Abstract
Baculovirus infection has extended the capabilities for transfection of exogenous genes into a variety of mammalian cell types. Because rat hepatocytes plated on collagen-coated dishes and maintained in dimethyl sulfoxide (DMSO)-supplemented chemically defined medium are an excellent model system for studying liver function in vitro, we investigated the ability of baculoviruses to infect and deliver exogenous genes to cells in this culture system. Efficient delivery to hepatocytes in short-term culture becomes restricted to peripheral cells, or “edge” cells, as the hepatocytes acquire intercellular junctions and form islands with time in culture. This barrier to baculovirus entry can be overcome, and the percentage of internal cells within the hepatocyte islands that are infected with the baculovirus can be increased more than 100-fold, when cells are subjected to transient calcium depletion before and during infection. These findings suggest that at least in some cell types, such as hepatocytes, baculovirus entry may require contact with the basolateral surface. We conclude from this study that recombinant baculovirus infection following transient depletion of extracellular calcium results in delivery of exogenous genes to at least 75% of hepatocytes in long-term DMSO culture, thereby making it possible for the first time to carry out gain-of-function and loss-of-function studies in this cell system.
Primary rat hepatocytes, plated on collagen-coated plastic dishes and fed chemically defined medium supplemented with dimethyl sulfoxide (DMSO), can be maintained in a differentiated state for at least 60 days (28, 29). The morphological and biochemical characteristics of these cells are similar to those of hepatocytes in rat liver in vivo. Hepatocytes in culture secrete albumin and retain steady-state mRNA expression of liver-specific and common genes. As in the uninduced in vivo liver, the level of DNA synthesis in these hepatocyte cultures is low (approximately 2.2% of cultured cells) and the hepatocytes do not undergo cell division (29). During the first 3 days following plating, primary rat hepatocytes exist as a single-cell monolayer. Shortly thereafter, the hepatocytes move on the collagen matrix into multicellular islands (28, 29; H. C. Isom, unpublished data). This dynamic motility is observed throughout the lifetime of the culture. As the culture ages, hepatocytes within the islands become tightly packed together and intercellular junctions between neighboring cells are observed by electron microscopy (7, 29).
A barrier to the use of primary hepatocytes for gain-of-function and loss-of-function analyses has been the inability to efficiently transfect DNA into hepatocytes in in vitro culture systems. Although a large number of cationic lipid-based DNA transfection methods are available (6, 16, 17, 24, 30, 39), those that we have tested on primary rat hepatocyte cultures result in very low transgene expression (approximately 1 to 3% [H. C. Isom, unpublished data]). The use of viral gene transfer vectors in primary hepatocyte cultures is promising, but each method has drawbacks. Retrovirus-mediated gene transfer requires division of target cells following viral inoculation (19, 33). Use of this gene delivery system in hepatocytes is severely limited by the nondividing nature of hepatocytes in vivo and in vitro. Artificially stimulating hepatocytes in the liver in vivo to exit the G0 phase of the cell cycle significantly improves retroviral gene transfer efficiency (18, 31, 32). Expression of transgenes from retroviral vectors requires stable insertion of vector sequences into the dividing target cell genome (18). Adenovirus gene transfer vectors have been used for transient gene delivery to quiescent hepatocytes in culture (35). However, it has been demonstrated that adenovirus vectors are toxic at multiplicities of infection (MOI) greater than 100 in the established human hepatic cell line Huh7 (26).
The baculovirus Autographa californica multiple nuclear polyhedrosis virus (AcMNPV), which has been used to generate recombinant proteins in insect cells, is capable of efficiently delivering genes to numerous mammalian cell types (42). The transgene delivered must be under the transcriptional control of a mammalian promoter for gene expression to occur. Several studies have demonstrated that recombinant baculoviruses can deliver transgenes to hepatic cells at efficiencies approaching 100% (4, 10, 12, 25, 26, 40, 42). Previous reports have also demonstrated that baculoviruses can mediate gene delivery into primary human, mouse, rabbit, and rat hepatocytes in culture (4, 26, 40) and to perfused ex vivo human liver segments (25, 40). Injection of baculovirus directly into the mouse liver parenchyma also results in successful gene transfer localized to the injection site (25). In addition, gene transfer to human liver cell tumors in nude mice (generated by transplantation of Huh7 cells) can be achieved by injection of baculovirus (25). The results presented in this report characterize baculovirus-mediated gene delivery into primary rat hepatocytes maintained in a chemically defined medium supplemented with DMSO.
MATERIALS AND METHODS
Cell culture.
Sf21 insect cells (Invitrogen, Carlsbad, Calif.) were maintained in Grace's insect medium supplemented with Yeastolate, lactalbumin hydrolysate (Gibco BRL, Gaithersburg, Md.), and 10% fetal bovine serum (FBS; HyClone, Logan, Utah) in a nonhumidified incubator at 28°C without CO2. Primary rat hepatocytes were maintained in Dulbecco's modified Eagle medium (DMEM)–F12 or RPMI 1640 (Gibco BRL) supplemented with insulin (0.06 μg/ml), glucagon (0.04 μg/ml), dexamethasone (0.4 μg/ml), transferrin (100 μg/ml), epidermal growth factor (EGF; 25 ng/ml; Sigma Chemical Co., St. Louis, Mo.), 1 μM [+]-α-tocopherol (Sigma), and 2% DMSO (Sigma) in a humidified incubator at 37°C with 5% CO2 (28, 29). At designated days postseeding, primary rat hepatocyte cultures were infected with cytomegalovirus (CMV)-lacZ baculovirus at the indicated PFU per cell in either (i) RPMI 1640 supplemented as described above; (ii) DMEM–F12 supplemented as described above (referred to below as control medium) after a 1-h pretreatment in the same medium, or (iii) calcium-free DMEM (Gibco BRL) supplemented in the same manner as the control medium without or with various concentrations of EGTA (Sigma) after 1 h of pretreatment in the same medium. Cultures were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Fisher Biotech, Fair Lawn, N.J.) to assay for β-galactosidase (β-Gal) activity.
Construction of the CMV-lacZ baculovirus.
The pCMVlacZ transfer vector used in producing the CMV-lacZ recombinant baculovirus was constructed based on a derivative of pBacPAK9 (Clontech; Palo Alto, Calif.) with a modified polylinker. This plasmid contains a 758-bp HindIII-XbaI fragment of the human CMV immediate-early promoter excised from pCMV-EBNA (Invitrogen), a 3.0-kb NotI-NotI lacZ fragment, and an 850-bp BamHI-BglII fragment derived from pRSVneo (23) containing simian virus 40 (SV40) splice and polyadenylation sequences. The transfer plasmid was recombined with linear viral DNA from BakPAK6 viral DNA (Clontech) after lipofection of Sf21 cells as described previously (5). The virus was plaque purified three times prior to amplification.
Infection of primary rat hepatocytes with recombinant CMV-lacZ baculovirus.
Primary rat hepatocytes from male Fischer F344 rats (180 to 200 g; Charles River Breeding Laboratories) were isolated and plated as described by Isom et al. (29). At designated days of infection, duplicate plates of cells were trypsinized, and the viable cell number was determined with a hemocytometer using trypan blue exclusion. Average cell counts were calculated and used to determine the volume of high-titer CMV-lacZ baculovirus stock necessary to infect cells at the indicated MOI. Baculovirus stocks and titers were prepared in Sf21 insect cells as described by Delaney and Isom (11). Baculovirus was diluted in either RPMI 1640, DMEM–F12 (control medium), calcium-free DMEM, or calcium-free DMEM supplemented with a designated concentration of EGTA to a final volume of 0.5 ml. Incubation of cultures with baculovirus for less than 40 min resulted in decreased gene transfer efficiencies, whereas no difference was observed if cultures were incubated for ≥1 h (data not shown). Therefore, baculovirus was absorbed to primary rat hepatocytes for 1 h at 37°C with gentle rocking every 15 min to ensure even distribution of the inoculum. Following infection, cultures were washed twice in phosphate-buffered saline (PBS) and then refed with either RPMI 1640 or DMEM–F12 control medium containing calcium.
In situ β-Gal staining with X-Gal.
At designated times following infection with the CMV-lacZ baculovirus, primary rat hepatocyte cultures were washed once with PBS containing 2 mM MgCl2. Cultures were then fixed for 5 min in PBS containing 2% formaldehyde and 0.05% glutaraldehyde. Cultures were washed twice in PBS. Substrate-stain solution (PBS containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, and 1 mg of X-Gal solubilized in dimethyl formamide [DMF]/ml) was applied, and cultures were incubated in a 37°C humidified incubator containing 5% CO2 for appropriate times to allow the appearance of β-Gal activity. Cultures were then rinsed once in PBS and fixed at room temperature for 10 min in 10% phosphate-buffered formalin. Following fixation, cultures were rinsed once in PBS and stored at 4°C in PBS containing 0.2% sodium azide. Cultures were observed for β-Gal activity on an inverted microscope.
Immunohistochemistry.
Twenty-one days after seeding, primary rat hepatocyte cultures were infected with the CMV-lacZ baculovirus as described above. At 3 h postinfection (p.i.), cultures were washed three times in PBS and fixed in 3.7% zinc-buffered formalin for 10 min at room temperature. The following immunohistochemical staining procedure was carried out at room temperature. Cultures were rinsed three times in PBS and stained for the AcMNPV virus using the peroxidase LSAB+ Kit as described by the manufacturer (Dako Corporation, Carpinteria, Calif.). Briefly, endogenous peroxidase was quenched by incubation of cultures with 10% hydrogen peroxide in methanol for 20 min. Cultures were then rinsed three times in PBS. Endogenous biotin was blocked using the Dako Biotin Blocking System according to the manufacturer's instructions. Cultures were incubated in primary anti-AcMNPV antibody or control rabbit immunoglobulin G (IgG) antibody (Vector Laboratories, Inc., Burlingame, Calif.) for 30 min, followed by three washes in PBS. The primary AcMNPV antibody reacts predominantly with the major viral coat protein and nucleocapsid (F. M. Boyce, unpublished data). Cultures were then incubated with a biotinylated anti-rabbit secondary antibody for 30 min and washed four times in PBS. Streptavidin peroxidase was applied to the cultures and incubated for 15 min, followed by five washes in PBS. Cultures were incubated in substrate-chromogen solution until the appearance of brown staining in the anti-AcMNPV specimens. Staining was terminated by five rinses in distilled water. Cultures were counterstained with hematoxylin (Immunon, Pittsburgh, Pa.) for 3 min, rinsed five times in distilled water, incubated in 0.2% ammonia water for 5 min, and rinsed five times in distilled water. Coverslips were applied, and specimens were viewed by standard light-field microscopy.
RESULTS
Baculovirus-mediated gene transfer into primary rat hepatocytes in short-term culture.
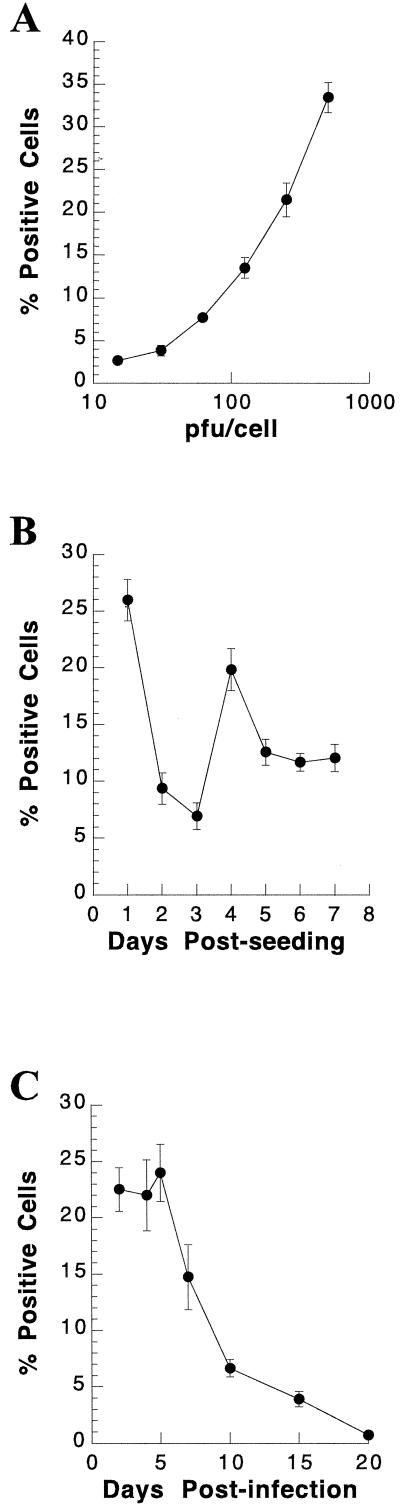
Previously, Rous sarcoma virus (RSV)-lacZ AcMNPV was used to efficiently transfer the lacZ reporter gene to primary rat hepatocytes in short-term culture (4). It was necessary for us to first test whether these studies could be reproduced under the conditions used in our laboratory for short-term culture of primary rat hepatocytes. In addition, we chose to use a baculovirus in which the lacZ gene was driven by the CMV promoter instead of the RSV promoter. CMV-lacZ AcMNPV, generated as described in Materials and Methods, was used for these studies. We assessed the ability of CMV-lacZ AcMNPV to mediate gene transfer into short-term primary hepatocytes. The percentage of cells expressing the reporter gene was directly proportional to the MOI (Fig. 1A). When hepatocytes were seeded at plating densities of 1.0 × 106, 0.5 × 106, 0.25 × 106, and 0.125 × 106 cells/60-mm dish, infected with CMV-lacZ baculovirus, and stained 1 day p.i., no significant change in the percentage of β-Gal-positive cells was observed as seeding density was decreased, suggesting that seeding density had no effect on the efficiency of baculovirus-mediated gene delivery (data not shown). Hepatocytes infected with CMV-lacZ baculovirus at 1, 2, 3, 4, 5, 6, and 7 days postseeding and stained for β-Gal activity 24 h p.i. were most susceptible to baculovirus gene transfer at day 1 postseeding (approximately 25% positive [Fig. 1B]). The reporter gene was detected in 20 to 25% of the cells through day 5 in culture, at which time the percentage of positive cells began to decline (Fig. 1C).
FIG. 1.
Baculovirus-mediated gene transfer into primary rat hepatocytes in short-term culture. (A) Dose dependence of baculovirus-mediated gene transfer into short-term culture. Primary rat hepatocytes were perfused and seeded on collagen-coated dishes. Cultures were infected with CMV-lacZ baculovirus at the indicated multiplicities 24 h postseeding. At 24 h p.i., primary hepatocyte cultures were stained for β-Gal activity as described in Materials and Methods, and the percent β-Gal-positive cells was determined. The mean percent β-Gal-positive cells, with the standard deviation, is shown as a function of the PFU of CMV-lacZ baculovirus/cell used to infect primary rat hepatocyte cultures. (B) Effect of time in culture on susceptibility of primary rat hepatocytes to baculovirus-mediated gene transfer. Primary rat hepatocytes were perfused, seeded on collagen-coated dishes, and infected with 300 PFU of CMV-lacZ baculovirus/cell on the indicated days postseeding. At 24 h p.i., cultures were stained for β-Gal activity and the percent positive cells was determined. Each symbol represents the mean percentage of primary rat hepatocytes at the indicated day postseeding that were β-Gal positive, with the standard deviation. (C) Detection of β-Gal activity at various times after infection of hepatocytes in short-term culture. Primary rat hepatocytes were seeded on collagen-coated dishes and infected with 200 PFU of CMV-lacZ baculovirus/cell 24 h postseeding. Cultures were stained for β-Gal activity at the indicated number of days p.i., and the percent β-Gal-positive cells was determined. Each symbol represents the mean percentage of primary rat hepatocytes that stained β-Gal positive at the indicated day p.i., with the standard deviation.
Dose-dependent baculovirus-mediated gene transfer into primary rat hepatocytes in long-term DMSO culture.
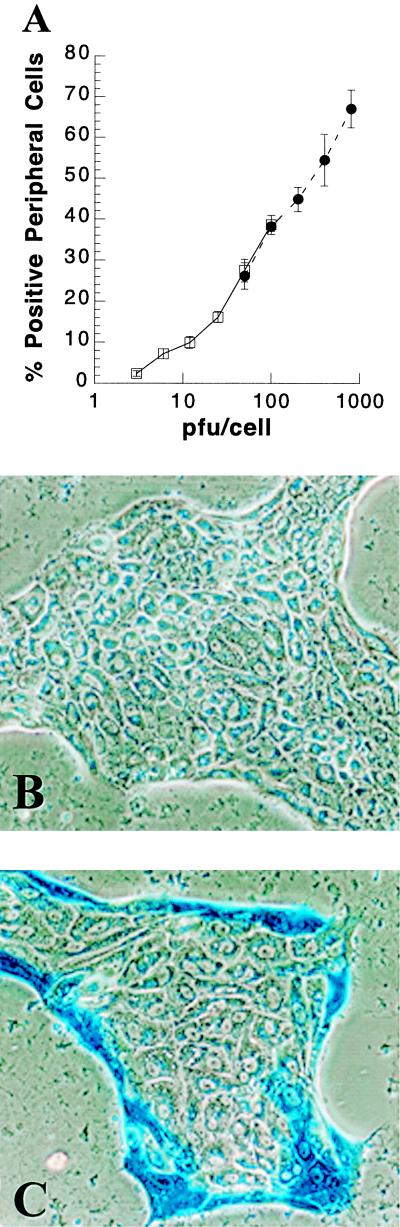
We next assessed the ability of the baculovirus to transfer genes into primary hepatocytes in long-term DMSO culture. During the first week of culture in a chemically defined medium supplemented with DMSO, hepatocytes are evenly distributed on collagen-coated dishes. After 8 to 10 days of culture, the spaces between individual cells begin to disappear and the cells coalesce to form islands. This process continues until approximately day 15 postseeding, after which the overall appearance of the culture does not change for months. The timing of these changes can vary by several days among cultures prepared independently from different rats. Primary hepatocytes in culture for 55 days were infected with CMV-lacZ baculovirus at MOI ranging from 3 to 100. At 24 h p.i., hepatocytes were stained for β-Gal activity and the percent positive cells was determined. Remarkably, while cells on the peripheries of hepatocyte islands expressed β-Gal in a multiplicity-dependent fashion, very few hepatocytes in the interiors of islands stained positive for β-Gal. Because only the peripheral cells, or “edge” cells, expressed the reporter gene, the data were expressed as the percent positive peripheral cells (Fig. 2A). To determine whether the “edge” cell effect could be resolved by increasing the multiplicity, primary hepatocytes in culture for 56 days were infected with higher multiplicities up to 800 PFU of CMV-lacZ baculovirus/cell (Fig. 2A). Approximately 70% of the “edge” or peripheral cells in long-term DMSO culture efficiently expressed the lacZ reporter gene after treatment with 800 PFU of CMV-lacZ baculovirus/cell. Although the percent positive peripheral cells was dose dependent, increasing the MOI did not result in gene delivery to internal hepatocytes (Fig. 2A and B).
FIG. 2.
Dose-dependent baculovirus-mediated gene transfer into primary rat hepatocytes in long-term DMSO culture. (A) Fifty-five days postseeding, primary rat hepatocytes in long-term DMSO culture were infected with 3, 6, 12, 25, 50, or 100 PFU of CMV-lacZ baculovirus/cell. At 24 h p.i., cultures were stained for β-Gal activity and the percent positive peripheral (edge) cells was determined (□). In a separate experiment, primary rat hepatocytes in long-term DMSO culture for 56 days were infected with 50, 100, 200, 400, or 800 PFU of CMV-lacZ baculovirus/cell (●). At 24 h p.i., cultures were stained for β-Gal activity. Results show the percentage of peripheral hepatocytes positive for β-Gal activity, with the standard deviation, at the indicated PFU of CMV-lacZ baculovirus/cell. (B) Primary rat hepatocytes in long-term DMSO culture for 56 days were mock infected and stained for β-Gal activity 24 h later. (C) Primary hepatocytes in long-term DMSO culture for 56 days were infected with 800 PFU of CMV-lacZ baculovirus/cell and stained for β-Gal activity at 24 h p.i.
Detection of β-Gal activity at various times after infection of long-term cultures of primary hepatocytes.
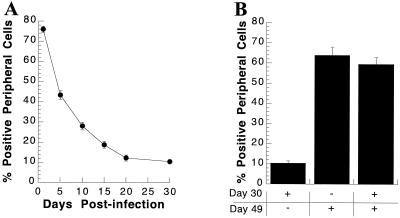
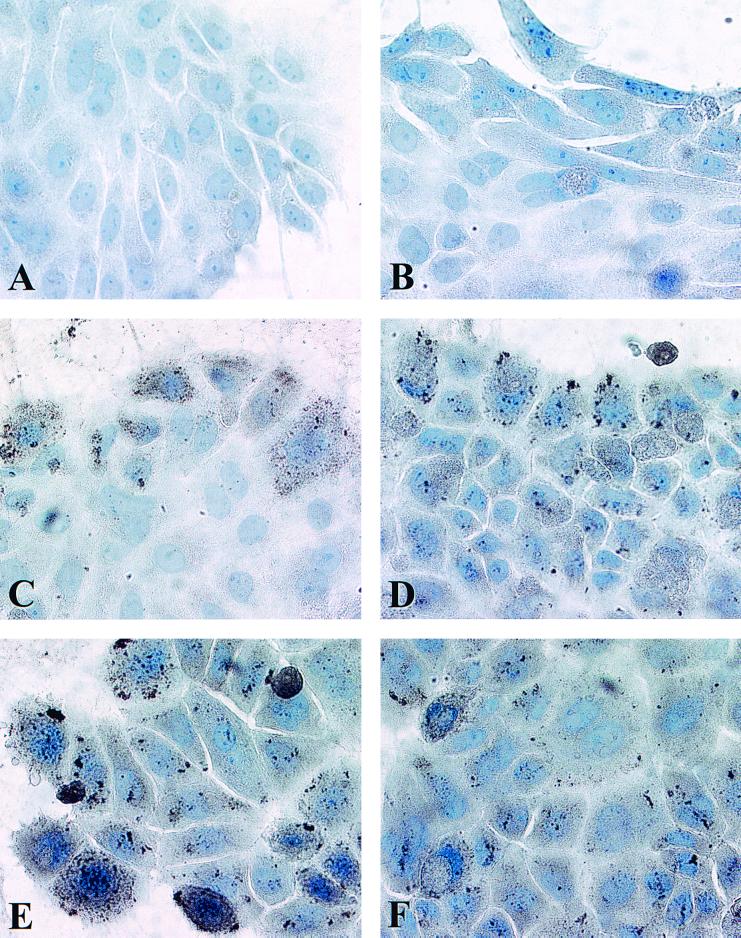
Hepatocytes in culture for 30 days were infected with 400 PFU of CMV-lacZ baculovirus/cell and maintained for an additional 30 days p.i. (Fig. 3A and Fig. 4). Hepatocytes were stained for β-Gal activity, and the percent positive peripheral cells was determined at various times p.i. The percent positive peripheral cells was approximately 75% at 24 h p.i. and decreased progressively with time in culture. However, by day 20, the decrease reached a plateau; on day 20, 11.8% of peripheral island cells stained positive for β-Gal activity, while on day 30, approximately 10% remained positive.
FIG. 3.
Detection of β-Gal activity at various times after infection of long-term cultures of primary hepatocytes and susceptibility of peripheral cells to reinfection with CMV-lacZ baculovirus. (A) Detection of β-Gal activity at various times after infection of a long-term DMSO culture. Thirty days postseeding, primary rat hepatocytes in DMSO culture were infected with 400 PFU of CMV-lacZ baculovirus/cell. At the indicated time p.i., cultures were stained for β-Gal activity and the percent β-Gal-positive peripheral (edge) cells was determined. The results show the percentage of peripheral cells positive for β-Gal activity at the indicated time p.i., with the standard deviation. Where no error bar is shown, the error falls within the size of the symbol. (B) Susceptibility of peripheral cells to reinfection. At day 30 postseeding, hepatocytes were either mock infected or infected with 400 PFU of CMV-lacZ baculovirus/cell. Cultures were divided into three groups. One group of cultures infected on day 30 was mock infected at day 49 (+, −), one group of cultures infected at day 30 was reinfected at day 49 (+, +), and the cultures mock infected at day 30 were infected at day 49 (−, +). All cultures were fixed and stained for β-Gal activity at 50 days postseeding. Each bar represents the mean percentage of peripheral (edge) hepatocytes positive for β-Gal activity, with the standard deviation, following infection at the indicated day postseeding.
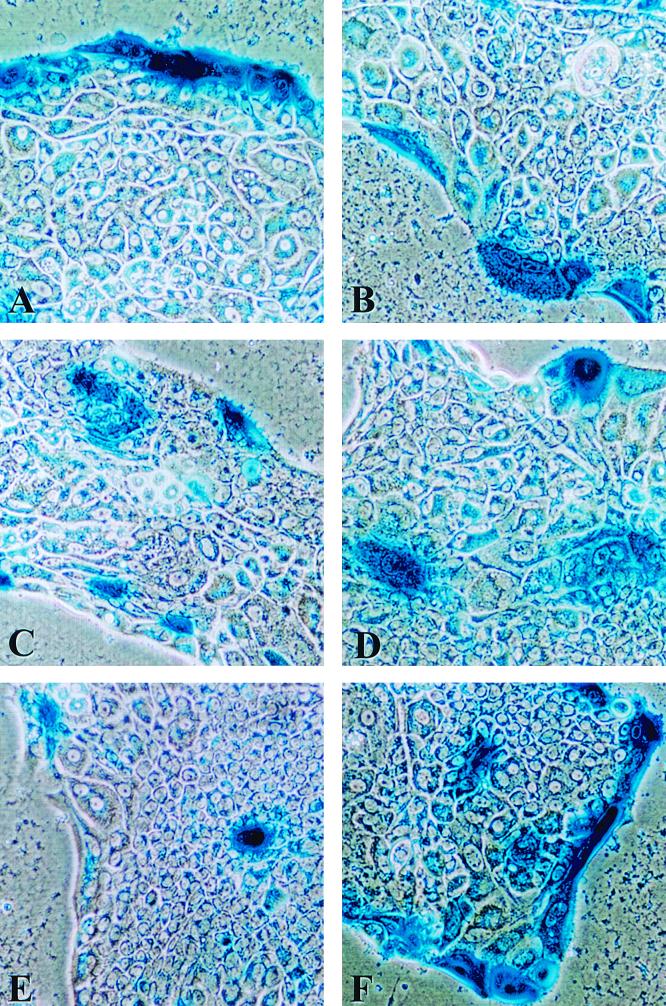
FIG. 4.
Motility of primary hepatocytes in long-term DMSO culture. Primary rat hepatocytes in long-term DMSO culture for 30 days were infected with 400 PFU of CMV-lacZ baculovirus/cell. Cultures were stained for β-Gal activity after 1 (A), 5 (B), 10 (C), 15 (D), and 30 (E) days p.i. (F) Susceptibility to reinfection of peripheral (edge) cells in long-term primary rat hepatocyte cultures maintained in DMSO. A culture infected on day 30 postseeding was reinfected with an additional 400 PFU of CMV-lacZ baculovirus/cell on day 49 and was stained for β-Gal activity at 24 h p.i.
Examination of the cultures also revealed that although the percent β-Gal-positive peripheral cells decreased with time p.i., cells positive for β-Gal also became apparent in the interiors of hepatocyte islands (Fig. 4). The distances between the peripheries of hepatocyte islands and the locations of the β-Gal-positive cells within the islands increased with time. These findings support the concept that even though hepatocytes in long-term DMSO culture do not proliferate, they exhibit motility. Infection with CMV-lacZ baculovirus thus makes it possible to genetically mark cells on the peripheries of hepatocyte islands and monitor their migration with time.
Reinfection of long-term DMSO-cultured hepatocytes with CMV-lacZ baculovirus.
The results from our previous experiment suggested that cells located within hepatocyte islands are resistant to infection with CMV-lacZ but can move to the periphery and become new edge cells with time. It is also likely that many of the initially positive edge cells failed to migrate and simply lost reporter gene expression with time. A question that arose from these studies was whether edge cells that were no longer positive for β-Gal activity remained susceptible to baculovirus-mediated gene transfer. Parallel dishes of primary hepatocytes in culture for 30 days were mock infected or infected with 400 PFU of CMV-lacZ baculovirus/cell. Nineteen days after the initial infection (49 days postseeding), the cells were mock infected or reinfected with another 400 PFU of CMV-lacZ baculovirus/cell. Twenty-four hours after the second infection, hepatocytes were stained for β-Gal activity and the percent positive peripheral cells was determined (Fig. 3B and 4F). Hepatocytes that were not reinfected expressed the reporter gene in only 10% of the edge-cell population. Hepatocytes that were reinfected expressed the reporter gene in 60% of the peripheral cells. When hepatocytes that had been mock infected at day 30 were infected at day 49 for the first time with 400 PFU of CMV-lacZ baculovirus/cell, 63% of the peripheral cells were positive for reporter gene expression. These results demonstrate that essentially the same percentage of peripheral cells was susceptible to baculovirus-mediated gene transfer regardless of whether the infection was an initial infection or a second infection.
Effect of calcium depletion on baculovirus-mediated gene delivery of CMV-lacZ to primary rat hepatocyte cultures.
To use the baculovirus for efficient gene delivery to primary rat hepatocytes in long-term DMSO culture, it was necessary to establish a method that would overcome the “edge” cell effect and ensure homogeneous expression of the exogenously delivered gene within hepatocyte islands. It has recently been reported that infection of Caco-2 intestinal epithelial cell cultures by CMV is inefficient and limited to peripheral cells (15). To overcome this limitation, the investigators pretreated the cultures with 100 mM EGTA in Krebs Ringer solution. The efficiency of CMV infection increased and was homogeneous throughout the culture. When we treated primary rat hepatocytes at EGTA concentrations of 25, 100, 250, or 500 μM in Krebs Ringer solution, the cells displayed a high level of cytopathology (data not shown). However if EGTA was diluted in calcium-free cell culture medium instead of Krebs Ringer solution, no cytopathology was observed at EGTA concentrations below 100 μM.
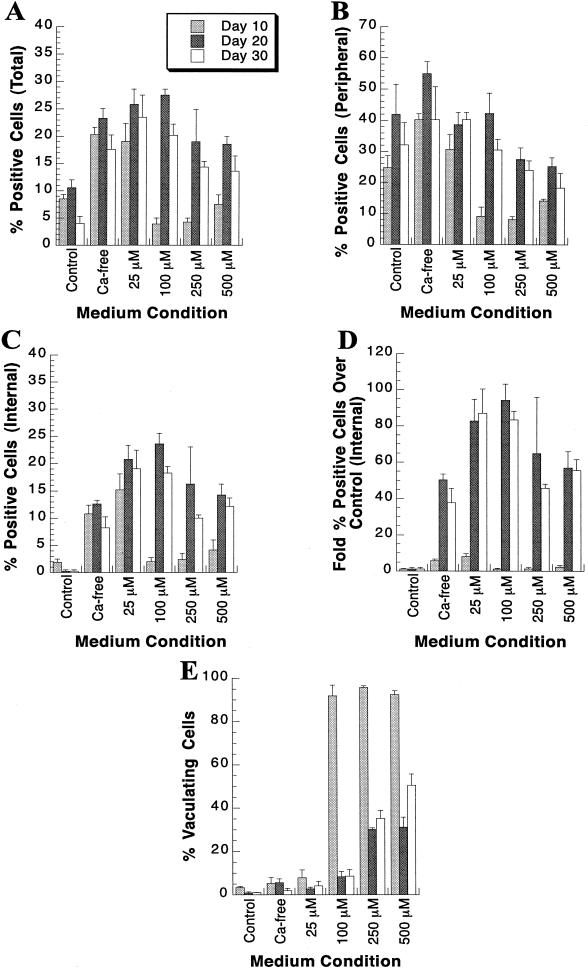
To examine the effect of transient calcium depletion on baculovirus infection of hepatocytes in long-term DMSO culture, three different culture media were tested. Cultures were treated 1 h prior to baculovirus infection and during the 1-h infection with baculovirus diluted in either (i) control medium (as described in Materials and Methods), (ii) calcium-free DMEM (supplemented with the same additives as control medium including DMSO, EGF, and α-tocopherol), referred to below as Ca-free medium, and (iii) calcium-free DMEM supplemented not only with the normal additives but also with varying concentrations of EGTA and referred to subsequently as 25, 100, 250, and 500 μM, respectively. Experiments were carried out with these varying pretreatment conditions on hepatocytes that had been in culture for 10, 20, or 30 days. For all conditions, the MOI was 400 PFU of CMV-lacZ/cell. Several points were readily apparent. Whether hepatocytes were in culture for 10, 20, or 30 days, baculovirus-mediated gene delivery was markedly enhanced when cultures were pretreated and infected in calcium-free medium or calcium-free medium supplemented with 25 μM EGTA (Fig. 5A). In addition, the effects of increasing the EGTA concentration depended on the length of time the hepatocytes were in culture. For cells in culture for 10 days, maximum efficiency was achieved at 25 μM EGTA, while for cells in culture for 20 or 30 days, maximum efficiency was achieved at 100 μM EGTA. Maintenance of DMSO, EGF, and tocopherol in the media was required for maximal gene transfer efficiency (data not shown).
FIG. 5.
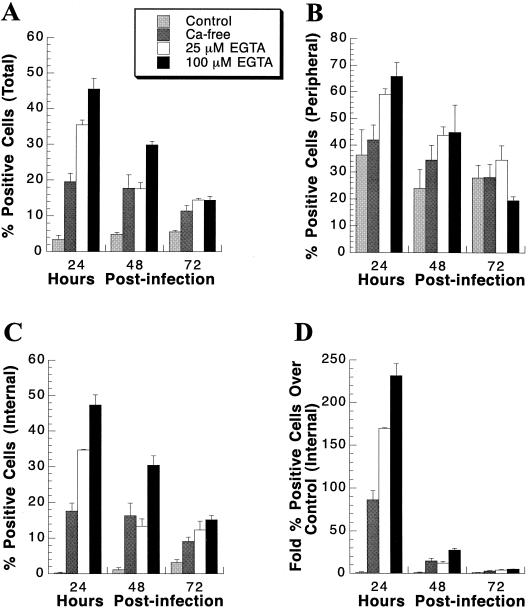
Effect of calcium depletion on baculovirus-mediated gene delivery of CMV-lacZ to primary rat hepatocyte cultures. At the indicated days postseeding, primary rat hepatocytes were pretreated in either control medium (DMEM–F12; designated Control), calcium-free DMEM (Ca-free), or calcium-free DMEM supplemented with either 25 (25 μM), 100 (100 μM), 250 (250 μM), or 500 (500 μM) μM EGTA for 1 h. Subsequently, cultures were infected for 1 h with 400 PFU of CMV-lacZ baculovirus/cell diluted in either control medium, calcium-free DMEM, or calcium-free DMEM supplemented with either 25, 100, 250, or 500 μM EGTA. At 24 h p.i., cultures were stained for β-Gal activity. Percent β-Gal-positive cells was determined for the total culture (A), the peripheral cells (B), and the internal cells (C). (D) Fold increase in internal β-Gal-positive cells following extracellular calcium depletion relative to the control medium. Each bar represents the mean percent β-Gal-positive primary rat hepatocytes, with the standard deviation, at the indicated medium condition. (E) Percent cytopathic cells present in the primary rat hepatocyte cultures following calcium depletion prior to and during baculovirus infection. Each bar represents the mean percent cytopathic cells at the indicated medium condition, with the standard deviation.
Examination of the cultures following staining for β-Gal activity revealed that upon calcium depletion, gene transfer was no longer limited to peripheral cells and was homogeneous throughout the hepatocyte islands (Fig. 6). To quantify the changes in gene delivery characteristics upon calcium depletion, the percentages of peripheral and internal cells expressing β-Gal activity were determined. The difference in the percent β-Gal-positive peripheral cells upon calcium depletion, compared to that for cells pretreated and infected with control medium, was not significant at day 20 or 30 (Fig. 5B). A decrease in positive peripheral cells was observed at day 10 when EGTA concentrations of 100 μM or higher were used (Fig. 5B). Conversely, a significant increase in the percent β-Gal-positive internal cells was observed following pretreatment and infection under calcium-depleted conditions (Fig. 5C). The data were also calculated in terms of fold increase over control medium (Fig. 5D). For example, for cells in culture for 20 days, the level of β-Gal-positive internal cells was 94-fold higher when cells were pretreated and infected in calcium-free medium supplemented with 100 μM EGTA than when they were pretreated and infected with control medium.
FIG. 6.
Effect of calcium depletion on baculovirus-mediated gene delivery to internal hepatocytes. At day 20 postseeding, primary rat hepatocytes were pretreated in either control medium, calcium-free DMEM, or calcium-free DMEM supplemented with either 25 or 100 μM EGTA for 1 h. Subsequently, cultures were infected for 1 h with 400 PFU of CMV-lacZ baculovirus/cell diluted in either control medium (A), calcium-free DMEM (B), or calcium-free DMEM supplemented with either 25 (C) or 100 (D) μM EGTA. At 24 h p.i., cultures were stained for β-Gal activity.
To evaluate if the calcium depletion conditions employed in our baculovirus-mediated gene transfer system were cytopathic, the percentage of vacuolating cells was determined following pretreatment and infection under calcium-free or EGTA calcium chelation conditions. No cytopathology was observed when control medium, calcium-free medium , or 25 μM EGTA medium was used (Fig. 5E). However, concentrations of EGTA greater than 25 μM were cytopathic to day-10 cultures. Cytopathology was not observed in day-20 or day-30 cultures until concentrations of EGTA exceeded 100 μM. Thus, to achieve maximal efficiency of baculovirus-mediated gene transfer to hepatocytes in culture for less than 20 days, cultures should be pretreated and infected in calcium-free DMEM supplemented with 25 μM EGTA. Hepatocytes in culture for more than 20 days should be pretreated and infected at an EGTA concentration of 100 μM to achieve maximal gene transfer efficiency.
Localization of baculovirus to internal cells within hepatocyte islands as a function of transient calcium depletion.
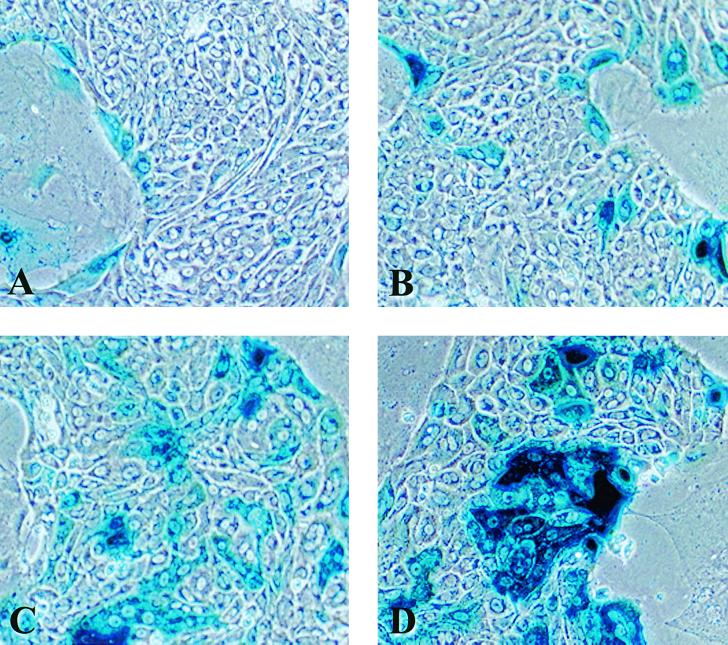
In the studies described above, the ability of CMV-lacZ baculovirus to “infect” hepatocytes was determined indirectly by assaying for β-Gal activity. We next directly measured the effect of calcium depletion on the ability of baculoviruses to be taken-up by internal cells of hepatocyte islands. Primary rat hepatocyte cultures infected with CMV-lacZ baculovirus were fixed and stained for AcMNPV. Because no viral progeny are produced after “infection” into primary hepatocytes, staining detects localization of the inoculated virus. Primary rat hepatocytes at 21 days postseeding were pretreated and either infected with 400 PFU of CMV-lacZ baculovirus/cell or mock infected (Fig. 7). Cultures were then stained for AcMNPV at 3 h p.i. Mock-infected cultures incubated with primary antibody to AcMNPV (Fig. 7A) and CMV-lacZ-infected cultures in control medium incubated with a rabbit IgG control antibody (Fig. 7B) were negative for immunoperoxidase staining. In cultures pretreated and infected in control medium, staining was restricted to peripheral cells (Fig. 7C). When cells were pretreated and infected in calcium-free DMEM, staining for CMV-lacZ baculovirus was present in the peripheral cells, but internal cells were also stained (Fig. 7D). Cultures pretreated and infected under stringent calcium depletion conditions of 25 and 100 μM EGTA displayed increased staining of peripheral cells and homogeneous staining throughout the culture (Fig. 7E and F). Therefore, the increased efficiency and homogeneity of β-Gal activity can be attributed to the increased availability of the internal cells to bind and internalize the CMV-lacZ baculovirus.
FIG. 7.
Immunoperoxidase staining for AcMNPV in primary rat hepatocytes. Twenty-one days postseeding, primary rat hepatocytes were pretreated in either control medium, calcium-free DMEM, or calcium-free DMEM supplemented with either 25 or 100 μM EGTA for 1 h. Subsequently, cultures were infected for 1 h with 400 PFU of CMV-lacZ baculovirus/cell diluted in either control medium, calcium-free DMEM, or calcium-free DMEM supplemented with either 25 or 100 μM EGTA. At 3 h p.i., cultures were fixed and incubated with an antibody to AcMNPV (A, C, D, E, and F) or a control antibody (B) as described in Materials and Methods. (A) Primary rat hepatocytes at 21 days postseeding that were pretreated and mock infected in control medium. (B) Cultures pretreated and infected with the CMV-lacZ baculovirus in control medium and incubated with a rabbit IgG control antibody. (C) Cultures pretreated and infected in control medium. (D) Cultures pretreated and infected in calcium-free DMEM. (E and F) Cultures pretreated and infected under calcium depletion conditions of 25 and 100 μM, respectively.
Determination of the proper pretreatment time prior to infection affording the maximal gene delivery efficiency.
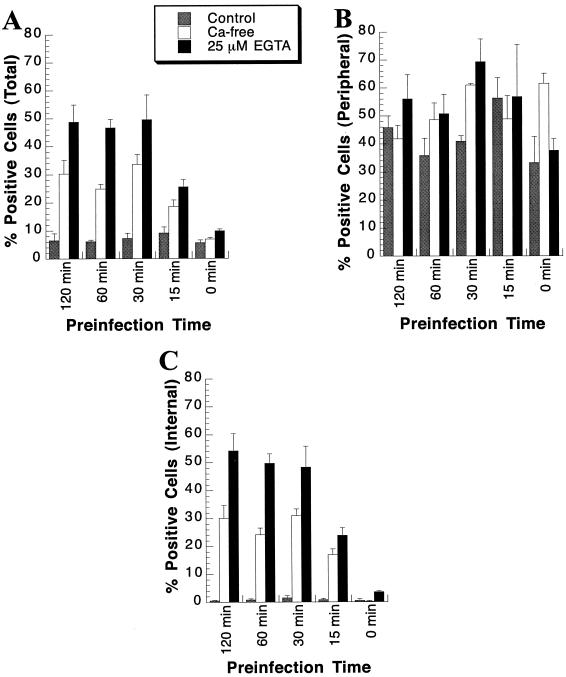
In the calcium depletion experiments described above, a 1-h preincubation time in calcium-free medium with or without EGTA supplementation was used. To ensure that the 1-h preincubation was sufficient to allow for enhanced efficiency of baculovirus-mediated gene transfer, a time course experiment with varying pretreatment times was performed on day-20 cultures. A multiplicity of 400 PFU of CMV-lacZ baculovirus/cell was used for the studies whether cells were preincubated in control medium or calcium-free DMEM with or without 25 μM EGTA supplementation. Gene transfer efficiency reached a maximum by 30 min and stayed at a plateau thereafter (Fig. 8A). As indicated in Fig. 8B, the percent β-Gal-positive peripheral hepatocytes was not significantly affected by varying the calcium depletion pretreatment times. The percentage of internal hepatocytes expressing β-Gal following gene delivery was maximal when calcium was depleted 30 min prior to infection and plateaued thereafter (Fig 8C). Since no distinction was observed between the 30-, 60-, and 120-min calcium depletion pretreatments, the 60-min pretreatment time was selected as the optimum time.
FIG. 8.
Determination of the proper pretreatment time prior to infection affording the maximal baculovirus-mediated gene delivery efficiency. At 20 days postseeding, primary rat hepatocytes were pretreated for the indicated times in either control medium (shaded bars), calcium-free DMEM (open bars), or calcium-free DMEM supplemented with 25 μM EGTA (solid bars). Subsequently, cultures were infected for 1 h with 400 PFU of CMV-lacZ baculovirus/cell diluted in either control medium, calcium-free DMEM, or calcium-free DMEM supplemented with 25 μM EGTA. At 24 h p.i., cultures were stained for β-Gal activity. The percent β-Gal-positive cells was determined for the total culture (A), the peripheral cells (B), and the internal cells (C). Each bar represents the mean percent β-Gal-positive primary rat hepatocytes, with the standard deviation, at the indicated pretreatment time prior to CMV-lacZ baculovirus infection.
Detection of β-Gal activity at various times after infection of long-term cultures of primary rat hepatocytes following calcium depletion.
Transfection under nonselective conditions via chemical methods results in short-term gene expression that decreases within hours or days of gene transfer. This response depends on the cell type used, the gene expressed, and the promoter that drives the expression of the transgene. Baculovirus-mediated gene delivery to mammalian cells represents a transient system of gene transfection. To determine the β-Gal activity at various times p.i. following calcium depletion in long-term primary rat hepatocytes at day 20 postseeding, the following experiment was performed. Cultures were pretreated in control medium, calcium-free medium, or calcium-free medium supplemented with EGTA, infected with 400 PFU of CMV-lacZ baculovirus/cell for 1 h in the corresponding medium condition, and refed with control medium containing calcium. At 24, 48, and 72 h p.i, cultures were fixed and stained for β-Gal activity. Maximal β-Gal activity was observed at 24 h p.i. (Fig. 9A). In all pretreatment and infection medium conditions, β-Gal activity decreased over time p.i. Although a slight increase in the percentage of peripheral cells positive for β-Gal activity was observed upon calcium depletion at 24 h p.i (Fig. 9B), the major effect of calcium depletion on β-Gal activity was observed in internal hepatocytes (Fig 9C). In agreement with previous results, the percent β-Gal-positive internal cells at 24 h p.i. increased from 0.2% for the control medium condition to approximately 17.6, 34.7, and 47.4% for the calcium-free medium and 25 and 100 μM EGTA concentrations, respectively. At 24 h p.i., the percent β-Gal-positive internal hepatocytes was 232-fold higher when cultures were pretreated and infected in calcium-free medium supplemented with 100 μM EGTA than when they were pretreated and infected in control medium (Fig. 9D). This induction decreased by 48 h and even further by 72 h.
FIG. 9.
Detection of β-Gal activity at various times after infection of long-term cultures of primary hepatocytes following calcium depletion. Twenty days postseeding, primary rat hepatocytes were pretreated in either control medium (light shaded bars), calcium-free DMEM (dark shaded bars), or calcium-free DMEM supplemented with either 25 (open bars) or 100 (solid bars) μM EGTA for 1 h. Subsequently, cultures were infected for 1 h with 400 PFU of CMV-lacZ baculovirus/cell diluted in either control medium, calcium-free DMEM, or calcium-free DMEM supplemented with either 25 or 100 μM EGTA. At the indicated times p.i., cultures were stained for β-Gal activity. The percent β-Gal-positive cells was determined for the total culture (A), the peripheral cells (B), and the internal cells (C). (D) Fold increase in internal β-Gal-positive cells following extracellular calcium depletion relative to β-Gal-positive internal cells in the control medium. Each bar represents the mean percent β-Gal-positive primary rat hepatocytes at the indicated time p.i., with the standard deviation.
Increased dose-dependent gene transfer into primary rat hepatocytes under calcium-depleted conditions.
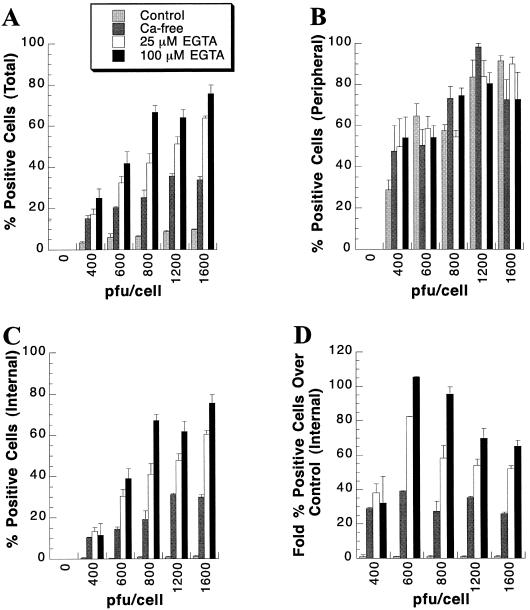
As shown in Fig. 2, we observed that baculovirus-mediated gene transfer into long-term primary rat hepatocytes was dose dependent, but limited to peripheral cells, in medium containing calcium. Similarly, we observed that, upon calcium depletion, the efficiency of gene transfer was markedly increased in hepatocyte islands; however, the latter studies were performed at an input multiplicity of 400 PFU of CMV-lacZ baculovirus/cell. To determine whether even greater gene transfer efficiency could be achieved upon calcium depletion by increasing the dose of baculovirus inoculation, primary rat hepatocyte cultures were pretreated with control medium, calcium-free DMEM, or calcium-free DMEM supplemented with 25 or 100 μM EGTA for 1 h. Cultures were then infected with increasing MOI of CMV-lacZ baculovirus diluted in the corresponding medium for 1 h and refed with control medium containing calcium. Cultures were stained for β-Gal activity 24 h p.i. As shown in Fig. 10A, a dose-dependent increase in baculovirus-mediated gene transfer was observed. At each MOI, the percent β-Gal-positive cells was greater with increased calcium depletion stringency. The highest percent β-Gal-positive cells was observed in the 100 μM EGTA medium condition infected with 1,600 PFU of CMV-lacZ baculovirus/cell. Whereas only approximately 9.7% of cells were β-Gal positive in the control medium condition when infection was at 1,600 PFU of CMV-lacZ baculovirus/cell, approximately 75.6% of hepatocytes were positive in the 100 μM EGTA pretreatment and infection condition. No cytopathology was observed when cultures were infected with 1,600 PFU of CMV-lacZ baculovirus/cell (data not shown).
FIG. 10.
Increased dose-dependent gene transfer into primary rat hepatocytes under calcium-depleted conditions. Twenty-one days postseeding, primary rat hepatocytes were pretreated in either control medium (light shaded bars), calcium-free DMEM (dark shaded bars), or calcium-free DMEM supplemented with either 25 (open bars) or 100 (solid bars) μM EGTA for 1 h. Subsequently, cultures were infected for 1 h with the indicated MOI of CMV-lacZ baculovirus/cell diluted in either control medium, calcium-free DMEM, or calcium-free DMEM supplemented with either 25 or 100 μM EGTA. At 24 h p.i., cultures were stained for β-Gal activity. The percent β-Gal-positive cells was determined for the total culture (A), the peripheral cells (B), and the internal cells (C). (D) Fold increase in internal β-Gal-positive cells following extracellular calcium depletion over the level in control medium. Each bar represents the mean percent β-Gal-positive primary rat hepatocytes at the indicated PFU of CMV-lacZ baculovirus/cell, with the standard deviation.
The percent β-Gal-positive peripheral cells increased slightly with increasing MOI, but no significant difference was observed between control and calcium depletion pretreatment and infection medium conditions (Fig. 10B). In contrast, the percent β-Gal-positive internal hepatocytes clearly increased in a dose-dependent manner (Fig. 10C). As expected, the percentage of internal hepatocytes expressing β-Gal after gene transfer also increased significantly with increased calcium depletion stringency. For example, at 1,600 PFU of CMV-lacZ baculovirus/cell, the percent positive internal cells under control medium conditions was approximately 1.2%, but it increased to approximately 75.3% in calcium-free DMEM supplemented with 100 μM EGTA. This represents a 65-fold increase in internal cells positive for baculovirus-mediated gene transfer (Fig. 10D). An even greater increase in internal cells positive for baculovirus-mediated gene transfer compared to the control medium condition of 105-fold was observed when cultures were infected at 600 PFU of CMV-lacZ baculovirus/cell. As no significant difference in the percent β-Gal-positive peripheral cells was observed between medium conditions, the increase in total cells positive for gene transfer is attributed to the increase in internal hepatocytes expressing the delivered transgene.
DISCUSSION
Based on the results presented in this paper, we conclude the following. (i) The previously reported findings that baculovirus can mediate efficient gene transfer into primary hepatocytes in short-term culture (4, 26, 40) have been reproduced using a system in which primary rat hepatocytes are plated on rat tail collagen-coated plates and fed a chemically defined medium supplemented with DMSO. (ii) When hepatocytes in DMSO culture migrate to form islands (approximately 8 to 10 days postseeding and thereafter), baculovirus-mediated gene delivery is restricted to the peripheral (edge) cells. Reporter gene expression is observed only in peripheral cells because the baculovirus is able to gain entry only into peripheral cells. (iii) Hepatocytes in islands in long-term DMSO culture are in a dynamic state; that is, peripheral cells move to become external cells and vice versa. (iv) Baculovirus-mediated gene transfer efficiency in hepatocytes in long-term DMSO culture can be enhanced by transient calcium depletion. The enhanced efficiency is directly related to uptake of baculovirus by internal cells in hepatocyte islands. The optimal method for calcium depletion without toxicity varies depending on whether the hepatocytes have been in culture for 10 days or for 20 to 30 days. (v) Baculovirus-mediated gene delivery is dose dependent both with regard to peripheral cells and with regard to internal cells. (vi) The findings described in this study make it possible for the first time to use baculoviruses to deliver genes at high efficiency to primary rat hepatocytes in long-term DMSO culture; specifically, at a multiplicity of 1,600 PFU of CMV-lacZ baculovirus/hepatocyte following transient calcium depletion, β-Gal activity was detected in 75% of the cells. No significant cytopathology was observed at the EGTA concentrations or MOIs affording the maximal gene transfer efficiency. A previous study reported that cytopathology was not observed when HepG2 cells were infected at a multiplicity of 5,000 PFU of baculovirus/cell (14). Therefore, it is possible that the percentage of primary rat hepatocytes positive for baculovirus-mediated gene transfer may be increased to >75% if the MOI is increased to more than 1,600 PFU/cell.
It has previously been reported that recombinant baculovirus containing a gene under the control of a mammalian promoter could mediate delivery of that gene to primary rat hepatocytes in short-term culture (4, 26, 40). Therefore, before addressing the question for hepatocytes in long-term culture, it was necessary to reinvestigate this finding in short-term primary rat hepatocytes plated on rat tail collagen and fed a chemically defined medium supplemented with DMSO. In addition, the results of the studies in short-term cultures led to some important novel conclusions. We determined that (i) baculovirus is an efficient mechanism for gene delivery to primary rat hepatocytes fed a chemically defined medium supplemented with DMSO, (ii) this process is dose dependent and is independent of the initial seeding density of the hepatocytes, (iii) baculovirus-mediated gene transfer in short-term primary rat hepatocytes is transient, and (iv) β-Gal activity is detectable in 20 to 25% of the cells for at least 5 days after recombinant baculovirus infection.
In carrying out experiments to determine the effect of time in culture of hepatocytes in the DMSO culture system on susceptibility to baculovirus-mediated gene transfer, it became readily apparent that the dynamic movement of the hepatocytes into islands markedly affected gene transfer efficiency. Time lapse photomicroscopy of hepatocytes plated on rat tail collagen-coated dishes etched with gridlines had previously indicated that hepatocytes in long-term DMSO culture exhibit motility (H. C. Isom, unpublished data). The data reported in this study, in particular those shown in Fig. 4, further support this concept. Specifically, in studying the detection of β-Gal activity over time p.i. in peripheral hepatocytes in long-term DMSO culture, we found that cells expressing β-Gal disappeared from the island periphery and cells expressing β-Gal appeared internally, suggesting that the peripheral cells had migrated and become internal cells and vice versa. In addition, the superinfection study (Fig. 3 and 4) indicated that once an internal cell assumed the position of being a peripheral cell, it became capable of being infected by baculovirus and hence a recipient of the gene being transferred. This system should also make it possible to monitor hepatocyte movement. Specifically, if hepatocytes in long-term culture are infected with recombinant baculovirus containing the gene for the green fluorescence protein (GFP), it should be possible with time lapse fluorescence microscopy to monitor the movement of hepatocytes.
Although the ability to efficiently deliver exogenous genes to hepatocytes in short-term DMSO culture using baculovirus is important because it makes possible many gain-of-function and loss-of-function studies that could not otherwise be achieved, our goal was to pursue baculovirus-mediated gene delivery to hepatocytes in long-term culture because of the many advantageous characteristics of the long-term culture system. Primary rat hepatocytes plated on rat tail collagen-coated plates can be maintained in serum-free, chemically defined medium supplemented with DMSO for more than 1 year (28, 29). As in the in vivo liver, hepatocytes under routine culture conditions retain hepatocyte morphology, secrete high levels of albumin, and do not synthesize DNA or proliferate (27, 28). The cells are mononucleated and binucleated, arranging themselves in specific patterns within multicellular islands that do not completely fill the culture dish. Ultrastructural analysis has shown that the hepatocytes contain large numbers of mitochondria and extensive rough and smooth endoplasmic reticulum, indicating that the cells are intact and metabolically active (29). Cell-cell junctions with desmosomes are apparent, as are numerous bile canaliculi with microvilli. The cells contain regular circular nuclei with prominent nucleoli and a low nucleus-to-cytoplasm ratio (7). This long-term culture system has been used to study molecular mechanisms of albumin expression (28, 29), immortalization and transformation of hepatocytes (49–51), and DNA synthesis (8, 41). This system also lends itself to studies on cell death because there are essentially no background apoptotic cells (3). The ability of hepatocytes in long-term DMSO culture to retain liver-specific gene expression and structural integrity at a plateau for a long time is advantageous for many types of analyses. For example, the cells can be treated with drugs, growth factors, or other agents for extended times prior to the transient introduction of exogenous genes.
Restricted expression of the reporter gene to only the peripheral cells of cell islands in hepatocytes in long-term DMSO culture was unexpected. Previously, we characterized the peripheral cells of hepatocytes in long-term culture and demonstrated that they are the same as hepatocytes within the islands with regard to albumin expression and ultrastructure (8). Therefore, there was no a priori reason to believe that these cells would differ with regard to gene expression or membrane protein receptor composition. One possibility was that the CMV promoter could function only in peripheral cells. This option seemed unlikely because we had previously demonstrated, using transfection with plasmid DNA, that a CMV promoter was capable of driving the lacZ gene in hepatocytes within islands as well as cells at the periphery (data not shown). Although the transfection efficiencies are extremely low (1% for calcium phosphate and 3% for lipofectin), we observed that the distribution of β-Gal-positive cells was random. An alternative possibility was that the baculovirus was able to get into peripheral cells only. Immunohistochemical analysis using an antibody to the AcMNPV baculovirus indicated that this was the case (Fig. 7). Our initial concern was that there was some physical limitation causing preferential attachment of the baculovirus to the peripheral cells. However, altering several technical variables such as the volume of the virus inoculum used for adsorption, time of adsorption, etc., had no effect (data not shown).
We hypothesized that the baculovirus was only able to bind to, and deliver its genome to, peripheral cells because cell-cell junctions masked the baculovirus binding motif, preventing baculovirus attachment to, and entry into, internal hepatocytes. Several studies have determined that disruption of calcium-dependent intercellular junctions by EGTA, a calcium chelator, improves viral infection efficiency in various tissues and polarized cell cultures (15, 46–48). We can conclude from this study that baculovirus, at least with regard to infection of hepatocytes, can now be added to this list. Specifically, we showed that the efficiency of gene delivery by a recombinant baculovirus increased significantly when extracellular calcium was depleted using EGTA. Transient depletion of extracellular calcium appears to overcome the inefficiency of baculovirus-mediated gene transfer at the basolateral surface by opening intercellular epithelial junctions. This technique may be applicable for delivery of other vectors or drugs to polarized epithelia or other tissues. In addition, this finding provides further support for the concept that hepatocytes in long-term DMSO culture have intercellular junctional complexes.
Intercellular junctional complexes separate the apical and basal regions and enable epithelial cells to maintain cell polarity and an electrically tight barrier between epithelial and endothelial cells. Junctional complexes found in rat hepatocytes include the calcium-dependent tight junctions, adherens junctions, and desmosomes, as well as gap junctions, the activity of which is independent of extracellular calcium stores (2, 21, 22, 38, 43, 44). Tight junctions, partially composed of occludin and zona occludens among other proteins, are the apical-most complexes that form continuous contacts and limit paracellular movement of molecules between neighboring cells (13, 37, 48). Adherens junctions are specialized cadherin-based adhesive contacts located below the tight junction in the junctional complex. The binding of cadherins to cytoplasmic catenins, in association with the actin cytoskeleton, couples cell-cell adhesion to tissue morphology (52). Below the adherens junctions are the desmosomes, which are button-like structures composed of the calcium-binding desmoglein and desmocollin glycoproteins. Desmosomes mediate cell-cell adhesion and provide anchoring sites for cytoplasmic intermediate filaments (13, 48). The importance of calcium in the synthesis and maintenance of epithelial tight junctions, adherens junctions, and desmosomes has been well established (1, 9, 13, 48). Therefore, depletion of extracellular calcium by chelators, such as EGTA, opens the interhepatocellular epithelial junctional complexes. This exposes the basolateral surfaces of internal hepatocytes within islands in long-term DMSO culture to baculovirus infection and subsequent gene delivery.
The mechanism by which baculovirus DNA is introduced into a mammalian cell has been partially elucidated. Although the binding motif responsible for baculovirus endocytosis in mammalian cells is currently unknown, heparan sulfate is required for efficient gene transfer (14). The data presented in this study demonstrate that at least in some cell types, such as hepatocytes, entry of the baculovirus may require contact with the basolateral surface. After endocytosis, the acidification of the endosome releases the baculovirus into the cytoplasm, where it localizes to the nucleus in an actin-dependent manner. Baculovirus then enters through the nuclear pores in nonmitotic cells, and hence baculovirus can enter and release its genome into the nuclei of nondividing cells (45).
The question of whether baculovirus can infect nondividing mammalian cells has been addressed recently. Van Loo et al. (45) demonstrated that baculovirus infects nondividing mammalian epithelial pig kidney cells (Pk-1) arrested in S phase. Pk-1 cells were infected with baculovirus expressing GFP 12 h after seeding in the presence of aphidicolin, a reversible inhibitor of DNA polymerase, and were analyzed for GFP expression 12 h after infection. Pk-1 cells arrested in G1/S were infected as efficiently as unarrested cells. Electron microscopy showed that the baculovirus nucleocapsids, containing the electron-dense genome, appear to dock on the nuclear pores of infected cells and enter the nuclei of the cell cycle-arrested Pk-1 cells. While this evidence indicated that baculovirus is able to infect nondividing cells, cell division of Pk-1 cells was inhibited by aphidicolin treatment. Primary rat hepatocytes plated on rat tail collagen-coated plates and maintained in a serum-free, chemically defined medium supplemented with DMSO do not synthesize DNA or proliferate under routine culture conditions (27–29) and, as such, represent a more natural system which does not require the use of a cell cycle inhibitor to address this question. Our data show that baculovirus infects nondividing primary rat hepatocytes, presumably releasing its genome into the nucleus, as evidenced by expression of the recombinant lacZ transgene contained within the baculovirus genome. Therefore our findings confirm and extend previous work showing that nondividing mammalian cells can be infected by baculovirus.
Initial studies suggested that baculovirus-mediated gene transfer to mammalian cells could be achieved predominantly in hepatic cells (4, 26). Continued analysis has shown that baculovirus can infect and mediate gene transfer to cell types other than hepatic cells (14, 36, 42, 45). However, this system has not proven applicable to all cell types. Our finding regarding the need for transient breakage of intercellular linkages to allow baculovirus to enter hepatocytes may be applicable to other cell types previously thought not to be infectible by baculovirus.
The baculovirus has several features that make it attractive as a vehicle for gene transfer: (i) it can harbor very large pieces of DNA (20, 34), (ii) large quantities of recombinant baculovirus can easily be produced and purified from cultured insect cells, (iii) baculovirus can efficiently infect nondividing cells, and (iv) baculovirus is not cytopathic. For these reasons, it was particularly important to establish whether baculovirus could be used to deliver genes to hepatocytes in long-term DMSO culture. We conclude from this study that recombinant baculovirus infection following transient depletion of extracellular calcium results in delivery of exogenous genes to at least 75% of hepatocytes in long-term DMSO culture, thereby making it possible for the first time to carry out gain-of-function and loss-of-function studies in this cell system.
ACKNOWLEDGMENTS
We thank Colleen Kelley, Tom Miller, Michelle Wible, Rick Yeager, Michele Yon, and Nicole Zandy for excellent technical assistance. We also thank Edward E. Cable for helpful discussions and assistance in preparation of the manuscript.
This work was supported in part by research grants from the National Institutes of Health (CA23931, CA73045, DK53430, and DK54482 to H.C.I. and DK49088 to F.M.B.).
REFERENCES
- 1.Bhat M, Toledo-Velasquez D, Wang L, Malanga C J, Ma J K H, Rojanasakul Y. Regulation of tight junction permeability by calcium mediators and cell cytoskeleton in rabbit tracheal epithelium. Pharm Res. 1993;10:991–997. doi: 10.1023/a:1018906504944. [DOI] [PubMed] [Google Scholar]
- 2.Borrmann C M, Mertens C, Schmidt A, Langbein L, Kuhn C, Franke W W. Molecular diversity of plaques of epithelial-adhering junctions. Ann N Y Acad Sci. 2000;915:144–150. doi: 10.1111/j.1749-6632.2000.tb05237.x. [DOI] [PubMed] [Google Scholar]
- 3.Bour E S, Ward L K, Cornman G A, Isom H C. Tumor necrosis factor-alpha-induced apoptosis in hepatocytes in long-term culture. Am J Pathol. 1996;148:485–495. [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce F M, Bucher N L. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce F M, Franco E A. High-efficiency transduction of mammalian cells using baculovirus: practical aspects. In: Cid-Arrequi A, Garcia-Carranca A, editors. Viral vectors: basic science and gene therapy. Natick, Mass: Eaton Publishing; 2000. pp. 359–367. [Google Scholar]
- 6.Breiner K M, Urban S, Schaller H. Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses. J Virol. 1998;72:8098–8104. doi: 10.1128/jvi.72.10.8098-8104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cable E E, Connor J R, Isom H C. Accumulation of iron by primary rat hepatocytes in long-term culture: changes in nuclear shape mediated by non-transferrin-bound forms of iron. Am J Pathol. 1998;152:781–792. [PMC free article] [PubMed] [Google Scholar]
- 8.Cable E E, Isom H C. Exposure of primary rat hepatocytes in long-term DMSO culture to selected transition metals induces hepatocyte proliferation and formation of duct-like structures. Hepatology. 1997;26:1444–1457. doi: 10.1002/hep.510260611. [DOI] [PubMed] [Google Scholar]
- 9.Cereijido M, Valdes J, Shoshani L, Contreras R G. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161–177. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- 10.Condreay J P, Witherspoon S M, Clay W C, Kost T A. Transient and stable gene expression in mammalian cells transduced with recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaney W E, IV, Isom H C. Hepatitis B virus replication in human HepG2 cells mediated by hepatitis B virus recombinant baculovirus. Hepatology. 1998;28:1134–1146. doi: 10.1002/hep.510280432. [DOI] [PubMed] [Google Scholar]
- 12.Delaney W E, IV, Miller T G, Isom H C. Use of the hepatitis B virus recombinant baculovirus-HepG2 system to study the effects of (−)-β-2′,3′-dideoxy-3′-thiacytidine on replication of hepatitis B virus and accumulation of covalently closed circular DNA. Antimicrob Agents Chemother. 1999;43:2017–2026. doi: 10.1128/aac.43.8.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denker B M, Nigam S K. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 14.Duisit G, Saleun S, Douthe S, Barsoum J, Chadeuf G, Moullier P. Baculovirus vector requires electrostatic interactions including heparan sulfate for efficient gene transfer in mammalian cells. J Gene Med. 1999;1:93–102. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<93::AID-JGM19>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Esclatine A, Lemullois M, Servin A L, Quero A M, Geniteau-Legendre M. Human cytomegalovirus infects Caco-2 intestinal epithelial cells basolaterally regardless of the differentiation state. J Virol. 2000;74:513–517. doi: 10.1128/jvi.74.1.513-517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felgner P L, Gadek T R, Holm M, Roman R, Chan H W, Wenz M, Northrop J P, Ringold G M, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felgner P L, Ringold G M. Cationic liposome-mediated transfection. Nature. 1989;337:387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 18.Ferry N, Duplessis O, Houssin D, Danos O, Heard J M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci USA. 1991;88:8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferry N, Heard J M. Liver-directed gene transfer vectors. Hum Gene Ther. 1998;9:1975–1981. doi: 10.1089/hum.1998.9.14-1975. [DOI] [PubMed] [Google Scholar]
- 20.Fraser M J. Ultrastructural observations of virion maturation in Autographa californica nuclear polyhedrosis virus-infected Spodoptera frugiperda cell cultures. J Ultrastruct Mol Struct Res. 1986;95:189–195. [Google Scholar]
- 21.Fujikura Y, Ohta H, Hirai T, Fukumoto T. Immunohistochemical analysis of rat liver using a monoclonal antibody (HAM8) against gap junction. Anat Rec. 1993;235:335–341. doi: 10.1002/ar.1092350302. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- 23.Gorman C, Padmanabhan R, Howard B H. High-efficiency DNA-mediated transformation of primate cells. Science. 1983;221:551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- 24.Harada Y, Iwai M, Tanaka S, Okanoue T, Kashima K, Maruyama-Tabata H, Hirai H, Imanishi J, Mazda O. Highly efficient suicide gene expression in hepatocellular carcinoma cells by Epstein-Barr virus-based plasmid vectors combined with polyamidoamine dendrimer. Cancer Gene Ther. 2000;7:27–36. doi: 10.1038/sj.cgt.7700079. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann C, Lehnert W, Strauss M. The baculovirus vector system for gene delivery into hepatocytes. Gene Ther Mol Biol. 1998;1:231–239. [Google Scholar]
- 26.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J M, Camper S A, Tilghman S M, Miller T, Georgoff I, Serra R, Isom H C. Functional analyses of albumin expression in a series of hepatocyte cell lines and in primary hepatocytes. Cell Growth Differ. 1992;3:577–588. [PubMed] [Google Scholar]
- 28.Isom H, Georgoff I, Salditt-Georgieff M, Darnell J E., Jr Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J Cell Biol. 1987;105:2877–2885. doi: 10.1083/jcb.105.6.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isom H C, Secott T, Georgoff I, Woodworth C, Mummaw J. Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci USA. 1985;82:3252–3256. doi: 10.1073/pnas.82.10.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarnagin W R, Debs R J, Wang S S, Bissell D M. Cationic lipid-mediated transfection of liver cells in primary culture. Nucleic Acids Res. 1992;20:4205–4211. doi: 10.1093/nar/20.16.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kay M A, Li Q, Liu T J, Leland F, Toman C, Finegold M, Woo S L. Hepatic gene therapy: persistent expression of human α1-antitrypsin in mice after direct gene delivery in vivo. Hum Gene Ther. 1992;3:641–647. doi: 10.1089/hum.1992.3.6-641. [DOI] [PubMed] [Google Scholar]
- 32.Kay M A, Rothenberg S, Landen C N, Bellinger D A, Leland F, Toman C, Finegold M, Thompson A R, Read M S, Brinkhous K M, Woo S L C. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- 33.Kitten O, Cosset F L, Ferry N. Highly efficient retrovirus-mediated gene transfer into rat hepatocytes in vivo. Hum Gene Ther. 1997;8:1491–1494. doi: 10.1089/hum.1997.8.12-1491. [DOI] [PubMed] [Google Scholar]
- 34.Kool M, Voncken J W, van Lier F L, Tramper J, Vlak J M. Detection and analysis of Autographa californica nuclear polyhedrosis virus mutants with defective-interfering properties. Virology. 1991;183:739–746. doi: 10.1016/0042-6822(91)91003-y. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Kay M A, Finegold M, Stratford-Perricaudet L D, Woo S L. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- 36.Merrihew R V, Clay W C, Condreay J P, Witherspoon S M, Dallas W S, Kost T A. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J Virol. 2001;75:903–909. doi: 10.1128/JVI.75.2.903-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitic L L, Anderson J M. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 38.Novikoff P M, Ikeda T, Hixson D C, Yam A. Characterizations of and interactions between bile ductule cells and hepatocytes in early stages of rat hepatocarcinogenesis induced by ethionine. Am J Pathol. 1991;139:1351–1368. [PMC free article] [PubMed] [Google Scholar]
- 39.Rippe R A, Brenner D A, Leffert H L. DNA-mediated gene transfer into adult rat hepatocytes in primary culture. Mol Cell Biol. 1990;10:689–695. doi: 10.1128/mcb.10.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandig V, Hofmann C, Steinert S, Jennings G, Schlag P, Strauss M. Gene transfer into hepatocytes and human liver tissue by baculovirus vectors. Hum Gene Ther. 1996;7:1937–1945. doi: 10.1089/hum.1996.7.16-1937. [DOI] [PubMed] [Google Scholar]
- 41.Serra R, Isom H C. Stimulation of DNA synthesis and protooncogene expression in primary rat hepatocytes in long-term DMSO culture. J Cell Physiol. 1993;154:543–553. doi: 10.1002/jcp.1041540313. [DOI] [PubMed] [Google Scholar]
- 42.Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, Miyamura T, Matsuura Y. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J Gen Virol. 1997;78:2657–2664. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- 43.Tateno C, Yoshizato K. Long-term cultivation of adult rat hepatocytes that undergo multiple cell divisions and express normal parenchymal phenotypes. Am J Pathol. 1996;148:383–392. [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukita S, Tsukita S. Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol. 1989;108:31–41. doi: 10.1083/jcb.108.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Loo N, Fortunati E, Ehlert E, Rebelink M, Grosveld F, Sholte B J. Baculovirus infection of nondividing mammalian cells: mechanisms of entry and nuclear transport of capsids. J Virol. 2001;75:961–970. doi: 10.1128/JVI.75.2.961-970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Davidson B L, Melchert P, Slepushkin V A, van Es H H, Bodner M, Jolly D J, McCray P B., Jr Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly D J, Davidson B L, McCray P B., Jr Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Respir Cell Mol Biol. 2000;22:129–138. doi: 10.1165/ajrcmb.22.2.3938. [DOI] [PubMed] [Google Scholar]
- 49.Woodworth C, Secott T, Isom H C. Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res. 1986;46:4018–4026. [PubMed] [Google Scholar]
- 50.Woodworth C D, Isom H C. Regulation of albumin gene expression in a series of rat hepatocyte cell lines immortalized by simian virus 40 and maintained in chemically defined medium. Mol Cell Biol. 1987;7:3740–3748. doi: 10.1128/mcb.7.10.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodworth C D, Isom H C. Transformation of differentiated rat hepatocytes with adenovirus and adenovirus DNA. J Virol. 1987;61:3570–3579. doi: 10.1128/jvi.61.11.3570-3579.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yap A S, Brieher W M, Gumbiner B M. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]