Abstract
Mounting evidence has implicated the RNA m6A methylation catalyzed by METTL3 in a wide range of physiological and pathological processes, including tumorigenesis. The detailed m6A landscape and molecular mechanism of METTL3 in prostate cancer (PCa) remains ill-defined. We find that METTL3 is overexpressed in PCa and correlates with worse patient survival. Functional studies establish METTL3 as an oncoprotein dependent on its m6A enzymatic activity in both AR+ and AR− PCa cells. To dissect the regulatory network of m6A pathway in PCa, we map the m6A landscape in clinical tumor samples using m6A-seq and identify genome-wide METTL3-binding transcripts via RIP-seq. Mechanistically, we discover RRBP1 as a direct METTL3 target in which METTL3 stabilizes RRBP1 mRNA in an m6A-dependent manner. RRBP1 positively correlates with METTL3 expression in PCa cohorts and exerts an oncogenic role in aggressive PCa cells. Leveraging the 3D structural protein-protein interaction between METTL3 and METTL14, we successfully develop two potential METTL3 peptide inhibitors (RM3 and RSM3) that effectively suppress cancer cell proliferation in vitro and tumor growth in vivo. Collectively, our study reveals a novel METTL3/m6A/RRBP1 axis in enhancing aggressive traits of PCa, which can be therapeutically targeted by small-peptide METTL3 antagonists.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-024-05418-6.
Keywords: Prostate cancer, METTL3, Peptide inhibitor, m6A, RRBP1
Introduction
Prostate cancer (PCa) is a major cause of cancer-related deaths in men globally [1]. The treatment options are currently limited for advanced PCa patients, and androgen deprivation therapy (ADT) is the mainstay of therapy. Unfortunately, ADT usually fails to endow a durable response and most patients eventually develop castration-resistant PCa (CRPC), which is currently incurable [2]. Therefore, development of new therapeutics for aggressive PCa variants, especially CRPC, is urgently needed. So far, our understanding of PCa progression is incomplete, warranting further detailed mechanistic studies.
N6-Methyladenosine (m6A) is the most prevalent internal modification for eukaryotic RNA and influences nearly every stage of RNA metabolism, including transcription, splicing, decay, export and translation [3, 4]. As a dynamic and reversible process, m6A modification is decorated by Writer complex (i.e., methyltransferase including mainly METTL3, METTL14 and WTAP), removed by Erasers (i.e., demethylases FTO and ALKBH5) and recognized by Readers (i.e., m6A-binding proteins including YTHDF1/2/3, IGF2BP1/2/3, HNRNPA2B1 and others) [5, 6]. Increasing evidence has firmly implicated m6A pathway in a variety of cancer development, but in a context-dependent manner. For example, METTL3 has been reported to function as an oncogenic factor in glioblastoma [7], colorectal carcinoma [8], leukemia [9], breast cancer [10], and bladder cancer [11]. Whereas, a tumor suppressive role for METTL3 has also been suggested in ocular melanoma [12]. Consequently, METTL3 has been recently emerged as an attractive target for anticancer drug development.
Conceptually, bioinformatic analyses have suggested an important role for METTL3-mediated m6A pathway in prostate tumorigenesis [13–16]. Experimentally, there are few papers reported the biological role of METTL3 in PCa cells and identified few m6A targets on an individual gene basis such as GLI1 [17], MYC [18], KIF3C [19], ITGB1 [20], and USP4 [21]. However, several key questions remain unanswered: 1) a landscape of m6A methylation on PCa transcriptome in clinical tumors is uncharacterized; 2) the genome-wide targets of METTL3 in PCa cells remain elusive; 3) evidence supporting METTL3 as a valid therapeutic target for treating PCa lacks, in that previous studies mainly used genetic knockdown approaches instead of therapeutic interference with m6A signaling modulators. Here, we systematically investigate METTL3 expression, functions, and its direct targets in PCa. We find that METTL3 is oncogenic in human PCa by methylating and thus stabilizing RRBP1 mRNA in an m6A-dependent manner. Using N6-methyladenosine sequencing (m6A-seq) and RNA immunoprecipitation sequencing (RIP-seq) in clinical samples, we further reveal a gene-regulatory network governed by METTL3-mediated m6A signaling. Importantly, although a small-molecule METTL3 inhibitor STM2457 has been recently introduced for treating blood cancers [9], its efficacy against solid PCa is less effective. Alternatively, we develop for the first-time peptide inhibitors (linear RM3 and stapled form RSM3) that drastically inhibit METTL3 enzymatic activity and induce METTL3 degradation, leading to a strong anticancer toxicity in aggressive PCa both in vitro and in vivo.
Results
Upregulation of METTL3 in PCa correlates with poor survival
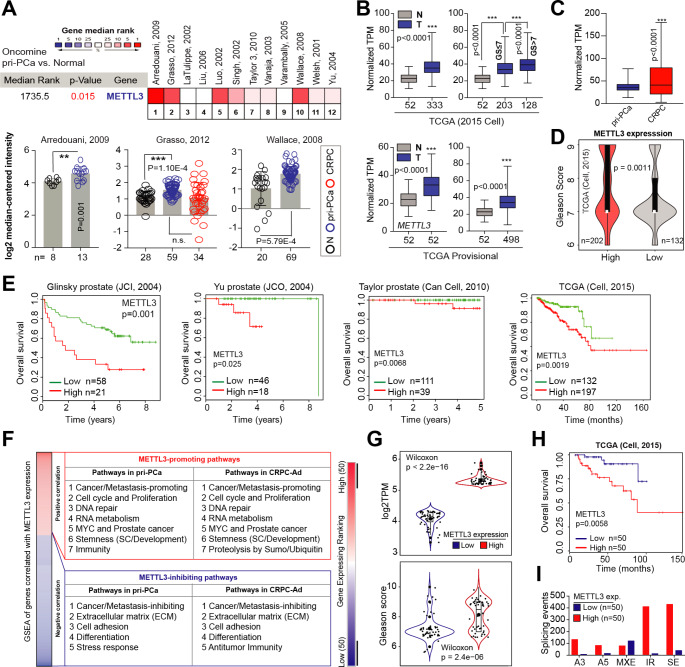
Recently, we have comprehensively dissected, genomically and transcriptomically, the m6A pathway (24 genes) as a whole in PCa using TCGA cohort [22]. We found that many of the m6A pathway genes were upregulated, while a few genes (including eraser FTO) downregulated, in primary PCa (pri-PCa) versus (vs.) normal tissues [22], indicating an overactivation of m6A pathway. To further investigate the potential roles of m6A modification in PCa evolution in detail, we focused on METTL3 as it’s the only enzymatic subunit in the Writer complex. Analysis of METTL3 mRNA levels in Oncomine database revealed that METTL3 was overexpressed in pri-PCa vs. normal/benign prostate tissues (Fig. 1A). This result was further confirmed by analyzing both the curated [23] and noncurated (i.e., provisional) TCGA cohorts (Fig. 1B). Interestingly, examination of large clinical RNA-seq datasets indicated a further increase of METTL3 levels in CRPC va. pri-PCa (Fig. 1C). In an attempt to understand the molecular basis underpinning METTL3’s dysregulation in PCa, we surveyed its mutational landscape. Although we did see an increase in METTL3 alteration in CRPC vs. pri-PCa cohorts, it was in total mutated at a very low frequency (Fig. S1A), suggesting that its mis-expression was not due to genomic alterations. Clinically, a strong positive correlation between METTL3 expression and tumor Gleason Score (GS) was observed (Fig. 1D), consistent with a gradual upregulation of METTL3 in GS high (> 7) vs. low (≤ 7) tumors (Fig. 1B). Also, the METTL3 mRNA levels adversely associated with PCa patients’ overall survival in multiple datasets (Fig. 1E). Experimentally, we determined METTL3 expression at both protein and mRNA levels in a panel of prostate normal and cancerous cell lines, finding a general overexpression in PCa cells vs. immortalized normal prostatic epithelial cells RWPE1 (Fig. S1B). Altogether, our data established a potential oncogenic role of METTL3 in PCa initiation and castration-resistant progression.
Fig. 1.
Increased METTL3 expression in PCa correlates with multiple oncogenic pathways and worse patient survival. (A) Oncomine analysis showing increased METTL3 expression in PCa vs. normal or benign tissues. The bottom illustrates the details in three representative datasets. (B) Overexpression of METTL3 at mRNA level in both curated (2015 Cell) and noncurated pan-cancer PCa TCGA cohorts. (C) Comparison of METTL3 expression reveals an upregulation in CRPC vs. pri-PCa samples. D and E. High METTL3 mRNA levels correlate with increase GS (D) and worse patient overall survival (E) in indicated datasets. Survival p-value was determined using the Log-Rank test. F. GSEA of genes co-expressed with METTL3 in curated pri-PCa (TCGA) and CRPC (SU2C-PCF) cohorts. G-I. Fractionation of TCGA pri-PCa cohort (2015 Cell) into METTL3 high and low groups, and comparison of METTL3 expression (G, up), GS (G, bottom), patient’s survival outcome (H), and splicing landscape (I) showing METTL3 high group being more aggressive
A protumorigenic role of METTL3 is associated with multiple cancer-related pathways
Many roles of METTL3-mediated m6A signaling have been proposed in development and cancer [3, 4]. To comprehensively dissect the molecular functions of METTL3 in PCa, we performed a gene-coexpression analysis, coupled with gene set enrichment analysis (GSEA), to identify biological gene signatures and pathways that may be regulated by METTL3. Both curated TCGA pri-PCa [23] and CRPC [24] cohorts were examined and similar repertoires of biological pathways were identified (Fig. 1F and Table S1), indicating conserved roles of METTL3 in both treatment-naïve and treatment-resistant PCa. Globally, METTL3 positively regulated proliferation (evidenced by enrichment of pathways tied to cell cycle progression and DNA repair), MYC and PCa-related signatures, RNA metabolism (such as transcription and splicing), and stemness to eventually promote cancer development and progression (Fig. 1F and Fig. S1C). Supporting our analyses, the involvement of m6A pathway in all these processes have been documented in diverse biological contexts [4, 25, 26]. For example, it has been reported that METTL3 plays an oncogenic role in multiple cancer types [25] by regulating tumor stemness [27] and RNA stability and splicing [28, 29], among other mechanisms. Conversely, genes negatively correlated with METTL3 expression were enriched in pathways associated with extracellular matrix (ECM) and cell adhesion, differentiation, stress response and/or anti-tumor immunity (Fig. 1F and Table S1), indicating a cancer/metastasis-inhibiting function. To further corroborate these results, we fractionated TCGA pri-PCa cohort into two extremes (Fig. 1G) and asked whether high or low METTL3 levels in PCa was indeed associated with tumor aggressiveness. Expectedly, tumors expressing highly (vs. lowly) the METTL3 exhibited elevated GS (Fig. 1G, bottom), worse survival outcome (Fig. 1H), and distinct splicing landscapes (Fig. 1I). The total dysregulated splicing events (5.77-fold) and intron retention (IR; 31.62-fold) were specifically upregulated in the METTL3 high group (Fig. 1I). We have recently shown that the severity of RNA splicing disruption correlates with increased aggressiveness [30]. Collectively, higher METTL3 expression predicted a molecularly aggressive phenotype in PCa, consistent with recent reports [18, 21].
Attenuation of METTL3 inhibits PCa progression in vitro and in vivo
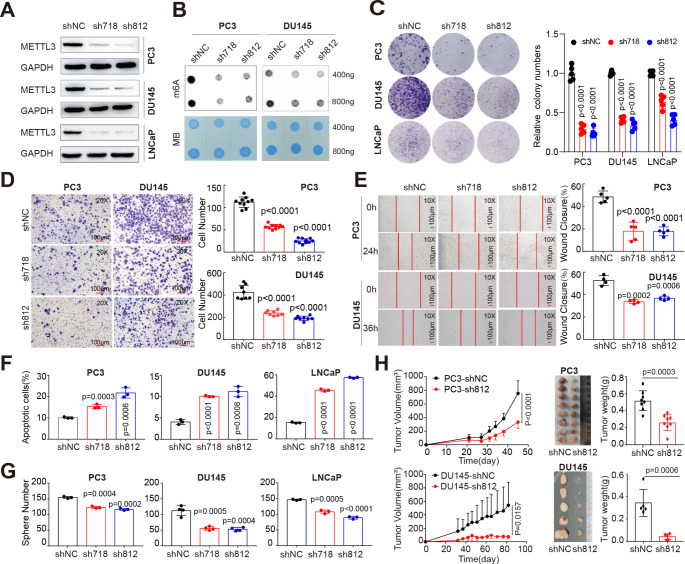
A critical role of METTL3 in mobility of AR− CRPC cells (i.e., DU145 and PC3) has been reported recently [21]. To comprehensively dissect the roles of METTL3-mediated m6A pathway in both AR+ and AR− cells, we knocked down METTL3 in LNCaP and PC3 cells. Two independent shRNA-mediated depletion of METTL3 (Fig. 2A and Fig. S2A) significantly reduced the total m6A levels in PCa cells (Fig. 2B), and concurrently led to a remarkable impairment of cell growth in these lines as evidenced by the colony formation assay (Fig. 2C). Moreover, trans-well (Fig. 2D) and wound-healing (Fig. 2E) assays revealed that attenuation of METTL3 significantly decreased cell mobility in PC3 and DU145 cells. Quantitation of apoptosis via Annexin V staining indicated that loss of METTL3 caused a significant increase in apoptosis (Fig. 2F and Fig. S2B) in both AR+ LNCaP and AR− lines, validating the reduced proliferation phenotype (Fig. 2C). Moreover, knockdown (KD) of METTL3 significantly inhibited PCa sphere formation (Fig. 2G), as well as the sphere size (Fig. S2C and Fig. S2D), indicating a requirement of METTL3 for optimal PCa stemness. Alternatively, we obtained similar results by depleting METTL3 with two independent siRNAs in PCa cells. Briefly, siRNA treatment significantly reduced METTL3 expression both at mRNA (Fig. S2E) and protein (Fig. S2F) levels, leading to a global decrease in RNA m6A methylation (Fig. S2G), clonal development capacity (Fig. S2H), viability (Fig. S2I), and migration (Fig. S2J) in both DU145 and PC3 cells. All these abovementioned in vitro assays strongly established a protumorigenic role of METTL3 in PCa. To next demonstrate the role of METTL3 in vivo, we generated subcutaneous tumors in BALB/c athymic nu−/nu− (nude) male mice implanted with PCa cells stably expressing control or METTL3-targeting shRNAs. As shown in Fig. 2H, METTL3 KD inhibited the growth of both PC3 and DU145 xenograft models. Quantitative real-time RT-PCR (qPCR; Fig. S2K) and immunohistochemistry analysis (Fig. S2L) confirmed the depletion of METTL3 in these tumors. Collectively, these data convincedly demonstrated that METTL3 is oncogenic in PCa development and progression.
Fig. 2.
Knock-down of METTL3 significantly inhibits PCa progression in vitro and in vivo.A. Western blot analysis showing METTL3 abundance in three PCa cell lines transduced with scrambled (shNC) or METTL3-specific hairpin shRNAs (sh718 and sh812) and probed with indicated antibodies. GAPDH served as a loading control. B. Dot blot assay showing the global m6A levels in indicated cells treated with different shRNAs. Methylene blue (MB) staining served as a loading control. C. Knocking down METTL3 inhibits clonal development in indicated PCa cells. Representative images and relative quantification are shown for each cell line. Experimental details are as follows: LNCaP (30 K/well for 14 days), DU145 (8 K/well for 9 days), PC3 (5 K/well for 9 days). D and E. Cell mobility evaluated by Trans-well (40 K PC3 per well for 24 h and 70 K DU145 per well for 30 h; D) and wound-healing (24 h for PC3 cells and 36 h for DU145 cells; E) assays showing that METTL3 knock-down inhibits cancer cell migration. F. Flow cytometry analysis showing an increased cellular apoptosis in indicated PCa cell lines upon METTL3 depletion. G. Knocking down METTL3 inhibits sphere formation in both AR + and AR- PCa cell lines. H. Depletion of METTL3 inhibits the growth of PC3 and DU145 xenograft tumors in vivo (n = 5 for each group). Shown are the tumor growth curves (left), endpoint tumor images (middle), and tumor weight (right) of indicated PCa models
Interestingly, we noticed that, in multiple assays (e.g., Fig. 2G and Fig. S2I), DU145 (vs. PC3) cells appeared to be more susceptible to METTL3 loss, especially in the tumor regeneration assay (Fig. 2H). Although both cell lines are AR−, PC3 is PTEN-null whereas DU145 expresses wide-type (WT) PTEN. We next investigated whether tumor suppressor PTEN plays a role in dictating biological response of these two lines to METTL3 depletion. Recently, there were two studies providing indirect link between METTL3/m6A signaling and PTEN regulation. Li et al. reported that METTL3-mediated m6A modification of HOXC10 in liver cancer promoted its expression, which in turn suppressed the transcription of PTEN [31]. In Lu et al. study, both METTL3 and m6A methylation were found markedly upregulated in lung resident mesenchymal stem cells (LR-MSCs), leading to an aberrant differentiation of LR-MSCs into myofibroblasts [32]. Molecularly, METTL3 binds the primary transcripts of miR21 (pri-miR21) to promote its m6A modification and maturation in LR-MSCs, causing elevation of mature miR-21. Resultantly, miR-21 targets PTEN mRNA for expression inhibition [32]. Notably, there was another study providing direct evidence that METTL3 regulates PTEN transcripts in prostate tissue. Li et al. indicated that METTL3 was aberrant upregulated in benign prostatic hyperplasia (BPH) samples and methylated PTEN mRNA for degradation through reader YTHDF2 [33].
Examination of gene expression changes in PTEN− PC3 cells upon METTL3 KD showed that PTEN and the two reported effectors (HOXC10 and miR-21) were not affected transcriptionally (Fig. S3A). Interestingly, in PTEN+ DU145 cells, METTL3 KD further increased the expression of PTEN but without affecting HOXC10 and miR-21 (Fig. S3B). Western blot assay confirmed the upregulation of PTEN protein only in DU145 cells upon METTL3 depletion (Fig. S3C). These data indicated that METTL3 loss-mediated inhibition of DU145 fitness might be, at least partially, through upregulation of PTEN. Next, we performed functional rescue experiments to see whether restoration of PTEN would sensitize PC3 cells to METTL3 loss. Overexpression (OE) of PTEN in METTL3-depleted cells significantly increased the expression of PTEN but not METTL3 (Fig. S3D). Functional colony formation (measuring cell growth; Fig. S3E) and sphere assay (measuring cancer stemness; Fig. S3F) showed that while METTL3 KD cells infected with or without Control-OE lentivirus displayed similar inhibitory effect on cell aggressiveness (comparing lane 2 and 3), PTEN-OE in PC3 cells further exacerbated the cell growth inhibition upon METTL3 KD (comparing lane 3 and 4). Importantly, the inhibition rates in colony formation and sphere assays increased from average 35% to 53% and 28% to 49%, respectively, in METTL3 KD PC3 cells without or with PTEN-OE (Fig. S3E and S3F). These data, together, strongly indicated that restoration of PTEN in PC3 made it more vulnerable to METTL3 loss. To further validate this idea that PTEN is functionally involved in the METTL3-mediated oncogenic effects in PCa, we also performed the same set of experiments in PTEN+ DU145 cells. As expected, similar results were observed, as PTEN-OE only increased the expression of PTEN but not the METTL3 mRNAs (Fig. S3G) and further sensitized DU145 to METTL3 KD in both colony formation (Fig. S3H) and sphere assay (Fig. S3I). Of clinical relevance, we analyzed the largest pri-PCa [23] and CRPC [34] cohorts to reveal that METTL3 is negatively correlated with PTEN at mRNA level in pri-PCa (spearman ρ = -0.22) samples (Fig. S3J). No correlation in CRPC samples was found (Fig. S3J), which was explainable by the fact that PTEN is frequently lost or mutated in CRPC patients (> 33% mutation rate; Fig. S3K). Altogether, our data established PTEN as a determinant in PCa cells with differential responses to METTL3 loss, in line with the previous report that PTEN is a downstream target of METTL3/m6A axis in prostate tissue [33].
METTL3 promotes prostate tumorigenesis in an m6A-dependent manner
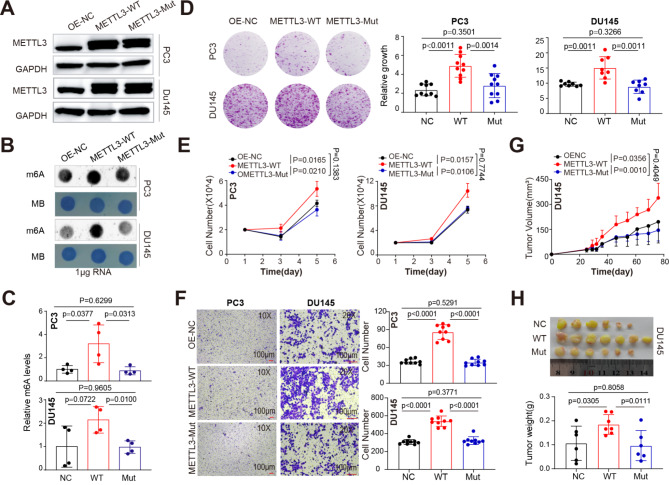
METTL3 forms an obligate heterodimer with METTL14 to exert its methyltransferase activity [3]. To determine whether METTL3’s function of accelerating PCa aggressiveness was dependent on its m6A catalytic activity, we successfully established stable cell lines expressing WT METTL3 or its catalytic-dead mutant (aa395-398, DPPW→APPA). A lack of methyltransferase activity of this mutant has been previously described [35, 36]. Overexpression of the WT, but not the mutant, METTL3 (Fig. 3A) resulted in a significant increase in global m6A levels in both DU145 and PC3 cells by dot-blot assay (Fig. 3B), which was further confirmed by quantification of RNA methylation status via EpiQuik m6A Quantification Kit (Fig. 3C). A series of cell-based assays in two different PCa cell lines showed that only transduction of WT (compared to the mock or mutant-expressing) METTL3 lentivirus further promoted cell clonal development (Fig. 3D), proliferation (cell counting; Fig. 3E), and migration ability (trans-well assay; Fig. 3F). Importantly, xenograft tumor assay indicated that the catalytic-dead METTL3 (vs. WT) failed to promote DU145 tumor development in vivo, as evidenced by indistinguishable difference in tumor growth dynamics (Fig. 3G) and weight (Fig. 3H) between mutant and control groups. Collectively, these results strongly indicated that the m6A catalytic activity of METTL3 is required for its role in advancing prostate tumorigenesis.
Fig. 3.
METTL3 overexpression promotes PCa progression in a m6A-dependent way. (A) Western blot analysis showing overexpression of WT or catalytically dead (i.e., mutant) METTL3 in PCa cells. GAPDH served as a loading control. (B) Dot blot assay showing the global m6A levels in indicated cells treated with different overexpressing constructs. Methylene blue (MB) staining served as a loading control. (C) Relative m6A levels in indicated conditions detected via EpiQuik m6A quantification kit. D-F. Overexpression of WT, but not the mutant, METTL3 promotes CRPC clonal development (D), proliferation (E), and Trans-well migration (F) in both DU145 and PC3 cells in vitro. G and H. The m6A enzymatic activity is required for METTL3 in enhancing PCa development in vivo. Shown are the tumor growth curves (G), endpoint tumor images and tumor weight (H) of DU145 model. In D, 5 K PC3 and 8 K DU145 were initially seeded and visualized at day 10. In F, 40 K PC3 per well for 24 h and 70 K DU145 per well for 30 h were used
Identification of global METTL3 targets in PCa cells
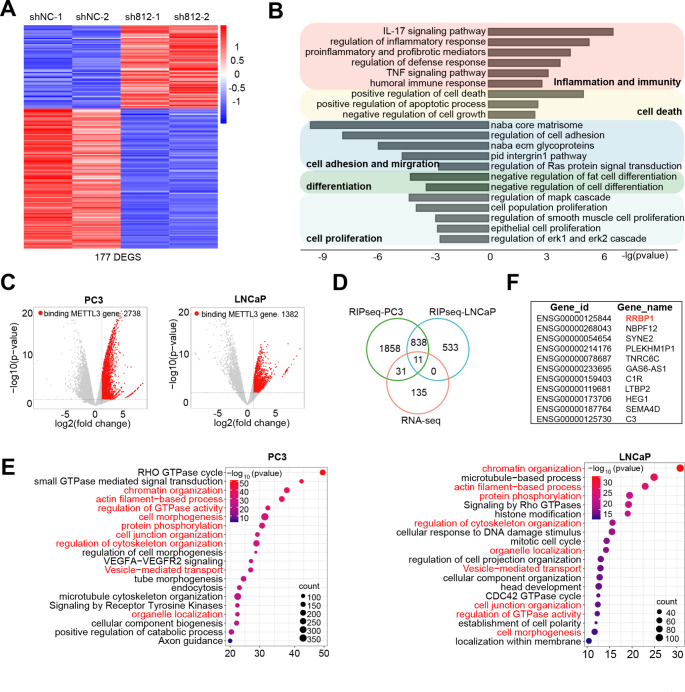
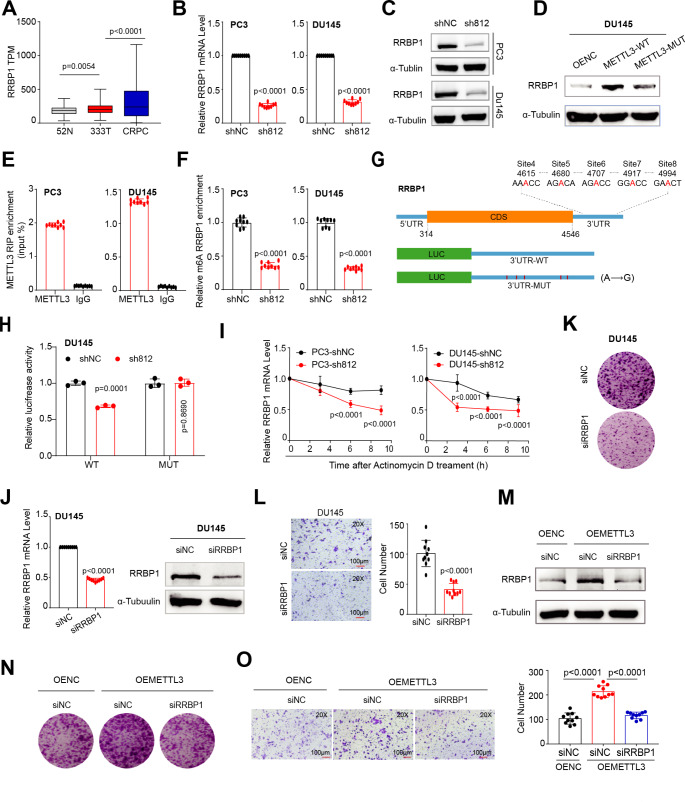
To directly unravel the mechanisms of action of METTL3 in PCa cells, we utilized a multi-omics approach. We first performed RNA-seq analysis in PC3 cells with or without METTL3-KD, finding a set of 177 differentially expressed genes (DEGs; 66 up- and 111 down-regulated) (Fig. 4A and Table S2). Gene ontology (GO) analysis indicated that genes upregulated in METTL3-KD cells were enriched in pathways tied to inflammation/immunity and cell death (Fig. 4B), whereas genes downregulated were enriched in pathways associated with migration/adhesion, differentiation and proliferation, in line with an oncogenic role of METTL3. The m6A writer complex binds RNA to decorate m6A modification [26]. Next, we performed RIP-seq experiments in both AR+ LNCaP and AR− PC3 cells with an METTL3-specific antibody. As shown in Figs. 4C, 382 and 738 potential METTL3 target genes were identified in PC3 and LNCaP, respectively, with 849 genes being shared between these two cell lines (Fig. 4D and Table S3). Consistently, GO analysis of genes bound by METTL3 revealed, overall, a similar pattern of biological pathways enriched in LNCaP and PC3 cells (Fig. 4E), highlighting that METTL3 may play similar functional roles in both AR+ and AR− cells. To further identify genes that were directly regulated by METTL3 in PCa cells, we integrated the RNA-seq and RIP-seq data by overlapping DEGs and genes bound by METTL3 protein (Fig. 4D), and identified 11 key genes (Fig. 4F). Among them, SYNE2 [37] and HEG1 [38] have been reported to be regulated by m6A modification in different context, indicating validity of our data. We chose RRBP1 (reticulum ribosome-binding protein 1) for further investigation as it showed the most significant reduction in mRNA expression upon METTL3-KD.
Fig. 4.
Identification of the genome-wide METTL3 targets in PCa cells. (A) Heatmap of differentially expressed genes (DEGs) identified in PC3 cells by RNA-seq (fold change (FC) ≥ 1.5 and FDR < 0.1). (B) GO analysis of DEGs as shown in A. (C) Volcano plot of METTL3-bound genes identified in AR + LNCaP and AR− PC3 cells by RIP-seq (FC ≥ 2 and FDR < 0.1). (D) Overlap between DEGs and METTL3-bound genes in indicated contexts. (E) GO analysis of METTL3-bound genes showing that a significant proportion of enriched pathways are commonly enriched in both LNCaP and PC3 cells. (F) List of the 11 overlapped genes from D
METTL3 accelerates PCa progression through upregulating oncogenic RRBP1
In line with an upregulation of METTL3 in pri-PCa and CRPC, similar pattern was found for RRBP1 mRNA levels (Fig. 5A), implying a positive relationship between them. qPCR analysis confirmed a reduction in RRBP1 expression at both mRNA (Fig. 5B) and protein (Fig. 5C) levels upon METTL3-KD. Notably, the enzymatic activity of METTL3 was required for this phenotype, as only the exogenous expression of WT, but not the mutant, METTL3 strongly upregulated RRBP1 protein expression (Fig. 5D). To further demonstrate RRBP1 as a direct substrate bound by METTL3, we performed RIP-qPCR to reveal that METTL3 strongly bound RRBP1 transcripts in both DU145 and PC3 cells (Fig. 5E) and the m6A modification on RRBP1 mRNA by m6A-qPCR was significantly reduced upon METTL3-KD (Fig. 5F). Analysis of RRBP1 sequences and METTL3 RIP-seq peaks unraveled multiple consensus m6A motifs (RRACH) in its 3’-UTR region (Fig. 5G). Mutagenesis assay was performed with luciferase (Luc) reporters containing either a WT or a mutated (MUT) 3’-UTR placed after the coding region of a firefly luciferase. Results indicated that the luciferase activity of WT, but not the MUT, reporter was significantly reduced in DU145 cells when METTL3 was knocked down (vs. shNC control) (Fig. 5H), suggesting a positive regulation of m6A on RRBP1 mRNA. To further dissect the fate of RRBP1 mRNA upon m6A methylation, we determined its mRNA stability after transcriptional inhibition with Actinomycin D. Attenuation of METTL3 markedly accelerated RRBP1 mRNA decay in both DU145 and PC3 cells (Fig. 5I), suggesting that METTL3 upregulated RRBP1 through mRNA stability in a m6A-dependent manner. Altogether, these results established RRBP1 as a direct target of METTL3 in PCa cells.
Fig. 5.
Oncogenic RRBP1 is a direct and functional target of METTL3-mediated m6A signaling in promoting PCa aggressiveness. A. Overexpression of RRBP1 at mRNA level during PCa progression, as analyzed in TCGA (pri-PCa vs. N) and CRPC (vs. pri-PCa) cohorts. B. qPCR analysis showing a reduced RRBP1 expression in PC3 and DU145 cells upon METTL3 depletion. C and D. Western blot analysis of RRBP1 protein levels in indicated PCa cells with METTL3-KD (C) or METTL3 overexpression (D). Tubulin served as a loading control. E. RIP-qPCR analysis showing an enrichment of METTL3 binding at RRBP1 transcripts in PCa cells. F. m6A-qPCR analysis showing much reduced m6A modifications in RRBP1 transcripts in PCa cells upon METTL3-KD. G. Schematic of the potential m6A sites in the RRBP1 3’-UTR. Shown below is the experimental design of constructing luciferase reporters containing WT or mutant 3’-UTR sequences of RRBP1.H. Luciferase reporter assay using the WT or mutated 3’-UTR constructs in DU145 cells with or without METTL3-KD. The firefly luciferase activity was normalized to Renilla luciferase activity. I. mRNA stability assay showing the kinetics of RRBP1 expression in PCa cells with or without METTL3-KD after treatment with actinomycin D (10 µg/mL) for indicated time points. J. Efficient siRNA-mediated KD of RRBP1 both at the mRNA levels (left) and protein levels (right) in DU145 cells. Tubulin served as a loading control. K and L. Knocking down RRBP1 inhibits clonal development (8 K/well for 10 days, K) and migration (50 K/well for 30 h, L) in DU145 cells. M-O. RRBP1 is a key downstream effector of METTL3/m6A pathway in PCa. Reducing the RRBP1 protein into baseline level (M) significantly counteracts the pro-proliferative (N) and pro-migratory (O) effects of METTL3 overexpression in PCa cells. Experiments were performed in METTL3 overexpressing DU145 cells treated with or without siRNA targeting RRBP1. Empty vector-overexpressing cells (OE-NC) transfected with siNC was used as a baseline control
Next, we investigated the role of RRBP1 in PCa. siRNA-mediated KD of RRBP1 significantly reduced its expression at both mRNA and protein levels (Fig. 5J), and concurrently suppressed cell proliferation (Fig. 5K) and migration (Fig. 5L). These results suggested an oncogenic role for RRBP1 in PCa progression. To further test whether RRBP1 was a key downstream effector of METTL3-mediated m6A pathway, we performed RRBP1-KD experiments in METTL3-overexpresssing PCa cells. Our data clearly showed that reducing the RRBP1 protein expression to a baseline level (comparable to cells without METTL3 overexpression; Fig. 5M) significantly counteracted the pro-proliferative (Fig. 5N) and pro-migratory (Fig. 5O) effects of METTL3 overexpression in DU145 cells. Altogether, our findings highlighted that METTL3 accelerates PCa progression via targeting RRBP1 mRNA for stabilization in a m6A-dependent manner.
An m6A landscape in human PCa tissues reveals clinical significance of the METTL3/RRBP1 axis
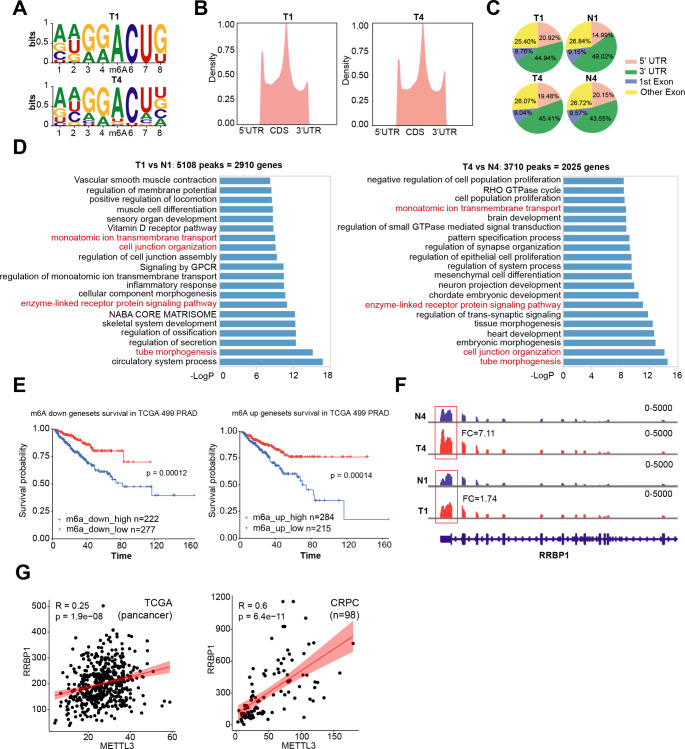
A global m6A landscape in clinical PCa samples is lacking, although there are few studies that have identified multiple METTL3 targets at individual gene basis, with the aid of general m6A methylation patterns in particular PCa cell lines. Here, we for the first time performed m6A-seq on two pairs of matched cancer/paracancer tissues. By HOMER algorithm, we showed that transcripts pulled-down by an m6A-specific antibody markedly enriched for the known m6A consensus RRACH motif (Fig. 6A), validating our experimental pipeline. Moreover, m6A peaks were predominately located in the vicinity of 5’UTR to 1st Exon and CDS to 3’UTR regions (Fig. 6B), similar to previously reported m6A maps generated in other tissues [39]. No obvious difference was found in the global m6A distribution pattern among cancer vs. paracancer tissues (Fig. 6C). Pair-wise comparison of m6A peaks in tumor vs. nontumor tissues identified 5180 methylated peaks in 2,910 genes and 3710 peaks in 2025 genes for patient 1 and 4, respectively (Fig. 6D and Table S4). To determine the cellular pathways that m6A might influence in clinical PCa, we conducted GO analysis on genes containing differential m6A peaks. Globally, m6A regulated mRNAs encoded a variety of pathways linked to a spectrum of important biological functions including development, cell adhesion and ECM (Fig. 6D), consistent with reported diverse roles of m6A pathway [3]. Specifically, besides that several GO terms were commonly enriched in two patients, a large part of enriched GO terms was still quite distinct, suggesting heterogeneity of m6A landscape among PCa patients. This is in line with the intrinsic property of heterogeneity of PCa. Our data therefore provides a useful resource for further mining m6A pattern in clinical PCa.
Fig. 6.
The clinical significance of the METTL3/RRBP1 axis in human PCa. A. Two paris of matched PCa and paracancer tissues were used for m6A-seq analysis. Shown are the concesus m6A RRACH motifs identified in two clinical prostate tumor samples. B and C. Statistics of m6A-seq peaks. Shown are the profiles of m6A peak density along mRNA transcript (B) and the genomic peak locations (C). D. GO analysis of genes bearing differential m6A peaks in tumor vs. nontumor tissues. Shown are the top 20 enriched pathways in two patient tumors, with a small proportion of GO pathways being commonly shared. E. Survival analysis of TCGA pan-cancer cohort based on genes that were co-upregulated or co-downregulated in both mRNA expression and m6A levels in tumor tissues. F. Distribution and differential enrichment of m6A peaks across RRBP1 transcripts in tumor (T) and normal (N) samples. G. Positive correlations between RRBP1 and METTL3 mRNA levels in TCGA pri-PCa (left) and CRPC (right) cohorts
To further demonstrate the clinical relevance of RNA m6A modification in PCa, we established signatures denoting to genes co-upregulated or co-downregulated in both mRNA expression and m6A levels in these two tumor tissues (Table S5). To our surprise, survival analysis of TCGA cohort showed that patients with higher expression of these co-regulated genes had a much worse prognosis (Fig. 6E). Considering that m6A methylation can both either promote or repress gene expression in an sustrate-dependent maner [4, 40], this data not only highlighted an involement of m6A modification in PCa biology but also pointed the complexcity of such regulatory mechnisam in regulating cancer behavior. Furthermore, in line with our cell line-derived data (Fig. 5G), we observed a greater enrichment of m6A siganls in RRBP1 mRNA (mainly the 3’-UTR region) in tumor vs. non-tumor tissues (Fig. 6F). In addition, correlation analysis in both TCGA pri-PCa and CRPC datasets showed a strong positive correlation between RRBP1 and METTL3 at mRNA levels (Fig. 6G), further comfirming our in vitro experimental data that METTL3 upregulated RRBP1 expression.
Peptide targeting METTL3-METTL14 interacting interface inhibites PCa in vivo
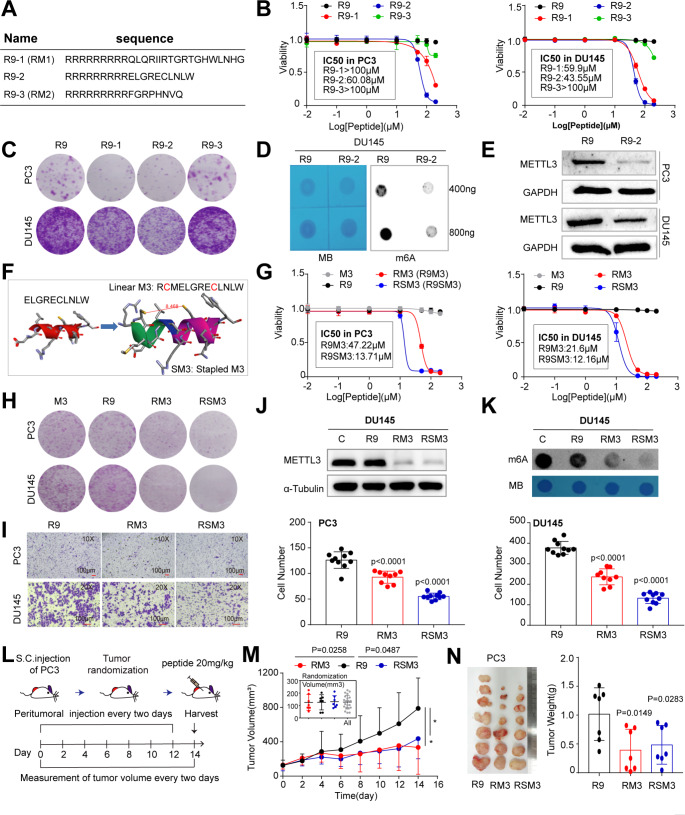
The protein-protein interaction (PPI) between METTL3 and METTL14, and the S-adenosyl methionine (AdoMet)-binding site of METTL3 are all crucial to the enzymatic activity of Writer complex [35]. With an aim to develop novel small-peptide METTL3 inhibitors, we designed two peptides corresponding to two interfaces of METTL3-METTL14 complex and one peptide to the AdoMet site of METTL3 (Fig. S4A), based on their structures elucidated previously [35]. Linking an arginine-9 motif (R9), a typical cationic and cell-penetrating peptide (CPP), to the N-terminal of these peptides made them potential inhibitors for m6A pathway (Fig. 7A). Using R9 as a negative control, we performed in vitro assays to show that peptides targeting interface 1 and 2 (i.e., R9-1 and R9-2) exhibited inhibitory effect on cell viability (Fig. 7B) and clonal development (Fig. 7C), with R9-2 being the superior one. Notably, peptide targeting the AdoMet site had no obvious impact on cell growth compared to R9 only (Fig. 7B and C). We therefore focused on R9-2 peptide for further investigation. Treatment of PCa cells with R9-2 significantly reduced the global m6A modification (Fig. 7D), which might be attributed to the reduced level of METTL3 protein after peptide treatment (Fig. 7E). Such reduction of METTL3 protein was consistent with the concept that a subunit is usually unstable if it is not properly assembled into a complex [41].
Fig. 7.
Small peptides targeting METTL3-METTL14 interaction inhibites PCa in vitro and in vivo. (A) Sequence of peptides R9-1, R9-2, and R9-3. R9 denotes the cell penetrable arginine-9 motif. (B) CCK8 cell viability (3 days) assays in indicated cells treated with different peptides at escalating doses. Data represent mean ± SD from a representative experiment with four technical repeats and the experiment was replicated three times with similar results. (C) Colony formation assay in PC3 and DU145 cells treated with indicated peptides at IC50 dosages as revealed in B. 5 K PC3 and 8 K DU145 were initially seeded and visualized at day 10. D and E. Peptide R9-2 relative to control R9 treatment significantly reduces the global m6A levels (D) and METTL3 protein expression (E) in PC3 and DU145 cells. F. Schematic of devising a staple form of peptide R9-2. G. CCK8 cell viability (3 days) assays in indicated cells treated with different peptides at escalating doses. M3, linear form of the staple peptide. SM3, the stapled M3 peptide. H and I. PCa cells treated with RM3 and RSM3, compared to R9, peptides at IC50 markedly inhibits cell proliferation (5 K PC3 and 8 K DU145 for 8 days; H) and migration (40 K PC3 per well for 20 h and 70 K DU145 per well for 30 h; I) in vitro. J and K. Reduced METTL3 protein expression (J) and the global m6A levels (K) in DU145 cells treated with RM3 and RSM3, compared to R9 or vehicle, peptides at IC50. The relative global m6A status was detected via dot blot assay. L. Schematic of in vivo peptide treatment. M and N. Inhibitory effects of METTL3-targeting peptides on the growth of PC3 CRPC model in vivo. Shown are the tumor growth curve (M; insets present tumor randomizations), endpoint tumor image and tumor weight (N) of xenografts (n = 7 for each group)
Although peptides were intrinsically safer than small molecule inhibitors, the IC50 of R9-2 in PC3 (60.08 µM) and DU145 (43.55 µM) cells were relatively high. Thus, we next sought to optimize it by modifying its sequence (i.e., increased 3 aa for the purpose of designing staple peptide) and changing it from linear form to a stapled form (Figs. 7F and S4A), which could increase peptide uptake and stability [42]. Using R9 or the linear sequence (before stapled; M3) as negative controls (which displayed no noticeable effect; Fig. 7G), we performed a series of cell-based assays to show that, relative to the linear RM3, stapled RSM3 displayed stronger inhibitory effect on cell proliferation (Fig. 7G) and clonal development (Fig. 7H) in two CRPC cell lines. In migration assay, both RM3 and RSM3 halted cell mobility, with RSM3 being more effective than RM3 (Fig. 7I) in PCa cells. As expected, RM3 or RSM3 treatment at IC50 concentration markedly reduced METTL3 protein abundance (Fig. 7J) and total m6A level (Fig. 7K). It is noteworthy that a first-in-class small-molecule METTL3 inhibitor STM2457 was recently discovered [9]. Albeit peptide vs. small molecule possessed intrinsically distinct properties, we compared the IC50s of STM2457 and our peptide inhibitors in PCa cells. STM2457 was initially described to be effective against multiple leukemia cell lines (IC50 ≈ 1.26 µM) [9], but we found that it was much less effective in killing solid PCa cells in vitro. The IC50s of STM2457 in both DU145 and PC3 cells were greater than 50 µM, whereas peptide inhibitors (RM3 and RSM3) comparatively behaved more superiorly (Fig. S4B), suggesting potential advantage of our peptide inhibitors over STM2457 in different biological context.
Last, we asked that whether an in vitro growth-inhibiting effect of METTL3-targeting peptides could be translated into therapeutic benefit in vivo. Using androgen-insensitive PC3 xenograft in immunodeficient mice as a CRPC model, we treated similar-sized tumors with indicated peptides at a dose of 20 mg/Kg via peritumoral injection once every other day for 14 days (Fig. 7L). Results clearly showed that both RM3 and RSM3 alone effectively inhibited tumor growth in terms of tumor volume (Fig. 7M) and weight (Fig. 7N). Notably, no toxicity was noticed as evidenced by stable body weights during treatment course (Fig. S4C). Collectively, we have devised METTL3-targeting peptide inhibitors that may offer a new avenue for anticancer therapy.
Discussion
Compelling evidence has established METTL3 and/or m6A as critical players in variety of cancers in a context-dependent manner [4, 40], with majority of papers positioned METTL3 as an oncogene. Hence, METTL3 is widely pursued as a new target for anticancer drug development. Several reports have suggested an important role of METTL3 in PCa cell lines [17–21], with identification of a few downstream METTL3 targets on an individual gene basis. However, our understanding of m6A signaling in PCa is incomplete and a comprehensive and in-depth study of METTL3 is lacking. Here, we take an integrative and complementary approach to systematically dissect METTL3-mediated m6A pathway in PCa development and progression. Using both relatively indolent AR+ and aggressive AR− PCa models and human clinical samples, we perform in vitro and in vivo biological assays, mutagenesis, multi-omics sequencing, novel drug development, and preclinical therapeutic studies. First, to directly show the clinical relevance of m6A modification, we perform m6A-seq in clinical prostate tumors to provide the actual m6A landscape, finding that m6A-modified transcripts are heterogeneously involved in many cancer-related pathways. Second, METTL3 binds RNA to deposit m6A. We identify the global targets of METTL3 in PCa cells by RIP-seq technique. Third, integrating the m6A-seq, RIP-seq, and regular RNA-seq data, we reveal a gene regulatory network centered on m6A signaling in aggressive PCa cells. Fourth, using genetic KD and rescue experiments, we identify RRBP1 as not only a direct target, but also a key functional downstream effector, of METTL3 in an m6A-dependent manner. A higher expression of RRBP1 predicts a worse patient outcome. Interestingly, we also show that the PTEN expression status is likely a functional determinant in the responses of PCa cells to METTL3 loss. Fifth, we develop novel small-peptide METTL3 inhibitors that demonstrate robust PCa-inhibiting activity in vitro and in vivo. These peptide inhibitors present a previously unexplored avenue for treating advanced PCa, especially CRPC.
Besides revealing the global m6A substrates in clinical tumor samples, we herein identify RRBP1 as a new and direct substrate of METTL3 through multi-omics analysis. Mechanistically, we further show that upregulation of RRBP1 in PCa is attributed, at least partially, to m6A-dependent stabilization of RRBP1 mRNA catalyzed by METTL3. RRBP1 appears to be a key effector of METTL3’s oncogenic function in PCa, as RRBP1-KD abolishes the pro-proliferation impact caused by METTL3 overexpression. RRBP1 is an endoplasmic reticulum (ER) membrane protein and is essential for ribosome binding and translocation of nascent proteins across the rough ER membrane [43–45]. Previous studies have linked an increased RRBP1 expression to pathology of various tumor types, including lung [46], breast [47], and bladder [48] cancers. These reports suggest RRBP1 as a potential oncogene, but little is known about its role in PCa. In this study, we experimentally show that depletion of RRBP1 significantly inhibits aggressive features of PCa cells, indicating that RRBP1 indeed functions oncogenically in PCa. Notably, beside the oncogenic substrates, tumor suppressor PTEN is also reported as a downstream target of METTL3 in prostate tissue [33]. We find that METTL3 is negatively correlated with PTEN transcriptionally in TCGA pri-PCa cohort, and restoration of PTEN in PTEN-null PC3 model made it more vulnerable to METTL3 loss.
Another innovative and significant finding of our study is the development of small-peptide METTL3 inhibitors (i.e., linear RM3 and stapled RSM3). Based on the 3D structure of METTL3-METTL14 complex [35], we design multiple peptides and find that one targeting the interface 2 of PPI exhibits a good anticancer activity in PCa cells in vitro. Further sequence modification and stapling leads to the discovery of RSM3 (Fig. S4), which displays a superior toxicity against PCa both in vitro and in vivo. The design, detailed chemical characterization, and biological effects of such peptide inhibitor peptides on PPI between METTL3 and METTL14 and on other cancer types are recently presented in our parallel paper [49]. We have shown that RSM3 can significantly reduce the interaction of METTL3 with METTL14 [49]. While we are preparing the manuscript, a small-molecule METTL3 inhibitor STM2457 is reported to treat blood cancers [9], whereas its application in solid tumors remains unstudied. Interestingly, our in vitro cell-killing assays show that the IC50 of STM2457 in PC3 and DU145 cells are both over 50 µM and 20 µM, respectively. Comparatively, the IC50 of peptide inhibitor RSM3 in PC3 and DU145 are 13.71 µM and 12.11 µM, respectively. Moreover, it is worth noting that although peptide (aiming to block METTL3-METTL14 interaction) and small molecule inhibitor (aiming to block SAM domain) function differently and possess distinct physicochemical properties, peptide drug is generally believed to deliver a higher selectivity and lower toxicity [42]. Our in vivo tumor assay also indicates RSM3 yields a better effect of restraining PC3 xenograft growth than STM2457 [49]. We also test the anticancer activity of RSM3 in some other cancer types such as pancreatic cancer [49], indicating a potential general application of METTL3 peptide inhibitor in many other METTL3-driven tumors. Collectively, our study reveals a novel METTL3/m6A/RRBP1 axis in aggressive PCa, which can be therapeutically targeted by small-peptide METTL3 antagonists.
Materials and methods
Cell culture
Human normal prostate epithelial cell line RWPE-1 and four prostate cancer cell lines PC3, DU145, LNCaP and VCaP were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. RWPE-1 was cultured in K-SFM medium (Gibco, 17005-042). The four kinds of PCa cells were cultured in RPMI-1640 medium (Gibco, 8123209) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Excell, FSP500) and 1X antibiotic (penicillin/streptomycin). 293T cells were cultured in DMEM medium supplemented with 8% fetal bovine serum and 1X antibiotic (penicillin/streptomycin). The incubator conditions were set at 37℃ and 5% CO2.
RNA sequencing (RNA-seq)
The RNA-seq analysis was performed in collaboration with LC Sciences. First, we used the Omega total RNA Midi Kit (Omega, R6834-02) to extract total RNA from PC3-shNC and PC3-shMETTL3 cells. We then purified the Poly(A) RNA to construct a cDNA library. The CDNA library samples were sequenced on illumina Novaseq™ 6000. For data analysis, we first compared clean reads with the Human reference genome (GRCh38) using STAR v2.7.3a [50]. Then, differential expression genes (DEGs) between PC3-shNC and PC3-shMETTL3 were identified using DESeq2 package [51]. We defined genes with false discovery rate (FDR) < 0.05, fold change (FC) ≥ 1.5, and base-mean (readcounts) > 10 as differentially expressed genes (DEGs).
RNA immunoprecipitation and high-throughput sequencing (RIP-seq)
RIP experiments, high-throughput sequencing and data analysis were conducted by Seqhealth Technology (Wuhan, China). The following is a brief description of the experimental procedure. First, PC3 and LNCaP cell lines were treated with cell lysis buffer. Then 10% lysate was separated as “Input”, and 80% lysate was used for immunoprecipitation reactions with anti-METTL3 antibody (Abcam, ab195352), named “IP” group, and the remaining 10% lysate was incubated with IgG (Cell Signaling Technology) antibody as negative control IgG group. Subsequently, the RNA of Input, IP and IgG groups was extracted with Trizol reagent (Invitrogen, USA), and finally the RNA was purified to generate cDNA library. For data analysis, the resulting clean reads were compared with the human reference genome (GRCh38) by STAR v2.7.3a [50]. The exomePeak (Version 3.8) [52] and ChIPseeker package [53] were used for peak calling and annotation.
Methylated RNA immunoprecipitation and sequencing (MeRIP-seq)
MeRIP-seq was performed in collaboration with LC Sciences company. First, we used Trizol to extract total RNA from prostate and PCa tissues. Then, the total RNA was sent to LC Sciences company for sequencing and analysis. For data analysis, the resulting clean reads were compared with the human reference genome (GRCh38) by STAR v2.7.3a [50], The tool HOMER [54] was used for de novo and known motif identification. The Integrative Genomics Viewer (IGV) software was utilized to visualize selected peaks.
MeRIP-RT-qPCR
The procedure of MeRIP RT-qPCR assay is described as follows: First, total RNA was extracted by Trizol, and then m6A-RIP assay was performed by MeRIP kit (Epigentek, p9018). Finally, the enriched RNA was used for MeRIP RT-qPCR validation. The relative m6A modification level of RRBP1 is normalized to the input. The primers for MeRIP RT-qPCR were shown as following:
RRBP1-F: 5’-GCAGCCAGTTCTCAAAGG-3’ and RRBP1-R: 5’-AACACCCAGGAGGTCACA-3’.
Plasmid construction and shRNA or siRNA transfection
We used Plko.1-puro-shRNA lentiviral vector to construct METTL3 knockdown stable cell lines. The two shRNA sequences are shMETTL3-718 (5’-TGCACTTCAGACGAATTAT-3’) and shMETTL3-812 (5’-TCAGTATCTTGGGCAAGTT-3’). In addition, PCDH plasmid was used to construct METTL3-overexpressing wild type and mutant stable cell lines. The mutation sequence of METTL3 catalytic domain was as follows (aa395-398, DPPW→APPA). The specific construction method of stable cell lines was carried out according to previous descriptions [55]. The PTEN-OE plasmid (pUC-CMV backbone) was a gift from Dr. Liming Wang (Hunan University). For siRNA-mediated KD experiments, the siRNA sequences were designed and synthesized by Gene Parma (Shanghai, China). The target sequences of siRNA are shown as follows:
METTL3-siRNA1 (5’-GCUACCUGGACGUCAGUAU-3’); METTL3-siRNA2 (5’-GGUUGGUGUCAAAGGAAAU-3’) and RRBP1-siRNA (5’-AUGUCGGUUACAAGAAGAA-3’).
Quantitative real-time PCR (qPCR)
RNA was isolated using RNA-easy Isolation Reagents (Vazyme, R701-01, China) following the manufacturer’s instructions. cDNA was generated by the Reverse Transcription Kit (Vazyme, R233-01). The qRT-PCR assay was performed using ChamQ SYBR Master Mix (Vazyme, Q311-02) on Bio-rad CFX96 real-time PCR system (Biorad, USA). All primers used in the present study were listed as following: GAPDH (F: 5’-ACTTTGGTATCGTGGAAGGACT-3’ and R: 5’- GCCTTGGCAGCGCCAGTAG-3’), METTL3 (F: 5’-GAGTGCATGAAAGCCAGTGA-3’ and R: 5’-ACTGGAATCACCTCCGACAC-3’), RRBP1 (F: 5’-AGTTCGGACCAGGTGAGGGAGCAC-3’ and R: 5’-GCGTCTTCAGCTGAACGGGGTC-3’).
RIP-qPCR
The RIP assay was performed according to the previous description [56]. First, PC3 and LNCaP cell lines were treated with cell lysis buffer. Then the lysate was immunoprecipitation reactions with anti-METTL3 antibody (Abcam, ab195352) and control IgG antibody. RNA enriched with antibodies was extracted for qPCR assay. The primers for RIP RT-qPCR were RRBP1-F (5’-GCAGCCAGTTCTCAAAGG-3’) and RRBP1-R (5’-AACACCCAGGAGGTCACA-3’).
Luciferase report assay
The luciferase reporter assay was conducted as previous [57]. DU145 cells were first seeded in 24-well plates (BKMAM, Changde), then the RRBP1-3’UTR pmirGLO fusion plasmid was transfected into the cells, finally the activities of firefly luciferase and renilla luciferase were measured according to Dual-Luciferase® Reporter Assay (Promega E1910) instructions.
RNA stability analyses
The RNA stability was determined according to a previous protoocol [58]. The cells were first seeded in 12-well plates (BKMAM, Changde) 24 h in advance. Next, 110 µg/ml actinomycin D (M4881, AbMole, USA) was added to cells at different time points. Finally, RNA at different time points was extracted by trizol for RT-qPCR.
Western blot
The proteins were denatured by SDS and fractionated by SDS PAGE gel, then transferred onto PVDF membrane, blocked in 5% nonfat milk, and incubated with specific primary antibody at 4 °C overnight. After washing with PBST, the membrane was incubated with secondary antibody, exposed by Hyperfilm ECL kit and finally images were acquired. Antibodies against METTL3 (Abcam; ab195352), RRBP1 (Abclonal; A12239), PTEN (Cell signaling; #9559S), GAPDH (Proteintech; 60004-1-Ig), α-Tubulin (Proteintech; 11224-1-AP) were all diluted at 1:1000 ratio.
Cell proliferation and apoptosis assays
For cell proliferation assay, 2 × 104 PCa cells were plated in 12-well plates (BKMAM, Changde) and incubation at 37 °C. The number of cells were counted after 72 h, 120 h and 168 h. For colony formation assay, 5 × 103 DU145, 8 × 103 PC3, or 30 × 103 LNCaP cells were seeded in each well of 6-well plates, and DU145, PC3, and LNCaP cell colonies were subjected to methanol fixation and crystal violet staining after 7 ~ 15 days. For cell viability assay, the cell culture of 96-well plates was added with 10% CCK8 (APExBIO, K1018, USA) and incubation at 37 °C for 3 h. The absorbance of each well was measured by a microplate reader set at 450 nm. For cell apoptosis assay, Annexin V-PI apoptosis detection kit (Bioscience, A6030L) and Flow cytometry (Beckman, USA) were used according to the manufacturer’s instruction.
Trans-well migration assay
Trans-well assay was used to asses cell migration ability. After adding 700ul RPMI1640 medium with 20% FBS into the bottom of wells of 24-well plate, the suspension of 50 ~ 70 × 103 DU145 or 40 ~ 50 × 103 PC3 cells in RPMI1640 containing 1% FBS were placed into the trans-well chamber. After incubation for 1 ~ 2 days at 37 °C, the trans-well chambers were subjected to methanol fixation and crystal violet staining.
Wound-healing assay
After the cell density grew to 90%, the sterilized tip was used to scratch a “wound” of the same width on the bottom of the dish. The width of the wound was recorded by taking pictures through a microscope at different time points, and the degree of wound closure was subsequently calculated.
RNA m6A dot-blot assay
Total RNA was extracted by Trizol, denatured for 95 min, and then cross-linked on Hybond-N+ membrane. Then, the membrane was blocked with 5% nonfat milk, followed by incubation with an m6A antibody (Abcam, ab151230, USA) at 4 °C overnight. After washing with PBST for 3 times, the membrane was incubated with secondary antibody at room temperature for 1 h. Finally, the membrane was exposed via Hyperfilm ECL kit and images were acquired. Methylene blue (MB) was used to stain mRNA and also function as a loading control.
Animal experiments
Approximately 1 × 106 PCa cells (PC3-shNC, PC3-sh812 cell lines) or 2 × 106 DU145 cells (DU145-shNC, DU145-sh812, DU145-OENC, DU145-OEMETTL3-WT, DU145-OEMETTL3-Mut cell lines) per injection were suspended in a mixture of 100 µL PBS and Matrigel (1:1), and then injected subcutaneously into male BALB/c nude mice aged around 6 ~ 7 weeks old. Tumor volume was monitored at regular intervals and the volume was calculated as (length × width2)/2. Finally, the mice were sacrificed and the tumors were dissected and weighed.
Peptide inhibitor treatment
A xenograft model was established by subcutaneous injection of approximately 1 × 106 PC3 cells into male nude mice (n = 21). When the tumor size reached ~ 100mm3, the tumor-bearing mice were randomly divided into three groups. Mice in the three groups were injected with peptide R9, RM3 and RSM3, respectively, at the dosage of 20 mg/Kg once every 2 days via orthotopic injection (total of 7 times). The size of the tumors was measured every two days and the mice were collected at day 28 post implantation. The endpoint tumors were dissected and weighed.
Statistical analysis
We used the Log-Rank test to calculate P values for survival analyses. The co-expression analysis was performed based Spearman correlation. Alternatively, Student’s t-test, paired or unpaired two-tailed t-test were used to calculate the statistical significance between comparisons depending on the data type. P < 0.05 is considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
DZ and JS conceived and designed the study, interpreted data, and finalized the manuscript. YF and ZL performed most experiments. JW and CZ conducted Bioinformatic analysis. YT, JX, QH, WL, and LL provided assistance in in vitro cell-based assays. HX and BX provided reagents and clinical samples, respectively. All authors read and approved the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81972418 to D.Z. and 21975068 to J.S.), the National Youth Talent Support Program (202309460011 to J.S.), the Excellent Youth Foundation of Hunan Province (2021JJ10028 to D.Z., 2022JJ10008 to J.S., and 2024JJJCQN0337 to L.L), the Shenzhen Science and Technology Program (JCYJ20220530160410024 to D.Z. and JCYJ20210324122403010 to J.S.), Guangdong Basic and Applied Basic Research Foundation (2024A1515012312 to D.Z.) and the Fundamental Research Funds for the Central Universities. C.Z. was supported, in part, by the Hunan Province Natural Science Foundation (2022JJ40111) and the Changsha Natural Science Foundation (KQ2202182). J.X. was supported, in part, by China Postdoctoral Science Foundation (2022M721100) and the Changsha Natural Science Foundation (KQ2202154). Y.F. was supported, in part, by the Postgraduate Scientific Research Innovation Project of Hunan Province (CX20230415).
Data availability
All data generated or analysed during this study are included in this published article. The RNA-seq, RIP-seq and m6A-seq data have been deposited in GEO database under accession code GSE241315, GSE240667, GSE240668, GSE240669, and GSE240672. The data analysed in the current study are available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
All animal studies were approved by the Ethics Committee for Animal Experiments at HNU University (Approval No: HNU-IACUC-2021-101). Human prostate specimens were from Zhongda Hospital of Southeast University and the study were approved by the Ethics Committee of Zhongda Hospital, Southeast University. The approval ID is 2022ZDKYSB099. All human PCa samples were collected in accordance with the national and institutional ethical guidelines.
Consent for publication
All authors consent for publication.
Conflict of interest
We declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuqing Feng and Zenghui Li contributed equally to this work.
Contributor Information
Junfeng Shi, Email: Jeff-shi@hnu.edu.cn.
Dingxiao Zhang, Email: zdx1980@hnu.edu.cn.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48 [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Liu J, Zhu X, Yang B, He Z, Yao X (2023) AZGP1P2/UBA1/RBM15 Cascade mediates the fate determinations of prostate cancer stem cells and promotes therapeutic effect of Docetaxel in Castration-resistant prostate Cancer via TPM1 m6A modification. Res (Wash DC) 6:0252 [DOI] [PMC free article] [PubMed]
- 3.Shi H, Wei J, He C (2019) Where, when, and how: context-dependent functions of RNA methylation writers, readers, and Erasers. Mol Cell 74(4):640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han X, Wang M, Zhao YL, Yang Y, Yang YG (2020) RNA methylations in human cancers. Semin Cancer Biol 75:97–115. [DOI] [PubMed]
- 5.Wang Z, Zhou J, Zhang H, Ge L, Li J, Wang H (2023) RNA m(6) a methylation in cancer. Mol Oncol 17(2):195–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Hu X, Yu H, Sun H, Zhang L, Shao C (2024) The FTO mediated N6-Methyladenosine modification of DDIT4 regulation with tumorigenesis and metastasis in prostate Cancer. Res (Wash D C) 7:0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Yi Y, Miao Y, Long W, Long T, Chen S, Cheng W, Zou C, Zheng Y, Wu X et al (2019) N(6)-Methyladenosine modulates nonsense-mediated mRNA decay in human glioblastoma. Cancer Res 79(22):5785–5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, Chen ZH, Zeng ZL, Wang F, Zheng J et al (2019) METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer 18(1):112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, Pilka ES, Aspris D, Leggate D, Hendrick AG et al (2021) Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593(7860):597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Chen Z, Guan T, Zhou Y, Ge L, Zhang H, Wu Y, Jiang GM, He W, Li J et al (2021) N(6) -Methyladenosine regulates mRNA Stability and Translation Efficiency of KRT7 to promote breast Cancer lung metastasis. Cancer Res 81(11):2847–2860 [DOI] [PubMed] [Google Scholar]
- 11.Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q et al (2019) METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer 18(1):110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia R, Chai P, Wang S, Sun B, Xu Y, Yang Y, Ge S, Jia R, Yang YG, Fan X (2019) M(6)a modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol Cancer 18(1):161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji G, Huang C, He S, Gong Y, Song G, Li X, Zhou L (2020) Comprehensive analysis of m6A regulators prognostic value in prostate cancer. Aging 12(14):14863–14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Lu Y, Duan D, Wang H, Man G, Kang C, Abulimiti K, Li Y (2020) Systematic Investigation of mRNA N (6)-Methyladenosine Machinery in Primary Prostate Cancer. Dis Markers 2020:8833438 [DOI] [PMC free article] [PubMed]
- 15.Wang J, Lin H, Zhou M, Xiang Q, Deng Y, Luo L, Liu Y, Zhu Z, Zhao Z (2020) The m6A methylation regulator-based signature for predicting the prognosis of prostate cancer. Future Oncol 16(30):2421–2432 [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Liu Y, Liu J, Xu T, Cheng G, Shou Y, Tong J, Liu L, Zhou L, Xiao W et al (2020) The identification of critical m(6)a RNA methylation regulators as malignant prognosis factors in prostate adenocarcinoma. Front Genet 11:602485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai J, Yang F, Zhan H, Situ J, Li W, Mao Y, Luo Y (2019) RNA m(6)a methyltransferase METTL3 promotes the growth of prostate Cancer by regulating hedgehog pathway. Onco Targets Ther 12:9143–9152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Y, Du Y, Wang L, Liu X (2020) The M6A methyltransferase METTL3 promotes the development and progression of prostate carcinoma via mediating MYC methylation. J Cancer 11(12):3588–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H, Zhang F, Zhong Q, Hou J (2021) METTL3-mediated m6A modification of KIF3C-mRNA promotes prostate cancer progression and is negatively regulated by miR-320d. Aging 13(18):22332–22344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li E, Wei B, Wang X, Kang R (2020) METTL3 enhances cell adhesion through stabilizing integrin beta1 mRNA via an m6A-HuR-dependent mechanism in prostatic carcinoma. Am J Cancer Res 10(3):1012–1025 [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Pan C, Wang X, Xu D, Ma Y, Hu J, Chen P, Xiang Z, Rao Q, Han X (2021) Silencing of METTL3 effectively hinders invasion and metastasis of prostate cancer cells. Theranostics 11(16):7640–7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou C, He Q, Feng Y, Chen M, Zhang D (2022) A m(6)Avalue predictive of prostate cancer stemness, tumor immune landscape and immunotherapy response. NAR Cancer 4(1):zcac010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research N (2015) The Molecular Taxonomy of primary prostate Cancer. Cell 163(4):1011–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G et al (2015) Integrative clinical genomics of advanced prostate cancer. Cell 161(5):1215–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen XY, Zhang J, Zhu JS (2019) The role of m(6)a RNA methylation in human cancer. Mol Cancer 18(1):103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaccara S, Ries RJ, Jaffrey SR (2019) Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol 20(10):608–624 [DOI] [PubMed] [Google Scholar]
- 27.Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG et al (2017) M(6)a RNA methylation regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem cells. Cell Rep 18(11):2622–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G et al (2014) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505(7481):117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY et al (2016) Nuclear m(6)a reader YTHDC1 regulates mRNA splicing. Mol Cell 61(4):507–519 [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Hu Q, Liu X, Ji Y, Chao HP, Liu Y, Tracz A, Kirk J, Buonamici S, Zhu P et al (2020) Intron retention is a hallmark and spliceosome represents a therapeutic vulnerability in aggressive prostate cancer. Nat Commun 11(1):2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Guo Q, Shi Q, Rao Y, Dong Y, Chen F, Qi X (2023) M(6)A-mediated upregulation of HOXC10 promotes human hepatocellular carcinoma development through PTEN/AKT/mTOR signaling pathway. Discov Oncol 14(1):175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Liu Z, Zhang Y, Wu X, Bian W, Shan S, Yang D, Ren T (2023) METTL3-mediated m6A RNA methylation induces the differentiation of lung resident mesenchymal stem cells into myofibroblasts via the miR-21/PTEN pathway. Respir Res 24(1):300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Yao H, Huang J, Li C, Zhang Y, Xu R, Wang Z, Long Z, Tang J, Wang L (2022) METTL3 promotes prostatic hyperplasia by regulating PTEN expression in an m(6)A-YTHDF2-dependent manner. Cell Death Dis 13(8):723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, Cieslik M, Benelli M, Robinson D, Van Allen EM et al (2019) Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 116(23):11428–11436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C et al (2016) Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature 534(7608):575–578 [DOI] [PubMed] [Google Scholar]
- 36.Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M et al (2017) The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med 23(11):1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Tan Y, Yao Y, Gu L, Huang L, Song T (2023) Genome-wide detection of m6A-associated SNPs in atrial fibrillation pathogenesis. Front Cardiovasc Med 10:1152851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li XC, Jin F, Wang BY, Yin XJ, Hong W, Tian FJ (2019) The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 9(13):3853–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Shi X, Huang T, Zhao X, Chen W, Gu N, Zhang R (2020) Dynamic landscape and evolution of m6A methylation in human. Nucleic Acids Res 48(11):6251–6264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He L, Li H, Wu A, Peng Y, Shu G, Yin G (2019) Functions of N6-methyladenosine and its role in cancer. Mol Cancer 18(1):176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory B, Rahman N, Bommakanti A, Shamsuzzaman M, Thapa M, Lescure A, Zengel JM, Lindahl L (2019) The small and large ribosomal subunits depend on each other for stability and accumulation. Life Sci Alliance 2(2) [DOI] [PMC free article] [PubMed]
- 42.Muttenthaler M, King GF, Adams DJ, Alewood PF (2021) Trends in peptide drug discovery. Nat Rev Drug Discov 20(4):309–325 [DOI] [PubMed] [Google Scholar]
- 43.Savitz AJ, Meyer DI (1993) 180-kD ribosome receptor is essential for both ribosome binding and protein translocation. J Cell Biol 120(4):853–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benyamini P, Webster P, Meyer DI (2009) Knockdown of p180 eliminates the terminal differentiation of a secretory cell line. Mol Biol Cell 20(2):732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueno T, Tanaka K, Kaneko K, Taga Y, Sata T, Irie S, Hattori S, Ogawa-Goto K (2010) Enhancement of procollagen biosynthesis by p180 through augmented ribosome association on the endoplasmic reticulum in response to stimulated secretion. J Biol Chem 285(39):29941–29950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai HY, Yang YF, Wu AT, Yang CJ, Liu YP, Jan YH, Lee CH, Hsiao YW, Yeh CT, Shen CN et al (2013) Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene 32(41):4921–4931 [DOI] [PubMed] [Google Scholar]
- 47.Telikicherla D, Marimuthu A, Kashyap MK, Ramachandra YL, Mohan S, Roa JC, Maharudraiah J, Pandey A (2012) Overexpression of ribosome binding protein 1 (RRBP1) in breast cancer. Clin Proteom 9(1):7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang M, Liu S, Zhou B, Wang J, Ping H, Xing N (2020) RRBP1 is highly expressed in bladder cancer and is associated with migration and invasion. Oncol Lett 20(5):203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Feng Y, Han H, Jiang X, Chen W, Ma X, Mei Y, Yuan D, Zhang D, Shi J (2024) A stapled peptide inhibitor targeting the binding interface of N6-Adenosine-methyltransferase subunits METTL3 and METTL14 for Cancer Therapy. Angew Chem Int Ed Engl :e202402611 [DOI] [PubMed]
- 50.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11(10):R106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng J, Lu Z, Liu H, Zhang L, Zhang S, Chen Y, Rao MK, Huang Y (2014) A protocol for RNA methylation differential analysis with MeRIP-Seq data and exomePeak R/Bioconductor package. Methods 69(3):274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu G, Wang LG, He QY (2015) ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31(14):2382–2383 [DOI] [PubMed] [Google Scholar]
- 54.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38(4):576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang D, Jiang P, Xu Q, Zhang X (2011) Arginine and glutamate-rich 1 (ARGLU1) interacts with mediator subunit 1 (MED1) and is required for estrogen receptor-mediated gene transcription and breast cancer cell growth. J Biol Chem 286(20):17746–17754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gagliardi M, Matarazzo MR (2016) RIP: RNA immunoprecipitation. Methods Mol Biol 1480:73–86 [DOI] [PubMed] [Google Scholar]
- 57.Yu J, Liu D, Sun X, Yang K, Yao J, Cheng C, Wang C, Zheng J (2019) CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/beta-catenin signaling via transactivation of GSK-3beta and Axin2 expression. Cell Death Dis 10(1):26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Wang J, Xu D, Xiang Z, Ding J, Yang X, Li D, Han X (2021) M(6)a mRNA methylation regulates testosterone synthesis through modulating autophagy in Leydig cells. Autophagy 17(2):457–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article. The RNA-seq, RIP-seq and m6A-seq data have been deposited in GEO database under accession code GSE241315, GSE240667, GSE240668, GSE240669, and GSE240672. The data analysed in the current study are available from the corresponding authors upon reasonable request.