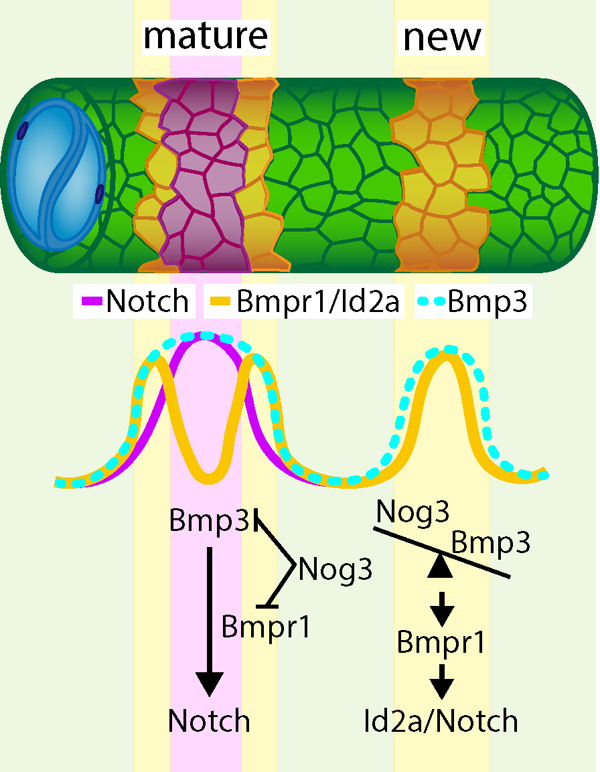
Summary
The vertebrate spine is a metameric structure composed of alternating vertebral bodies (centra) and intervertebral discs1. Recent studies in zebrafish have shown that the epithelial sheath surrounding the notochord differentiates into alternating cartilage-like (col2a1/col9a2+) and mineralizing (entpd5a+) segments which serve as a blueprint for centra formation2–5. This process also defines the trajectories of migrating sclerotomal cells that form the mature vertebral bodies4. Previous work demonstrated that notochord segmentation is typically sequential and involves the segmented activation of Notch signaling2. However, it is unclear how Notch is activated in an alternating and sequential fashion. Furthermore, the molecular components that define segment size, regulate segment growth, and produce sharp segment boundaries have not been identified. In this study, we uncover that a BMP signaling wave acts upstream of Notch during zebrafish notochord segmentation. Using genetically encoded reporters of BMP activity and signaling pathway components, we show that BMP signaling is dynamic as axial patterning progresses, leading to the sequential formation of mineralizing domains in the notochord sheath. Genetic manipulations reveal that type I BMP receptor activation is sufficient to ectopically trigger Notch signaling. Moreover, loss of Bmpr1ba and Bmpr1aa or Bmp3 function disrupts ordered segment formation and growth, which is recapitulated by notochord-specific overexpression of the BMP antagonist, Noggin3. Our data suggest that BMP signaling in the notochord sheath precedes Notch activation and instructs segment growth, facilitating proper spine morphogenesis.
Graphical Abstract
Results and Discussion
In zebrafish, the outer epithelial layer of the notochord sheath initially expresses col2a1a and col9a2 (col9a2+ henceforth) uniformly. Around 5 days post-fertilization (dpf), alternating domains of sheath cells differentiate into entpd5a+ cells sequentially along the anterior-posterior (AP) axis2,3. This process requires Notch activity2 and induces a mineralization program5 that forms rings of chordacentra around the entpd5a+ domains to which osteoblast precursors are then recruited to form vertebral centra. Cells that continue to express col9a2 are confined to the prospective intervertebral discs (IVDs)2–4. Sequential and segmented activation of Notch in precise regions along the notochord sheath is, therefore, crucial to establish properly spaced centra2. However, Notch activates simultaneously with entpd5a, suggesting it is a late marker of notochord segmentation. To identify upstream activators of Notch during notochord segmentation, we turned to RNA sequencing (RNAseq) data we previously generated from three populations of purified sheath cells (col9a2+, entpd5a+, and double+) at 13dpf2. Reviewing the RNAseq data, we identified several differentially expressed BMP signaling components (Figures 1A and S1A). BMPs are known to regulate cartilage and bone growth during development, and repair6,7. In mice, knockout of type I BMP receptors leads to irregular chondrogenesis and endochondral ossification8 and centra formation is disrupted in Bmpr1b−/− mice in which Acvr1, another BMP receptor, is conditionally knocked out from Col2 expressing cells9. This suggests a role for BMPs in centra ossification. Moreover, in zebrafish, overexpression of Bmp2b induces hyperossification10. However, whether BMP signaling plays a role in establishing the location of centra domains or in the relative timing of centra formation along the AP axis is unknown.
Figure 1: BMP activity is dynamic during notochord segmentation.
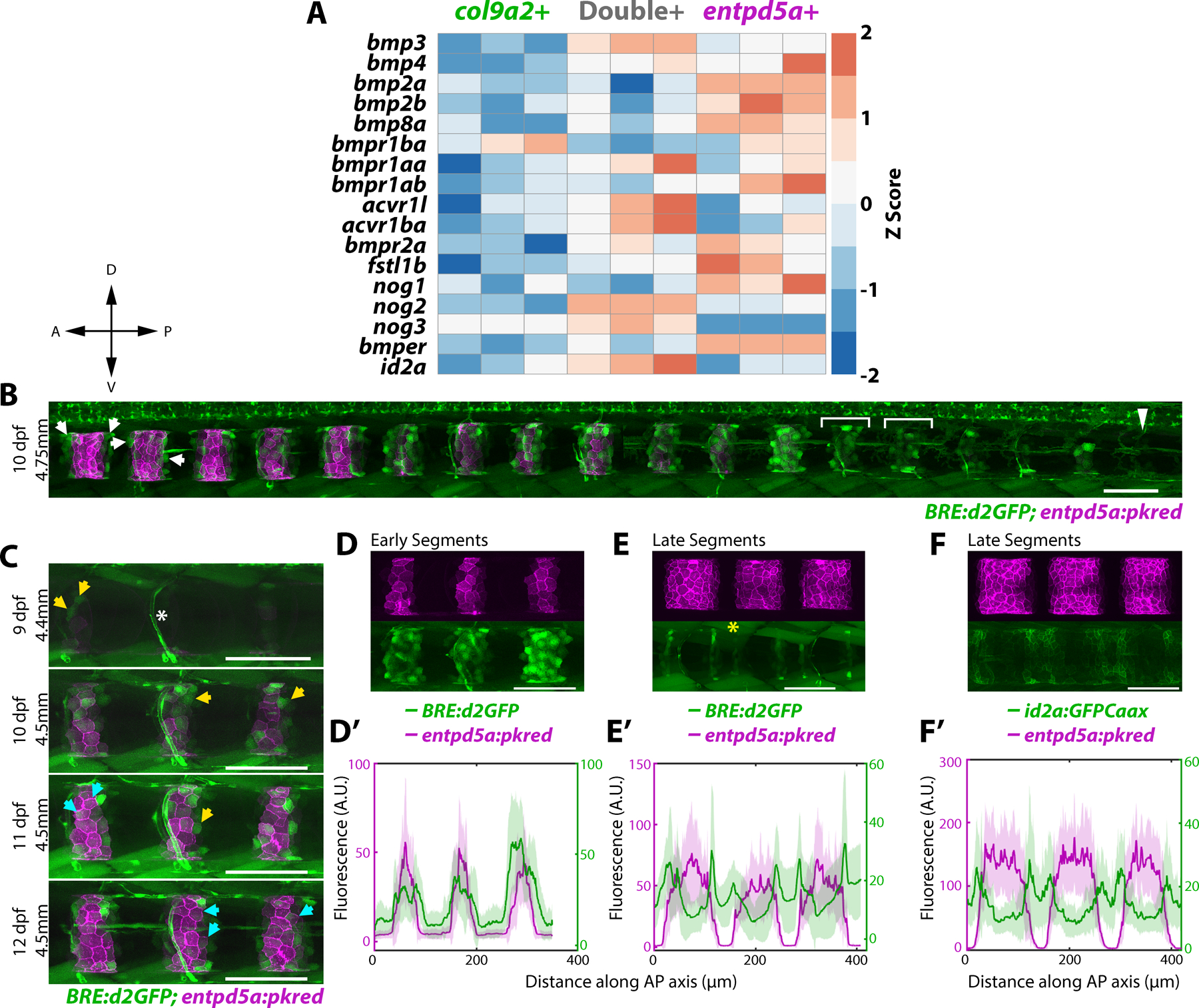
A) Heatmap of differentially expressed genes in three notochord domains collected from 13 dpf larvae. Primary data is from Wopat et al. 2018. Z-score values were calculated from normalized counts.
B) 10 dpf larva expressing BRE:d2GFP and entpd5a:pkred. BMP activity is ON at the center of prospective mineralizing domains (brackets) and gets restricted to segment boundaries in mature entpd5a+ domains (arrows). Arrowhead indicates unsegmented region. n=6 fish. Compass indicates A = anterior, P = posterior, D = dorsal, V = ventral
C) BRE:d2GFP and entpd5a:pkred expression in the same fish over 4 days. Yellow arrows mark cells that have turned on BRE:d2GFP, cyan arrows mark cells that have subsequently decreased BRE:d2GFP expression and activated entpd5a:pkred. n=3 fish. Asterisk marks blood vessel expression.
D) Newly formed segments expressing both BRE:d2GFP and entpd5a:pkred. n=6 fish.
D’) Fluorescence intensity plot showing overlap of the two expression patterns in (D). BRE:d2GFP peaks extending more broadly along the AP axis.
E) Mature segments expressing both BRE:d2GFP and entpd5a:pkred transgenes. BRE:d2GFP expression decreased at the center of mineralizing domains and is enriched at segment boundaries. Asterisk denotes muscle expression. n=6 fish.
E’) Fluorescence intensity plot of (E) showing BRE:d2GFP peaks flanking entpd5a:pkred expression.
F) Mature segments expressing entpd5a:pkred and id2a:GFPCaax. n=3 fish.
F’) Fluorescence intensity plot of (F).
See Figure S1
Dynamic activation of BMP signaling during notochord segmentation
To investigate the role of BMP signaling during notochord segmentation, we first imaged a previously generated BMP activity reporter, BRE:d2GFP11. This reporter contains Smad 1/5 binding sites (BMP Responsive Elements) upstream of a minimal promoter and destabilized green fluorescent protein (d2GFP). Live confocal imaging revealed that BRE:d2GFP expression is highly dynamic in the notochord sheath as segmentation progresses (Figure 1B). To analyze the temporal dynamics of BMP signaling, we imaged 9 dpf larvae co-expressing entpd5a:pkred every 24 hours for 4 days (Figure 1C). Tracking individual cells, we found that sheath cells activate BMP signaling prior to expressing entpd5a (Figure 1C, yellow arrows). Once cells begin to express entpd5a, BRE:d2GFP expression decreases (Figure 1C cyan arrows). This generates a pattern of d2GFP fluorescence that peaks in the center of newly formed segments (Figure 1B brackets, 1D,D’), while in more mature segments expression is enriched on the edges of entpd5a+ domains (Figure 1B arrows, 1E,E’). Thus, BMP signaling is active at the center of newly differentiating segments and activity travels outward within each segment as mineralizing domains expand laterally.
Next, we examined the expression of id2a, a target of BMP signaling. Inhibitors of differentiation/DNA binding (ID) proteins are basic helix-loop-helix proteins that are direct, early targets of BMP 2/4 signaling7,12. Additionally, ID proteins are known to regulate Notch pathway activity in various tissues13,14. We found that the expression pattern of id2a:GFPCaax, a transgene we previously established2, closely mirrors that of the BMP reporter (Figure 1F, F’, and S1B). Together, these data show that BMP signaling is dynamic during sheath cell differentiation prior to entpd5a expression.
Bmp3 signaling precedes entpd5a activation and is required to specify the mineralizing cell fate
The BRE:d2GFP reporter contains binding sites for only Smads 1 and 511. To obtain a more complete picture of BMP signaling in the notochord, we searched for additional BMPs in our RNAseq data and identified bmp3 as the most highly expressed BMP ligand in sheath cells. bmp3 is enriched in double+ cells (Figure 1A and S1A), corresponding to the newly differentiated mineralizing population. Bmp3, is a unique member of the BMP signaling family that is thought to oppose the osteogenic activity of canonical BMP (Bmp2/4) signaling in some contexts15,16. During endochondral ossification in mice, knockout of Bmp3 can lead to increased ossification in trabecular bone15,17. However, overexpression of Bmp3 has also been shown to increase the region of hypertrophic chondrocytes in mice18, which share similarities with entpd5a+ notochord sheath cells such as expression of collagen type X, mmp13a/b, and runx2b2,19. These conflicting data from mice suggest that the role of BMP3 in ossification may be context dependent, making it difficult to predict how Bmp3 may function during notochord segmentation.
Bmp3 is known to activate Smads 2 and 320, which would not be detected by the BRE:d2GFP reporter. To visualize bmp3 expression dynamics during notochord segmentation, we adapted a promoter knock-in method from previous work21 to insert a destabilized fluorescent protein (VenusPest) into the bmp3 locus. Fluorescence intensity analysis of bmp3:VenusPest in entpd5a:pkred expressing larvae revealed that bmp3 is progressively expressed in sheath cells along the AP axis, ahead of entpd5a (Figure 2A, A’ and B, B’; Figure S2A). During early stages of notochord segmentation (i.e., in the unsegmented notochord), bmp3 expression turns on several segments ahead of entpd5a (Figure 2A, A’ arrows, and Figures S2A), whereas in mature, anterior segments (Figure 2A, A’ arrowheads), and at later stages (Figure 2B, B’), the two expression patterns overlap. Together with the BRE:d2GFP reporter (Figure 1B–E’), these data indicate that various BMP signaling components are active in the notochord sheath prior to the activation of Notch and entpd5a.
Figure 2: Bmp3 signaling precedes entpd5a activation and is required to specify mineralizing cells.
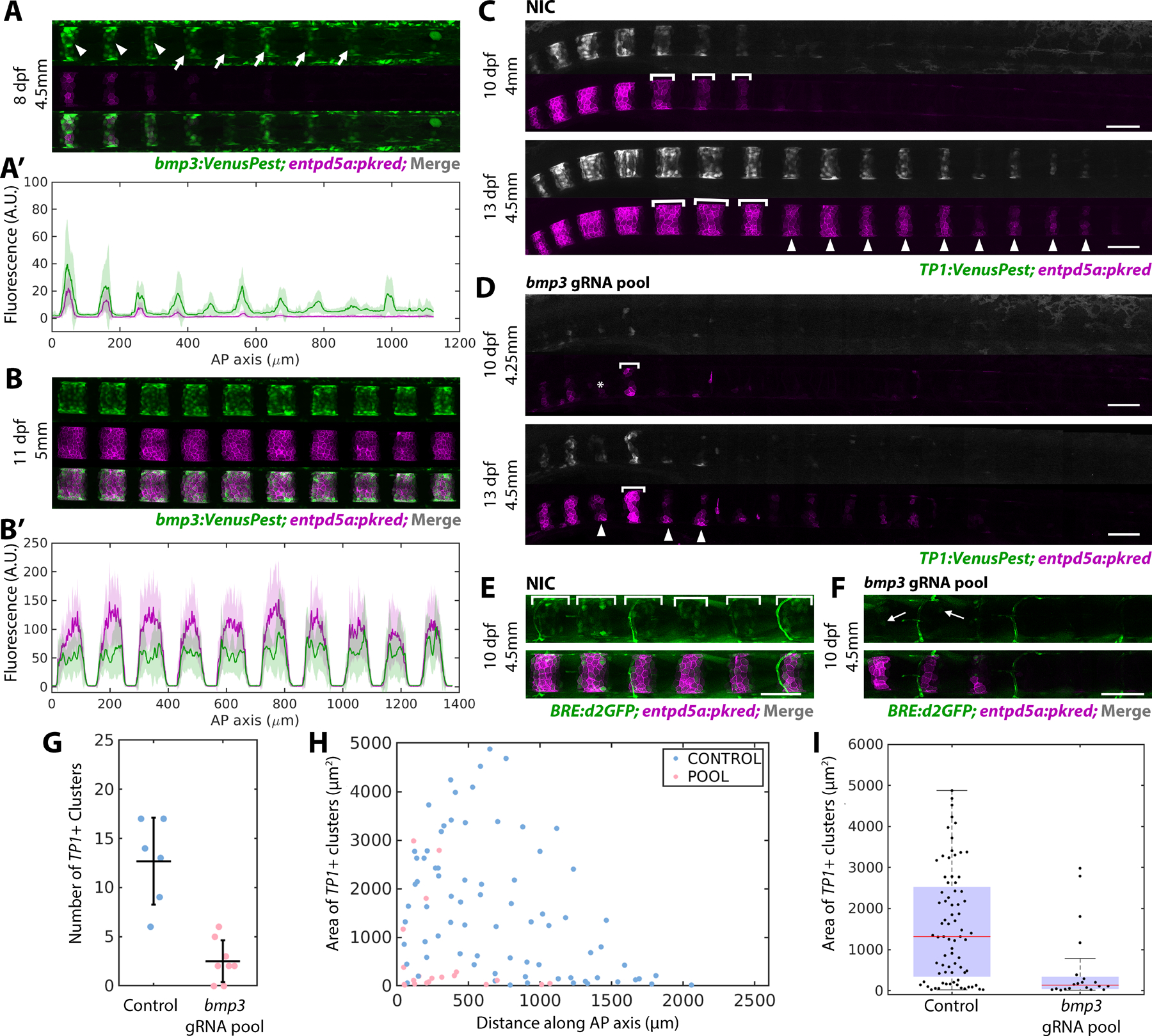
A) bmp3:VenusPest expression in the posterior of an 8 dpf larva. Arrowheads mark segments that have activated both bmp3:VenusPest and entpd5a:pkred, arrows mark bmp3:VenusPest+ segments without entpd5a:pkred. n=3 fish
A’) Fluorescence intensity plot of (A).
B) Mature segments from an 11 dpf larva expressing bmp3:VenusPest and entpd5a:pkred. n=4 fish.
B’) Fluorescence intensity plot of (B) reveals overlapping expression domains.
C) Non-injected control (NIC) larva at 10 and 13 dpf expressing TP1:VenusPest and entpd5a:pkred. Brackets indicate lateral growth between the two timepoints. Arrowheads mark new segments at 13 dpf.
D) 10 (top) and 13 dpf (bottom) larva injected with bmp3 gRNA pool. Segments are smaller, irregularly shaped, and fewer in number compared to controls. Asterisk marks a skipped segment, brackets indicate lateral growth between the two timepoints, and arrowheads point to newly formed segments at 13 dpf.
E) WT control 10 dpf larva expressing both entdp5a:pkred and BRE:d2GFP. Brackets indicate regions of BMP activity. n=6 fish/condition.
F) Larva at the same age and standard length as (E) injected with bmp3 CRISPR pool. Expression of BRE:d2GFP and entpd5a:pkred are reduced. Arrow indicates cell still expressing BRE:d2GFP. n=6 fish.
G) Number of segments at 10 dpf in fish injected with bmp3 gRNAs compared to controls (horizontal line representing mean ± standard deviation). Data was compiled using MATLAB. Loss of bmp3 caused a significant decrease in segment number. NIC n = 6, bmp3 gRNA pools n = 8, p =0.0036.
H) Area of TP1:VenusPest+ clusters measured along the AP axis in larvae injected with bmp3 gRNAs and controls at 10 dpf.
I) Distribution of segment areas from (H). Loss of bmp3 leads to decreased segment area along the AP axis. NIC n = 6, bmp3 gRNA pools n = 8, p = 6.71 × 10−5
See Figure S2
To determine whether bmp3 is required for notochord segmentation, we followed an F0 CRISPR/Cas9 targeting strategy22 and designed a pool of gRNAs targeting 4 regions of the bmp3 gene in all three exons (Figure S2B). We chose this approach because BMPs play important roles during early embryo development23,24 and this helps bypass those early requirements. The pool of gRNAs and Cas9 protein was injected into one-cell stage embryos containing entpd5a:pkred and a TP1:VenusPest25 reporter to mark Notch activity. To determine the effects of bmp3 loss on segment initiation and growth, individual fish were imaged at 10 and 13 dpf. Upon loss of bmp3, notochord segmentation was severely impaired, as indicated by both a lack of Notch activation and impaired entpd5a:pkred expression (Figure 2C, D). Compared to control siblings of the same developmental stage (standard length26), fish injected with the bmp3 gRNAs had significantly fewer segments at 10 dpf (Figure 2G) and segments were smaller in area along the AP axis (Figure 2H, I). At 13 dpf, larvae showed a decrease in both dorsoventral (DV) and lateral segment growth compared to control siblings (Figure 2D), with some individuals failing to form any TP1/enptd5a+ segments (Figure S2E, E’). To determine the effect of bmp3 loss on canonical BMP signaling, we also analyzed BRE:d2GFP expression. Interestingly, loss of bmp3 decreased BRE:d2GFP expression (Figure 2E, F), suggesting bmp3 functions upstream of canonical BMP signaling during sheath cell differentiation. However, the downregulation of BRE:d2GFP at the center of mature entpd5a+ domains in WT (Figure 1E) suggests that high expression of Bmp3 may eventually downregulate Bmp2/4. Together, these data indicate that bmp3 is essential for notochord segmentation, regulating segment growth in both the DV and AP dimensions.
BMP signaling induces Notch activation in the notochord sheath
The dynamics of BMP activity suggest it functions upstream of Notch during sheath cell differentiation. To test this possibility, we used the QF2/QUAS system27 to drive the expression of a constitutively active (CA) type I BMP receptor specifically in the notochord sheath. RNAseq data indicated that bmpr1ba is the most abundant of the four type-I BMP receptors expressed in the col9a2+ domain during segmentation (Figure 1A, S1A). We, therefore, generated a CAbmpr1ba DNA construct by introducing a Gln to Asp mutation in the GS domain of bmpr1ba, as used previously in other systems28, and used p2a-mCherry to mark expressing cells. The QUAS:CAbmpr1ba-p2a-mcherry construct was injected into zygotes containing a stable col9a2:QF2 transgene2 to specifically drive expression in col9a2+ cells. A QUAS:mcherry-NTR plasmid was used as a control and TP1:VenusPest25 marked Notch activation in the sheath as shown previously2. Mosaic expression of CAbmpr1ba in the sheath led to ectopic activation of Notch signaling, as indicated by TP1:VenusPest activity (Figure 3B and B’), while overexpression of the control construct did not (Figure 3A and A’). Ectopic Notch activation was detected in 91.6% of cells containing the CAbmpr1ba construct prior to notochord segmentation (Figure 3B arrows), as well as in 97.7% of cells at later stages within the IVD domains (Figure 3B’ arrows), compared to 10.1% and 8.25% in control fish, respectively.
Figure 3: BMP signaling induces Notch activation and establishes mineralizing domains.
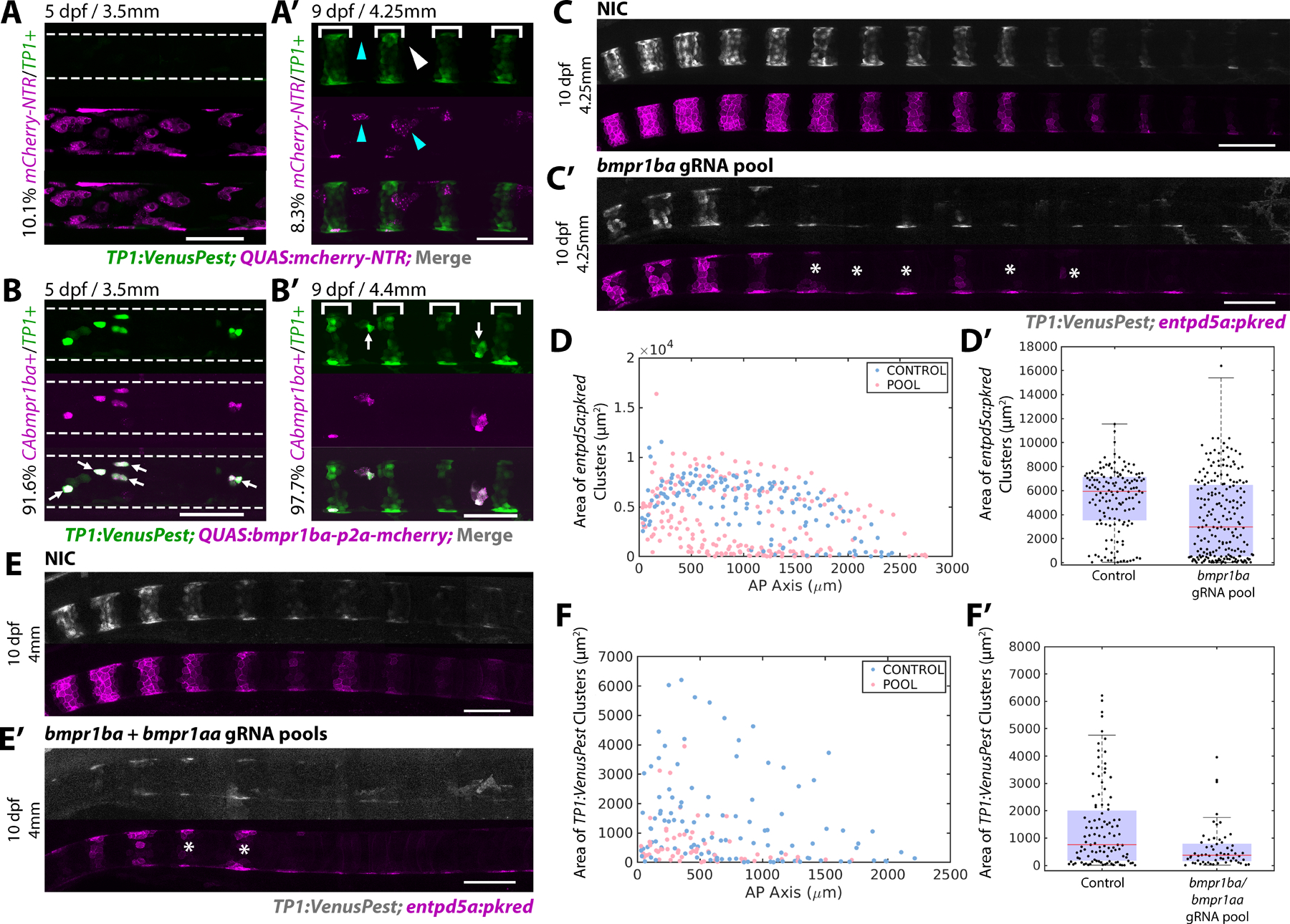
A, B) 5 dpf larvae injected with either QUAS:mCherry-NTR (A) or QUAS:CAbmpr1ba-p2a-mCherry (B) at the single-cell stage. Segmentation has not begun in the region analyzed. Overexpression of CAbmpr1ba ectopically activates Notch activity. Overexpression of the control construct does not. An average of 10.1% of cells overexpressing the control construct contained TP1:VenusPest expression (n=7 fish, 848 cells). 91.6% of cells overexpressing CAbmpr1ba ectopically activated Notch (arrows). (n=6 fish, 630 cells)
A’, B’) 9 dpf larvae overexpressing the control QUAS:mCherry-NTR (A’) or QUAS:CAbmpr1ba-p2a-mCherry (B’) in the sheath. TP1:VenusPest is activated in newly formed mineralizing segments (brackets). Of the cells in the IVD domains expressing QUAS:mCherry-NTR, 8.25% expressed TP1:VenusPest (n=4 fish, 479 cells). Arrowheads indicate cells expressing the control construct that have not activated Notch. QUAS:CAbmpr1ba-p2a-mCherry overexpression in the IVD domain caused ectopic Notch activation in 97.6% of cells (arrows). (n=4 fish, 71 cells)
C, C’) 10 dpf NIC fish (C) and fish injected with a gRNA pool targeting bmpr1ba (C’). Loss of bmpr1ba causes delayed segmentation and incomplete segment growth. Asterisks mark incomplete segments.
D) Segment areas plotted along the AP axis at 12 dpf. Loss of bmpr1ba decreased segment area compared to controls.
D’) Distribution of segment areas in NIC and bmpr1ba gRNA injected fish at 12 dpf . Loss of bmpr1ba decreased segment size at this stage, indicating disrupted sheath cell differentiation. (NIC n = 8 fish; bmpr1ba gRNA pool n = 12 fish; p value = 1.7945e-08).
E, E’) 10 dpf NIC fish (E) and fish injected with bmpr1ba/bmpr1aa gRNA pools (E’) expressing TP1:VenusPest and entpd5a:pkred show impaired segmentation. Asterisks mark incomplete segments.
F) Segment areas along the AP axis quantified at 10 dpf. (n=8 per condition)
F’) Box and whisker plot demonstrating the distribution of areas calculated in (F). (p value = 3.0413e-04).
See Figure S3A-F’
To test the requirement of bmpr1ba, we used the same F0 targeting strategy22 described above. Pools of gRNAs targeting the GS, ATP binding, and kinase domains of bmpr1ba were injected at the single cell-stage along with Cas9 protein (Figure S3A, B). Upon loss of bmpr1ba, sheath cell segmentation was delayed and enptd5a+ segment areas were reduced (Figure 3D, D’). At later developmental stages, lateral growth was also affected, periodically leading to jagged segment boundaries (Figure S3D). However, the effects of bmpr1ba loss were mild compared to the phenotype observed when bmp3 was targeted (Figure 2C–I and S2E, E’). We hypothesized this was due to compensation by other type I BMP receptors in the sheath (Figure 1A, S1A). To address this possibility, we simultaneously knocked down bmpr1ba and bmpr1aa. When targeted together, the phenotype observed was more severe and segment size was significantly reduced compared to controls (Figure 3E–F’).
Next, we investigated the downstream effector Id2a, which demonstrated similar expression dynamics to the BRE:d2GFP reporter (Figure 1F, S1B). Similar to bmpr1ba, we found that loss of id2a leads to delayed segmentation (Figure S3E–F’).
Together, these data show that BMP signaling is sufficient to activate Notch in the sheath and suggest BMP acts upstream of Notch during notochord segmentation.
BMP antagonism by Noggin3 limits segment growth
Several antagonists are known to block BMP pathway activation7,20,29. Examining our RNAseq data, we found that several noggin and follistatin genes are expressed in sheath cells (Figure 1A, S1A). Among them, noggin3 is the most highly upregulated BMP antagonist within the col9a2+ and double+ domains (Figure S1A). Upon secretion, Noggins directly bind and sequester BMP ligands, thereby preventing the interaction of BMP ligands with their target receptors20,30. To monitor noggin3 dynamics, we generated a promoter knock-in line (nog3:VenusPest) using the same strategy described above for bmp321. Live confocal imaging over time revealed that noggin3 expression in the sheath is highly dynamic. Initially, noggin3 is expressed in regions fated to become mineralizing domains, ahead of entpd5a activation (Figure 4A). As notochord segmentation progresses, the prospective IVD domains turn on noggin3 in an anterior to posterior fashion, essentially unsegmenting the expression pattern (Figure 4B). At later stages, noggin3 expression turns off in the entpd5a+ domain and is highly enriched in col9a2+ domain and at segment boundaries (Figure 4C).
Figure 4: Dynamic expression of the BMP antagonist Noggin3 regulates notochord segmentation.
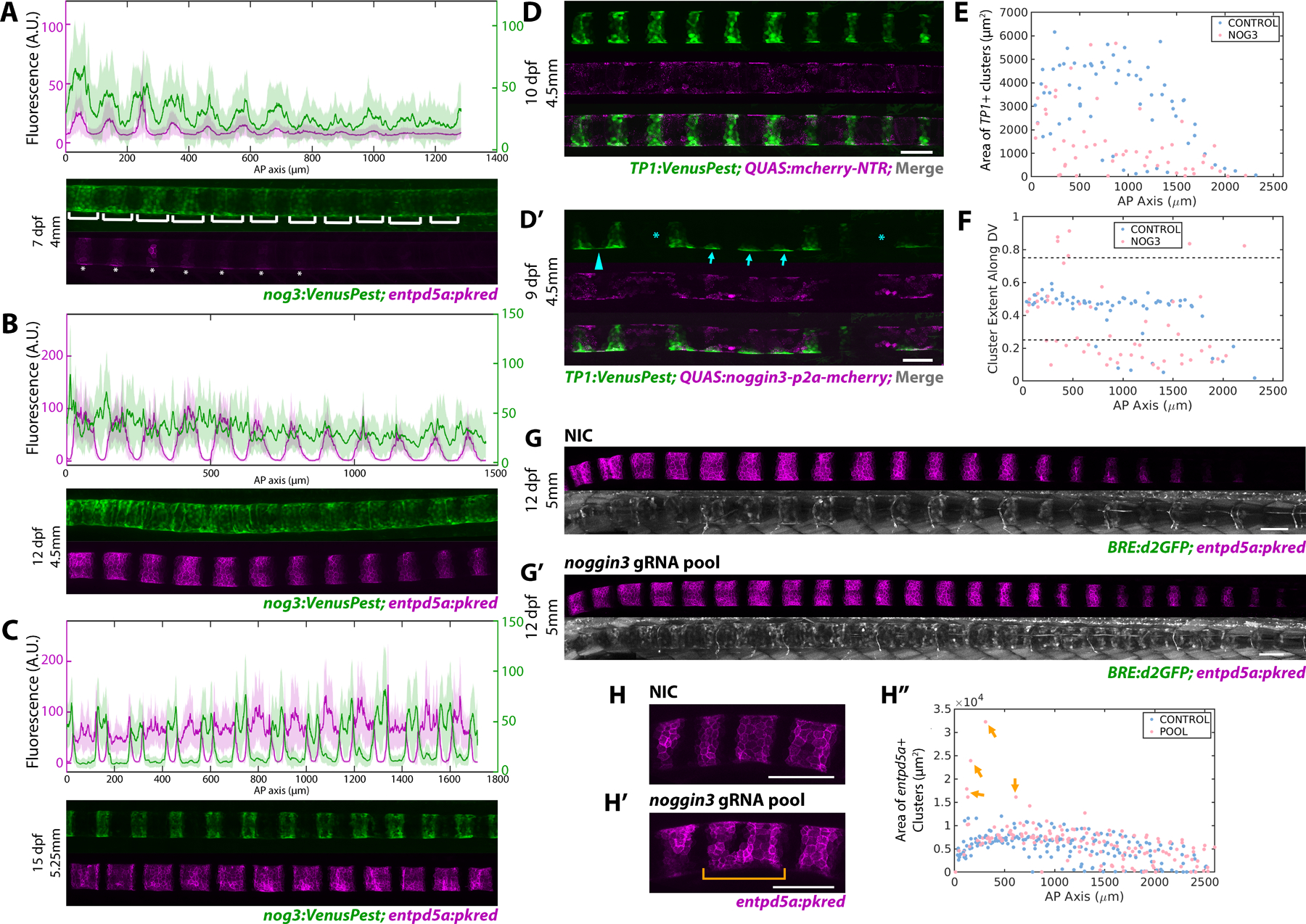
A) noggin3:VenusPest expression in the notochord sheath demonstrating expression in the prospective mineralizing domains prior to enptd5a. Brackets indicate noggin3+ segments, asterisks mark segments expressing entpd5a. n=4 fish. Florescence intensity analysis (top panel) shows noggin3:VenusPest peaking prior to entpd5a:pkred with broader expression domains.
B) 12 dpf fish expressing noggin3:VenusPest and entpd5a:pkred. entpd5a expression has expanded laterally as segments begin to mature, noggin3:VenusPest expression has begun in the prospective IVD domains, as indicated by the fluorescence intensity plots (top panel). n=3 fish.
C) Later stage (15 dpf) larva depicting mature entpd5a segments. noggin3 has turned off in the mineralizing domains and became restricted to the IVD domains. n=3 fish. Fluorescence intensity plot (top panel) shows noggin3 enriched at segment boundaries.
D, D’) Confocal image of 4.5mm larvae expressing either QUAS:mCherry-NTR (D) or QUAS:noggin3-p2a-mcherry (D’) in the sheath. TP1:VenusPest shows Notch+ mineralizing segments. Fish overexpressing noggin3 had skipped segments (asterisks), impaired DV and AP growth (arrows), and ventral fusions (arrowhead).
E) Area of TP1+ segments is markedly reduced in noggin3 overexpressing fish compared to controls (n = 4 mCherry control; n = 3 noggin3).
F) Plot of cluster centroid locations along the DV axis normalized along the DV width. Control fish develop segments with a ~0.5 DV value, indicating regularly shaped segments with even distribution of TP1:VenusPest signal. Fish overexpressing noggin3 have values above or below 0.75 and 0.25 respectively, indicating impaired growth in the DV dimension after segment initiation.
G, G’) 12 dpf, 5mm fish expressing BRE:d2GFP and entpd5a:pkred. Fish injected with noggin3 gRNAs (G’) demonstrates an increase in BRE:d2GFP expression in the IVD domain and more entpd5a+ segments compared to controls.
H, H”) Loss of noggin3 caused anterior segment fusions (H’ bracket), apparent in a segment area plot along the AP axis. (n = 9 NIC; n = 8 noggin3)
See Figure S3G,H
To assess Noggin3’s function in the sheath, we again used the QF2/QUAS system. A QUAS:nog3-p2a-mcherry construct was generated and injected into col9a2:QF2; TP1:VenusPest transgenic fish at the single cell stage. QUAS:mcherry-NTR was injected as a control. Live confocal imaging between 9 and 15 dpf revealed that overexpression of noggin3 in the notochord sheath strongly diminished Notch activity and caused dramatic notochord segmentation defects compared to control larvae of the same developmental stage (Figure 4D–F). In noggin3 overexpressing fish, notochord segments were frequently skipped, indicating that the periodicity of segmentation was altered (Figure 4D’ asterisks). Moreover, segment area along the AP axis was significantly decreased (Figure 4E), underscoring the importance of BMP signaling during segment expansion. Interestingly, TP1:VenusPest expression could still be detected in some cells on the dorsal and ventral sides where segments should have grown (Figure 4D’ arrows), which was apparent by plotting the DV extent of TP1+ segments along the AP axis (Figure 4F). These sites may represent segment seeds formed at notochord-myosepta contacts31. Furthermore, the ventral side of some presumed segment initiation sites were often fused with adjacent segments (Figure 4D’ arrowhead), suggesting that noggin3 plays a complex role in regulating boundary formation.
In parallel, we investigated the effects of knocking down noggin3. Following the F0 CRISPR strategy described previously22, we designed and injected 4 gRNAs targeting the noggin3 gene at the single cell stage (Figure S3G,H). Loss of noggin3 increased BMP activity along the length of the sheath, as indicated by BRE:d2GFP expression (Figure 4G,G’). In sharp contrast to the pooled gRNA experiments performed previously, targeting noggin3 caused fish to segment slightly faster, averaging roughly two entpd5a+ segments more than control siblings at 12 dpf. However, the overall effects of noggin3 loss seemed milder than anticipated. Unlike overexpressing CAbmpr1ba, we did not observe ectopic sheath cell differentiation in the IVD domains, except for some instances of anterior segment fusion (Figure 4H’), apparent in a plot of segment area along the AP axis (Figure 4H” arrows). We speculate that the relatively mild phenotype may be due to compensation from other BMP antagonists such as fstl1b, nog2, and bmper, which are also robustly expressed to varying degrees in all three domains (Figure 1A, S1A).
Altogether, our data show that BMP signaling regulates the induction of mineralizing domains upstream of Notch. This may occur via BMP upregulation of notch1a and/or notch2 expression. Our data also suggest that segment initiation and growth are differentially regulated, as illustrated by the continued presence of segment initiation sites when BMP signaling is blocked (Figure 4D’). Based on our functional data and the expression dynamics of the noggin3 and bmp3 transcriptional reporters, we propose that Noggin3 functions to prevent premature formation of mineralizing segments and that its inhibitory effect is then overcome as BMP ligand expression builds up along the AP axis. As entpd5a+ domains grow laterally, robust expression of Noggin3 in the col9a2+ domain may limit segment expansion, facilitating the formation of sharp segment boundaries.
Our data suggest that Bmp3 and Noggin3 are components of a differentiation wave in the notochord sheath that travels from anterior to posterior along the AP axis and from the center to the periphery of each notochord segment. The complex landscape of BMP/Notch signaling in the notochord sheath also suggests that other effectors in these pathways and additional spatial cues31 likely interact to refine the segmentation process. Future studies investigating these pathways may elucidate the mechanism underlying the robustness of notochord segmentation and spine patterning.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Information about resources and reagents used in this study can be directed towards our corresponding author, Michel Bagnat, PhD (michel.bagnat@duke.edu)
Materials availability
All zebrafish lines and plasmids generated during this study are available upon request without restriction.
Data code availability
RNAseq Data is originally from Wopat et al, 2018 (GEO: GSE109176). Microscopy data reported in this paper will be shared by the lead contact upon request.
MATLAB code generated for this study has been deposited on Github and can be viewed at the link listed in the Key Resources Table
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| N/A | ||
| Bacterial and virus strains | ||
| N/A | ||
| Biological samples | ||
| N/A | ||
| Chemicals, peptides, and recombinant proteins | ||
| N/A | ||
| Critical commercial assays | ||
| N/A | ||
| Deposited data | ||
| N/A | ||
| Experimental models: Cell lines | ||
| N/A | ||
| Experimental models: Organisms/strains | ||
| Tg(col9a2:GFPCaaX) pd1151 | 1,2 | N/A |
| Tg(col9a2:QF2) pd1163 | 2 | N/A |
| TgBAC(entpd5a:pkRED) hu7478 | 3 | N/A |
| TgKI(nog3:VenusPest) pd1262 | This work | N/A |
| TgKI(bmp3:VenusPest) pd1267 | This work | N/A |
| Tg(TP1:VenusPest) s940 | 4 | N/A |
| Tg(BRE-AAVmlp:d2GFP) mw30 | 5 | N/A |
| Oligonucleotides | ||
| QUAS:CAbmpr1ba-p2a-mCherry | This work | N/A |
| QUAS:noggin3-p2a-mCherry | This work | N/A |
| QUAS:mCherry-NTR | This work | N/A |
| Recombinant DNA | ||
| bmp3 sgRNAs: | This work | N/A |
| 5’ GGAGCGGGTTCTTGTGTCGTGGG 3’ | ||
| 5’ TGCTCGCAGATATTTAAAGGTGG 3’ | ||
| 5’ CGGGCAGTCGGTGTGGTGCCGGG 3’ | ||
| 5’ AGCGATCCATGCTGGAGGTGCGG | ||
| bmpr1ba sgRNAs: | This work | N/A |
| 5’ AGGGAGTCCTGAGCCAGAACCGG 3’ | ||
| 5’ TGGCTCAGGACTCCCTCTACTGg 3’ | ||
| 5’ GAGGACGGTCTGATAGATCTCGG 3’ | ||
| 5’ GATGGTGACACAGATCGGGAAGG 3’ | ||
| 5’ CGCTATGGAGAAGTGTGGATGGG 3’ | ||
| bmpr1aa sgRNAs: | This work | N/A |
| 5’ AGGTCTGAGACTGGTTGATGAGG 3’ | ||
| 5’ GAAGGTGTTCTTTACCCGAGAGG 3’ | ||
| 5’ TGGCCTGTGTCACCTGCATACGG 3’ | ||
| 5’ GTTCCATCTGTTGGATACGGTGG 3’ | ||
| noggin3 sgRNAs: | This work | N/A |
| 5’ GATATCACGGGGTACTGAACGGG 3’ | ||
| 5’ AGAGGACAAGCACGCGGGACAGG 3’ | ||
| 5’ GGAGGACCCGGATCCCGTACTGG 3’ | ||
| 5’ TGTCGCTTGGAATGGGACGGAGG 3’ | ||
| id2a sgRNAs: | This Work | N/A |
| 5’ GCCTGCATCACCCGCGAGCGGGG 3’ | ||
| 5’ GCTGAGAGGATCGTCCACGGGGG 3’ | ||
| 5’ CGAGATTCCCTGTTCGCGCTGGG 3’ | ||
| 5’ GATCGCGCTCGACTCCAATTCGG 3’ | ||
| Genotyping primers | ||
| bmpr1ba genotyping primers: | ||
| 5’ GCAGCGCACTATAGCGAAG 3’ | ||
| 5’ acctgcaataaggcctttca 3’ | ||
| bmp3 genotyping primers: | ||
| 5’ CCTACATGGGATGAGGCCAG 3’ | ||
| 5’ ACGACTTTGGTGAGATAATCCAC 3’ | ||
| noggin3 genotyping primers: | ||
| 5’ TACCGTATTTCCTGGCCACC 3’ | ||
| 5’ GCCGTCTTCTGAGCTTCTTG 3’ | ||
| Software and algorithms | ||
| ImageJ | https://imagej.nih.gov/ij/ | |
| Adobe Photoshop 2021 | https://www.adobe.com/creativecloud.html | |
| Adobe Illustrator 2021 | https://www.adobe.com/creativecloud.html | |
| MATLAB | https://www.mathworks.com/products/matlab.html | |
| Original Code | This work | https://github.com/padhyapok/notochord_area_plotter |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Zebrafish used in this study were raised and maintained under standard conditions32 in compliance with internal regulatory review at Duke University School of Medicine. Zebrafish larvae from an Ekkwill (EK) or AB/TL background were used for this study and wild type EK or AB/TL siblings were used as controls for all genetic manipulation experiments. Mutants and transgenic lines used in this study include: Tg(col9a2:GFPCaaX)pd1151 2,33, Tg(col9a2:QF2)2, TgBAC(entpd5a:pkRED)hu7478 3 , TgKI(nog3:VenusPest)pd1262, TgKI(bmp3:VenusPest)pd1267, Tg(TP1:VenusPest)s940 25, Tg(BRE-AAVmlp:d2GFP)mw30 11.
METHOD DETAILS
Knockdown of BMP signaling components
Targeting bmpr1ba
5 sgRNAs were pooled together targeting 3 functional domains of the bmpr1ba gene. These target sites include the GS domain, ATP binding domain, and kinase domain. A working solution containing 10ng/μL of each sgRNA and 250ng/μL Cas9 protein was injected at the single cell stage into wild type fish containing col9a2:GFPCaax and entpd5a:pkred transgenes.
The following target sites were used to generate each sgRNA: 5’ AGGGAGTCCTGAGCCAGAACCGG 3’; 5’ TGGCTCAGGACTCCCTCTACTGg 3’; 5’ GAGGACGGTCTGATAGATCTCGG 3’; 5’ GATGGTGACACAGATCGGGAAGG 3’; 5’ CGCTATGGAGAAGTGTGGATGGG 3’.
bmp3
4 sgRNAs were generated and pooled as mentioned previously. A working solution containing 10ng/μL of each sgRNA and 250ng/μL Cas9 protein was injected at the single cell stage into wild type fish containing TP1:VenusPest and entpd5a:pkred transgenes. Guides were designed to target all three exons of the bmp3 gene. The following target sites were used: 5’ AGCGATCCATGCTGGAGGTGCGG 3’; 5’ GGAGCGGGTTCTTGTGTCGTGGG 3’; 5’ TGCTCGCAGATATTTAAAGGTGG 3’; 5’ CGGGCAGTCGGTGTGGTGCCGGG 3’.
noggin3
4 sgRNAs were generated and pooled as mentioned previously. A working solution containing 10ng/μL of each sgRNA and 250ng/μL Cas9 protein was injected at the single cell stage into wild type fish containing BRE:d2GFP and entpd5a:pkred transgenes. Guides were designed to target multiple regions of the noggin3 coding region. The following target sites were used: 5’ GATATCACGGGGTACTGAACGGG 3’; 5’ AGAGGACAAGCACGCGGGACAGG 3’; 5’ GGAGGACCCGGATCCCGTACTGG 3’; 5’ TGTCGCTTGGAATGGGACGGAGG 3’
id2a
Injection solution was made as described above. Several regions of exon 1 were targeted. The following target sites were used: 5’ GCCTGCATCACCCGCGAGCGGGG 3’; 5’ GCTGAGAGGATCGTCCACGGGGG 3’; 5’ CGAGATTCCCTGTTCGCGCTGGG 3’; 5’ GATCGCGCTCGACTCCAATTCGG 3’
Generating endogenous promoter knock-in lines
Our strategy for generating endogenous reporters was adapted from a previous study21. A donor sequence containing a GFP gRNA target site (gbait), the heat shock promoter (hsp701), and destabilized Venus (VenusPest) was generated in a puc19 plasmid. The naturally occurring gbait site within VenusPest was mutated using site directed mutagenesis to yield the following sequence: 5’ GGtGAaGGaGAcGCaACtaAtGG 3’. A PCR donor was amplified from the plasmid using the following primers: puc19_F: 5’ gttttcccagtcacgacgtt 3’; puc19_R: 5’ tgtggaattgtgagcggata 3’. A stock injection solution containing the following was injected at the single cell stage: PCR donor (8ng/μL), Cas9 protein (166ng/μL), gbait gRNA (8ng/μL), target site gRNA (16ng/μL), phenol red
The following gRNA target sites were used to generate the endogenous bmp3 reporter: target_1: 5’ tgggcgcattcggacactattgg 3’; target_2: 5’ GCTGCATGAACGTCGATCTTTGG 3’
The following gRNA targets were used to generate a noggin3 reporter: target_1: 5’ AGGAGCCCGCGAGCTCACACAGG 3’; target_2: 5’ GGGTTGCATCGCGACCCGCGAGG 3’
Overexpression of a constitutively active BMP receptor
Cut and paste cloning was used to generate the QUAS:CAbmpr1ba-p2a-mCherry construct. The vector containing QUAS:p2a-mCherry was amplified using the following primers with restriction sites attached: 5’ SphI_p2a_mCherry_F: 5’ CGCGCATGCGACCCAGCTTTCTTGTAC 3’; NheI_QUAS_R: 5’ CGCGCTAGCGGAGCCTGCTTTTTTGTAC 3’. bmpr1ba cDNA was amplified using the following primers with NheI and SphI restriction sites attached: NheI_bmpr1ba_F: 5’ cgcGCTAGCTTACAACAACTCGCGGACAC 3’; SphI_bmpr1ba_R: 5’ cgcGCATGCGACTTTAATGTCCTGGGATTC 3’. Both insert and vector were digested with NheI-HF and SphI-HF (NEB) enzymes and ligated together using T4 DNA ligase (NEB).
The bmpr1ba sequence was subsequently mutated by site-directed mutagenesis to generate a constitutively active form. The C terminal glutamine within the GS domain was mutated to aspartic acid28. The following primer sets were used for this process: CA_bmpr1ba_F: 5’ TATAGCGAAGGACATCCAGATGGTGACAC 3’; CA_bmpr1ba_R: 5’ GTGCGCTGCACCAGTAGA 3’
Overexpression of noggin3
To generate a noggin3 overexpression construct, the QUAS:bmpr1ba-p2a-mCherry construct was used as a template. An InFusion (Takara) reaction was performed to swap the bmpr1ba sequence for noggin3 amplified from cDNA. The following primers were used for this process: IF_vector_F: 5’ TACAAAGTGGGGGGATCCGGA 3’; IF_vector_R: 5’ GCTAGCGGAGCCTGCTTT 3’; IF_nog3_F: 5’ GCAGGCTCCGCTAGCGCATTTACGCACCTCGGATAAG 3’; IF_nog3_R: 5’ TCCCCCCACTTTGTAGTTCGGGCAAGAGCATTTG 3’
Microscopy
Live confocal microscopy was performed using an SP8 inverted confocal microscope (Leica) with a 25x/0.95 Fluotar VISIR water immersion objective and Leica Application Suite software (Leica). Fish were anesthetized in 1X tricaine and then mounted onto glass bottom dishes in 1.3% or 0.9% low-melt agarose dissolved in egg water. Egg water was placed on top of the mounted fish to keep fish alive and prevent the agarose and fish from drying out. Digital stitching of tile scans was performed using Leica Application Suite (Leica). Images were false colored and minimally processed for brightness and contrast using ImageJ software (NIH). All images are oriented with the anterior end towards the left.
QUANTIFICATION AND STATISTICAL ANALYSIS
Quantifying CAbmpr1ba overexpression
The percent of cells expressing both CAbmpr1ba and TP1 was quantified using the ImageJ point tool to manually count the number of double positive cells compared to the number of cells solely containing CAbmpr1ba. For 9 dpf fish, only the IVD domains or unsegmented regions were quantified. The average percent of double positive cells was reported.
Area and Segment Number Quantification
Image processing and analysis was done in ImageJ and MATLAB. The 8-bit images were pre-processed, first by applying a gaussian blur operation. σ for the function was chosen to be 5 pixels for images containing VenusPest as a fluorescent label and 10 pixels for images containing pKRed. The images were passed with a rolling ball of radius 125 pixels for background subtraction. The notochord was then manually extracted, and the image was segmented using a fixed threshold of 5. After connecting regions of the binary image, a first check identifying the size of the resulting clusters was performed. Any cluster below 50 square pixels was identified as noise and discarded. Any groups close to each other within 80 pixels along the AP axis were also marked as belonging to the same cluster and areas and centroids were merged. The centroids along AP or DV axis were used as a marker of the cluster position. For noggin3 overexpression data, centroid position along the DV axis was rescaled to the local width of the notochord. Any cluster close to the Dorsal or Ventral side (below 0.25 or above 0.75 of the width) was identified as a seed. Area distributions were plotted using the MATLAB addon function CategoricalScatterplot. 1 pixel corresponded to 0.455 μm for these images.
Significance tests for segments and areas were calculated using the Kolmogorav-Smirnov test.
Fluorescence Intensity Profiles
For each pair of fluorescently images marked using pKRed and either VenusPest or GFP, a mask of the entire notochord was first generated manually using the pkRed label and used for both the images. Mean DV intensity values and the standard deviation were plotted along the AP axis.
Supplementary Material
Data S1: Donor plasmid for the generation of endogenous reporters. Related to Figures 2 and 4.
DNA sequence of donor plasmid containing a gBait gRNA site, the hsp701 promoter, and VenusPest. The gBait site within the Venus sequence has been mutated to prevent multiple cuts by Cas9.
Acknowledgments
We thank Brian Link for the BMP reporter line and members of the Di Talia and Bagnat labs for discussions. We would also like to thank Krissy Hays for her assistance in normalizing our RNA sequencing data. This work was supported in part by a Faculty Scholars grant from HHMI to M.B. and DST grant from Duke University to S.D.
Inclusion and Diversity
We support inclusive, diverse, and equitable conduct of research.
Footnotes
Declaration of interests
The authors declare no competing interests.
References
- 1.Bagnat M, and Gray RS (2020). Development of a straight vertebrate body axis. Development 147. 10.1242/dev.175794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wopat S, Bagwell J, Sumigray KD, Dickson AL, Huitema LFA, Poss KD, Schulte-Merker S, and Bagnat M (2018). Spine patterning is guided by segmentation of the notochord sheath. Cell Reports 22, 2026–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lleras Forero L, Narayanan R, Huitema LF, VanBergen M, Apschner A, Peterson-Maduro J, Logister I, Valentin G, Morelli LG, Oates AC, and Schulte-Merker S (2018). Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. eLife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peskin B, Henke K, Cumplido N, Treaster S, Harris MP, Bagnat M, and Arratia G (2020). Notochordal Signals Establish Phylogenetic Identity of the Teleost Spine. Current Biology 30, 2805–2814.e2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pogoda HM, Riedl-Quinkertz I, Löhr H, Waxman JS, Dale RM, Topczewski J, Schulte-Merker S, and Hammerschmidt M (2018). Direct activation of chordoblasts by retinoic acid is required for segmented centra mineralization during zebrafish spine development. Development 145. 10.1242/dev.159418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M, Chen G, and Li Y-P (2016). TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Research 4, 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowery JW, and Rosen V (2018). The BMP pathway and its inhibitors in the skeleton. Physiol Rev 98, 2431–2452. [DOI] [PubMed] [Google Scholar]
- 8.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, and Lyons KM (2005). Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. PNAS 102, 5062–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigueur D, Brugger S, Anbarchian T, Kim JK, Lee Y, and Lyons KM (2015). The Type I BMP Receptor ACVR1/ALK2 is Required for Chondrogenesis During Development. JBMR 30, 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laue K, Jänicke M, Plaster N, Sonntag C, and Hammerschmidt M (2008). Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development 135, 3775–3787. 10.1242/dev.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collery RF, and Link BA (2011). Dynamic smad-mediated BMP signaling revealed through transgenic zebrafish. Dev. Dyn 240, 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazono K, Maeda S, and Imamura T (2005). BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & Growth Factor Reviews 16, 251–263. [DOI] [PubMed] [Google Scholar]
- 13.Uribe RA, Kwon T, Marcotte EM, and Gross JM (2012). Id2a functions to limit Notch pathway activity and thereby influence the transition from proliferation to differentiation of retinoblasts during zebrafish retinogenesis. Dev Biol 371, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H-C, Perry SS, and Sun X-H (2009). Id1 attenuates Notch signaling and impairs T-cell commitment by elevating Deltex1 expression. Mol Cell Biol 29, 4640–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, and Lyons KM (2001). Bone morphogenetic protein-3 is a negative regulator of bone density. Nature Genetics 27, 84–88. 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 16.Gamer LW, Nove J, Levin M, and Rosen V (2005). BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Biol 285, 156–168. 10.1016/j.ydbio.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Banovac I, Grgurevic L, Rumenovic V, Vukicevic S, and Erjavec I (2022). BMP3 Affects Cortical and Trabecular Long Bone Development in Mice. International Journal of Molecular Sciences 23, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamer LW, Cox K, Carlo JM, and Rosen V (2009). Overexpression of BMP3 in the developing skeleton alters endochondral bone formation resulting in spontaneous rib fractures. Developmental Dynamics 238, 2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komori T (2022). Whole Aspect of Runx2 Functions in Skeletal Development. International Journal of Molecular Sciences 23, 5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katagiri T, and Watabe T (2016). Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol 8. 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura Y, Hisano Y, Kawahara A, and Higashijima S. i. (2014). Efficient generation of knock-in transgenic zebrafish carrying reporter/ driver genes by CRISPR/Cas9-mediated genome engineering. Scientific Reports 4, 6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu RS, Lam II, Clay H, Duong DN, Deo RC, and Coughlin SR (2018). A Rapid Method for Directed Gene Knockout for Screening in G0 Zebrafish. Developmental Cell 46, 112–125.e114. 10.1016/j.devcel.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Rogers KW, ElGamacy M, Jordan BM, and Müller P (2020). Optogenetic investigation of BMP target gene expression diversity. eLife 9, e58641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones WD, and Mullins MC (2022). Chapter Five - Cell signaling pathways controlling an axis organizing center in the zebrafish. In Current Topics in Developmental Biology, Kornberg T, ed. (Academic Press; ), pp. 149–209. [DOI] [PubMed] [Google Scholar]
- 25.Ninov N, Borius M, and Stainier DY (2012). Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development 139, 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parichy DM, Elizondo MR, Mills MG, Gordon TN, and Engeszer RE (2009). Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Developmental Dynamics 238, 2975–3015. 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subedi A, Macurak M, Gee ST, Monge E, Goll MG, Potter CJ, Parsons MJ, and Halpern ME (2014). Adoption of the Q transcriptional regulatory system for zebrafish transgenesis. Methods 66, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieser R, Wrana JL, and Massagué J (1995). GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. The EMBO Journal 14, 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correns A, Zimmermann LA, Baldock C, and Sengle G (2021). BMP antagonists in tissue development and disease. Matrix Biol Plus 11, 100071. 10.1016/j.mbplus.2021.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brazil DP, Church RH, Surae S, Godson C, and Martin F (2015). BMP signalling: agony and antagony in the family. Trends in Cell Biology 25, 249–264. 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Wopat S, Adhyapok P, Daga B, Crawford JM, Peskin B, Norman J, Bagwell J, Fogerson SM, Talia SD, Kiehart DP, et al. (2023). Axial segmentation by iterative mechanical signaling. bioRxiv, 2023.2003.2027.534101. 10.1101/2023.03.27.534101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunliffe VT (2003). Zebrafish: A Practical Approach. Edited by C. NÜSSLEIN-VOLHARD and R. DAHM. Oxford University Press. 2002. 322 pages. ISBN 0 19 963808 X. Price £40.00 (paperback). ISBN 0 19 963809 8. Price £80.00 (hardback). Genetics Research 82, 79–79. 10.1017/S0016672303216384. [DOI] [Google Scholar]
- 33.Garcia J, Bagwell J, Njaine B, Norman J, Levic DS, Wopat S, Miller SE, Liu X, Locasale JW, Stainier D, and Bagnat M (2017). Sheath cell invasion and trans-differentiation repair mechanical damage caused by loss of caveolae in the zebrafish notochord. Current Biology 27, 1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Donor plasmid for the generation of endogenous reporters. Related to Figures 2 and 4.
DNA sequence of donor plasmid containing a gBait gRNA site, the hsp701 promoter, and VenusPest. The gBait site within the Venus sequence has been mutated to prevent multiple cuts by Cas9.


