Abstract
Previous research has reported high occult nodal metastases rates for T3/T4 mucoepidermoid carcinoma (MEC) of the oropharynx (OP) and oral cavity (OC). Our study evaluates if there is a benefit of neck dissection (ND) in these patients. The 2004–2016 National Cancer Database was queried for cases of adult MEC of the OC and OP. Patients with clinical T3/T4 disease were included while those with metastatic disease were excluded. Patients were divided into two cohorts: those treated with and without ND. Univariate chi-square, Kaplan-Meier, and multivariable Cox regression analyses were implemented. A total of 243 patients met inclusion criteria, of which 79 (32.5%) underwent ND. The majority of patients were less than 60 years old (60.1%), White (76.2%), and male (53.5%). 92 (37.9%) patients had clinically node-positive (cN+) disease. ND patients had higher rates of cN + disease (53.2% vs. 30.5%, p = 0.002). Of patients undergoing ND, 35 (44.3%) had cN0 disease while 42 (53.2%) had cN + disease. ND patients more commonly had grade III/IV tumors (45.1% vs. 23.4%, p = 0.002). Upon examination of dissected nodes, 20.3% of cN0 patients undergoing ND were found to have occult nodal metastases. There was no significant difference in 5-year overall survival between patients with and without ND (61.8% vs. 53.6%, p = 0.610), even on multivariable Cox analysis (hazard ratio: 1.52, 95% confidence interval: 0.73–3.18, p = 0.269). Our study found patients with cN0 MEC of the OC and OP have a high rate (20.3%) of occult nodal metastasis. In this cohort, patients with ND were not found to have improved survival, possibly due to statistical underpowering. Further research is needed to evaluate the indications and benefit of ND for this rare tumor presentation.
Keywords: Mucoepidermoid Carcinoma, Oral Cavity, Oropharynx, NCDB, Survival, Neck Dissection
Keypoints
Previous research has reported high occult nodal metastases rates for T3/T4 mucoepidermoid carcinoma (MEC) of the oropharynx (OP) and oral cavity (OC).
Neck dissection patients had higher rates of cN + disease and more commonly had grade III/IV tumors.
Patients with MEC of the OP and OC have a high rate of occult nodal metastasis, suggesting neck dissection should be given strong consideration for these patients.
There was no significant difference in 5-year overall survival between patients treated with and without neck dissection.
Introduction
Salivary gland cancers (SGCs) represent 3–6% of all head and neck malignancies [1, 2]. Approximately 20% of SGCs arise in the minor salivary glands which are located in the upper aerodigestive tract and sinonasal cavity, comprising sites such as the palate and oral mucosa [2–4]. Tumors arising from these minor glands have a higher likelihood of malignancy than tumors of the major salivary glands [2, 4, 5]. While several histological subtypes of SGCs exist, mucoepidermoid carcinoma (MEC) is the most common minor salivary gland cancer (MiSGC), accounting for 16.5-45% of reported cases [4, 6].
Neck dissection is a potential treatment option performed to prophylactically remove lymph nodes that may be harboring cervical lymph node metastases [7–9]. Occult nodal metastasis in SGC occurs in 8–50% of patients, with high-grade histological subtypes having the highest incidence [10–12]. In the first report of occult nodal metastasis rates of MEC of the oral cavity (OC) and oropharynx (OP), Ellis et al. similarly found higher rates of occult disease in cT3/T4 tumors, suggesting potential benefit of prophylactic neck dissection in these patients [13]. Recent studies evaluating the benefit of elective neck dissection in SGC have recommended elective neck treatment for patients with high-grade histology, cT3/T4 tumors, or carcinoma arising from the submandibular, sublingual, or minor salivary glands [10–12, 14–16]. However, these studies often encompass a variety of histological subtypes and primary sites, limiting disease-specific information [10, 11]. The purpose of this study is to evaluate the benefit of neck dissection on survival in cT3/T4 MEC of the OC and OP. Given the low incidence of these tumors, our study leverages the National Cancer Database (NCDB) to ascertain this question.
Methods
The 2004–2016 NCDB was queried for all cases of adult MEC of the OC and OP. International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) codes were used to identify cases. MEC was identified with the following histology code: 8430. Primary sites of the OC and OP that were included were: C01.9, C02.2, C02.3, C02.4, C03.0, C03.1, C03.9, C04.0, C04.1, C04.8, C04.9, C05.0, C05,1, C05.2, C06.0, C06.1, C06.2, C09.0, C09.1, C09.8, C09.9, C10.2, C10.3, and C10.9. Only patients with cT3/T4 disease were included in analysis. Patients with multiple cancer diagnoses, metastatic disease, or unknown scope of regional lymph node surgery were excluded. As data from the NCDB is fully de-identified, our study was considered exempt from review by the Rutgers New Jersey Medical School Institutional Review Board.
Variables included in the analysis were age, race, sex, insurance status, facility type, primary site (OC or OP), clinical tumor (cT), clinical nodal (cN) stage, pathologic nodal (pN) stage, neck dissection status, and treatment. Neck dissection was defined as patients that underwent regional lymph node surgery with at least 18 lymph nodes examined. Age was reported as a binary variable (< 60 and 60 + years) for chi-square analysis and as a continuous variable for regression analysis. Insurance status groups were private insurance, Medicaid, Medicare, other government insurance, and no insurance. Facility types included comprehensive community cancer programs, community cancer program, academic/research program, and integrated network cancer program; these categories are defined by the NCDB. Surgical margin status was defined as positive or negative. High-grade tumors were defined as poorly differentiated or undifferentiated, as previously described [13, 17]. Treatment modalities evaluated were no treatment, surgery only, radiotherapy only, chemotherapy only, surgery with adjuvant radiation, and surgery with adjuvant chemoradiotherapy. Patients with other or unclear treatment regimens were not excluded from descriptive analyses but were excluded in relevant regression analyses.
Patients undergoing neck dissection were compared with those not undergoing neck dissection with the chi-square test and independent samples t test for categorical and continuous variables, respectively. The primary outcome for this study was overall survival (OS). Kaplan-Meier and multivariable Cox regression proportional hazards model were implemented for univariate and multivariate survival analyses, respectively. Statistical significance was set at p < 0.05. All statistical analyses were performed utilizing SPSS version 26 (IBM Corp, Armonk, New York).
Results
A total of 243 patients satisfied inclusion criteria, of which 79 (32.5%) underwent neck dissection (Table 1). The majority of patients were less than 60 years old (60.1%), White (76.2%), male (53.5%), had private insurance (51.7%), oral cavity disease (60.9%), and underwent treatment at an academic center (60.6%). 146 (60.1%) patients had cN0 disease and 92 (37.9%) had cN + disease. Neck dissection was performed at academic institutions more frequently than non-academic institutions (73.2% vs. 53.8%, p = 0.014).
Table 1.
Patient Clinical Characteristics
| No ND | ND | Total | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 164 | 67.5 | 79 | 32.5 | 243 | ||
| Age | 0.323 | ||||||
| Mean, years (SD) | 56.3 (17.6) | 55.5 (13.3) | 56.0 (16.3) | 0.732 | |||
| <60 | 95 | 57.9 | 51 | 64.6 | 146 | 60.1 | |
| 60+ | 69 | 42.1 | 28 | 35.4 | 97 | 39.9 | |
| Race | 0.013 | ||||||
| White | 120 | 74.1 | 62 | 80.5 | 182 | 76.2 | |
| Black | 40 | 24.7 | 10 | 13 | 50 | 20.9 | |
| Other | 2 | 1.2 | 5 | 6.5 | 7 | 2.9 | |
| Sex | 0.840 | ||||||
| Male | 87 | 53 | 43 | 54.4 | 130 | 53.5 | |
| Female | 77 | 47 | 36 | 45.6 | 113 | 46.5 | |
| Primary Site | 0.170 | ||||||
| Oral Cavity | 95 | 57.9 | 53 | 67.1 | 148 | 60.9 | |
| Oropharynx | 69 | 42.1 | 26 | 32.9 | 95 | 39.1 | |
| Insurance Type | 0.524 | ||||||
| Private Insurance | 82 | 51.9 | 40 | 51.3 | 122 | 51.7 | |
| Not Insured | 4 | 2.5 | 5 | 6.4 | 9 | 3.8 | |
| Medicaid | 19 | 12.0 | 10 | 12.8 | 29 | 12.3 | |
| Medicare | 51 | 32.3 | 23 | 29.5 | 74 | 31.4 | |
| Other Government | 2 | 1.3 | 0 | 0 | 2 | 0.8 | |
| Facility Type | 0.014 | ||||||
| Community Cancer Program | 2 | 1.5 | 3 | 4.2 | 5 | 2.5 | |
| Comprehensive CCP | 36 | 27.3 | 11 | 15.5 | 47 | 23.2 | |
| Academic | 71 | 53.8 | 52 | 73.2 | 123 | 60.6 | |
| Integrated Cancer Program | 23 | 17.4 | 5 | 7 | 28 | 13.8 | |
| N Stage | 0.006 | ||||||
| N0 | 111 | 67.7 | 35 | 44.3 | 146 | 60.1 | |
| N1 | 13 | 7.9 | 13 | 16.5 | 26 | 10.7 | |
| N2 | 35 | 21.3 | 29 | 36.7 | 64 | 26.3 | |
| N3 | 2 | 1.2 | 0 | 0 | 2 | 0.8 | |
| Nx | 3 | 1.8 | 2 | 2.5 | 5 | 2.1 | |
| Grade | 0.010 | ||||||
| Well Differentiated | 39 | 30.5 | 11 | 15.5 | 50 | 25.1 | |
| Moderately Differentiated | 59 | 46.1 | 28 | 39.4 | 87 | 43.7 | |
| Poorly Differentiated | 19 | 14.8 | 21 | 29.6 | 40 | 20.1 | |
| Undifferentiated | 11 | 8.6 | 11 | 15.5 | 22 | 11.1 | |
| Pathologic N Stage | < 0.001 | ||||||
| p0 | 29 | 20.4 | 29 | 37.2 | 58 | 26.4 | |
| p1 | 5 | 3.5 | 4 | 5.1 | 9 | 4.1 | |
| p2 | 10 | 7.0 | 32 | 41.0 | 42 | 19.1 | |
| pX | 98 | 69.0 | 13 | 16.7 | 111 | 50.5 | |
| Treatment Type | < 0.001 | ||||||
| No Treatment | 18 | 14.0 | 1 | 1.3 | 19 | 9.3 | |
| Surgery Only | 48 | 37.2 | 22 | 29.3 | 70 | 34.3 | |
| Radiation Only | 17 | 13.2 | 0 | 0 | 17 | 8.3 | |
| Surgery with aRT | 33 | 25.6 | 40 | 53.3 | 73 | 35.8 | |
| Surgery with aCRT | 11 | 8.5 | 11 | 14.7 | 22 | 10.8 | |
| Chemotherapy Only | 2 | 1.6 | 1 | 1.3 | 3 | 1.5 | |
| Surgical Margins | 0.445 | ||||||
| Negative Margins | 96 | 79.2 | 62 | 83.8 | 138 | 81.2 | |
| Positive Margins | 20 | 20.8 | 12 | 16.2 | 32 | 18.8 | |
Abbreviations; SD: Standard Deviation; ND: Neck Dissection; aRT: Adjuvant Radiotherapy; aCRT: Adjuvant chemoradiotherapy; CCP: Community Cancer Program
Of 79 patients undergoing neck dissection, 35 (44.3%) had cN0 disease and 42 (53.2%) had cN + disease. Of 164 patients not undergoing neck dissection, 111 (67.7%) had cN0 disease and 53 (32.3%) had cN + disease. Patients with cN + disease underwent neck dissection more frequently than those with cN0 disease (53.2% vs. 30.5%, p = 0.002). Patients undergoing neck dissection more commonly had high-grade tumors than those not undergoing neck dissection (45.1% vs. 23.4%, p = 0.010). Upon examination of dissected lymph nodes, 13 (20.3%) cN0 patients undergoing neck dissection had occult nodal metastases (Table 2).
Table 2.
Patient Nodal Disease Characteristics
| Pathologic N Stage | |||||
|---|---|---|---|---|---|
| Clinical N Stage | p0 | p+ | Total | ||
| All Patients | N | % | N | % | N |
| cN0 | 50 | 79.4 | 13 | 20.6 | 63 |
| cN+ | 7 | 15.9 | 37 | 84.1 | 44 |
| cNx | 1 | 50.0 | 1 | 50.0 | 2 |
| High-grade Patients | |||||
| cN0 | 6 | 66.7 | 3 | 33.3 | 9 |
| cN+ | 3 | 15.0 | 17 | 85.0 | 20 |
Of the 146 patients with cN0 disease, 55 (42.3%) underwent adjuvant therapy. Of 22 patients with cN0 disease and high-grade tumors, 14 (63.6%) underwent adjuvant treatment. 51 (68.0%) patients undergoing neck dissection also underwent adjuvant therapy; only 44 (34.1%) patients not undergoing neck dissection also underwent adjuvant therapy.
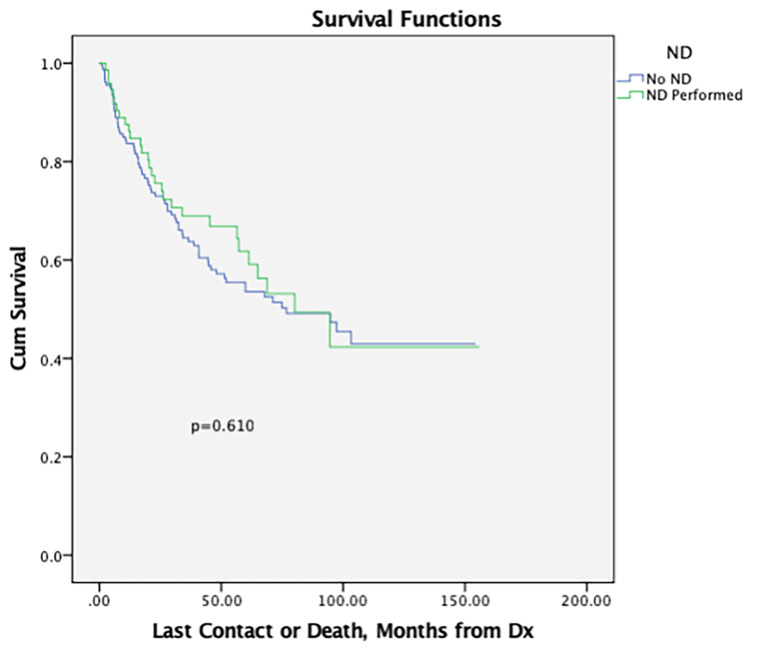
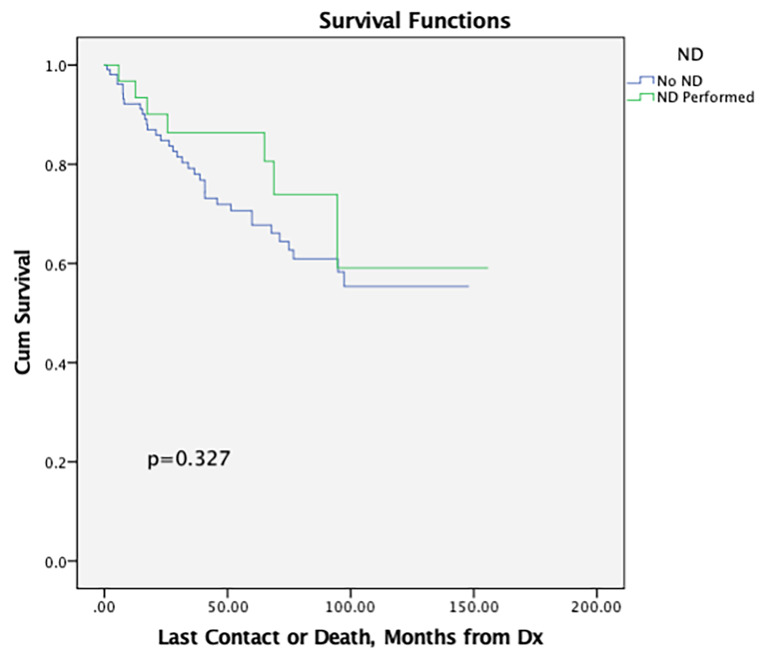
On Kaplan-Meier analysis, there was no significant difference in 5-year OS between patients undergoing and not undergoing neck dissection (61.8% vs. 53.6%, p = 0.610) (Table 3; Fig. 1). Among patients with cN0 disease, those undergoing neck dissection similar 5-year OS as those not undergoing neck dissection (86.3% vs. 67.7%, p = 0.327) (Fig. 2).
Table 3.
Kaplan Meier Analysis for Impact of Neck Dissection on Survival
| Variable | No. Patients | 1-Year OS (%) | 2-Year OS (%) | 5-Year OS (%) | p value |
|---|---|---|---|---|---|
| All Patients | 0.610 | ||||
| No ND | 157 | 83.7 | 73.0 | 53.6 | |
| ND Performed | 73 | 87.6 | 75.6 | 61.8 | |
| cN0 Patients Only | 0.327 | ||||
| No ND | 105 | 92.2 | 84.8 | 67.7 | |
| ND Performed | 31 | 96.8 | 90.1 | 86.3 | |
| cN + Patients Only | 0.091 | ||||
| No ND | 49 | 66.8 | 47.8 | 22.7 | |
| ND Performed | 41 | 80.2 | 63.8 | 45.9 | |
| High-grade Tumors Only in All Patients | 0.724 | ||||
| No ND | 30 | 63.3 | 48.4 | 36.3 | |
| ND Performed | 30 | 83.3 | 66.1 | 39.6 | |
| High-grade Tumors in cN0 Patients | 0.743 | ||||
| No ND | 14 | 71.4 | 57.1 | 49.9 | |
| ND | 7 | 85.7 | 71.4 | 57.1 |
Abbreviations: OS: Overall Survival; ND: Neck Dissection
Fig. 1.
Kaplan-Meier survival curve for all patients treated with and without neck dissection
Fig. 2.
Kaplan-Meier survival curve for patients with cN0 disease treated with and without neck dissection
Multivariable Cox regression proportional hazards model was implemented to evaluate the effect of neck dissection on survival in the context of other relevant clinicopathologic features (Table 4). Increasing age (hazard ratio [HR]: 1.05, 95% confidence interval [CI]: 1.03–1.07, p < 0.001), poorly differentiated (HR: 4.10, 95% CI: 1.63–10.31, p = 0.003), and undifferentiated tumors (HR: 4.25, 95% CI: 1.29–13.99, p = 0.017) were significantly associated with 5-year OS. Neck dissection, however, was not significantly associated with 5-year OS (HR: 1.52, 95% CI: 0.73–3.18, p = 0.269).
Table 4.
Cox Regression Proportional Hazards Model
| Variable | HR | p-value | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower | Higher | |||
| Age | 1.05 | < 0.001 | 1.03 | 1.07 |
| Race | ||||
| White | REF | |||
| Black | 1.29 | 0.502 | 0.62 | 2.69 |
| Other | 0 | 0.979 | 0 | . |
| Sex | ||||
| Male | REF | |||
| Female | 0.92 | 0.803 | 0.47 | 1.79 |
| Primary Site | ||||
| Oral Cavity | REF | |||
| Oropharynx | 1.40 | 0.369 | 0.68 | 2.89 |
| Clinical N Stage | ||||
| N0 | REF | |||
| N1 | 0.87 | 0.802 | 0.30 | 2.57 |
| N2 | 2.80 | 0.015 | 1.22 | 6.43 |
| N3 | 1.47 | 0.665 | 0.26 | 8.26 |
| Nx | 1.37 | 0.777 | 0.16 | 12.07 |
| Grade | ||||
| Well Differentiated | REF | |||
| Moderately Differentiated | 2.11 | 0.099 | 0.87 | 5.11 |
| Poorly Differentiated | 4.10 | 0.003 | 1.63 | 10.31 |
| Undifferentiated | 4.25 | 0.017 | 1.29 | 13.99 |
| Neck Dissection | ||||
| No ND | REF | |||
| ND Performed | 1.52 | 0.269 | 0.73 | 3.18 |
| Treatment Regimen | ||||
| No Treatment | REF | |||
| Surgery Only | 0.22 | 0.013 | 0.07 | 0.73 |
| Radiation Only | 0.66 | 0.549 | 0.17 | 2.58 |
| Surgery with aRT | 0.11 | 0.001 | 0.03 | 0.39 |
| Surgery with aCRT | 0.37 | 0.139 | 0.10 | 1.38 |
| Chemotherapy only | 4.23 | 0.157 | 0.57 | 31.23 |
Discussion
MEC of the OC and OP is a rare malignant tumor, contributing to the lack of randomized clinical trials and other high-quality evidence that can inform surgeons on the optimal management of these patients. Ellis et al. characterized prognostic factors and occult nodal metastasis for these patients using the 2004–2013 NCDB [13]. Their study elucidated the deleterious impact of increasing tumor size, nodal disease, and surgical margin status on survival. Similarly, they noted a 14.1% and 17.3% rate of occult disease in high-grade and cT3/4 tumors, respectively [13]. This study aimed to build upon their work and evaluate the benefit of neck dissection in these relatively high-risk patients. This analysis found that neck dissection was not significantly associated with improved survival but did report a higher rate of occult nodal metastasis (20.3%).
This study found that MEC of the OC and OP most occurred in White males in their mid-50s. These findings align with other studies which have corroborated an increased incidence of the OC and OP MEC in White individuals in their 50s [2, 6, 13, 18]. However, these analyses have reported a female predominance. Selecting for specifically cT3/T4 disease may be responsible as previous studies have found that males are more often diagnosed with higher grade and more advanced SGC [19, 20]. This may result in the majority male cohort. Surgical treatment with or without adjuvant therapy was the most common treatment modality (> 80%). The positive surgical margin rate for these patients was 18.8%. These findings are greater than those reported by Ellis et al., but lower than other studies analyzing MEC [13, 21, 22]. Ellis et al. reasoned that greater surgical access offered to tumors of the OC and OP compared to other locations in the upper aerodigestive tract likely translated to a lower positive margin rate [13]. Furthermore, this study only includes cT3/T4 tumors; the added bulk may contribute to this relatively higher positive margin rate as Ellis et. al’s study included cT1-4 MEC of the OC and OP.
This analysis found that 20.3% of patients with a clinically negative neck were found to have nodal disease on pathologic examination. The occult disease rate is higher than the 17.3% reported for cT3-4 MEC of the OC and OP by Ellis et al. and the 9.3% for MEC of the parotid gland reported by Xiao et al. [13, 23]. Cohort differences are likely a causal factor. For example, in an analysis of MEC of the parotid gland, Grasl et al. reported the risk for occult nodal metastases significantly increased with grade [24]. Other studies in the literature have characterized grade and other important risk factors such as tumor size and lymphovascular invasion as risk factors for occult nodal metastasis [13, 17, 24–26]. This cohort’s composition of only cT3/T4 patients and approximately 30% high-grade tumors (compared to 13.0% for Ellis et al.) likely drives the difference in occult nodal metastasis [13]. It is important to note that 33.3% of cN0 patients with a high-grade cT3/T4 tumor had occult nodal metastasis. Unfortunately, due to rarity of these cancers, the low sample size of this cohort does limit this finding’s utility (n = 9). Furthermore, in comparison to other studies analyzing MEC of alternate primary sites, it is important to consider the anatomic architecture of the OC and OP. Specifically, the OC and OP have rich lymphatic supplies that may result in higher risk of nodal metastases and more advanced disease burden [27, 28]. These findings indicate that elective neck dissection should be given strong consideration for patients with cT3/4 N0 MEC of the OC and OP.
The utility of elective neck dissection for SGC is controversial and necessitates consideration of many factors such as associated surgical morbidity, tumor histology, and adverse features [25]. On Kaplan-Meier analysis, while there did appear to be higher survival rates for patients undergoing neck dissection versus not, regardless of neck nodal status, there was no significant survival benefit for patients undergoing neck dissection. These findings align with several reports in the literature. Studying patients with cT1-2 N0 parotid MEC, Al-Qurayshi et al. found that elective neck dissection did not confer a survival benefit [25]. Nance et al. reported for MEC of the head and neck that the association of neck dissection with survival varied with tumor grade, finding no survival benefit for intermediate grade tumors and decreased survival in their high-grade group [29]. Herman et. al’s study of 59 patients with cN0 high-grade SGC did not find a difference in survival or locoregional control for patients treated with elective neck irradiation versus elective neck dissection. Similarly, analyzing cN0, high-grade parotid cancer, Harbison et al. also reported no statistically significant survival benefit for patients undergoing elective neck dissection, suggesting that radiation or elective neck dissection can be viable options to manage occult nodal metastases [30]. This study similarly failed to detect a significant difference in survival. A possible reason for this may be the use of adjuvant therapy in cN0 patients. Sample size limitations may also be a factor as the rarity of cT3/4 MEC of the OC and OP could have resulted in statistical underpowering, leading to a failure to detect a survival difference. As such, physicians should utilize patient-specific factors such as tumor size and grade when evaluating the decision for elective neck dissection as patients with larger and high-grade tumors appear to be at increased risk for occult nodal metastasis. Further prospective studies are necessary to better understand the optimal management of cN0 MiSGC.
Certain limitations inherent to all retrospective studies utilizing a national database need to be considered. Cases may have inaccurate or missing data, which are unverifiable due to lack of access to the original patient charts. Moreover, the NCDB is limited by the number of available variables; as such, there are likely potential confounders or important factors which our analysis could not capture such as specific comorbidities, tobacco use, imaging, quality of life, and multidisciplinary tumor board recommendations. In addition, certain studies have proposed the prognostic significance of several molecular markers in MEC which cannot be accounted for in this analysis [6]. We also are unable to analyze other relevant outcome variables such as disease-specific mortality, locoregional control, and recurrence-free survival. Despite these limitations, the NCDB has proven to be an invaluable resource for understanding survival outcomes of rare head and neck cancers.
Conclusion
The majority of patients with cT3/4 MEC of the OC and OP presented with cN0 disease, with a high rate of occult nodal metastasis. Patients with cN + disease and high-grade tumors more frequently underwent neck dissection. Neck dissection was not significantly associated with 5-year OS, possibly because of sample size limitations. The statistical insignificance of neck dissection persisted among patients with cN0 disease, cN + disease, and high-grade tumors. Neck dissection, however, should be given strong consideration for high-grade and cT3/4 MEC of the OC and OP because of the high rate of occult nodal metastasis. Further research and prospective studies are needed to better inform the decision to pursue neck dissection in this patient population.
Author Contributions
Rushi Patel: design, analysis, interpretation, manuscript writing; Aman M. Patel: design, analysis, interpretation, manuscript writing; Lucy Revercomb: design, analysis, interpretation, manuscript writing; Amy Patel: design, analysis, interpretation, manuscript writing; Christopher C. Tseng: design, analysis, interpretation, manuscript writing; Richard Chan Woo Park: design, analysis, interpretation, manuscript writing, final approval.
Declarations
Compliance with Ethical Standards
The National Cancer Database contains de-identified patient data and is therefore exempt from review based on the standing policy of the Rutgers New Jersey Medical School’s Institutional Review Board.
Statements and Declarations
No funding was received for conducting this study. The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borsetto D, Iocca O, De Virgilio A, Boscolo-Rizzo P, Phillips V, Nicolai P, Spriano G, Fussey J, Di Maio P (2021) Elective neck dissection in primary parotid carcinomas: a systematic review and meta-analysis. J Oral Pathol Med 50:136?144.10.1111/jop.13137 [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Wei R, Song X, Sun X, Wang H, Liu Q, Hu L, Yu H, Wang D (2021) Risk of second primary malignancy after minor salivary gland cancer: a Surveillance, Epidemiology, and end results database analysis. Head Neck 43:1769?1779.10.1002/hed.26641 [DOI] [PubMed] [Google Scholar]
- 3.Shi X, Huang N-S, Shi R-L, Wei W-J, Wang Y-L, Ji Q-H (2018) Prognostic value of primary tumor surgery in minor salivary-gland carcinoma patients with distant metastases at diagnosis: first evidence from a SEER-based study. Cancer Manag Res 10:2163?2172.10.2147/CMAR.S172725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poletto AG, Mello FW, Melo G, Rivero ERC (2020) Prevalence of mucoepidermoid carcinoma among intraoral minor salivary gland tumors: a systematic review and meta-analysis. J Oral Pathol Med 49:720?726.10.1111/jop.13073 [DOI] [PubMed] [Google Scholar]
- 5.Peraza A, Gómez R, Beltran J, Amarista FJ (2020) Mucoepidermoid carcinoma. An update and review of the literature. J Stomatol Oral Maxillofac Surg 121:713?720.10.1016/j.jormas.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Sarmento DJ, de Morais S, de LS de M, Costa A, de LL A, da Silveira ?JD (2016) Minor intraoral salivary gland tumors: a clinical-pathological study. Einstein (Sao Paulo) 14:508?512.10.1590/S1679-45082016AO3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan KY, Morgan PF, Neskey DM, Kim JJ, Huang AT, Garrett-Mayer E, Day TA (2018) Preoperative predictors of occult nodal disease in cT1N0 oral cavity squamous cell carcinoma: review of 2623 cases. Head Neck 40:1967?1976.10.1002/hed.25178 [DOI] [PubMed] [Google Scholar]
- 8.Patel AM et al (2024) Elective neck dissection in cT1-4 N0M0 head and neck basaloid carcinoma. Otolaryngol Head Neck Surg. 10.1002/ohn.757. Epub ahead of print. PMID: 38613196 [DOI] [PubMed]
- 9.Patel AM et al (2023) Elective neck dissection for cT1-4 N0M0 head and neck verrucous carcinoma. Otolaryngol Head Neck Surg 169(5):1187–1199. 10.1002/ohn.374. Epub 2023 Jun 6. PMID: 37278222 [DOI] [PubMed]
- 10.Westergaard-Nielsen M, Rosenberg T, Gerke O, Dyrvig A-K, Godballe C, Bjørndal K (2020) Elective neck dissection in patients with salivary gland carcinoma: a systematic review and meta-analysis. J Oral Pathol Med 49:606?616.10.1111/jop.13034 [DOI] [PubMed] [Google Scholar]
- 11.Westergaard-Nielsen M, Godballe C, Grau Eriksen J, Larsen SR, Kiss K, Agander T, Parm Ulh?i B, Wittenborg Charabi B, Ehlers Klug T, Jacobsen H, Johansen J, Kristensen CA, Andersen E, Andersen M, Bjørndal K (2021) Surgical treatment of the neck in patients with salivary gland carcinoma. Head Neck 43:1898?1911.10.1002/hed.26667 [DOI] [PubMed] [Google Scholar]
- 12.Yan F, Lao WP, Nguyen SA, Sharma AK, Day TA (2022) Elective neck dissection in salivary gland malignancies: systematic review and meta-analysis. Head Neck 44:505?517.10.1002/hed.26923 [DOI] [PubMed] [Google Scholar]
- 13.Ellis MA, Graboyes EM, Day TA, Neskey DM (2017) Prognostic factors and occult nodal disease in mucoepidermoid carcinoma of the oral cavity and oropharynx: an analysis of the National Cancer Database. Oral Oncol 72:174?178.10.1016/j.oraloncology.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel AM, Haleem A, Choudhry HS, Povolotskiy R, Roden DF (2024) Patterns and trends in adjuvant therapy for major salivary gland cancer. Otolaryngol Head Neck Surg. 10.1002/ohn.715. Epub ahead of print. PMID: 38482915 [DOI] [PubMed]
- 15.Patel AM et al (2024) Surgical resection improves overall survival in cT4b major salivary gland cancer. Otolaryngol Head Neck Surg 170(5):1349–1363. 10.1002/ohn.686. Epub 2024 Mar 1. PMID: 38426575 [DOI] [PubMed]
- 16.Patel AM et al (2024) Choice of adjuvant radiotherapy facility in major salivary gland cancer. Laryngoscope. 10.1002/lary.31352. Epub ahead of print. PMID: 38400788 [DOI] [PubMed]
- 17.Chen MM, Roman SA, Sosa JA, Judson BL (2014) Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head Neck 36:158?163.10.1002/hed.23256 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wang S, Zhang B (2021) A Population-based analysis of Mucoepidermoid Carcinoma of the oral cavity. Laryngoscope 131:E857?E863.10.1002/lary.28905 [DOI] [PubMed] [Google Scholar]
- 19.Cheung MC, Franzmann E, Sola JE, Pincus DJ, Koniaris LG (2011) A comprehensive analysis of parotid and salivary gland Cancer: worse outcomes for male gender. J Surg Res 171:151?158.10.1016/j.jss.2009.11.721 [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Ow A, Ruan M, Yang W, Zhang C, Wang L, Zhang C (2014) Prognostic factors in primary salivary gland mucoepidermoid carcinoma: an analysis of 376 cases in an eastern Chinese population. Int J Oral Maxillofac Surg 43:667?673.10.1016/j.ijom.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 21.McHugh CH, Roberts DB, El-Naggar AK, Hanna EY, Garden AS, Kies MS, Weber RS, Kupferman ME (2012) Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 118:3928?3936.10.1002/cncr.26697 [DOI] [PubMed] [Google Scholar]
- 22.Vander Poorten VL, Balm AJ, Hilgers FJ, Tan IB, Keus RB, Hart AA (2000) Stage as major long term outcome predictor in minor salivary gland carcinoma. Cancer 89:1195?1204.10.1002/1097-0142(20000915)89:6%3C1195::aid-cncr2%3E3.3.co;2-a [DOI] [PubMed]
- 23.Xiao CC, Zhan KY, White-Gilbertson SJ, Day TA (2016) Predictors of Nodal Metastasis in parotid malignancies: a National Cancer Data Base Study of 22,653 patients. Otolaryngol Head Neck Surg 154:121?130.10.1177/0194599815607449 [DOI] [PubMed] [Google Scholar]
- 24.Grasl S, Janik S, Faisal M, Grasl MC, Pammer J, Weinreb I, Fischer G, Kim J, Hosni A, de Almeida JR, Goldstein DP, Erovic BM (2023) Influence of Grading on Management and Outcome in Mucoepidermoid Carcinoma of the Parotid-A multi-institutional analysis. Laryngoscope 133:124?132.10.1002/lary.30135 [DOI] [PubMed] [Google Scholar]
- 25.Al-Qurayshi Z, Sullivan CB, Allison DB, Buchakjian MR (2022) Presentation and outcomes of patients with clinically T1-2, N0 parotid mucoepidermoid carcinoma: the roles of elective neck dissection and adjuvant radiotherapy. Head Neck 44:2151?2161.10.1002/hed.27128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen MP, Mitchell AO, Miles EF (2014) Postoperative Radiation Therapy for Parotid Mucoepidermoid Carcinoma. Case Rep Oncol Med 2014:345128.10.1155/2014/345128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tshering Vogel DW, Zbaeren P, Thoeny HC (2010) Cancer of the oral cavity and oropharynx. Cancer Imaging 10:62?72.10.1102/1470-7330.2010.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubek JE, Clayman L (2012) An update on squamous carcinoma of the oral cavity, oropharynx, and maxillary sinus. Oral Maxillofac Surg Clin North Am 24:307?316, x.10.1016/j.coms.2012.01.003 [DOI] [PubMed]
- 29.Nance MA, Seethala RR, Wang Y, Chiosea SI, Myers EN, Johnson JT, Lai SY (2008) Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer 113:2082?2089.10.1002/cncr.23825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harbison RA, Gray AJ, Westling T, Carone M, Rodriguez CP, Futran ND, Cannon RB, Houlton J (2020) The role of Elective Neck Dissection in High-Grade parotid malignancy: a hospital-based Cohort Study. Laryngoscope 130:1487?1495.10.1002/lary.28238 [DOI] [PMC free article] [PubMed] [Google Scholar]