Abstract
A major impediment to successful chemotherapy is the propensity for some tumor cells to undergo cell cycle arrest rather than apoptosis. It is well established, however, that the adenovirus E1A protein can sensitize these cells to the induction of apoptosis by anticancer agents. To further understand how E1A enhances chemosensitivity, we have made use of a human colon carcinoma cell line (HCT116) which typically undergoes cell cycle arrest in response to chemotherapeutic drugs. As seen by the analysis of E1A mutants, we show here that E1A can induce apoptosis in these cells by neutralizing the activities of the cyclin-dependent kinase inhibitor p21. E1A's ability to interact with p21 and thereby restore Cdk2 activity in DNA-damaged cells correlates with the reversal of G1 arrest, which in turn leads to apoptosis. Analysis of E1A mutants failing to bind p300 (also called CBP) or Rb shows that they are almost identical to wild-type E1A in their ability to initially overcome a G1 arrest in cells after DNA damage, while an E1A mutant failing to bind p21 is not. However, over time, this mutant, which can still target Rb, is far more efficient in accumulating cells with a DNA content greater than 4N but is similar to wild-type E1A and the other E1A mutants in releasing cells from a p53-mediated G2 block following chemotherapeutic treatment. Thus, we suggest that although E1A requires the binding of p21 to create an optimum environment for apoptosis to occur in DNA-damaged cells, E1A's involvement in other pathways may be contributing to this process as well. A model is proposed to explain the implications of these findings.
Chemotherapeutic drugs and γ radiation are the bases of most cancer treatments and work, for the most part, by damaging or inhibiting the synthesis of cellular DNA. As a rule, tumor cells that are sensitive to these forms of treatment undergo apoptosis, or autonomous cell death, whereas those that are resistant typically do not, owing in part to their inability to activate apoptotic programs (69). The ability of tumor cells to detect and respond to radiation or drug-induced DNA damage is not yet fully understood, but a connection between the p53 tumor suppressor protein and the capacity of these cells to initiate DNA damage-induced cell death has been well established (69).
In some cancers, the activation of p53 as a transcription factor in response to radiation or other DNA-damaging agents does not always lead to apoptosis but rather to cell cycle arrest (40, 58). The decision of a cell to enter cell cycle arrest or an apoptotic pathway rests heavily on a number of factors (69), and when the former prevails, the block usually occurs at both the G1/S and G2/M transitions of the cell cycle (70). These checkpoint responses are partially caused by the cyclin-dependent kinase inhibitor p21, which is transcriptionally induced by p53 following DNA damage (10, 17, 54, 78). This protein primarily binds to and inhibits cyclin E-, and A-dependent kinases (Cdks), both of which are important to S phase (53). Key substrates for cyclin E-Cdk2 include prereplication complexes and the retinoblastoma protein Rb, which acts to constrain the G1/S transition of normal cells while in its hypophosphorylated form (57). As with p53, the function of Rb is frequently lost in many human cancers (70), and this may be the reason why some cells fail to induce critical checkpoints after DNA damage, whether intrinsic or otherwise. For example, Rb−/− mouse embryo fibroblasts (MEFs) and Rb-negative human cell lines fail to arrest at the G1/S checkpoint after treatment with radiation or chemotherapeutic agents, notwithstanding the activation of p53 and an increase in the level of p21 (8, 29, 54). In addition, although the Rb-negative cells arrest in G2/M, a significant proportion of these cells undergo endoreduplication (a round of DNA replication without mitosis), indicating that Rb may also be important in preventing DNA replication in p21-induced G2/M-arrested cells (54). Finally, the notion that Rb acts downstream of p53 in response to DNA damage (8, 29, 54) is corroborated by the fact that the human papillomavirus E7 protein can override a p53-induced G1/S arrest (50, 72) without affecting the p53 → p21 response pathway (30), and this done is partly by interacting with Rb (74). Eventually these E7-expressing cells under conditions of drug-induced DNA damage undergo an apoptotic response with the retention of a phosphorylated Rb (30).
Nontumorigenic cells, such as fibroblasts (human or rat) and MEFs, do not readily undergo apoptosis when exposed to radiation or many anticancer agents, despite the accumulation of p53, which in this case functions to promote cell cycle arrest, or Bax (50), a proapoptotic member of the Bcl-2 family (64). These cells, however, can be sensitized to this form of treatment by the adenovirus E1A protein, making them susceptible to p53-mediated apoptosis (2, 43, 67). E1A can also promote apoptosis in normal fibroblasts or MEFs, and profoundly so under conditions of mitogen deficiency (2, 16, 42, 63). In this situation, E1A appears to stabilize p53 through a mechanism which is dependent on ARF (murine p19ARF; human p14ARF) (19, 42, 62). In effect, ARF, which is induced by the transcription factor E2F1 (5), inhibits the activity of MDM2 (59, 85), which negatively regulates p53 (61).
The signaling to p53 following DNA damage is separate and independent of ARF (41) and is mediated, in part, by kinases such as ATM and Chk2, which phosphorylate p53 on Ser-15 and Ser-20, respectively (3, 11, 12). The phosphorylation of p53 on Ser-20 apparently weakens the association between p53 and MDM2 (13, 31), and this in turn affects the stability of p53. The notion that E1A and DNA damage can activate p53 through distinct mechanisms is supported by the lack of phosphorylation of p53 on Ser-15 in cells expressing E1A (19).
The mechanisms by which E1A makes cultured cells sensitive to the induction of apoptosis by anticancer agents are not yet fully understood, although there is some evidence that the Rb protein and the transcriptional coactivator p300 (also called CBP) may be involved in this process (38, 67, 73). However, others have argued that the binding of p300 by E1A may be dispensable for apoptosis (15). It has also been reported that E1A-dependent apoptosis requires the activation of caspase-9 through the release of cytochrome c (24). However, it has been asserted that this pathway may not be sufficient for E1A to induce drug sensitivity and that a second pathway is also required, one that may involve cell cycle repressors (20). In considering this, it is interesting that the p21 protein has been shown to have a protective influence over apoptosis in DNA-damaged cells (2, 58, 79). With this in mind, and the fact that we and others have recently shown that E1A can bind directly to p21 (34, 36, 47) and its related inhibitor p27 (49), we examined this interaction in the context of E1A's ability to promote apoptosis and chemosensitivity in cells after treatment with a chemotherapeutic agent. In particular, we studied by flow cytometry a variety of E1A mutants (Table 1) in terms of their abilities to induce an apoptotic response in a human diploid carcinoma cell line after DNA damage. This detailed analysis enabled us to demonstrate for the first time that E1A can promote apoptosis in these cells by affecting the p21 pathway, although there are indications that other pathways may also be functioning in this process.
TABLE 1.
Binding properties of E1A mutants
| Mutant | Bindingc
|
||
|---|---|---|---|
| p300 | Rb | p21 | |
| E1A.928 | +ab | −ab | +b |
| E1A.RG2 | −ab | +ab | +b |
| E1A.dl26-35 | +ab | +ab | −b |
| E1A.dl26-35/928 | +b | −b | −b |
Data obtained from previous work (4,80).
Work described here and unpublished results of E1A interactions with p300 and Rb in HCT116 cells (D. Chattopadhyay).
+, positive for binding to a cellular protein; −, negative for binding to a cellular protein.
MATERIALS AND METHODS
Cell lines, transfections, and doxorubicin treatment.
HCT116 p21+/+, p21+/−, and p21−/− cells were kindly provided by B. Vogelstein (Johns Hopkins University School of Medicine, Baltimore, Md.) (78). These cell lines were maintained in McCoy's 5A medium supplemented with 10% fetal bovine serum and penicillin or streptomycin. For transient transfections, HCT116 cells seeded on 10-cm-diameter dishes were separately transfected with the indicated plasmids (15 μg) at a confluency of about 70%, using the Lipofectamine (GIBCO/BRL) method. The transfection efficiency under these conditions was about 20%.
Transfected and untransfected cells at 70% confluency were treated with the chemotherapeutic agent doxorubicin (Adriamycin) at a concentration of 0.2 μg/ml for various times.
Plasmids, protein purification, and transfections.
Mammalian expression vectors (pcDNA3; Invitrogen) containing cDNAs downstream of the cytomegalovirus promoter and encoding wild-type E1A12S, E1A.928, and E1A.RG2 have been described previously (47, 49). A cDNA encoding the E1A mutant dl26-35, derived from plasmid dl1102 (4), was subcloned into the pcDNA3 vector containing wild-type E1A12S. The pCMV expression vectors for CD20 (77), Cdk2DN (77), and glutathione S-transferase (GST)-Rb (379-928) (23) have also been described previously. Induction of the GST-Rb (379-928) fusion protein in Escherichia coli (strain BL21) by IPTG (isopropyl-β-d-thiogalactopyranoside) and its purification using glutathione-Sepharose beads (Pharmacia) was reported elsewhere (48). Once purified, GST-Rb (379-928) was quantitated by using the Bradford assay (Bio-Rad) and analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gels before use.
Antibodies, immunoblotting, and immunoprecipitations.
Anti-p21 (C19), anti-Cdk2 (M2), anti-cyclin E (M20), anti-cyclin A (H432), and anti-Cdk4 (C22) were from Santa Cruz Biotechnology. The monoclonal antibody M73 (28) and the fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody CD20Leu16 (Becton Dickinson) were used to identify the E1A proteins and the cell surface marker CD20, respectively. Anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase), which served as a control for protein loading, was purchased from BioDesign.
Immunoblot analysis was performed as previously described (47, 49). Briefly, whole-cell extracts (50 μg) were separated on SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. The membrane was then incubated with a primary antibody at a concentration specified by the manufacturer. After being washed in buffer, the membrane was incubated with a peroxidase-coupled secondary antibody and then developed by using the ECL chemiluminescence reagent (Amersham).
Preparation of whole-cell extract for immunoprecipitations was carried out as described previously (47, 49). To examine the amount of p21 bound to E1A or cyclin-Cdk2, 0.5 to 1.5 mg of transfected cell lysate was immunoprecipitated with anti-E1A (M73) or anti-Cdk2. Immune complexes of E1A or Cdk2 were then resolved on an SDS–12% polyacrylamide gel, and bound p21 was visualized by immunoblot analysis (described above) using antibodies against p21.
Kinase activity assays.
To determine kinase activity in doxorubicin-treated HCT116 cells with or without wild-type or mutant E1A, whole-cell extract (50 μg), prepared as previously described (47, 49), was immunoprecipitated with anti-Cdk2. Immune complexes of Cdk2 were washed as previously described (47, 49), and afterwards, the beads were resuspended in 15 μl of kinase buffer containing histone H1 (0.5 μg), 25 μM cold ATP, and 5 μCi of [γ-32P]ATP. After the mixtures were incubated for 25 min at 30°C, the reactions were terminated by 2× sample buffer, and the phosphorylated products were then analyzed on an SDS–8% polyacrylamide gel and visualized by autoradiography.
Flow cytometric analysis and deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays.
Total populations of transfected cells, including floating and adherent cells, were stained for CD20 expression, fixed in 80% ethanol, and stained for DNA content (with propidium iodide) as previously described (56). The staining of cells for E1A expression was performed according to published procedures (66). Briefly, cells were fixed in 80% methanol and then blocked with fetal bovine serum in phosphate-buffered saline. Afterwards, the cells were stained with anti-E1A (M73), washed twice in phosphate-buffered saline, and incubated with a goat anti-mouse FITC-conjugated secondary antibody. The cells were then treated with 50 μg of RNase A/ml and 50 μg of propidium iodide/ml. Samples were analyzed in a cell sorter (FACScan), and the cells were measured for their FITC (green channel) and propidium iodide (red channel) fluorescence intensities. Total populations were gated to remove doublets and small debris, and cells transfected with empty vector were used to establish the background levels of FITC fluorescence for unbiased analysis of E1A- or CD20-expressing cells. Cell cycle analysis of E1A-expressing cells against nonexpressing cells from each transfection was carried out using ModFit LT software (Verity Software House, Inc.). Analysis of cells for their sub-G1 DNA content or endoreduplication was performed with CellQuest software (Becton Dickinson).
TUNEL staining of the E1A-expressing cells was performed with the Apo-BrdU kit (Phoenix Flow Systems Inc.) as specified by the manufacturer's instructions. The cells were then processed for immunofluorescence using anti-E1A (M73), 4,6 diamidino-2-phenylindole (DAPI), and an anti-bromodeoxyuridine monoclonal antibody conjugated to FITC (Boehringer), as previously described (18, 47). The secondary antibody for E1A staining was Texas red-conjugated goat anti-mouse immunoglobulin G (Jackson Laboratory). Specimens for immunofluorescence were examined using a Nikon Optiphot-2 fluorescence microscope and then digitally captured (Oncor Video Imaging System).
RESULTS
Previous studies have indicated that the adenovirus E1A protein can sensitize cells to apoptosis following their exposure to ionizing radiation or other DNA-damaging agents (2, 43, 67). To determine whether the Cdk inhibitor p21 might be important to E1A in increasing cellular sensitivity to these chemotherapeutic agents, we used an isogenic set of human colon carcinoma cell lines (HCT116) which differ only in their p21 status due to homologous recombination (78). The parental HCT116 p21+/+ cell line (hereafter referred to as p21+/+) and the derivatives, HCT116 p21+/− (p21+/−) and HCT116 p21−/− (p21−/−), were chosen for the following reasons. Foremost, HCT116 cells are near diploid and express an apparent wild-type p53 and Rb protein (52, 75, 78). Furthermore, the p14ARF gene has been shown to be defective in these cells (52, 83), and although this protein does not appear to participate in the p53 response to DNA damage (41), it nevertheless eliminates the possibility of p14ARF promoting a p53-dependent cell cycle arrest or apoptosis in response to E1A, as has been previously shown in other cells lines in the absence of serum (19).
Doxorubicin affects the cell cycle distribution of p21+/+ and p21−/−.
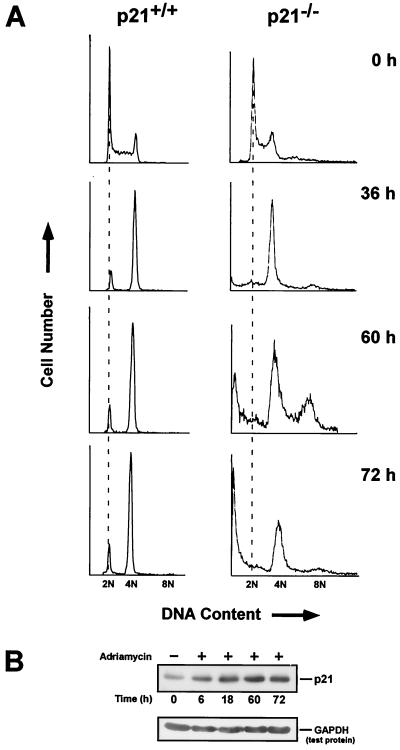
Doxorubicin is a chemotherapeutic drug which specifically interferes with the enzyme topoisomerase II and as a result stabilizes “cleavable complexes” of this enzyme with DNA (39). For purposes of standardization, we initially analyzed the effects of doxorubicin on p21+/+ and p21−/− cells as a function of time. Various studies have characterized the behavior of these cell lines toward this drug, and normally, they both exhibit a smaller number of cells in G1 than in G2 upon sustaining DNA damage (25, 78, 79). We found this to be the case as well (Fig. 1A). For example, after 36 h of doxorubicin treatment, the p21+/+ cells were exclusively blocked in G1 and G2, whereas the p21−/− cells were predominantly blocked in G2. With longer treatments (60 and 72 h), the cell cycle distribution of the p21+/+ cells remained relatively unchanged, while that of the p21−/− cells began to acquire a DNA content greater than 4N and to display evidence of undergoing apoptosis.
FIG. 1.
Cell cycle arrest of p21+/+ and p21−/− cells after exposure to doxorubicin. (A) Asynchronous cultures of p21+/+ and p21−/− cells were treated with doxorubicin for the indicated times and then stained with propidium iodide. The DNA content was examined by flow cytometry, and each plot represents the analysis of 10,000 events. The DNA contents of G1 and G2 are denoted as 2N and 4N, respectively. (B) Western blot analysis of p21+/+ cells after treatment with doxorubicin and probing with a p21-specific antibody. GAPDH served as a loading control. +, present; −, absent.
The pattern of accumulated p53 and p21 in DNA-damaged cells is also reflected in p21+/+ cells after treatment with doxorubicin for 14 h (22). However, upon closer examination, elevated levels of p21 protein can be seen as early as 6 h, reaching a maximum by 18 h and remaining at this level for the indicated times (Fig. 1B). As reported previously (79), expression of p21 did not affect the morphology of these cells, even after 60 h (data not shown).
The affect of doxorubicin on cell cycle proteins in p21+/+ and p21−/− cells.
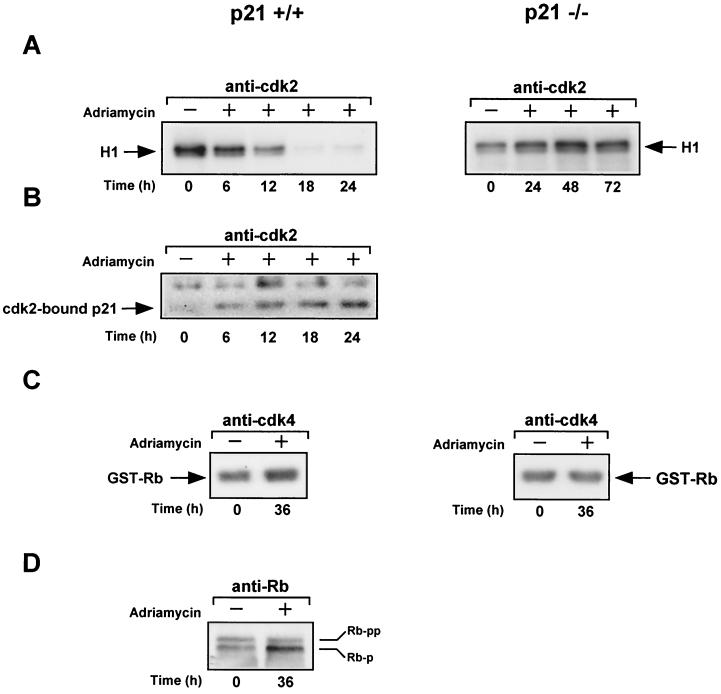
Because p21 universally inhibits cyclin-Cdk complexes (71), we compared the levels of Cdk2- and Cdk4-associated kinase activity in untreated and doxorubicin-treated p21+/+ cells. p21−/− cells, which were subject to the same protocol, were also evaluated for this activity. Cell extracts were prepared from both of these cell lines at different times after treatment with or without doxorubicin, and equal amounts were immunoprecipitated with either anti-Cdk2 or anti-Cdk4 antibody for the removal of cyclin-Cdk2 or -Cdk4 complexes. As shown in Fig. 2A, Cdk2-associated histone H1 kinase activity rapidly diminished (within 6 h) in the doxorubicin-treated p21+/+ cells but not in the p21−/− cells. The reduction in Cdk2-associated kinase activity observed in the p21+/+ cells correlates faithfully with an increase in immune complexes of cyclin E (22) or Cdk2 containing p21, which were obtained at different times after doxorubicin treatment (Fig. 2B). We suspect, therefore, that the loss of Cdk2-associated kinase activity in the doxorubicin-treated p21+/+ cells is most likely due to the accumulation of p21, particularly since the levels of cyclin E, cyclin A, and Cdk2 show no evidence of declining in these cells (data not shown). As expected, immune complexes of Cdk2 recovered from p21−/− cells after periods of doxorubicin treatment showed no evidence of Cdk2-bound p21 (data not shown). Finally, even though Cdk4-associated Rb kinase activity remained relatively unaffected in both the p21+/+ and p21−/− cell lines after exposure to doxorubicin (Fig. 2C), the p21+/+ cells displayed a prominent hypophosphorylated form of endogenous Rb (Fig. 2D), with little or no detectable change in the phosphorylation of Rb in p21−/− cells (data not shown). The observed increase in the amount of hypophosphorylated Rb is most likely because of the level of Cdk2-associated kinase activity, which becomes severely reduced in these cells. This result is mirrored by the finding that γ-irradiation of MEFs also leads to the inhibition of phosphorylated Rb, specifically at Cdk2 but not Cdk4 sites and in a p21-dependent manner (8). We conclude that the reduced levels of cyclin-Cdk2 activity observed in the p21+/+ cells after doxorubicin treatment is due to the binding of p21, and although we have no direct proof, this loss is likely to have an affect on the biochemical activities of Rb.
FIG. 2.
Effect of doxorubicin on the expression of G1 cyclin, Cdk2, and Cdk4 activities in p21+/+ and p21−/− cells after doxorubicin treatment. (A) Normalized extracts from untreated or doxorubicin-treated p21+/+ and p21−/− cells were immunoprecipitated with anti-Cdk2. The immune complexes were then assayed for associated kinase activity by incubation with [γ-32P]ATP and the substrate histone H1. (B) Same as described for panel A except the contents of immune complexes derived from p21+/+ cells were examined for p21 by Western blot analysis using anti-p21 as a probe. (C) Same as described for panel A except immune complexes of Cdk4 were examined for associated kinase activity using GST-Rb as a substrate. (D) Normalized extracts from untreated or doxorubicin-treated p21+/+ cells were assessed by Western blot analysis using anti-Rb as a probe after immunoprecipitation. +, present; −, absent.
E1A targets p21 in doxorubicin-treated p21+/+ cells.
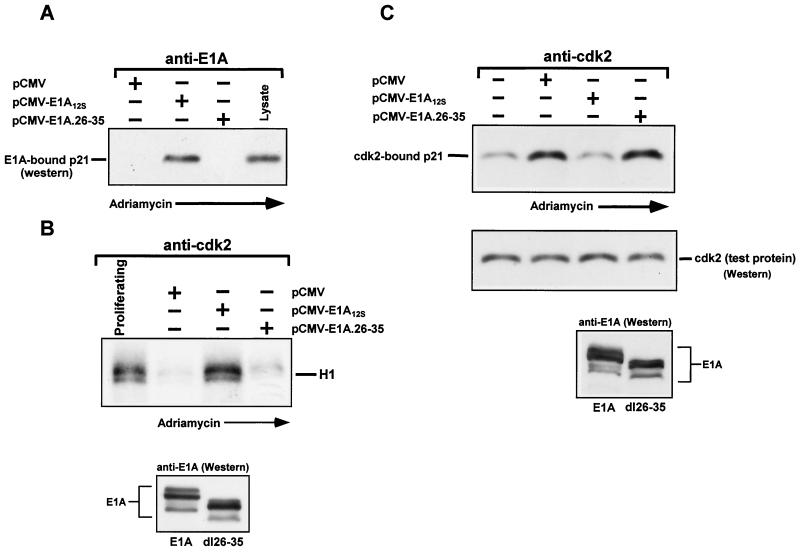
As we have shown previously, E1A can restore kinase activity by targeting the Cdk-inhibitory proteins p21 and p27 in terminally differentiated muscle cells or in epithelial cells treated with transforming growth factor β, respectively (47, 49). In view of this, and given the evidence presented above, we decided to examine whether E1A could also neutralize the inhibitory effect of p21 on Cdks in doxorubicin-treated p21+/+ cells. Therefore, p21+/+ cells were separately transfected with an empty vector (minus E1A) or with expression plasmids encoding either wild-type E1A or an E1A mutant (E1A.dl26-35) that showed no evidence of binding to p21 in vitro, as judged by a GST pull-down experiment (data not shown). Shortly thereafter, doxorubicin was added to the cultures for various periods. As shown in Fig. 3A, wild-type E1A was highly efficient in associating with p21 in doxorubicin-treated cells, whereas the E1A.dl26-35 mutant was not. Moreover, this mutant, as well as an E1A mutant failing to bind both p21 and Rb (E1A.dl26-35/928), was also unable to restore kinase activity to cyclin-Cdk2 complexes, unlike wild-type E1A (Fig. 3B) or the rest of the E1A mutants listed in Table 1 (data not shown). Finally, although wild-type E1A and the E1A mutants failing to bind p300 or Rb were repeatedly able to reduce the amount of p21 in association with cyclin-Cdk2 complexes in doxorubicin-treated p21+/+ cells, the E1A.dl26-35 mutant was unable to perform this activity (Fig. 3C and data not shown). It is important to note that E1A had no apparent effect on the accumulation of p21 in the doxorubicin-treated p21+/+ cells or, for that matter, on the levels of cyclin E, cyclin A, and Cdk2, which were identical to the levels observed in DNA-damaged cells without E1A (data not shown). The fact that increased levels of p21 remain virtually unperturbed by E1A in this context is highly consistent with what others have previously observed in that p53-mediated induction of p21 in cells stably expressing either an E1A or human papillomavirus type 16 E7 protein remain unaffected after DNA damage as well (9, 33). Likewise, the accumulation of p21 in an E1A-inducible murine cell line as a result of doxorubicin treatment also remains unaltered after E1A is expressed in these cells (M. Ghosh and M. L. Harter, unpublished results).
FIG. 3.
E1A expressed in doxorubicin-treated p21+/+ cells associates with p21 and reduces the amount of p21 in association with cyclin-Cdk2 complexes, thereby restoring their activity. (A) p21+/+ cells were transfected in parallel with pCMV-E1A12S, pCMV-E1A.26-35, or the control plasmid pCMV. Immediately after, doxorubicin was added to the cultures, and at 36 h posttransfection, the cells were collected and whole-cell extract was prepared. Normalized extracts were immunoprecipitated with anti-E1A, and immune complexes, along with lysate (to mark p21), were then subjected to Western blot analysis and enhanced chemiluminescence, using anti-p21 as a probe. (B) Cdk2-associated kinase activity in transfected p21+/+ cells with doxorubicin treatment for 36 h was determined as for Fig. 2. In the lower blot, the extracts used for panels A and B were subjected to Western blot analysis using anti-E1A as a probe. (C) Normalized extracts from transfected or untransfected p21+/+ cells with or without doxorubicin treatment for 36 h were immunoprecipitated with anti-Cdk2. The immune complexes were then examined for the presence of p21 by Western blot analysis using anti-p21 as a probe. The membrane was also probed with anti-Cdk2 (middle blot) to assure equal loading of the immunoprecipitated products. The same extracts were also subjected to Western blot and chemiluminescence analysis, using anti-E1A as a probe (bottom blot). +, present; −, absent.
G1 checkpoint established by doxorubicin in p21+/+ cells is abrogated by the expression of E1A.
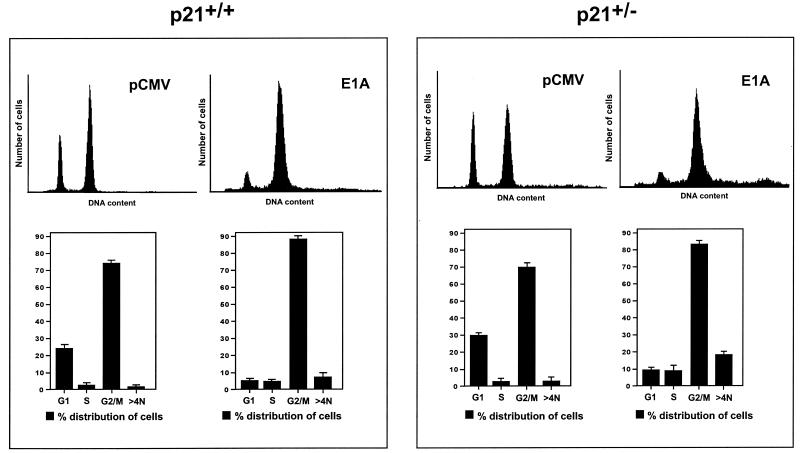
As described above, one of the consequences of p21 expression in doxorubicin-treated p21+/+ cells is the inhibition of Cdk2 activity (Fig. 2 and 3), a phenomenon that has also been observed in human diploid fibroblasts after γ-irradiation (21). Although direct evidence is lacking, the role of p21 in this context could partially explain its well-known ability to mediate a G1 arrest in DNA-damaged cells. In view of this corollary, and the fact that E1A can counter the inhibitory effect of p21 on Cdks, we determined whether E1A could overcome the G1 arrest observed in doxorubicin-treated p21+/+ cells (Fig. 1A). Therefore, p21+/+ or p21+/− cells transfected with wild-type E1A and treated with doxorubicin for 36 h were stained directly with an anti-E1A monoclonal antibody (M73) for E1A expression and with propidium iodide for DNA content; these cells were then assayed for cell cycle distribution by flow cytometry. As shown in Fig. 4, both of these cell lines, which were sorted on the basis of E1A expression, exhibited a dramatic reduction in the number of cells in G1 relative to cells transfected with the empty vector (minus E1A). Furthermore, a proportion of cells with a DNA content greater than 4N was reproducibly more evident in the population of p21+/− cells than in the p21+/+ cells. The ability of E1A to enhance what appears to be endoreduplication in the p21+/− cells may simply be a reflection of the fact that these cells have only one normal copy of the p21 gene (79) and therefore half the amount of p21 protein (78). This may also be one reason why these cells display a slight reduction in the proportion of cells in G2, as well as a broader G2 peak, than the E1A-expressing p21+/+ cells (Fig. 4). If this is true, it would suggest that when p21 is limited or minimal, its efficiency in negatively regulating the G2/M transition in DNA-damaged cells (10, 54) might be compromised. Finally, in contrast to the doxorubicin-treated p21+/+ and p21+/− cells, E1A had very little effect, if any, on the cell cycle distribution of p21−/− cells under the same conditions, and identical results were obtained when p21+/+ or p21+/− cells were cotransfected with the CD20 expression vector and sorted on the basis of CD20 expression (data not shown).
FIG. 4.
E1A overcomes G1 arrest induced in p21+/+ or p21+/− cells after doxorubicin treatment. p21+/+ and p21+/− cells were transfected in parallel with pCMV-E1A12S or the control plasmid pCMV, and immediately after, doxorubicin was added to the cultures. At 36 h posttransfection, the cells were collected and stained with propidium iodide and anti-E1A for DNA content and E1A expression, respectively. The cell cycle distribution of these cells was assessed by flow cytometry, as described in Materials and Methods. The gates were established with cells containing empty vector (pCMV), thereby allowing the analysis of only E1A-expressing cells. Each plot represents the analysis of 20,000 gated events. The data, from at least three different experiments, are presented in histograms which show the percentage of cells with G1 (2 N), S, G2/M (4N), and >4N DNA content, respectively. Note the presence of a sizeable population of doxorubicin-treated cells with greater-than-4N DNA content in the p21+/− cells transfected with E1A. The error bars indicate standard deviations.
E1A requires p21 to overcome a G1 arrest in doxorubicin-treated cells.
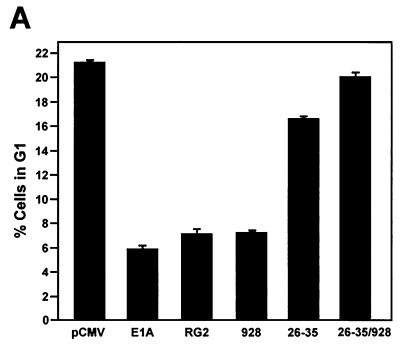
To elucidate the basis of E1A's ability to overcome a G1 arrest in doxorubicin-treated p21+/+ cells, cell cycle profiles of p21+/+ cells transiently transfected, in parallel, with empty vector and vectors expressing either wild-type E1A or one of the E1A mutants (Table 1) were examined after 36 h of doxorubicin treatment. Analysis of the E1A-expressing cells by flow cytometry clearly showed that the E1A mutants failing to bind only p300 and Rb were slightly less efficient than wild-type E1A in abrogating G1 arrest in these cells (Fig. 5A). By comparison, the E1A.26-35 mutant failing to bind only p21 was unsuccessful in overcoming a G1 arrest, and cells expressing a double E1A mutant (E1A.26-35/928) retained a cell cycle profile that was almost identical to that seen in cells transfected with empty vector. The fact that the G1 arrest in some cells could not be affected by E1A suggests that there may have been insufficient restoration of Cdk2 activity within a subpopulation, possibly due to cell-to-cell variation in E1A expressions levels.
FIG. 5.
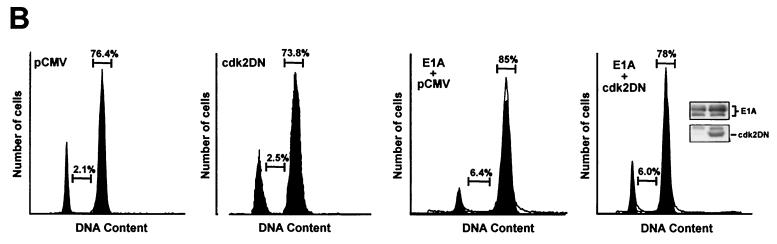
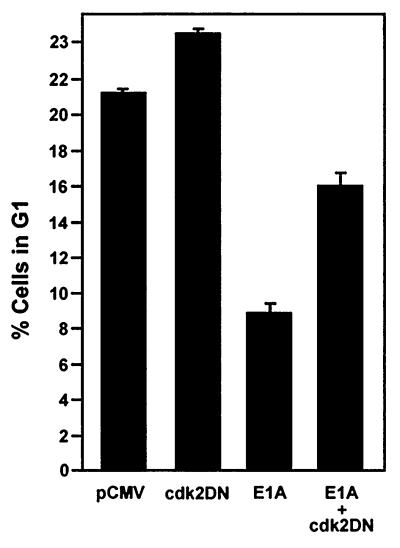
E1A requires p21 and the restoration of Cdk2 activity to overcome G1 arrest induced in p21+/+ cells after doxorubicin treatment. (A) p21+/+ cells were transfected in parallel with pCMV-E1A12S, E1A.RG2, E1A.928, E1A.dl26-35, E1A.dl26-35/928, or the control plasmid pCMV. Doxorubicin was added to the cultures immediately after, and at 36 h posttransfection, the cells were collected and stained with propidium iodide and anti-E1A for E1A expression. The cell cycle distribution of these cells was assessed by flow cytometry, as described in the legend to Fig. 4. (B) p21+/+ cells were transfected or cotransfected in parallel with a control plasmid (pCMV) and with pCMV and pCMV-E1A12S (4:1) or with Cdk2DN and pCMV-E1A12S (4:1), respectively. Afterwards, the cells were treated with doxorubicin, and at 36 h posttransfection, the cells were collected and processed for flow cytometry as described for panel A. The inset shows the expression of E1A and the coexpression of E1A and Cdk2DN in the doxorubicin-treated cells, as judged by Western blot analysis and the use of anti-E1A and anti-Cdk2. The histogram under the flow cytometry plots shows the percentage (+ standard deviation) of cells in a G1 population and is representative of three independent experiments.
The results of the experiments described above strongly indicate that E1A requires the p21 protein to overcome G1 arrest in doxorubicin-treated p21+/+ cells. In principle, this could be in harmony with E1A's ability to displace p21 from cyclin-Cdk2 complexes and thereby liberate their activity (Fig. 3), a function that could be sufficient for executing the initiation of DNA replication. If this is true, then coexpression of E1A with a dominant-negative form of Cdk2 (Cdk2DN), which has been previously shown to inhibit Cdk2 activity under a variety of conditions (46, 77), might counteract this effect. Therefore, p21+/+ cells were transfected with an expression vector for wild-type E1A together with either an empty vector or a vector encoding Cdk2DN. The cells were then treated with doxorubicin, and 36 h posttransfection were stained for the expression of E1A and DNA content for analysis by flow cytometry. As shown in Fig. 5B, cells expressing only E1A were again showing a reduction in the number of cells in G1 compared to cells without E1A. More importantly, though, cotransfection of Cdk2DN reversed the effects of E1A in the doxorubicin-treated p21+/+ cells: the arrest in G1 was largely restored, and in contrast to what was found in cells with E1A alone (Fig. 2), there was no significant increase in the number of cells in G2, at least at this time point. Nevertheless, there were indications that DNA synthesis may have initiated in these cells, with the proportion of cells distributed in S phase being almost identical to that seen in the doxorubicin-treated cells with E1A. Because Cdk2DN alone has no apparent effect on the number of cells in S phase (Fig. 5B), this observation could be due to a small number of cells which are capable of escaping the effects of Cdk2DN in reversing E1A-released Cdk2 activity, thus moving into S phase, but with a lower rate of DNA synthesis, since there was no evidence for a complete round of DNA replication. Alternatively, it could be a result of E1A functioning in yet another capacity, specifically in abrogating an S phase checkpoint which does not require p21 and which has been previously described in both normal and human cancer cells in response to DNA damage (1, 36, 82). Although we have no direct proof, doxorubicin-treated p21+/+ cells, apart from arresting in G1 and G2, could also be arresting in S phase. Hypothetically, if E1A were able to disrupt the checkpoint operating in S phase, then it would stand to reason that it would most likely require a cellular protein other than p21. It is possible, and indeed likely, that this protein is Rb, since it appears to function not only in arresting cells during G1 but during S phase as well, as evidenced under a variety of conditions (14, 35, 45). Taken together, these data indicate that although E1A cannot directly affect a G2 arrest in doxorubicin-treated p21+/+ cells, at least on a short-term basis, it can overcome a G1 arrest by restoring Cdk2 activity. Perhaps more importantly, though, the data provide the first direct evidence that the G1 arrest observed in DNA-damaged p21+/+ cells is mediated, at least in part, by the specific loss of Cdk2 activity.
Induction of apoptosis by E1A in doxorubicin-treated p21+/+ cells is largely dependent on p21.
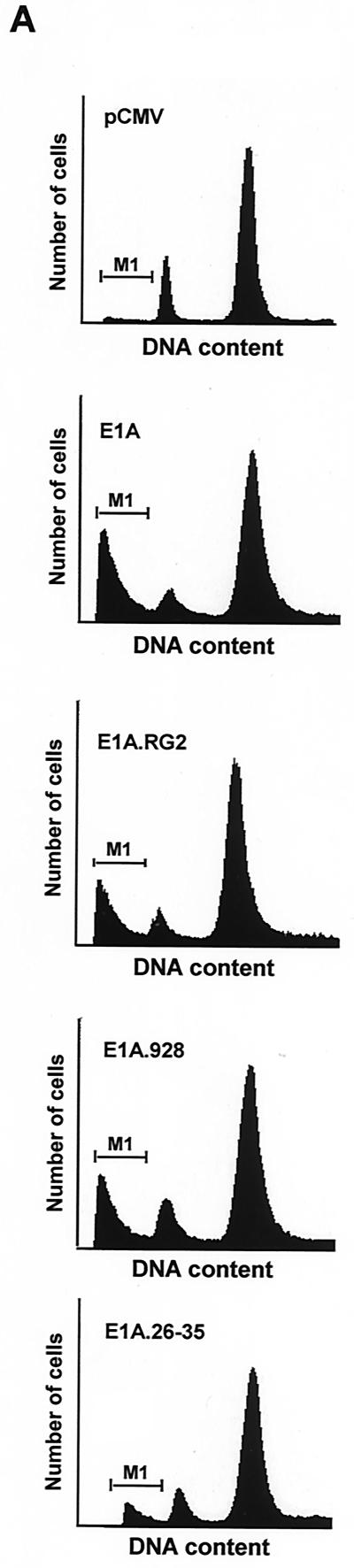
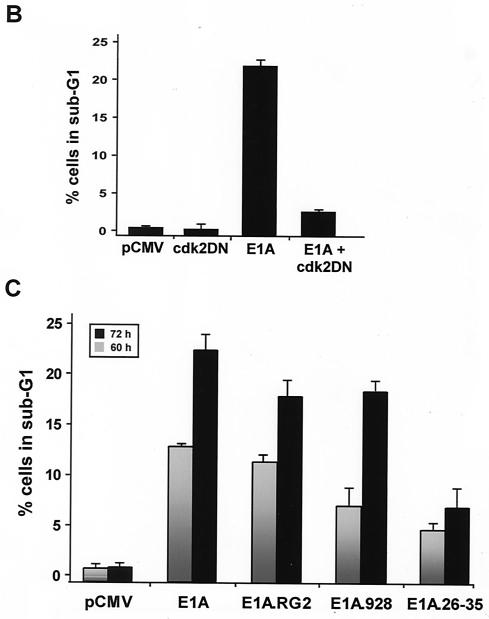
A major difference between p21+/+ cells and p21−/− cells in response to doxorubicin treatment is that the latter cells cannot sustain a G2 arrest beyond 36 h and eventually undergo apoptosis (Fig. 1) (79). In view of this result, and the fact that these two cell lines are virtually isogenic, others were quick to argue that p21 could function in suppressing p53-mediated apoptotic processes induced by DNA damage (79). Indeed, a large body of work (26, 44, 76), including the fact that p21 can protect skeletal muscle cells against apoptosis (81), has now rendered this notion real. From this perspective, and given E1A's ability to target and thereby affect the activity of p21 in DNA-damaged p21+/+ cells (Fig. 3 and 4), it was reasonable to assume that p21's function of inhibiting apoptosis may be compromised once E1A was expressed in these cells, principally as a function of time. Therefore, an analysis of the cell cycle distribution of DNA-damaged cells expressing either wild-type or mutant E1A for periods of 60 and 72 h was undertaken with the use of flow cytometry. In these experiments, an empty vector or plasmids encoding wild-type or mutant E1A were cotransfected with a plasmid expressing a cell surface marker (CD20), and the rate of apoptosis rather than total apoptosis was measured accordingly (66). Examples of the flow cytometric analysis of transfected doxorubicin-treated p21+/+ cells are shown and summarized in Fig. 6A and C. In keeping with E1A's ability to promote drug-induced apoptosis (43, 67), the percentage of cells with sub-G1 DNA contents (indicative of apoptosis) in cells expressing wild-type E1A for 60 and 72 h was substantially higher in cells without E1A. Notably, however, coexpression of the Cdk2DN mutant in this experimental setting had a prominent effect on E1A's ability to induce apoptosis in doxorubicin-treated p21+/+ cells in that there was a significant decrease in the population of sub-G1 cells (Fig. 6B). Key to this investigation is the fact that Cdk2DN alone had no effect on the number of apoptotic cells after doxorubicin treatment compared to the control.
FIG. 6.
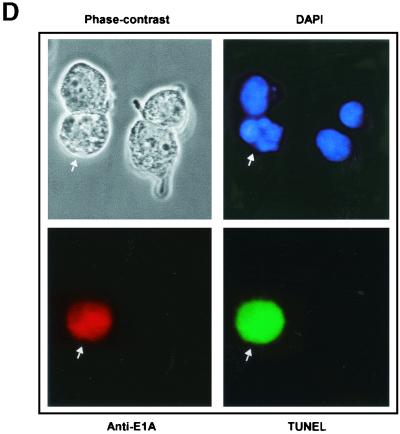
(facing page). Prolonged expression of E1A in doxorubicin-treated p21+/+ cells leads to apoptosis. (A) p21+/+ cells were cotransfected in parallel with the indicated plasmids (pCMV and pCMV-E1A12S, -E1A.RG2, -E1A.928, and -E1A.dl26-35) and a pCMV-CD20 expression vector. Immediately after, the cells were treated with doxorubicin, and at 60 and 72 h posttransfection, the DNA content was analyzed by propidium iodide staining and fluorescence-activated cell sorting analysis of CD20-positive cells. Only normal-size cells with slight loss of DNA content were analyzed, while doublets and debris, resulting from aggregates or necrotic death, were avoided. One representative experiment showing the percentage of cells with a sub-G1 DNA content (M1) at the 72-h time point is indicated. (B) p21+/+ cells were cotransfected with 12 μg of control plasmid (pCMV) or pCMV-Cdk2DN, pCMV and pCMV-E1A12S (8:4), or pCMV-E1A12S and pCMV-Cdk2DN (8:4) along with 2 μg of the pCMV-CD20 expression vector. Immediately after, the cells were treated with doxorubicin for 72 h. Apoptosis was measured as described for panel A, and the percentage of cells with a sub-G1 DNA content is summarized in a histogram. (C) Percentage of cells with a sub-G1 DNA content based on the data shown in panel A and derived from three separate experiments. (D) p21+/+ cells were transfected with pCMV-E1A12S and cultured in the presence of doxorubicin for 72 h. Afterwards, the cells were fixed and triple stained with DAPI and anti-E1A and for TUNEL. A fluorescence microscope analyzed a total of 100 cells, and the number of E1A-expressing cells undergoing apoptosis was about 25 to 30%. Phase contrast of the same field is also shown, and the arrows indicate an E1A-transfected cell in the advanced stages of apoptosis.
This result, together with our previous observations (Fig. 3 and 5), suggests that, in part, the release of Cdk2 activity by E1A is indeed an important requirement for rendering DNA-damaged cells sensitive to apoptosis.
At 60 and 72 h posttransfection, the E1A mutant failing to bind p300 (E1A.RG2) was only slightly less efficient than wild-type E1A in inducing apoptosis in the doxorubicin-treated p21+/+ cells, whereas the E1A mutant failing to bind p21 was quite ineffective (Fig. 6A and C). Of interest, however, is the fact that although the E1A mutant failing to bind Rb (E1A.928) was almost as effective as wild-type E1A in inducing apoptosis in these cells 72 h posttransfection, the kinetics of cell death as a result of these two proteins were not entirely identical, since early on the rate of apoptosis was about twofold greater in cells expressing wild-type E1A than in those expressing the E1A.928 mutant. However, over time these rates became almost identical. Among the possibilities in explaining this observation is the potential for some form of Rb to be functioning in a pathway other than that of p21 (8, 29). Thus, to become functionally inactivated, at least in this context, Rb may require a direct interaction with E1A. Indeed, we find that E1A is quite capable of associating with the partially phosphorylated form of Rb that is present in the doxorubicin-treated p21+/+ cells (Fig. 2D and data not shown). Nevertheless, these results provide a clear demonstration that E1A requires p21 to induce apoptosis in p21+/+ cells following treatment with a DNA-damaging agent.
Finally, That the cell death observed in the E1A-transfected cells was indeed a result of apoptosis is evidenced by the fact that many of these cells showed DNA fragmentation, as revealed by the TUNEL reaction, and a phenotype of nuclear blebbing and apoptotic bodies, as visualized by DAPI staining (Fig. 6D). Incidentally, neither of the E1A-transfected p21+/+ or p21+/− cells showed any sign of apoptosis under conditions of non-doxorubicin treatment, indicating that E1A alone cannot promote apoptosis in these cells lines without DNA damage or within this time frame (data not shown).
Expression of E1A in doxorubicin-treated cells leads to endoreduplication.
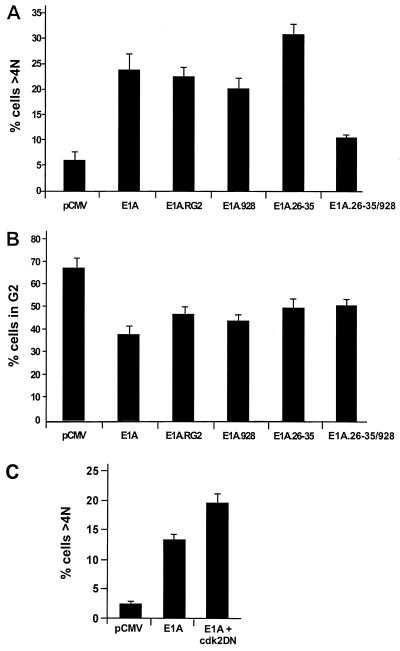
As described above, HCT116 cells lacking p21 eventually undergo endoreduplication followed by apoptosis after arresting in G2 in response to DNA-damaging drugs (Fig. 1A) (79). Since the parental cells with p21 do not behave similarly when subjected to the same regimen, it was of interest to determine whether E1A could cause these cells to undergo phenotypic changes analogous to those observed in the p21−/− cells after doxorubicin treatment. Therefore, p21+/+ cells transfected with CD20 together with empty vector and wild-type E1A or E1A mutants were subjected, as before, to pulse width analysis to allow only individual nuclei having greater than 4N DNA content to be measured. Compared to drug-treated p21+/+ cells without E1A, those expressing wild-type E1A or E1A mutants failing to bind only p300 or Rb exhibited similar increases in the number of cells with a greater-than-4N DNA content (Fig. 7A) and, perhaps more importantly, similar reductions in the number of cells in G2 (Fig 7B). Given this correspondence, the results suggest that any mutant form of E1A that is still capable of binding to p21 can induce doxorubicin-treated p21+/+ cells blocked in a G2-like state to initiate what appears to be a second round of DNA synthesis. Nonetheless, the E1A.26-35 mutant, which cannot bind to p21, was found to be more efficient than wild-type E1A or any of the other E1A mutants in increasing the number of cells with a DNA content of >4N (Fig. 7A). Still, it was almost equally efficient in reducing the number of cells in G2 (Fig. 7B). Because the E1A.26-35 mutant can target and thereby inactivate Rb, whereas the E1A.26-35/928 double mutant cannot (Fig. 7A), these results support a function for Rb in preventing the reinitiation of DNA synthesis in DNA-damaged cells that have arrested in G2 with elevated amounts of p21. This would be consistent with the fact that, compared to E1A alone, a much higher percentage of cells with a DNA content greater than 4N is exhibited in doxorubicin-treated p21+/+ cells after coexpression with the Cdk2DN mutant (Fig. 7C). As expected, there was no change when these cells were transfected with Cdk2DN alone (data not shown). Thus, these results in their entirety reflect the findings of previous experiments, demonstrating the tendency of Rb-negative cells when expressing high levels of p21 to undergo endoreduplication cycles after γ-irradiation (54). Apart from this, however, the marked elevation of endoreduplication observed in these cells after expression of the E1A.26-35 mutant may also coincide with the reported ability of Rb to function in an S phase checkpoint (see above).
FIG. 7.
Expression of E1A in doxorubicin-treated p21+/+ cells leads to a DNA content of >4N. p21+/+ cells were transfected with the indicated expression plasmids together with pCMV-CD20 and immediately after were treated with doxorubicin. At 72 h posttransfection, the cell cycle distribution of CD20-positive cells was analyzed by flow cytometry. An appropriate gating was applied to differentiate individual cells from aggregated cells, and visual inspection indicated that there were no mitotic cells in these populations. The histograms indicate the percentage of cells with DNA contents of 4N (G2) (B) and >4N (A and C), as calculated by Cell Quest software. The error bars indicate standard deviations.
DISCUSSION
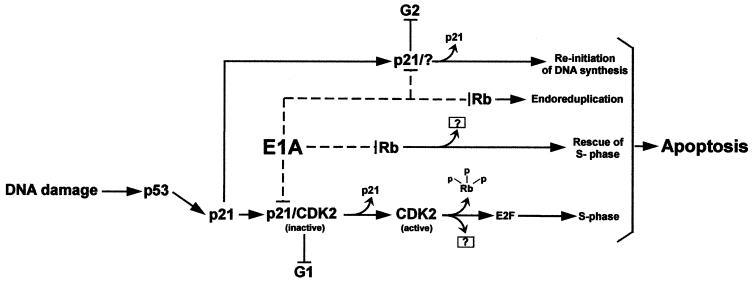
It is well established that the adenovirus E1A protein can enhance the sensitivity of tumor cells to chemotherapeutic agents by promoting apoptosis, but for the most part, the cellular proteins as well as the pathways required for this process are still relatively unknown. This is primarily because of conflicting evidence generated by differences in experimental design, cell types, and, perhaps more importantly, the use of E1A mutants, which were at the time uncharacterized in their ability to bind p21. As demonstrated by our earlier experiments, as well as the experiments presented here, the region on E1A to which p21 binds has now been defined (Fig. 3) (47) and in fact maps adjacent to the site which is responsible for binding p300. Thus, the use of E1A mutants with deletions spanning both of these regions may help to explain why others have argued for a role for p300 in E1A's ability to promote apoptosis and chemosensitivity in DNA-damaged cells, and for that matter, to inhibit p53-mediated p21 transactivation as well (67, 68, 73). However, by using a thoroughly characterized E1A mutant (E1A.RG2) that fails to bind only p300 (Table 1) (80), we were able to show that E1A does not require this protein to enhance chemosensitivity and promote drug-induced apoptosis. Instead, the results presented here clearly demonstrate a role for the p21 protein, as well as an indirect role for Rb (Fig. 8) in allowing E1A to perform this function, at least in a human tumor-derived diploid cell line.
FIG. 8.
Model of E1A-induced apoptosis in DNA-damaged HCT116 cells. Exposure to doxorubicin results in the activation of p53 and, consequently, an increase in the levels of p21. In this cell system, the induced inhibitor (p21) antagonizes Cdk2 activity, leading to G1 arrest, and presumably functions in maintaining G2 arrest as well. Once E1A is introduced into the arrested cells, it targets p21, resulting in the restoration of Cdk2 activity. This in turn completes the phosphorylation of Rb and any other downstream targets that may be involved in promoting inappropriate entry into S phase, followed by apoptosis. The release of E2F from Rb has not been demonstrated in this system, and the model only considers its separate function in inducing cell cycle progression and not apoptosis. The model also suggests a likely but distinct role for E1A in inducing apoptosis by binding directly to Rb. The release of G2 and subsequent apoptosis as a function of E1A's direct effect on p21 in this pathway is speculative, as are the other components of the model which are indicated with question marks.
E1A's requirement for p21 in inducing apoptosis in drug-treated cells raises important questions with regard to the pathways that may be involved in this activity. It is widely believed that p21 is a key player in arresting cells in G1 and G2 after DNA damage (54, 65, 69) and that this correlates, in part, to its inhibitory effect on Cdk2, but not Cdk4, as demonstrated here (Fig. 2) and also recently by others (8, 60). More importantly, inactivation of Cdks by p21 appears to coincide with its ability to protect cells from DNA damage-induced apoptosis (7, 37, 79). In light of this, we would argue that for E1A to create the proper environment for apoptosis to occur in cells that have decidedly arrested in G1 and G2 in response to chemotherapeutic drugs, it must first restore Cdk2 activity by neutralizing a p53-dependent p21. This notion is supported by previous experiments which have suggested that Cdk2 may be necessary for apoptosis. In particular, pharmacologic Cdk2 inhibitors or dominant-negatives Cdks can suppress apoptosis in a variety of cell types after growth factor deprivation (37, 51, 55). As shown here, E1A reduces early on the levels of p21 in association with cyclin-Cdk2 complexes in DNA-damaged cells, resulting in the induction of Cdk2 activity and an increase in the phosphorylation of Rb (Fig. 3 and data not shown). This in turn leads to a decrease in the number of cells in G1, entry into S phase, and an accumulation of cells in G2 (Fig. 4). We suggest that these events may be correlative, since they can be partially reversed by a dominant-negative Cdk2, which interferes directly with Cdk2 activity (Fig. 5). With these findings, therefore, we can now firmly establish a link between Cdk2 activation by E1A and the ability of this viral protein to create a setting for the occurrence of apoptosis in DNA-damaged cells. However, given the complexities of the biochemical pathways required for apoptosis, this is not to say that the restoration of Cdk2 is the only relevant activity for E1A in promoting cell death. Indeed, when considering p21's effect on the G2 checkpoint with respect to preventing the reinitiation of DNA synthesis in the absence of mitosis or cytokinesis (6, 79), we cannot rule out the possibility that E1A may also be perturbing the function of p21 in this context. Consistent with this notion is the fact that we find a decrease in the number of DNA-damaged cells in G2 with protracted periods of E1A expression (Fig. 7B).
The noticeable induction of apoptosis in drug-treated cells expressing an E1A mutant incapable of binding to Rb was somewhat unexpected, particularly since others have shown this protein, although not the other Rb-related proteins (p107 or p130), to be a requirement for E1A in promoting apoptosis and chemosensitivity (67). This incongruity is most likely explained by the fact that, although this mutant cannot bind directly to Rb, it can nevertheless restore Cdk2 activity (reference 47 and data not shown) and therefore indirectly affect various Rb functions because of additional phosphorylation. In this model, therefore, the theory of the role of Rb in the DNA damage response with respect to preventing the initiation of DNA replication (29) remains viable and may be supported by two recent findings. First, Cdk2 activity is apparently required to complete the phosphorylation of Rb (27, 46, 84); second, cyclin E-Cdk2 specifically phosphorylates Rb at a site which ostensibly induces a conformational change, resulting in the release of E2F, which in this case would function in the capacity of activating transcription (27) instead of apoptosis (56). The fact that the apoptotic function of E2F, which can also be inhibited by Rb, is apparently separate from its ability to activate transcription from genes relevant to DNA replication has recently been demonstrated, but only under conditions of p53-independent apoptosis (32, 56). Given these considerations and the dependency of p53 functions in DNA-damaged HCT116 cells, it is unclear whether E2F's apoptotic activity is contributing in any way to E1A's ability to induce apoptosis in these cells. However, since E1A is able to bind directly to Rb in drug-treated HCT116 cells (unpublished results), it is tempting to speculate that this interaction may be operative in alleviating Rb-E2F-mediated transcriptional repression in order to allow E2F-mediated apoptosis to occur in these cells. The notion that active transcriptional repression by an Rb-E2F complex regulates apoptosis has recently been suggested by others (32, 56). In this view, therefore, E1A's ability to promote apoptosis in DNA-damaged cells would not only involve the p21 protein but Rb as well. For that matter, the involvement of Rb might also be in the context of E1A having to interact with Rb in order to have an impact on its possible function in preventing DNA replication in p21 G2-arrested cells (54) and inhibiting S phase completion in DNA-damaged cells (82). Experiments to test this strategy in detail are under way and may provide information relevant to the therapy of cancer cells with a defective Rb and an intact p53 → p21 pathway.
ACKNOWLEDGMENTS
We thank J. Jacobberger for advice and stimulating discussions. We are also very grateful to S. van den Heuvel, S. Mymrik, P. Roychoudhury, and T. Hunter for reagents.
This work was supported by grants to M. L. Harter from the National Institutes of Health (GM54014) and the American Heart Association.
REFERENCES
- 1.Agarwal M L, Agarwal A, Taylor W R, Chernova O, Sharma Y, Stark G R. A p53-dependent S-phase checkpoint helps protect cells from DNA damage in response to starvation for pyrimidine nucleotides. Proc Natl Acad Sci USA. 1998;95:14775–14780. doi: 10.1073/pnas.95.25.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attardi L D, Lowe S W, Brugarolas J, Jacks T. Transcriptional activation by p53, but not induction of p21 gene, is essential for oncogene mediated apoptosis. EMBO J. 1996;15:3693–3701. [PMC free article] [PubMed] [Google Scholar]
- 3.Banin S, Moyal L, Khostravi R, Shieh S-Y, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 4.Barbeau D, Marcellus R C, Bacchetti S, Bayley S T, Branton P E. Quantitative analysis of regions of adenovirus E1A products involved in interactions with cellular proteins. Biochem Cell Biol. 1992;70:1123–1134. doi: 10.1139/o92-158. [DOI] [PubMed] [Google Scholar]
- 5.Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 6.Bates S, Ryan K M, Philips A C, Vousden K H. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–1703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- 7.Bissonnette N, Hunting D J. p21-induced cycle arrest in G1 protects cells from apoptosis induced by UV-irradiation or RNA polymerase 11 blockage. Oncogene. 1998;16:3461–3469. doi: 10.1038/sj.onc.1201899. [DOI] [PubMed] [Google Scholar]
- 8.Brugarolas J, Moberg K, Boyd S D, Taya Y, Jacks T, Lees J A. Inhibition of cyclin-dependent kinase 2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after γ-irradiation. Proc Natl Acad Sci USA. 1999;96:1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulavin D V, Tararova N D, Aksenov N D, Pospelov V A, Pospelova T. Deregulation of p53/p21Cip1/Waf1 pathway contributes to polyploidy and apoptosis of E1A + cHa-ras transformed cells after γ-irradiation. Oncogene. 1999;18:5611–5619. doi: 10.1038/sj.onc.1202945. [DOI] [PubMed] [Google Scholar]
- 10.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 11.Canman C E, Lim D S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siliciano J D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 12.Chehab N H, Malikzay A, Appel M, Halazonetis T D. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 13.Chehab N H, Malikzay A, Stavridi E S, Halazonetis T D. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew Y-P, Ellis M, Wilkie S, Mittnacht S. pRb phosphorylation mutants reveal role of pRB in regulating S phase completion by a mechanism independent of E2F. Oncogene. 1998;17:2177–2186. doi: 10.1038/sj.onc.1202443. [DOI] [PubMed] [Google Scholar]
- 15.Chiou S-K, White E. p300 binding by E1A cosegregates with p53 induction but is dispensable for apoptosis. J Virol. 1997;71:3515–3525. doi: 10.1128/jvi.71.5.3515-3525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 17.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 18.Der S D, Yang Y L, Weissmann C, Williams B R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Stanchina E, McCurrach M E, Zindy F, Shieh S-Y, Ferbeyre G, Samuelson A V, Prives C R M F, Sherr C J, Lowe S W. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duelli D M, Lazebnik Y A. Primary cells suppress oncogene-dependent apoptosis. Nat Cell Biol. 2000;2:859–862. doi: 10.1038/35041112. [DOI] [PubMed] [Google Scholar]
- 21.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 22.El-Deiry W S, Harper J W, O'Connor P M, Velculescu V E, Canman C E, Jackman J, Pietenpol J A, Burrell M, Hill D E, Wang Y, Wiman K G, Mercer W E, Kastan M B, Kohn K W, Elledge S J, Kinzler K W, Vogelstein B. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 23.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J-Y, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 24.Fearnhead H O, Rodriguez J, Govek E E, Guo W, Kobayashi R, Hannon G, Lazebnik Y A. Oncogene-dependent apoptosis is mediated by caspase-9. Proc Natl Acad Sci USA. 1998;95:13664–13669. doi: 10.1073/pnas.95.23.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flatt P M, Tang L J, Scatena C D, Szak S T, Pietenpol J A. p53 regulation of G2 checkpoint is retinoblastoma protein dependent. Mol Cell Biol. 2000;20:4210–4223. doi: 10.1128/mcb.20.12.4210-4223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorospe M, Wang X, Guyton K Z, Holbrook N J. Protective role of p21Waf1/Cip1 against prostaglandin A2-mediated apoptosis of human colorectal carcinoma cells. Mol Cell Biol. 1996;16:6654–6660. doi: 10.1128/mcb.16.12.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harbour J W, Luo R X, Santi A D, Postigo A A, Dean D C. cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:791–798. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 28.Harlow E, Franza B R, Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985;55:533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrington E A, Bruce J L H E, Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc Natl Acad Sci USA. 1998;95:11945–11950. doi: 10.1073/pnas.95.20.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hickman E S, Bates S, Vousden K H. Perturbation of the p53 response by human papillomavirus type 16 E7. J Virol. 1997;71:3710–3718. doi: 10.1128/jvi.71.5.3710-3718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirao A, Kong Y-Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge S J, Mak T W. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh J-K, Fredersdorf S, Kouzarides T, Martin K, Xin L. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- 33.Jones D L, Munger K. Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. J Virol. 1997;71:2905–2912. doi: 10.1128/jvi.71.4.2905-2912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keblusek P, Dorsman J C, Teunisse A F A S, Teunissen H A, van der Eb J, Zantema A. The adenoviral E1A oncoproteins interfere with the growth-inhibiting effect of the cdk-inhibitor p21CIP1/WAF1. J Gen Virol. 1999;80:381–390. doi: 10.1099/0022-1317-80-2-381. [DOI] [PubMed] [Google Scholar]
- 35.Knudsen E S, Buckmaster C, Chen T-T, Feramisco J R, Wang J Y J. Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev. 1998;12:2278–2292. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larner J, Lee H, Hamlin J. Radiation effects on DNA synthesis in a defined chromosomal replicon. Mol Cell Biol. 1994;14:1901–1908. doi: 10.1128/mcb.14.3.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levkau B, Koyama H, Raines E W, Clurman B E, Herren B, Orth K, Roberts J M, Ross R. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of cdk2: role of a caspase cascade. Mol Cell. 1998;1:553–563. doi: 10.1016/s1097-2765(00)80055-6. [DOI] [PubMed] [Google Scholar]
- 38.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 39.Liu L F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 40.Liu T J, el-Naggar A K, McDonnell T J, Steck K D, Wang M, Taylor D L, Clayman G L. Apoptosis induction mediated by wild-type p53 adenoviral gene transfer in squamous cell carcinoma of the head and neck. Cancer Res. 1995;55:3117–3122. [PubMed] [Google Scholar]
- 41.Lowe S W. Activation of p53 by oncogenes. Endocr Relat Cancer. 2000;6:45–48. doi: 10.1677/erc.0.0060045. [DOI] [PubMed] [Google Scholar]
- 42.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 43.Lowe S W, Ruley H E, Jacks T, Housman D E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 44.Lu Y, Yamagishi N, Yagi T, Takebe H. Mutated p21WAF1/CIP1/SDII lacking CDK-inhibitory activity fails to prevent apoptosis in human colorectal carcinoma. Oncogene. 1998;16:705–712. doi: 10.1038/sj.onc.1201585. [DOI] [PubMed] [Google Scholar]
- 45.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 46.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mal A, Chattopadhyay D, Ghosh M K, Poon R Y C, Hunter T, Harter M L. p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J Cell Biol. 2000;149:281–292. doi: 10.1083/jcb.149.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mal A, Piotrkowski A, Harter M L. Cyclin-dependent kinases phosphorylate the adenovirus E1A protein, enhancing its ability to bind pRb and disrupt pRb-E2F complexes. J Virol. 1996;70:2911–2921. doi: 10.1128/jvi.70.5.2911-2921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mal A, Poon R Y C, Howe P H, Toyoshima H, Hunter T, Harter M L. The E1A oncoprotein disables the CDK inhibitor p27Kip1 in TGF-β treated cells. Nature. 1996;380:262–265. doi: 10.1038/380262a0. [DOI] [PubMed] [Google Scholar]
- 50.McCurrach M E, Connor T M F, Knudson C M, Korsmeyer S J, Lowe S W. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meikrantz W, Schlegel R. Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases. J Biol Chem. 1996;271:10205–10209. doi: 10.1074/jbc.271.17.10205. [DOI] [PubMed] [Google Scholar]
- 52.Mitra J, Dai C Y, Somasundaram K, El-Deiry W, Satyamoorthy K S, Herlyn M, Enders G H. Induction of p21WAF1/CIP1 and inhibition of cdk2 mediated by the tumor suppressor p16INK4a. Mol Cell Biol. 1999;19:3916–3928. doi: 10.1128/mcb.19.5.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan D O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 54.Niculescu A B, III, Chen X, Smeets M, Hengst L, Prives C, Reed S I. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park D S, Farinelli S E, Greene L A. Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated PC12 cells and sympathetic neurons. J Biol Chem. 1996;271:8161–8169. doi: 10.1074/jbc.271.14.8161. [DOI] [PubMed] [Google Scholar]
- 56.Phillips A C, Bates S, Ryan K M, Helin K, Vousden K H. Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes Dev. 1997;11:1853–1863. doi: 10.1101/gad.11.14.1853. [DOI] [PubMed] [Google Scholar]
- 57.Planas-Silva M D, Weinberg R A. The restriction point and control of cell proliferation. Curr Opin Cell Biol. 1997;9:768–772. doi: 10.1016/s0955-0674(97)80076-2. [DOI] [PubMed] [Google Scholar]
- 58.Polyak K, Waldman T, He T-C, Kinzler K W, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996;10:1945–1952. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]
- 59.Pomerantz J, Schreiber-Angus N, Liegois N J, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H W, Cordon-Cardo C, DePinho R A. The Ink4a tumor suppressor gene product p19ARF interacts with mdm2 and neutralises mdm2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 60.Poon R, Jiang W, Toyoshima H, Hunter T. Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J Biol Chem. 1996;271:13283–13291. doi: 10.1074/jbc.271.22.13283. [DOI] [PubMed] [Google Scholar]
- 61.Prives C. Signaling to p53: breaking the MDM2–p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 62.Querido E, Teodoro J G, Branton P E. Accumulation of p53 induced by the adenovirus E1A protein requires regions involved in the stimulation of DNA synthesis. J Virol. 1997;71:3526–3533. doi: 10.1128/jvi.71.5.3526-3533.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao L, White E. Bcl-2 and the ICE family of apoptotic regulators: making a connection. Curr Opin Genet Dev. 1997;7:52–58. doi: 10.1016/s0959-437x(97)80109-8. [DOI] [PubMed] [Google Scholar]
- 65.Rigberg D A, Blinman T A, Kim F S, Cole M A, McFadden D W. Antisense blockade of p21/WAF1 decreases radiation-induced G2 arrest in esophageal squamous cell carcinoma. J Surg Res. 1999;81:6–10. doi: 10.1006/jsre.1998.5483. [DOI] [PubMed] [Google Scholar]
- 66.Rowan S, Ludwig R L, Haupt V, Bates S, Lu X, Oren M, Vousden K H. Specific loss of apoptotic but not cell-cycle arrest function in a human tumor derived p53 mutant. EMBO J. 1996;15:827–838. [PMC free article] [PubMed] [Google Scholar]
- 67.Samuelson A V, Lowe S W. Selective induction of p53 and chemosensitivity in RB-deficient cells by E1A mutants unable to bind the RB-related proteins. Proc Natl Acad Sci USA. 1997;94:12094–12099. doi: 10.1073/pnas.94.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez-Prieto R, Lleonart M, Ramon y Cajal S. Lack of correlation between p53 protein level and sensitivity to DNA-damaging agents in keratinocytes carrying adenovirus E1a mutants. Oncogene. 1996;11:675–682. [PubMed] [Google Scholar]
- 69.Schmitt C A, Lowe S W. Apoptosis and therapy. J Pathol. 1999;187:127–137. doi: 10.1002/(SICI)1096-9896(199901)187:1<127::AID-PATH251>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 70.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 71.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 72.Slebos R J, Lee M H, Plunkett B S, Kessis T D, Williams B O, Jacks T, Hedrick L, Kastan M B, Cho K R. p53-dependent G1 arrest involves pRb-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1994;91:5320–5324. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Somasundaram K, El-Deiry W S. Inhibition of p53-mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene. 1997;14:1047–1057. doi: 10.1038/sj.onc.1201002. [DOI] [PubMed] [Google Scholar]
- 74.Song S, Gulliver G A, Lambert P F. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci USA. 1998;95:2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart Z A, Leach S D, Pietenpol J A. p21Waf1/Cip1 inhibition of cyclin E/cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205–215. doi: 10.1128/mcb.19.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian H, Wittmack E K, Jorgensen T J. p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 2000;60:679–684. [PubMed] [Google Scholar]
- 77.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 78.Waldman T, Kinzler K W, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 79.Waldman T, Lengauer C, Kinzler K W, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 80.Wang H H-G, Rikitake Y, Carter M C, Yaciuk P, Abraham S E, Zerler B, Moran E. Identification of specific adenovirus E1A N-terminal residues critical to the binding of cellular proteins and the control of cell growth. J Virol. 1993;67:476–488. doi: 10.1128/jvi.67.1.476-488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Walsh K. Resistance to apoptosis conferred by cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wyllie F S, Haughton M F, Bond J A, Rowson J M, Jones C J, Wynford-Thomas D. S phase cell-cycle arrest following DNA damage is independent of the p53/p21WAF1 signalling pathway. Oncogene. 1996;12:1077–1082. [PubMed] [Google Scholar]
- 83.Yang C-T, You L, Yeh C-C, Chang J W W, Zhang F, McCormick F, Jablons D M. Adenovirus-mediated p14ARF gene transfer in human mesothelioma cells. J Natl Cancer Inst. 2000;92:636–640. doi: 10.1093/jnci/92.8.636. [DOI] [PubMed] [Google Scholar]
- 84.Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Xiong Y, Yarbrough W G. ARF promotes mdm2 degradation and stabilises p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]