Na3OV4 impaired early steps of virus internalization, suggesting a role for dynein- and tyrosine phosphatase-dependent pathways in early virus transport. It has recently been described that signal transduction pathways are activated to mediate early steps of viral infections (40, 50). Adenovirus was reported to enhance dynein-mediated nuclear targeting by activation of protein kinase A (PKA) and p38/mitogen-activated protein kinase (MAPK) (54). Also, the delivery of activated MAPK by incoming human immunodeficiency virus type 1 (HIV-1) enhances virus early infection (27).
In the presence of nocodazole, viral proteins were detected in a dispersed pattern and did not reach the perinuclear region, presumably because they are unable to move on microtubules. Although the microtubular network supports localization and maintenance of the Golgi apparatus, ASFV protein p54 was not redistributed by brefeldin A treatment. Similar results have been reported for an HSV-1 structural protein in Vero cells (14). The effect of specific LC8 inhibition (using its dominant negative) should be differentiated from microtubule inhibition due to colchicine (9) or nocodazole. Both inhibitions could be related but not identical and result in a different effect on viral infection. Cell shuttling of both proteins and vesicles requires cytoskeletal filaments and molecular motor proteins (3, 33). Similarly, cytosolic ASFV transport (bidirectional) seems to require microtubule filament integrity. Nevertheless, minus-end-directed or centripetal virus transport depends on dynein molecular motor activity and integrity of tyrosine phosphatase activity. Microtubules could also be involved in ASFV exit, since in the presence of nocodazole microtubule depolymerization inhibits virus production at both early and late times postinfection. These results are coincident with previous studies of Carvalho et al. (7). ASFV virions must move from factory areas to the plasma membrane to be released by budding, and it is possible that microtubules play a role in virus particle transport from factory areas to the cell membrane (9; this study).
Viral proteins encoded by enveloped animal viruses interact with host molecules to influence factors such as cellular tropism, the site of assembly and release of viral progeny, and the immune response of the host to infection. Understanding how viruses exploit cell-based transport could yield basic information relevant to the normal recruitment of specific microtubule-associated proteins by organelles in the cytoplasm. Viruses provide interesting model systems for basic studies on cytoskeleton-based intracellular transport and definition of the molecular machinery involved in motor protein recruitment and cargo selection. Since dynein inhibition blocks viral infection, the identification of the specific amino acid sequences required for viral protein transport could be of value in the design of new antiviral drugs targeting the dynein multicomplex.
ACKNOWLEDGMENTS
We thank A. Alvarez-Barrientos for confocal microscopy assistance. We thank Stephen M. King for the generous gift of the anti-LC8 polyclonal antibody and Richard Vallee and Chin-Yin Tai for the p50/dynamitin construct.
This work was supported by EU QLK3-2000-00362, BMC 2000-1003, BIO 98-307, and PB 96-105 from Programa de Promoción General del Conocimiento and SC 00-049 Programa Sectorial INIA grants.
REFERENCES
- 1.Aizawa H, Emori Y. Molecular cloning of a ubiquitously distributed microtubule-associated protein with Mr 190.000. J Biol Chem. 1990;265:13849–13855. [PubMed] [Google Scholar]
- 2.Alcami A, Carrascosa A L, Viñuela E. The entry of African swine fever virus into Vero cells. Virology. 1989;171:68–75. doi: 10.1016/0042-6822(89)90511-4. [DOI] [PubMed] [Google Scholar]
- 3.Allan V J, Schroer T A. Membrane motors. Curr Opin Cell Biol. 1999;11:476–482. doi: 10.1016/s0955-0674(99)80068-4. [DOI] [PubMed] [Google Scholar]
- 4.Benashski S H, Harrison A, Patel-King R S, King S M. Dimerization of the highly conserved light chain shared by dynein and myosin V. J Biol Chem. 1997;272:20929–20935. doi: 10.1074/jbc.272.33.20929. [DOI] [PubMed] [Google Scholar]
- 5.Brun A, Rodríguez F, Escribano J M, Alonso C. Functionality and cell anchorage dependence in insect cells of the African swine fever virus gene A179L, a viral bcl-2 homolog. J Virol. 1998;72:10227–10233. doi: 10.1128/jvi.72.12.10227-10233.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brun A, Rivas C, Esteban M, Escribano J M, Alonso C. African swine fever virus gene A179L, a viral homologue of bcl-2, protects cells from programmed cell death. Virology. 1996;225:227–230. doi: 10.1006/viro.1996.0592. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho Z G, De Matos A P, Rodrigues-Pousada C. Association of African swine fever virus with the cytoskeleton. Virus Res. 1988;11:175–192. doi: 10.1016/0168-1702(88)90042-1. [DOI] [PubMed] [Google Scholar]
- 8.Crépieux P, Kwon H, Leclerc N, Spencer W, Richard S, Lin R, Hiscott J. IκBα physically interacts with a cytoskeleton-associated protein through its signal response domain. Mol Cell Biol. 1997;17:7375–7385. doi: 10.1128/mcb.17.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Matos A P A, Carvalho Z G. African swine fever virus interaction with microtubules. Biol Cell. 1993;78:229–234. doi: 10.1016/0248-4900(93)90134-z. [DOI] [PubMed] [Google Scholar]
- 10.De Matteis M A, Morrow J S. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 11.Dillman J F, III, Pfister K K. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon L K, Costa J V, Escribano J M, Rock D L, Vinuela E, Wilkinson P J. Asfarviridae. In: Van Regenmortle M, et al., editors. Seventh report of the International Committee on Taxonomy of Viruses. New York, N.Y: Academic Press; 2000. pp. 159–165. [Google Scholar]
- 13.Echeverri C J, Paschal B M, Vaughan K T, Valee R B. Molecular characterization of the 50 Kd subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliot G, Ohare P. Intracellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 15.Epstein E, Sela-Brown A, Ringel I, Kilav R, King S M, Benashski S E, Yisraeli J K, Silver J, Naveh-Many T. Dynein light chain binding to a 3′-untranslated sequence mediates parathyroid hormone mRNA association with microtubules. J Clin Investig. 2000;105:505–512. doi: 10.1172/JCI8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 17.García-Beato R, Salas M L, Viñuela E, Salas J. Role of the host cell nucleus in the replication of African swine fever virus DNA. Virology. 1992;188:637–649. doi: 10.1016/0042-6822(92)90518-t. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons I R, Cosson M P, Evans J A, Gibbons B H, Houck B, Martinson K H, Sale W S, Tang W. Potent inhibition of dynein adenosinetriphosphatase and of the motility of cilia and sperm flagella by vanadate. Proc Natl Acad Sci USA. 1978;75:2220–2224. doi: 10.1073/pnas.75.5.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez-Puertas P, Rodriguez F, Oviedo J M, Ramiro-Ibáñez F, Ruiz-Gonzalvo F, Alonso C, Escribano J M. Neutralizing antibodies to African swine fever virus inhibit through different proteins both virus attachment and internalization. Influence of passage history of the virus. J Virol. 1996;70:5689–5694. doi: 10.1128/jvi.70.8.5689-5694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Puertas P, Rodríguez F, Oviedo J M, Brun A, Alonso C, Escribano J M. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology. 1998;243:461–471. doi: 10.1006/viro.1998.9068. [DOI] [PubMed] [Google Scholar]
- 21.Goval J J, Van Cauwenberge A, Alexandre H. Respective roles of protein tyrosine kinases and protein kinases C in the upregulation of beta-catenin distribution, and compaction in mouse preimplantation embryos: a pharmacological approach. Biol Cell. 2000;92:513–526. doi: 10.1016/s0248-4900(00)01105-9. [DOI] [PubMed] [Google Scholar]
- 22.Habermann A, Schroer T A, Griffiths G, Burkhardt J K. Immunolocalization of cytoplasmic dynein and dynactin subunits in cultured macrophages: enrichment on early endocytic organelles. J Cell Sci. 2001;114:229–240. doi: 10.1242/jcs.114.1.229. [DOI] [PubMed] [Google Scholar]
- 23.Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzbaur E L F, Vallee R B. Dyneins, molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- 25.Itin C, Ulitzur N, Mühlbauer B, Pfeffer S R. Mapmodulin, cytoplasmic dynein and microtubules enhance the transport of mannose-6-phosphate receptors from endosomes to the trans-Golgi network. Mol Biol Cell. 1999;10:2191–2197. doi: 10.1091/mbc.10.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob Y, Badrane H, Ceccaldi P-E, Tordo N. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J Virol. 2000;74:10217–10222. doi: 10.1128/jvi.74.21.10217-10222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacqué J-M, Mann A, Enslen H, Sharova N, Brichacek B, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaffrey S R, Snyder S H. PIN, an associated protein inhibitor of neuronal nitric oxide synthetase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- 29.Kaelin K, Dezelee S, Masee M J, Bras F, Flamand A. The UL25 protein of pseudorabies virus associates with capsids and localizes to the nucleus and microtubules. J Virol. 2000;74:474–482. doi: 10.1128/jvi.74.1.474-482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King S. The dynein microtubule motor. Biochim Biophys Acta. 2000;1496:60–75. doi: 10.1016/s0167-4889(00)00009-4. [DOI] [PubMed] [Google Scholar]
- 31.King S M, Patel-King R S. The M(r) = 8,000 and 11,000 outer arm dynein light chains from Chlamydomonas flagella have cytoplasmic homologs. J Biol Chem. 1995;270:11445–11452. doi: 10.1074/jbc.270.19.11445. [DOI] [PubMed] [Google Scholar]
- 32.King M, Barbarese E, Dillman III J F, Patel-King R S, Carson J H, Pfister K. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem. 1996;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- 33.Klopfenstein D R, Vale R D, Rogers L. Motor protein receptors: moonlighting on other jobs. Cell. 2000;10:537–540. doi: 10.1016/s0092-8674(00)00144-6. [DOI] [PubMed] [Google Scholar]
- 34.Leopold P L, Kreitzer G, Miyazawa N, Rempel S, Pfister K K, Rodriguez-Boulan E, Crystal R G. Dynein-and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11:151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 35.Liang J, Jaffrey S R, Guo W, Snyder S H, Clardy J. Structure of the PIN/LC8 dimer with a bound peptide. Nat Struct Biol. 1999;6:735–740. doi: 10.1038/11501. [DOI] [PubMed] [Google Scholar]
- 36.Lukashok S A, Tarassishin L, Li Y, Horwitz M. An adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis complexes with dynein and a small GTPase. J Virol. 2000;74:4705–4709. doi: 10.1128/jvi.74.10.4705-4709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miskin J E, Abrams C C, Goatley L C, Dixon L K. A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science. 1998;281:562–565. doi: 10.1126/science.281.5376.562. [DOI] [PubMed] [Google Scholar]
- 38.Naisbitt S, Valtschanoff J, Allison D W, Sala C, Kim E, Craig A M, Weimberg R J, Sheng M. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin V and dynein. J Neurosci. 2000;20:4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell. 2000;103:569–581. doi: 10.1016/s0092-8674(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 40.Nemerow G R, Stewart P L. Role of α(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ploubidou A, Moreau V, Ashman K, Reckman I, González C, Way M. Vaccinia infection disrupts microtubule organization and centrosome function. EMBO J. 2000;19:3932–3944. doi: 10.1093/emboj/19.15.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthalakath H, Huang D C S, O'Reilly L A, King S M, Strasser A. The proapoptotic activity of the bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 43.Quintyne N J, Gill S R, Eckley D M, Crego C L, Compton D A, Schroer T A. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramiro-Ibañez F, Ortega A, Brun A, Escribano J M, Alonso C. Apoptosis, a mechanism of cell killing and lymphoid organ impairment during acute African swine fever virus infection. J Gen Virol. 1996;77:2209–2219. doi: 10.1099/0022-1317-77-9-2209. [DOI] [PubMed] [Google Scholar]
- 45.Ramiro-Ibáñez F, Ortega A, Ruiz-Gonzalvo F, Escribano J M, Alonso C. Modulation of immune cell populations and activation markers in the pathogenesis of African swine fever virus infection. Virus Res. 1997;47:31–40. doi: 10.1016/s0168-1702(96)01403-7. [DOI] [PubMed] [Google Scholar]
- 46.Raux H, Flammand A, Blondel D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J Virol. 2000;74:10212–10216. doi: 10.1128/jvi.74.21.10212-10216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez F, Alcaraz C, Eiras A, Yañez R J, Rodriguez J M, Alonso C, Rodriguez J F, Escribano J M. Characterization and molecular basis of heterogeneity of the African swine fever virus envelope protein p54. J Virol. 1994;68:7244–7252. doi: 10.1128/jvi.68.11.7244-7252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez F, Ley V, Gomez-Puertas P, García R, Rodríguez J F, Escribano J M. The structural protein p54 is essential for African swine fever virus viability. Virus Res. 1996;40:161–167. doi: 10.1016/0168-1702(95)01268-0. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Crespo I, Straub W, Gavilanes F, Ortiz de Montellano P R. Binding of dynein light chain to neuronal nitric oxide synthase in the absence of inhibition. Arch Biochem Biophys. 1998;359:297–304. doi: 10.1006/abbi.1998.0928. [DOI] [PubMed] [Google Scholar]
- 50.Sanglioglu S, Benson P K, Yang J, Morrey Atkinson E, Reynolds T, Engelhardt J F. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by Rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–9189. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnorrer F, Bohmann K, Nüsslein-Volhard C. The molecular motor dynein is involved in targeting Swallow and bicoid RNA to the anterior pole of Drosophila oocytes. Nat Cell Biol. 2000;21:85–190. doi: 10.1038/35008601. [DOI] [PubMed] [Google Scholar]
- 52.Sodeik B, Ebershold M W, Helenius A. Microtubule mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;144:657–672. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suomalainen M, Nakano M Y, Keller S, Boucke K, Stidwill R P, Greber U F. Microtubule dependent plus- and minus end directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999;14:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suomalainen M, Nakano M Y, Boucke K, Keller S, Greber U F. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 2001;20:1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valle M, Muñoz M, Kremer L, Valpuesta J M, Martinez-A C, Carrascosa J L, Albar J P. Selection of antibody probes to correlate protein sequence domains with their structural distribution. Protein Sci. 1999;8:883–889. doi: 10.1110/ps.8.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viñuela E. African swine fever virus. Curr Top Microbiol Immunol. 1985;116:155–170. doi: 10.1007/978-3-642-70280-8_8. [DOI] [PubMed] [Google Scholar]
- 57.Whittaker G R, Kann M, Helenius A. Viral entry into the nucleus. Annu Rev Cell Dev Biol. 2000;16:627–651. doi: 10.1146/annurev.cellbio.16.1.627. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson P J. African swine fever virus. In: Pensaert M B, editor. Virus infections of porcines. Amsterdam, The Netherlands: Elsevier; 1989. pp. 17–35. [Google Scholar]
- 59.Wu H, Maciejewski M W, Marintchev A, Benashski S E, Mullen G P, King S M. Solution structure of a dynein motor domain associated light chain. Nat Struct Biol. 2000;7:575–579. doi: 10.1038/76804. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y X, Uezato T, Fujita M. Tyrosine phosphorylation and cellular redistribution of ezrin in MDCK cells treated with pervanadate. J Cell Biochem. 2000;79:311–321. doi: 10.1002/1097-4644(20001101)79:2<311::aid-jcb140>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 61.Yañez R J, Rodriguez J M, Nogal M L, Yuste L, Enríquez C, Rodriguez J F, Viñuela E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology. 1995;208:249. doi: 10.1006/viro.1995.1149. [DOI] [PubMed] [Google Scholar]
- 62.Ye G-J, Vaughan K T, Vallee R B, Roizman B. The herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye G-J, Roizman B. The essential protein encoded by the UL31 gene of herpes simplex virus 1 depends for its stability on the presence of UL34 protein. Proc Natl Acad Sci USA. 2000;97:11002–11007. doi: 10.1073/pnas.97.20.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]