Abstract
Background:
Estimating the burden of disease averted by vaccination can assist policymakers to implement, adjust, and communicate the value of vaccination programs. Demonstrating the use of a newly available modeling tool, we estimated the burden of influenza illnesses averted by seasonal influenza vaccination in El Salvador, Panama, and Peru during 2011–2018 among two influenza vaccine target populations: children aged 6–23 months and pregnant women.
Methods:
We derived model inputs, including incidence, vaccine coverage, vaccine effectiveness, and multipliers from publicly available country-level influenza surveillance data and cohort studies. We also estimated changes in illnesses averted when countries’ vaccine coverage was achieved using four different vaccine deployment strategies.
Results:
Among children aged 6–23 months, influenza vaccination averted an estimated cumulative 2,161 hospitalizations, 81,907 medically-attended illnesses, and 126,987 overall illnesses during the study period, with a prevented fraction ranging from 0.3 % to 12.5 %. Among pregnant women, influenza vaccination averted an estimated cumulative 173 hospitalizations, 6,122 medically attended illnesses, and 16,412 overall illnesses, with a prevented fraction ranging from 0.2 % to 10.9 %. Compared to an influenza vaccine campaign with equal vaccine distribution during March—June, scenarios in which total cumulative coverage was achieved in March and April consistently resulted in the greatest increase in averted illness (23 %−3,129 % increase among young children and 22 %−3,260 % increase among pregnant women).
Discussion:
Influenza vaccination campaigns in El Salvador, Panama, and Peru conducted between 2011 and 2018 prevented hundreds to thousands of influenza-associated hospitalizations and illnesses in young children and pregnant women. Existing vaccination programs could prevent additional illnesses, using the same number of vaccines, by achieving the highest possible coverage within the first two months of an influenza vaccine campaign.
Keywords: Influenza epidemiology, Influenza vaccine, Averted illness, Vaccine effectiveness, Compartment model
1. Introduction
Influenza disease burden estimation is a strategic priority under the World Health Organization’s (WHO) Pandemic Influenza Preparedness Partnership Contribution High-Level Implementation Plans [1–3], and is important for evidence-informed decision-making about prevention strategies, including vaccination. To support this global priority, in 2015, WHO published A Manual for Estimating Disease Burden Associated with Seasonal Influenza [4] that is designed for use with sentinel surveillance data or hospital discharge data. Since the manual’s publication, there has been considerable progress in influenza disease burden estimation, particularly in low- and middle-income countries [5]. However, rates and absolute counts of influenza-associated disease and mortality provide only partial information to support interventions. Expressing disease burden in terms of the burden averted or potentially averted through available interventions (e.g., vaccination) can provide more direct evidence to support decisions about vaccine introduction, expansion, and timing for greatest impact.
To that end, the WHO Burden of Disease manual is being updated to include a chapter about how to estimate the disease burden averted through influenza vaccination [4]. In countries where influenza vaccination programs are already in place, estimates of the burden averted will demonstrate the value of existing vaccination programs and highlight ways that changes in timing and deployment of vaccines could lead to additional reductions in disease burden. It also provides valuable information to conduct economic evaluations. Where influenza vaccination programs are not in place, estimates of the burden that could have been averted through vaccination can inform decision-making about the potential value of vaccine introduction. Consequently, WHO, Pan American Health Organization (PAHO), and the United States Centers for Disease Control and Prevention (U.S. CDC) collaborated to develop a Microsoft Excel-based tool and R-Shiny App [6] to complement the chapter and support such vaccine impact analyses.
The objective of this evaluation was to pilot the chapter’s methods and accompanying tools by estimating the burden of influenza disease averted through existing influenza vaccination programs for pregnant women and children aged 6–23 months in El Salvador, Panama, and Peru, using available influenza surveillance data and data from cohort studies from multiple influenza seasons. Additionally, these countries have seasonal influenza vaccination campaigns that target young children (aged 6–23 months), pregnant women, individuals with chronic illness, healthcare workers, and older adults (aged ≥ 65 years) [7]. These estimates of influenza illnesses and hospitalizations averted from established influenza vaccine campaigns or with modified campaigns can help guide governments, non-governmental organizations, and policy makers to implement, adjust, and communicate the value of influenza vaccination programs in target populations.
2. Methods
2.1. Data inputs
The evaluation period spanned the 2011–2018 influenza seasons. In El Salvador and Panama, the evaluation period was 2015–2017 for children 6–23 months of age and pregnant women. In Peru, the evaluation period was 2011–2015 for children 6–23 months of age and 2017–2018 for pregnant women.
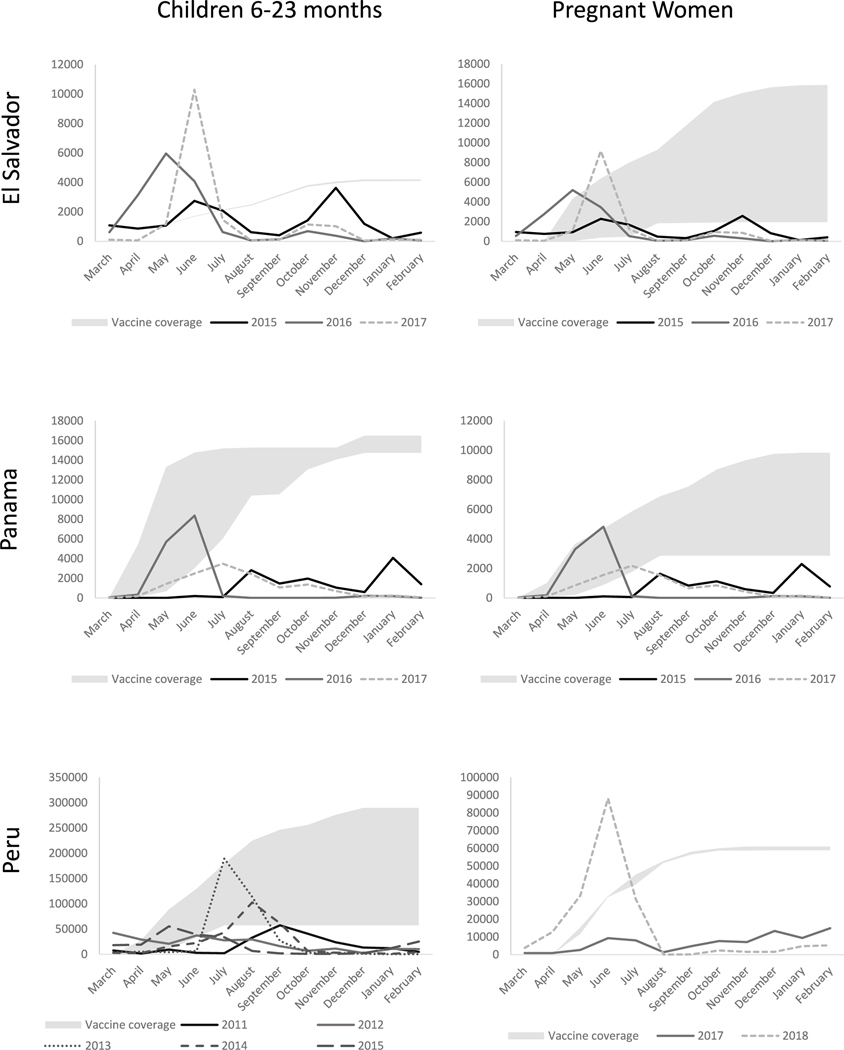
Data inputs for each country, target population, and year are described in Table 1. Vaccine effectiveness estimates and vaccine coverage were derived from published cohort studies in target populations and countries, when available, and from other target population-specific literature when unavailable [9–12]. The monthly number of hospitalizations and multipliers to estimate the number of individuals hospitalized with influenza from the number of people not hospitalized were estimated using data available in FluNet [16] and influenza incidence rates from published cohorts [11,12,14], using methods described in the WHO Burden of Disease manual [4] (Supplemental Fig. 1). Briefly, to estimate the timing of influenza incidence (Fig. 1), we calculated the monthly proportion of annual influenza cases by dividing the number of cases reported in FluNet in a given month by the total number of cases in the year. We then estimated the number of hospitalized influenza illnesses by month by multiplying the monthly proportion of annual cases by respective countries’ influenza-associated hospitalization incidence rate, and susceptible target population size, estimated as those who have not been previously infected or effectively vaccinated. A similar approach was used to estimate the number of outpatient influenza illnesses by month, using respective countries’ influenza incidence rates. Monthly hospitalized illnesses and outpatient illnesses were summed to estimate monthly medically-attended illnesses. We used a multiplier to estimate the number of influenza-associated illnesses that were not medically attended [14]. The number of outpatient illnesses and non-medically attended illnesses were summed to estimate the number of non-hospitalized illnesses (i.e., symptomatic cases without hospitalization). The country- and population-specific burden of disease estimates were used to generate ratios of non-hospitalized influenza illnesses to hospitalized illnesses for each season under investigation.
Table 1.
Model input variables and their data sources per country, population, and year.
| Variable | Children 6–23 months |
Pregnant women |
||
|---|---|---|---|---|
| Input | Data source | Input | Data source | |
|
| ||||
|
El Salvador
Population size |
2015: 214,172 2016: 211,886 2017: 208,175 |
[8] - Estimated as 3/8 of the population of children aged 0–4 | 2015: 92,170 2016: 92,170 2017: 92,170 |
[8] - Estimate from live births (2018) |
| Vaccine effectiveness | 53 % (28 %−70 %) | [9] | 22 % (−64.1 % to 62.9 %) | [10] |
| Vaccine coverage | 2015: 3.7 % 2016: 3.7 % 2017: 3.7 % |
INFLUMIKA cohort, unpublished data | 2015: 78.3 % 2016: 11.1 % 2017: 9.6 % |
INFLUMIKA cohort, unpublished data |
| Influenza incidence | 4.9 per 100 PY | [11] | 5.7 per 100 PY | [12] |
| Hospitalization incidence | 0.10 per 100 PY | [11] | 0.20 per 100 PY | [13] - Peru site-specific estimate |
| Proportion non-medically attended illness | 0.355 | [14] - Estimate from children < 2 | 0.627 | [14] - Estimate from adults aged 18–49 |
| Hospitalization multiplier | 76.52 | # non-hospitalized illnesses / hospitalized illnesses | 76.59 | # non-hospitalized illnesses / hospitalized illnesses |
|
Panama
Population size |
2015: 145,702 2016: 146,544 2017: 146,822 |
[8] - Estimated as 3/8 of the population of children aged 0–4 | 2015: 75,901 2016: 75,184 2017: 76,166 |
[8] - Estimate from live births |
| Vaccine effectiveness | 53 % (28 %−70 %) | [9] | 22 % (−64.1 % to 62.9 %) | [10] |
| Vaccine coverage | 2015: 19.1 % 2016: 21.2 % 2017: 19.6 % |
INFLUMIKA cohort, unpublished data | 2015: 58.9 % 2016: 31.1 % 2017: 17.0 % |
INFLUMIKA cohort, unpublished data |
| Influenza incidence | 6.80 per 100 PY | [11] | 4.30 per 100 PY | [12] |
| Hospitalization incidence | 0.10 per 100 PY | [11] | 0.20 per 100 PY | [13] - Peru site-specific estimate |
| Proportion non-medically attended illness | 0.355 | [14] - Estimate from children < 2 | 0.627 | [14] - Estimate from adults aged 18–49 |
| Hospitalization multiplier | 105.98 | # non-hospitalized illnesses / hospitalized illnesses | 58.19 | # non-hospitalized illnesses / hospitalized illnesses |
|
Peru
Population size |
2011: 1,143,371 2012: 1,135,911 2013: 1,126,155 2014: 1,114,653 2015: 1,102,017 |
[8] - Estimated as 3/8 of the population of children aged 0–4 | 2017: 610,316 2018: 601,781 |
[15] - Estimate from live births |
| Vaccine effectiveness | 53 % (28 %−70 %) | [9] | 22 % (− 64.1 % to 62.9 %) | [10] |
| Vaccine coverage | 2011: 34 % 2012: 48.1 % 2013: 27.1 % 2014: 17.6 % 2015: 9.8 % |
Peru Community Cohort, unpublished data | 2017: 45.4 % 2018: 44.4 % |
PRIME cohort, unpublished data |
| Influenza incidence | 2011: 14.66 per 100 PY 2012: 17.37 per 100 PY 2013: 24.21 per 100 PY 2014: 18.12 per 100 PY 2015: 13.46 per 100 PY |
Peru Community Cohort, unpublished data | 2017: 5.51 per 100 PY 2018: 13.32 per 100 PY |
[13] - Peru site-specific estimate |
| Hospitalization incidence | 0.48 per 100 PY | [14] - Estimate from children < 2 | 0.20 per 100 PY | [13] - Peru site-specific estimate |
| Proportion non-medically attended illness | 0.355 | [14] – Estimate from children < 2 | 0.627 | [14] - Estimate from adults aged 18–49 |
| Hospitalization multiplier | 2011: 47.92 2012: 56.67 2013: 78.76 2014: 59.06 2015: 44.03 |
# non-hospitalized illnesses / hospitalized illnesses | 2017: 74.07 2018: 180.23 |
# non-hospitalized illnesses / hospitalized illnesses |
PY = person-year.
Fig. 1.
Influenza illness and vaccine coverage among children 6–23 months and pregnant women, El Salvador, Panama, Peru, 2011–2018 Vaccine coverage is the range of cumulative vaccine coverage (number of target population vaccinated) during analytical period; lines indicate the overall number of influenza illnesses in the target population during specified year.
2.2. Base model
The mathematical model of influenza vaccine impact is based on a static compartmental model derived from Tokars et al [17] and requires estimates of influenza-associated disease burden, multipliers for non-hospitalized illnesses and medically-attended illnesses, national vaccination coverage, demographic data, and vaccine effectiveness. Methods are described in detail in the WHO Burden of Disease Manual [4] and were operationalized using the accompanying MS Excel Tool and R Shiny App. Briefly, to estimate the impact of countries’ influenza vaccination campaigns, the model compares the estimate of the burden of disease averted through an observed/actual vaccination scenario with a hypothetical modeled scenario of the burden of disease that would have occurred in the absence of a vaccination campaign. To estimate the number of influenza-associated events (i.e., non-hospitalized illnesses, hospitalized illnesses, and medically attended illnesses) in a given month, the model multiplies the influenza incidence rate by the target population susceptible to influenza virus infection, again estimated as those who have not been previously infected or effectively vaccinated. In the hypothetical absence of vaccination, the number of susceptible individuals is defined as those in a given month who have not previously been infected.
Additionally, the model estimates the number of individuals needed to vaccinate (NNV) to prevent an influenza-associated event, calculated as the reciprocal of the risk reduction between the unvaccinated population and the vaccinated population. It also estimates the prevented fraction, estimated as the number of events avoided among the vaccinated population divided by the estimated number of events that would have occurred in the absence of a vaccination program.
To estimate the 95 % confidence intervals (CIs) for averted illnesses (hospitalized, non-hospitalized, and medically attended), prevented fraction, and NNV, we used an R script to perform Montecarlo simulations with 5000 iterations [18]. The total number of hospitalizations, non-hospitalizations and medically attended illnesses were each simulated using a Poisson distribution to estimate simulated multipliers. The monthly distribution of the burden of disease was maintained. Vaccine coverage was simulated using a binomial distribution, maintaining the monthly distribution. Vaccine effectiveness was simulated using a beta distribution, restricting the lower bound used for generating the shape parameters to values above zero [19].
All modelled countries use the Southern Hemisphere influenza vaccine formulation [20], which is typically available as early as March, two months prior to the start of the typical influenza season in May [21,22]. Therefore, for methodological consistency across countries and years, and as to not account for vaccination in months the vaccine is not yet available, the 12 months of the base model spanned from March until February of the following year (e.g., March 2015–February 2016).
2.3. Additional vaccine deployment scenarios
In addition to the base model, we evaluated changes in the burden of disease averted using countries’ actual annual coverage but administered using four different vaccine deployment strategies. In each scenario, vaccine campaigns began in March. Briefly, scenario one reflected equal distribution of the annual cumulative coverage over the first four months of the influenza vaccine campaign (e.g., March, April, May, June), whereas scenarios two through four had increasingly greater coverage in earlier months. The four different scenarios are described in Table 2.
Table 2.
Description of scenarios modelling different vaccine deployment strategies.
| Scenario | Distribution of annual coverage | Example: Annual cumulative coverage is 20 % of target population |
|||
|---|---|---|---|---|---|
| March* | April | May | June | ||
|
| |||||
| Scenario 1 | Equal distribution across all four months of the vaccine campaign (March-June) | 5 % | 5 % | 5 % | 5 % |
| Scenario 2 | 50 % of the annual cumulative coverage is achieved in March, 25 % in April, 25 % in May | 10 % | 5 % | 5 % | 0 % |
| Scenario 3 | Equal distribution in March and April | 10 % | 10 % | 0 % | 0 % |
| Scenario 4 | 75 % of the annual cumulative coverage is achieved in March, 25 % April | 15 % | 5 % | 0 % | 0 % |
The vaccine campaign begins in March, two months prior to the start of a typical Southern Hemisphere influenza season and when seasonal vaccines are typically made available.
These scenarios were applied to each country, target population, and influenza season under investigation. Estimates of the prevented fraction, hospitalizations averted, and overall illnesses (defined as the sum of all hospitalized and non-hospitalized illnesses) averted were calculated for each scenario with an estimate of the percent change from the respective base scenario result (calculated as [Scenario-Base]/Base).
All calculations were performed in Microsoft Office Excel and R version 4.3.0, per manual and tool instructions.
2.4. Ethics considerations
This project was reviewed by the U.S. Centers for Disease Control and Prevention, WHO, and PAHO and determined to not constitute research with human subjects. Data from the Peru Community Cohort and the PRIME cohort came from protocols approved by the NAMRU-SOUTH Institutional Review Board (Protocols NMRCD.2009.0005 and NAMRU6.2016.0015, respectively).
3. Results
3.1. Children: base model
During the 2015–2017 influenza seasons in El Salvador, among a median annual population of 211,886 children aged 6–23 months, full vaccination (two doses of influenza vaccine at least four weeks apart if previously unvaccinated, or one single dose if previously vaccinated) with annual coverage of 3.7 % averted a total of 4 hospitalized illnesses, 220 medically attended illnesses, and 340 overall illnesses (Table 3). The greatest prevented fraction was observed in 2015 (1.1 %; 95 % CI = 0.6–1.4 %) and the lowest was observed in 2016 (0.3 %; 95 % CI = 0.2–0.5 %).
Table 3.
Estimated influenza illnesses averted through influenza vaccination among children aged 6–23 months, El Salvador (2015–2017), Panama (2015–2017), Peru (2011–2015).
| Year | Prevented fraction1 | Hospitalized illnesses |
Medically-attended illnesses |
Overall illnesses2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # illnesses | # averted (95 % CI) | NNV (95 % CI) | # illnesses | averted (95 % CI) | NNV (95 % CI) | # illnesses | # averted (95 % CI) | NNV (95 % CI) | ||
|
| ||||||||||
|
El Salvador 2015 |
1.1 % (0.6 %, 1.4 %) | 205 | 2 (1, 3) | 1951 (1441, 3591) | 10,242 | 112 (60, 147) | 39 (30, 73) | 15,880 | 173 (96, 226) | 25 (19, 46) |
| 2016 | 0.3 % (0.2 %, 0.5 %) | 205 | 1 (0, 1) | 1943 (1338, 3168) | 10,254 | 35 (22, 47) | 39 (29, 61) | 15,898 | 54 (34, 74) | 25 (18, 40) |
| 2017 | 0.7 % (0.4 %, 1.0 %) | 202 | 1 (1, 2) | 1928 (1336, 3227) | 10,113 | 73 (43, 101) | 39 (28, 66) | 15,680 | 113 (66, 156) | 25 (18, 43) |
|
Panama 2015 |
8.5 % (5.4 %, 12.0 %) | 128 | 12 (7, 18) | 1971 (1332, 3497) | 8,804 | 821 (508, 1199) | 29 (20, 47) | 13,649 | 1273 (784, 1862) | 18 (13, 30) |
| 2016 | 0.7 % (0.4 %, 1.0 %) | 141 | 1 (1, 1) | 1944 (1380, 3372) | 9,745 | 70 (37, 94) | 28 (21, 51) | 15,109 | 108 (57, 146) | 18 (14, 33) |
| 2017 Peru 2011 |
9.4 % (5.1 %, 12.3 %) | 127 | 13 (7, 19) | 1973 (1364, 3829) | 8,780 | 907 (474, 1220) | 29 (21, 55) | 13,612 | 1406 (730, 1927) | 18 (13, 35) |
| 14.8 % (8.7 %, 20.4 %) | 4243 | 734 (404, 1089) | 433 (295, 781) | 133,859 | 23,173 (12748, 34355) | 14 (9, 25) | 207,534 | 35,927 (19757, 53191) | 9 (6, 16) | |
| 2012 | 12.5 % (7.4 %, 18.4 %) | 4219 | 603 (339, 952) | 444 (297, 754) | 156,941 | 22,419 (12552, 35261) | 12 (8, 20) | 243,319 | 34,759 (19486, 54637) | 8 (5, 13) |
| 2013 | 7.5 % (4.7 %, 10.4 %) | 4414 | 358 (219, 533) | 445 (302, 724) | 227,064 | 18,441 (11284, 26795) | 9 (6, 14) | 352,037 | 28,590 (17540, 41627) | 6 (4, 9) |
| 2014 | 6.9 % (4.4 %, 9.7 %) | 4461 | 333 (206, 489) | 439 (299, 708) | 172,823 | 12,891 (8008, 18562) | 11 (8, 18) | 267,943 | 19,986 (12411, 28850) | 7 (5, 12) |
| 2015 | 2.1 % (1.2 %, 2.9 %) | 4727 | 102 (58, 145) | 431 (307, 745) | 137,307 | 2965 (1719, 4141) | 15 (11, 25) | 212,879 | 4596 (2665, 6460) | 10 (7, 16) |
CI = confidence interval; NNV = number needed to vaccinate to prevent one influenza illness; overall illness is defined as all influenza illnesses (both medically-attended and non-medically-attended).
Prevented fraction calculated as the number of events avoided among the vaccinated population divided by the estimated number of events that would have occurred in the absence of a vaccination program.
Overall illnesses include medically and non-medically attended illnesses.
Additionally, during the 2015–2017 influenza seasons in Panama, among a median annual population of 146,544 children aged 6–23 months, vaccination with annual coverage ranging from 19.2 to 21.2 % averted a total of 26 hospitalized illnesses, 1,798 medically attended illnesses, and 2,787 overall illnesses. The greatest prevented fraction was observed in 2017 (9.4 %; 95 % CI = 5.1–12.3 %) and the lowest was observed in 2016 (0.7 %; 95 % CI = 0.4–1.0 %).
During the 2011–2015 influenza seasons in Peru, among a median annual population of 1,135,911 children aged 6–23 months, vaccination with annual coverage ranging from 9.8 to 48.1 % averted a total of 2,130 hospitalized illnesses, 79,889 medically attended illnesses, and 123,859 overall illnesses. The greatest prevented fraction was observed in 2011 (14.8 %; 95 % CI = 8.7–20.4 %) and the lowest was observed in 2015 (2.1 %; 95 % CI = 1.2–2.9 %).
3.2. Children: additional vaccine deployment scenarios
All modelled scenarios showed an increase in the prevented fraction and number of averted illnesses compared with the base scenario. In El Salvador, scenario four (March-April vaccination period with greater coverage distribution in March) generated the greatest improvement from the base scenario across all years (138–865 % increase). For Panama, scenarios three (March-April vaccination period with equal coverage distribution) and four generated the greatest improvement from the base scenario, with scenario four (23–3,129 % annual increase from base) performing slightly better than scenario three (22–3,112 % increase) (Table 4). Finally, in Peru, scenarios three (42–246 % increase) and four (42–256 % increase) again generated the greatest improvement from the base scenario, with scenario four performing slightly better than scenario three.
Table 4.
Estimated influenza illnesses averted through influenza vaccination among children aged 6–23 months under different vaccination scenarios, El Salvador (2015–2017), Panama (2015–2017), Peru (2011–2015).
| Scenario | Prevented fraction1 |
Hospitalizations averted |
Overall illnesses averted2 |
Percent change from base scenario |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | 1 | 2 | 3 | 4 | Base | 1 | 2 | 3 | 4 | Base | 1 | 2 | 3 | 4 | Base | 1 | 2 | 3 | |
|
| |||||||||||||||||||
|
El Salvador 2015 |
1.1 % | 2.2% | 2.4% | 2.5% | 2.5% | 2 | 5 | 5 | 5 | 5 | 173 | 350 | 394 | 404 | 412 | 102% | 127% | 133 % | 138% |
| 2016 | 0.3 % | 1.9% | 2.7% | 3.1 % | 3.2% | 1 | 4 | 6 | 6 | 7 | 54 | 307 | 437 | 496 | 525 | 464% | 703 % | 811 % | 865% |
| 2017 | 0.7% | 2.4% | 3.1 % | 3.2% | 3.2% | 1 | 5 | 6 | 7 | 7 | 113 | 383 | 497 | 508 | 508 | 240% | 341 % | 350% | 351 % |
|
Panama 2015 |
8.5% | 11.6% | 11.7% | 11.7% | 11.7% | 12 | 16 | 17 | 17 | 17 | 1273 | 1763 | 1773 | 1773 | 1773 | 38% | 39% | 39% | 39% |
| 2016 | 0.7% | 13.8 % | 18.7 % | 20.4 % | 20.5 % | 1 | 21 | 29 | 32 | 33 | 108 | 2248 | 3137 | 3472 | 3490 | 1980 % | 2802 % | 3112% | 3129% |
| 2017 | 9.4% | 9.5% | 10.9 % | 11.3 % | 11.4% | 13 | 13 | 15 | 16 | 16 | 1406 | 1421 | 1644 | 1720 | 1729 | 1 % | 17% | 22% | 23 % |
|
Peru 2011 |
14.8 % | 19.6 % | 19.9 % | 20.2% | 20.3 % | 734 | 1002 | 1025 | 1041 | 1043 | 3592 | 48,998 | 50,120 | 50,911 | 51,034 | 36% | 40% | 42% | 42% |
| 2012 | 12.5% | 25.4 % | 28.8 % | 29.6 % | 30.6 % | 603 | 1330 | 1545 | 1598 | 1663 | 3475 | 76,672 | 89,070 | 92,172 | 95,892 | 121 % | 156% | 165% | 176% |
| 2013 | 7.5% | 20.2 % | 20.4 % | 20.4 % | 20.5 % | 358 | 1038 | 1049 | 1051 | 1055 | 28,590 | 82,824 | 83,645 | 83,866 | 84,126 | 190% | 193 % | 193 % | 194% |
| 2014 | 6.9% | 10.8 % | 11.3 % | 11.5% | 11.5% | 333 | 527 | 554 | 564 | 565 | 1998 | 31,660 | 33,256 | 33,868 | 33,950 | 58% | 66% | 69% | 70% |
| 2015 | 2.1 % | 5.3 % | 6.5% | 7.1 % | 7.3 % | 102 | 261 | 323 | 354 | 363 | 4596 | 11,741 | 14,566 | 15,926 | 16,353 | 155% | 217% | 246% | 256% |
Scenario 1 = Equal distribution of annual cumulative coverage across all four months of the vaccine campaign (March-June); Scenario 2 = 50 % of the annual cumulative coverage achieved in March, 25 % in April, 25 % in May; Scenario 3 = equal distribution of annual cumulative coverage achieved in March and April; Scenario 4 = 75 % of the annual cumulative coverage is achieved in March, 25 % April
Prevented fraction calculated as the number of events avoided among the vaccinated population divided by the estimated number of events that would have occurred in the absence of a vaccination program.
Overall illnesses include medically and non-medically attended illnesses.
3.3. Pregnant women: base model
During the 2015–2017 influenza seasons in El Salvador, among a median annual population of 92,170 pregnant women, vaccination with annual coverage ranging from 9.6 to 77.1 % averted a total of 20 hospitalized illnesses, 570 medically attended illnesses, and 1,528 overall illnesses (Table 5). The greatest prevented fraction was observed in 2015 (9.5 %; 95 % CI = 2.2–31.5 %), the same year as for children, and the lowest was observed in 2016 (0.2 %; 95 % CI = 0.1–0.7 %).
Table 5.
Estimated influenza illnesses averted through influenza vaccination among pregnant women, El Salvador (2015–2017), Panama (2015–2017), and Peru (2017–2018).
| Year | Prevented fraction1 | Hospitalized illnesses |
Medically-attended illnesses |
Overall illnesses2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # illnesses | # averted (95 % Cl) | NNV (95 % Cl) | # illnesses | # averted (95 % Cl) | NNV (95 % Cl) | # illnesses | # averted (95 % Cl) | NNV (95 % Cl) | ||
|
| ||||||||||
|
El Salvador 2015 |
9.5 % (2.2 %, 31.5 %) | 159 | 17 (3, 73) | 2386 (625, 10944) | 4600 | 482 (102, 2124) | 82 (21, 375) | 12,331 | 1291 (271, 5670) | 31 (8, 140) |
| 2016 | 0.2 % (0.1 %, 0.7 %) | 177 | 0 (0, 1) | 2365 (813, 9850) | 5114 | 13 (3, 37) | 82 (28, 343) | 13,711 | 34 (8, 100) | 30 (11, 129) |
| 2017 | 1.5 % (0.3 %, 4.2 %) | 177 | 3 (1, 8) | 2329 (771, 10287) | 5131 | 76 (17, 226) | 80 (27, 352) | 13,756 | 203 (46, 604) | 30 (10, 133) |
|
Panama 2015 |
10.9 % (2.5 %, 32.1 %) | 131 | 16 (3, 64) | 2340 (601, 10963) | 2901 | 353 (75, 1377) | 106 (28, 491) | 7778 | 947 (202, 3677) | 40 (10, 184) |
| 2016 | 0.4 % (0.1 %, 1.3 %) | 148 | 1 (0, 2) | 2304 (760, 10210) | 3261 | 13 (3, 43) | 104 (35, 452) | 8742 | 36 (8, 114) | 39 (13, 171) |
| 2017 | 3.4 % (0.8 %, 9.9 %) | 143 | 5 (1, 16) | 2344 (737, 10025) | 3151 | 110 (26, 344) | 106 (34, 442) | 8449 | 295 (71, 927) | 40 (13, 166) |
|
Peru 2017 |
8.2 % (1.9 %, 24.7 %) | 1069 | 96 (21, 354) | 2381 (665, 10580) | 29,933 | 2685 (590, 9825) | 85 (24, 383) | 80,248 | 7198 (1590, 26317) | 32 (9, 142) |
| 2018 | 3.3 % (0.8 %, 11.0 %) | 1040 | 36 (8, 127) | 2542 (809, 11127) | 69,000 | 2390 (524, 8490) | 38 (12, 169) | 184,987 | 6408 (1409, 22842) | 14 (5, 63) |
CI = confidence interval; NNV = number needed to vaccinate; overall illness is defined as all influenza illnesses (both medically-attended and non-medically-attended).
Prevented fraction calculated as the number of events avoided among the vaccinated population divided by the estimated number of events that would have occurred in the absence of a vaccination program.
Overall illnesses include medically and non-medically attended illnesses.
Additionally, during the 2015–2017 influenza seasons in Panama, among a median annual population of 75,901 pregnant women, vaccination with annual coverage ranging from 19.0 to 66.5 % averted a total of 22 hospitalized illnesses, 477 medically attended illnesses, and 1,278 overall illnesses. The greatest prevented fraction was observed in 2015 (10.9 %; 95 % CI = 2.5–32.1 %) and the lowest was again observed in 2016 (0.4 %; 95 % CI = 0.1–1.3 %).
During the 2017 and 2018 influenza seasons in Peru, among a median annual population of 606,049 pregnant women, vaccination with annual coverage ranging from 44.4 to 45.4 % averted a total of 132 hospitalized illnesses, 5,075 medically attended illnesses, and 13,606 overall illnesses. Prevented fractions were 8.2 % (95 % CI = 1.9–24.7 %) in 2017 and 3.3 % (95 % CI = 0.8–11.0 %) in 2018.
3.4. Pregnant women: additional vaccine deployment scenarios
As with the scenarios for children, all modelled scenarios that try to optimize the timing of vaccination showed an increase in the prevented fraction and number of averted illnesses compared with the base scenario. In all three countries, scenario four generated the greatest improvement from the base scenario across all years, with an 89–1,657 % annual increase from base in El Salvador, a 22–3260 % annual increase from base in Panama, and a 42–389 % annual increase from base in Peru (Table 6). However, in El Salvador, scenario three performed similarly in 2017 (89 % increase for both scenarios three and four); in Panama, all scenarios performed equally in 2015 (41 % increase for all scenarios); and in Peru, scenario three performed similarly in 2017 (42 % increase for both scenarios three and four).
Table 6.
Estimated influenza illnesses averted through influenza vaccination among pregnant women under different vaccination scenarios, El Salvador (2015–2017), Panama (2015–2017), and Peru (2017–2018).
| Scenario | Prevented fraction1 |
Hospitalizations averted |
Overall illnesses averted2 |
Percent change from base scenario |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | 1 | 2 | 3 | 4 | Base | 1 | 2 | 3 | 4 | Base | 1 | 2 | 3 | 4 | Base | 1 | 2 | 3 | |
|
| |||||||||||||||||||
|
El Salvador 2015 |
9.5% | 19.0 % | 21.2% | 21.7% | 22.1 % | 17 | 35 | 40 | 41 | 42 | 1291 | 2736 | 3099 | 3184 | 3248 | 112% | 140% | 147% | 151 % |
| 2016 | 0.2% | 2.6% | 3.6% | 4.0% | 4.2% | 0 | 5 | 6 | 7 | 8 | 34 | 359 | 498 | 561 | 591 | 965% | 1379 % | 1567 % | 1657 % |
| 2017 | 1.5% | 1.9% | 2.7% | 2.7% | 2.7% | 3 | 3 | 5 | 5 | 5 | 203 | 267 | 374 | 384 | 384 | 32% | 84% | 89% | 89% |
|
Panama 2015 |
10.9 % | 14.9% | 15.0 % | 15.0 % | 15.0 % | 16 | 23 | 23 | 23 | 23 | 947 | 1332 | 1340 | 1340 | 1340 | 41 % | 41 % | 41 % | 41 % |
| 2016 | 0.4% | 8.5% | 11.6% | 12.7% | 12.8% | 1 | 13 | 18 | 20 | 20 | 36 | 779 | 1079 | 1191 | 1197 | 2087 % | 2929 % | 3243 % | 3260 % |
| 2017 | 3.4% | 3.4% | 3.9% | 4.1 % | 4.1 % | 5 | 5 | 6 | 6 | 6 | 295 | 298 | 343 | 358 | 360 | 1 % | 16% | 21 % | 22% |
|
Peru 2017 |
8.2% | 10.7 % | 11.3 % | 11.5% | 11.5% | 96 | 126 | 134 | 136 | 137 | 7198 | 9466 | 1008 | 1021 | 1025 | 32% | 40% | 42% | 42% |
| 2018 | 3.3 % | 11.5% | 14.5 % | 15.1 % | 15.3 % | 36 | 130 | 166 | 174 | 176 | 6408 | 2310 | 2952 | 3087 | 3133 | 261 % | 361 % | 382% | 389% |
Scenario 1 = Equal distribution of annual cumulative coverage across all four months of the vaccine campaign (March-June); Scenario 2 = 50 % of the annual cumulative coverage achieved in March, 25 % in April, 25 % in May; Scenario 3 = equal distribution of annual cumulative coverage achieved in March and April; Scenario 4 = 75 % of the annual cumulative coverage is achieved in March, 25 % April.
Prevented fraction calculated as the number of events avoided among the vaccinated population divided by the estimated number of events that would have occurred in the absence of a vaccination program.
Overall illnesses include medically and non-medically attended illnesses.
4. Discussion
With a newly available tool, public health decision makers can estimate what is often a challenge in public health — quantifying the number of illnesses and hospitalizations prevented by a public health intervention. Using this tool, we estimated that influenza vaccination campaigns in El Salvador, Panama, and Peru conducted between 2011 and 2018 made a large difference in key target populations, preventing hundreds to thousands of hospitalizations and tens of thousands of influenza illnesses in young children and pregnant women. Moreover, expressing vaccine impact in terms of the number and proportion of illnesses prevented by vaccination (i.e., averted illnesses and prevented fraction, respectively) might be more understandable messages to the general public than disease risk reduction from vaccination (i.e., estimates of vaccine effectiveness).
The tool also demonstrates how optimizing the timing and deployment of vaccine programs could prevent further illnesses and hospitalizations. While we could have modelled how increasing coverage would have increased the seasonal impact, as has been shown in prior analyses [23], we instead focused on demonstrating how changes using the same amount of vaccine doses in a different deployment strategy would impact the number of illnesses and hospitalizations prevented. These scenarios model a common reality to public health decision makers: with a fixed budget and constrained resources, when should countries start a vaccine campaign and how many people should be targeted each month to have the greatest impact in the target populations? In our scenarios for both young children and pregnant women, the largest improvements in the proportion of influenza cases prevented were estimated when vaccine campaigns achieved the highest possible coverage within the first two months after the Southern Hemisphere formulation typically becomes available (i.e., March and April), and approximately one to two months before the May start of a typical influenza season [21]. Similar scenario models have also demonstrated that focusing influenza vaccination during the early months of typical influenza virus circulation also prevented additional hospitalizations compared with models when vaccination occurred later, after influenza virus circulation had begun [22,24–26]. These types of modelled scenarios can help countries better utilize finite vaccine supplies and optimally plan and time procurement of influenza vaccines prior to seasonal campaigns.
These modelling efforts for two vaccine target populations in El Salvador, Panama, and Peru highlighted the benefit to modelling the impact of influenza vaccination, but also demonstrated gaps in routinely available data that are needed as inputs. The critical data inputs for the model include the influenza incidence rates, vaccine coverage, and vaccine effectiveness. As these inputs can vary widely from season to season, it is important for countries to try to model contemporary inputs to best understand the impact of their influenza vaccination campaigns. These three countries were chosen, in part, because influenza incidence data and vaccine coverage estimates were available from community cohort studies conducted for several years, and vaccine effectiveness estimates were available for overlapping years. Such specialized cohort studies are expensive and require substantial resources, and many countries may not have this level of data routinely available. Instead, most countries may need to leverage routine surveillance data or make assumptions that inputs for their country are represented by available data from other similar countries, as we did when assuming that the incidence of influenza-associated hospitalizations in pregnant women in El Salvador and Panama were similar to estimates observed in Peru or that Southern Hemisphere vaccine effectiveness estimates in young children in these countries was similar to the vaccine effectiveness estimated for Northern Hemisphere vaccines in the United States. Alternatively, countries could strive to fill selected data gaps by setting up local systems or participating in regional networks such as REVELAC-i [27] to capture information to estimate influenza disease burden, vaccine effectiveness, or vaccine coverage, if such data would motivate vaccine policy makers.
The models to estimate the impact of influenza vaccination and our implementation of the model in this pilot have limitations. First, the model is sensitive to its inputs, and we did not have all the necessary inputs for each specific year, country, and target population. When an input was not available for a specific stratum, we assumed that available inputs from elsewhere (e.g., other countries, other target groups, or other years) were similar. Relatedly, we used estimates of influenza disease burden and vaccine coverage from subnational cohorts, but these cohorts might not be representative of the target population at large [28]. Second, the model does not account for waning vaccine-induced immunity, which may be important to consider when modelling the impact of year-round vaccination versus seasonal vaccine campaigns or when modelling campaigns to vaccinate prior to seasonal influenza activity. Additionally, the model does not consider differential VE for severe versus non-severe influenza illness. Third, estimates of burden of influenza prevented by vaccinating pregnant women does not include protection conferred to infants younger than six months and therefore are an underestimation of the full impact of influenza vaccination [29]. Similarly, these models do not consider the possibility that influenza vaccines indirectly protect against infection among the unvaccinated through herd immunity [30]. Fourth, the confidence interval estimation restricted the distribution used for simulation to positive values, therefore estimations do not account for scenarios where VE or its variance may be negative.
In conclusion, we have demonstrated the use of a new tool developed by WHO, PAHO, and U.S. CDC to estimate the number of influenza-associated illnesses and hospitalizations prevented by seasonal influenza vaccination. Future work could build upon this model, adding costs associated with averted illnesses to further the relevance for policymaking and investment [31] and expanding the model to estimate averted deaths as well as the burden of disease averted in countries with year-round influenza circulation. However, in its current form, this model of prevented influenza burden, and the accompanying tools, can be useful for countries that are evaluating existing influenza vaccine programs or considering influenza vaccination strategies.
5. Disclaimer
The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the of the U.S. Centers for Disease Control and Prevention, U.S. Department of the Navy, U.S. Government, the World Health Organization, or the Pan American Health Organization. ANC, RG, FSD, JDC, KEL, EAB, MR and YT are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. § 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by an employee of the U.S. Government as part of that person’s official duties.
Supplementary Material
Acknowledgements
Thank you to Dr. Ashley Fowlkes (U.S. CDC) for review of model structure and inputs. The authors thank the Pregnancy and Influenza Multinational Epidemiologic (PRIME) Study investigators in Peru and Abt Associates for contributing data about influenza incidence rates among pregnant women in Peru. Thank you to Dr. César Munayco-Escate from the Peruvian Centers for Disease and Control, and participants and collaborators of the PRIME cohort in Hospital Nacional Docente Madre Niño San Bartolomé, Instituto Nacional Materno Perinatal and Hospital Nacional Arzobispo Loayza as well as to the Peru Community Cohort participants and Peruvian Ministry of Health collaborators in Cusco, Puerto Maldonado, Tumbes and Lima. Finally, we thank the International Reagent Resource (https://www.internationalreagentresource.org/) for providing reagents for influenza testing in all three cohorts.
Funding
This work was supported in part by a grant from the U.S. Centers for Disease Control and Prevention (CDC) through a cooperative agreement (Award # NU51IP000940) with the Pan American Health Organization (PAHO). The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC, PAHO, or WHO.
Footnotes
CRediT authorship contribution statement
Anna N. Chard: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Chiedza Machingaidze: Writing – review & editing, Validation, Software, Methodology, Conceptualization. Sergio Loayza: Writing – review & editing, Validation, Methodology, Conceptualization. Radhika Gharpure: Writing – original draft. Francisco Nogareda: Writing – review & editing. Rosalba González: Writing – review & editing, Data curation. Rhina Domínguez: Writing – review & editing, Data curation. Yeny O Tinoco: Data curation, Writing – review & editing. Fatimah S. Dawood: Data curation, Writing – review & editing. Joseph Daniel Carreon: Data curation, Writing – review & editing. Kathryn E. Lafond: Writing – review & editing. Jorge Jara: Writing – review & editing. Eduardo Azziz-Baumgartner: Writing – review & editing, Supervision, Data curation, Conceptualization. Vanessa Cozza: Writing – review & editing. Paula Couto: Writing – review & editing, Supervision, Methodology, Conceptualization. Melissa A. Rolfes: Writing – original draft, Methodology. Stefano Tempia: Supervision, Methodology, Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2024.04.007.
Data availability
Data will be made available on request.
References
- [1].World Health Organization, Pandemic Influenza Preparedness Framework: Partnership contribution preparedness high-level implementation plan II 2018–2023. 2021: Geneva. [Google Scholar]
- [2].World Health Organization, Pandemic Influenza Preparedness Framework Partnership Contribution Implementation Plan 2013 – 2016. 2014. [Google Scholar]
- [3].World Health Organization, Pandemic Influenza Preparedness Framework: Partnership Contribution High-Level Implementation Plan III 2024–2030. 2023: Geneva. [Google Scholar]
- [4].World Health Organization, A manual for estimating disease burden associated with seasonal influenza. 2015. [Google Scholar]
- [5].Lee VJ, et al. Advances in measuring influenza burden of disease. Influenza Other Respir Viruses 2018;12(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].World Health Organization. Influenza-Burden-Burden-Averted. 2024. March 14 2024; Available from: https://github.com/ChiedzaWHO/Influenza-Burden-Burden-Averted.
- [7].Ropero-Alvarez AM, et al. Expansion of seasonal influenza vaccination in the Americas. BMC Public Health 2009;9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].United Nations. UNdata. 2022. [cited 2023 January 11]; Available from: https://data.un.org/Default.aspx.
- [9].Chung JR, et al. Patterns of influenza vaccination and vaccine effectiveness among young US children who receive outpatient Care for Acute Respiratory Tract Illness. JAMA Pediatr 2020;174(7):705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Owusu D, et al. Effectiveness of maternal influenza vaccination in Peru PRIME cohort. open forum. Infect Dis 2023;10(2):p. ofad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Azziz-Baumgartner E, et al. , Incidence of respiratory virus illness and hospitalizations in a Panama and El Salvador birth cohort, 2014–2018. Lancet Reg Health Am, 2022. 13: p. None. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Azziz-Baumgartner E, et al. Incidence of influenza and other respiratory viruses among pregnant women: a multi-country, multiyear cohort. Int J Gynaecol Obstet 2022;158(2):359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dawood FS, et al. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis 2021;21(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tinoco YO, et al. Burden of influenza in 4 ecologically distinct regions of Peru: household active surveillance of a community cohort, 2009–2015. Clin Infect Dis 2017;65(9):1532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Peru National Institute of Statistics and Informatics. Vital Statistics. [cited 2023 January 11]; Available from: https://www.inei.gob.pe/estadisticas/indice-tematico/poblacion-y-vivienda/.
- [16].World Health Organization. FluNet. 2023. [cited 2023 21 June]; Available from: https://www.who.int/tools/flunet.
- [17].Tokars JI, et al. An evaluation and update of methods for estimating the number of influenza cases averted by vaccination in the United States. Vaccine 2018;36(48): 7331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kostova D, et al. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PLoS One 2013;8(6):e66312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Branscum AJ, Gardner IA, Johnson WO. Estimation of diagnostic-test sensitivity and specificity through bayesian modeling. Prev Vet Med 2005;68(2–4):145–63. [DOI] [PubMed] [Google Scholar]
- [20].Pan-American Health Organization. Influenza Vaccine. [cited 2023 17 May]; Available from: https://www.paho.org/en/influenza-vaccine.
- [21].Cox N. Influenza seasonality: timing and formulation of vaccines. Bull World Health Organ 2014;92(5):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Durand LO, et al. Timing of influenza epidemics and vaccines in the American tropics, 2002–2008, 2011–2014. Influenza Other Respir Viruses 2016;10(3):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hughes MM, et al. Projected population benefit of increased effectiveness and coverage of influenza vaccination on influenza burden in the United States. Clin Infect Dis 2020;70(12):2496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferdinands JM, et al. Waning of influenza vaccine protection: exploring the trade-offs of changes in vaccination timing among older adults. Clin Infect Dis 2020;70 (8):1550–9. [DOI] [PubMed] [Google Scholar]
- [25].Costantino V, Trent M, MacIntyre CR. Modelling of optimal timing for influenza vaccination as a function of intraseasonal waning of immunity and vaccine coverage. Vaccine 2019;37(44):6768–75. [DOI] [PubMed] [Google Scholar]
- [26].Lutz CS, et al. Estimating the number of averted illnesses and deaths as a result of vaccination against an influenza pandemic in nine low- and middle-income countries. Vaccine 2021;39(30):4219–30. [DOI] [PubMed] [Google Scholar]
- [27].Pan American Health Organization. Network for the Evaluation of Vaccine Effectiveness in Latin America and the Caribbean - influenza, (REVELAC-i). January 20, 2023]; Available from: https://www.paho.org/en/network-evaluation-vaccine-effectiveness-latin-america-and-caribbean-influenza-revelac-i.
- [28].Ropero-Álvarez AM, et al. Influenza vaccination in the Americas: Progress and challenges after the 2009 A(H1N1) influenza pandemic. Hum Vaccin Immunother 2016;12(8):2206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Azziz-Baumgartner E, Grohskopf L, Patel M. Realizing the potential of maternal influenza vaccination. JAMA 2021;325(22):2257–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eichner M, et al. Direct and indirect effects of influenza vaccination. BMC Infect Dis 2017;17(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McMorrow ML, et al. Prioritization of risk groups for influenza vaccination in resource limited settings - a case study from South Africa. Vaccine 2019;37(1): 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.