Abstract
Intraepithelial lymphocytes (IELs) reside in the epithelial layer and protect against foreign pathogens, maintaining the epithelial barrier function in the intestine. Interactions between IEL and epithelial cells are required for IELs to function effectively; however, the underlying molecular machinery remains to be elucidated. In this study, we found that intestinal epithelium-specific deficiency of the clathrin adaptor protein (AP)-1B, which regulates basolateral protein sorting, led to a massive reduction in IELs. Quantitative proteomics demonstrated that dozens of proteins, including known IEL-interacting proteins (E-cadherin, butyrophilin-like 2, and plexin B2), were decreased in the basolateral membrane of AP-1B-deficient epithelial cells. Among these proteins, CD166 interacted with CD6 on the surface of induced IEL. CD166 knockdown, using shRNA in intestinal organoid cultures, significantly inhibited IEL recruitment to the epithelial layer. These findings highlight the essential role of AP-1B-mediated basolateral sorting in IEL maintenance and survival within the epithelial layer. This study reveals a novel function of AP-1B in the intestinal immune system.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-024-05455-1.
Keywords: Alcam, Iodixanol density gradient ultracentrifugation, Induced IEL, Natural IEL, TMT-based quantitative proteome analysis
Introduction
The intestinal epithelium serves as both a conduit for absorbing food nutrients and a vital biological barrier that segregates commensal microorganisms and potentially harmful luminal materials from the inner sanctum of the body. These epithelial cells exhibit distinct protein localization at the apical and basolateral membranes, a polarized arrangement essential for conducting specialized functions. The apical membrane, which faces the intestinal lumen, facilitates the absorption of nutrients, ions, and water, while secreting mucins, antibacterial substances, and detecting chemicals in the lumen. The basolateral membrane, facing the lamina propria, establishes cell–cell and cell-basement membrane junctions to maintain epithelial integrity, while secreting hormones and cytokines into the lamina propria in response to the dynamic luminal environment.
Clathrin adaptor protein (AP) complexes, which are heterotetrameric protein assemblies, mediate the sorting of membrane proteins between the trans-Golgi network, endosomes, lysosomes, and the plasma membrane. Among these AP complexes, AP-1, which includes the ubiquitously expressed AP-1A and the epithelium-specific AP-1B, regulates polarized sorting at the trans-Golgi network and/or at the recycling endosomes [1, 2]. AP-1B consists of four subunits: γ, β1, σ1, and μ1B. Three of these subunits (γ, β1, and σ1) are shared with the AP-1A complex, which contains ubiquitously expressed μ1A. The μ1B subunit, encoded by Ap1m2, recognizes the tyrosine motif (YXXØ, X: any amino acid; Ø: a large hydrophobic amino acid) present in the cytoplasmic tails of membrane proteins and facilitates the selective transport of cargo proteins to the basolateral membrane [3–5].
Mutations in genes encoding AP-1 subunits are associated with human diseases, including a congenital enteropathy accompanying diarrhea via an intestinal epithelial dysfunction [6, 7]. We and other research groups have reported the critical role of AP-1B in maintaining intestinal homeostasis. AP-1B deficiency in intestinal epithelial cells disrupts basolateral protein sorting, resulting in several abnormalities in intestinal epithelial homeostasis and immune functions, including crypt hyperplasia, impaired secretory lineage differentiation, and reduced cytokine production upon viral infection [8–11]. Germline knockout (KO) of AP-1B (Ap1m2−/−) causes nutritional dysfunction, resulting in 50% of the Ap1m2−/− mice dying in the early stages of life [9]. Survived Ap1m2−/− mice develop spontaneous chronic colitis, accompanied by dysbiosis, caused by epithelial barrier and immune dysfunctions [8, 12]. These data demonstrated the importance of AP-1B in maintaining intestinal homeostasis. However, the regulation of polarized protein sorting by AP-1B is not fully understood.
Intestinal intraepithelial lymphocytes (IELs) are a specialized population of immune cells localized above the basement membrane within the intestinal epithelium. IELs closely adhere to the basement membrane to fortify the intestinal barrier, patrol the intestinal epithelium, promote wound repair, and defend against pathogens [13, 14]. IELs include innate lymphoid cells but mostly consist of T lymphocytes [15] and are classified into two primary groups: natural and induced. Natural IELs are characterized by the surface expression of the gd or ab T-cell receptors (TCRs) and often express CD8αα homodimers that lack TCR coreceptor function [14, 16]. TCRαβ+ precursors of natural IELs undergo a thymic selection process based on self-antigen or agonist stimuli [17]. Natural IELs play pleiotropic roles in host defense, tissue repair, and the regulation of immune responses [18, 19]. In contrast, induced IELs express αβTCR together with either CD4 or CD8αβ. Induced IELs originate from antigen-primed conventional CD4+ or CD8αβ+ T cells and predominantly contribute to the host’s defense against mucosal pathogens [16]. Moreover, some induced IELs serve as tissue-resident memory cells [20].
Interactions between IELs and epithelial cells via the basolateral membrane are critical for the differentiation, function, and maintenance of IELs. However, little is known about the molecular machinery underlying these interactions. Previous studies have identified several molecules that mediate IEL-epithelial cell interactions. For instance, E-cadherin interacts with αEβ7 integrin on the IELs and maintains IELs at the epithelial layer [21–23]. The interaction between epithelial cells and γδ-IELs, which is a subset of natural IEL expressing γδ TCR, activates γδ-IELs through plexin B2-semaphorin 4D. This activation plays a crucial role in repairing the colonic epithelium damaged by colitis, as it enhances the production of keratinocyte growth factor-1 by γδ-IELs [24]. Butyrophilin-like 2 (Btnl2) also interacts with gd-IELs and suppresses the homeostatic proliferation of γδ-IELs [25]. A recent study further demonstrated that the herpes virus entry mediator expressed in intestinal epithelial cells interacts with Tnfsf14 on IELs, representing a critical interaction for the survival and patrolling ability of intraepithelial CD8α T cells [26]. Considering that the absence of each molecule results in a partial decrease in IELs, further elucidation is required to understand the molecular basis of IEL-epithelial cell interactions.
In this study, we investigated the underlying molecular mechanisms behind the IEL and epithelial cell interactions which are required for IELs to function effectively. We found that intestinal epithelium-specific deficiency in AP-1B severely affects the recruitment and maintenance of IELs. We further revealed, using a density gradient ultracentrifugation with quantitative proteomic analysis, that AP-1B fine-tunes appropriate delivery of known IEL-regulating proteins, including E-cadherin, plexin B2, and Btnl2, to the basolateral surface. Furthermore, we discovered that CD166-CD6 acts as a novel regulator of IEL-intestinal epithelial interaction. These findings suggest the emerging concept that AP-1B plays an important role in regulating IEL maintenance by properly sorting multiple membrane proteins within intestinal epithelial cells.
Materials and methods
Animals
VillinCreERT2 Ap1m2flox/flox mice backcrossed onto C57BL6/J genetic background were bred and maintained under specific pathogen-free conditions in the animal facility of Faculty of Pharmacy, Keio University. VillinCreERT2 Ap1m2flox/flox mice or Ap1m2flox/flox littermate mice were orally administrated 10 mg/ml tamoxifen at 100 µL (1 mg/mouse/day) for 5 consecutive days. After 2 days of interval, the mice were sacrificed by cervical dislocation under deep anesthesia. C57BL6/J mice were purchased from CLEA Japan and Sankyo Laboratory Services (SLC). Generation of Ap1m2 germline knockout mouse was previously described and defined as AP-1B KO mice in the text [8, 9]. All animal experiments were approved by the Animal Studies Committees of Keio University.
Cell preparation
Mouse intestinal tissues were treated with Hank’s Balanced Salt Solutions (HBSS) containing 1 mM dithiothreitol and 20 mM EDTA (all from Nacalai-Tesque, Kyoto, Japan) for 20 min in a shaking incubator (37 °C, 200 rpm) to isolate epithelial cells. Epithelial cells were collected into 50 ml tubes through 100 µm cell strainer and treated the remaining tissue once more with HBSS containing 20 mM EDTA for 20 min. After collecting the epithelial cells into 50 ml tubes through 100 µm cell strainer, we isolated IELs from epithelial cells by centrifuging in 40% percoll (Cytiva, Tokyo, Japan).
For isolation of lamina propria (LP) lymphocytes, the remaining tissues were minced and dissociated with RPMI1640 medium containing, 0.5 mg/ml Collagenase (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), 0.5 mg/ml DNaseI (Sigma Aldrich, St. Louis, MO, USA), 2% new born calf serum (NBCS, Thermo Fisher Scientific, Waltham, MA, USA), 100 µg/ml penicillin, and streptomycin, and 20 mM HEPES (Nacalai Tesque) at 37 °C for 30 min. The cell suspensions were then filtered to obtain single-cell suspensions for flow cytometry [27].
Flow cytometry
The monoclonal antibodies used in flow cytometry were showed in Supplementary Table 1. These antibodies were used with Fixable Viability Stain 780 (BD Bioscience). Precision Count Beads (BioLegend) was used for cell counting. The stained samples were analyzed using a FACS LSRII or, a FACS Celesta (BD Bioscience). Data were analyzed with Flowjo software version 10 (BD Bioscience).
Iodixanol density gradient ultracentrifugation
From euthanized AP-1BΔIEC and control mice, a 10 cm long small intestine was cut from just below the stomach. After cutting open and washing off the luminal contents by ice-cold Hank’s Balanced Salt Solutions (HBSS), mouse intestinal tissues were treated with ice-cold HBSS containing 1 mM dithiothreitol and 30 mM EDTA (all from Nacalai-Tesque, Kyoto, Japan) for 10 min. Epithelium was carefully separated from the lamina propria by manipulation with fine needles under microscopic monitoring. The separated epithelium was washed with ice-cold HBSS one time and lysed by nitrogen cavitation, using nitrogen gas and cell disruption vessels (Parr Instrument Company, Moline, IL, USA) [28, 29]. Cell lysate was centrifuged at 3000 rpm for 5 min to remove nucleus and cell debris. Supernatant was ultracentrifuged at 160,000×g for 1 h to concentrate cell membrane. After discarding the supernatant, the pellet was suspended by 25 mM NaCl, 50 mM NaF, 20 mM HEPES 50% iodixanol solution. For iodixanol density gradient formation, 10, 12.5, 15, 17.5, 20, 22.5, 25, 27.5, and 30% iodixanol solutions were prepared by using 25 mM NaCl, 50 mM NaF, 20 mM HEPES [30]. Iodixanol gradient (10–30%) was made in 13 PA tube (Eppendorf Himac Technologies, Ibaraki, Japan) by gently adding the prepared solutions with micro syringe with sharp needle (both from Ito Corporation). The suspended sample was carefully added to the top of the gradient. The gradient was set to the P40ST rotor and himac Ultracentrifuge CP 80NX (Eppendorf Himac Technologies) and centrifuged at 64,000×g on average for 90 min without deceleration. After centrifuge, samples were slowly taken from the rotor, and dispensed the fractionated samples into protein low bind tubes (Eppendorf, Hamburg, Germany).
Western blotting
Separating gel for SDS-PAGE were made from, ×4 separating gel buffer solution, acrylamide/bis solution, TEMED, APS (all from Nacalai Tesque) and distilled water. Stacking gel was made from, ×4 stacking gel buffer solution, acrylamide/bis solution, TEMED, APS (all from Nacalai Tesque) and distilled water. Samples were boiled in ×2 sample buffer with 2-melcaptoethanol (Nacalai Tesque) at 95 °C for 5 min. Samples were separated in created polyacrylamide gels or precast gels (BIO-RAD) and transferred into PVDF membranes. The 5% skim milk in TBS-T or PVDF Blocking Buffer for Can Get Signal (TOYOBO) was used for PVDF membrane blocking, and Can Get Signal Immunoreaction Enhancer Solution (TOYOBO) was used for both primary and secondary antibodies listed in Supplementary Tables 2 and 3. Immunoreactivities were detected by Chemi-Lumi One series (Nacalai Tesque) and Amersham ImageQuant 600 and 800 (Cytiva). Densitometry of band was measured by ImageJ Fiji [31].
TMT-based quantitative proteome analysis
Fractionated samples were collected by trichloroacetic acid (TCA) precipitation. Briefly, 20% TCA was added to samples equivalently and mixed. After 30 min on ice, samples were centrifuged by 15,000×g, 4 °C for 10 min. Supernatants were discarded and pellets were suspended in cold acetone for wash. Pellets were dried and reduced with 10 mM DTT for 30 min, alkylated with 50 mM iodoacetamide for 30 min, and samples were digested with trypsin. Each sample was labeled with the tandem mass tag (TMT) reagents (6 plex, Thermo Fisher Scientific) [32]. After fractionation by strong cation exchange (SCX) chromatography, LC/MS/MS analysis was performed on an Orbitrap Fusion Lumos (Thermo Fisher Scientific) in MS3 mode [32]. Peptides and proteins were identified by means of automated database searching using MaxQuant (ver.1.6.17.0) against UniprotKB/Swissprot release 2017_04 with Trypsin/P specificity allowing for up to 2 missed cleavages. TMT6plex (N-Term), TMT6plex (K) and Carbamidomethyl (C) were set as fixed modifications. Acetyl (Protein N-term) and Oxidation (M) were allowed as variable modifications. 1% false discovery rate (FDR) cutoffs (peptide spectrum match (PSM) and protein) were employed for identifications. The search result was processed with the Perseus ver (1.6.14.0) software for the quantitative analysis. Subcellular location of the proteins obtained was annotated by UniProtR package (ver. 2.2.0) in R software (2022.02.3).
Immunofluorescence analysis
For frozen blocks, samples from mouse intestine were embedded into OCT compound (Sakura Finetech, Tokyo, Japan) without fixation and freeze by liquid nitrogen, and kept in −80 °C deep freezer. Samples were cut into 5 µm sections and dried for overnight. Samples were fixed by 4% paraformaldehyde (PFA) for 10 min or cold acetone in −30 °C for 10 min. Samples were blocked with 5% donkey serum (Merck, Darmstadt, Germany) and added primary antibody showed in Supplementary Table 4 were diluted in 0.2% BSA/PBS. After incubation overnight, samples were washed with PBS for 3 times, and added secondary antibodies diluted in 0.2% BSA/PBS. After 1 h incubation in room temperature, samples were washed with PBS for 3 times and added ProLong™ Gold antifade mountant (Thermo Fisher Scientific) and embedded on cover glass.
For whole mount staining, epithelium from mouse intestine was isolated by stripping with 30 mM EDTA in HBSS. Epithelial samples were fixed by 4% PFA for 30 min and blocked with 5% donkey serum. Samples were stained with antibodies in Supplementary Table 4 diluted in 0.2% saponin/0.2% BSA/PBS. After incubation overnight, samples were washed with PBS for 3 times, and added secondary antibodies diluted in 0.2% BSA/PBS. After 1 h incubation in room temperature, samples were washed with PBS for 3 times with ProLong™ gold antifade mountant (Thermo Fisher Scientific) and embedded on cover glass.
Samples were blocked with 5% donkey serum (Merck, Darmstadt, Germany) and added primary antibody were diluted in 0.2% BSA/PBS. After incubation overnight, samples were washed with PBS for 3 times, and added secondary antibodies diluted in 0.2% BSA/PBS. After one hour incubation in room temperature, samples were washed with PBS for 3 times and added ProLong™ Gold antifade mountant (Thermo Fisher Scientific) and embedded on slide glass. The samples were observed using fluorescence microscope BZ-X810 (Keyence, Osaka, Japan) with BZ-X810 Viewer application or confocal laser microscope FV3000 (Olympus, Tokyo, Japan) with FV31S-SW application. Obtained data were analyzed by ImageJ Fiji. Primary and secondary antibodies are showed in Supplementary Tables 4 and 5.
Preparation of recombinant Fc-fusion proteins
The cDNA was synthesized from total RNA of mouse thymus or intestinal epithelium by RT-PCR reaction (ReverTraAce qPCR master mix, TOYOBO, or SuperScript IV RT, Thermo Fisher Scientific). cDNAs encoding extracellular domain of CD166 and Btnl2 were amplified by PCR using primer pairs listed in Supplementary Table 6. PCR products were cleaved at the restriction enzyme recognition site added to the PCR primers and cloned into pFUSE-IgG1e2-Fc2, which was treated with the corresponding restriction enzyme (InvivoGen, San Diego, CA, USA). The DNA sequence of cloned genes was confirmed by Sanger DNA sequencing (Eurofins Japan). Plasmids were multiplied by E coli DH5α strain, purified by Midiprep system (Promega), and concentrated to 1 mg/mL by ethanol precipitation. Plasmid vector was transfected into Expi293F cell by using ExpiFectamine 293 transfection system (Thermo Fisher Scientific). Fc-fusion proteins were purified from culture supernatant by HiTrap rProtein A FF (Cytiva). Isolated IELs were incubated with purified Fc proteins (0.25 µg/106 cells) for 30 min at 4 °C. After washing, Fc proteins bound to the cell surface were detected by PE-conjugated anti-human IgG Fc antibodies.
Mouse small intestinal organoid culture
Small intestinal organoids were derived from C57BL/6N mice (Clea Japan) according to a previous report [33]. The duodenum was removed from mice and cut into 5-mm segments. The segments were incubated cold 2 mM EDTA in PBS for 5 min and washed by pipetting. The segments were incubated in cold 2 mM EDTA in PBS for 30 min, and crypts were isolated by pipetting with cold HBSS. Dissociated crypts were passed through a 70 µm cell strainer. The crypts were resuspended in DMEM/F12 medium (Themo Fisher Scientific); then, the number of crypts were counted, and the crypts were resuspended in Matrigel (Corning) or Geltrix (Thermo Fisher Scientific). Next, the crypts were plated in a 48-well plate with IntestiCult organoid growth medium (Stemcell Technologies). Media were changed every 2 days.
Lentiviral infection
The sequence for the shRNA was designed by using VectorBuilder. The shRNA target sequence is shown in Supplementary Table 7. The oligo DNA of the target sequence with linker and restriction enzyme recognition site shown in Supplementary Table 8 was first inserted to pENTR4-H1 and then transferred to CS-EF-Rfa-mRFP by using Gateway LR Clonase (Thermo Fisher Scientific). Lenti-X 293T cells were propagated in DMEM containing 10% (vol:vol) FBS, 1% GlutaMAX (Thermo Fisher Scientific), 100 U/ml penicillin, and 100 µg/ml streptomycin (Nacalai Tesque). Lentiviral supernatants were generated by transient transfection of shRNA containing SIN vector (17 µg) or control vector (CS2-EF-mRFP1, 17 µg), pCAG-HIVgp (10 µg), and pCMV-VSV-G-Rev (10 µg) into Lenti-X 293 T (5 × 106/10 ml; Clontech) using Polyethylenimine Max (Polysciences, Warrinton, PA, USA), according to the manufacturer’s protocol. All vectors were kindly provided by Dr. H. Miyoshi and RIKEN Bioresource Research Center. Transfected host cells were incubated for 24 h; after culturing for another 48 h, viruses were purified after removal of host cells and centrifugation. Dissociated organoids were infected by mixing with 250 µl of virus-including medium, followed by centrifugation at 600×g for 1 h at 32 °C. Then, cells were embedded into Matrigel and plated in 48 well plate.
Coculture of IELs and intestinal organoids
Isolated CD8β and CD4 IELs were positively selected by using biotin conjugated anti-mouse CD8b (YTS156.7.7; BioLegendd) and anti-CD4 (RM4-5; BioLegend) and Streptavidin particles. After IEL selection, organoids cultured more than a week were released from Matrigel by using Gentle dissociation buffer (Stem Cell Technologies). 100 organoids and 1 × 105 IELs were mixed in 400 µl of DMEM including 10 µM Y27632 [34]. After 30 min in 37 °C 5% CO2, samples were centrifuged for 1 min at 200 × g. Samples were resuspended in Matrigel or Geltrix. Next, the crypts were plated in a 48-well plate with organoid growth medium supplemented with 10 ng/ml IL-2, 10 ng/ml IL-7 and 10 ng/ml IL-15/Ra complex (all from BioLegend). Media were changed every 2 days.
After 4 days of co-culture, the samples were subjected to immunofluorescence analysis. The samples were fixed with 4% PFA for 20 min and then collected into protein low bind tubes. After three-times washing with PBS and incubation with 5% donkey serum for 15 min, samples were stained with Alexa Fluor 488 conjugated anti-CD45.2 (104; BioLegend) and Alexa Fluor 647 conjugated anti-EpCAM (CO17-1A; BioLegend) and Hoechst 33342 (Molecular Probes, Eugene, OR, USA) in the presence of 0.2% BSA in PBS for overnight at 4 °C. After wash with PBS, samples were suspended in antifade mountant (Thermo Fisher Scientific) and embedded on slide glass. The samples were observed using confocal laser microscope FV3000 (Olympus, Tokyo, Japan). Obtained data were opened with ImageJ software. Interactions between the IEL and the epithelium were quantified by analyzing each confocal image individually. Cells that appeared in the same location across consecutive images were identified as the same cells. The area of the organoids was determined from the projection images.
Quantitative RT-PCR
Total RNA was prepared using TRIzol (Life Technologies) from epithelium isolated from mice or organoids. First-strand cDNA synthesis was completed using the Revertra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan). The qPCR reactions were conducted in CFX-connect (Bio-Rad, Hercules, CA, USA) using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). The specific primers used are shown in Supplementary Table 9.
Statistical analysis
Statistical significance between two groups was calculated by Student’s t test, t test Welch’s correction, or Mann–Whitney’s U test. Statistical significance between the control group and other multiple groups was calculated by Dunnet’s T3 multiple comparison test. P < 0.05 indicated statistical significance. Data were output by Prism version 9 (GraphPad, San Diego, CA, USA).
Data availability
The raw MS data and analysis files have been deposited with the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the jPOST partner repository (https://jpostdb.org) with the data set identifier PXD042900 [35].
Supplemental materials
Supplementary Tables 1–9 are primers and antibodies lists. Supplementary Table 10, associated with Fig. 7, is a list of proteins that were changed in AP-1B deficient epithelium. Supplementary Table 11, associated with Fig. 7D, lists basolateral proteins that decreased in AP-1B-deficient epithelium and increased in the apical region.
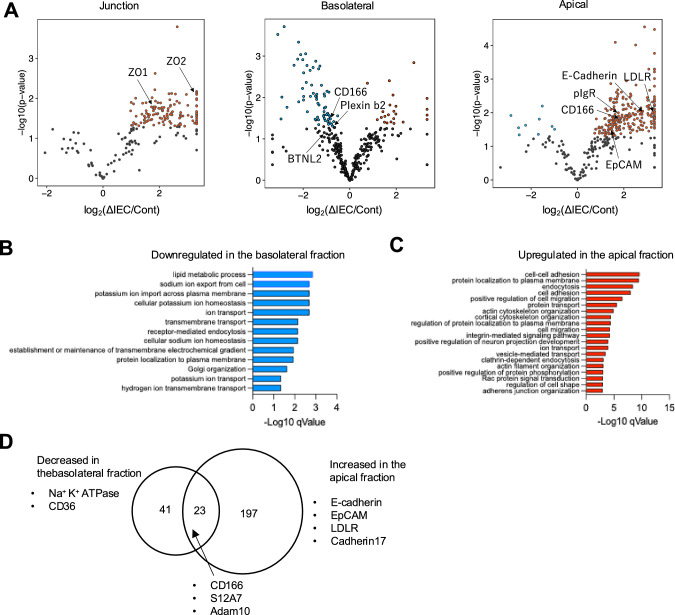
Fig. 7.
Protein sorting in the intestinal epithelial cells is disturbed by AP-1B deficiency. A Volcano plots based on proteomic analysis show differences in membrane protein expression levels for each membrane fraction of AP-1B-deficient epithelial cells and controls. Each dot represents a protein. Proteins with log2(AP-1BΔIEC/Control) > 1.3 and P < 0.05 are indicated by red dots, and proteins with log2(AP-1BΔIEC/Control) < −1.3 and P < 0.05 are shown by blue dots. Plasma membrane protein was filtered by subcellular location information from UniProt. Gene Ontology analysis (biological process) was performed on the basolateral (B) or apical (C) membrane proteins that were altered between AP-1BΔIEC and control mice. Proteins that decreased or increased in AP-1BΔIEC mice (P < 0.05) were listed and subjected to analysis using the DAVID database. Biological processes that were significantly decreased in the basolateral fraction or increased in the apical fractions were represented by blue and red bars, respectively. D The Venn diagram illustrates proteins that decreased in the basolateral fraction and increased in the apical fraction of AP-1BΔIEC mice compared to that of control mice
Results
AP-1B deficiency in intestinal epithelial cells reduces the number of IELs
During our study, we found that CD8α+ lymphocytes associated with the epithelial layer nearly disappeared in the small intestine of AP-1B deficient mice (Ap1m2−/−) (Fig. 1A). Flow cytometric analysis of the epithelial compartment also revealed that AP-1B deficiency led to a massive reduction in both γσ-IELs and TCRβ+ IELs (Fig. 1B). This new finding implies that AP-1B is indispensable for IEL-epithelial cell interactions in the intestine.
Fig. 1.

The number of IELs is decreased in the intestine of AP-1B germ line knockout mice. A Immunofluorescence images of CD8α (green) and EpCAM (magenta) in the small intestine of AP-1B germline knockout mice (AP-1B KO) and control littermates (WT). Scale bars are 100 µm. B The gating strategy for identifying IEL subsets using flow cytometry
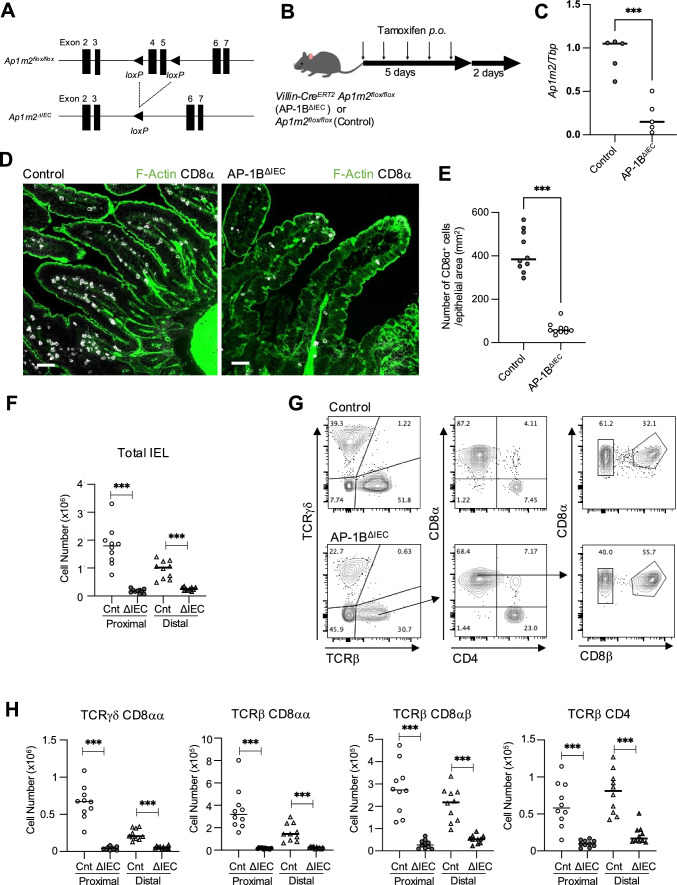
Ap1m2−/− mice tend to succumb to malnutrition during infancy, and Ap1m2 is expressed in extraintestinal tissues, such as the renal and alveolar epithelia [4, 9]. Therefore, we generated a tamoxifen-inducible and intestinal epithelial-specific gene targeting mice (Villin-CreERT2AP1m2Flox/Flox) (Fig. 2A) [9, 36]. To induce Ap1m2 deletion in intestinal epithelial cells, Villin-CreERT2Ap1m2flox/flox mice were orally administered tamoxifen daily for 5 days (Fig. 2B). Two days after the final administration of tamoxifen, we confirmed that the expression of Ap1m2 in Villin-CreERT2Ap1m2flox/flox was reduced to approximately 20% compared to that of a littermate that was of genotype Ap1m2flox/flox (Fig. 2C). No obvious weight loss or diarrhea was observed under these experimental conditions. Hereafter, we define the Villin-CreERT2Ap1m2flox/flox mouse strain after tamoxifen administration as the AP-1BΔIEC mice and Ap1m2flox/ flox mice as the control mice.
Fig. 2.
AP-1B deficiency in intestinal epithelial cells results in decreased IEL numbers. A The scheme for generating Villin-CreERT2 Ap1m2flox/flox mice (AP-1BΔIEC mice). B Schedule of tamoxifen administration to AP-1BΔIEC and Ap1m2flox/flox mice (control). Mice were orally administrated 10 mg/ml tamoxifen at 100 µL for 5 consecutive days. Two days after the final administration, mice were used for the subsequent experiments. C Ap1m2 gene expression in the intestine of AP-1BΔIEC after tamoxifen was administered. D Immunofluorescence images of CD8α (gray), and F-actin (green) in the small intestine of AP-1BΔIEC and control mice. Scale bars: 50 µm. E The number of CD8α+ cells residing in the epithelium was counted across at least three sections from ten AP-1BΔIEC and control mice, and the data were normalized to the epithelial area using ImageJ. *** P < 0.001 calculated using the t test with Weltch’s correction. F Flow cytometry data of the number of live IEL (CD45+ FVS780−) in AP-1BΔIEC and control mice. IELs were isolated from the small intestine, 10 cm below the stomach (proximal) and 10 cm from the cecum (distal). The total cell number was counted by using cell counting beads. * P < 0.05, ** P < 0.01, *** P < 0.001 calculated using Dunnett’s T3 multiple comparison test (n = 10). The representative data from three independent experiments are shown. G Gating strategy of CD45+ FVS780− IELs. H Flow cytometry data of the IEL number in AP-1BΔIEC and control mice. * P < 0.05, ** P < 0.01, *** P < 0.001 calculated by Dunnett’s T3 multiple comparison test (n = 10). The representative data from three independent experiments are shown. In each graph, dots represent values obtained from individual mice, and horizontal bars represent the average values
Consistent with germline KO mice, AP-1BΔIEC mice exhibited a marked reduction in IELs in the upper small intestine (Fig. 2D, E). Flow cytometry analysis confirmed that the total percentage of IELs was significantly decreased in both the upper and lower small intestines of AP-1BΔIEC mice compared to that observed in the control mice (Fig. 2F). We performed a more detailed analysis of the IEL subsets (Fig. 2G, H). The number of two natural IEL subsets, γδ-IELs and TCRβ+ CD8αα+ IELs, was significantly lower in AP-1BΔIEC mice compared to that in control mice (P < 0.001 calculated by Dunnett’s T3 multiple comparison test, Fig. 2H). Similarly, the number of IELs expressing TCRβ+ CD8αβ+ and TCRβ+ CD4+, known as induced IEL subsets, also decreased in AP-1BΔIEC mice (Fig. 2H).
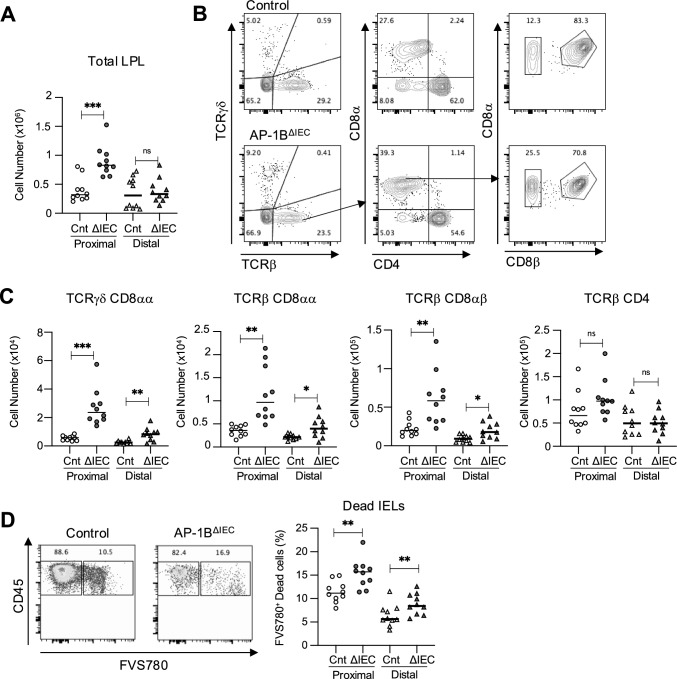
On the other hand, in the lamina propria of proximal intestine, the total number of CD45+ lymphocytes was significantly higher in AP-1BΔIEC mice compared to that of the control littermates (Fig. 3A). Further analysis showed that the increased IEL subsets in the proximal lamina propria were γδ-IELs, TCRβ+ CD8αα+, and TCRβ+ CD8αβ+ cells, but not TCRβ+ CD4 cells (Fig. 3B, C). These expansions of T-cell subsets in the lamina propria may be attributable to the liberation of IELs from the epithelial layer. In contrast, AP-1B deficiency increased the number of dead cells among the IELs remaining in the epithelial layer (Fig. 3D). Based on these observations, we concluded that AP-1B-dependent protein sorting secures the epithelial-IEL interaction to anchor all IEL subsets in the epithelial compartment of the small intestine.
Fig. 3.
The proportion of T cells in the lamina propria is increased in AP-1BΔIEC mice. A The number of lamina propria lymphocytes (LPLs) in AP-1BΔIEC and control mice. The total cell number was counted by using cell counting beads. These experiments were performed three times independently. * P < 0.05, ** P < 0.01, *** P < 0.001 calculated using Dunnett’s T3 multiple comparison test (n = 10). B Gating strategy for isolation of the LPLs from the AP-1BΔIEC and control mice. The parent gate is CD45+ live singlet cells. C The cell number of each LPL subsets in AP-1BΔIEC and control mice were measured by flow cytometry analysis and calculated by multiplying the total cell number by frequency. D IELs were separated as described in the “Materials and methods” section. The frequency of FVS780+ dead cells in CD45+ cells is shown. These experiments were performed three times independently. * P < 0.05, ** P < 0.01, *** P < 0.001 calculated using Dunnett’s T3 multiple comparison test (n = 10). In each graph, dots represent values obtained from individual mice, and horizontal bars represent the average values
AP-1B maintains polarized membrane proteins of intestinal epithelial cells
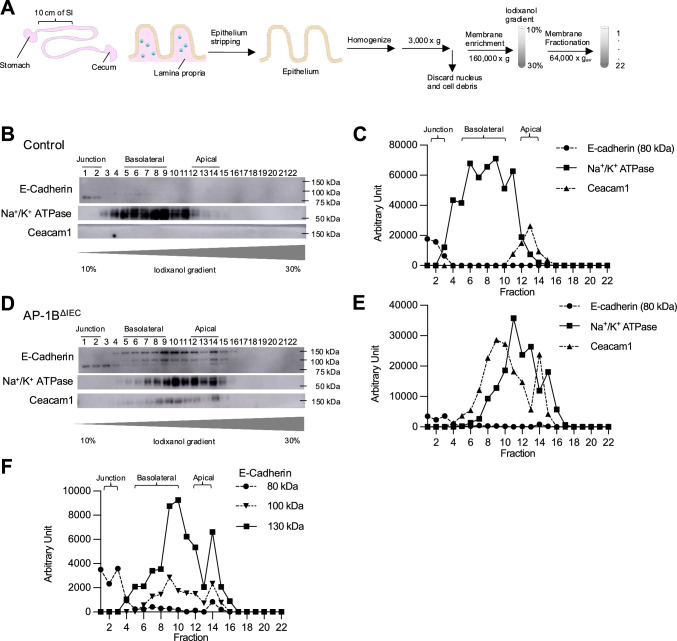
Given that AP-1B controls protein sorting toward the basolateral membrane, the disappearance of IELs in AP-1BΔIEC mice may result from the mislocalization of the basolateral proteins that interact with IELs. To investigate this hypothesis, we performed a quantitative proteomic analysis of plasma membrane proteins. We established a method to separate the epithelial tissue into three fractions, basement, apical, and junctional regions, by iodixanol-based density gradient ultracentrifugation (Fig. 4A). Using the intestinal epithelium tissue obtained from control mice, the junction (Fraction [Fr.] 1–2), basement (Fr. 5–9), and apical (Fr. 12–14) regions were separated and identified by western blot analysis using E-cadherin, Na+/K+ ATPase, and Carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) as the representative markers, respectively (Fig. 4B, C).
Fig. 4.
Fractionation of the plasma membrane of intestinal epithelial cells by iodixanol density gradient ultracentrifugation. A An experimental scheme of plasma membrane fractionation from isolated intestinal epithelia. A 10 cm duodenum was removed from each mouse below the stomach. Intestinal epithelium was stripped off from the mucus layer and homogenized by nitrogen cavitation. After discarding the nucleus by low-speed centrifugation, the cell membrane was enriched using ultracentrifugation. The enriched cell membrane was added to the top of a 10–30% iodixanol gradient and was fractionated by ultracentrifugation. Fractionation of the plasma membranes of the control (B) and AP-1BΔIEC (D) was confirmed by western blotting using specific membrane markers. C,E The line graphs show the intensities of each marker’s western blot signal, quantitated by densitometry and plotted against the fraction number. F The graph is a line graph showing the band intensities of E-cadherin that appeared at three molecular weights in AP-1BΔIEC mice
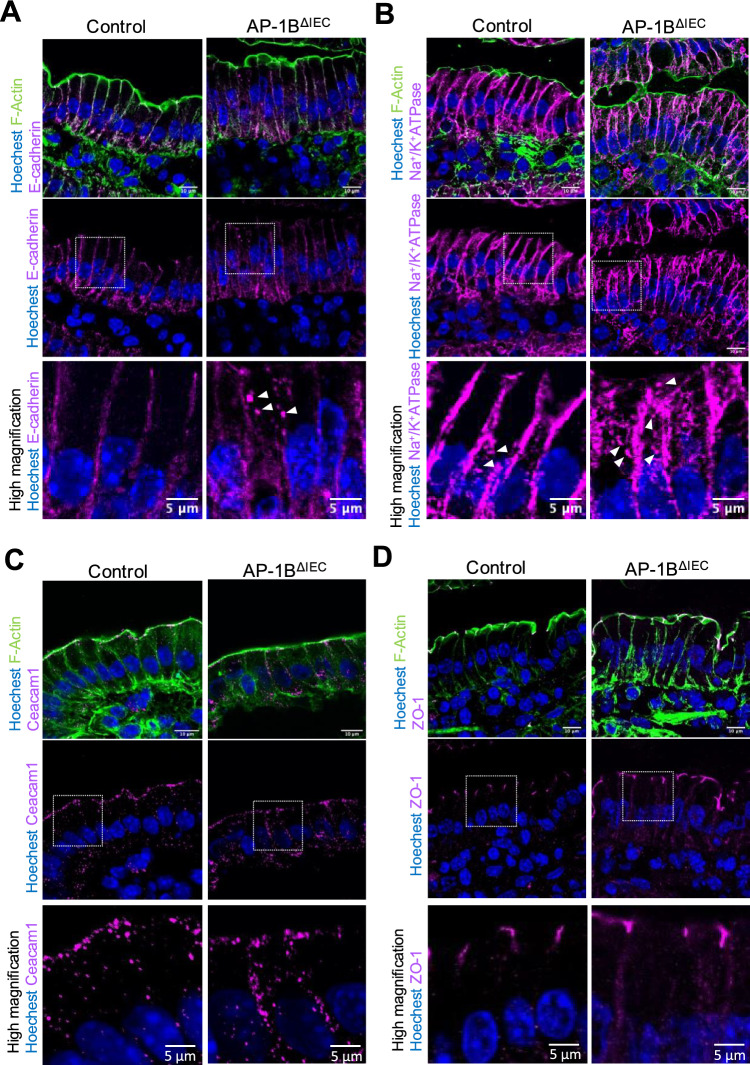
Membrane segregation of the AP-1B-deficient epithelium showed altered localization of each membrane marker protein (Fig. 4D–F). Na+/K+-ATPase and Ceacam1 were detected broadly from the apical to basolateral regions. E-cadherin, with three different molecular weights (130, 100, and 80 kDa), was distributed throughout the three compartments (Fig. 4F). As E-cadherin undergoes post-translational processing, its high molecular weights (130 and 100 kDa) are considered precursors of the 80 kDa mature protein [37, 38]. E-cadherin precursors, 100 and 130 kDa, were detected in the non-junctional fractions, suggesting that the post-translational modification of E-cadherin may be affected by AP-1B deficiency (Fig. 4F). Mislocalization of marker proteins in AP-1BΔIEC mice was confirmed by fluorescence immunostaining. E-cadherin was detected as abnormal cytoplasmic puncta, in addition to the basolateral membrane of the intestinal epithelial cells of AP-1BΔIEC mice (Fig. 5A). Na+/K+-ATPase was primally localized to the basolateral membrane but was also detected as intracellular granules in both the control and AP-1BΔIEC mice. This localization pattern may be due to the shuttling of Na+/K+-ATPase between the endosome and the basolateral plasma membrane (Fig. 5B). Ceacam1 was detected in the basolateral and apical membranes (Fig. 5C). These results are most likely attributed to AP-1B deficiency, which impedes the accurate transport of these proteins, resulting in an abnormal polarity or accumulation of precursors. In contrast, the localization of Zonula occludens-1 (ZO-1) to tight junctions was not altered by AP-1B deficiency (Fig. 5D).
Fig. 5.
Immunofluorescence images for membrane proteins in intestinal epithelial cells. Immunofluorescence images of the small intestine for E-cadherin (A), Na+/K+-ATPase (B), Ceacam 1 (C), and ZO-1 (D). Proteins are indicated in magenta in each panel. Nuclei were stained with Hoechst 33342 and shown in blue. F-actin was stained with phalloidin and shown in green. All images were taken with an FV3000 confocal microscope using FV31S-SW software. Scale bars are 10 µm in upper two panels, and 5 µm in high magnified images. Representative images from at least three independent experiments are shown
Polarized proteome analysis of intestinal epithelial cells
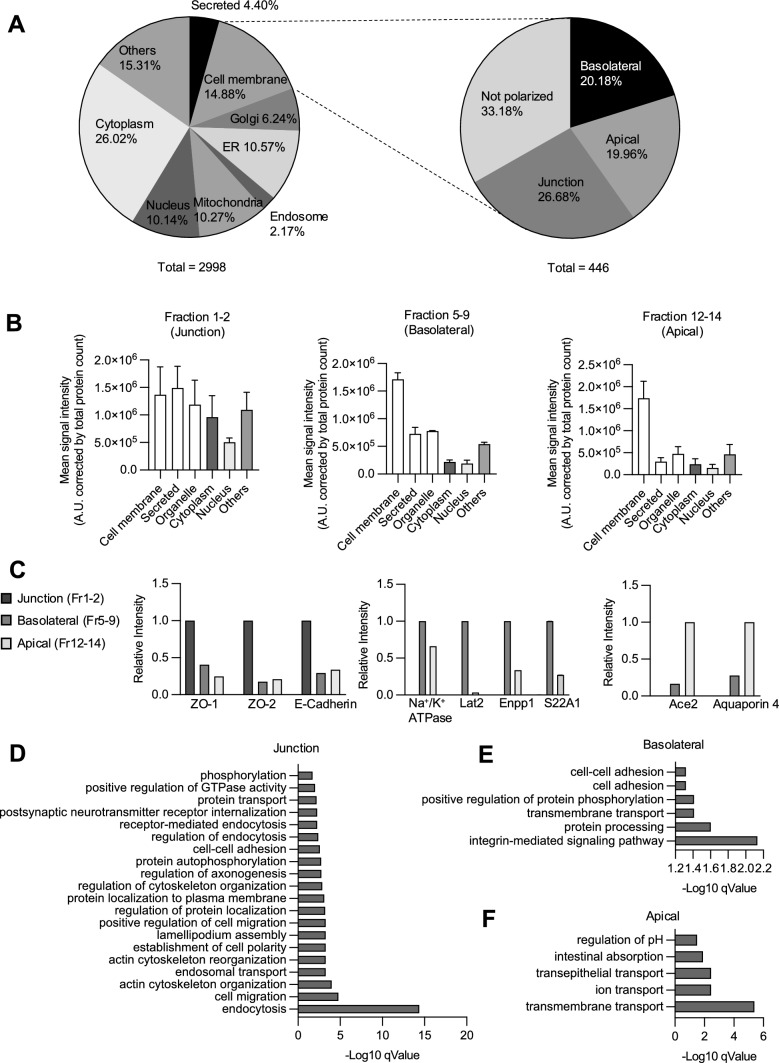
For proteomic analysis, the three membrane fractions were subjected to TMT-based quantitative mass spectrometry [32]. We analyzed Ap1m2 flox/ flox mice after administration of tamoxifen, which were used as control mice. This analysis identified 2998 proteins, among which 446 proteins, containing transmembrane, GPI-anchored, or peripheral extracellular domains, were classified as cell membrane proteins in the subcellular localization of UniProt knowledgebase (Fig. 6A). The proteins in the basolateral and apical fractions were mainly cell membrane proteins, whereas those in the junctional fraction were more diverse (Fig. 6B).
Fig. 6.
Quantitative proteome analysis of membrane fractions of intestinal epithelial cells. A Pie charts showing the subcellular localization of all 2998 proteins identified by mass spectrometry (left), of which 446 were plasma membrane proteins and were classified as apical, basolateral, junction, or not polarized (right). Subcellular localizations were referenced from the UniProt release on 7/23/2022. B Quantification of the signal intensity obtained from proteins that are annotated as cellular components indicated in the x-axis normalized by the total number of proteins in each fraction. C Relative signal intensities of marker proteins of each fraction. ZO-1, ZO-2, and E-cadherin were used for junction area markers. Na+/K+ ATPase, large neutral amino acids transporter small subunit 2 (Lat2), ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Enpp1) and solute carrier family 22 member 1 (S22A1) were used as the basolateral membrane markers. Ace2 and Aquaporin 4 were used as the apical membrane markers. Gene ontology (GO) analysis (biological process) by DAVID database were performed on the membrane proteins detected in the junction region (D), the basolateral region (E), and the apical region (F) of the control mice
Furthermore, comparative quantification of peptide signal intensities revealed that ZO-1, ZO-2, and E-cadherin, known as junctional proteins, were enriched in the junctional fraction; whereas the basolateral membrane markers Na+/K+-ATPase, large neutral amino acid transporter small subunit 2 (Lat2), ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Enpp1), and solute carrier family 22 member 1 (S22A1) were mainly detected in the basolateral fraction. The apical membrane markers Ace2 and Aquaporin 4 were enriched in the apical region (Fig. 6C). Gene Ontology (GO) analyses of each membrane region by DAVID database demonstrated that proteins associated with integrin-mediated signaling pathways were enriched in the basolateral region, whereas transmembrane transport, ion transport, and intestinal absorption molecules were enriched in the apical region (Fig. 6D–F) [39]. These observations are consistent with the expected functions of each membrane region of the intestinal epithelium.
Comprehensive analysis of AP-1B-dependent cargo candidates
Subsequently, we sought to identify AP-1B-dependent cargo candidates. Comparative proteomic analysis showed that 64 basolateral and 9 apical proteins, accounting for 14.3% and 2.02% of the 446 proteins classified into the cell membrane (Fig. 6A), respectively, were less abundant in the AP-1B-deficient epithelium compared to control (Fig. 7A; Supplementary Table 10). In contrast, the abundances of 23 basolateral, 220 apical, and 128 junctional proteins were enhanced by AP-1B deficiency (Fig. 7A; Supplementary Table 10). GO analysis revealed that several ion transport proteins were downregulated in the basolateral region of AP-1B-deficient epithelial cells (Fig. 7B). Moreover, proteins associated with cell–cell adhesion, cell migration, and integrin-mediated signaling pathways were abnormally enriched in the apical region of AP-1B-deficient epithelial cells (Fig. 7C). Of note, biological processes that were significantly increased in the basolateral fraction or decreased were not detected. We found that several known IEL-associated molecules were missorted in the absence of AP-1B. For instance, E-cadherin, which is required for IEL binding to the intestinal epithelium, was increased in the apical region of intestinal epithelial cells in AP-1BΔIEC mice compared to controls (Fig. 7A) [21, 22].
Of the 64 basolateral proteins decreased in AP-1B-deficient epithelium, 23 increased in the apical region (Fig. 7D). Among these basolateral-to-apical-shifted proteins, eight proteins were transmembrane proteins with few extracellular domains or membrane-associated domains on the cytoplasmic side, six proteins were ER-resident proteins, and seven proteins were transporters and enzymes whose ligands were unknown (Supplementary Table 11). After excluding these proteins, two molecules, plexin B2 (PLXB2) and CD166, remained as candidate regulators of IEL-epithelial cell interactions. PLXB2 has already been reported as an activator of the γδ-IEL, which interacts with Semaphorin 4D on the γδ-IEL [24]. However, the role of CD166, a type 1 transmembrane protein, in IEL-epithelial cell interactions has not been explored.
AP-1B regulates the basolateral sorting of CD166
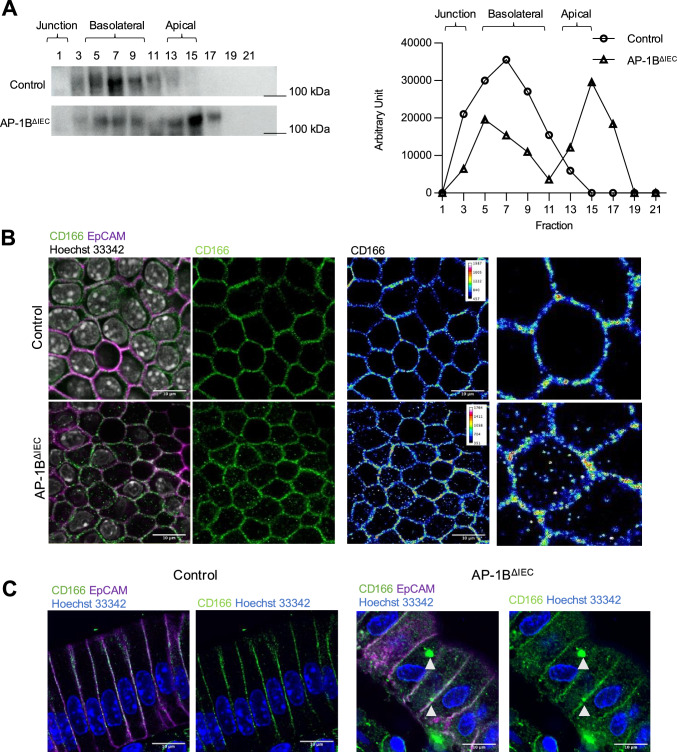
The results of the proteomic analysis were confirmed by iodixanol density gradient fractionation and western blotting. Consistent with the proteomic analysis, CD166 was detected in the basolateral fraction of control mice and both the basolateral and apical fractions of AP-1B-deficient epithelium (Fig. 8A). Whole-mount immunofluorescence analysis of the intestinal epithelium further demonstrated that CD166 was localized not only on the lateral membrane but also in the cytoplasmic granular structures in the AP-1B-deficient epithelium (Fig. 8B, C). These data suggest that AP-1B contributed to the polarized sorting of CD166 to the basolateral membrane.
Fig. 8.
AP-1B deficiency causes mislocalization of CD166 in the small intestine. A Western blot analysis of the expression of membrane fractions from AP-1B deficient epithelial cells and control epithelial cells. The representative data from three independent experiments are shown. B Whole-mount immunofluorescence staining of the small intestinal epithelium from AP-1BΔIEC and control mice. The right panels show pseudo-color images depicting signal intensities of CD166 immunostaining. The representative data from two independent experiments is shown. Gray indicates Hoechst 33342, green is CD166, and magenta is EpCAM. C Immunofluorescence images of the small intestine cryosections from control and AP-1BΔIEC mice. Blue indicates Hoechst 33342, green is CD166, and magenta is EpCAM. All pictures were taken with the FV3000 confocal microscope and FV31S-SW application with a 60× objective lens. Obtained pictures were analyzed by using ImageJ software with the OlympusViewer plugin. Scale bars are 10 µm
CD166 attached to CD6+ induces IELs
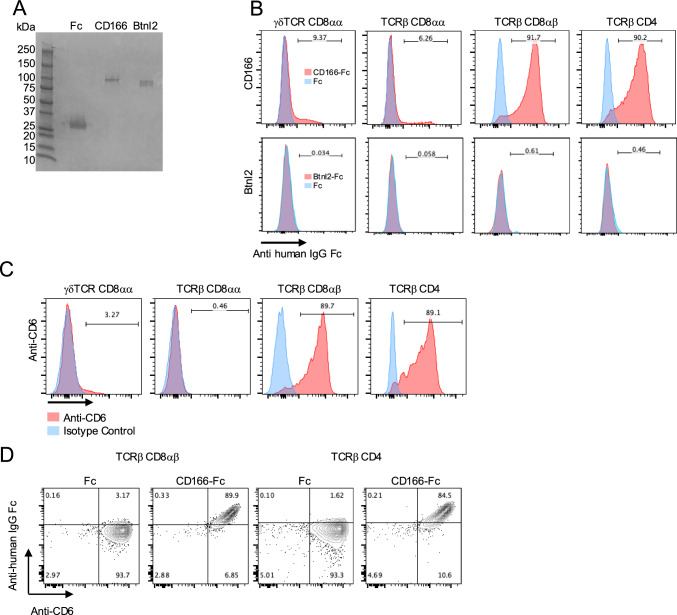
To investigate the association between CD166 and IELs, IELs isolated from the small intestine were incubated with a recombinant mouse CD166 extracellular domain fused to human IgG Fc (CD166-Fc) (Fig. 9A). CD166-Fc adheres selectively to TCRβ+ CD8αβ+ and TCRβ+ CD4+ IELs, but not to γδ-TCR+ CD8αα+ IELs nor TCRβ+ CD8αα+ IELs (Fig. 9B). Consistently, the expression of CD6, the ligand for CD166, was restricted to the surface of TCRβ+ CD8αβ+ and TCRβ+ CD4+ IELs and was not detected in other IEL subsets (Fig. 9C) [40]. Furthermore, nearly all the CD6-expressing IEL subsets are bound to CD166-Fc (Fig. 9 D).
Fig. 9.
CD166 attaches to CD6+ IELs. A Coomassie brilliant blue staining after SDS-PAGE of Fc recombinant proteins. From the right, human IgG1 Fc fragment protein (Fc) was used as a control, and CD166-Fc and Btnl2-Fc were applied. B Binding assay of CD166-Fc and Btnl-Fc recombinant protein to IELs. IELs were incubated with CD166-Fc and Btnl-Fc (red) or Fc fragment protein (control, blue) for 30 min at 4 °C. Fc proteins were detected by fluorescence-activated cell sorting (FACS) using anti-human IgG Fc antibody. The numbers represent the frequency of cells attached to Fc-fusion proteins. C FACS analysis of CD6 expression on IELs. Anti-mouse CD6 (red) or isotype control (blue) were used. The number represents the frequency of CD6+ IELs in each IEL subset. D Co-staining experiment of anti-CD6 antibody and CD166-Fc proteins for induced IELs
CD166 is involved in the attachment of induced IEL to the epithelium
We evaluated whether CD166 was involved in IEL-intestinal epithelial interactions. We established a co-culture system of isolated mouse CD4+ and CD8β+ IELs, and intestinal organoids of C57BL/6J mice [34]. We confirmed that CD166 was expressed in the organoid cultures (Fig. 10A) and that IELs adhered to the epithelium in this co-culture system (Fig. 10B, C). Pretreatment of induced IELs with the CD166-Fc-fusion protein significantly inhibited IEL adhesion to the epithelium, whereas the control Fc protein did not show such an inhibitory effect (Fig. 10C). To confirm the importance of CD166 in the epithelial-IEL interaction, we introduced shRNA targeting CD166 into the organoid culture system using a lentiviral vector (Fig. 10D). Downregulation of Alcam, which encodes CD166, was observed in the organoids. CD166 knock-down had no obvious effect on organoid proliferation or morphology. The number of IELs attached to CD166-knockdown was significantly lower than that attached to control organoids (Fig. 10E). These data suggest that CD166 is a novel regulator of IEL-epithelial cell interactions.
Fig. 10.
CD166 gene knockdown decreases the interactions between IELs and small intestinal organoids. A Intestinal organoids were stained with EpCAM (magenta) and CD166 (green). Scale bars are 50 µm or 20 µm. B IELs were co-cultured with intestinal organoids. IELs (green) attached to organoids (EpCAM, magenta) were counted in panels C and E. Scale bars represent 50 µm. C IELs were pretreated with CD166-Fc or control Fc and subsequently co-cultured with small intestinal organoids. Organoids were co-cultured for 7 days. IELs (green) attached to the organoid epithelium (magenta) were counted as described in the “Materials and methods” section. The images presented in the figure are two-dimensional projections of three-dimensional stacked images acquired using a confocal microscope. Student’s t test, * P < 0.05. Scale bars are 50 µm. This experiment was repeated three times. D Quantitative PCR of the relative expression level of Alcam, encoding CD166. *** P < 0.001, Dunnet’s T3 multiple comparison test used, E IELs were co-cultured with CD166-knockdown organoids for 7 days. After fixation, IEL (green) attached to the organoid epithelium (magenta) were counted. Dunnet’s T3 multiple comparison test: ** P < 0.01, *** P < 0.001. Scale bars are 50 µm. This experiment was repeated twice. All pictures were taken with the FV3000 confocal microscope and FV31S-SW application with a 20× lens or 60× lens. Obtained pictures were analyzed by using ImageJ software with the OlympusViewer plugin. In each graph, the dots represent the values obtained from each culture well and the bars represent the average
Discussion
We demonstrated that AP-1B-mediated polarized sorting of proteins in the intestinal epithelium is essential for IELs to interact with the intestinal epithelium and for their survival. A comprehensive analysis of polarized proteins in AP-1B-deficient epithelial cells suggested that AP-1B may regulate the basolateral sorting of several key molecules, namely, E-cadherin, PLXB2, Btnl2, and CD166, which mediate IEL-epithelial interactions.
Many studies on AP-1B-dependent membrane trafficking utilized Madin–Darby canine kidney (MDCK) cells expressing shRNA against Ap1m2 mRNA and LLC-PK1 cells naturally lacking AP1m2 [5, 41–43]. One of these studies conducted proteomic analysis in AP-1B-knockdown MDCK cells [43]. They distinguished between the apical and basolateral membranes of MDCK cells by labeling each membrane with biotin. However, this biotinylation-based approach is unsuitable for intestinal epithelial cells because the intestinal lumen is rich in biotin from microbial and dietary sources. In this study, we adopted membrane fractionation using the iodixanol density gradient method and succeeded in separating the apical and basolateral membranes of intestinal epithelial cells. Moreover, quantitative proteomic analysis showed that several hundred proteins, including E-cadherin and LDLR, which have been reported as AP-1B cargo proteins, were missorted in the intestinal epithelial cells of AP-1BΔIEC mice [5, 9, 44]. AP-1B deficiency caused a decrease in numerous membrane proteins in the basolateral fraction, confirming that AP-1B is a main regulator of basolateral protein sorting.
Notably, in the present study, AP-1B deficiency significantly increased the number of proteins in the apical fraction. Of these proteins, some proteins decreased in the basolateral fraction, indicating that these proteins were missorted from basolateral to apical membranes as previously described [43]. However, the reason underlying the increase in missorted proteins in the apical fraction remains unclear. It is important to consider that the density gradient centrifugation method used in this study does not exclusively fractionate proteins on the plasma membrane surface. In this study, Na+/K+ ATPase, utilized as a marker for the basolateral membrane fraction, was detected across a broad range of fractions by Western blotting. Immunofluorescence staining revealed its presence not only at the basolateral membrane but also within intracellular granules, even in control mice. These findings suggest that intracellular vesicles of similar density may also be present in the same fraction during the density gradient experiment. The proteomic analysis further supports this, indicating that the basolateral fraction contains intracellular membrane proteins to some extent, a pattern also observed in the apical fraction. These observations highlight the complexity of membrane fractionation and suggest that our proteomic analysis results could serve as a foundation for identifying more specific marker molecules and refining fractionation techniques in future studies.
CD166, E-cadherin, and Na+/K+-ATPase, which were abundant in the apical fraction in AP-1B deficient epithelial cells, exhibited distinct localization not only on the basolateral membrane but also as the intracellular granules by observing in immunofluorescence analysis. Given that AP-1B can sort basolateral membrane proteins at recycling endosomes, these intracellular granules may be associated with recycling endosomes. Our proteome data also suggest that transferrin receptors present on recycling endosomes are increased in the apical membranes fraction of AP-1BΔIEC mice. However, CD166-positive intracellular granules did not colocalize with Myo5b, a recycling endosome marker, in our immunostaining study [41, 45]. In addition, late endosomal markers including Lamp1, Lamp2, and VAMP8, and early endosomal markers, Rab5A and C, were also increased in the apical membrane fraction of AP-1BΔIEC mice, indicating that these aberrant intracellular granules are endosome-derived structures. Elucidating the nature of CD166-positive intracellular structures by using these proteins as organelle markers may provide new insights into the transport mechanism mediated by AP-1B. Nonetheless, our study provides the first comprehensive information on the proteins sorted by AP-1B in the intestinal epithelial cells and will help uncover the regulation mechanisms of membrane protein sorting in vivo.
We identified CD166, expressed by intestinal epithelial cells, as a potential new regulator of interactions with induced IELs. CD166, also known as an activated leukocyte cell adhesion molecule, ALCAM, is a member of the immunoglobulin superfamily and participates in homotypic and heterotypic cell–cell adhesion [46, 47] CD166 is expressed on the basolateral membranes of intestinal epithelial cells. Loss of AP-1B caused the missorting of CD166 toward the apical fraction of the intestinal epithelium and thus reduced CD166 in the basolateral fraction. Notably, CD6, a ligand for CD166, is selectively expressed on the surface of induced but not natural IELs. These data imply that IELs interact with intestinal epithelial cells via CD166-CD6 heterophilic interactions. Indeed, CD166 knockdown, in intestinal organoid cultures, decreases the interaction between IELs and epithelial cells. CD166 maintains the stemness of intestinal stem cells (ISCs), which has been attributed to the CD166-CD166 homophilic interactions of ISCs with Paneth and stromal cells [46]. Given that the affinity of the heterophilic interaction is 100-fold higher than the homophilic interaction [47], the CD166-CD6 heterophilic interaction between the induced IELs and ISCs may also contribute to the maintenance of stemness. Although γδ-IEL cells are known to support the proliferation of intestinal epithelial cells by producing KGF-1 [48], the role of induced IELs remains unknown. Further investigation of the CD166-CD6 heterotypic interaction may lead to the discovery of new functions of induced IELs in the maintenance of intestinal epithelial cells.
CD6-dependent signaling plays a role in T-cell activation. CD6 binds CD166 to antigen-presenting cells at the immunological synapse and functions as a co-stimulator of T-cell activation. The CD6-CD166 interaction is essential for the establishment and stabilization of T-cell-antigen-presenting cell contacts and sustained TCR-induced T-cell proliferation [49]. In contrast, CD6 attenuates early and late T-cell responses in a context-dependent manner. Therefore, CD6 may be a rheostat-type regulator of activation that fine-tunes T-cell activity in response to signal strength [50]. As intestinal epithelial cells constitutively express MHC class I and class II molecules upon infection and inflammation [51], epithelial CD166 may contribute to the antigen-specific responses of induced IELs under certain physiological and pathological conditions. Collectively, our study suggests that interactions between CD166 and CD6 may regulate not only the adhesion of intraepithelial lymphocytes (IELs) to the intestinal epithelium but also their mutual functional coordination. However, further research is required to verify these hypotheses.
In this study, we employed a tamoxifen-induced gene deletion system to investigate the role of AP-1B in the intestine, allowing for a relatively short period of analysis post-gene-deletion. However, we did not determine whether the membrane proteins undergoing mislocalization are direct cargo proteins of AP-1B or if their missorting is influenced indirectly. Specifically, the tyrosine motif essential for recognition by AP-1B was not detected in the cytoplasmic region of CD166. Further investigation is warranted to identify the specific cargo proteins that directly interact with AP-1B. Moreover, in our proteome analysis, 64 proteins were decreased in the basolateral fraction by AP-1B deficiency. These candidate proteins were narrowed down to CD166 because it has a sufficient length of the extracellular domain, and its ligand expresses on the surface of IELs. In the future, nonbiased loss-of-function screening of these 64 proteins using siRNA or CRISPR-Cas9 systems should be conducted. This approach may highlight additional regulatory molecules involved in IEL-epithelial interactions, providing a more comprehensive understanding of the mechanisms underlying this cellular process.
In conclusion, AP-1B ensures IEL-epithelial cell interaction by sorting integral membrane proteins into the basolateral membrane. AP-1B is essential for the proper localization of hundreds of proteins, including E-cadherin, plexin B2, and Btnl2, as well as CD166, in the plasma membrane, which may be essential for the interaction between IELs and the intestinal epithelium. Our findings provide new insights into IEL homeostasis in the small intestine.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by JSPS Grant-in-Aid for Scientific Research, JST CREST grant number, JST Moonshot R&D, AMED CREST, Keio University Program for the Advancement of Next Generation Research Projects, The Asahi Grass Foundation, Secom Science and Technology Foundation, Fuji Foundation for Protein Research, the “Kibou Projects” scholarship for doctoral students from Japanese Society for Immunology, and the Keio University Doctorate Student Grant-in-Aid Program from Ushioda Memorial Fund.
Abbreviations
- AP
Adaptor protein
- Btnl2
Butyrophilin-like 2
- Enpp1
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
- Fr.
Fraction
- GO
Gene ontology
- IEL
Intraepithelial lymphocytes
- ISC
Intestinal stem cell
- KO
Knockout
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Lat2
Large neutral amino acid transporter small subunit 2
- MDCK
Madin–Darby canine kidney
- PLXB2
Plexin B2
- TCR
T-cell receptor
- S22A1
Solute carrier family 22 member 1
Author contributions
Conceptualization: Shunsuke Kimura, Koji Hase, Daisuke Takahashi; Methodology: Ryohtaro Matsumoto, Shunsuke Kimura, Koji Hase, Daisuke Takahashi, Yasushi Ishihama; Formal analysis and investigation: Ryotaro Matsumoto, Kosuke Ogata, Yusuke Kinashi, Takahiro Yamada, Ryo Morita, Keisuke Tanaka, Kouya Hattori, Mayumi Endo, Yumiko Fujimura; Writing—original draft preparation: Ryohtaro Matsumoto; Writing—review and editing: Shunsuke Kimura, Koji Hase; Funding acquisition: Ryohtaro Matsumoto, Shunsuke Kimura, Koji Hase; Resources: Nobuo Sasaki, Hiroshi Ohno; Supervision: Shunsuke Kimura, Koji Hase.
Funding
This study was supported by JSPS Grant-in-Aid for Scientific Research 19K07239, 23H02739 (S.K.), 20H05876, 20H00509, 22K19445, and 23H05482 (K.H.); JST CREST grant number JPMJPR19H3, JPMJCR19H3 (S.K.), and JPMJCR19H1 (K.H.); JST Moonshot R&D grant number JPMJMS2025 (K.H.); AMED CREST grant numbers 22gm1310009h0003 (K.H.); Keio University Program for the Advancement of Next Generation Research Projects (S.K.); The Asahi Grass Foundation (K.H.); Secom Science and Technology Foundation (K.H.); Fuji Foundation for Protein Research (K.H); the “Kibou Projects” scholarship for doctoral students from Japanese Society for Immunology (R.M.); and the Keio University Doctorate Student Grant-in-Aid Program from Ushioda Memorial Fund (R.M.).
Data availability
Data were output by Prism version 9 (GraphPad, San Diego, CA, USA).
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to publish
This manuscript does not contain any individual person’s data in any form.
Ethics approval
All animal experiments were approved by the Animal Studies Committees of Keio University (No. A2022-282).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shunsuke Kimura, Email: kimura-sn@keio.jp.
Koji Hase, Email: hase.a6@keio.jp.
References
- 1.Nakatsu F, Hase K, Ohno H (2014) The role of the clathrin adaptor AP-1: polarized sorting and beyond. Membranes 4:747–763. 10.3390/membranes4040747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonifacino JS (2014) Adaptor proteins involved in polarized sorting. J Cell Biol 204(1):7–17. 10.1083/jcb.201310021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269:1872–1875. 10.1126/science.7569928 [DOI] [PubMed] [Google Scholar]
- 4.Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS (1999) μ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett 449(2–3):215–220. 10.1016/s0014-5793(99)00432-9 [DOI] [PubMed] [Google Scholar]
- 5.Fölsch H, Ohno H, Bonifacino JS, Mellman I (1999) A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99:189–198. 10.1016/s0092-8674(00)81650-5 [DOI] [PubMed] [Google Scholar]
- 6.Klee KMC, Janecke AR, Civan HA, Rosipal Š, Heinz-Erian P, Huber LA, Müller T, Vogel GF (2020) AP1S1 missense mutations cause a congenital enteropathy via an epithelial barrier defect. Hum Genet 139(10):1247–1259. 10.1007/s00439-020-02168-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan MC (2022) New directions for the clathrin adaptor AP-1 in cell biology and human disease. Curr Opin Cell Biol 76:102079. 10.1016/j.ceb.2022.102079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi D, Hase K, Kimura S, Nakatsu F, Ohmae M, Mandai Y, Sato T, Date Y, Ebisawa M, Kato T, Obata Y, Fukuda S, Kawamura YI, Dohi T, Katsuno T, Yokosuka O, Waguri S, Ohno H (2011) The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology 141:621–632. 10.1053/j.gastro.2011.04.056 [DOI] [PubMed] [Google Scholar]
- 9.Hase K, Nakatsu F, Ohmae M, Sugihara K, Shioda N, Takahashi D, Obata Y, Furusawa Y, Fujimura Y, Yamashita T, Fukuda S, Okamoto H, Asano M, Yonemura S, Ohno H (2013) AP-1B-mediated protein sorting regulates polarity and proliferation of intestinal epithelial cells in mice. Gastroenterology 145:625–635. 10.1053/j.gastro.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 10.Stanifer ML, Mukenhirn M, Muenchau S, Pervolaraki K, Kanaya T, Albrecht D, Odendall C, Hielscher T, Haucke V, Kagan JC, Bartfeld S, Ohno H, Boulant S (2020) Asymmetric distribution of TLR3 leads to a polarized immune response in human intestinal epithelial cells. Nat Microbiol 5(1):181–191. 10.1038/s41564-019-0594-3 [DOI] [PubMed] [Google Scholar]
- 11.Duclos M, Bourdais A, Nicolle O, Michaux G, Bidaud-Meynard A (2023) The clathrin adaptor AP-1B independently controls proliferation and differentiation in the mammalian intestine. bioRxiv. 10.1101/2023.05.12.540539 [Google Scholar]
- 12.Jangid A, Fukuda S, Seki M, Horiuchi T, Suzuki Y, Taylor TD, Ohno H, Prakash T (2020) Association of colitis with gut-microbiota dysbiosis in clathrin adapter AP-1B knockout mice. PLoS ONE 15(3):e0228358. 10.1371/journal.pone.0228358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayassi T, Jabri B (2018) Human intraepithelial lymphocytes. Mucosal Immunol 11(5):1281–1289. 10.1038/s41385-018-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma H, Qiu Y, Yang H (2021) Intestinal intraepithelial lymphocytes: maintainers of intestinal immune tolerance and regulators of intestinal immunity. J Leukoc Biol 109(2):339–347. 10.1002/JLB.3RU0220-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Kaer L, Olivares-Villagómez D (2018) Development, homeostasis, and functions of intestinal intraepithelial lymphocytes. J Immunol 200(7):2235–2244. 10.4049/jimmunol.1701704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheroutre H, Lambolez F (2008) Doubting the TCR coreceptor function of CD8alphaalpha. Immunity 28:149–159. 10.1016/j.immuni.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Joannou K, Baldwin TA (2023) Destined for the intestine—thymic selection of TCRαβ CD8αα intestinal intraepithelial lymphocytes. Clin Exp Immunol 213(1):67–75. 10.1093/cei/uxad049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald BD, Jabri B, Bendelac A (2018) Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat Rev Immunol 18(8):514–525. 10.1038/s41577-018-0013-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gui Y, Cheng H, Zhou J, Xu H, Han J, Zhang D (2022) Development and function of natural TCR+ CD8αα+ intraepithelial lymphocytes. Front Immunol 13:1059042. 10.3389/fimmu.2022.1059042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R (2006) Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol 176(4):2079–2083. 10.4049/jimmunol.176.4.2079 [DOI] [PubMed] [Google Scholar]
- 21.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB (1994) Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372:190–193. 10.1038/372190a0 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, Nelson WJ, Parker CM, Brenner MB (1998) Direct and regulated interaction of integrin αEβ7 with E-cadherin. J Cell Biol 140:197–210. 10.1083/jcb.140.1.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM (1999) T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol 162:6641–6649. 10.4049/jimmunol.162.11.6641 [PubMed] [Google Scholar]
- 24.Meehan TF, Witherden DA, Kim CH, Sendaydiego K, Ye I, Garijo O, Komori HK, Kumanogoh A, Kikutani H, Eckmann L, Havran WL (2014) Protection against colitis by CD100-dependent modulation of intraepithelial γδ T lymphocyte function. Mucosal Immunol 7(1):134–142. 10.1038/mi.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panea C, Zhang R, VanValkenburgh J, Ni M, Adler C, Wei Y, Ochoa F, Schmahl J, Tang Y, Siao CJ, Poueymirou W, Espert J, Lim WK, Atwal GS, Murphy AJ, Sleeman MA, Hovhannisyan Z, Haxhinasto S (2021) Butyrophilin-like 2 regulates site-specific adaptations of intestinal γδ intraepithelial lymphocytes. Commun Biol 4(1):913. 10.1038/s42003-021-02438-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo GY, Takahashi D, Wang Q, Mikulski Z, Chen A, Chou TF, Marcovecchio P, McArdle S, Sethi A, Shui JW, Takahashi M, Surh CD, Cheroutre H, Kronenberg M (2022) Epithelial HVEM maintains intraepithelial T cell survival and contributes to host protection. Sci Immunol 7(73):eabm6931. 10.1126/sciimmunol.abm6931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodyear AW, Kumar A, Dow S, Ryan EP (2014) Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis. J Immunol Methods 405:97–108. 10.1016/j.jim.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 28.Simpson RJ (2011) Disruption of cultured cells by nitrogen cavitation. Cold Spring Harb Protoc 2010(11):pdb.prot5513. 10.1101/pdb.prot5513 [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Philips MR (2017) Nitrogen cavitation and differential centrifugation allows for monitoring the distribution of peripheral membrane proteins in cultured cells. J Vis Exp 126:56037. 10.3791/56037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Galli T, Leu S, Wade JB, Weinman EJ, Leung G, Cheong A, Louvard D, Donowitz M (2001) Na+–H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol 537(Pt 2):537–552. 10.1111/j.1469-7793.2001.00537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogata K, Ishihama Y (2020) Extending the separation space with trapped ion mobility spectrometry improves the accuracy of isobaric tag-based quantitation in proteomic LC/MS/MS. Anal Chem 92(12):8037–8040. 10.1021/acs.analchem.0c01695 [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265. 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- 34.Nozaki K, Mochizuki W, Matsumoto Y, Matsumoto T, Fukuda M, Mizutani T, Watanabe M, Nakamura T (2016) Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol 51(3):206–213. 10.1007/s00535-016-1170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuda S, Watanabe Y, Moriya Y, Kawano S, Yamamoto T, Matsumoto M, Takami T, Kobayashi D, Araki N, Yoshizawa AC, Tabata T, Sugiyama N, Goto S, Ishihama Y (2017) jPOSTrepo: an international standard data repository for proteomes. Nucleic Acids Res 45(D1):D1107–D1111. 10.1093/nar/gkw1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fel Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39(3):186–193. 10.1002/gene.20042 [DOI] [PubMed] [Google Scholar]
- 37.Posthaus H, Dubois CM, Laprise MH, Grondin F, Suter MM, Müller E (1998) Proprotein cleavage of E-cadherin by furin in baculovirus over-expression system: potential role of other convertases in mammalian cells. FEBS Lett 438(3):306–310. 10.1016/s0014-5793(98)01330-1 [DOI] [PubMed] [Google Scholar]
- 38.Geng F, Zhu W, Anderson RA, Leber B, Andrews DW (2012) Multiple post-translational modifications regulate E-cadherin transport during apoptosis. J Cell Sci 125(Pt 11):2615–2625. 10.1242/jcs.096735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, Imamichi T, Chang W (2022) DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res 50(W1):W216–W221. 10.1093/nar/gkac194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel DD, Wee SF, Whichard LP, Bowen MA, Pesando JM, Aruffo A, Haynes BF (1995) Identification and characterization of a 100-kD ligand for CD6 on human thymic epithelial cells. J Exp Med 181(4):1563–1568. 10.1084/jem.181.4.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E (2007) AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA 104(5):1564–1569. 10.1073/pnas.0610700104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillon GA, Burriat-Couleru P, Abegg D, Criado Santos N, Watanabe R (2018) Clathrin and AP1 are required for apical sorting of glycosyl phosphatidyl inositol-anchored proteins in biosynthetic and recycling routes in Madin-Darby canine kidney cells. Traffic 19(3):215–228. 10.1111/tra.12548 [DOI] [PubMed] [Google Scholar]
- 43.Caceres PS, Gravotta D, Zager PJ, Dephoure N, Rodriguez-Boulan E (2019) Quantitative proteomics of MDCK cells identify unrecognized roles of clathrin adaptor AP-1 in polarized distribution of surface proteins. Proc Natl Acad Sci USA 116:11796–11805. 10.1073/pnas.1821076116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA (2007) Type I gamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. J Cell Biol 176(3):343–353. 10.1083/jcb.200606023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Edwards JG, Riley N, Provance DW Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD (2008) Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 35(3):535–548. 10.1016/j.cell.2008.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith NR, Davies PS, Levin TG, Gallagher AC, Keene DR, Sengupta SK, Wieghard N, El Rassi E, Wong MH (2017) Cell adhesion molecule CD166/ALCAM functions within the crypt to orchestrate murine intestinal stem cell homeostasis. Cell Mol Gastroenterol Hepatol 3(3):389–409. 10.1016/j.jcmgh.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan NJ, Barclay AN, Brown MH (2004) Frontline: optimal T cell activation requires the engagement of CD6 and CD166. Eur J Immunol 34(4):930–940. 10.1002/eji.200424856 [DOI] [PubMed] [Google Scholar]
- 48.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH (2004) Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 172:4151–4158. 10.4049/jimmunol.172.7.4151 [DOI] [PubMed] [Google Scholar]
- 49.Zimmerman AW, Joosten B, Torensma R, Parnes JR, van Leeuwen FN, Figdor CG (2006) Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 107(8):3212–3220. 10.1182/blood-2005-09-3881 [DOI] [PubMed] [Google Scholar]
- 50.Oliveira MI, Gonçalves CM, Pinto M, Fabre S, Santos AM, Lee SF, Castro MA, Nunes RJ, Barbosa RR, Parnes JR, Yu C, Davis SJ, Moreira A, Bismuth G, Carmo AM (2012) CD6 attenuates early and late signaling events, setting thresholds for T-cell activation. Eur J Immunol 42(1):195–205. 10.1002/eji.201040528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geuking MB (2023) Expanding the role of MHC class II on intestinal epithelial cells. Mucosal Immunol 16(4):548–550. 10.1016/j.mucimm.2023.05.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw MS data and analysis files have been deposited with the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the jPOST partner repository (https://jpostdb.org) with the data set identifier PXD042900 [35].
Data were output by Prism version 9 (GraphPad, San Diego, CA, USA).