Abstract
Purpose
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) have improved patient survival in hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) metastatic breast cancer (mBC) in clinical trials and real-world studies. However, investigations of survival gains in broader HR+/HER2− mBC populations using epidemiological approaches are limited.
Methods
This retrospective study used SEER registry data to assess breast cancer-specific survival (BCSS) in patients diagnosed with HR+/HER2− de novo mBC from 2010 to 2019. Kaplan–Meier and Cox proportional hazards models were used to compare BCSS in patients diagnosed before (2010‒2013 with follow-up to 2014) and after (2015‒2018 with follow-up to 2019) the 2015 guideline recommendations for CDK4/6i use. A comparison was made to patients with HR+/HER2-positive (HER2+) de novo mBC, for which no major guideline changes occurred during 2015–2018.
Results
Data from 11,467 women with HR+/HER2− mBC and 3260 women with HR+/HER2+ mBC were included. After baseline characteristic adjustment, patients with HR+/HER2− mBC diagnosed post-2015 (n = 6163), had an approximately 10% reduction in risk of BC-specific death compared with patients diagnosed pre-2015 (n = 5304; HR = 0.895, p < 0.0001). Conversely, no significant change was observed in HR+/HER2+ BCSS post-2015 (n = 1798) versus pre-2015 (n = 1462). Similar results were found in patients aged ≥ 65 years.
Conclusion
Using one of the largest US population-based longitudinal cancer databases, significant improvements in BCSS were noted in patients with HR+/HER2− mBC post-2015 versus pre-2015, potentially due to the introduction of CDK4/6i post-2015. No significant improvement in BCSS was observed in patients with HR+/HER2+ mBC post-2015 versus pre-2015, likely due to the availability of HER2-directed therapies in both time periods.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-024-07469-6.
Keywords: Metastatic breast cancer, CDK4/6 inhibitor, HER2, Elderly, SEER database
Introduction
Despite treatment advances over the past decade, fewer than one third of patients with metastatic breast cancer (mBC) survive 5 years after diagnosis [1]. Survival rates are associated in part with tumor molecular subtype [2]. Using the Surveillance Epidemiology, and End Results Program (SEER) database, considered the gold standard for data quality amongst cancer registries globally, Howlader et al. [3] assessed survival outcomes of women diagnosed with breast cancer between 2010 and 2013 and reported that breast cancer-specific survival (BCSS) was longer in patients with HR+/HER2+ compared with HR+/HER2− de novo stage IV breast cancer (or mBC). Four-year BCSS rates were 45.5% vs 35.9%, respectively, corresponding to a significant difference of approximately 10 percentage points at the population level [3]. The authors concluded that this difference was likely due, in part, to major advances in HER2-targeted treatments such as monoclonal antibodies targeting HER2 (trastuzumab was approved by the US Food and Drug Administration [FDA] in 1998, followed by approval of pertuzumab in 2012) [3–6].
More recently, cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, in combination with endocrine therapy (ET), have been approved for the treatment of HR+/HER2− mBC disease (palbociclib was FDA-approved in 2015 [7], followed by ribociclib and abemaciclib, both in 2017 [8, 9]). CDK4/6 inhibitors prevent cell-cycle progression by blocking the formation of cyclin D-CDK4/6 complexes and preventing the inactivation of the tumor suppressor retinoblastoma 1 [10]. Multiple randomized clinical trials (RCTs) and real-world studies have shown that use of CDK4/6 inhibitors plus ET approximately doubles patient progression-free survival (PFS) and can improve overall survival (OS) in patients with HR+/HER2− mBC [11–23]. These regimens have now become the clinical guideline-recommended standard of care in patients with HR+/HER2− mBC [24–27].
Understanding whether these improvements are also seen at the population level in the years since regulatory approval is crucial for assessing the impact of CDK4/6 inhibitors on patient survival outside of RCTs. Thus, a more expansive view of outcome trends for patients with HR+/HER2− mBC in the US would yield valuable insight into the broad effectiveness of current standards of care for mBC.
Early indications of population-level improvements in OS in patients with HR+/HER2− mBC were seen in a study by Alvarez et al. using SEER data [28], which reported a 2-year OS rate of 65% in the post-CDK4/6 inhibitor (2015–2017) cohort versus 62% in the pre-CDK4/6 inhibitor (2011–2013) cohort. However, in using a 2-year OS metric and a relatively short patient follow-up time for assessing differences in survival where median OS was not reached, this study was limited in its ability to capture the clinical benefit derived from the increasing uptake of CDK4/6 inhibitors following market authorization.
To address these data gaps, we conducted a SEER registry-based, retrospective study investigating BCSS in women with HR+/HER2− de novo stage IV breast cancer diagnosed before and after the adoption in 2015 of CDK4/6 inhibitors plus ET as the standard of care. As SEER captures the cause of death, BCSS was used as the survival outcome, in line with Howlader [3]. We also expanded on the approach taken by Alvarez et al. [28] by adding an additional year of data both preceding and following the guideline inclusion of CDK4/6 inhibitors, assessing 4-year BCSS, and comparing outcomes with those of patients with HR+/HER2+ de novo stage IV breast cancer, a population for which CDK4/6 inhibitors are not currently indicated, over the same periods. Including a HR+/HER2+ comparator cohort served two purposes: First, by including patients with HR+/HER2− and with HR+/HER2+ mBC, we reduced the risk of assigning a potential improvement in HR+/HER2− BCSS to CDK4/6 inhibitors that could alternatively be explained by a general improvement in BCSS over time across molecular subtypes, for instance due to higher rates of breast cancer screening. Secondly, novel HER2-targeted therapies were introduced earlier than CDK4/6 inhibitors and would, therefore, not necessarily be expected to demonstrate similar population-level survival improvements in the HER2+ population over the time periods assessed in this study. Therefore, improved BCSS in the HR+/HER2− population, together with no significant improvement in the HR+/HER2+ population and a reduction in the survival difference between the two populations after 2015 would lend support to BCSS improvements with CDK4/6 inhibitors in the HR+/HER2− population. Survival results are reported for the overall population and for a subgroup of women aged ≥ 65 years, given the large number of women at risk of poor outcomes in this age group [2].
Methods
Study design and patients
This retrospective study used data from the SEER 17 Research Plus database [29]. Patient inclusion and exclusion criteria mirrored those used by Howlader et al. [3], except our analyses were limited to patients with HR+/HER2− or HR+/HER2+ de novo stage IV breast cancer. Patient data from 2010 to 2019 were included in this study. Patients were women aged ≥ 18 years with a diagnosis of invasive tumor, node, metastasis (TNM) stage IV breast cancer as their first cancer, and HR+/HER2− or HR+/HER2+ breast cancer subtype as determined by the SEER breast subtype variable. Exclusion criteria included missing cause of death among patients who died, missing data on molecular subtype (based on estrogen receptor/progesterone and HER2 status), cases identified postmortem, patient alive but with no survival time, and invalid or missing date of diagnosis.
Patients were classified into 2 cohorts: patients diagnosed from 2010 to 2013 before the approval of CDK4/6 inhibitors with follow-up to 2014 (minimum of 1 year) or death, whichever occurred earlier, and patients diagnosed from 2015 to 2018 after the approval of CDK4/6 inhibitors with follow-up to 2019 (minimum of 1 year) or death, whichever occurred earlier. These time periods were selected to compare survival outcomes in HR+/HER2− mBC before and after the approval of the first CDK4/6 inhibitor, palbociclib, in February 2015 [10]. The length of time that the pre- and post-2015 cohorts were monitored was identical to permit valid inter-cohort comparisons in survival outcomes.
Outcomes
The SEER Cause-Specific Death Classification variable was used to capture death from breast cancer and determine BCSS, defined as the number of absolute months from breast cancer diagnosis to the date of death attributed to breast cancer [30]. Deaths resulting from breast cancer were treated as events while deaths due to other causes were censored. Patients who were still alive at the end of the follow-up period were also censored.
Statistical analyses
Patient demographics and clinical characteristics, including disease stage, age at diagnosis, race and ethnicity, SEER geographic region (i.e, urban vs rural), SEER registry site, median household income (by county of residence), marital status, and index year were summarized using descriptive statistics (Table 1). All analyses were stratified by breast cancer molecular subtype (HR+/HER2− and HR+/HER2+ mBC). A subgroup analysis was conducted for patients aged ≥ 65 years.
Table 1.
Baseline demographics and clinical characteristics of women with HR+/HER2− or HR+/HER2+ mBC (SEER-17, excluding Alaska Native Tumor Registry, 2010–2013 and 2015–2018)
| Characteristic | HR+/HER2− cohort | HR+/HER2+ cohort | ||||
|---|---|---|---|---|---|---|
| Pre-2015a (n = 5304) | Post-2015b (n = 6163) | p-value | Pre-2015a (n = 1462) | Post-2015b (n = 1798) | p-value | |
| Age at diagnosis, years | < 0.001 | 0.007 | ||||
| Mean (SD) | 61.7 (13.9) | 62.8 (14.2) | 57.4 (13.9) | 58.5 (14.4) | ||
| Median (range) | 62 (19−100) | 63 (21−100) | 57 (23–96) | 59 (18–100) | ||
| Age at diagnosis, n (%) | < 0.001 | 0.002 | ||||
| 18–44 years | 596 (11.2) | 677 (11.0) | 273 (18.7) | 313 (17.4) | ||
| 45–64 years | 2510 (47.3) | 2656 (43.1) | 770 (52.7) | 862 (47.9) | ||
| 65–84 years | 1903 (35.9) | 2443 (39.6) | 364 (24.9) | 558 (31.0) | ||
| ≥ 85 years | 295 (5.6) | 387 (6.3) | 55 (3.8) | 65 (3.6) | ||
| Race and ethnicity, n (%) | < 0.001 | 0.001 | ||||
| Non-Hispanic White | 3580 (67.5) | 4003 (65.0) | 919 (62.9) | 1085 (60.3) | ||
| Non-Hispanic Black | 718 (13.5) | 834 (13.5) | 239 (16.3) | 253 (14.1) | ||
| Non-Hispanic Asian or Pacific Islander | 394 (7.4) | 526 (8.5) | 99 (6.8) | 199 (11.1) | ||
| Hispanic | 584 (11.0) | 727 (11.8) | 193 (13.2) | 245 (13.6) | ||
| American Indian/Alaska Native/Unknown | 28 (0.5) | 73 (1.2) | 12 (0.8) | 16 (0.9) | ||
| SEER geographic regionc, n (%) | 0.337 | 0.370 | ||||
| Big metro/metro/urban | 4729 (89.2) | 5460 (88.6) | 1288 (88.1) | 1602 (89.1) | ||
| Less urban/rural | 575 (10.8) | 703 (11.4) | 174 (11.9) | 196 (10.9) | ||
| SEER registry sited, n (%) | 0.569 | 0.060 | ||||
| California | 2133 (40.2) | 2459 (39.9) | 564 (38.6) | 725 (40.3) | ||
| Connecticut | 269 (5.1) | 311 (5.0) | 76 (5.2) | 65 (3.6) | ||
| Georgia | 641 (12.1) | 783 (12.7) | 225 (15.4) | 270 (15.0) | ||
| Hawaii | 97 (1.8) | 110 (1.8) | 22 (1.5) | 26 (1.4) | ||
| Iowa | 211 (4.0) | 236 (3.8) | 49 (3.4) | 70 (3.9) | ||
| Kentucky | 324 (6.1) | 332 (5.4) | 76 (5.2) | 110 (6.1) | ||
| Louisiana | 334 (6.3) | 439 (7.1) | 110 (7.5) | 114 (6.3) | ||
| New Jersey | 744 (14.0) | 843 (13.7) | 186 (12.7) | 258 (14.3) | ||
| New Mexico | 115 (2.2) | 136 (2.2) | 42 (2.9) | 36 (2.0) | ||
| Seattle (Puget Sound) | 317 (6.0) | 354 (5.7) | 74 (5.1) | 66 (3.7) | ||
| Utah | 119 (2.2) | 160 (2.6) | 38 (2.6) | 58 (3.2) | ||
| Median household income | < 0.001 | < 0.001 | ||||
| < $55,000 | 1332 (25.1) | 1357 (22.0) | 406 (27.8) | 395 (22.0) | ||
| $55,000—$64,999 | 1606 (30.3) | 1039 (16.9) | 406 (27.8) | 275 (15.3) | ||
| $65,000—$74,999 | 896 (16.9) | 1633 (26.5) | 241 (16.5) | 473 (26.3) | ||
| ≥ $75,000 | 1470 (27.7) | 2134 (34.6) | 409 (28.0) | 655 (36.4) | ||
| Marital Status, n (%) | 0.950 | 0.344 | ||||
| Single (never married) | 1130 (21.3) | 1338 (21.7) | 352 (24.1) | 449 (25.0) | ||
| Married/unmarried living with domestic partner | 2335 (44.0) | 2709 (44.0) | 671 (45.9) | 840 (46.7) | ||
| Divorced/separated/widowed | 1558 (29.4) | 1788 (29.0) | 358 (24.5) | 433 (24.1) | ||
| Unknown | 281 (5.3) | 328 (5.3) | 81 (5.5) | 76 (4.2) | ||
HER2 human epidermal growth factor receptor 2, HR hormone receptor, mBC metastatic breast cancer, SD standard deviation, SEER Surveillance, Epidemiology, and End Results
aPre-2015 consists of patients diagnosed between 2010 and 2013 (with follow-up to December 31, 2014)
bPost-2015 consists of patients diagnosed between 2015 and 2018 (with follow-up to December 31, 2019)
c“Missing/unknown” excluded in each model due to low number of patients in this category. Geographic sites within California and Georgia were combined into their respective states for a total of 11 states instead of 16 unique registries
d“Alaska Natives” excluded in each model due to low number of patients in this category; category coefficients and standard errors could not be estimated
Unadjusted Kaplan–Meier analyses were conducted to assess BCSS in both the pre- and post-2015 groups. Landmark survival probabilities were assessed at 6, 12, 18, 24, 36, and 48 months. Relative risk of death and corresponding 95% confidence intervals (CIs) were determined using Cox proportional hazards models. Multivariable regression analyses were adjusted for age at diagnosis, race-ethnicity, SEER geographic region, SEER registry site, median household income, and marital status. These analyses were performed for the overall population and for patients aged ≥ 65 years, and separately for patients with HR+/HER2− and HR+/HER2+ mBC. In addition, BCSS was compared between groups with HR+/HER2− and HR+/HER2+ mBC in each year cohort to capture changes in survival differences between the molecular subtypes over time. Median BCSS was also assessed for the entire period (2000–2019) by age, race and ethnicity, and median household income. All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute, Cary, North Carolina).
Results
Patient characteristics
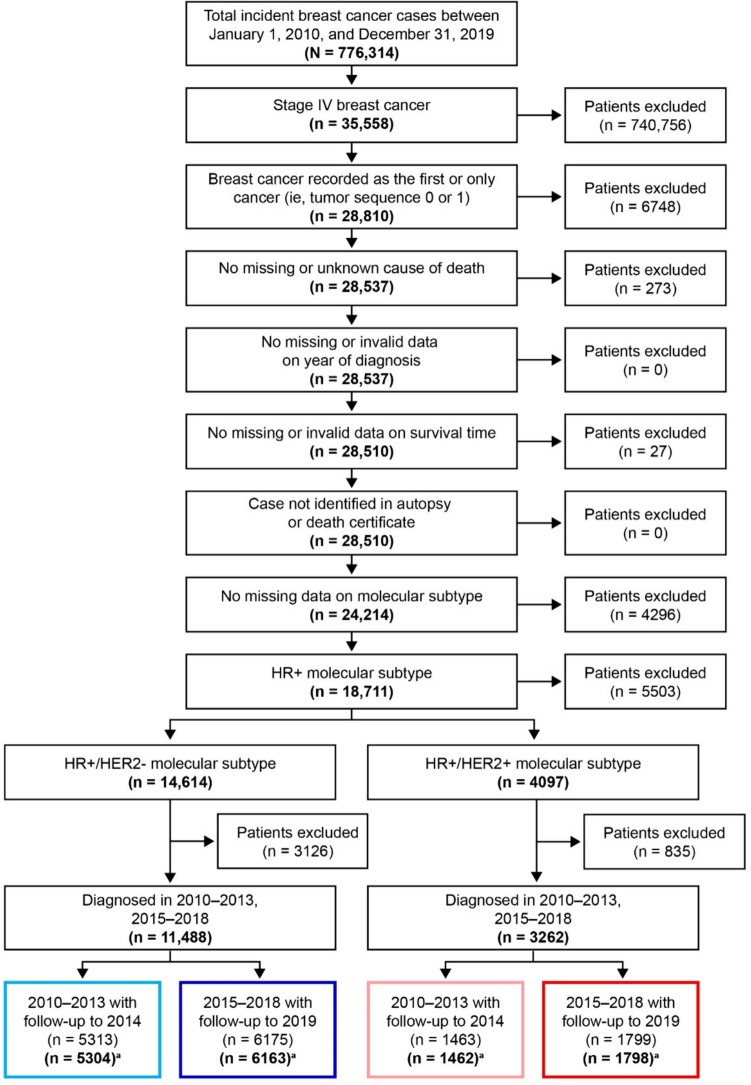
Data from 11,467 women with HR+/HER2− mBC and 3260 women with HR+/HER2+ mBC were included in this study. Patient selection is detailed in Fig. 1. Of patients diagnosed with HR+/HER2− mBC, 5304 were included in the pre-2015 group and 6163 were included in the post-2015 group. Compared with the pre-2015 cohort, patients in the post-2015 cohort were less likely to be non-Hispanic White (67.5% vs 65.0%, p < 0.001), tended to be older (mean age [standard deviation, SD]: 61.7 years [13.9] vs 62.8 years [14.2]; p < 0.001] and to reside in areas with higher median household income (p < 0.001). No differences were seen in other baseline characteristics (Table 1).
Fig. 1.
Patient selection diagram. a Excludes “missing/unknown” from SEER Geographic Region AND “Alaska Natives” from SEER registry site. HER2 human epidermal growth factor receptor 2, HR hormone receptor, SEER Surveillance, Epidemiology, and End Results
Among patients diagnosed with HR+/HER2+ mBC, 1462 were included in the pre-2015 group and 1798 in the post-2015 group. Similar to the HR+/HER2− population, patients in the post-2015 group compared with the pre-2015 cohort were less likely to be non-Hispanic White (60.3% vs 62.9%; p < 0.001), tended to be older (mean age [SD]: 58.5 years [14.4] vs 57.4 years [13.9]; p = 0.007) and to reside in areas with higher median household income (p < 0.001). No differences were observed in other baseline characteristics (Table 1). Descriptive statistics for patients aged ≥ 65 years are shown in Online Resource Table S1.
Breast cancer-specific survival in patients with HR+/HER2− mBC
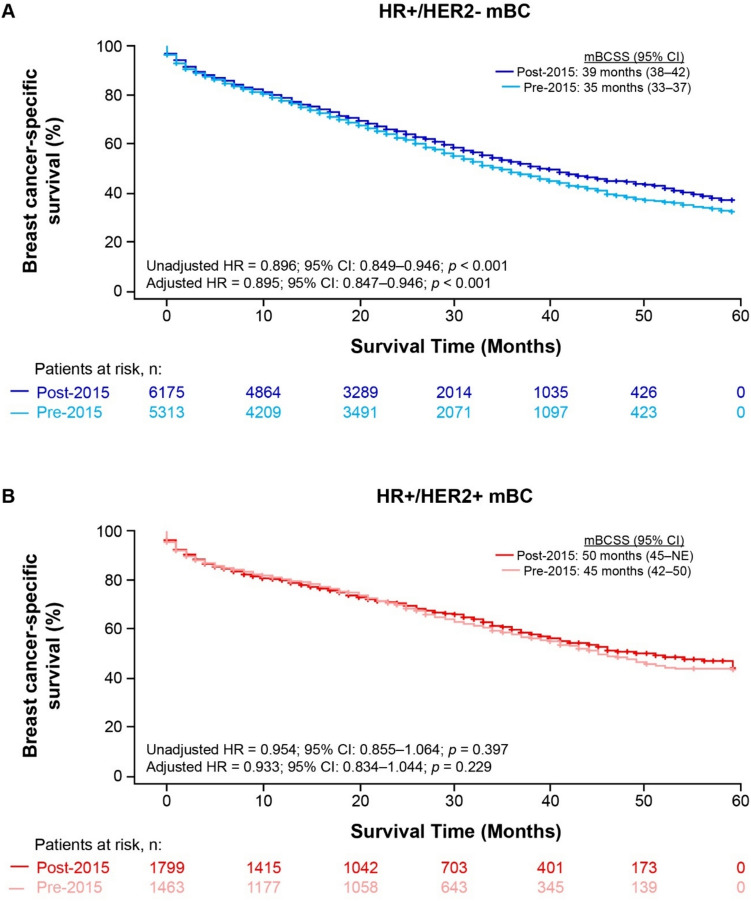
In the unadjusted analysis of patients with HR+/HER2− mBC, BCSS was significantly longer among patients in the post-2015 group versus the pre-2015 group (median BCSS: 39 [95% CI 38–42] vs 35 [95% CI 33–37] months; hazard ratio [HR] = 0.896, 95% CI 0.849–0.946; p < 0.001; Table 2, Fig. 2A). The 48-month BCSS rate was 44.5% for the post-2015 group and 38.5% for the pre-2015 group (Online Resource Table S2). After adjusting for baseline characteristics in the multivariable regression analysis, patients in the post-2015 group had significantly reduced risk of BC-specific death than those in the pre-2015 group (adjusted HR = 0.895, 95% CI 0.847–0.946; p < 0.001; Table 2).
Table 2.
Multivariable Cox regression analysis of breast cancer-specific survival (BCSS) in patients with HR+/HER2− or HR+/HER2+ mBC
| Characteristic | HR+/HER2− cohort (n = 11,467) | HR+/HER2+ cohort (n = 3260) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Study period | ||||||
| Pre-2015a | Reference | Reference | ||||
| Post-2015b | 0.896 | 0.849–0.946 | < 0.001 | 0.954 | 0.855–1.064 | 0.397 |
| Adjusted post-2015b,c | 0.895 | 0.847–0.946 | < 0.001 | 0.933 | 0.834–1.044 | 0.229 |
| Age at diagnosis | ||||||
| 18–44 years | Reference | Reference | ||||
| 45–64 years | 1.29 | 1.16–1.42 | < 0.001 | 1.69 | 1.41–2.03 | < 0.001 |
| 65–84 years | 1.56 | 1.41–1.73 | < 0.001 | 2.52 | 2.08–3.06 | < 0.001 |
| ≥ 85 years | 2.47 | 2.15–2.84 | < 0.001 | 4.28 | 3.19–5.74 | < 0.001 |
| Race and ethnicity | ||||||
| Non-Hispanic White | Reference | Reference | ||||
| Non-Hispanic Black | 1.33 | 1.22–1.44 | < 0.001 | 1.36 | 1.15–1.59 | < 0.001 |
| Non-Hispanic Asian or Pacific Islander | 0.94 | 0.84–1.06 | 0.302 | 1.09 | 0.88–1.35 | 0.447 |
| Hispanic | 0.99 | 0.91–1.09 | 0.907 | 1.06 | 0.89–1.27 | 0.509 |
| American Indian/Alaska Native/Unknown | 0.59 | 0.40–0.87 | 0.008 | 0.70 | 0.33–1.47 | 0.344 |
| SEER geographic regiond | ||||||
| Big metro/metro/urban | Reference | Reference | ||||
| Less urban/rural | 1.03 | 0.93–1.14 | 0.576 | 1.01 | 0.83–1.24 | 0.891 |
| SEER registry sitee | ||||||
| Alaska natives | – | – | – | – | – | – |
| California | Reference | Reference | ||||
| Connecticut | 0.92 | 0.80–1.06 | 0.259 | 0.63 | 0.45–0.88 | 0.007 |
| Georgia | 1.06 | 0.96–1.17 | 0.238 | 1.14 | 0.95–1.36 | 0.161 |
| Hawaii | 1.13 | 0.92–1.41 | 0.252 | 1.52 | 1.00–2.31 | 0.049 |
| Iowa | 1.03 | 0.89–1.20 | 0.707 | 0.68 | 0.48–0.98 | 0.036 |
| Kentucky | 1.07 | 0.93–1.22 | 0.336 | 1.11 | 0.86–1.44 | 0.434 |
| Louisiana | 1.01 | 0.89–1.15 | 0.896 | 1.02 | 0.80–1.30 | 0.887 |
| New Jersey | 1.06 | 0.97–1.16 | 0.210 | 0.95 | 0.79–1.14 | 0.559 |
| New Mexico | 1.16 | 0.96–1.41 | 0.132 | 1.07 | 0.74–1.54 | 0.723 |
| Seattle (Puget Sound) | 1.00 | 0.88–1.13 | 0.980 | 0.84 | 0.62–1.14 | 0.250 |
| Utah | 1.10 | 0.92–1.32 | 0.297 | 1.01 | 0.72–1.42 | 0.956 |
| Median household income | ||||||
| < $55,000 | Reference | Reference | ||||
| $55,000–$64,999 | 0.97 | 0.89–1.06 | 0.523 | 0.99 | 0.82–1.20 | 0.952 |
| $65,000–$74,999 | 0.96 | 0.86–1.06 | 0.400 | 0.94 | 0.77–1.15 | 0.558 |
| ≥ $75,000 | 0.88 | 0.79–0.97 | 0.011 | 0.93 | 0.76–1.14 | 0.490 |
| Marital status | ||||||
| Single (never married) | Reference | Reference | ||||
| Married/unmarried living with domestic partner | 0.80 | 0.74–0.86 | < 0.001 | 0.83 | 0.72–0.96 | 0.010 |
| Divorced/separated/ widowed | 1.03 | 0.95–1.11 | 0.501 | 1.14 | 0.97–1.34 | 0.105 |
| Unknown | 0.92 | 0.80–1.05 | 0.194 | 0.98 | 0.75–1.27 | 0.849 |
BCSS breast cancer-specific survival, CI confidence interval, HER2 human epidermal growth factor receptor 2, HR hazard ratio, HR+hormone receptor, mBC, metastatic breast cancer, SEER Surveillance, Epidemiology, and End Results
aPre-2015 consists of patients diagnosed between 2010 and 2013 (with follow-up to December 31, 2014)
bPost-2015 consists of patients diagnosed between 2015 and 2018 (with follow-up to December 31, 2019)
cMultivariable adjusted hazard ratio. Results were adjusted for age at diagnosis, race and ethnicity, SEER geographic region, SEER registry site, median household income, and marital status
d“Missing/unknown” excluded in each model due to low number of patients in this category
e“Alaska Natives” excluded in each model due to low number of patients in this category
Fig. 2.
Kaplan–Meier breast cancer-specific survival (BCSS) curves for patients diagnosed with HR+/HER2− (A) or HR+/HER2+ (B) mBC pre- or post-2015 (SEER-17, excluding Alaska Native Tumor Registry). CI confidence interval, HER2 human epidermal growth factor receptor 2, HR hazard ratio, HR+hormone receptor-positive, mBC metastatic breast cancer, mBCSS median breast cancer-specific survival, NE not estimable
Multivariable analyses (Table 2) showed that age impacted the risk of BC-specific death, with older patients experiencing greater risk than younger patients. Compared with patients aged 18–44 years, patients aged ≥ 85 years, 65–84 years, or 45–65 years had a 147% (HR = 2.47, 95% CI 2.15–2.84; p < 0.001), 56% (HR = 1.56, 95% CI 1.41–1.73; p < 0.001), and 29% (HR = 1.29, 95% CI 1.16–1.42; p < 0.001) higher risk of BC-specific death, respectively. Non-Hispanic Black patients had a 33% higher risk of BC-specific death compared with non-Hispanic White patients (HR = 1.33, 95% CI 1.22–1.44; p < 0.001). Patients residing in areas with median household income of at least $75,000 had reduced risk of BC-specific death relative to patients residing in areas with household income under $55,000 (HR = 0.88, 95% CI 0.79–0.97; p = 0.011), as did patients who were married/living with a domestic partner compared with single (never married) patients (HR = 0.80, 95% CI 0.74–0.86; p < 0.001). SEER geographic region and SEER registry site were not associated with BCSS. Median BCSS during the period from 2010 to 2019 by age, race and ethnicity, and household income are reported in Online Resource Table S3.
Similarly to the overall study population, BCSS of patients with HR+/HER2− mBC in the subgroup aged ≥ 65 years (n = 5028) was significantly longer in the post-2015 group with a median survival of 34 months (95% CI 32–37) versus 29 months (95% CI 27–31) in the pre-2015 group (adjusted HR = 0.893, 95% CI 0.824–0.967; p = 0.006) (Online Resource Table S4, Online Resource Fig. S1A). Factors associated with BCSS in this subgroup were similar to the overall study population, except for income, which was not significant.
Breast cancer-specific survival in patients with HR+/HER2+ mBC
In the unadjusted analysis of patients with HR+/HER2+ mBC, BCSS was longer among patients diagnosed post-2015 versus pre-2015, but the difference was not statistically significant (median BCSS: 50 months [95% CI 45–not estimable] vs 45 months [95% CI 42–50]; unadjusted HR = 0.954, 95% CI 0.855–1.064; p = 0.397; Table 2, Fig. 2B). BCSS rates at 48 months were 50.7% and 47.7% for the post- and pre-2015 groups, respectively (Online Resource Table S2). After adjusting for baseline characteristics, patients in the post-2015 group did not differ significantly in the risk of BC-specific death compared with those in the pre-2015 group (adjusted HR = 0.933, 95% CI 0.834–1.044; p = 0.229, Table 2).
Similar to the HR+/HER2− cohort, BCSS also varied with age, race, and ethnicity: patients aged ≥ 85 years had a > fourfold higher risk of BC-specific death compared with patients aged 18–44 years (HR = 4.28, 95% CI 3.19–5.74; p < 0.001), and non-Hispanic Black patients had a 36% increased risk of death compared with non-Hispanic White patients (HR = 1.36, 95% CI 1.15–1.59; p < 0.001; Table 2). Differences in BCSS were also seen in SEER registry site and marital status, but not SEER geographic region or household income. In the subgroup of patients aged ≥ 65 years there was no difference in BCSS between the pre- and post- 2015 groups, and only age was associated with BCSS (Online Resource Table S4, Online Resource Fig. S1B).
Comparison of BCSS trends between HR+/HER2– versus HR+/HER2+ mBC
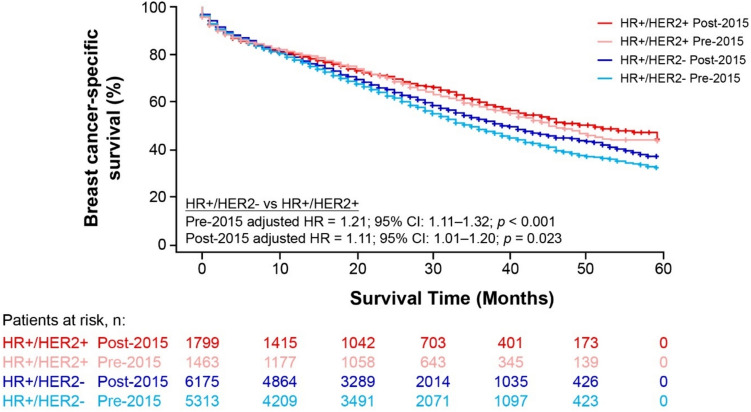
When comparing BCSS between the different HER2 cohorts, survival differences were smaller between the post-2015 groups than those of the pre-2015 groups. Specifically, 48-month BCSS was 9.2 percentage points lower in patients with HR+/HER2− (38.5%) versus HR+/HER2+ (47.7%) mBC in the pre-2015 period, but the difference was reduced to 6.2 percentage point after 2015 (44.5% vs 50.7%) (Fig. 3, Online Resource Table S2). After adjusting for baseline characteristics, patients with HR+/HER2− mBC had a 21% higher risk of BC-specific death compared with patients with HR+/HER2+ mBC in the pre-2015 group (adjusted HR = 1.21, 95% CI 1.11–1.32; p < 0.001; Fig. 3). For the post-2015 group, patients with HR+/HER2− mBC had an 11% higher risk of BC-specific death compared with patients with HR+/HER2+ mBC after adjustment (adjusted HR = 1.11, 95% CI 1.01–1.20; p = 0.023. For patients aged ≥ 65 years, there was no significant difference in BCSS between HR+/HER2− and HR+/HER2+ mBC pre-2015 (adjusted HR = 1.04, 95% CI 0.90–1.20; p = 0.608) and post-2015 (adjusted HR = 0.88, 95% CI 0.78–1.00; p = 0.056).
Fig. 3.
Kaplan–Meier breast cancer-specific survival curves for patients diagnosed with HR+/HER2− or HR+/HER2+ mBC, pre- and post-2015 (SEER-17, excluding Alaska Native Tumor Registry). CI confidence interval, HER2 human epidermal growth factor receptor 2, HR hazard ratio, HR+hormone receptor-positive, mBC metastatic breast cancer
Discussion
The advent of CDK4/6 inhibitors has fundamentally altered the HR+/HER2− mBC treatment landscape over the past 8 years [31]. Multiple RCTs and real-world studies have supported the efficacy and effectiveness of palbociclib, ribociclib, and abemaciclib, in combination with ET, as first- and second-line therapies for extending PFS, with manageable side effects compared to ET alone [11, 12, 16, 18, 20, 32, 33]. The OS benefit conferred by adding a CDK4/6 inhibitor to ET has been less clear in RCTs. A statistically significant OS benefit has been shown for ribociclib [19] but not for palbociclib and abemaciclib relative to ET alone in the first-line mBC setting [15, 23]. Although median OS for patients taking palbociclib plus letrozole (53.9 months) was not significantly longer than placebo plus letrozole in a phase 3 RCT (PALOMA-2), an independent phase 2 RCT in an endocrine sensitive population reported a relatively long median OS for patients taking palbociclib plus letrozole or fulvestrant (PARSIFAL-LONG, 65.4 months) [23, 34]. In addition, real-world comparative-effectiveness studies of CDK4/6 inhibitors plus ET versus ET alone have reported significant OS improvements with CDK4/6 inhibitor treatment in the US [17, 22]. Taken together, this evidence suggests significant survival improvement for patients taking CDK4/6 inhibitors for their HR+/HER2− mBC.
Understanding whether these survival improvements have been seen in a population-based setting is crucial. RCTs are restricted to specific populations that are often younger, healthier, and less diverse, and real-world comparative-effectiveness studies, although essential for assessing effectiveness in routine clinical practice and in more diverse populations, are often by design limited by different inclusion criteria and exclude patients not receiving the treatments being studied [35]. As such, population-based studies are unique in their comprehensive scope and ability to assess broad epidemiology trends.
Using the SEER database, we compared BCSS in patients diagnosed with HR+/HER2− mBC during the periods 2010–2013 (with follow-up to 2014) and 2015–2018 (with follow-up to 2019). These cohorts were based on the first CDK4/6 inhibitor approval of palbociclib in 2015 and the subsequent adoption of a CDK4/6 inhibitor plus ET as the standard of care for patients with HR+/HER2− mBC. We found a clinically meaningful (approximately 10%) improvement in BCSS for patients diagnosed with HR+/HER2− mBC after 2015 versus those diagnosed before 2015, a finding that remained statistically significant following multivariable adjustment for baseline patient demographics. Our BCSS findings are consistent with those of the Alvarez et al. study, which compared OS before and after 2015 for patients with HR+/HER2− mBC in the SEER database; despite analyzing shorter time-based cohorts where median OS was not reached, including fewer years post-2015 than in our study, and using a different survival endpoint, the authors noted a significant increase in 2-year OS after 2015 [28].
In contrast to the trends that we and others [28] have observed for patients with HR+/HER2− mBC, we found numerical but not significant improvements in BCSS for patients with HR+/HER2+ mBC post- versus pre-2015, a population for which CDK4/6 inhibitors are not currently indicated. The lack of a significant improvement in BCSS for patients with HR+/HER2+ post- versus pre-2015 may be related to differences when novel treatments were introduced; pertuzumab was approved as a targeted therapy for HER2+ tumors in 2012, with an OS benefit demonstrated by the phase 3 CLEOPATRA trial [36]. Therefore, the pertuzumab approval and subsequent clinical adoption overlapped both our pre- and post-2015 groups, potentially prolonging survival in both groups, with no significant difference as a result. A different grouping of patients by year (e.g., pre- and post-pertuzumab introduction) may have shown a difference over time; indeed, real-world OS improvements were observed post-pertuzumab/trastuzumab emtansine authorization in the Dutch SONABRE registry and French ESME-MBC database [4, 37].
In our study, the HR+/HER2+ cohort, for which CDK4/6 inhibitors are not currently approved, served as a comparison group to the HR+/HER2− cohort. We found that BCSS was lower in the HR+/HER2− cohort compared with the HR+/HER2+ cohort in the pre-2015 period (21% higher risk of death with HR+/HER2−). This finding is consistent with Howlader et al. [3] who also found shorter BCSS in patients with HR+/HER2− compared with HR+/HER2+ de novo stage IV during the same time period in the SEER database. Despite being drawn from the same database over the same time period, the BCSS estimates in Howlader et al. are slightly smaller (35.9% and 45.5%) than those here (38.5% and 47.7%). This difference could be explained by the fact that Howlader et al. used multiple imputation for patients with missing HER2 status, whereas our study did not. The authors attributed the difference in BCSS between the 2 molecular subtypes in part to the major advances in HER2-targeted treatments such as trastuzumab, FDA-approved in 1998, followed by approval of pertuzumab in 2012 [3–6].
Importantly, we also found that the difference in BCSS was lower in the post-2015 period (11% higher risk of death) in the HR+/HER2− cohort compared with the HR+/HER2+ cohort. Hence, despite a numerically improved survival observed in the HR+/HER2+ cohort, the survival gap between molecular subtypes was reduced post-2015, lending support to the idea that treatment advances were uniquely effective for the HR+/HER2− group during this time period. In addition, the use of the HER2+ group as an anchor reduces the possibility that the 10% BCSS improvement in the HR+/HER2− group was associated with a general improvement in BCSS independent of molecular subtype. The comparison to the HR+/HER2+ group therefore provides support that the introduction of CDK4/6 inhibitors for treating HR+/HER2− mBC may have prolonged BCSS in the HR+/HER2− population.
Similar patterns of results were seen for patients aged ≥ 65 years: patients with HR+/HER2− mBC saw prolonged survival post-2015 compared with pre-2015, but those with HR+/HER2+ mBC did not. This is an important observation because half of breast cancer deaths occur in women aged ≥ 70 years [38]. Furthermore, older patients have been proportionally underrepresented in clinical trials assessing anticancer drug efficacy [39, 40]; therefore, studies supporting the effectiveness of therapies for older patients address a key knowledge gap.
The population-level 10% reduction in risk of HR+/HER2− breast cancer-related death observed in our study for the post-2015 versus pre-2015 group can be considered clinically meaningful, even by RCT standards [41, 42]. However, the extent to which this finding can be attributed to treatment with CDK4/6 inhibitors cannot be determined, because SEER data do not include detailed information on treatments received by patients; in particular, the database contains no information on the uptake of CDK4/6 inhibitors or in which line of therapy patients received these treatments. Comprehensive data on the adoption of CDK4/6 inhibitors in the US are scarce. While one study using the Flatiron Health database suggests that although rapid adoption of palbociclib occurred in 2015, the percentage of patients with HR+/HER2− mBC taking first-line palbociclib plus ET did not exceed 30% between 2015 and 2019 [43]; another small 2017 survey of 64 invited oncologists reported that 52.4% and 42.9% of patients were receiving a CDK4/6 inhibitor-based regimen in the first and second lines of therapy, respectively [44]. Ribociclib and abemaciclib prescriptions would have added to these totals beginning in 2017, but it is unclear if uptake was large enough to prolong BCSS substantially before 2019. In addition, a study from a single institution of patients treated between 2015 and 2017 reported that only 42 of 230 (18.3%) patients received palbociclib as a first-line therapy, with the remainder receiving it in later lines [45]. If CDK4/6 inhibitor use was not widespread and biased toward later lines of therapy from 2015 to 2019, the 10% improvement in BCSS reported in our study is reasonable and may represent a lower bound for potential survival gains at the population level. More widespread use and proportionally greater first-line use over time may result in survival improvements approaching the approximate 25%-40% improvement reported in real-world studies and a meta-analysis of RCTs [17, 22, 46, 47]. Future studies with a longer follow-up may reveal greater relative improvements in post-2015 survival.
A major strength of this study is that it utilized one of the largest US population-based longitudinal databases (SEER 17), which covered up to 26.5% of the US population [48], allowing for a large cohort size. However, this study has several limitations. As mentioned above, CDK4/6 inhibitor use is not tracked in SEER data, thus the direct effects of CDK4/6 inhibitor treatment on BCSS could not be assessed. Also, the exact dates of diagnosis and death are not provided in the SEER database, potentially affecting the BCSS results. In addition, though our follow-up time was longer than in previous studies, it may be of insufficient length to fully capture the survival benefit provided by CDK4/6 inhibitors. Further, this was an observational study and subject to unmeasured confounding, because the SEER database also does not collect key clinical variables such as Eastern Cooperative Oncology Group performance status and the presence of genetic biomarkers. However, unmeasured confounders are unlikely to systematically affect the relative differences over time and between subtypes. While we excluded a sizeable proportion of patients (4296, approximately 15%) due to missing information on molecular subtype, previous efforts at molecular subtype imputation using SEER registry data found that women with missing subtypes tended to be older and had poorer outcomes than their peers with known subtypes [3]. Although nonimputed data may be biased toward BCSS overestimation, the BCSS ratio between HR+/HER2− and HR+/HER2+ cohorts is unlikely to be affected. Lastly, the analysis was limited to patients with de novo metastatic disease (ie, incident stage IV breast cancer cases) because SEER registries do not track recurrences in patients who had an initial early-stage breast cancer diagnosis but later progressed to mBC.
Conclusion
Using one of the largest US population-based longitudinal datasets, we observed significant improvements in BCSS post-2015 versus pre-2015 in patients with HR+/HER2− de novo mBC in contrast with nonsignificant improvements in BCSS post-2015 in patients with HR+/HER2+ de novo mBC. Similar results were found in the subgroup of patients aged ≥ 65 years. Although the limitations of the current analysis prevent attribution of the change in BCSS to specific treatments, advances such as the introduction of CDK4/6 inhibitors for treating HR+/HER2− mBC may have contributed to population-level improvement in BCSS over time. As the current study assessed survival early after CDK4/6 inhibitor introduction, there is a potential for further survival improvement as the use of these treatments becomes more widespread. Future studies that include more recent years, longer follow-up periods, and treatment-pattern data are needed to verify the effectiveness of CDK4/6 inhibitors in patients with HR+/HER2− mBC on a US population level.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors assisted with the development, review and approval of the submitted manuscript.
Funding
This work was supported by Pfizer Inc. Medical writing support was provided by Kevin Woolfrey, PhD, of Oxford PharmaGenesis Inc., (Newtown, PA, USA) and was funded by Pfizer Inc.
Data availability
The data that support the findings of this study are available from the National Cancer Institute and can be accessed with permission under a data use agreement.
Declarations
Competing interest
AB reports consultancy fees from AstraZeneca, Pfizer, Novartis, Lilly, Genentech/Roche, Seagen, Daiichi-Sankyo, Merck, Agendia, Sanofi, and Puma, and provides research support to Agendia and AstraZeneca MLK reports ongoing collaboration on a Pfizer-funded study. SS, RS, AC, DM are employees and stockholders of Pfizer Inc. SK and RKG are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which was a paid consultant to Pfizer in connection with the development of this manuscript. Their compensation is unconnected to the studies on which they work.
Consent to participate
Patient consent was not required for this anonymized, non-interventional study.
Ethical approval
The study was reviewed by the RTI International Institutional Review Board and received a determination of “not research involving human subjects.”
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.SEER Explorer (2020) Surveillance Research Program, National Cancer Institute; 2023 Apr 19. Available from: https://seer.cancer.gov/statistics-network/explorer. Accessed Sep 2023
- 2.SEER (2020) Cancer stat facts: Female breast cancer subtypes. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed Sep 2023
- 3.Howlader N, Cronin KA, Kurian AW, Andridge R (2018) Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomark Prev 27:619–626. 10.1158/1055-9965.EPI-17-0627 [DOI] [PubMed] [Google Scholar]
- 4.Grinda T, Antoine A, Jacot W, Blaye C, Cottu PH, Dieras V, Dalenc F, Goncalves A, Debled M, Patsouris A, Mouret-Reynier MA, Mailliez A, Clatot F, Levy C, Ferrero JM, Desmoulins I, Uwer L, Petit T, Jouannaud C, Lacroix-Triki M, Deluche E, Robain M, Courtinard C, Bachelot T, Brain E, Perol D, Delaloge S (2021) Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008–2017 ESME cohort. ESMO Open 6:100114. 10.1016/j.esmoop.2021.100114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwase T, Shrimanker TV, Rodriguez-Bautista R, Sahin O, James A, Wu J, Shen Y, Ueno NT (2021) Changes in overall survival over time for patients with de novo metastatic breast cancer. Cancers (Basel). 10.3390/cancers13112650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundquist M, Brudin L, Tejler G (2017) Improved survival in metastatic breast cancer 1985–2016. Breast 31:46–50. 10.1016/j.breast.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 7.US Food & Drug Administration (2015) Palbociclib (IBRANCE). https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance
- 8.US Food & Drug Administration (2017) Ribociclib (Kisqali). https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali
- 9.US Food & Drug Administration (2018) FDA approves new treatment (Verzenio, abemaciclib) for certain advanced or metastatic breast cancers. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-advanced-or-metastatic-breast-cancers
- 10.Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, Zirkelbach JF, Yu J, Liu Q, Zhao L, Crich J, Chen XH, Hughes M, Bloomquist E, Tang S, Sridhara R, Kluetz PG, Kim G, Ibrahim A, Pazdur R, Cortazar P (2015) FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 21:4760–4766. 10.1158/1078-0432.ccr-15-1185 [DOI] [PubMed] [Google Scholar]
- 11.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17:425–439. 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925–1936. 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 13.Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H, Amiri-Kordestani L, Ibrahim A, Sridhara R, Goldberg KB, Theoret MR, Kluetz PG, Blumenthal GM, Pazdur R, Beaver JA, Prowell TM (2020) CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol 21:250–260. 10.1016/s1470-2045(19)30804-6 [DOI] [PubMed] [Google Scholar]
- 14.Gao JJ, Cheng J, Prowell TM, Bloomquist E, Tang S, Wedam SB, Royce M, Krol D, Osgood C, Ison G, Sridhara R, Pazdur R, Beaver JA, Amiri-Kordestani L (2021) Overall survival in patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer treated with a cyclin-dependent kinase 4/6 inhibitor plus fulvestrant: a US Food and Drug Administration pooled analysis. Lancet Oncol 22:1573–1581. 10.1016/S1470-2045(21)00472-1 [DOI] [PubMed] [Google Scholar]
- 15.Goetz MP (2023) MONARCH 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy in patients with HR+, HER2− advanced breast cancer. Cancer Res Presentation at SABCS 2023. Abstract GS01-12.
- 16.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 17.Goyal RK, Chen H, Abughosh SM, Holmes HM, Candrilli SD, Johnson ML (2023) Overall survival associated with CDK4/6 inhibitors in patients with HR+/HER2- metastatic breast cancer in the United States: A SEER-Medicare population-based study. Cancer 129:1051–1063. 10.1002/cncr.34675 [DOI] [PubMed] [Google Scholar]
- 18.Harbeck N, Bartlett M, Spurden D, Hooper B, Zhan L, Rosta E, Cameron C, Mitra D, Zhou A (2021) CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol 17:2107–2122. 10.2217/fon-2020-1264 [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, Conte P, Cameron DA, Andre F, Arteaga CL, Zarate JP, Chakravartty A, Taran T, Le Gac F, Serra P, O’Shaughnessy J (2022) Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 386:942–950. 10.1056/NEJMoa2114663 [DOI] [PubMed] [Google Scholar]
- 20.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, Andre F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738–1748. 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 21.Llombart-Cussac A, Sledge GW, Masakazu T, Neven P, Sohn JH, Inoue K, Pivot X, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Conte P, Andre V, Bian Y, Shahir A, van Hal G (2023) PD13–11 Final overall survival analysis of Monarch 2: A phase 3 trial of abemaciclib plus fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer. Cancer Res 83:13–11. 10.1158/1538-7445.SABCS22-PD13-11 [Google Scholar]
- 22.Rugo HS, Brufsky A, Liu X, Li B, McRoy L, Chen C, Layman RM, Cristofanilli M, Torres MA, Curigliano G, Finn RS, DeMichele A (2022) Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer 8:114. 10.1038/s41523-022-00479-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slamon D, Dieras V, Rugo HS, Harbeck N, Im SA, Gelmon K, Lipatov O, Walshe JM, Martin M, Mac Gregor MC, Bananis E, Gauthier E, Lu DR, Kim S, Finn RS (2023) Overall survival with palbociclib plus letrozole in advanced breast cancer. J Clin Oncol. 10.1200/JCO.23.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, Boyle F, Cardoso MJ, Carey LA, Cortes J, El Saghir NS, Elzayat M, Eniu A, Fallowfield L, Francis PA, Gelmon K, Gligorov J, Haidinger R, Harbeck N, Hu X, Kaufman B, Kaur R, Kiely BE, Kim SB, Lin NU, Mertz SA, Neciosup S, Offersen BV, Ohno S, Pagani O, Prat A, Penault-Llorca F, Rugo HS, Sledge GW, Thomssen C, Vorobiof DA, Wiseman T, Xu B, Norton L, Costa A, Winer EP (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623–1649. 10.1016/j.annonc.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAndrew NP, Finn RS (2022) Clinical review on the management of hormone receptor-positive metastatic breast cancer. JCO Oncol Pract 18:319–327. 10.1200/OP.21.00384 [DOI] [PubMed] [Google Scholar]
- 26.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 34:3069–3103. 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- 27.Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321:288–300. 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- 28.Alvarez A, Bernal AM, Anampa J (2023) Racial disparities in overall survival after the introduction of cyclin-dependent kinase 4/6 inhibitors for patients with hormone receptor-positive, HER2-negative metastatic breast cancer. Breast Cancer Res Treat 198:75–88. 10.1007/s10549-022-06847-2 [DOI] [PubMed] [Google Scholar]
- 29.DCCPS. SRP (2020) National Cancer Institute. Bethesda, MD. Available from: https://seer.cancer.gov/registries/terms.html.
- 30.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA (2010) Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102:1584–1598. 10.1093/jnci/djq366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang JC, O’Regan R (2018) Use of novel combination therapies in the treatment of advanced HR+/HER2− breast cancer. J Natl Compr Canc Netw 16:S5–S17. 10.6004/jnccn.2018.0200 [DOI] [PubMed] [Google Scholar]
- 32.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Pivot X, Vidam G, Wang Y, Rodriguez Lorenc K, Miller M, Taran T, Jerusalem G (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36:2465–2472. 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 33.Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A (2017) MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35:2875–2884. 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 34.Llombart-Cussac A, Perez-Garcia JM, Bellet M, Dalenc F, Gil-Gil M, Ruiz-Borrego M, Gavila J, Schmid P, Zamora P, Wheatley D, Martinez-de Deunas E, Amillano K, Anton A, Cottu P, Vinas G, Petit T, Tesarova P, Ceuva J, Colleoni M, Martinez del Prado MP, Andres R, Aguirre E, Diaz M, Vitorino S, Sampayo-Cordero M, Cortes J (2023) PARSIFAL-LONG: Extended follow-up of hormone receptorpositive/HER2-negative advanced breast cancer patients treated with fulvestrant and palbociclib vs letrozole and palbociclib in the PARSIFAL study. Cancer Res Presentation at SABCS 2023.
- 35.Cottu P, Ramsey SD, Sola-Morales O, Spears PA, Taylor L (2022) The emerging role of real-world data in advanced breast cancer therapy: Recommendations for collaborative decision-making. Breast 61:118–122. 10.1016/j.breast.2021.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, Clark E, Knott A, Restuccia E, Benyunes MC, Cortes J, group Cs (2020) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21:519–530. 10.1016/S1470-2045(19)30863-0 [DOI] [PubMed] [Google Scholar]
- 37.Ibragimova KIE, Geurts SME, Croes S, Erdkamp F, Heijns JB, Tol J, Vriens B, Aaldering KNA, Dercksen MW, Pepels M, Peters N, van de Winkel L, Tilli DJP, Vriens IJH, de Boer M, Tjan-Heijnen VCG (2021) Survival before and after the introduction of pertuzumab and T-DM1 in HER2-positive advanced breast cancer, a study of the SONABRE Registry. Breast Cancer Res Treat 188:571–581. 10.1007/s10549-021-06178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL (2022) Breast cancer statistics, 2022. CA Cancer J Clin 72:524–541. 10.3322/caac.21754 [DOI] [PubMed] [Google Scholar]
- 39.Singh H, Kanapuru B, Smith C, Fashoyin-Aje LA, Myers A, Kim G, Pazdur R (2017) FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the US Food and Drug Administration. J Clin Oncol 35:10009. 10.1200/JCO.2017.35.15_suppl.10009 [Google Scholar]
- 40.Talarico L, Chen G, Pazdur R (2004) Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 22:4626–4631. 10.1200/JCO.2004.02.175 [DOI] [PubMed] [Google Scholar]
- 41.Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, de Vries EG, Piccart MJ (2015) A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 26:1547–1573. 10.1093/annonc/mdv249 [DOI] [PubMed] [Google Scholar]
- 42.Ellis LM, Bernstein DS, Voest EE, Berlin JD, Sargent D, Cortazar P, Garrett-Mayer E, Herbst RS, Lilenbaum RC, Sima C, Venook AP, Gonen M, Schilsky RL, Meropol NJ, Schnipper LE (2014) American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol 32:1277–1280. 10.1200/JCO.2013.53.8009 [DOI] [PubMed] [Google Scholar]
- 43.Jaber Chehayeb R, Hood A, Wang X, Miksad R, Schellhorn Mougalian S, Lustberg MB, Wang SY, Greenup RA, Pusztai L, Kunst N (2022) Treatment sequencing patterns and associated direct medical costs of metastatic breast cancer care in the United States, 2011 to 2021. JAMA Netw Open 5:e2244204. 10.1001/jamanetworkopen.2022.44204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldschmidt D, Dalal AA, Romdhani H, Kelkar S, Guerin A, Gauthier G, Wu EQ, Niravath P, Small T (2018) Current treatment patterns among postmenopausal women with HR+/HER2− metastatic breast cancer in US community oncology practices: an observational study. Adv Ther 35:482–493. 10.1007/s12325-018-0676-2 [DOI] [PubMed] [Google Scholar]
- 45.Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, Bose R, Cherian M, Hernandez-Aya L, Frith A, Peterson L, Luo J, Krishnamurthy J, Ma CX (2019) Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Canc Netw 17:141–147. 10.6004/jnccn.2018.7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ha MJ, Singareeka Raghavendra A, Kettner NM, Qiao W, Damodaran S, Layman RM, Hunt KK, Shen Y, Tripathy D, Keyomarsi K (2022) Palbociclib plus endocrine therapy significantly enhances overall survival of HR+/HER2- metastatic breast cancer patients compared to endocrine therapy alone in the second-line setting: a large institutional study. Int J Cancer 150:2025–2037. 10.1002/ijc.33959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Wu J, Ji Z, Chen L, Zou J, Zheng J, Lin W, Cai J, Chen Y, Zheng D, Chen Y, Li Z (2023) Comparative efficacy and safety of different combinations of three CDK4/6 inhibitors with endocrine therapies in HR+/HER-2 - metastatic or advanced breast cancer patients: a network meta-analysis. BMC Cancer 23:816. 10.1186/s12885-023-11322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.SEER (2020) SEER registries: Number of persons by race and Hispanic ethnicity for SEER participants (2020 census data). Available from: https://seer.cancer.gov/registries/data.html. Accessed Feb 2024
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the National Cancer Institute and can be accessed with permission under a data use agreement.