Abstract
Background
Thromboembolism (TE) is a well-known complication during chemotherapy in cancer patients. However, the risk of TE associated with immune checkpoint inhibitors (ICIs) is unknown. This study was performed to investigate the incidence of TE and associated risk factors in patients treated with ICIs.
Methods
We conducted a retrospective chart survey of patients receiving at least one ICI at Shinshu University Hospital between September 2014 and October 2021. Age, sex, cancer type, body mass index, medical history, laboratory data at commencement of treatment, and medication data were obtained from electronic medical records. TE events (venous thromboembolism [VTE], arterial thromboembolism [ATE]) were identified after ICI initiation.
Results
The study population consisted of 548 patients with a median age of 70.0 (19–89) years, 71.4% men, and a median follow-up of 15.1 months (range; 0.16–72.0 months). Nivolumab was the most commonly used ICI (45.8%), followed by pembrolizumab (23.9%), pembrolizumab plus anticancer drugs (7.8%), and nivolumab plus ipilimumab (5.1%). Thirty-eight cases of TE (6.9%) occurred (22 VTE, 16 ATE). Risk factors significantly associated with TE in multivariate logistic analysis were dyslipidemia (OR 2.44; 95% CI 1.17–5.09; p = 0.017), Khorana score ≥ 2 (HR 2.40; 95% CI 1.14–5.04; p = 0.021). Overall survival was not significantly different from patients without TE (p = 0.963).
Conclusion
These results suggested that the frequency of TE is higher than expected and should be considered and monitored in patients treated with ICIs.
Keywords: Venous thromboembolism, Arterial thromboembolism, Thrombosis, Immune checkpoint inhibitor, Risk factor
Introduction
Cancer patients are at higher risk of thromboembolism (TE) as a complication [1, 2]. For example, the risk of venous thromboembolism (VTE) is 4–7 times higher in cancer patients compared to the general population [3–5]. Moreover, patients with VTE and cancer have a higher mortality rate than patients with VTE alone or with cancer alone [6, 7]. Similarly, the incidence of arterial thromboembolism (ATE) is also increased in cancer patients compared to the general population [3, 4, 7, 8].
Immune checkpoint inhibitors (ICIs) have significantly improved the clinical outcomes in patients with various malignancies [9–13]. However, ICIs are known to be associated with a number of immune-related adverse events (irAEs), including gastrointestinal, cutaneous, thyroid and hematologic findings, such as autoimmune hemolytic anemia and thrombocytopenia. Phase III clinical trials using ICIs reported the frequencies of these irAEs, but did not include TE as an irAE. Several recent clinical studies and case reports presented details of TE events in patients treated with ICI monotherapy [14] or in combination with chemotherapy [15–19]. Anticancer drugs, such as platinum-based agents, angiogenesis inhibitors, and hormonal agents, have been shown to increase TE risk in cancer patients [20–24]. However, it remains unclear whether ICIs actually increase the risk of TE compared to conventional chemotherapy. In addition, the TE risk associated with ICI treatment and its effects on patient prognosis are also unknown.
This study was performed to investigate the frequency of TE in patients treated with ICIs. Furthermore, we examined the potential risk factors for developing TE and compared the prognosis between patients with and without TE.
Methods
Subjects
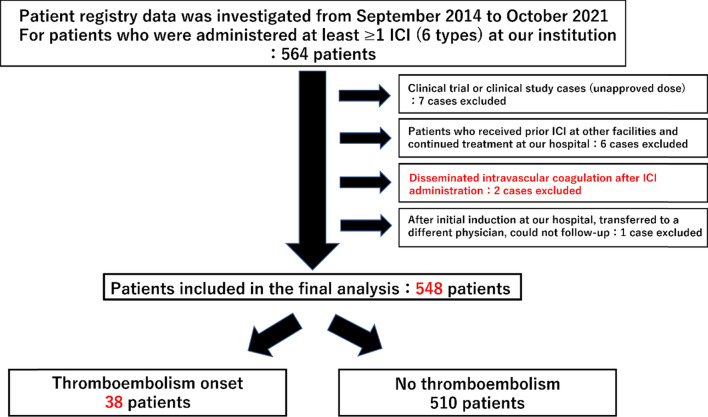
Patients treated with ICIs at Shinshu University Hospital between September 1, 2014, and October 31, 2021, were retrospectively surveyed. To ensure a minimum observation period of 6 months, the data cutoff was set as June 30, 2022. The list of treated patients was extracted from the medication history database of our hospital pharmacy department. The eligibility criterion was at least one cycle of ICI treatment at our institution, including monotherapy as well as combination therapy. Baseline clinical information and laboratory data were defined as within 2 weeks prior to the induction of ICI. Patients in clinical trials or clinical studies were excluded (Fig. 1).
Fig. 1.
Flow diagram and clinical characteristics of the study population. A total of 564 patients were initially selected, 16 of whom were excluded, and finally 548 patients were included in the analysis. Thromboembolism occurred in 38 patients
Investigations
This was a retrospective study to explore factors associated with the development of TE after ICI administration. All baseline and subsequent clinical data were extracted from the electronic medical records.
Patient background data
The following data at the start of initial ICI treatment were investigated. Age, sex, Eastern Cooperative Oncology Group performance status (ECOG-PS), body mass index (BMI), smoking history, cancer type, treatment line, contents of treatment regimen, comorbidities (presence of cancer other than main disease, hypertension, dyslipidemia, diabetes, coronary artery disease, cerebrovascular disease, peripheral vascular disease, pulmonary disease, liver disease, renal disease), history of myocardial infarction and history of surgical procedures.
Clinical laboratory test values
Blood biomarkers—white blood cell (WBC), neutrophil, lymphocyte, monocyte, eosinophil, hemoglobin (Hb), platelet (Plt), serum creatinine (Cre), lactate dehydrogenase, blood urea nitrogen, albumin, D-dimer, fibrinogen, C-reactive protein (CRP), thyroid-stimulating hormone, free thyroid hormone 3 and free thyroid hormone 4—were retrospectively examined based on the electronic medical records. Blood laboratory data were used for the most recent data prior to ICI administration.
Calculation of Khorana score
The Khorana VTE risk assessment score (Khorana score) was calculated based on cancer type and biomarkers, such as WBC count, Plt count, Hb level, and BMI. A score 0 indicates low risk (VTE incidence 0.3%); score 1–2 indicates intermediate risk (VTE incidence 2%); and a score ≥ 3 is classified as high risk (VTE incidence 6.7%) [25].
Concomitant medications
The following medications were investigated at the time of ICI initiation. Anticoagulants, antiplatelet agents, oral corticosteroid hormones, thyroid hormone, non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, opioid analgesics, angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACE-Is), diuretics, diabetes medications, anti-hyperlipidemic agents, proton pump inhibitors, histamine H2 receptor blockers, and antibiotics were investigated.
In addition, we investigated the history of treatment with bevacizumab (BEV), ramucirumab (RAM), and axitinib before and after the first dose of ICI.
Methods for evaluation of thromboembolism
TE events, including VTE and ATE and events, after initiation of ICI treatment were investigated by chart review using electronic medical records.
VTE was defined as cases diagnosed by computed tomography (CT) or ultrasound echocardiography for deep vein thrombosis, pulmonary embolism, and visceral vein thrombosis after the first ICI administration. ATE was defined as myocardial infarction or cerebral infarction diagnosed by magnetic resonance imaging, CT, or cardiac catheterization. VTE and ATE cases collected according to these definitions were defined as “thromboembolism onset.” The time to TE onset was defined as the period from ICI induction to TE onset or until censored observation at the last follow-up. The observation period for the occurrence of VTE and ATE began after the first administration of the ICI and ended 6 months later, between October 2021, the last day of the ICI administration study period, and June 2022.
Statistical analysis
The cohort was divided into the TE group and non-TE group according to the presence or absence of TE, respectively, and baseline characteristics were compared between groups to search for risk factors for the development of TE.
The Mann–Whitney U-test was used to compare continuous variables (quantitative data) that did not follow a normal distribution. For comparisons of nominal variables (qualitative data), the Chi-squared test or Fisher’s exact test was used, depending on the expected value. These univariate analyses were performed as exploratory analyses.
The presence or absence of TE was then used as the objective variable, and factors with p < 0.05 in univariate analysis and age, smoking history, and surgical history were included as explanatory variables in multivariate logistic regression analysis to explore risk factors for the development of TE after initial ICI administration.
Overall survival (OS) was defined as the period from the start of ICI administration until death or censored observation at the last follow-up, using the log-rank test with the Kaplan–Meier method. The cumulative incidence of TE was determined by the Gray test using the cumulative incidence method.
All statistical analyses were performed using EZR [26] Ver. 1.55 (Saitama Medical Center, Jichi Medical University, Japan). In all analyses, p < 0.05 was taken to indicate statistical significance.
Results
Clinical characteristics of the study population
Figure 1 shows a flow diagram for patient selection in this study. A total of 564 patients who received at least one ICI at our institution were initially selected. Seven of these patients were excluded because they had been included in a clinical trial or clinical study. Six patients who had already been confirmed to have received some form of ICI at another medical facility were excluded. Two patients who developed disseminated intravascular coagulation after ICI administration were excluded. In addition, one patient who was initially treated and then followed up at another institution was also excluded. Therefore, a total of 548 patients were finally included in the analysis (Fig. 1).
The clinical characteristics of the enrolled subjects are summarized in Table 1. The study population consisted of 391 men (71.4%) and 157 women (28.6%) with a mean age of 70.0 years (IQR; 63.0–76.0). The most common carcinomas in the study population were non-small cell lung cancer (NSCLC) (n = 198, 36.1%), followed by malignant melanoma (n = 109, 19.9%), head and neck cancers (n = 71, 13.0%), and urothelial cancers (n = 47, 8.6%). Single ICI regimens were administered in 419 cases (76.5%) and combination regimens were administered in 129 cases (23.5%). The most common regimen was nivolumab (n = 251, 45.8%), followed by pembrolizumab (n = 131, 23.9%), pembrolizumab + chemotherapy (n = 43, 7.8%), and nivolumab + ipilimumab (n = 28, 5.1%). The 1st (n = 222) and 2nd (n = 201) lines of treatment accounted for 77.2% of cases (n = 423).
Table 1.
Patient characteristics (n = 548)
| Variable | Median | [IQR] | < Min–Max > |
|---|---|---|---|
| Age at start of ICIs | 70.0 | [63.0–76.0] | < 19.0–89.0 > |
| BMI | 21.9 | [19.6–24.2] | < 12.6–38.1 > |
| n | (%) | (% missing) | |
| Sex | (0) | ||
| Male | 391 | 71.4 | |
| Female | 157 | 28.6 | |
| ECOG-PS | (0) | ||
| 0–1 | 475 | 86.7 | |
| ≥ 2 | 73 | 13.3 | |
| Khorana score at start of ICIs | (0) | ||
| 0 | 219 | 40.0 | |
| 1 | 215 | 39.2 | |
| 2 | 83 | 15.1 | |
| 3 | 25 | 4.6 | |
| 4 | 5 | 0.9 | |
| 5 | 1 | 0.2 | |
| Cancer types | (0) | ||
| Non-small cell lung cancer | 198 | 36.1 | |
| Malignant melanoma | 109 | 19.9 | |
| Head and neck cancers | 71 | 13.0 | |
| Urothelial cancers | 47 | 8.6 | |
| Renal cell cancer | 28 | 5.1 | |
| Hepatic cell cancer | 27 | 4.9 | |
| Esophageal cancer | 17 | 3.1 | |
| Gastric cancer | 14 | 2.6 | |
| Small cell lung cancer | 9 | 1.6 | |
| Malignant pleural mesothelioma | 9 | 1.6 | |
| Gynecologic cancers | 5 | 0.9 | |
| Other cancersa | 14 | 2.6 | |
| Therapeutic management | (0) | ||
| Monotherapy | |||
| Nivolumab | 251 | 45.8 | |
| Pembrolizumab | 131 | 23.9 | |
| Durvalumab | 16 | 2.9 | |
| Atezolizumab | 13 | 2.4 | |
| Avelumab | 7 | 1.3 | |
| Ipilimumab | 1 | 0.2 | |
| Combination therapy | |||
| Pembrolizumab + chemotherapy | 43 | 7.8 | |
| Nivolumab + ipilimumab | 28 | 5.1 | |
| Atezolizumab + bevacizumab | 26 | 4.7 | |
| Atezolizumab + chemotherapy | 18 | 3.3 | |
| Nivolumab + ipilimumab + chemotherapy | 9 | 1.6 | |
| Durvalumab + chemotherapy | 4 | 0.7 | |
| Pembrolizumab + target therapy | 1 | 0.2 | |
| Therapeutic line of ICIs | (0) | ||
| 1st | 222 | 40.5 | |
| 2nd | 201 | 36.7 | |
| 3rd | 74 | 13.5 | |
| 4th | 27 | 4.9 | |
| ≥5th (Max:15th) | 24 | 4.4 |
IQR interquartile range, Min minimum, Max maximum, ICIs immune checkpoint inhibitors, BMI body mass index, ECOG-PS Eastern Cooperative Oncology Group performance status
a Breast cancer (n = 3), Hodgkin lymphoma (n = 2), Pancreatic cancer (n = 2), Thymic cancer (n = 2), Merkel cell cancer (n = 2), Cancer of unknown primary (n = 2), Cholangiocellular cancer (n = 1)
Frequency of thromboembolism events
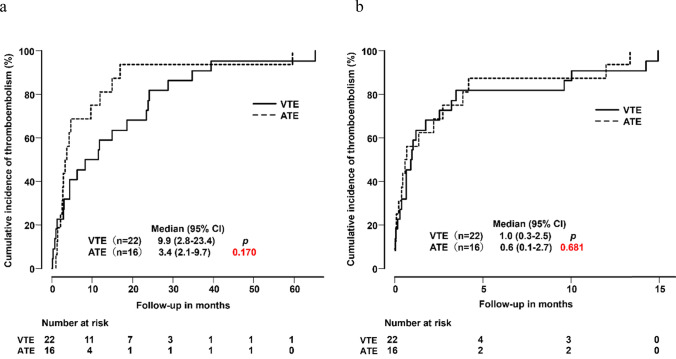
The frequencies of TE events according to cancer types are summarized in Table 2. The mean follow-up observation period was 15.1 months (range; 0.16–72.0 months) after the first administration of ICI. Thirty-eight of the 548 patients developed TE during the follow-up period consisting of VTE (n = 22) and ATE (n = 16). The frequency of TE was 6.9%. The cancer type most commonly associated with TE was NSCLC (n = 15), followed by malignant melanoma (n = 9), and urothelial cancers (n = 7). With regard to the cumulative incidence and time course, there were no statistically significant differences between incidences of VTE and ATE after the first or last administration of ICI (Fig. 2a, b).
Table 2.
Frequency of thromboembolism by cancer types
| Patients with thromboembolism | Event rate | (95% CI) | VTE | Incidence of VTE rate | (95% CI) | ATE | Incidence of ATE rate | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | (%) | n | (%) | n | (%) | ||||
| Total | 548 | (100) | 38 | (100) | 6.9 | (5.0–9.4) | 22 | 4.0 | (2.5–6.0) | 16 | 2.9 | (1.7–4.7) |
| Cancer types | ||||||||||||
| Non-small cell lung cancer | 198 | (36.1) | 15 | (39.5) | 7.6 | (4.3–12.2) | 7 | 3.5 | (1.4–7.1) | 8 | 4.0 | (1.8–7.8) |
| Malignant melanoma | 109 | (19.9) | 9 | (23.7) | 8.3 | (3.8–15.1) | 7 | 6.4 | (2.6–12.8) | 2 | 1.8 | (0.2–6.5) |
| Head and neck cancers | 71 | (13.0) | 3 | (7.9) | 4.2 | (0.9–11.9) | 2 | 2.8 | (0.3–9.8) | 1 | 1.4 | (0.0–7.6) |
| Urothelial cancers | 47 | (8.6) | 7 | (18.4) | 14.9 | (6.2–28.3) | 4 | 8.4 | (2.4–20.4) | 3 | 6.4 | (1.3–17.5) |
| Renal cell cancer | 28 | (5.1) | 1 | (2.6) | 3.6 | (0.1–18.3) | 1 | 3.6 | (0.1–18.3) | 0 | – | |
| Hepatic cell cancer | 27 | (4.9) | 1 | (2.6) | 3.7 | (0.1–19.0) | 1 | 3.7 | (0.1–19.0) | 0 | – | |
| Esophageal cancer | 17 | (3.1) | – | – | – | – | – | – | ||||
| Gastric cancer | 14 | (2.6) | 1 | (2.6) | 7.1 | (0.2–33.9) | 1 | 7.1 | (0.2–33.9) | 0 | – | |
| Small cell lung cancer | 9 | (1.6) | 1 | (2.6) | 11.1 | (0.3–48.2) | 0 | – | 1 | 11.1 | (0.3–48.2) | |
| Malignant pleural mesothelioma | 9 | (1.6) | – | – | – | – | ||||||
| Gynecologic cancers | 5 | (0.9) | – | – | – | – | ||||||
| Other cancersa | 14 | (2.6) | – | – | – | – | ||||||
CI confidence interval
a Breast cancer (n = 3), Hodgkin lymphoma (n = 2), Pancreatic cancer (n = 2), Thymic cancer (n = 2), Merkel cell cancer (n = 2), Cancer of unknown primary (n = 2), Cholangiocellular cancer (n = 1)
Fig. 2.
Cumulative incidence of thromboembolism from the initial ICI administration to onset (a). Cumulative incidence of thromboembolism from the final ICI administration to onset (b)
Clinical risk factors for thromboembolism during ICI therapy
The results of univariate analysis of baseline clinical characteristics for the development of TE are summarized in Table 3. There were no statistically significant differences in clinical backgrounds, including age, sex, BMI, ECOG-PS, co-existing multiple cancers, smoking history, or surgical history between the TE group and non-TE group. In addition, concomitant therapies (history of BEV, RAM or axitinib use) during the study period were also not related to the development of TE. Urothelial cancers were significantly related to the occurrence of TE (p = 0.035) (Table 3).
Table 3.
Univariative analysis of patient backgrounds
| Thromboembolism (+) (n = 38) | Thromboembolism (−) (n = 510) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | (%) | < Min–Max > | n | (%) | < Min–Max > | p | |
| Age (Median [IQR]) | 71.0 | [68.3–77.8] | < 51–83 > | 70.0 | [62.3–76.0] | < 19–89 > | 0.0502 | |
| < 70 vs. ≥ 70 | < 70 | 13 | 34.2 | 250 | 49.0 | 0.111 | ||
| < 65 vs. ≥ 65 | < 65 | 6 | 15.8 | 156 | 30.6 | 0.081 | ||
| Sex | Female | 13 | 34.2 | 144 | 28.2 | 0.549 | ||
| BMI (Median [IQR]) | 22.90 | [20.25–24.38] | < 12.6–31.2 > | 21.83 | [19.53–24.22] | < 13.25–38.13 > | 0.141 | |
| < 24.99 vs. ≥ 25.00 | < 24.99 | 29 | 76.3 | 410 | 80.4 | 0.692 | ||
| ECOG-PS | 0.519 | |||||||
| 0–1 vs. 2–4 | 0–1 | 34 | 89.5 | 441 | 86.5 | 0.781 | ||
| Use of BEV, RAM, or axitinib | Yes | 6 | 15.8 | 115 | 22.7 | 0.443 | ||
| Co-existence of two or more cancers | Yes | 5 | 13.2 | 83 | 16.3 | 0.783 | ||
| Smoking History | Yes | 21 | 55.3 | 338 | 66.3 | 0.230 | ||
| Surgical History | Yes | 18 | 47.4 | 274 | 53.7 | 0.556 | ||
| Therapeutic line of ICIs | ||||||||
| 1st vs. 2nd or more | 1st | 17 | 44.7 | 205 | 40.2 | 0.705 | ||
| 1, 2nd vs. 3rd or more | 1, 2nd | 30 | 78.9 | 392 | 76.9 | 0.924 | ||
| Chemotherapy | ||||||||
| ICI single vs. ICI + Chemotherapy | ICI single | 31 | 81.6 | 416 | 81.6 | 1.000 | ||
| Cancer types | ||||||||
| NSCLC vs. Others | NSCLC | 15 | 39.5 | 183 | 35.9 | 0.787 | ||
| Malignant melanoma vs. Others | Malignant melanoma | 9 | 23.7 | 100 | 19.6 | 0.692 | ||
| Head and neck cancers vs. Others | Head and neck cancers | 3 | 7.9 | 68 | 13.3 | 0.476 | ||
| Urothelial cancers vs. Others | Urothelial cancers | 7 | 18.4 | 40 | 7.8 | 0.035* | ||
Min minimum, Max maximum, IQR interquartile range, BMI body mass index, ECOG-PS Eastern Cooperative Oncology Group performance status, BEV bevacizumab, RAM ramucirumab, ICIs immune checkpoint inhibitors, NSCLC non-small cell lung cancer
* p < 0.05
The results of univariate analysis of Khorana score and laboratory data are shown in Table 4. There was a significant difference in the overall Khorana score between the TE and non-TE groups (p = 0.023). Furthermore, Khorana score ≥ 2 (p = 0.021) and each 1-point increase (p = 0.037) showed significant correlations with the development of TE. In the laboratory findings, higher WBC count (p = 0.038), lower Hb level (p = 0.026), neutrophil count (p = 0.008), D-dimer level (p = 0.016), and CRP level (p = 0.031) were significant risk factors for TE. In terms of comorbidities (Table 5), there were significant differences in incidence of TE between in both patients with hypertension (p = 0.013) and dyslipidemia (p = 0.001) respectively.
Table 4.
Univariate analysis of Khorana score and blood data
| Variable | Thromboembolism ( +) (n = 38) | Thromboembolism (-) (n = 510) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (% missing) | Median | [IQR] | < Min–Max > | n | (% missing) | Median | [IQR] | < Min–Max > | p | ||
| Khorana score MEAN [±S.D.] | 38 | (0) | 1.26 [± 1.13] | 510 | (0) | 0.85 [± 0.90] | 0.023* | |||||
| ≥ 1 vs. 0 | ≥ 1 | 27 | 302 | 0.206 | ||||||||
| ≥ 2 vs. 0–1 | ≥ 2 | 14 | 100 | 0.021* | ||||||||
| ≥ 3 vs. 0–2 | ≥ 3 | 5 | 26 | 0.087 | ||||||||
| per 1-point increase | 0.037* | |||||||||||
| Component of Khorana score | ||||||||||||
| WBC | 38 | (0) | 6.70 | [5.71–8.40] | < 2.63–22.97 > | 510 | (0) | 5.94 | [4.63–7.67] | < 1.91–46.38 > | 0.038* | |
| Hb | 38 | (0) | 11.3 | [9.9–12.9] | < 7.2–16.7 > | 510 | (0) | 12.2 | [10.9–13.5] | < 6.3–17.9 > | 0.026* | |
| Plt | 38 | (0) | 26.3 | [20.2–34.1] | < 15.2–92.4 > | 510 | (0) | 23.6 | [18.5–30.1] | < 0.5–77.1 > | 0.088 | |
| BMI ≥ 35.0 kg/m2 | 0 | (0) | – | – | – | 2 | (0) | – | – | – | 1.000 | |
| Cancer typesa | 1 | (0) | – | – | – | 15 | (0) | – | – | – | 1.000 | |
| Cancer typesb | 22 | (0) | – | – | – | 234 | (0) | – | – | – | 0.206 | |
| Blood data | ||||||||||||
| Nut | 38 | (0) | 4.71 | [3.96–6.42] | < 1.68–20.90 > | 510 | (0) | 4.01 | [2.97–5.43] | < 0.73–41.74 > | 0.008* | |
| Lym | 38 | (0) | 1124.0 | [898.5–1497.5] | – | 510 | (0) | 1158.5 | [811.8–1627.8] | – | 0.917 | |
| Mon | 38 | (0) | 374.5 | [302.0–609.8] | – | 510 | (0) | 399.0 | [300.0–507.8] | – | 0.795 | |
| Eos | 38 | (0) | 143.0 | [82.0–222.3] | – | 510 | (0) | 109.0 | [58.3–195.3] | – | 0.255 | |
| ALB | 38 | (0) | 3.5 | [3.3–4.0] | < 2.5–4.6 > | 510 | (0) | 3.8 | [3.4–4.2] | < 0.9–5.1 > | 0.069 | |
| BUN | 38 | (0) | 17.5 | [14.4–20.6] | < 9.0–33.6 > | 510 | (0) | 16.4 | [13.6–20.6] | < 5.5–49.5 > | 0.335 | |
| Cre | 38 | (0) | 0.91 | [0.72–1.06] | < 0.43–2.08 > | 510 | (0) | 0.86 | [0.71–1.06] | < 0.33–9.47 > | 0.533 | |
| LDH | 38 | (0) | 203 | [183–279] | < 125–737 > | 508 | (0.4) | 204 | [172–257] | < 92–4038 > | 0.513 | |
| D-Dimer | 24 | (36.8) | 2.2 | [1.1–6.5] | < 0.7–18.2 > | 238 | (53.3) | 1.1 | [0.8–2.5] | < 0.4–90.0 > | 0.016* | |
| Fibg | 15 | (60.5) | 401.0 | [357.0–556.0] | – | 199 | (39.0) | 411.0 | [339.5–495.0] | – | 0.387 | |
| CRP | 37 | (2.6) | 0.70 | [0.22–3.75] | < 0.02–15.3 > | 504 | (1.2) | 0.44 | [0.11–2.00] | < 0.01–24.30 > | 0.031* | |
| TSH | 31 | (18.4) | 1.93 | [1.47–3.60] | – | 382 | (25.1) | 1.96 | [1.21–3.28] | – | 0.483 | |
| T3 | 31 | (18.4) | 2.49 | [2.32–2.90] | – | 378 | (25.9) | 2.68 | [2.39–2.99] | – | 0.106 | |
| T4 | 31 | (18.4) | 1.23 | [1.12–1.35] | – | 383 | (25.0) | 1.23 | [1.11–1.37] | – | 0.937 | |
IQR interquartile range, Min minimum, Max maximum, S.D. standard deviation, WBC white blood cell, Hb hemoglobin, Plt platelet, BMI body mass index, Nut neutrophil, Lym lymphocyte, Mon monocyte, Eos eosinophil, ALB albumin, BUN blood urea nitrogen, Cre serum creatinine, LDH lactate dehydrogenase, Fbig fibrinogen, CRP C-reactive protein, TSH thyroid-stimulating hormone, FT3 free thyroid hormone 3, FT4 free thyroid hormone 4
aGastric cancer or Pancreatic cancer
bLung cancers or Gynecologic cancers or Urothelial cancers or Lymphoma
* p < 0.05
Table 5.
Univariate analysis of comorbidity/previous medical history
| History or comorbidity disease | n | (%) | p | ||
|---|---|---|---|---|---|
| Thromboembolism | |||||
| (+) | (−) | ||||
| Hypertension | Yes | 23 | 197 | 10.5 | 0.013* |
| Dyslipidemia | Yes | 15 | 85 | 15.0 | 0.001* |
| Diabetes | Yes | 7 | 77 | 8.3 | 0.753 |
| Atrial fibrillation | Yes | 2 | 25 | 7.4 | 0.682 |
| Chronic lung disease | Yes | 4 | 60 | 6.3 | 1.000 |
| Interstitial lung disease | Yes | 1 | 21 | 4.5 | 1.000 |
| Chronic liver disease | Yes | 2 | 45 | 4.3 | 0.762 |
| Chronic renal disease | Yes | 3 | 28 | 9.7 | 0.467 |
| Congestive heart failure | Yes | 1 | 7 | 12.5 | 0.439 |
| History of myocardial infarction | Yes | 4 | 22 | 15.4 | 0.097 |
| Peripheral vascular disease | Yes | 1 | 10 | 9.1 | 0.550 |
| Cerebrovascular disease | Yes | 4 | 31 | 11.4 | 0.292 |
* p < 0.05
Table 6 shows the results of univariate analysis of the effects of concomitant medications on TE. There were no significant differences in incidence of TE between patients treated with or without anticoagulants, corticosteroids, or thyroid hormones. However, there were significant differences according to the use of antihypertensives, including ARBs and ACE-Is (p = 0.009), and antihyperlipidemic agents (p = 0.023).
Table 6.
Univariate analysis of concomitant medications at the start of ICIs
| Medication Status | n | (%) | p | ||
|---|---|---|---|---|---|
| Thromboembolism | |||||
| (+) | (−) | ||||
| Anti-thrombotic agents | |||||
| Anticoagulants | Yes | 6 | 48 | 11.1 | 0.322 |
| Antiplatelets | Yes | 6 | 54 | 10.0 | 0.289 |
| Endocrine drugs | |||||
| Corticosteroid hormones | Yes | 1 | 26 | 3.7 | 1.000 |
| Thyroid hormone | Yes | 4 | 34 | 10.5 | 0.324 |
| NSAIDs | Yes | 11 | 94 | 10.5 | 0.169 |
| Acetaminophen | Yes | 5 | 89 | 5.3 | 0.650 |
| Opioid analgesics | Yes | 7 | 114 | 5.8 | 0.459 |
| ARBs/ACE-Is | Yes | 16 | 113 | 12.4 | 0.009* |
| Diuretics | Yes | 5 | 45 | 10.0 | 0.377 |
| Diabetes medications | Yes | 7 | 60 | 10.4 | 0.218 |
| Anti-hyperlipidemic agents | Yes | 13 | 91 | 12.5 | 0.023* |
| PPIs | Yes | 13 | 210 | 5.8 | 0.502 |
| H2-receptor blockers | Yes | 0 | 13 | 0.0 | 1.000 |
| Antibiotics | Yes | 1 | 20 | 4.8 | 1.000 |
ICIs immune checkpoint inhibitors, NSAIDs non-steroidal anti-inflammatory drugs, ARBs angiotensin receptor blockers, ACE-Is angiotensin-converting enzyme inhibitors, PPIs proton pump inhibitors
* p < 0.05
Multivariate logistic regression analysis was performed on a total of 7 variables. Four variables were urothelial cancer, dyslipidemia, hypertension, and Khorana score ≥ 2 with p < 0.05 in univariate analysis results. Remaining three factors were included for the purpose of excluding from bias in ICI initiation "age," "surgical history," and "smoking history," which are already reported as TE risk factors in general. The analysis results showed that dyslipidemia (OR 2.44; 95% CI 1.17–5.09; p = 0.017), and Khorana score ≥ 2 (OR 2.40; 95% CI 1.14–5.04; p = 0.021) were independent risk factors for TE (Table 7).
Table 7.
Multivariate analysis for associations between clinical factors and TE
| Variable | Odds ratio | (95% CI) | p |
|---|---|---|---|
| Dyslipidemia | 2.44 | (1.17–5.09) | 0.017* |
| Khorana score ≥ 2 | 2.40 | (1.14–5.04) | 0.021* |
| Urothelial cancers | 2.61 | (0.99–6.92) | 0.053 |
| Hypertension | 1.71 | (0.82–3.57) | 0.154 |
| Age | 1.03 | (0.99–1.07) | 0.206 |
| Operation history | 1.59 | (0.75–3.37) | 0.225 |
| Smoking history | 1.71 | (0.84–3.50) | 0.142 |
TE thromboembolism, CI confidence interval
* p < 0.05
Impact of thromboembolism on overall survival
We examined survival according to the development of TE in the present study population. There was no significant difference in OS between the TE group (median OS, 20.9 months; 95% CI 10.7–46.8) and non-TE group (median OS, 20.1 months; 95% CI 17.4–24.4; p = 0.963) during the observation period (Fig. 3). In addition, there were also no differences in OS between the VTE group (median OS, 34.8 months; 95% CI 10.7–47.0) and non-VTE group (median OS, 20.1 months; 95% CI 17.4–24.4; p = 0.498) or between the ATE group (median OS, 15.2 months; 95% CI, 5.2–18.5) and non-ATE group (median OS, 20.1 months; 95% CI 17.4–24.4; p = 0.369) (data not shown).
Fig. 3.
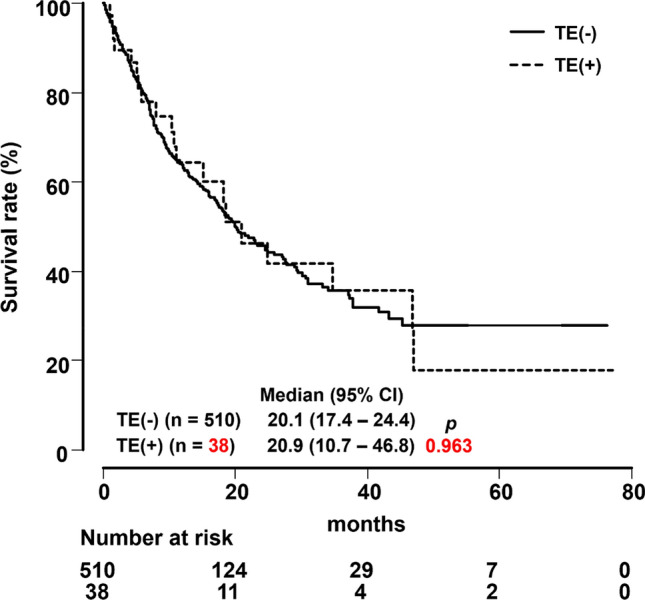
Overall survival curves for patients with or without thromboembolism
Discussion
TE is an important complication in cancer patients. The 1-year cumulative frequencies of VTE for various cancers were reported to be 2.4% for lung cancer, 1.5% for bladder cancer, and 0.5% for malignant melanoma [27]. Seng et al. [21] reported that the frequency of TE during anticancer chemotherapy was 1.92% for VTE, and showed that the incidence of VTE was 1.67-fold higher for cisplatin-containing regimens compared with non-cisplatin regimens. In addition, the frequency of VTE was also reported to be 11.9% with the use of BEV. In a comparison of standard anticancer therapy with or without BEV, the incidence of VTE was reported to be 1.33-fold higher in the BEV group [23].
In this study, we found that the overall frequency of TE after ICI administration was 6.9%, with VTE and ATE occurring at rates of 4.0 and 2.9%, respectively. Previous studies [14–19, 28, 29] showed that the frequency of TE after ICI ranged from 2.0 to 12.9% for VTE and 1.3 to 4.9% for ATE. Hsu et al. [15] reported one case of VTE after ICI administration in a total of 50 cases (2.0%). In contrast, the highest frequency of 12.9% was reported in a total of 672 cases [19]. Similarly, ATE was reported in 1.3% of 552 cases [14] and 4.9% of 122 cases [28]. The total number of cases in our study was higher than in these previous reports. The frequency of TE observed here was comparable with other studies, and suggested that the occurrence of TE is more common than reported in clinical trials. Therefore, our results suggest that the occurrence rate of TE during ICI therapy has been underestimated in the past.
We found that patients with comorbid lipid abnormalities had a significantly increased rate of TE with ICI administration. Although the mechanisms of TE associated with ICI are not yet understood, it has been demonstrated that ICI administration is associated with a proinflammatory state and elevated inflammatory cytokine levels [30]. Systemic inflammatory responses induced by ICI may enhance the prothrombotic state by activating platelets and impairing the fibrinolytic system [31–33]. Furthermore, experiments in mice have shown that programmed death-1 (PD-1) is involved in the downregulation of atherosclerosis-promoting T cells and that inhibition of PD-1 or cytotoxic T-lymphocyte-associated protein-4 increases macrophage and inflammatory T cell infiltration in atherosclerotic plaques, promoting vascular inflammation and atherosclerosis [34, 35]. This promotion of atherosclerotic plaque after ICI use may contribute to an increase in TE events [36]. Lipid abnormalities may further promote atherosclerosis and thus increase the incidence of TE [37].
The Khorana score quantifies the predicted risk of VTE prior to administration of anticancer drugs in patients with cancer. In previous study mentioned that patients with BMI ≥ 35 kg/m2 accounted for only a small percentage of the cancer cohort, which may have results in underestimation of the Khorana score level by 1 point [38]. Only 2 of 548 patients had BMI ≥ 35 kg/m2 in this study. We included BMI in Khorana score in the present study it is unclear that BMI could be contributed to a possible factor for TE in the Japanese population. In Japanese patients with Khorana score ≥ 2, WBC count ≥ 11,000 and Hb level < 10 g/dL, may be the main factors of concern regarding the development of TE.
Several studies indicated that poor ECOG-PS was a potential risk factor for developing TE in patients treated with immunotherapy [18, 39], however, we found no association with ECOG-PS and TE in the present study.
It has been shown that survival was longer in patients with irAE than in non-irAE patients [40–42]. With regard to prognosis, there were no differences in OS between patients who developed TE after receiving ICI and those who did not in the present study. Although several reports suggested that VTE development after ICI was associated with shorter OS [13, 19], others found no association with OS or PFS [43]. The relation of TE after ICI to OS remains unclear, and further investigations are required.
There present study had some limitations. First, most of the patients with dyslipidemia and hypertension as comorbidities were on medications for these conditions, but information about control during ICI therapy and history of medication in each patient were not fully examined. Second, pretreatment D-dimer and CRP levels were significantly related to the development of TE in univariate analysis, but 36.8% of D-dimer levels and 2.6% of CRP levels were missing. Therefore, the evaluation may have been insufficient. However, these data could be valuable markers of the development of TE, and further prospective studies are needed for accurate evaluation of our results.
Conclusion
In conclusion, our findings indicated that TE should be considered and monitored in patients treated with ICIs. In addition, we showed that cancer patients with Khorana score ≥ 2 and/or comorbid lipid abnormalities were at higher risk of developing TE associated with ICI administration.
Acknowledgements
We thank all patients and their families who participated in this study and the health care providers who made the effort.
Abbreviations
- TE
Thromboembolism
- VTE
Venous thromboembolism
- ATE
Arterial thromboembolism
- ICIs
Immune checkpoint inhibitors
- irAEs
Immune-related adverse events
- ECOG-PS
Eastern Cooperative Oncology Group performance status
- BMI
Body mass index
- WBC
White blood cell
- Hb
Hemoglobin
- Plt
Platelet
- Cre
Serum creatinine
- CRP
C-reactive protein
- NSAIDs
Non-steroidal anti-inflammatory drugs
- ARBs
Angiotensin receptor blockers
- ACE-Is
Angiotensin-converting enzyme inhibitors
- BEV
Bevacizumab
- RAM
Ramucirumab
- CT
Computed tomography
- OS
Overall survival
- NSCLC
Non-small cell lung cancer
- OR
Odds ratio
Author contributions
TI conceptualization, methodology, investigation, formal analysis, writing – original draft. TA investigation, data curation, writing – review and editing. TK validation, writing – review and editing, project administration.
Funding
Not applicable.
Data availability
Data supporting the results of this study are available from the corresponding author (TI) upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Ethical Guidelines for Medical Research Involving Human Subjects and was approved by the Shinshu University School of Medicine Biological and Medical Research Ethics Committee (approval number: 5550). As this was a retrospective and observational study, the requirement for informed consent from individual patients was waived by the Shinshu University School of Medicine Biological and Medical Research Ethics Committee (approval number: 5550). Instead, an opt-out document was posted on the Shinshu University Hospital website. In addition, all procedures were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
TI and TA have no conflicts of interest to declare related to this article. TK has received personal fees from AstraZeneca, Bristol – Myers Squibb, MSD, Ono, Chugai, Daiichi – Sankyo Pharmaceutical.LTDs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. Thromb Haemost. 2017;117:57–65. 10.1160/TH15-08-0686. [DOI] [PubMed] [Google Scholar]
- 2.Grilz E, Königsbrügge O, Posch F, Schmidinger M, Pirker R, Lang IM, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103:1549–56. 10.3324/haematol.2018.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–46. 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 4.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 5.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 6.Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine. 1999;78:285–91. 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50. 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Eckert L, Wang Y, Wang H, Cohen A. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist. 2013;18:1321–9. 10.1634/theoncologist.2013-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345–56. 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, Angelis FD, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kewan T, Ko T, Flores M, Sallam Y, Haddad A, Daw H. Prognostic impact and risk factors of cancer-associated thrombosis events in stage-IV cancer patients treated with immune checkpoint inhibitors. Eur J Haematol. 2021;106:682–8. 10.1111/ejh.13598. [DOI] [PubMed] [Google Scholar]
- 15.Hsu JC, Lin J-Y, Hsu M-Y, Lin PC. Effectiveness and safety of immune checkpoint inhibitors: A retrospective study in Taiwan. PLoS ONE. 2018;13: e0202725. 10.1371/journal.pone.0202725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–311. 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez-Sainz L, Martinez-Marin V, Viñal D, Martinez-Perez D, Pedregosa J, Garcia-Cuesta JA, et al. Incidence of venous thromboembolic events in cancer patients receiving immunotherapy: a single-institution experience. Clin Transl Oncol. 2021;23:1245–52. 10.1007/s12094-020-02515-3. [DOI] [PubMed] [Google Scholar]
- 18.Guven DC, Aksun MS, Sahin TK, Aktepe OH, Yildirim HC, Taban H, et al. Poorer baseline performance status is associated with increased thromboembolism risk in metastatic cancer patients treated with immunotherapy. Support Care Cancer. 2021;29:5417–23. 10.1007/s00520-021-06139-3. [DOI] [PubMed] [Google Scholar]
- 19.Moik F, Chan W-SE, Wiedemann S, Hoeller C, Tuchmann F, Aretin M-B, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137:1669–78. 10.1182/blood.2020007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117:219–30. 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
- 21.Seng S, Liu Z, Chiu SK, Proverbs-Singh T, Sonpavde G, Choueiri TK, et al. Risk of venous thromboembolism in patients with cancer treated with Cisplatin: a systematic review and meta-analysis. J Clin Oncol. 2012;30:4416–26. 10.1200/JCO.2012.42.4358. [DOI] [PubMed] [Google Scholar]
- 22.Proverbs-Singh T, Chiu SK, Liu Z, Seng S, Sonpavde G, Choueiri TK, et al. Arterial thromboembolism in cancer patients treated with cisplatin: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1837–40. 10.1093/jnci/djs435. [DOI] [PubMed] [Google Scholar]
- 23.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–85. 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 24.Totzeck M, Mincu RI, Rassaf T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: A meta-analysis of more than 20 000 patients. J Am Heart Assoc. 2017;6: e006278. 10.1161/JAHA.117.006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8. 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grover SP, Hisada YM, Kasthuri RS, Reeves BN, Mackman N. Cancer therapy-associated thrombosis. Arterioscler Thromb Vasc Biol. 2021;41:1291–305. 10.1161/ATVBAHA.120.314378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando Y, Hayashi T, Sugimoto R, Nishibe S, Ito K, Kawada K, et al. Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs. 2020;38:1200–6. 10.1007/s10637-019-00881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulder FI, Horváth-Puhó E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137:1959–69. 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 30.Urwyler P, Earnshaw I, Bermudez M, Perucha E, Wu W, Ryan S, et al. Mechanisms of checkpoint inhibition-induced adverse events. Clin Exp Immunol. 2020;200:141–54. 10.1111/cei.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–8. 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 32.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 33.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–8. 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein H-H, Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS ONE. 2014;9: e93280. 10.1371/journal.pone.0093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bu D-X, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1100–7. 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inno A, Chiampan A, Lanzoni L, Verzè M, Molon G, Gori S. Immune checkpoint inhibitors and atherosclerotic vascular events in cancer patients. Front Cardiovasc Med. 2021;8: 652186. 10.3389/fcvm.2021.652186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray JG, Rosendaal FR. The role of dyslipidemia and statins in venous thromboembolism. Curr Control Trials Cardiovasc Med. 2001;2:165–70. 10.1186/cvm-2-4-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overvad TF, Ording AG, Nielsen PB, Skjøth F, Albertsen IE, Noble S, et al. Validation of the Khorana score for predicting venous thromboembolism in 40 218 patients with cancer initiating chemotherapy. Blood Adv. 2022;6:2967–76. 10.1182/bloodadvances.2021006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endo S, Honda T, Kawahara T, Sakakibara R, Mitsumura T, Okamoto T, et al. Profile of metastatic lung cancer patients susceptible to development of thromboembolism during immunotherapy. Cancer Treat Res Commun. 2022;31: 100547. 10.1016/j.ctarc.2022.100547. [DOI] [PubMed] [Google Scholar]
- 40.Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5:1043–7. 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–8. 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–6. 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deschênes-Simard X, Richard C, Galland L, Blais F, Desilets A, Malo J, et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: A retrospective multicentric cohort study. Thromb Res. 2021;205:29–39. 10.1016/j.thromres.2021.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the results of this study are available from the corresponding author (TI) upon reasonable request.