Abstract
Indoxyl sulfate (IS), a uremic toxin, is a harmful factor that damages kidneys. Chronic heat stress in laying hens causes renal injury; however, whether IS accumulation is involved in this injury remains unknown. We selected 20 Boris brown laying hens (27 weeks old) and randomly assigned them to two groups (n = 10), one group was exposed to chronic heat stress (32 °C for 4 weeks), whereas the other was maintained at 24 °C. Chronic heat exposure significantly increased plasma and renal IS concentrations (P < 0.05). Exposure to heat also increased renal expression of the aryl hydrocarbon receptor (AhR) and its target genes (CYP1A4 and CYP1B1). Furthermore, chronic heat exposure tended to increase the 2-thiobarbituric acid reactive substances content (P = 0.08) and significantly decreased the antioxidant capacity in the kidney, while increasing the transcription levels of nuclear factor κB and fibrosis-related genes (COLA1A1, αSMA, TGF-β, Smad3, and VCAM-1) and the area of renal fibrosis. Our results indicate that chronic heat exposure induces systemic and renal IS accumulation in laying hens. This accumulated IS may activate the AhR pathway and chronically disrupt the oxidative stress status and antioxidant activity, thus promoting renal fibrosis and dysfunction in laying hens.
Keywords: Laying hens, Fibrosis, Heat stress, Indoxyl sulfate, Kidney, Uremic toxins
Subject terms: Kidney, Animal physiology
Introduction
Heat stress is one of the worst environmental stressors in poultry, having negative consequences for productivity and product quality1, resulting in substantial economic losses. In laying hens, heat stress disrupts the balance between plasma calcium and phosphorus levels, impairing egg production and eggshell quality2,3. Furthermore, our previous study showed that heat stress in laying hens can cause serious kidney damage4. Although this renal damage was proposed as a contributing factor to poor productivity in heat-stressed laying hens, this relationship requires detailed clarification.
In humans, renal dysfunction leads to uremia associated with the accumulation of uremic toxins in the body5.This induces oxidative stress, leading to cytotoxic and inflammatory effects, which contribute to the progression of renal damage and are involved in various renal failure-related complications6. Indoxyl sulfate (IS), the end product of dietary tryptophan degradation through the indole pathway, is among the most potent uremic toxins7,8. Under normal conditions, uremic toxins are excreted in urine; however, they accumulate in the blood when the kidneys fail9. In patients with kidney failure, plasma IS levels increase with disease progression10,11. Proximal tubular cells accumulate IS via organic anion transporters (OATs), and this accumulation induces nephrotoxicity by chronically stimulating oxidative stress and inflammation via aryl hydrocarbon receptor (AhR) activation12,13. Because heat exposure induces renal dysfunction in laying hens4, it is possible that heat-stressed hens have elevated systemic and renal IS concentrations and that IS accumulation can further promote renal damage. However, no reports have investigated whether heat exposure affects plasma IS concentrations in laying hens or whether it is associated with kidney injury.
In this study, we hypothesized that heat stress-induced renal dysfunction causes IS accumulation in the body and that the accumulated IS further contributes to the progression of renal dysfunction in heat-stressed laying hens. To verify these hypotheses and elucidate the underlying mechanism, we investigated the effects of heat stress on IS accumulation in the plasma and kidneys of laying hens and the expression of IS-mediated activation genes such as OATs and AhR in the kidney. In addition, we examined the effects of IS accumulation on renal oxidative stress status in heat-stressed hens and its association with progressive renal fibrosis and dysfunction.
Results
Chronic heat stress causes renal dysfunction and IS accumulation in laying hens
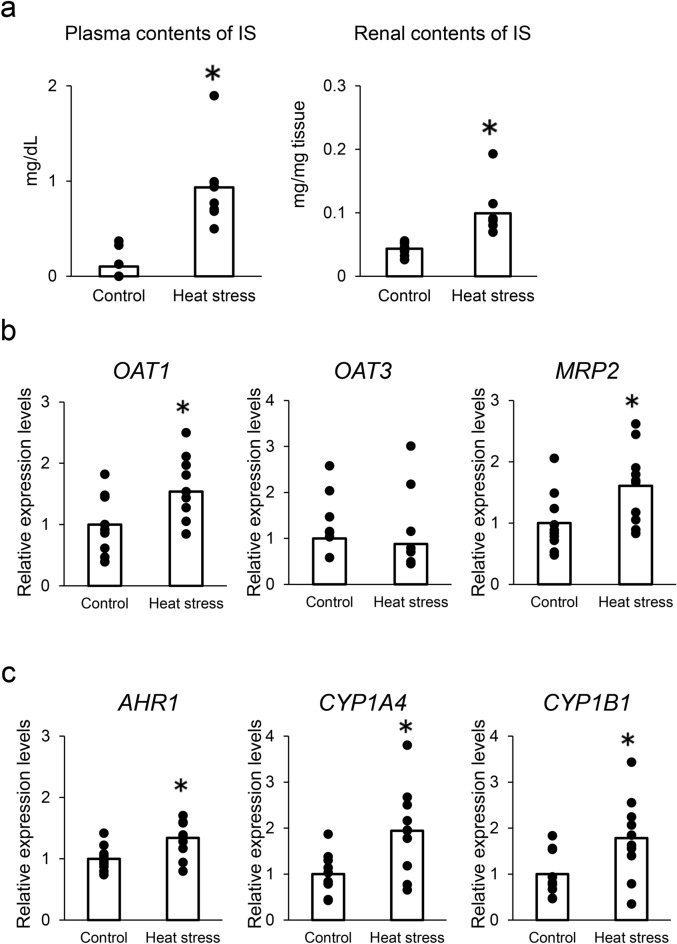
As shown in Table 1, chronic heat stress reduced body weight gain (g/day), daily feed intake (g/day), egg-laying rate (%), egg weight (g), and daily egg production (g/day) (P < 0.05). Heat stress also reduced eggshell strength (kg/cm2) and eggshell weight (g) and thickness (mm). These results showed that heat stress was induced in our study. In comparison with the control group, plasma calcium and phosphate concentrations decreased when the birds were exposed to heat stress (Table 2). While blood urea nitrogen levels in plasma were unaffected, heat exposure significantly decreased plasma albumin levels and increased plasma creatinine levels (Table 2). These results indicate that chronic heat exposure (32 °C for 4 weeks) impaired egg-laying performance and renal function. As shown in Fig. 1a, chronic heat exposure significantly increased IS concentrations in both plasma and renal tissues. Quantitative polymerase chain reaction (PCR) analysis was used to determine the expression of OATs and multidrug resistance-associated proteins (MRPs) in the renal tissues of laying hens. OAT1 and MRP2 expression significantly increased in the heat-stressed group compared with that in the control group (Fig. 1b). Similarly, AhR1, CYP1A4, and CYP1B1 expression significantly increased in heat-stressed hens compared with that in the control group (Fig. 1c).
Table 1.
Laying performance and egg quality.
| Control | Heat stress | Pooled SEM | Significance | |
|---|---|---|---|---|
| Body weight gain, g/day | 1.7 | − 10.7 | 1.53 | * |
| Daily feed intake, g/day | 150.8 | 89.9 | 8.58 | * |
| Laying ratea, % | 97.2 | 65.5 | 3.94 | * |
| Average egg weighta, g | 61.9 | 55.0 | 1.12 | * |
| Daily egg productiona, g/day | 61.9 | 53.0 | 1.13 | * |
| Eggshell strengthb, kg/cm2 | 4.1 | 3.5 | 0.14 | * |
| Eggshell weightb, g | 6.5 | 5.6 | 0.19 | * |
| Eggshell thicknessb, mm | 0.40 | 0.36 | 0.001 | * |
aLaying performance refers to the average data of each group of hens (n = 10 for each week).
bEggshell quality refers to the average data of each group of eggs (n = 10 for each week).
*P < 0.05 indicates statistical significance.
Table 2.
Biochemical parameters in plasma.
| Control | Heat stress | Pooled SEM | Significance | |
|---|---|---|---|---|
| Calcium, mg/dL | 23.8 | 17.7 | 1.32 | * |
| Phosphate, mg/dL | 4.75 | 2.57 | 0.33 | * |
| Blood urea nitrogen, mg/dL | 0.71 | 0.59 | 0.038 | - |
| Albumin, mg/dL | 2.32 | 2.02 | 0.058 | * |
| Creatinine, mg/dL | 0.037 | 0.056 | 0.003 | * |
*P < 0.05 indicates statistical significance.
Plasma contents refers to the average of each hen group (n = 10).
Fig. 1.
Effect of heat stress on indoxyl sulfate (IS) accumulation and IS-related gene expression in laying hens. (a) IS plasma levels and renal contents in control (n = 10) and heat stress (n = 10) hen groups for 4 weeks. Statistical analysis was performed using Student’s t-test. *P < 0.05 was considered statistically significant. (b) mRNA expression levels of OAT1, OAT3, and MRP2, (c) mRNA levels of IS-mediated activation genes (AhR, CYP1A4, and CYP1B1) in renal tissue. mRNA expression levels were quantified by real-time quantitative PCR and normalized to 18srRNA. n = 10 in each group. Statistical analysis was performed using Student’s t-test.
IS accumulation affects oxidant and antioxidant status in the kidneys of heat-stressed laying hens
As shown in Fig. 2a, the renal contents of thiobarbituric acid reactive substances (TBARS; a marker of oxidative stress) tended to increase (P = 0.08) in heat-stressed hens compared with that in the control group. Additionally, the renal gene expression of NF-kB, which is upregulated by oxidative stress, significantly increased in the heat-stressed group compared with that in the control group (Fig. 2b). However, the total antioxidant capacity in the renal tissue of heat-stressed hens was significantly lower than that in the control group (Fig. 2c). Similarly, CAT and GPX4 mRNA expressions were significantly downregulated by heat exposure, whereas the expression levels of SOD1 and SOD2 were not altered by heat exposure (Fig. 2d).
Fig. 2.
Effect of IS accumulation on the oxidant and antioxidant status in the kidneys of laying hens under heat stress. (a) TBARS content in renal tissue. n = 10 in each group. Statistical analysis was performed using Student’s t-test. (b) NF-kB mRNA expression levels in the kidney quantified by real-time quantitative PCR and normalized to 18srRNA. n = 10 in each group. Statistical analysis was performed using Student’s t-test. (c) Total antioxidant capacity in renal tissue. n = 10 in each group. Statistical analysis was performed using Student’s t-test. (d) Quantitative analysis of antioxidant enzyme genes in the kidney. CAT, SOD1, SOD2, and GPX4 mRNA expression levels measured by real-time PCR and normalized to 18srRNA. Statistical analysis was performed using Student’s t-test.
IS accumulation induces renal fibrosis in heat-stressed laying hens
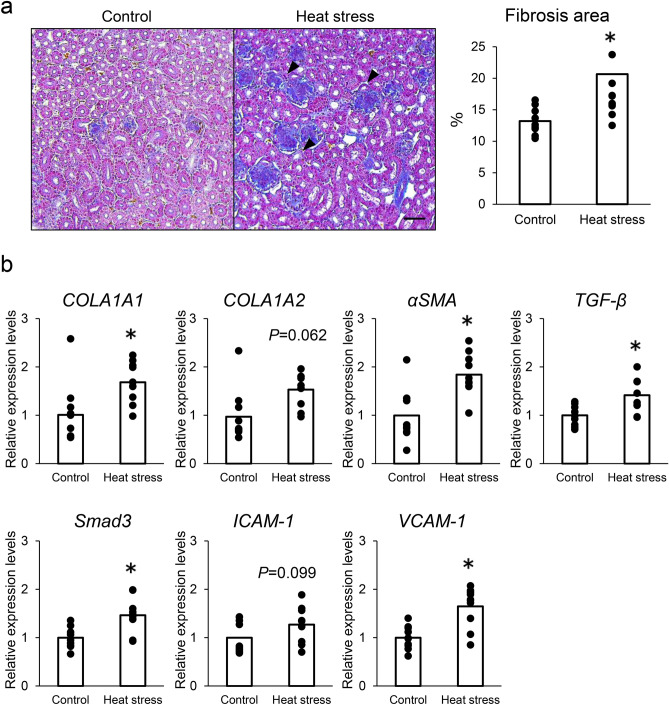
As shown in Fig. 3a, histopathological analysis revealed that the renal tubules were severely damaged (black arrows), and the fibrotic area significantly increased in heat-stressed hens. A fibrotic area was observed in the interstitial region of the renal cortex (Fig. 3a). Furthermore, the proximal tubule collapsed, indicating proximal tubular injury (Fig. 3a, black arrow). In the heat-stressed group, the gene expression levels of collagen type I alpha 1 (COL1A1), α-smooth muscle actin (αSMA), transforming growth factor-β (TGF-β), and Smad3, the intracellular effectors of TGF-β signaling, were significantly increased, and collagen type I alpha 2 (COL1A2) expression levels increased (P = 0.062; Fig. 3b). Furthermore, the expression of vascular cell adhesion molecule-1 (VCAM-1) significantly increased and that of intercellular adhesion molecule-1 (ICAM-1) tended to increase (P = 0.099) in the heat-stressed group compared with that in the control group (Fig. 3b).
Fig. 3.
Effects of IS accumulation on renal fibrosis and expression of fibrotic-related genes. (a) Representative Masson’s trichrome-stained kidney sections of control and heat-stressed hens (Left). Arrows indicate proximal tubular injury. Morphometric analysis of the fibrotic area using picrosirius red-stained kidney (Right). n = 10 for each group. Data are presented as percentages of the total cortex. Statistical analysis was performed using Student’s t-test. Bars = 100 μm. (b) Quantitative analysis of fibrotic genes. mRNA levels of COLA1A1, COLA1A2, αSMA, TGF-β, Smad3, ICAM-1, and VCAM-1 quantified by real-time quantitative PCR and normalized to 18srRNA. n = 10 in each group. Statistical analysis was performed using Student’s t-test.
Discussion
In the present study, we examined the effect of heat exposure on IS accumulation in laying hens and the involvement of IS accumulation in renal fibrosis and dysfunction. Consistent with our previous study, chronic heat exposure in laying hens increased plasma creatinine and decreased albumin levels, confirming that chronic heat stress causes renal failure4. Notably, heat stress in laying hens significantly increased IS concentrations in the plasma and kidneys. To the best of our knowledge, this is the first report showing that heat exposure induces IS accumulation in the blood and kidneys of laying hens.
Circulating IS accelerates the progression of renal failure and induces its own accumulation, resulting in a negative chain of renal dysfunction14. Because cells import IS via OAT1 and OAT3 and cellular excretion is mediated by MRTs15,16, the target cells for IS may be proximal tubular cells expressing these transporters17,18. The accumulation of uremic toxins in tubular cells plays a particularly important role in progressive renal failure19. In this study, we observed the upregulation of OAT1 and MRT2 in the renal tissue of heat-stressed hens. This finding is consistent with those of previous studies in mammals, which revealed that the accumulated IS in proximal tubular cells upregulated OAT1and increased OAT1-mediated IS uptake in chronic renal disease models17,20.
AhR activation by IS can induce the progression of glomerular injury and renal inflammation21. A recent study using microarray analysis demonstrated that IS upregulated AhR client genes, including CYP1A1 and CYP1B 1, in human umbilical vein endothelial cells22. In addition, avian CYP1A4 is orthologous to mammalian CYP1A123. Consistent with this view, our data revealed that AhR and its target genes CYP1A4 and CYP1B1 are expressed at high levels in the kidneys. Thus, our results indicate that under chronic heat stress, the AhR pathway is activated in the kidneys of laying hens when IS accumulates in the blood and kidneys.
Systemic IS plays an important role in the progression of renal fibrosis by inducing reactive oxygen species (ROS) production and inflammatory responses13,24. In this study, the TBARS content tended to be elevated in the renal tissue of heat-stressed hens, and the expression of NF-kB, an inflammatory modulator regulated by ROS, was significantly elevated in heat-stressed hens. In contrast, a decrease in the total antioxidant capacity and expression of antioxidant-related genes was observed in the renal tissue of heat-stressed laying hens. These results are consistent with those of previous studies illustrating that IS induces oxidative stress and damages the antioxidant defense system through coordinated regulation by ROS and NF-kB7,18,25. The cytochrome P450 family promotes ROS generation and causes cell damage, contributing to disease development26. Considering that we observed elevated renal expression of CYP450 family genes and NF-kB in heat-stressed hens, oxidative damage in heat-stressed hens may be caused by ROS generation via IS-induced activation of the CYP450 family and NF-kB. However, heat stress can enhance ROS generation and induce oxidative damage by mitochondrial dysfunction27–29. Indeed, our previous studies demonstrated that chronic heat exposure led to mitochondrial dysfunction, which is indicated by decreased mitochondrial DNA copy numbers and ATP levels in the kidneys of heat-stressed laying hens4. Consequently, our current findings indicated that increased oxidative damage in the kidneys of laying hens under heat exposure was induced by IS-induced activation of the CYP450 family and NF-kB and possibly by mitochondrial dysfunction.
In renal failure models, IS accumulates in the proximal tubular cells and causes cytotoxicity, ultimately leading to renal fibrosis and dysfunction7. We observed extensive multicellular interstitial fibrosis with cortical atrophy and tubular loss in the kidneys of heat-stressed hens, indicating cytotoxicity induced by IS accumulation in the proximal tubules. Consistent with our previous findings4, the renal cortical fibrotic area and the expression of profibrotic genes were significantly increased in heat-stressed hens. Interestingly, the expression of Smad3 and VCAM-1, which are induced through NF-κBactivation by IS and stimulated by ROS production30,31, was also elevated. Thus, in laying hens, systemic and renal IS accumulation due to chronic heat exposure leads to further tubulointerstitial damage via increased oxidative stress and disruption of the antioxidant balance, resulting in renal dysfunction and progressive renal fibrosis.
Our findings indicate that IS is an important inducer of renal fibrosis and dysfunction in laying hens exposed to chronic heat stress. IS is produced when tryptophan is converted to indole by intestinal bacteria and is oxidized and sulfated in the liver32,33. Thus, IS accumulation could be controlled by altering the levels of amino acids and proteins in the feed provided to hens under heat stress. Suppressing IS accumulation could help alleviate renal dysfunction in heat-stressed hens, ultimately contributing to improved egg productivity. However, the mechanism of IS accumulation in the systemic and renal systems of laying hens under chronic heat stress remains unclear. Heat stress reportedly induces dysbiosis in the gut microbiota34, increases oxidative stress, and alters the expression of various metabolic enzymes in the liver35,36. Considering the importance of gut bacteria and liver metabolism in IS production, future investigations of the gut–liver–kidney relationship are required to clarify the underlying mechanism of IS accumulation in laying hens under heat stress.
In conclusion, we demonstrated that chronic heat exposure causes renal dysfunction, which in turn induces systemic and renal IS accumulation in laying hens. IS accumulation due to heat exposure may activate the AhR pathway and chronically disrupt oxidative stress status and antioxidant activity in the proximal tubules, which can promote further progression of renal fibrosis and dysfunction in heat-stressed laying hens. Further investigations are necessary to validate our findings and examine the causal relationship between IS production/accumulation and renal injuries in a heat-stressed laying hen model.
Methods
Ethics statement
All procedures were approved by the Animal Care Committee of the Institute of Livestock and Grassland Science, National Agriculture and Food Research Organization (NARO), Japan (Approval number: 21C118ILGS) and in accordance with the ARRIVE guidelines. All methods were performed in accordance with relevant guidelines and regulations.
Birds
Animal experiments were performed in accordance with a previous report4. The experiment comprised a 4-week preliminary breeding period for adaptation and a 4-week experimental period. During the preliminary breeding period, the egg production status of 96 hens was recorded, and 20 Boris Brown laying hens (27 weeks old, peak time of egg laying) weighing approximately 2006 ± 27 g, with the same feed consumption and egg production status (average laying rate: 98.4%), were used in this experiment. The hens were raised individually in wire-floored cages (measuring 33 × 45 × 40 cm3 [width × height × depth]) and fed a corn-soybean meal-based diet (containing 0.3% non-phytate P, 3.3% calcium, 500 IU/kg vitamin D, 2800 kcal/kg ME, and 15.5% crude protein: Table 3) designed to meet the Japanese feeding standard for poultry37. The birds had free access to feed and fresh water. The animals were randomly divided into two groups (n= 10). One group was exposed to heat stress in a controlled environment chamber at 32 °C and 55% ± 5% relative humidity (RH) for 4 weeks. The other group was maintained at 24 °C and 55% ± 5% RH. The light regimen was 14 L:10 D, and the dark period was from 19:00 to 05:00 h. The number of eggs and egg weight of each laying hen were recorded daily throughout the experiment. The laying rate, average egg weight, and average daily egg production were calculated weekly. Once a week, eggs were collected to determine the eggshell breaking strength, eggshell thickness, and egg weight using a digital egg tester (Model DET6500, NABEL Co., Ltd., Kyoto, Japan). Body weight and feed intake were recorded once a week and on the final day of the experimental period. All birds were humanely euthanized via rapid decapitation technique followed by exsanguination using appropriate equipment according to the procedures described in the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition38. Renal tissue samples from all birds were immediately stored in microtubes at − 80 °C or in RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) at − 20 °C until further use.
Table 3.
Composition of the basal diet.
| Ingredients, % | |
| Corn | 67.23 |
| Soybean meal | 22.97 |
| Vegetable oil | 0.42 |
| Calcium carbonate | 7.86 |
| Dibasic calcium phosphate hydrate | 0.91 |
| Sodium chloride | 0.27 |
| dl-Methionine | 0.09 |
| Vitamin mixturea | 0.10 |
| Mineral mixtureb | 0.10 |
| Selenium | 0.05 |
| Calculated value | |
| Crude protein, % | 15.50 |
| Metabolizable energy, Mcal/kg | 2.80 |
| Non-phytate P, % | 0.30 |
| Calcium, % | 3.30 |
| Vitamin D, IU/kg | 500.00 |
aVitamin mixture contained the following components (per kilogram of diet): vitamin A (from retinyl acetate), 10,000 IU; cholecalciferol, 500 IU; vitamin E (from dl-α-tocopheryl acetate), 15 IU; vitamin K (menadione sodium bisulfate), 0.8 mg; riboflavin, 7 mg; d-calcium pantothenate, 5 mg; nicotinic acid, 25 mg; choline chloride, 400 mg; pyridoxine hydrochloride, 3 mg; folic acid, 1.5 mg; thiamine mononitrate, 1.5 mg; biotin, 0.2 mg; and vitamin B12, (cyanocobalamin), 10 μg.
bMineral mixture contained the following components (per kilogram of diet): iron (FeSO4⋅7H2O), 80 mg; manganese (MnCO3⋅nH2O), 60 mg; zinc (ZnO), 40 mg; copper (CuSO4⋅5H2O), 8 mg; and iodine (calcium iodate), 0.5 mg.
Blood collection and analysis
At the final day of the experimental period, blood samples were collected from all birds (10 birds/ group) by venipuncture from the branchial vein. Blood samples were centrifuged at 3000 × g for 15 min at 10 °C to separate plasma from the whole blood. Plasma was then transferred into 1.5 mL labelled vials and stored at − 20 °C until further use. Plasma levels of calcium, phosphate, blood urea nitrogen, creatinine, and albumin were analyzed by Kotobiken Medical Laboratories (Ibaraki, Japan).
IS content in plasma and kidney
Plasma and renal IS contents were determined using the indican assay kit (DIDC-100, BioAssay Systems, Hayward, CA). 100 mg of frozen kidney tissues were homogenized in 0.9 mL of ice-cold phosphate-buffered saline (PBS) by bead beating at 4200 rpm for 30 s using a Cell Destroyer (Pro Sense Inc., Tokyo, Japan), and then centrifuged at 1000 × g for 10 min at 4 °C. Subsequently, the supernatant was used for the indican assay. The indican concentration was quantitated using an improved Curzon and Walsh method with chromogen reaction according to the manufacturer’s (BioAssay Systems) instructions. Each assay was performed in duplicate.
Total RNA isolation, cDNA synthesis, and real-time PCR
Total RNA was extracted from kidney samples using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 1-μg total RNA using random primers (TOYOBO, Tokyo, Japan) and ReverTra Ace (TOYOBO). Real-time PCR was performed in duplicate to measure mRNA expression using a QuantStudio 5 Real-time PCR system (Applied Biosystems, Foster City, CA, USA) and THUNDERBIRD SYBR qPCR Master Mix (TOYOBO, Tokyo, Japan). Table 4 lists the primer sequences for the target and reference genes. PCR primers for chicken nuclear factor (NF)-κB, catalase (CAT), superoxide dismutase (SOD) 1, SOD2, and glutathione peroxidase (GPX) 4 were purchased from Qiagen (Venlo, Netherlands).
Table 4.
Primer sequences used for quantitative real-time PCR.
| Genea | Primer sequences (5′–3′)b | Accession no. | References |
|---|---|---|---|
| OAT1 | F: TGGTTCTCCACCAGCTTTGC | BBSRC Chick EST ID 603807902F1 | 39 |
| R: TTCAGGGAGGAAAAGAGCAGCG | |||
| OAT3 | F: CCCTTCTTCCTCTTCTTCCTCG | BBSRC Chick EST ID 603812145F1 | 39 |
| R: TGGATCAGATAAATGCTGACCCC | |||
| MRP2 | F: AAATCCTCCCTCACCAACTGCC | XM_421698 | 39 |
| R: TTCGCCTTGCAGAGAAGACG | |||
| AhR1 | F: GCTGTGATGCAAAAGGAAAGATTGTC | NM204118.1 | 40 |
| R: ATTCCACTCTCACCCGTCTTC | |||
| CYP1A4 | F: ACTGCCAGGAGAAAAGGACAG | NM_205147.1 | 40 |
| R: TCAAAGCCTGCCCCAAACAG | |||
| CYP1B1 | CATCTTCCTCATCAGGTATCCAAAAGT | XM_419515.3 | 40 |
| GTACAGGAAAGCCACGATGTAG | |||
| COL1A1 | F: ACCTCAGCAAGAACCCCAAG | XM_025144131.2 | 41 |
| R: CTCACCGCCGTACTCAAACT | |||
| COL1A2 | F: GCGGTTTCTACTGGATTGA | NM_001079714.2 | 41 |
| R: AGCGAGACGGCTTATTTG | |||
| αSMA | F: AAGCACCACTGAATCCCAAAG | NM_001031229.1 | 41 |
| R: CCAGAGTCAAGCACAATCCCT | |||
| TGF-β | F: GCAAACTGCGTCTGACCG | NM_001318456.1 | 41 |
| R: ACGAAGAAGATGCTGTGGC | |||
| Smad3 | F: GTTTCACTGACCCGTCGAAT | AY391265 | 42 |
| R: GGTGGGATCTTGCAGACTGT | |||
| ICAM-1 | F: CACGTTCCAAGCAAGACTGA | XM 003642866.3 | 43 |
| R: CCACAGCAAGCTGATGAAGA | |||
| VCAM-1 | F: ACCCAAATGGACTACCCCCT | XM 422310.3 | 43 |
| R: AGGATCACTGGGAAAAGAGTAAAGT | |||
| 18srRNA | F: TCAGATACCGTCGTAGTTCC | HQ873432.1 | 44 |
| R: TTCCGTCAATTCCTTTAAGTT |
aOAT1 organic anion transporter 1, OAT3 organic anion transporter 3, MRP2 multidrug resistance-associated protein 2, AhR1 aryl hydrocarbon receptor 1, CYP1A4 cytochrome P450 family 1A4, CYP1B1 cytochrome P450 family 1B1, COL1A1 collagen type I alpha 1, COL1A2 collagen type I alpha 2, αSMA α-smooth muscle actin, TGF-β transforming growth factor-β, Smad3 SMAD family member 3, ICAM-1 intercellular adhesion molecule-1, VCAM-1 vascular cell adhesion molecule-1, 18srRNA 18S ribosomal RNA.
bF, forward, R, reverse.
Determination of 2-thiobarbituric acid reactive substances (TBARS) content
100 mg of frozen kidney tissues were homogenized in 0.9 mL of ice-cold potassium chloride buffer (1.15%, w/v) by bead beating at 4200 rpm for 30 s using a Cell Destroyer (Pro Sense Inc., Tokyo, Japan). Lipid peroxidation in renal tissue was measured using the TBARS assay45. Briefly, the reaction mixture contained 1.5-mL acetic acid (20%, w/v, pH 3.5), 1.5-mL 2-thiobarbituric acid (0.8% w/v), 0.2-mL sodium dodecyl sulfate (8.1%, w/v), 0.3-mL distilled water, and 0.2-mL tissue homogenate. The mixture was incubated at 95 °C for 60 min. Samples were then cooled and extracted into a 2.5-mL mixture of n-butanol and pyridine (15:1, v/v) and 0.5-mL distilled water. After centrifugation, the organic layer was collected, fluorescence was measured at a wavelength of 532 nm (Shimadzu UV-1800; Shimadzu, Kyoto, Japan), and quantified as malondialdehyde equivalent. The protein concentrations of the homogenate samples were determined by BCA assay using the TaKaRa BCA protein assay kit (Takara Bio Inc., Shiga, Japan). Each assay was performed in duplicate.
Total antioxidant capacity in the kidney
The total antioxidant capacity in the kidney was determined using an antioxidant capacity assay kit (PAO KPA-050, Japan Institute for the Control of Aging, Japan) according to the manufacturer’s instructions. 100 mg frozen renal tissues were homogenized in 0.9 mL of ice-cold PBS and centrifuged at 1000 × g for 10 min. Subsequently, the supernatant was used for this assay. Briefly, 10 µL of supernatant or standard reagent of uric acid of known concentration was mixed with Cu++ solution. When Cu++ is reduced by antioxidants, it forms Cu+. The reduced Cu+ then reacts with a chromatic solution, and its absorbance at 480–490 nm can be measured to calculate the antioxidant capacity, which can be expressed as μmol antioxidant capacity per ml tissue homogenate. Each assay was performed in duplicate.
Histological and fibrotic area analyses
Renal tissues were fixed in 4% paraformaldehyde, dehydrated in a graded series of 70, 80, 90, 95, and 100% ethanol, and embedded in paraffin. Paraffin-embedded tissues were cut into 4-μm sections, rehydrated in xylene and ethanol, and stained with Masson’s trichrome and picrosirius red46. Light microscopy (Leica, Nusslock, Germany) was used to observe histopathological changes in kidney. Fibrosis was quantified using picrosirius red staining. Eight fields per sample were randomly selected for fibrotic area quantification using ImageJ software version 6.0 (Media Cybernetics, Inc., Rockville, MD). Images were acquired at the same magnification under identical conditions, and the fibrotic area was expressed as a percentage of the captured image area.
Statistical analysis
All data were analyzed using Student’s t-test considering individual laying hens as the experimental unit. Data are shown as the means and pooled standard error of the mean (SEM). Differences were considered significant at P < 0.05 and a trend at 0.05 ≤ P < 0.1.
Acknowledgements
We would like to thank Dr. Hitoshi Murakami for his cooperation in the animal experiments. We would also like to thank Enago (https://www.enago.jp/) for the English language review.
Author contributions
F.H. wrote the main text of the manuscript and collected the data, contributed to the design and the drafting of the paper. F.H. and H.O. carried out the animal experiments. F.H. and H.O. contributed to sample collection. All authors read and approved the final manuscript.
Funding
This study was supported by Japan Society for the Promotion of Science KAKENHI (Grant Number: JP21K14966) to FH and the Environment Research and Technology Development Fund (JPMEERF20S11820) of the Environmental Restoration and Conservation Agency of Japan.
Data availability
The datasets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wasti, S., Sah, N. & Mishra, B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals10, 1266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allahverdi, A., Feizi, A., Takhtfooladi, H. A. & Nikpiran, H. Effects of heat stress on acid-base imbalance, plasma calcium concentration, egg production and egg quality in commercial layers. Glob. Vet.10, 203–207 (2013). [Google Scholar]
- 3.Mignon-Grasteau, S. et al. Robustness to chronic heat stress in laying hens: A meta-analysis. Poult. Sci.94, 586–600 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Nanto-Hara, F., Yamazaki, M., Murakami, H. & Ohtsu, H. Chronic heat stress induces renal fibrosis and mitochondrial dysfunction in laying hens. J. Anim. Sci. Biotechnol.14, 81 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosner, M. H. et al. Classification of uremic toxins and their role in kidney failure. Clin. J. Am. Soc. Nephrol.16, 1918–1928 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pieniazek, A., Bernasinska-Slomczewska, J. & Gwozdzinski, L. Uremic toxins and their relation with oxidative stress induced in patients with CKD. Int. J. Mol. Sci.22, 6196 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, T.-H. et al. Indoxyl sulfate, a tubular toxin, contributes to the development of chronic kidney disease. Toxins12, 684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg, A. H., Kumar, S. & Karumanchi, S. A. Indoxyl sulfate in uremia: An old idea with updated concepts. J. Clin. Invest.132, e155860 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii, H., Goto, S. & Fukagawa, M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins10, 202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holle, J. et al. Serum indoxyl sulfate concentrations associate with progression of chronic kidney disease in children. PLoS ONE15, e0240446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, C. H. et al. Indoxyl sulfate, homocysteine, and antioxidant capacities in patients at different stages of chronic kidney disease. Nutr. Res. Pract.16, 464–475 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakamatsu, T. et al. Indoxyl sulfate promotes macrophage IL-1β production by activating aryl hydrocarbon receptor/NF-κ/MAPK cascades, but the NLRP3 inflammasome was not activated. Toxins10, 124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano, T. et al. Indoxyl sulfate contributes to mTORC1-induced renal fibrosis via the OAT/NADPH oxidase/ROS pathway. Toxins13, 909 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan, H. et al. Defining therapeutic targets for renal fibrosis: Exploiting the biology of pathogenesis. Biomed. Pharmacother.143, 112115 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Deguchi, T. et al. Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int.65, 162–174 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Lin, S.-P. et al. Transporter-mediated interaction of indican and methotrexate in rats. J. Food Drug Anal.26, S133–S140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enomoto, A. et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J. Am. Soc. Nephrol.13, 1711–1720 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Motojima, M., Hosokawa, A., Yamato, H., Muraki, T. & Yoshioka, T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-κB and free radical in proximal tubular cells. Kidney Int.63, 1671–1680 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Lim, Y. J., Sidor, N. A., Tonial, N. C., Che, A. & Urquhart, B. L. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: Mechanisms and therapeutic targets. Toxins13, 142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mihajlovic, M. et al. Protein-bound uremic toxins induce reactive oxygen species-dependent and inflammasome-mediated IL-1β production in kidney proximal tubule cells. Biomedicines9, 1326 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichii, O. et al. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl-hydrocarbon receptor ligand. PLoS ONE9, e108448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondouin, B. et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int.84, 733–744 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Goldstone, H. M. H. & Stegeman, J. J. A revised evolutionary history of the CYP1A subfamily: Gene duplication, gene conversion, and positive selection. J. Mol. Evol.62, 708–717 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Yu, M., Kim, Y. J. & Kang, D.-H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol.6, 30–39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu, H. et al. NF-κB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am. J. Physiol. Cell Physiol.301, C1201–C1212 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Veith, A. & Moorthy, B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol.7, 44–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kikusato, M. & Toyomizu, M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS ONE8, e64412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbarian, A. et al. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol.7, 37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao, H., Zhang, L., Li, J., Xing, T. & Gao, F. Acute stress deteriorates breast meat quality of Ross 308 broiler chickens by inducing redox imbalance and mitochondrial dysfunction. J. Anim. Sci.100, skac221 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu, H., Saito, S., Higashiyama, Y., Nishijima, F. & Niwa, T. CREB, NF-κB, and NADPH oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. Am. J. Physiol. Cell Physiol.304, C685–C692 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Inami, Y. et al. Effect of AST-120 on endothelial dysfunction in adenine-induced uremic rats. Int. J. Nephrol.2014, 164125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gryp, T. et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int.97, 1230–1242 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Madella, A. M., Van Bergenhenegouwen, J., Garssen, J., Masereeuw, R. & Overbeek, S. A. Microbial-derived tryptophan catabolites, kidney disease and gut inflammation. Toxins14, 645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringseis, R. & Eder, K. Heat stress in pigs and broilers: Role of gut dysbiosis in the impairment of the gut-liver axis and restoration of these effects by probiotics, prebiotics and synbiotics. J. Anim. Sci. Biotechnol.13, 126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emami, N. K., Jung, U., Voy, B. & Dridi, S. Radical response: Effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants10, 35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim, D. Y., Lim, B., Kim, J. M. & Kil, D. Y. Integrated transcriptome analysis for the hepatic and jejunal mucosa tissues of broiler chickens raised under heat stress conditions. J. Anim. Sci. Biotechnol.13, 79 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NARO. Japanese Feeding Standard for Poultry, 2011 (2012).
- 38.Leary, S. et al. AVMA Guidelines for the Euthanasia of Animals 2020 edn.
- 39.Dudas, P. L., Pelis, R. M., Braun, E. J. & Renfro, J. L. Transepithelial urate transport by avian renal proximal tubule epithelium in primary culture. J. Exp. Biol.208, 4305–4315 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Jönsson, M. E., Woodin, B. R., Stegeman, J. J. & Brunström, B. Cytochrome P450 1 Genes in birds: Evolutionary relationships and transcription profiles in chicken and Japanese quail embryos. PLoS ONE6, e28257 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng, Y. et al. Chronic corticosterone exposure induces liver inflammation and fibrosis in association with m6A-linked post-transcriptional suppression of heat shock proteins in chicken. Cell Stress Chaperones25, 47–56 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, X., McFarland, D. C. & Velleman, S. G. Effect of smad3-mediated transforming growth factor-β1 signaling on satellite cell proliferation and differentiation in chickens. Poult. Sci.87, 1823–1833 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Su, L. et al. Suppresses of astragalus polysaccharide on E. coli-induced injured intestinal microvascular through TLR4-NF-κB signal pathways in chickens. Braz. J. Poult. Sci.21, 300. 10.1590/1806-9061-2018 (2019). [Google Scholar]
- 44.Li, Y. P., Bang, D. D., Handberg, K. J., Jorgensen, P. H. & Zhang, M. F. Evaluation of the suitability of six host genes as internal control in real-time RT-PCR assays in chicken embryo cell cultures infected with infectious bursal disease virus. Vet. Microbiol.110, 155–165 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.95, 351–358 (1979). [DOI] [PubMed] [Google Scholar]
- 46.Nanto-Hara, F. et al. The guanylate cyclase C agonist linaclotide ameliorates the gut–cardio–renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol. Dial. Transplant.35, 250–264 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.