Abstract
Cullin-RING ubiquitin ligase 4 (CRL4) is closely correlated with the incidence and progression of ovarian cancer. DDB1- and CUL4-associated factor 13 (DCAF13), a substrate-recognition protein in the CRL4 E3 ubiquitin ligase complex, is involved in the occurrence and development of ovarian cancer. However, its precise function and the underlying molecular mechanism in this disease remain unclear. In this study, we confirmed that DCAF13 is highly expressed in human ovarian cancer and its expression is negatively correlated with the overall survival rate of patients with ovarian cancer. We then used CRISPR/Cas9 to knockout DCAF13 and found that its deletion significantly inhibited the proliferation, colony formation, and migration of human ovarian cancer cells. In addition, DCAF13 deficiency inhibited tumor proliferation in nude mice. Mechanistically, CRL4-DCAF13 targeted Fraser extracellular matrix complex subunit 1 (FRAS1) for polyubiquitination and proteasomal degradation. FRAS1 influenced the proliferation and migration of ovarian cancer cell through induction of the focal adhesion kinase (FAK) signaling pathway. These findings collectively show that DCAF13 is an important oncogene that promotes tumorigenesis in ovarian cancer cells by mediating FRAS1/FAK signaling. Our findings provide a foundation for the development of targeted therapeutics for ovarian cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-024-05446-2.
Keywords: Ovarian cancer, DCAF13, FRAS1, CRL4 E3 ubiquitin ligase, FAK
Introduction
Ovarian cancer is a common malignant tumor of the female reproductive system and has a high mortality rate among gynecological cancers [1]. Ovarian cancer typically proceeds undetected and lacks specific clinical symptoms in early stages [2]. Accordingly, more than 70% of patients with ovarian cancer are diagnosed in late stages, which are associated with extensive metastasis and poor prognosis [3]. Currently, surgical resection combined with chemotherapy is the standard treatment for ovarian cancer. However, the 5-year survival rate of patients with advanced disease remains less than 40% [4]. With prolonged chemotherapy cycles and multiple relapses, the sensitivity of most ovarian cancers to chemotherapeutic drugs gradually decreases, ultimately leading to therapeutic recalcitrance [5]. Therefore, studies are urgently needed to understand the pathogenesis of ovarian cancer to improve prognosis and reduce mortality.
Cullin-RING ubiquitin ligase 4 (CRL4) is an important member of the E3 ubiquitin ligase family. The CRL4 E3 ubiquitin ligase consists of three components, the scaffold protein CUL4, RING finger protein RBX1 (also known as ROC1 or HRT1), and DNA damage binding protein 1 (DDB1) [6]. Numerous studies showed that the CRL4 complex plays an important role in ovarian cancer [7–9]. DDB1- and CUL4-associated factor 13 (DCAF13), a substrate recognition protein for the CRL4 E3 ubiquitin ligase complex, is highly amplified in breast, liver, and lung cancer [10–14]. An early discovery by our group suggested that DCAF13 is involved in cell cycle regulation, apoptosis, tumor-related signaling pathways, and other processes in breast cancer, and thus promotes breast cancer cell proliferation [11]. Moreover, DCAF13 overexpression in breast and lung cancer is significantly associated with low survival rates, and therefore shows potential for use as a tumor biomarker [11, 14]. However, the function of CRL4 in ovarian cancer as well as the underlying molecular mechanism remain unclear.
Here, we report that DCAF13 is a CRL4 adaptor that is prominently expressed in human ovarian cancer and is associated with poor prognosis. DCAF13 knockout inhibited the proliferation and migration of ovarian cancer cells in vitro and the growth of xenografted tumors in vivo. In addition, we demonstrated that DCAF13 regulated ovarian cancer cell proliferation and migration by affecting the ubiquitination of the Fraser extracellular matrix complex subunit 1 (FRAS1) and activating the focal adhesion kinase (FAK) signaling pathway. Our findings provide new biomarker options and potential strategies for targeted molecular therapy for ovarian cancer.
Results
DCAF13 overexpression is positively correlated with histological grade and overall survival of patients with ovarian cancer
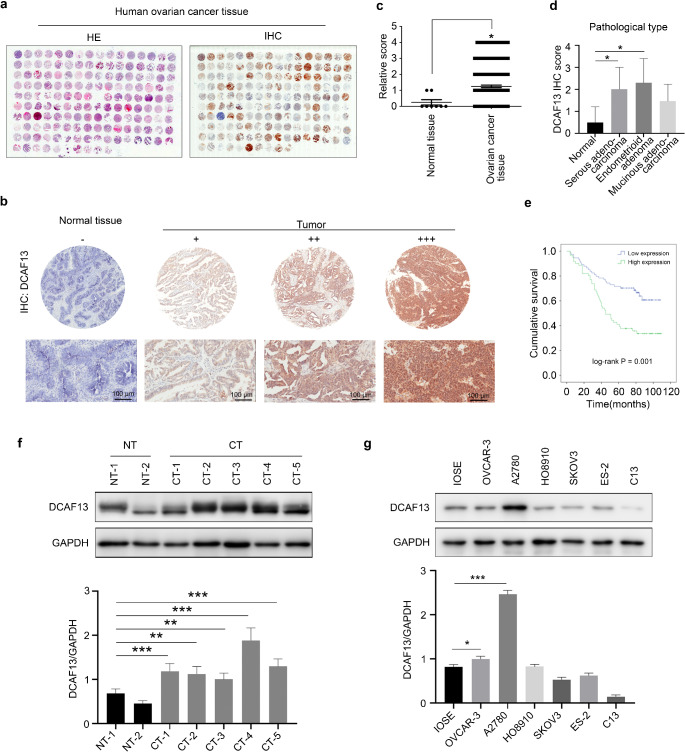
To investigate whether DCAF13 is involved in ovarian cancer, we conducted H&E staining and immunohistochemical analyses of ovarian cancer tissue microarrays to determine the DCAF13 expression level. The tissue microarrays included 8 normal ovarian tissue samples and 146 ovarian cancer tissue samples from patients aged 20–75 years (mean, 48 years) (Fig. 1a). Based on the staining intensity and positivity rate, we divided the stained ovarian tissues into negative (−), positive (+), moderate (++) and strong positive (+++) classes (Fig. 1b). The positive rates of DCAF13 staining in these different ovarian cancer type tissues, such as serous adenocarcinoma, mucinous adenocarcinoma, endometrial carcinoma, and clear cell carcinoma, were 98.9% (87/88), 97.4% (37/38), 100.0% (17/17), and 100.0% (3/3), respectively, whereas the strong positive rates reached as high as 95.5% (84/88), 84.2% (32/38), 100.0% (17/17), and 100.0% (3/3), respectively (Table 1). Positive staining of DCAF13 staining was not observed in normal ovarian tissue (0%; Table 1). We found that DCAF13 protein expression was higher in ovarian cancer tissues than in normal ovarian tissues (P < 0.05, Fig. 1c).
Fig. 1.
Expression pattern of DCAF13 in ovarian cancer tissues and ovarian cancer cells. a H&E staining and immunohistochemistry of DCAF13 protein expression in ovarian cancer tissue microarrays. b Immunohistochemistry for DCAF13 protein expression in normal ovarian tissue and ovarian cancer tissue in tissue microarray. The results were divided into negative (-), moderate (++) and strong positive (+++) staining pattern. c Statistical analysis of DCAF13 expression in normal ovarian tissue and ovarian cancer tissue of tissue microarray, *, P < 0.05. d Statistical analysis of DCAF13 expression in different pathological types of ovarian cancer tissue microarray, *, P < 0.05. e Kaplan-Meier analysis of cumulative survival rate of ovarian cancer tissue microarray, log-rank statistical test, P = 0.001. f Western blot (upper panel) and quantification (lower panel) of DCAF13 protein expression in patient-derived ovarian cancer tissues and paracancerous tissues (NT represents paracancerous tissue, n = 2; CT refers to cancerous tissue, n = 5). g Western blot (upper panel) and quantification (lower panel) of DCAF13 expression in ovarian cancer cells (OVCAR-3, A2780, HO8910, SKOV3, ES-2, and C13), and immortalized mouse ovarian surface epithelium (IOSE). GAPDH was used as the loading control
Table 1.
Detailed pathological types and grades of ovarian cancer from the patient tissue microarray
| Ovarian cancer type | Case | Positive n (%) | P | Strongly positive n (%) | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| (n) | +++ | ++ | + | − | |||||
|
Normal ovarian tissue |
8 | 0 (0) | 0 (0) | 0 (0) |
8 (100.0%) |
0 (0) | < 0.0001 | 0 (0) | < 0.0001 |
| Serous adenocar-cinoma | 88 |
71 (80.7%) |
13 (14.8%) |
3 (3.4%) |
1 (1.1%) |
87 (98.9%) |
84 (95.5%) |
||
| Mucinous adenocar-cinoma | 38 |
24 (63.2%) |
8 (21.1%) |
5 (13.2%) |
1 (2.6%) |
37 (97.4%) |
32 (84.2%) |
||
| Endome-trioid adenoma | 17 |
13 (76.5%) |
4 (23.5%) |
0 (0) | 0 (0) |
17 (100.0%) |
17 (100.0%) |
||
| Clear cell carcinoma | 3 |
3 (100.0%) |
0(0) | 0 (0) | 0 (0) |
3 (100.0%) |
3 (100.0%) |
||
To assess the correlation between DCAF13 protein expression and ovarian cancer progression, we analyzed the relationship between its overexpression and the clinicopathological features of ovarian cancers. The rate of DCAF13 positivity was significantly higher in grade III ovarian cancers than in grade I/II ovarian cancers (P = 0.008; Table 2). Similarly, the strong positive rate of DCAF13 protein expression was significantly higher in serous adenocarcinomas than in non-serous adenocarcinomas (P = 0.001; Table 2). This rate was also higher in pathological stage II disease than in pathological stage I disease (P < 0.001; Table 2). Based on the IHC scores, DCAF13 protein expression was higher in serous and endometrioid adenomas than in normal ovarian tissues (Fig. 1d). Subsequent analysis of tissue microarray data revealed that patients with elevated DCAF13 expression had a lower overall survival rate compared with those who showed lower DCAF13 expression (P = 0.001, Fig. 1e).
Table 2.
Correlation between DCAF13 expression and clinicopathological characteristics
| Variables | DCAF13 expression | Total | χ2 | P | ||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Age (years) | 0.54 | 0.464 | ||||
| ≤ 50 | 38 | 27 | 65 | |||
| > 51 | 36 | 33 | 69 | |||
| null | ||||||
| T stage | 2.20 | 0.137 | ||||
| T1/T2 | 27 | 15 | 42 | |||
| T3 | 47 | 46 | 93 | |||
| TNM stage | 2.20 | 0.137 | ||||
| Ι/II | 27 | 15 | 42 | |||
| III/IV | 47 | 46 | 93 | |||
| null | ||||||
| N stage | 2.05 | 0.152 | ||||
| N0 | 61 | 44 | 105 | |||
| N1 | 13 | 17 | 30 | |||
| null | ||||||
| M stage | 0.52 | 0.471 | ||||
| M0 | 64 | 50 | 114 | |||
| M1 | 10 | 11 | 21 | |||
| null | ||||||
| Grade | 7.08 | 0.008 | ||||
| I/II | 24 | 11 | 35 | |||
| III | 31 | 44 | 75 | |||
| Pathological subtype | 11.8 | 0.001 | ||||
| Non-serous adenocarcinoma | 45 | 19 | 64 | |||
| Serous adenocarcinoma | 29 | 42 | 71 | |||
| Pathological subtype | 19.3 | 0 | ||||
| I | 52 | 24 | 76 | |||
| II | 13 | 34 | 47 | |||
Human ovarian cancer tissues and adjacent tissues were also collected from patients. Western blot analysis showed that DCAF13 protein expression was higher in ovarian cancer tissues than in paracancerous tissues (Fig. 1f). In addition, western blotting and Q-PCR revealed that DCAF13 was highly expressed in the ovarian cancer cell lines A2780, OVCAR-3, and HO8910 (Fig. 1g and Fig. S1a). These results indicate that human ovarian cancer tissues have high expression levels of DCAF13 protein and that the DCAF13 expression intensity is related to the survival rate of patients and pathological type of the disease.
DCAF13 mediates ovarian cancer cell proliferation, colony formation, and migration
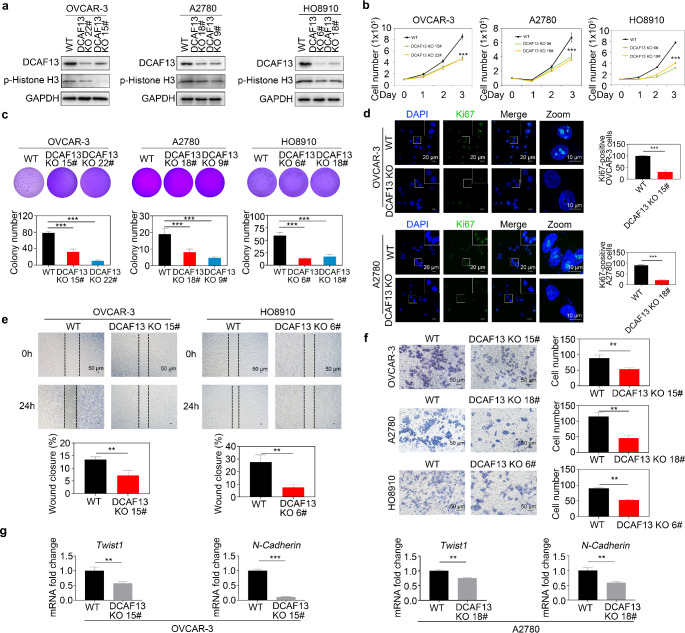
To further clarify the role of DCAF13 in ovarian cancer, we employed CRISPR/Cas9 technology to delete DCAF13 in the ovarian cancer cell lines OVCAR-3, A2780, and HO8910. We generated several DCAF13-partial-knockout cell lines and used immunoblotting to confirm the DCAF13 knockout efficacy as well as select clones with obvious DCAF13 knockout (Fig. 2a and Fig. S1b, c). Cell proliferation assays showed that partial DCAF13 knockout resulted in a consistent and significant decrease in cell proliferation (Fig. 2b). Colony formation assays demonstrated that deletion of DCAF13 resulted in a decreased number of formed colonies (Fig. 2c). Similarly, the expression of p-histone H3, Ki67 and p-AKT, i.e., established markers of cell proliferation, was significantly reduced in DCAF13-deficient ovarian cancer cells based on both immunoblotting and immunofluorescence (Fig. 2a, d and Fig. S1d, e). In addition, scratch and transwell experiments revealed that DCAF13 deletion significantly inhibited ovarian cancer cell migration (Fig. 2e, f). Consistently, qRT-PCR data showed that DCAF13 deletion decreased expression of the cell migration markers Twist1 and N-cadherin in OVCAR-3 and A2780 cells (Fig. 2g). These findings demonstrate the crucial role of DCAF13 in promoting the proliferation and migration of ovarian cancer cells.
Fig. 2.
DCAF13 deletion inhibits ovarian cancer cell proliferation, clone formation, and metastatic ability. a Detection of DCAF13 knockout efficiency in OVCAR-3, A2780 and HO8910 by immunoblotting. Two independent clones (22# and 15# in OVCAR-3; 18# and 9# in A2780; 6# and 18# in HO8910) are shown. b Growth curve of DCAF13 knockout ovarian cancer cells in OVCAR-3, A2780 and HO8910. This experiment was replicated three times. The error bars represent SD. Two-way ANOVA test was applied. ***, P < 0.001. c Soft agar assay detection colony formation in DCAF13-deleted OVCAR-3, A2780 and HO8910 cells. Data are presented as mean ± SD. ***, P < 0.001. d Immunofluorescence analysis of Ki67 (green) levels in WT and DCAF13-deficient OVCAR-3 and A2780 cells. Nuclei are counterstained with DAPI (blue). Scale bar, 20 μm. The white box area is enlarged.e Wound-healing assay for the migration ability of WT and DCAF13-deleted cells. Data are presented as mean ± SD for n = 3 per cell line. Student’s t-test was applied. **, P < 0.01. Scale bar, 50 μm. f Representative images (left panel) and quantification (right panel) of Transwell assay results showed that DCAF13 deletion inhibits ovarian cancer cell migration. This experiment was replicated three times. The error bars represent SD. **, P < 0.01, two-way ANOVA test. Scale bar, 50 μm. g qRT-PCR detection of cell migration-related genes in WT and DCAF13-deleted cells in OVCAR-3 and A2780. The error bars represent SD. Student’s t-test was applied. **, P < 0.01; ***, P < 0.001
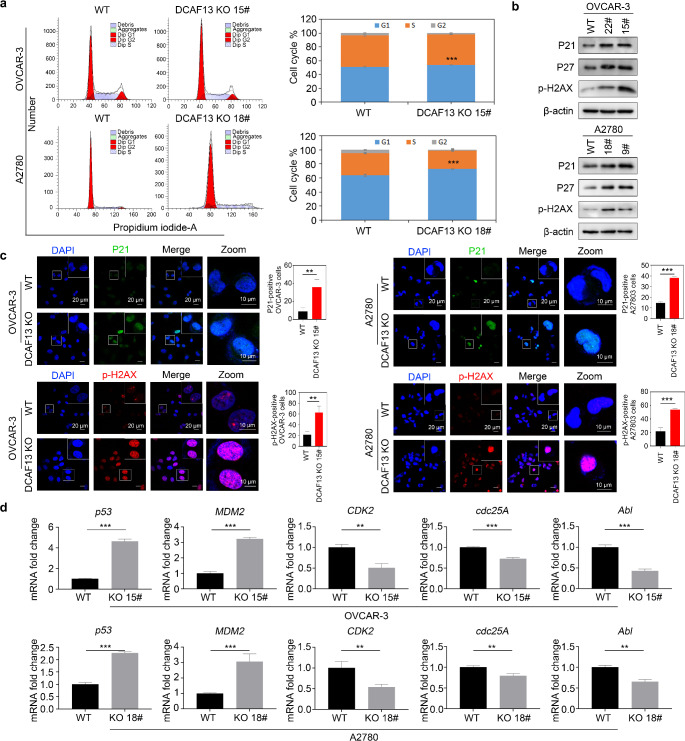
DCAF13 deletion causes cell cycle arrests
We further investigated the mechanism by which DCAF13 deletion inhibits ovarian cancer cells. Flow cytometry demonstrated that DCAF13-deleted cells were halted in G1 phase of the cell cycle (Fig. 3a). Cell cycle regulation is closely associated with DNA damage and senescence [15, 16]. Western blotting further showed that expression of the cyclin-dependent kinase inhibitors P21 and P27 and the DNA damage marker p-H2AX were increased in DCAF13-deleted cells, indicating that DCAF13 deletion caused DNA damage in ovarian cancer cells (Fig. 3b and Fig. S1f). Immunofluorescence results also showed that the expression of P21 and p-H2AX protein was increased in DCAF13-deleted cells (Fig. 3c), which is consistent with the western blot results (Fig. 3b). Furthermore, qRT-PCR analysis revealed that DCAF13 deletion boosted the expression of p53 and the p53-downstream gene MDM2, whereas the expression of CDK2, cdc25A, and Abl was decreased, indicating that the DNA damage mechanism was activated at the G1/S checkpoint (Fig. 3d). These findings indicate that DCAF13 deletion causes cell cycle arrest.
Fig. 3.
DCAF13 deletion affects the cell cycle and promotes cell senescence. a Flow cytometry was employed to analyze the changes in the cell cycle in DCAF13-deleted OVCAR-3 and A2780 cells. **, P < 0.01 (nonparametric test). b Western blot analysis of the expression of P21, P27 and p-H2AX in WT and DCAF13-deleted OVCAR-3 and A2780 cells. c Representative images (left panel) and quantification (right panel) of immunofluorescence analysis of P21 (green) and p-H2AX (red) levels in WT and DCAF13-deleted OVCAR-3 and A2780 cells. Cells were counterstained with DAPI (blue). Scale bar, 20 μm. The white box area is enlarged. **, P < 0.01; ***, P < 0.001 d qRT-PCR detection of cell cycle related genes in WT and DCAF13-deleted cells in OVCAR-3 and A2780. The error bars represent SD. Student’s t-test was applied. **, P < 0.01; ***, P < 0.001
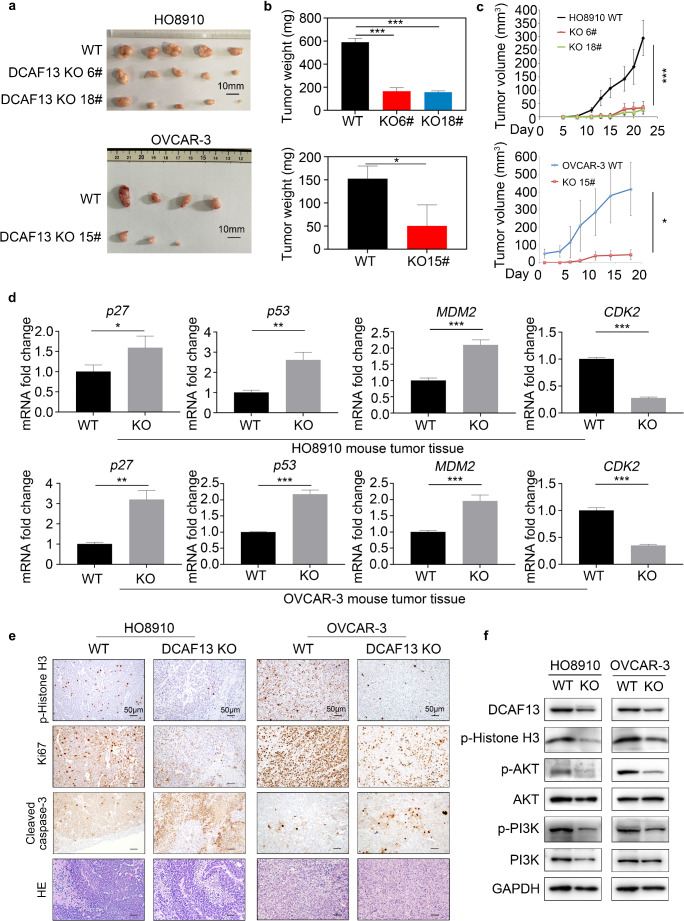
DCAF13 deletion inhibits tumor growth in vivo
To determine the impact of DCAF13 on ovarian cancer cell proliferation in vivo, we subcutaneously transplanted equal numbers of WT or DCAF13-deleted ovarian cancer cells into the left and right flanks of nude mice, respectively, and measured the tumor volume during xenograft development. Tumor size and weight were lower in nude mice transplanted with DCAF13-deleted ovarian cancer cells than in the WT group (Fig. 4a–c). The qRT-PCR results revealed that expression of the cell cycle-related genes p27, p53, MDM2, and CDK2 were altered in DCAF13-deficient tumor tissues (Fig. 4d), which is consistent with the in vitro results (Fig. 3d). Immunohistochemistry results revealed that protein expression of the cell proliferation markers p-histone H3 and Ki67 were decreased, whereas that of the apoptosis marker cleaved caspase-3 was increased in DCAF13-deleted tumor tissue (Fig. 4e). Western blotting revealed that the protein levels of p-histone H3, p-AKT, and p-PI3K were decreased in the DCAF13-deleted group (Fig. 4f and Fig. S1g). These results indicate that DCAF13 deletion inhibited tumor proliferation in vivo.
Fig. 4.
DCAF13 deletion inhibits tumor growth in vivo. a Tumor size of HO8910 and OVCAR-3 wild-type and DCAF13 deletion ovarian cancer cells in nude mice. 5 × 106 cells were injected subcutaneously into flank of nude mice (n = 6). Some nude mice failed to form tumors. Scale bar, 10 mm. b Deletion of DCAF13 in HO8910 and OVCAR-3 cells inhibits tumor weight in vivo. *, P < 0.05; ***, P < 0.001, according to two-way ANOVA. c Tumor growth curve of nude mice. Tumor volume in each group was measured every 2–3 days. *, P < 0.05; ***, P < 0.001, according to two-way ANOVA. d qRT-PCR detection of cell cycle-related factors in tumors. The error bars represent SD. Student’s t-test was applied. *, P < 0.05; **, P < 0.01; ***, P < 0.001. e Histochemistry and immunohistochemical analysis of p-Histone H3, Ki67 and Cleaved Caspase-3 in wild-type and DCAF13 deletion xenografts. Scale bar, 50 μm. Objective magnification 20x. f Western blot analysis of DCAF13, p-Histone H3, p-AKT, AKT, p-PI3K and PI3K expression in WT and DCAF13-deleted HO8910 and OVCAR-3 mouse tumor tissue. GAPDH was used as control
CRL4DCAF13 regulates cell proliferation by targeting FRAS1 for polyubiquitination and degradation
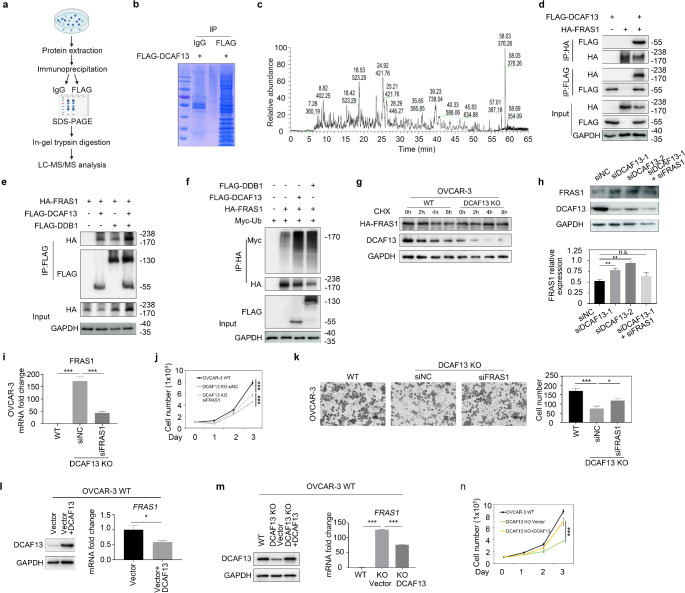
To identify the specifically targeted substrate of DCAF13, we isolated the DCAF13-associated protein complex in HEK293T cells using tandem affinity purification and then performed mass spectrometry analysis (Fig. 5a). Coomassie brilliant blue staining showed that the proteins were pulled down with IgG and FLAG antibodies (Fig. 5b). Mass spectrometry analysis suggested that DCAF13 interacts with FRAS1 (Fig. 5c). FRAS1 is an extracellular matrix protein that plays a significant role in tumor invasion and migration [17–20]. In agreement with the mass spectrometry results, co-immunoprecipitation assays demonstrated that DCAF13 interacts directly with FRAS1 (Fig. 5d). To identify the interaction domain of DCAF13 with FRAS1, we used two DCAF13 truncations, in which either the conserved SOF or WD domains of DCAF13 were deleted. The results showed that both the DCAF13 SOF△ and WD△ truncations interacted with FRAS1, suggesting that both domains are involved in the interaction (Fig. S1h). Given that DCAF13 functions as a substrate receptor of CRL4 E3 ubiquitin ligase, we analyzed whether FRAS1 acts as a substrate of CRL4 E3 ubiquitin ligase. We examined the association between FRAS1 and DDB1, which is the linker protein of CRL4 E3 ubiquitin ligase, and found that FRAS1 also directly interacts with DDB1, and that DDB1 overexpression strengthened the interaction between FRAS1 and DCAF13 (Fig. 5e), suggesting that FRAS1 can form complexes with CRL4 E3 ligase. Further evaluation of whether FRAS1 can be ubiquitinated by CRL4DCAF13 E3 ligase showed that the levels of FRAS1 polyubiquitination significantly increased after DCAF13 or DDB1 overexpression (Fig. 5f), indicating that CRL4DCAF13 E3 ligase targeted FRAS1 for polyubiquitination. Furthermore, we examined the degradation rates of FRAS1 by using the protein synthesis inhibitor cycloheximide (CHX). FRAS1 protein was mostly degraded upon CHX treatment but was stable in DCAF13-deficient cells (Fig. 5g and Fig. S2a). Moreover, when DCAF13 was depleted using siRNAs, the level of FRAS1 protein was significantly increased (Fig. 5h). ROC1, a component of the CRL4 E3 ubiquitin ligase, was depleted by siRNA oligos, which similarly increased FRAS1 expression (Fig. S2b). These results indicate that CRL4DCAF13 E3 ligase targets FRAS1 for ubiquitination and proteasomal degradation.
Fig. 5.
FRAS1 is the key target of DCAF13 in ovarian cancer cells. a Schematic representation of immunoprecipitation combined with liquid chromatography-mass spectrometry. b Coomassie brilliant blue staining of proteins pulled with IgG antibody and FLAG antibody. FLAG-DCAF13 plasmid was overexpressed in this experiment. c The base peak of protein sample by mass spectrometry. d Co-immunoprecipitation results showing FRAS1 interacts with DCAF13. 293T cells transfected with plasmids encoding the indicated proteins were lysed and subjected to IP with anti-FLAG or anti-HA beads. Input lysates were immunoblotted with antibodies against HA and FLAG. e Co-immunoprecipitation results showing FRAS1 interacts with DDB1 and DCAF13. The overexpression of DDB1 enhances the interaction between FRAS1 and DCAF13. f Co-immunoprecipitation experiments showing overexpression of DCAF13 and DDB1 increase FRAS1 polyubiquitination. g The Cycloheximide (CHX)-chasing experiment revealed the FRAS1 stability. OVCAR-3 WT and DCAF13-deleted cells were transfected with HA-FRAS1 plasmid. 10 µM CHX was used to inhibit protein synthesis in OVCAR-3. At the specified time points after CHX treatment, the cells were lysed for immunoblotting. h Western blot shows the expression of FRAS1 in OVCAR-3 cells transfected with control siRNA (siNC), siDCAF13 or siFRAS1(upper panel) and relative quantitative analysis (lower panel). **, P < 0.01; ns, P > 0.05. i mRNA expression level of FRAS1 in WT or DCAF13 deficient OVCAR-3 cells transfected with control siRNA (siNC) or siFRAS1 ***, P < 0.001. j FRAS1 deletion rescues cell proliferation defects in DCAF13 deficient OVCAR-3 cells. ***, P < 0.001. k FRAS1 deletion rescues cell migration ability in OVCAR-3 DCAF13 KO cells. Representative images from the Transwell assay (left panel) and quantification analysis (right panel) were shown. *, P < 0.05, ***, P < 0.001. l Western blot results of DCAF13 protein expression (left panel) and qRT-PCR results of FRAS1 mRNA expression in wild-type OVCAR-3 transfected with DCAF13 plasmids. *, P < 0.05. m Western blot results of DCAF13 protein expression (left panel) and qRT-PCR results of FRAS1 mRNA expression in WT, DCAF13 KO and DCAF13 KO transfected with DCAF13 plasmids. ***, P < 0.001. n Overexpression of DCAF13 rescues the ability of cell proliferation. ***, P < 0.001
To determine whether the observed decrease in DCAF13-deficient ovarian cancer cell proliferation was due to DCAF13 dependent expression of FRAS1, we performed FRAS1 knockdown experiments using RNA interference. The qRT-PCR results showed that FRAS1 mRNA was suppressed in OVCAR-3 DCAF13 knockout cells (Fig. 5i). Cell counting assays and transwell assays showed that silencing of FRAS1 partially rescued ovarian cancer cell proliferation and migration defects caused by DCAF13 deficiency (Fig. 5j–k). To further explore the relationship between DCAF13 and FRAS1, we constructed a stable WT ovarian cancer cell line overexpressing DCAF13 using the Lenti-X VSVG lentivirus packaging system. According to the qRT-PCR results, FRAS1 expression was reduced in DCAF13-overexpressing cells (Fig. 5l and Fig. S2c). We then overexpressed DCAF13 in DCAF13-deletion cells, which decreased FRAS1 expression (Fig. 5m and Fig. S2d). The cell proliferative capacity was also rescued in cells overexpressing DCAF13 (Fig. 5n), confirming that DCAF13 is crucial for cellular proliferation. These findings demonstrate that CRL4DCAF13 regulates the proliferation and migration of ovarian cancer cells by targeting FRAS1 for polyubiquitination and degradation.
CRL4DCAF13-mediated FRAS1/FAK signaling pathway is necessary for ovarian cancer cell proliferation
Kiyozumi et al. showed that FRAS1 contains an RGD-motif that mediates binding to integrins [21]. FAK is the key tyrosine kinase in the integrin signaling pathway [22]. FAK is a cytoplasmic protein tyrosine kinase that is highly expressed and overactivated in many advanced solid tumors and is associated with tumor growth and metastasis [23]. Thus, we hypothesized that FRAS1 affects ovarian cancer cell proliferation and migration by regulating the FAK signaling pathway.
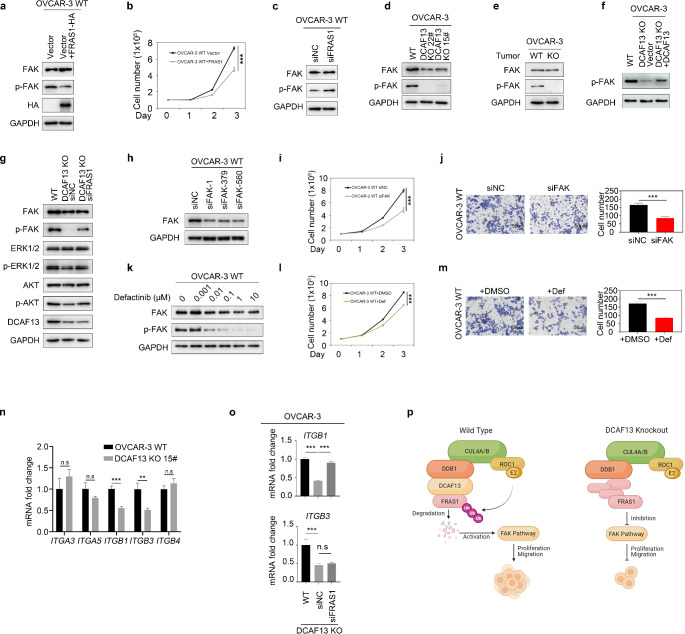
We examined whether FRAS1 affects the phosphorylation level of FAK, indicating activity of the FAK signaling pathway. We found that overexpression of HA-FRAS1 significantly decreased p-FAK (Fig. 6a and Fig. S2e), whereas silencing of FRAS1 significantly increased p-FAK (Fig. 6c and Fig. S2f), indicating that FRAS1 regulates FAK signaling pathway activity. Cell proliferation assays showed that overexpression FRAS1 inhibited ovarian cancer cell proliferation (Fig. 6b). These results demonstrate that FRAS1 negatively regulated the FAK signaling pathway.
Fig. 6.
FRAS1 affects ovarian cancer cell proliferation and migration by mediating the FAK signaling pathway. a Western blot detection FAK, p-FAK, HA protein expression in cells. OVCAR-3 cells were transfected with HA-FRAS1 vector and or with control empty vector. GAPDH was used as control. b Cell proliferation assay of overexpressed HA-FRAS1 and control ovarian cancer cells. ***, P < 0.001. c Western blot results showing the FAK signaling pathway was activated after interfering with FRAS1. GAPDH was used as control. d Western blot analysis of FAK and p-FAK expression in WT and DCAF13-deleted OVCAR-3 cells. GAPDH was used as control. e Western blot analysis of FAK and p-FAK expression in WT and DCAF13-deleted OVCAR-3 mouse tumor tissue. f Western blot analysis of p-FAK expression in WT, DCAF13-deleted OVCAR-3 cells and DCAF13-deleted OVCAR-3 cell tansfected with DCAF13 plasmids. g Western blot analysis of p-FAK, p-ERK1/2 and p-AKT expression in WT, DCAF13-deleted OVCAR-3 cells, DCAF13 and FRAS1 double knockdown cells. h Western blot results for FAK siRNA interference efficiency. i FAK silencing inhibiting ovarian cancer cell proliferation. ***, P < 0.001. j FAK deletion inhibiting cell migration of ovarian cancer cells. Representative images from the Transwell assay (upper panel) and quantification analysis (lower panel) were shown. **, P < 0.01. k OVCAR-3 cells were exposed to increasing doses of the FAK inhibitor Defactinib (Def) for 24 h. Western blot analysis of FAK and p-FAK. l Ovarian cancer cell proliferation, after treatment with 1 µM Defactinib for 24 h. ***, P < 0.001. m Defactinib inhibiting cell migration of ovarian cancer cells. Representative images from the Transwell assay (upper panel) and quantification analysis (lower panel) were shown. **, P < 0.01. n Alterations in integrin expression in wild-type and DCAF13 deletion OVCAR-3 cells were analyzed by qRT-PCR. n.s, P > 0.05, ** P < 0.01, *** P < 0.001. o qRT-PCR detection the expression level of ITGB1 after interfering with FRAS1. *** P < 0.001. p A working model explaining how DCAF13 promotes ovarian cancer cell proliferation and migration. In wild-type ovarian cancer cells, DCAF13 ubiquitin degrades the FRAS1 substrate and activates the FAK signaling pathway, thus promoting the proliferation and migration of ovarian cancer cells. When DCAF13 is knocked out, FRAS1 accumulation leads to inhibition of the FAK signaling pathway and suppression of proliferation and migration of ovarian cancer cells
As described above, we showed that silencing of DCAF13 resulted in an increase in the level of FRAS1 (Fig. 5h). We evaluated whether DCAF13 affects the activity of the FAK signaling pathway via FRAS1 and found that the level of p-FAK was significantly reduced in DCAF13-deficient ovarian cancer OVCAR-3 and A2780 cells (Fig. 6d and Fig. S2g–h). p-FAK expression was also reduced in DCAF13-deficient tumor tissue (Fig. 6e and Fig. S2i). Moreover, silencing of FRAS1 in DCAF13-deficient ovarian cancer cell partially rescued the expression level of p-FAK and p-AKT, which are downstream activity indicators of the FAK signaling pathway (Fig. 6g and Fig. S2j). Overexpression of DCAF13 in the DCAF13 KO OVCAR-3 cell lines could rescue the low FAK phosphorylation phenotypes (Fig. 6f). These results demonstrate that DCAF13 regulates the FAK signaling pathway via FRAS1.
To examine whether the FAK signaling pathway regulates ovarian cancer cell proliferation and migration, we silenced the expression of FAK using RNA interference (Fig. 6h and Fig. S2k). Silencing of FAK significantly inhibited ovarian cancer cell proliferation and migration (Fig. 6i, j). Moreover, treatment of ovarian cancer cells with the FAK inhibitor defactinib (Def) at concentrations ranging from 0.001 to 10 µM significantly decreased the level of p-FAK, indicating that Def inhibited the activity of the FAK signaling pathway (Fig. 6k and Fig. S2l). Def also inhibited ovarian cancer cell proliferation and migration (Fig. 6l–m). These results indicate that the FAK signaling pathway regulates ovarian cancer cell proliferation and migration.
We next explored how FAK affects the proliferation and migration of ovarian cancer cells. FAK is a non-receptor tyrosine kinase that is overexpressed in various tumor cells [22]. Serine/threonine protein kinase B, also known as Akt, is a downstream cytokine in the FAK signaling pathway [22]. For example, in ovarian cancer, tumor ascites prevents death-inducing signals via activation of an αvβ5 integrin–FAK–AKT signaling pathway [24]. Thus, FAK may affect the proliferation and migration of ovarian cancer via the AKT signaling pathway. Our results showed that deletion of DCAF13 reduced the expression of p-FAK and p-AKT, whereas silencing of FRAS1 in DCAF13-deficient cells partially rescued the expression of p-FAK and p-AKT (Fig. 6g). Moreover, compared with that in subcutaneous tumors formed by WT ovarian cancer cells, the expression level of p-AKT and p-PI3K was significantly reduced in subcutaneous tumors formed by DCAF13 knockout ovarian cancer cells (Fig. 4f). These results indicate that DCAF13 affects ovarian cancer cell proliferation and migration by regulating the DCAF13-FRAS1-FAK-AKT signaling pathway.
Integrins activate FAK signaling, and the integrin/FAK pathway promotes tumorigenesis [25, 26]. Lahlou et al. found that the deletion of FAK or ITGB1, which encodes β1 integrin, inhibits tumor initiation [27]. In a small-scale screening experiment, we found that DCAF13 deletion reduced ITGB1 and ITGB3 expression (Fig. 6n). Moreover, the ITGB1 expression pattern was reversed after FRAS1 silencing, whereas that of ITGB3 was not (Fig. 6o). These results indicate that FRAS1 inhibited ITGB1 expression, which inactivates the FAK signaling pathway.
In summary, in WT ovarian cancer cells, the CRL4DCAF13 ubiquitin ligase complex target FRAS1 for polyubiquitination and degradation, resulting in activation of the FAK signaling pathway, thus promoting ovarian cancer cell proliferation and migration (Fig. 6p).
Discussion
Our study demonstrates that DCAF13 plays a critical role in ovarian cancer carcinogenesis. Previous studies have revealed that the increase of DCAF13 expression in breast cancer and hepatocellular carcinoma leads to poor prognosis [10–12]. In this research, we found that DCAF13 affects ovarian cancer, indicating that it might play a role in promoting pan-cancer development. Based on our findings, it appears that the deletion of DCAF13 suppresses both the proliferation and migration of ovarian cancer cells, which is consistent with previous findings in breast cancer [28]. Detailedly, DCAF13 deletion led to cell cycle arrest and enhanced expression of P21 and P27. DCAF13 deletion also increased the expression of DNA damage marker p-H2AX. Sun et al.found a crucial role of DCAF13 in DNA damage response and repair in tumor cells, which supports the proposed role of DCAF13 in our study [29]. In addition, our research revealed that DCAF13 not only promotes cancer cell proliferation but also contributes to cell migration and senescence. More importantly, we elucidated a novel molecular mechanism in which the CRL4DCAF13 E3 ligase regulated the FAK signaling pathway by the ubiquitin-mediating degradation of FRAS1.
MLN4924 is a selective inhibitor of CUL neddylation, a prerequisite for the activity of Cullin (CUL)-RING E3 ligase (CRL4 E3 ubiquitin ligase). MLN4924 exhibits potent toxicity and side effects [30, 31]. Therefore, there is a need to identify superior target proteins to develop potential therapeutic drugs. DCAF13, a CRL4 E3 ubiquitin ligase substrate-binding protein, is more specific than CRL because of its targeting specificity, which theoretically would reduce side effects. DCAF13 might influence tumor cell proliferation via the PI3K–PTEN and P53 pathways [11]. However, our findings suggest that DCAF13 could also affect the FAK signaling pathway through FRAS1. Previous research has suggested that the FAK signaling pathway could be affected by ubiquitin ligases, potentially contributing to tumor occurrence and metastasis [32, 33]. Our research revealed that DCAF13, the key protein in the CRL4 complex, might have an impact on the proliferation and migration of ovarian cancer cells by activating the FAK signaling pathway, further confirming this assumption. Our study suggests that DCAF13 is a potential target for ovarian cancer diagnosis and treatment. Silencing the expression of DCAF13 can be a novel treatment for ovarian cancer. In cancer therapy, small interfering RNAs (siRNAs) are considered powerful tools for gene silencing (knockdown), enabling the suppression of oncogene factors in cancer [34]. Our findings provide evidence for future ovarian cancer therapy by inhibiting DCAF13 with siRNAs. Although siRNA interventions have been experimentally applied to treat ovarian cancer, siRNA-based therapies have not yet reached the clinic; however, further development of multiple targets, improved delivery systems, and combination treatments will enable a breakthrough.
Website prediction analysis identified potential ubiquitin modification sites in FRAS1. Furthermore, our study revealed that the CRL4 complex affects the ubiquitination of FRAS1. Co-immunoprecipitation and ubiquitin co-immunoprecipitation assays revealed that DDB1 and DCAF13, the pivotal components of CRL4, modulate the ubiquitination of FRAS1. Moreover, FRAS1 affects the biological phenotype of cells via ubiquitin-mediated degradation. Our results, which involved the overexpression and knockdown of FRAS1, revealed its effect on the proliferation and migration of ovarian cells. Recent research indicated that mutations in FRAS1 might cause Fraser syndrome, a rare chromosomal disease with cryptic malformations and multiple organ hypoplasia [33]. FRAS1 has also been associated with various cancers, and its silencing suppresses the migration and invasion of non-small-cell lung cancer cells [35] and promotes liver metastasis in gastric cancer [36]. FRAS1 has also been identified as a promising diagnostic marker for endometrial carcinoma [37] and it is implicated in ovarian cancer resistance to carboplatin [18]. Moreover, FRAS1 expression in renal clear cell carcinoma tissues are significantly higher than those in normal tissues. Patients with reduced FRAS1 expression in tumors show an increased incidence of metastasis and a poor prognosis, highlighting it as a prospective target for treatment and a valuable prognostic biomarker for clear cell carcinoma [17]. Our results similarly showed that FRAS1 expression was increased after DCAF13 knockout, supporting these results.
The effect of the FAK signaling pathway on both cell migration and proliferation has been consistently validated in numerous studies. In ovarian, breast, and gastric cancers, FAK is overactivated and promotes cell proliferation and migration [38–40]. The FAK protein is considered a potential target for anti-cancer drugs and is overexpressed in ovarian cancer [41, 42]. We found that the FAK signaling pathway is suppressed after DCAF13 knockout in ovarian cancer cells. Moreover, the capacity of ovarian cancer cells to proliferate and migrate was reduced after DCAF13 knockout, which might also be connected to suppression of the FAK signaling pathway. Previous studies have shown that FRAS1 knockdown in lung cancer cells inhibits the FAK signaling pathway [42]. Our results differed from this, which could be due to tissue specificity or transcriptional differences. In addition, our results showed that the FAK protein was dephosphorylated, indicating that FRAS1 affects FAK phosphorylation. FAK activity increases after its phosphorylation, and this protein participates in multiple signaling pathways, such as PI3K/AKT and MAPK/ERK, to regulate cell growth and affect tumor occurrence and migration. Our results showed that FRAS1 affects ovarian cancer cell proliferation and migration through the FAK/PI3K/AKT signaling pathway, which is consistent with the aforementioned view. FAK has Y397 and Y925 phosphorylation sites, but only Y397, a common phosphorylation site, was detected in our experiment, whereas the other phosphorylation sites could be detected individually in follow-up experiments to explore how FRAS1 affects FAK dephosphorylation, highlighting the significant involvement of DCAF13 in the development of ovarian cancer. However, there are currently no available data on the effect of the DCAF13-FRAS1-FAK pathway on the proliferation of ovarian cancer cells. Thus, our findings indicate that DCAF13 is a promising target for ovarian cancer therapy.
Materials and methods
References to supplementary tables and figures are indicated with prefix ‘S’. A comprehensive list of antibodies used in this study is presented in Table S1.
Cell culture and stable cell line generation with CRISPR/Cas9
Human ovarian cancer cell lines A2780, C13, ES-2, HO8910, OVCAR-3, and SKOV3 were purchased the American Type Culture Collection (ATCC, Manassas, VA, USA). Human normal ovarian epithelial cell line IOSE was supplied by Heng-Yu Fan, Zhejiang University [43]. Cells were grown in DMEM (Gibco | Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco | Thermo Fisher Scientific) and 1% penicillin-streptomycin (Gibco | Thermo Fisher Scientific) at 37 ℃ in humidified atmosphere composed of 5% CO2 and 95% air (standard culture conditions).
DCAF13-deficient cells were established using CRISPR/Cas9 technology. The guide RNA sequences used for targeting human DCAF13 were: human DCAF13–1: 5’- AGCGGGACAGCAGTGAGCCC-3’; human DCAF13–2: 5’-GATGTGGATTACTCTCCCAC-3’. The guide RNA sequences were inserted into LentiCRISPRv2 (addgene, #98290). The 293T cells were seeded into 6-well plates at a density of 100,000 cells/well. After 24 h, the medium was replaced with fresh DMEM supplemented with 10% FBS. To make lentivirus, 293T cells/well were cotransfected with a recombinant lentiviral vector LentiCRISPRv2 (400 ng) with the packaging plasmids pMD2.G (200 ng) and psPAX2 (200 ng) by using poly-jet (SignaGen, Rockville, MD, USA). After 48 h transfection, the 293T cell supernatant containing virus was filtered through a 0.45–µm hydrophilic polyvinylidene difluoride (PVDF) membrane (Millex-HV, Millipore, Burlington, MA, USA) to remove cell debris. The filtered 2 mL lentivirus were transfected into 4T1, 4T07, and MCF-7 cells by using polybrene (final concentration 8 ug/mL). Twenty-four hours after transfection, 1–2 ug/mL puromycin (Gibco) was continuously added to the cells for 3 days to sort transfected cells. Viable cells were sorted into 96-well plates with a single cell in each well. Colonies were grown and clones were screened by Western blot analysis using antibodies against DCAF13.
Cell proliferation and colony formation assays
A total of 1 × 105 cells were seeded per well in a 6-wells plate (Corning, NY, USA) (n = 3 per group). The cells were counted by hemocytometer at 24, 48, and 72 h after seeding. Cell count was plotted as a function of time after seeding.
The colony formation assay was conduct on soft agar. Six-wells plates were coated with 1.5 mL of 0.5% agar (Sigma-Aldrich, St. Louis, MO, USA) base layer. Subsequently, a suspension of 2 × 103 cells in 1.5 mL of 0.35% top agar was carefully added. To provide nutrients to the cells, 2 mL of cell culture medium was transferred onto the top layer twice a week. After a period of 3 weeks following plating, colonies were stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) dissolved in PBS and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Scratch and transwell assays
A total of 5 × 105 cells were seeded in 6-wells plates in medium supplemented with 10% FBS. Once cells reached 90% confluence, the monolayer was scraped with a with 10-µL pipette tip across the center of each well and the cells were washed once with PBS. Next, 2 mL of fresh serum-free medium was added to each well to starve the cells. The plates were imaged immediately after scratching and washing (baseline) and at 24 h using an inverted phase contrast microscope (CKX53, Olympus, Tokyo, Japan). The extent of cell migration was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and data are presented as percentage of cell-occupied area in the scratch channel at 24 h versus baseline.
Migration experiments were conducted in 24-wells plates using Transwell chambers equipped with 8-µm pore filters (Corning, NY, USA). Cells (1 × 104) were resuspended in 300 µL of FBS-free medium and transferred into the upper chamber. Next, 500 µL of medium containing 10% FBS was added to the lower chamber. After 24 h, cells were fixed in methanol for 5 min. Stationary cells in the upper chamber were removed with cotton swabs. The cells that had migrated were stained with hematoxylin and quantified using ImageJ software (National Institutes of Health).
Western blotting
Protein from cells and tissues was extracted using RIPA lysis buffer (Beyotime Biotechnology, Haimen, China) and quantified with a bicinchoninic acid assay (BCA assay kit; Beyotime Biotechnology). 20 µg protein per well was separated using SDS-PAGE, transferred to PVDF membranes (Merck | Millipore, Burlington, MA, USA), and blocked using 5% powdered milk for 1 h at RT. PVDF membranes were incubated overnight at 4 °C with primary antibodies against proteins of interest. Subsequently, the samples were incubated with a secondary antibody, specifically an anti-rabbit IgG HRP-linked antibody (Cell Signaling Technology, Danvers, MA, USA). The resulting bands were then visualized using an enhanced chemiluminescence detection kit (Merck | Millipore). Data acquisition was carried out using an Imager 680 (Amersham | GE Healthcare, Chicago, IL, USA). Image analysis and quantification using ImageJ software (National Institutes of Health).
Co-immunoprecipitation assay
Cells were lysed in cell lysis buffer for Western and IP (Beyotime Biotechnology). Cell lysates were spiked with 50 µL of Protein A/G magnetic beads (MCE Magnetic, Mianyang, China) and washed 3 × with 400 µL binding/washing buffer (1 × PBS + 0.5% Tween-20). The corresponding primary antibody was incubated with magnetic beads at 4 ℃ for 4 h, and subsequently the cell lysates were transferred into antibody-magnetic bead complex solution and incubated overnight at 4 ℃. The beads were thoroughly washed 5 × with a binding/washing buffer, followed by suspension in 1 × loading buffer, and heated at 95 °C for 5 min. The resulting samples were then analyzed by Western blot.
Protein identification analysis
This experiment was performed using 293T cells. DCAF13 was overexpressed by transfecting the DCAF13-FLAG plasmid into 293T cells. When the cells grew to 80%, the cells were collected and lysed in cell lysis buffer for Western and IP (Beyotime Biotechnology). The cell lysates were supplemented with 50 µL of Protein A/G magnetic beads (MCE Magnetic) and subjected to three washes using 400 µL of binding/washing buffer (1 × PBS + 0.5% Tween-20). Sequently, the corresponding primary antibody was incubated with the magnetic beads 4 h. Following this, the cell lysates were transferred into a solution containing the antibody-magnetic bead complex and incubated overnight at 4 ℃. The beads were thoroughly washed five times with a binding/washing buffer, followed by suspension in 1 × loading buffer, and heated at 95 °C for 5 min. Control experiments using IgG antibodies. Proteins were separated using SDS-PAGE. The gel was stained with Coomassie Brilliant Blue for 30 min and destained by deionized water to remove background. The obtained samples were subjected to protein identification using LC-MS/MS by APT Biotechnology (Shanghai, China).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cultured cells or murine tumor tissues using TRIzol reagent (Invitrogen | Thermo Fisher Scientific) (n = 3 per group). Extracted RNA was converted to cDNA through reverse transcription using Prime Script RT Reagent Kit (Takara Bio, Shiga, Japan). Real-time PCR analysis was performed with TB Green Master Mix Kit (Takara Bio) on a Realplex2 PCR System (Eppendorf, Hamburg, Germany). The mRNA levels of each gene were standardized to the expression levels of the housekeeping gene β-actin. Primer information is presented in Table S2.
Plasmids and RNA interference
Expression constructs coding for mouse Dcaf13 cDNA (Flag-DCAF13), Flag-DCAF13 SOF∆, Flag-DCAF13 WD∆, Flag-DDB1, and Myc-Ub plasmids were kindly provided by Dr. Heng-Yu Fan [44]. This protein is characterized by the presence of seven WD40 repeats at its N terminus and a SOF1 domain located at the C terminus. Human FRAS1 cDNA was cloned by Miaoling Biotechnology (Wuhan, China). FRAS1 is a remarkably conserved protein consisting of 1976 amino acid residues, with a molecular weight of 217 kDa.
A total of 2 × 105 cells were seeded in six-well plates for 24 h. Lipofectamine RNAiMAX reagent (Invitrogen) was used for siRNA transfection. After 48 h of transfection (final siRNA concentration 80 nM), the cells were collected and analyzed by qRT-PCR or Western blot to assess interference efficiency. The siRNA sequences are listed in Table S3.
Flow cytometry analysis
Cells (1 × 106) were fixed with 70% ethanol for 24 h. After centrifugation and a dual washing step with PBS, the cells were resuspended in 500 µL of PI + RNase staining buffer (BD Biosciences, Franklin Lakes, NJ, USA) and incubated at 37 ℃ in the dark for 30 min. Cells were assayed by flow cytometry (model flow cytometer; BD Biosciences). Data were analyzed using ModFit software (Verity Software House, Topsham, ME, USA).
Mouse xenograft models
All animal experiments were approved by the Jiaxing University’s institutional review board (registration no. JUMC2020-069). Specific pathogen free female BALB/c nude mice, aged 6–8 weeks, were obtained from Jiangsu Jicui Yaokang Biotechnology Co. (Nanjing, China). Animals were housed in a room with 12-hour light/dark cycles in individually ventilated cages with ad libitum access to sterilized food and water. The animals were treated in accordance with institutional guidelines and the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (8th edition).
The mice were randomly assigned to three groups and anesthetized with ether. Subcutaneously, One group was injected with wild-type ovarian cancer cells in the right dorsal flank (n = 6/group), and the other two groups was injected with DCAF13-deleted cells (single bolus of 5 × 106 cells in PBS) in the right dorsal flank (n = 6/group). The tumor size of the mice was measured with a caliper every 2–3 days. The tumor volume was calculated using the formula: (width)2 × height × 0.523 [45]. Nude mice were killed by cervical dislocation. A tumor diameter of > 15 mm constituted a human endpoint. Resected tumor tissue was fixed in 4% paraformaldehyde or stored at -80 ℃ until further use.
Histochemistry and immunohistochemistry
Paraffin-embedded human tissue samples from ovarian cancer tissue and paracancerous tissues were provided by the Affiliated Hospital of Jiaxing University (approval no. LS2020-148). Human ovarian cancer tissue microarrays were purchased from Shanghai Outdo Biotech Co. (Shanghai, China). Clinical information was provided by Shanghai Outdo Biotech Co. The clinical information of the ovarian cancer tissue microarrays was presented in Table S4 which includes the detailed information of the patients such as sex, age, pathology, nature, Grade, Stage, etc. The clinical information of ovarian cancer patients enrolled at Jiaxing University hospital was presented in Table S5.
Fixed mouse-derived tumor tissue was thawed, embedded in paraffin, sliced into 5-µm thick sections (Leica, Wetzlar, Germany), deparaffinized, and stained with hematoxylin and eosin (H&E). For immunohistochemistry (IHC), the deparaffinized sections underwent 10-minute incubation in 0.3% H2O2. Antigen retrieval was performed using 10 mM sodium citrate (pH = 6.0) for 15 min. Subsequently, the sections were incubated overnight at 4 °C with primary antibodies against Ki67 (1:400), p-histone H3 (1:200), and cleaved caspase-3 (1:200) (Table S1). Next, a biotinylated and peroxidase-conjugated secondary antibody was applied for 30 min (1:400, Cell Signaling Technology). The sections were counterstained utilizing a Vectastain ABC kit and a 3,3′-diaminobenzidine peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA).
In Fig. 1b, immunohistochemistry was performed on the paraffin-embedded tissue microarray using anti-DCAF13 antibodies (Table S1). Protein expression levels were scored by multiplying the percentage of positive cells and immunostaining intensity. The percentage was scored as follows: non-positive cells = 0 points, 1–25% = 1 point, 26–50% = 2 points, 51–75% = 3 points, and 76–100% = 4 points. The staining intensity was scored as follows: no positive staining = 0 points, weak staining = 1 point, moderate staining = 2 points, and strong staining = 3 points. The final scores were obtained according to the above terms: 0 was negative expression (negative), 1–3 was weak expression (+), 4–8 was moderate expression (++), and 9–12 was high expression (+++). In Fig. 1e, by performing immunohistochemistry on ovarian cancer tissue microarrays, the DCAF13 staining intensity and positivity rate, i.e. DCAF13 expression level, of each tissue spot in the chip were obtained. According to Table S4, the survival period of patients corresponding to each tissue point in the ovarian cancer chip was obtained. By using Kaplan Meier survival analysis and log rank statistics, the relationship between the expression level of DCAF13 and patient survival rate was analyzed.
Immunofluorescence staining
Cells (5 × 104) were seeded in 24-well plates, fixed with 4% paraformaldehyde for 30 min, and blocked using 5% bovine serum albumin for 1 h. Subsequently, the cells were incubated with primary antibodies against Ki67 (1:400), p21 (1:800), and p-H2AX (1:400) (Table S1). Primary antibodies were removed with a single washing step, and cells were incubated with secondary antibodies labeled with Alexa488 or Alexa594 (Abcam, Cambridge, UK). Next, cells were counterstained with DAPI (Beijing Solarbio Science & Technology Co., Beijing, China). Digital images were captured with a confocal laser scanning microscope (FV3000, Olympus).
Statistical analysis
GraphPad Prism (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Mean ± standard deviation represented the data and their differences. Samples with n < 8 were subjected to analysis using nonparametric tests. The normal distribution of data was assessed using ANOVA. The correlation between DCAF13 expression and clinicopathological characteristics was assessed using both the Chi-square test and Fisher’s exact tests. Using the Kaplan-Meier method, survival rates after tumor removal were calculated, and differences in survival curves were evaluated using the Log-rank test. Additionally, a multivariate survival analysis using the Cox proportional hazard regression model was performed, integrating all relevant traits found in the univariate survival study. A P-value of < 0.05 was considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our gratitude to Yuan-Yuan Gao, Jing-Ya Zhong, Jing-Jian Dong, and Li-Li Shi for their invaluable technical support. We also acknowledge Dr. Kun-Liang Guan for providing the CRISPR/Cas9 plasmid and Dr. Heng-Yu Fan for the IOSE cells. This research was made possible through the generous support of the Key Laboratory of Medical Electronics and Digital Health of Zhejiang Province and Engineering Research Center of Intelligent Human Health Situation Awareness of Zhejiang Province, Jiaxing University, 314001, China. This work was supported by the Jiaxing talent pioneer innovation team, Jiaxing.
Author contributions
W-WP, Z-JZ, and S-QC were responsible for the overall conception and design of this experiment. W-WP and Z-JZ collated and summarized the experimental results and wrote the manuscript. Z-YT and X-MW participated in most of the experimental procedures in this study. J-YZ and Z-YW participated in primer design. Q-QS, Y-XH, and Q-YZ were involved in the management and sampling of experimental animals. H-YH was involved in the immunohistochemical experiments. X C, X Z, and A-JL participated in the collection of clinicopathological specimens. C-WX, S-BL, X-CZ, Y-JG, A-JL, and MH revised the final manuscript. All the authors participated in the analysis of the results and provided critical input.
Funding
This work was supported by grants from National Natural Science Foundation of China (31871402), the Natural Science Foundation of Zhejiang Province (LY21H160047, LGD21H160003, LQ23C070001, LY17H160060, Z20H160031, LGF20H160031, LGD22H030004) and Zhejiang Province Traditional Chinese Medicine Science and Technology Plan Project (2020AZ114). This work was supported by grants from Zhejiang Provincial Foreign Expert Grant (12.2018). This work was supported by Jiaxing Key Laboratory for Photonanomedicine and Experimental Therapeutics (12.2019), and Jiaxing Science and Technology Planning Project (2023AY40005, 2020AD30073). This work was supported by the Dutch Cancer Foundation (KWF, project 10666) and the Top-level Talent Project of Zhejiang Province. This work was supported by the Jiaxing talent pioneer innovation team (6.2021).
Data availability
No new datasets were generated during the current study.
Declarations
Ethical approval
All clinical samples used in this study were approved by the Human Research Ethics Committee of the Affiliated Hospital of Jiaxing University (approval number: LS2021-KY-292). All patients provided written informed consent before enrollment. All archived samples were approved by the Institutional Review Board of Jiaxing University. All animal experiments involved in this study were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals following approval by the Laboratory Animal Ethics Committee of Jiaxing University (approval number: JUMC2021-151).
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ze-Yi Tang, Xiao-Min Wang and Chun-Wei Xu contributed equally to this work.
Contributor Information
Zha-Jun Zhan, Email: zjnpr@zjut.edu.cn.
Shu-Qun Cheng, Email: chengshuqun@aliyun.com.
Wei-Wei Pan, Email: wwpan@mail.zjxu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Qi S, Shi C, Han X, Yu J, Zhang L et al (2020) Identification of metastasis and prognosis-associated genes for serous ovarian cancer. Biosci Rep 40(6):BSR20194324. 10.1042/BSR20194324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C et al (2021) Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 397(10290):2182–2193. 10.1016/S0140-6736(21)00731-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Zyl B, Tang D, Bowden NA (2018) Biomarkers of platinum resistance in ovarian cancer: what can we use to improve treatment. Endocr Relat Cancer 25(5):R303–R318. 10.1530/ERC-17-0336 [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Xie HJ, Li YY, Wang X, Liu XX, Mai J (2022) Molecular mechanisms of platinum–based chemotherapy resistance in ovarian cancer. Oncol Rep 47(4):82–93. 10.3892/or.2022.8293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu K, Hopkins BD, Sanchez R, DeVita RJ, Pan ZQ (2021) Targeting Cullin-RING E3 ubiquitin ligase 4 by small molecule modulators. J Cell Signal 2(3):195–205. 10.33696/Signaling.2.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu X, Meng Y, Xu L, Qiu L, Wei M, Su D et al (2019) Cul4 E3 ubiquitin ligase regulates ovarian cancer drug resistance by targeting the antiapoptotic protein BIRC3. Cell Death Dis 10(2):104. 10.1038/s41419-018-1200-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Guo J, North BJ, Tao K, Zhou P, Wei W (2019) The emerging role for Cullin 4 family of E3 ligases in tumorigenesis. Biochim et Biophys Acta Reviews Cancer 1871(1):138–159. 10.1016/j.bbcan.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng Y, Qiu L, Zeng X, Hu X, Zhang Y, Wan X et al (2022) Targeting CRL4 suppresses chemoresistant ovarian cancer growth by inducing mitophagy. Signal Transduct Target Therapy 7(1):388. 10.1038/s41392-022-01253-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Li L, Fu L, Yuan Y, Dai H, Zhu T et al (2019) Integrated Bioinformatics Analysis the function of RNA binding proteins (RBPs) and their prognostic value in breast Cancer. Front Pharmacol 10:140. 10.3389/fphar.2019.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan BQ, Wang XM, Zheng L, Han Y, Gao J, Lv MD et al (2022) DCAF13 promotes breast cancer cell proliferation by ubiquitin inhibiting PERP expression. Cancer Sci 113(5):1587–1600. 10.1111/cas.15300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J, Hou P, Chen J, Wang P, Wang W, Liu W et al (2017) The overexpression and prognostic role of DCAF13 in hepatocellular carcinoma. Tumour Biology: J Int Soc Oncodevelopmental Biology Med 39(6):1–9. 10.1177/1010428317705753 [DOI] [PubMed] [Google Scholar]
- 13.Yan H, Bi L, Wang Y, Zhang X, Hou Z, Wang Q et al (2017) Integrative analysis of multi-omics data reveals distinct impacts of DDB1-CUL4 associated factors in human lung adenocarcinomas. Sci Rep 7(1):333. 10.1038/s41598-017-00512-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei S, Lu K, Xing J, Yu W (2023) A multidimensional pan-cancer analysis of DCAF13 and its protumorigenic effect in lung adenocarcinoma. FASEB J 37(4):e22849. 10.1096/fj.202201022RRR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z, Dong Q, Liu X, Wei L, Liu L, Li Y et al (2020) Dynamic transcriptome profiling in DNA damage-induced cellular senescence and transient cell-cycle arrest. Genomics 112(2):1309–1317. 10.1016/j.ygeno.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Ogrodnik M, Salmonowicz H, Jurk D, Passos JF (2019) Expansion and cell-cycle arrest: common denominators of Cellular Senescence. Trends Biochem Sci 44(12):996–1008. 10.1016/j.tibs.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 17.Wang V, Geybels MS, Jordahl KM, Gerke T, Hamid A, Penney KL et al (2021) A polymorphism in the promoter of FRAS1 is a candidate SNP associated with metastatic prostate cancer. Prostate 81(10):683–693. 10.1002/pros.24148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talbot JC, Nichols JT, Yan YL, Leonard IF, BreMiller RA, Amacher SL et al (2016) Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial-mesenchymal interaction. Dev Biol 416(1):136–148. 10.1016/j.ydbio.2016.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrou P, Makrygiannis AK, Chalepakis G (2008) The Fras1/Frem family of extracellular matrix proteins: structure, function, and association with Fraser syndrome and the mouse bleb phenotype. Connect Tissue Res 49(3):277–282. 10.1080/03008200802148025 [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Wang Z, Lu H, Zhao Z, Guo L, Kong F et al (2022) Comprehensive analysis of FRAS1/FREM family as potential biomarkers and therapeutic targets in renal clear cell carcinoma. Front Pharmacol 13:972934. 10.3389/fphar.2022.972934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyozumi D, Osada A, Sugimoto N, Weber CN, Ono Y, Imai T et al (2005) Identification of a novel cell-adhesive protein spatiotemporally expressed in the basement membrane of mouse developing hair follicle. Exp Cell Res 306(1):9–23. 10.1016/j.yexcr.2005.01.020 [DOI] [PubMed] [Google Scholar]
- 22.Sulzmaier FJ, Jean C, Schlaepfer DD (2014) FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14(9):598–610. 10.1038/nrc3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Yi Q, Tang L (2019) The roles of nuclear focal adhesion kinase (FAK) on Cancer: a focused review. J Experimental Clin cancer Research: CR 38(1):250. 10.1186/s13046-019-1265-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane D, Goncharenko-Khaider N, Rancourt C, Piché A (2010) Ovarian cancer ascites protects from TRAIL-induced cell death through alphavbeta5 integrin-mediated focal adhesion kinase and akt activation. Oncogene 29(24):3519–3531. 10.1038/onc.2010.107 [DOI] [PubMed] [Google Scholar]
- 25.Cooper J, Giancotti FG (2019) Integrin signaling in Cancer: mechanotransduction, stemness, epithelial plasticity, and Therapeutic Resistance. Cancer Cell 35:347–367. 10.1016/j.ccell.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen D et al (2020) Periostin promotes colorectal tumorigenesis through Integrin-FAK-Src pathway-mediated YAP/TAZ activation. Cell Rep 30:793–806e6. 10.1016/j.celrep.2019.12.075 [DOI] [PubMed] [Google Scholar]
- 27.Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC et al (2007) Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A 104:20302–20307. 10.1073/pnas.0710091104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Li H, Mao A, Lu J, Liu W, Qie J et al (2020) DCAF13 promotes triple-negative breast cancer metastasis by mediating DTX3 mRNA degradation. Cell Cycle (Georgetown Tex) 19(24):3622–3631. 10.1080/15384101.2020.1859196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Baechler SA, Zhang X, Kumar S, Factor VM, Arakawa Y et al (2023) Targeting neddylation sensitizes colorectal cancer to topoisomerase I inhibitors by inactivating the DCAF13-CRL4 ubiquitin ligase complex. Nat Commun 14(1):3762. 10.1038/s41467-023-39374-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, You X, Xu T, Chen Q, Li H, Dou L et al (2022) PD-L1 induction via the MEK-JNK-AP1 axis by a neddylation inhibitor promotes cancer-associated immunosuppression. Cell Death Dis 13(10):844. 10.1038/s41419-022-05292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo MS, Subhash VV, Suda K, Balcıoğlu HE, Zhou S, Thuya WL et al (2019) FBXW5 promotes tumorigenesis and metastasis in gastric Cancer via activation of the FAK-Src signaling pathway. Cancers (Basel) 11(6):836. 10.3390/cancers11060836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao G, Gong L, Su D, Jin Y, Guo C, Yue M et al (2019) Cullin5 deficiency promotes small-cell lung cancer metastasis by stabilizing integrin β1. J Clin Investig 129(3):972–987. 10.1172/JCI122779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlakis E, Chiotaki R, Chalepakis G (2011) The role of Fras1/Frem proteins in the structure and function of basement membrane. Int J Biochem Cell Biol 43(4):487–495. 10.1016/j.biocel.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 34.Ashrafizadeh M, Hushmandi K, Rahmani Moghadam E et al (2020) Progress in delivery of siRNA-Based therapeutics employing Nano-vehicles for treatment of prostate Cancer. Bioeng (Basel) 7:91. 10.3390/bioengineering7030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan Q, Huang RF, Liang XH, Ge MX, Jiang JW, Lin H et al (2014) FRAS1 knockdown reduces A549 cells migration and invasion through downregulation of FAK signaling. Int J Clin Exp Med 7(7):1692–1697 eCollection 2014 [PMC free article] [PubMed] [Google Scholar]
- 36.Umeda S, Kanda M, Miwa T, Tanaka H, Tanaka C, Kobayashi D et al (2020) Fraser extracellular matrix complex subunit 1 promotes liver metastasis of gastric cancer. Int J Cancer 146(10):2865–2876. 10.1002/ijc.32705 [DOI] [PubMed] [Google Scholar]
- 37.Boscaro C, Baggio C, Carotti M, Sandonà D, Trevisi L, Cignarella A et al (2022) Targeting of PFKFB3 with miR-206 but not mir-26b inhibits ovarian cancer cell proliferation and migration involving FAK downregulation. FASEB Journal: Official Publication Federation Am Soc Experimental Biology 36(3):e22140. 10.1096/fj.202101222R [DOI] [PubMed] [Google Scholar]
- 38.Kim H, Son S, Ko Y, Shin I (2021) CTGF regulates cell proliferation, migration, and glucose metabolism through activation of FAK signaling in triple-negative breast cancer. Oncogene 40(15):2667–2681. 10.1038/s41388-021-01731-7 [DOI] [PubMed] [Google Scholar]
- 39.Ma Y, Fu Y, Fan X, Ji Q, Duan X, Wang Y et al (2023) FAK/IL-8 axis promotes the proliferation and migration of gastric cancer cells. Gastric Cancer: Official J Int Gastric Cancer Association Japanese Gastric Cancer Association 26(4):528–541. 10.1007/s10120-023-01384-3 [DOI] [PubMed] [Google Scholar]
- 40.Ozmadenci D, Shankara Narayanan JS, Andrew J, Ojalill M, Barrie AM, Jiang S et al (2022) Tumor FAK orchestrates immunosuppression in ovarian cancer via the CD155/TIGIT axis. Proc Natl Acad Sci U S A 119(17):e2117065119. 10.1073/pnas.2117065119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan R, Yu Y, Zhu H, Zhang W, Qin Y, Ye L et al (2022) RSPO2 promotes progression of ovarian cancer through dual receptor-mediated FAK/Src signaling activation. iScience 25(10):105184. 10.1016/j.isci.2022.105184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray U, Jung DB, Jin L, Xiao Y, Dasari S, Sarkar Bhattacharya S et al (2022) Targeting LRRC15 inhibits metastatic dissemination of Ovarian Cancer. Cancer Res 82(6):1038–1054. 10.1158/0008-5472.CAN-21-0622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullany LK, Fan HY, Liu Z, White LD, Marshall A, Gunaratne P et al (2011) Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene 30(32):3522–3536. 10.1038/onc.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang YL, Zhao LW, Zhang J, Le R, Ji SY, Chen C et al (2022) DCAF13 promotes pluripotency by negatively regulating SUV39H1 stability during early embryonic development. EMBO J 37(18):e98981. 10.15252/embj.201898981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J et al (2016) The Hippo Pathway Kinases LATS1/2 suppress Cancer Immunity. Cell 167(6):1525–1539 e17. 10.1016/j.cell.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new datasets were generated during the current study.