Abstract
This report analyzes the role of vascular endothelial growth factor (VEGF)-induced angiogenesis in the immunoinflammatory lesion stromal keratitis induced by ocular infection with herpes simplex virus (HSV). Our results show that infection with replication-competent, but not mutant, viruses results in the expression of VEGF mRNA and protein in the cornea. This a rapid event, with VEGF mRNA detectable by 12 h postinfection (p.i.) and proteins detectable by 24 h p.i. VEGF production occurred both in the virus-infected corneal epithelium and in the underlying stroma, in which viral antigens were undetectable. In the stroma, VEGF was produced by inflammatory cells; these initially were predominantly polymorphonuclear leukocytes (PMN), but at later time points both PMN and macrophage-like cells were VEGF producers. In the epithelium, the major site of VEGF-expressing cells in early infection, the infected cells themselves were usually negative for VEGF. Similarly, in vitro infection studies indicated that the cells which produced VEGF were not those which expressed virus. Attesting to the possible role of VEGF-induced angiogenesis in the pathogenesis of herpetic stromal keratitis were experiments showing that VEGF inhibition with mFlt(1–3)-immunoglobulin G diminished angiogenesis and the severity of lesions after HSV infection. These observations are the first to evaluate VEGF-induced angiogenesis in the pathogenesis of stromal keratitis. Our results indicate that the control of angiogenesis represents a useful adjunct to therapy of herpetic ocular disease, an important cause of human blindness.
Immunoinflammatory lesions in the corneal stroma resulting from ocular infection with herpes simplex virus (HSV) are one of the most common infectious causes of blindness in the western world (28). Studies in animal models have revealed a complex pathogenesis, with the principal event being invasion by CD4+ T cells that orchestrate the chronic inflammatory process (26, 27, 28, 31, 34, 37, 41). Whereas the normal cornea is avascular, in herpetic stromal keratitis (HSK) neovascularization may be prominent, even reaching the central cornea. Inflammatory cells readily escape from such vessels, giving rise to haze and vision impairment. Indeed, neovascularization anywhere along the visual axis may impair vision, with vessel removal being highly problematic.
The mechanism by which HSV ocular infection results in corneal angiogenesis is not understood. Unlike some viruses, most notably human herpesvirus 8 and Orf virus, HSV appears not to encode proteins which themselves are directly angiogenic. Thus, human herpesvirus 8 encodes at least five such proteins. These include the three CC chemokine-homologous proteins vMIP-I, -II, and -III; an angiogenic viral interleukin-6; and G-protein-coupled receptor as an angiogenesis activator (2, 3, 21, 33, 35). The Orf virus also encodes a protein homologous to vascular endothelial growth factor (VEGF), the family of glycoproteins that are the most important specific mediators of angiogenesis (18, 24, 42). The four known isoforms of VEGF bind to tyrosine kinase receptors on vascular endothelial cells, causing their division and migration (14, 25, 42). The VEGF molecules, along with members of the angiopoietin family and at least one ephrin, are the major growth factors responsible for normal vasculogenesis and angiogenic remodeling (42). Furthermore, VEGF proteins also appear as important mediators of pathological angiogenesis, with such neovessels (NV) often being leaky and unstable (7, 16). VEGF expression has been related to the regulation of certain immunoinflammatory diseases but has not, to our knowledge, been investigated for its possible role in the pathogenesis of HSK. In the present report, we describe changes in angiogenesis that occur in the mouse cornea in response to HSV infection. We demonstrate that VEGF is induced by HSV infection but that its primary cellular source appears not to be cells directly infected by HSV. We also demonstrated that VEGF inhibition by administration of a chimeric murine soluble VEGF receptor protein, mFlt(1–3)-immunoglobulin G [mFlt(1–3)-IgG], decreases the severity of HSK. Procedures which fully suppress angiogenesis may represent a valuable therapeutic approach to control HSK.
MATERIALS AND METHODS
Viruses.
The HSV type 1 (HSV-1) KOS strain used was passaged and assayed on Vero cells for measurement of PFU by standard protocols. Virus stocks were aliquoted and stored at −80°C. Replication-defective viruses and the complementing cell lines for ICP4−/− and ICP8−/− were kindly provided by J. C. Glorioso, Department of Neurology, University of Pittsburgh School of Medicine, Pittsburgh, Pa., and David A. Knipe, Microbiology and Molecular Genetics Department, Harvard Medical School, Boston, Mass., respectively. Green fluorescent protein (GFP)–HSV-1 KOS (GFP is driven under control of the promoter of the UL35 gene, encoding the basic phosphorylated capsid protein) was a kind gift of S. Person, Virology Laboratories, Department of Pharmacology and Molecular Science, Johns Hopkins University School of Medicine, Baltimore, Md.
Mice.
Five- to 6-week-old female BALB/c mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were used. All experiments were conducted in compliance with the guide for the care and use of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. The facilities used were accredited by the American Association for Accreditation of Laboratory Animal Care. All ocular experimental procedures were conducted according to the Association for Research in Vision and Ophthalmology resolution on the use and care of laboratory animals.
Corneal HSV infection and alkali burn model.
Mice were infected on the slightly scratched cornea by 5 × 105 or 5 × 107 HSV-1 KOS or 5 × 105 ICP4−/−, ICP8−/−, and GFP-HSV under Avertin anesthesia (250 mg/kg by intraperitoneal injection). In the alkali burn model, Whatman filter papers (diameter, 1 mm) were soaked in 0.5 N NaOH for 1 min and then were put onto the central region of the mouse cornea under anesthesia for 1 min. The excess NaOH was removed by rinsing the eyes with 5 ml of phosphate-buffered saline and then gently blotting them with tissue. In the trauma control group, the corneas were slightly scratched only. The mice were observed every other day, and angiogenesis and clinical lesion scores were documented.
Clinical scoring system.
Mice were examined at different time points after infection for the development of clinical lesions by slit lamp microscopy (Kowa Co., Nagoya, Japan) and stereomicroscopy (Leica Microscopy Systems Ltd., Heerbrugg, Switzerland) with an image system (Hamamatsu Photonics K.K., Hamamatsu City, Japan). The severity of stromal keratitis (19) and the degree of angiogenesis (10) were measured as described previously. Briefly, the clinical lesion score of HSK was described as follows: 0, normal cornea; 1, mild haze; 2, moderate haze with iris visible; 3, severe haze with iris not visible; 4, severe haze and corneal ulcer; and 5, corneal rupture. In reference to the angiogenesis scoring system, the method relied on quantifying the degree of NV formation based on three primary parameters: (i) the circumferential extent of NV (as the angiogenic response is not uniformly circumferential in all cases), (ii) the centripetal growth of the longest vessels in each quadrant of the circle, and (iii) the length of the longest NV in each quadrant, which was graded between 0 (no NV) and 4 (NV in the corneal center) in increments of about 0.4 mm (the radius of the cornea is about 1.5 mm). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.2 to 1.5 mm toward the corneal center. The final angiogenesis scores of the four quadrants of the cornea were then summed to derive the NV index (range, 0 to 16) for each eye at a given time point. The extent of the NV ingrowth was also recorded by direct measurement using calipers (Biomedical Research Instruments, Rockville, Md.) under stereomicroscopy.
Corneal NV India ink perfusion technique.
The method for corneal NV India ink perfusion was described previously (39). Briefly, the mice were anesthetized with Avertin at day 1 postinfection (p.i.). The descending aorta was clamped, the right atrium was cut, and 1 ml of waterproof drawing ink (India ink) (no. 4465; Eberhard Faber Inc., Lewisburg, Tenn.) was infused through the left ventricle. Five minutes later, the eyes were removed. The corneas (with limbal regions) were isolated, radially cut from the edge to the center of the cornea, and flat mounted on glass slides. The NV were viewed under the light microscope, and the images were taken by the image system.
Histopathological and immunohistochemical staining.
At various times after infection, whole eyes were fixed in 10% buffered neutral formalin and embedded in paraffin, and tissue sections were stained with hematoxylin and eosin as described previously (19). Sections were observed for thickness of the cornea, presence of inflammatory infiltrates, neovascularization, and corneal perforation. For immunohistochemistry, eyes were removed and snap frozen in OCT compound (Miles, Elkhart, Ind.). Sections (6 μm) were cut, air dried, and fixed in cold acetone for 10 min. The sections were then blocked with 3% bovine serum albumin and stained with biotinylated anti-mVEGF164 (R & D Systems Inc., Minneapolis, Minn.). Sections were then treated with horseradish peroxidase-conjugated streptavidin (1:1,000) and 3,3′-diaminobenzidine (Vector, Burlingame, Calif.) and counterstained with hematoxylin. Cellular infiltration was quantified by enumerating the infiltrating cells in the corneal stroma. Each number was derived from four central corneal sections from two eyes. The infiltrating cells were counted in the whole corneal section.
Corneal lysate VEGF ELISA.
Corneas at different time points were removed from whole eyes immediately after sacrifice, put into RPMI 1640 without serum, and stored at −80°C. The corneas were homogenized in an ice bath with a tissue homogenizer (PRO Scientific Inc., Monroe, Conn.) for 1 min. The resulting lysates were collected and assayed by a standard sandwich enzyme-linked immunosorbent assay (ELISA) protocol. The anti-VEGF capture biotinylated detection antibodies and standard mVEGF164 were from R & D Systems. The color reaction was measured with an ELISA reader (Spectramax 340; Molecular Devices) at 460 nm. The detection limit was 2 pg/ml. Quantification was performed with Spectramax ELISA reader software version 1.2.
Fluorescence-activated cell sorter (FACS) staining for Gr-1-, Mac-1-, and VEGF-positive cells from inflamed corneal cells.
Four corneas each at days 1, 8, and 15 p.i. were isolated from the eyeball. The corneas were digested with collagenase (at pH 7.0) as described elsewhere, with some modifications (9, 31). Briefly, the corneas were put into collagenase IV (Sigma Chemical Co., St. Louis, Mo.) at a concentration of 60 U/ml in Hanks balanced salt solution. The corneas were incubated for 1 h at 37°C in cell incubator. After incubation, the corneas were disrupted by passage through a cell strainer and grinding with a syringe plunger. The cells were collected, washed, and resuspended in RPMI 1640 with 5% fetal calf serum (FCS). The cells were blocked with 100% FCS for 30 min and stained for either Gr-1 (BD PharMingen, San Diego, Calif.) or Mac-1 (CD11b) (BD PharMingen) and mVEGF164.
In vitro experiments with thioglycolate-elicited neutrophils and macrophages.
For isolation of polymorphonuclear leukocytes (PMN), thioglycolate-induced peritoneal exudate was used as a source of recently extravasated PMN. Mice received an intraperitoneal injection of 1 ml of thioglycolate. Three hours later, the peritoneal exudate cells (PEC) were removed, washed once in RPMI 1640 medium supplemented with 5% FCS, and then incubated on a plastic surface for 1 h at 37°C in the same medium. The nonadherent cells were removed and shown to be >98% PMN based on nuclear morphology. For macrophage preparation, the peritoneal exudate was removed 4 days after the injection of thioglycolate. The PEC were incubated either in two-well chamber slides (for later immunohistochemical study) or in 24-well plates (for later RT-PCR) and infected at a multiplicity of infection (MOI) of 2 for GFP–HSV-1, and immunohistochemical staining for VEGF was conducted at 18 h p.i.
RNA extraction and RT-PCR.
At different time points after ocular infection, the corneas were isolated and four at each time point were immediately excised and transferred to Tri-reagent (Molecular Biology Inc., Cincinnati, Ohio). The total RNA was extracted according to the manufacturer's directions. Total RNA (10 μg) was reverse transcribed, and aliquots of cDNA were used in a 25-μl PCR mixture as previously described (21). The primer sequences for VEGF were 5′-GCGGGCTGCCTCGCAGTC-3′ (sense) and 5′-TCACCGCCTTGGCTTGTCAC-3′ (antisense), corresponding to bp 16 to 33 and 659 to 640, respectively, of the mouse VEGF cDNA sequence (numbering is according to reference 8). Reverse transcription-PCR (RT-PCR) products were 716 bp (mVEGF188), 644 bp (mVEGF164), and 512 bp (mVEGF120). The amplification profile was 94°C for 1 min, 65°C for 1 min, and 72°C for 15 min for 30 cycles.
Statistical analysis.
Significant differences between groups were evaluated using Student's t test. A P value of ≤0.05 was regarded as indicating a significant difference between two groups.
RESULTS
HSV ocular infection results in corneal angiogenesis.
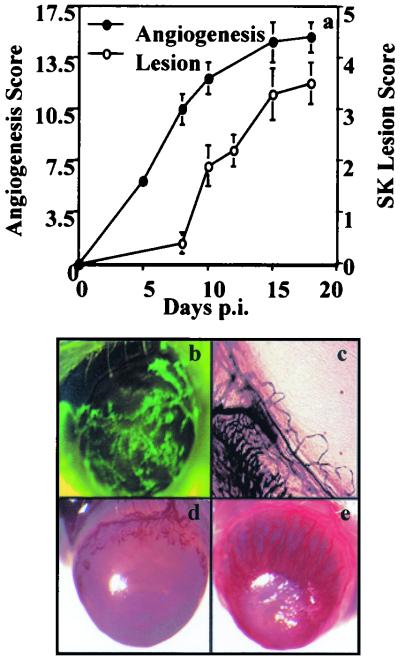
Following mild scarification and infection of the cornea with GFP–HSV-1 (KOS background), virus replicates in the epithelium for up to 4 to 5 days p.i. As shown in Fig. 1b and c, evidence of angiogenic sprouting from limbal vessels was present at 24 h p.i., when the virus was replicating vigorously in the corneal epithelium. At around day 8 p.i., when clinical HSK becomes discernible, angiogenesis had advanced approximately halfway across the cornea (Fig. 1a and d), reaching the central region and its maximal extent at around 15 days p.i. (Fig. 1e). Lesions of HSK in this model are usually at their peak at between 15 and 21 days p.i. In trauma control animals, mild angiogenesis was evident at 24 h p.i. (score of 2.5 ± 0.3), but this had resolved within 2 to 3 days postinjury. A similar transient level of mild corneal angiogenesis was evident in mice exposed to UV-inactivated HSV KOS (the titer prior to inactivation was the same as or 20-fold higher than that used for active infection). Such data indicate that HSV-induced angiogenesis requires viral replication.
FIG. 1.
Angiogenesis and lesion scores at different times after HSV infection. BALB/c mice were infected on the slightly scarified corneal surface with HSV KOS or GFP-HSV (KOS background). Angiogenesis and HSK lesion scores were documented at different time points p.i. as described in Materials and Methods. (a) Correlation of angiogenesis and HSK lesion scores in the process of HSK. (b) GFP-HSV replicates on the infected corneal surface at day 1 p.i. The image was taken under a GFP filter by stereomicroscopy and an image system. Magnification, ×20. (c) Angiogenic sprouting was evident at day 1 p.i. as shown by the India ink perfusion technique. Magnification, ×50. (d) At day 8 p.i., neovascularization was around halfway toward the center of the cornea. (e) Intense neovascularization was seen in HSV-infected cornea at day 16 p.i. Magnification, ×20.
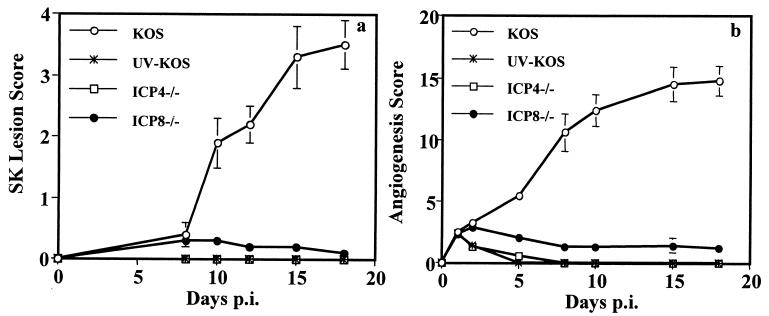
Similar experiments were done with HSV-1 deletion mutants that were replication defective. Thus, infection with 5 × 105 or 5 × 107 PFU of the immediate-early α gene mutant ICP4−/− or early β gene mutant ICP8−/−, neither of which generated progeny virus in the eye, failed to produce significant angiogenesis beyond levels seen in trauma controls (Fig. 2b). These virus infections also failed to induce HSK (Fig. 2a). In other experiments, mice were exposed three times at 2-day intervals to 5 × 105 or 5 × 107 PFU of either mutant virus, but in no instance was angiogenesis that was any greater than that occurring in trauma controls observed. Such experiments are consistent with the notion that angiogenesis resulting from HSV infection is the consequence of viral replication.
FIG. 2.
Necessity for replicating virus to induce angiogenesis and HSK. BALB/c mice were infected (with wt KOS, UV-inactivated KOS [UV-KOS], ICP4−/−, or ICP8−/−) or only scratched on the corneal epithelium as a trauma control. Angiogenesis scores (a) and HSK lesion scores (b) were recorded as described in the text. Mild angiogenesis was observed at day 1 postinjury and then faded away in trauma control eyes (data not shown).
Induction of VEGF by HSV infection.
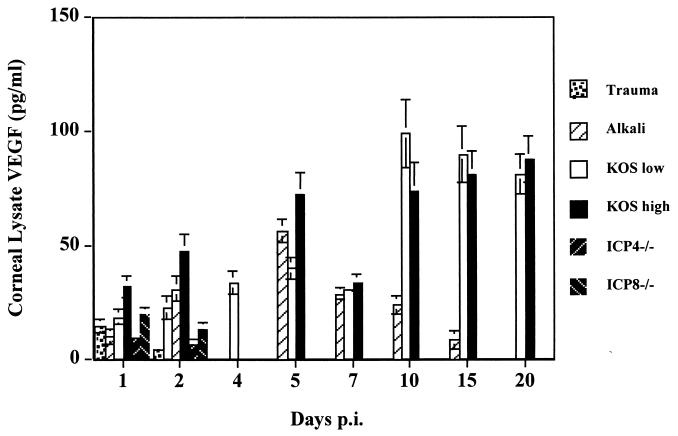
The mechanism by which HSV results in angiogenesis has not been defined. However, the most potent natural angiogenesis factors are members of the VEGF family (17, 42). To determine if such molecules were induced after HSV ocular infection, several experimental approaches were pursued. In the first series of experiments, corneal lysates from animals infected for different time periods with wild-type (wt)or mutant viruses were measured for VEGF expression by ELISA. As a positive control, animals were exposed to an alkali burn, which causes VEGF production by inflammatory cell infiltrates (11). As shown in Fig. 3, using two different doses of HSV KOS (5 × 105 and 5 × 107 PFU), VEGF levels during the preclinical phase (5 days) correlated with the titer of viruses used for infection. (72 ± 11 and 44 ± 5 pg/ml for the higher- and lower-titer groups, respectively; P ≤ 0.05). In the clinical phase (8 to 21 days), VEGF levels were the same in both groups, peaked at 10 days, and maintained similar levels (on days 7, 10, 15, and 20 p.i., the P value was ≥0.05). In trauma control animals, low levels of VEGF (9 to 20 pg/ml) were detectable on day 1, but none was detectable beyond day 2 postinjury. The alkali burn positive controls had peak VEGF levels at 54 ± 7 pg/ml at around day 5 p.i., and this was comparable to the average VEGF level (58 ± 19 pg/ml) of the groups with lower and higher viral titers at the same time point (P ≥ 0.05).
FIG. 3.
Presence of VEGF in corneal lysates at different times after HSV infection. At different time points p.i., the infected (wt KOS [low, 5 × 105 PFU; high, 5 × 107 PFU], ICP4−/−, or ICP8−/−) and control (trauma or alkali) corneas (n = 4) were isolated and stored at −80°C until further use. The corneal lysates were assayed by ELISA for detection of mVEGF164. VEGF levels in the wt virus-infected corneas were correlated with the titers of the infected virus in the preclinical phase (P ≤ 0.05). VEGF in trauma control corneas could not be detected beyond 48 h. VEGF levels in UV-inactivated-HSV-infected corneas at all time points were similar to those in trauma control and ICP4−/−- and ICP8−/−-infected corneas (data not shown).
Whereas wt virus infection induced VEGF, infection with mutant viruses induced only low levels (10 to 19 pg/ml) which were not significantly above those observed in trauma control animals at the same time points at days 1 and 2 p.i. (P ≥ 0.05). In separate experiments, animals were infected with wt virus, and at several time points, starting at 12 h p.i., samples were processed to measure VEGF mRNA levels by semiquantitative RT-PCR. By this analysis, two out of three murine VEGF isoforms (16), mVEGF120 and mVEGF164, were detected beginning as early as 12 h p.i. (Fig. 4).
FIG. 4.
Expression of corneal VEGF mRNA at various times after HSV infection. Mice were infected as described in the text. At different time points p.i., the eyes were enucleated and the corneas (n = 4) were isolated and stored at −80°C in Tri-reagent. Total RNA was extracted from the samples and reverse transcribed into cDNA. PCR was performed to detect the isoforms (VEGF120, -164, and -188) of VEGF in HSV-infected corneas. β-Actin served as the positive control and standard for semiquantitative RT-PCR.
Cell source of VEGF.
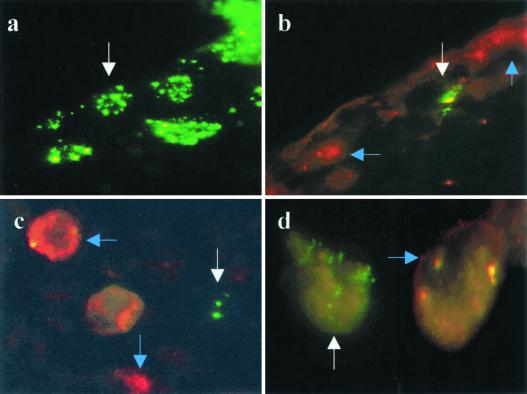
In an attempt to determine which cells in the eye were responsible for VEGF production, two types of experiments were performed. Initially, eyes were infected with wt virus or recombinant virus expressing GFP. At different times p.i., corneal sections were examined by immunohistochemical staining for VEGF expression. In the second experiment, infected animals were killed at various times p.i., the corneas were collected and collagenase digested, and the isolated cells were analyzed by FACS for phenotype and VEGF expression. The results of immunohistochemical staining revealed that virus-expressing cells (Fig. 5a) were confined to the epithelium. Virus detected in the epithelium was present in occasional cells for up to 5 days. However, cells that expressed VEGF were located in both the epithelium and the stroma. Of interest is that VEGF-expressing cells in the epithelium were mostly cells that were not detectably infected with virus but were usually close to virus-infected cells. Such VEGF-positive epithelial cells were abundant at 24 h, but by 48 h only occasional cells were VEGF positive (Fig. 5b).
FIG. 5.
Demonstration of virus-infected and VEGF-expressing cells in vivo and in vitro. (a and b) Frozen sections of corneas 24 h p.i. with GFP-HSV. (a) Corneal epithelial cells infected with virus. (b) A different area of the same cornea stained for VEGF. The white arrow indicates a virus-infected cell lacking VEGF expression. The blue arrows indicate VEGF-expressing cells with no virus infection. (c and d) Thioglycolate-elicited PEC infected with GFP-HSV at an MOI of 2 for 18 h and stained with VEGF. Virus-infected cells (white arrows) and VEGF-expressing cells (blue arrows) are distinct. (c) Neutrophils; (d) macrophages.
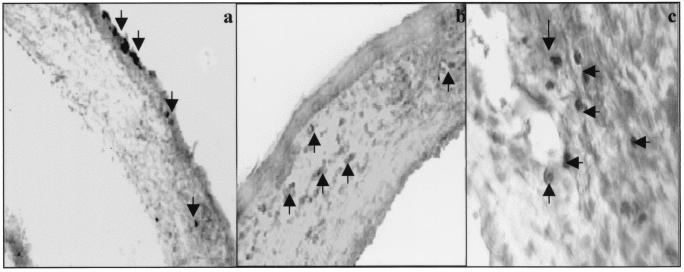
In the stroma, VEGF-positive cells were observed at 24 h p.i., and such cells were far more abundant during the clinical phase (8 to 21 days) when virus was no longer present (Fig. 6). At no stage was virus (GFP-HSV) detectable in cells in the stroma. Furthermore, whereas only a minority of stromal inflammatory cells were VEGF positive at 24 h (<5%; 7 ± 5 positive cells/central corneal section), at 7 days and beyond (beginning of the clinical phase) >15% (35 ± 9 [day 7 p.i.] and 60 ± 19 [day 10 p.i.] positive cells/central corneal section) of cells were VEGF positive. At the early phase almost all inflammatory cells appeared to be neutrophils, whereas at 7 days and later inflammatory cells were both neutrophils and macrophage-like cells. Curiously, the VEGF-positive inflammatory cells were more abundant near the sites of neovascularization (Fig. 6c). Control uninfected corneas never contained VEGF-positive cells in the epithelium or stroma.
FIG. 6.
VEGF expression in corneal sections at different times after HSV infection. Eyes were collected at different times p.i., and frozen sections were processed for VEGF detection as described in Materials and Methods. (a) VEGF-positive corneal epithelial cells and a few infiltrating cells in the corneal stroma at day 1 p.i.; (b) absence of VEGF-positive cells in the epithelium and both negative and positive (arrow) cells in the stroma (day 8 p.i.); (c) VEGF-positive cells tend to gather at sites of the neovascularization (day 15 p.i.).
In the second series of experiments, cells isolated from HSV-infected corneas were analyzed by FACS to determine the percentage of inflammatory and noninflammatory cells that were VEGF positive. In such studies inflammatory cells were identified by expression of either the Gr-1 (mainly on neutrophils) or Mac-1 (mainly on macrophages) marker. At 24 h, the majority of cells were Gr-1 and Mac-1 negative, and only 2.3% (1,150 ± 250 cells/cornea [average for four mice]) were VEGF positive. By day 8 and beyond, the majority of cells recovered from infected corneas were inflammatory and expressed either Gr-1 or Mac-1. At day 8, around 15% of cells (16,000 ± 5,650 cells/cornea [mean value for four mice]) were VEGF positive, and by day 12 p.i., 31% (22,500 ± 4,780 cells/cornea [mean value for four mice]) were VEGF positive.
The above data indicate that HSV infection of the cornea causes apparently uninfected cells to produce VEGF. Initially the predominant producer cell type appeared to be noninflammatory cells in the epithelium, but subsequently, in the clinical phase, inflammatory cells in the stroma were the only sites of VEGF production. In both phases, cells which produce VEGF appeared not to be infected by virus. To further analyze this issue, thioglycolate-induced inflammatory cells were infected in vitro at an MOI of 2 with GFP–HSV-1. Eighteen hours later, cells were fixed, stained with biotinylated VEGF antibody and streptavidin-phycoerythrin, and then analyzed by fluorescence microscopy. The data (Fig. 5c and d) indicate that most of the VEGF-producing cells were not themselves virus-infected cells. Such virus-infected cultures contained both PMN and macrophage-like cells, and both cell types were shown to be VEGF producers upon virus infection. In the absence of virus infection, a very low percentage (around 2%) of the inflammatory cells produced detectable levels of VEGF.
mFlt(1–3)-IgG ameliorates angiogenesis and HSK lesions.
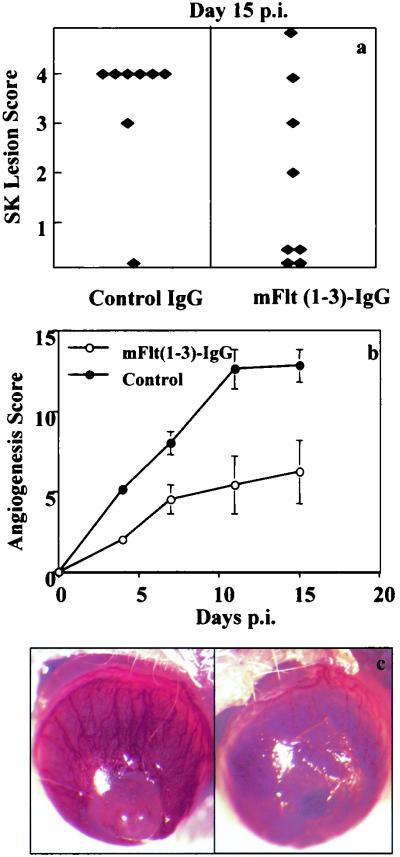
The above data indicated that HSV-1 infection of the cornea upregulates VEGF expression, which in turn could act as the principal mediator of HSV-induced angiogenesis. Consequently, we expected that if VEGF activity was inhibited, this could reduce angiogenesis and modulate the severity of HSK. To test such a notion, BALB/c mice were infected with HSV and were treated systemically either with an mVEGF antagonist [the recombinant soluble form of the VEGF receptor Flt extracellular domain (1–3)-IgG] or with control mouse IgG. The severity and incidence of lesions were measured at days 11, 15, and 18. These were significantly reduced in mice treated with the VEGF antagonist, although the treated group showed severe periocular skin lesions compared with the control mouse IgG-treated group. Clinical lesion scores of ≤2.0 were evident in 45 and 12.5% of treated and control mice, respectively, at day 15 p.i. (Fig. 7a and c). Angiogenesis scores in treated mice were reduced at all time points observed, with scores of 6.2 ± 5.0 and 14.9 ± 3.0 in mFlt(1–3)-IgG-treated and control corneas, respectively, at day 15 p.i. (Fig. 7b and c). The data indicate that treatment with the VEGF antagonist inhibited both angiogenesis and HSK lesion severity; however, the effect was usually not complete.
FIG. 7.
Effect of VEGF inhibition on severity of HSK. Mice (n = 5) were treated every other day with mFlt(1–3)-IgG at 12.5 mg/kg starting 1 h prior to HSV-1 infection. Mouse IgG served as a control. (a and b) The HSK lesion scores (a) and angiogenesis scores (b) were determined as described in Materials and Methods. (c) Corneal images of control (left) and mFlt(1–3)-IgG-treated (right) mice at day 15 p.i. The experiments were done twice with similar results, and the results of one such experiment are shown.
DISCUSSION
This report analyzes the role of VEGF-induced angiogenesis in the immunoinflammatory lesion stromal keratitis induced by ocular infection with HSV. Our results show that infection with replication-competent, but not mutant, virus results in the expression of VEGF mRNA and protein in the cornea. This a rapid event, with VEGF mRNA detectable by 12 h p.i. and proteins detectable by 24 h p.i. VEGF production occurred both in the virus-infected corneal epithelium and in the underlying stroma, in which viral antigens were undetectable. In the stroma, VEGF was produced by inflammatory cells which initially were predominantly PMN, but at later time points both PMN and macrophage-like cells were VEGF producers. In the epithelium, the major site of VEGF-expressing cells in early infection, the infected cells were almost always negative for VEGF. Similarly, in vitro infection studies indicated that the VEGF-producing cells themselves were not those which expressed virus. Attesting to the possible role of VEGF-induced angiogenesis in the pathogenesis of HSK were experiments showing that VEGF inhibition with mFlt(1–3)-IgG diminished angiogenesis and the severity of lesions after HSV infection. These observations are the first to evaluate VEGF-induced angiogenesis in the pathogenesis of stromal keratitis.
Angiogenesis and vasculogenesis are topics of notable interest to ophthalmology and tumor biology. Such processes in adults occur only in pathological states such as wound healing and inflammation (17, 32). Neovascularization in the eye is usually an unwanted event, since usually the consequence is interference with the passage of light along the visual axis. Abnormal angiogenesis in the retina induced by the hypoxia associated with diabetes often results in marked impairment in vision (6, 23). In HSK, as shown in this report, angiogenesis is a prominent and early feature of the pathogenesis. The uninfected eye lacks blood vessels in the cornea, but angiogenenic sprouting and vasculogenesis invade from the limbus soon after HSV infection. The likely multiple stimuli which induce such a response have yet to be identified. However, the present results show that the VEGF family of proteins are involved. These molecules are important angiogenic factors during development (5, 15) and are frequently involved in pathological neovascularization such as occurs in several tumor systems (17, 20). Our data show that at least two of the known VEGF isoforms are produced by ocular cells following HSV infection. This is a rapid event, but the mechanism by which HSV causes VEGF expression remains to be elucidated. Accordingly, when we looked for producer cells following infection with GFP-marked virus, VEGF-producing cells themselves were usually not those which could be shown to be infected with virus. In fact, the VEGF producers appeared to be two major cell types, noninflammatory cells and inflammatory cells. Early after infection most producing cells were noninflammatory and were assumed to be mainly corneal epithelial cells. As seen in corneal tissue sections, the VEGF-producing cells were close to infected cells but were seldom infected themselves.
The second VEGF-producing cell type was inflammatory cells in the corneal stroma. These were the major VEGF producers in the clinical phase, with both PMN and macrophage-like cells involved. Since virus is usually undetectable in the stroma and is absent in the cornea after the first few days of infection (19, 38, 40), virus itself would seem not to be the stimulus for VEGF production. This was also noted to be the situation with in vitro infection experiments with inflammatory exudate cells, where it was observed that VEGF-producing and virus-producing cells were distinct.
The above observations raise the question as to the mechanism by which HSV infection of a cell triggers another cell to turn on VEGF gene expression. Presently it is unclear if this paracrine event is mediated by some HSV-encoded component released from infected cells or if it is the consequence of infected-cell-derived host proteins released from such cells. Since productive HSV infection eliminates most host cell protein synthesis (29, 30), newly expressed host proteins such as cytokines or chemokines are unlikely candidates. Nevertheless, some HSV-infected cells may turn on interleukin-6 synthesis (22, 36), and this cytokine represents a possible candidate. The virus itself, unlike some other herpesviruses (4), is not known to encode proteins that act as angiokine mimics, but some viral proteins are released from infected cells and these can even access nearby cells. The VP22 protein is the best known example of this event (12, 13). We are currently analyzing the role of VP22 along with some potential host cell-derived proteins as candidates to explain VEGF triggering.
Although our studies represent the first to document that VEGF production occurs during ocular infection with HSV, it remains to be proven if VEGF is the principal mediator of HSV-induced angiogenesis or if such an event is a necessary component of HSK pathogenesis. Some data presented in this report do support a major role of VEGF-induced angiogenesis in HSK. Accordingly, when infected mice were treated with the specific anti-VEGF antagonist mFlt(1–3)-IgG (1, 18, 20), angiogenesis was minimized and HSK lesions diminished. However, the effect was not complete, indicating either that the treatment itself failed to eliminate all VEGF activity or, perhaps more likely, that other angiogenesis factors were also involved. These issues are under further investigation.
In conclusion, our studies demonstrate a role for VEGF-induced angiogenesis in the pathogenesis of a virus-induced immunoinflammatory lesion. Such observations could mean that the therapeutic control of HSK, a distressing cause of human blindness, might benefit from measures which target angiogenesis.
ACKNOWLEDGMENTS
Our work was supported by NIH grant EY05093.
We thank Teresa Sobhani for technical help and for frequently acting as a cheerleader. We thank Tommy Jordan for his excellent computer skills.
REFERENCES
- 1.Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little R F, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001;97:2173–2176. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- 3.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A, Gerhengorn M C. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a v oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 4.Boshoff C. Kaposi's sarcoma. Coupling herpesvirus to angiogenesis. Nature. 1998;391:24–25. doi: 10.1038/34054. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Dor Y, Herbert J M, Fukumura D, Brusselmans K, Dewerchin M, Neeman, Bono F, Abramovitch R, Maxwell P, Koch C J, Ratcliffe P, Moons L, Jain R K, Collen D, Keshet E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate K H, Foidart J M, Schaper W, Charnock-Jones D S, Hicklin D J, Herbert J M, Collen D, Persico M G. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 8.Claffey K P, Wilkison W O, Spigelman B M. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992;267:16317–16322. [PubMed] [Google Scholar]
- 9.Cousins S W, Strelein J W. Flow cytometric detection of lymphocyte proliferation in eyes with immunogenic inflammation. Investig Ophthalmol Vis Sci. 1990;31:2111–2115. [PubMed] [Google Scholar]
- 10.Dana M R, Zhu S, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409. doi: 10.1097/00003226-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Edelman J L, Castro M R, Wen Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Investig Ophthalmol Vis Sci. 1999;40:1112–1123. [PubMed] [Google Scholar]
- 12.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 13.Elliott G, O'Hare P. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J Virol. 1999;73:4110–4119. doi: 10.1128/jvi.73.5.4110-4119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson U, Alitalo K. Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr Top Microbiol Immunol. 1999;237:41–57. doi: 10.1007/978-3-642-59953-8_3. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. In: Claesson-Welsh L, editor. Vascular growth factors and angiogenesis. Berlin, Germany: Springer-Verlag; 1996. pp. 1–30. [Google Scholar]
- 17.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. Vascular endothelial growth factor: molecule and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 19.Gangappa S P, Babu J S, Thomas J, Daheshia M, Rouse B T. Virus induced immunoinflammatory lesions in the absence of viral antigen recognition. J Immunol. 1998;161:4289–4300. [PubMed] [Google Scholar]
- 20.Gerber H P, Kowalski J, Sherman D, Eberhard D A, Ferrara N. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res. 2000;60:6253–6258. [PubMed] [Google Scholar]
- 21.Haque N S, Fallon J T, Taubman M B, Harpel P C. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood. 2001;97:39–45. doi: 10.1182/blood.v97.1.39. [DOI] [PubMed] [Google Scholar]
- 22.Kanangat S, Babu J S, Knipe D M, Rouse B T. HSV-1-mediated modulation of cytokine gene expression in a permissive cell line: selective upregulation of IL-6 gene expression. Virology. 1996;219:295–300. doi: 10.1006/viro.1996.0250. [DOI] [PubMed] [Google Scholar]
- 23.Kwak N, Okamoto N, Wood J M, Campochiaro P A. VEGF is major stimulator in model of choroidal neovascularization. Investig Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- 24.Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin H G, Ziche M, Lanz C, Buttner M, Rziha H J, Dehio C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signaling through VEGF-2 (KDR) but not VEGF (flt-1) receptor tyrosine kinase. EMBO J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 26.Newell C K, Martin S, Sendele D, Mercadel C M, Rouse B T. Herpes simplex virus-induced stromal keratitis: role of T lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niemialtowski M G, Rouse B T. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 28.Pepose J S, Leib D A, Stuart P M, Easty D L. Herpes simplex virus disease. In: Pepose J S, Holland G N, Wilhelmus K R, editors. Ocular infection & immunity. St. Louis, Mo: Mosby-Year Book, Inc.; 1996. pp. 905–932. [Google Scholar]
- 29.Roizman, B., and A. E. Sears. Herpes simplex viruses and their replication, p. 2231–2295. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 30.Rouse B T, Massoud D, Schmid D S. Herpes simplex virus and the immune response: a balance of power. In: Cunningham M, Fujinami R, editors. Effects of microbes on the immune system. 1st ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2000. pp. 387–397. [Google Scholar]
- 31.Russell R G, Nassisse M P, Larsen H S, Rouse B T. Role of T lymphocytes in the pathogenesis of herpetic stromal keratitis. Investig Ophthalmol Vis Sci. 1984;25:938–944. [PubMed] [Google Scholar]
- 32.Shiro, A., R. Rohan, M. Kuroki, M. Tolentino, and A. P. Adamis. 1998. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization 39:18–22. [PubMed]
- 33.Stine J T, Wood C, Hill M, Epp A, Raport C J, Schweickart V L, Endo Y, Sasaki T, Simmons G, Boshoff C, Clapham P, Chang Y, Moore P, Gray P W, Chantry D. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis and selectively chemoattracts TH2 cells. Blood. 2000;95:1151–1157. [PubMed] [Google Scholar]
- 34.Streilein J W, Dana M R, Ksander B R. Immunity causing blindness. Five different paths to herpes stromal keratitis. Immunol Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 35.Sun R, Lin S F, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas J, Gangappa S P, Chun S, Daheshia M, Rouse B T. Herpes simplex replication induces expression of chemokines and proinflammatory cytokines in eye implications in herpetic stromal keratitis. J Interferon Cytokine Res. 1998;18:681–690. doi: 10.1089/jir.1998.18.681. [DOI] [PubMed] [Google Scholar]
- 37.Thomas J, Rouse B T. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 38.Thomas J, Gangappa S P, Kanagat S, Rouse B T. On the essential involvement of neutrophils in the immunopathological disease herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 39.Tobe T, Okamoto N, Vinores M A, Derevjanik N L, Vinores S A, Zack D J, Campochiaro P A. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Investig Vis Sci. 1998;39:180–188. [PubMed] [Google Scholar]
- 40.Tumpey T M, Chen S H, Oakes J E, Lausch R N. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verjans G M, Remeijer L, Mooy C M, Osterhaus A D. Herpes simplex virus-specific T cells infiltrate the cornea of patients with herpetic stromal keratitis: no evidence for autoreactive T cells. Investig Ophthalmol Vis Sci. 2000;41:2607–2612. [PubMed] [Google Scholar]
- 42.Yancopoulos G D, Davis S, Gale N W, Rudge J, Wiegand S J, Holash J. Vascular-specific growth factor and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]