Abstract
Mood disorders, including depression, remain a significant global health concern, necessitating continuous efforts to develop novel and more effective antidepressant therapies. Although there have been significant advancements in comprehending the biology of Major Depressive Disorder (MDD), a considerable number of people suffering from depression do not exhibit positive responses to the pharmacologic treatments now available. This study specifically examines emerging targets and potential future approaches for pharmaceutical interventions in the treatment of MDD. The discussion revolves around novel therapeutic agents and their effectiveness in treating depression. The focus is on the specific pathophysiological pathways targeted by these agents and the amount of evidence supporting their use. While conventional antidepressants are anticipated to continue being the primary treatment for MDD in the foreseeable future, there is currently extensive research being conducted on numerous new compounds to determine their effectiveness in treating MDD. Many of these compounds have shown encouraging results. This review highlighted the recent advances in the synthesis of antidepressant derivatives and explores their pharmacologic insights for the treatment of mood disorders.
Keywords: Antidepressant derivatives, Depression, Dopamine, Mood disorders, Pharmacological insights, Serotonin
Introduction
Depression is a significant mood disease characterized by a continuous sense of sadness, severe low mood, impaired thinking, hopelessness, and lack of interest. Major depressive disorder (MDD) is a significant public health issue that causes substantial impairment in occupational, psychologic, and social functioning (Kupferberg et al. 2023). Over time, it may also encompass incapacity to feel pleasure, impairment in motor function, alterations in sleep and eating patterns, trouble in focusing, and contemplation of suicide (Clack 2019). Depression is classified as one of a diverse set of illnesses, which are broadly categorized in the International Classification of Diseases (ICD) issued by the World Health Organization (WHO). The current version of the ICD-11 categorizes depressive disorders into several types: single episode depressive disorder and recurrent depressive disorder. In addition, there are various other types of depression, including anxiety and mixed depressive disorder, other defined depressive disorders, dysthymic disorder (persistent depressive disorder), and nonspecific depression (Reed et al. 2019).
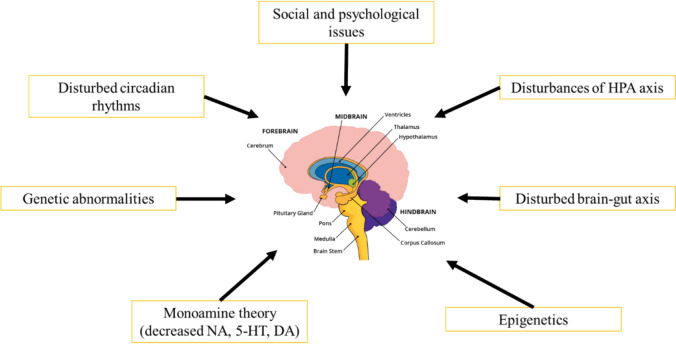
The etiology of this sickness remains elusive, and it was not until the mid-1960s that a molecular rationale for the condition gained acceptance (Fig. 1). The monoamine theory, which is widely recognized, posits that depression is caused by a lack of brain monoamine activity, namely noradrenaline (NA) and/or serotonin (5-HT) (Hindmarch et al. 2002). According to this idea, one way to cure depression is to increase this monoaminergic activity. In a typical brain, neurotransmitters such as NA or 5-HT are released into the synapse by the presynaptic neuron (Elhwvegi et al. 2004).
Fig. 1.
Main mechanism of depression where the primary anatomical components which encompasses the amygdala and the hippocampus
Given that it influences neurogenesis and synaptic plasticity, vascular endothelial growth factor may also have a role in the pathophysiology of depression (Fournier and Duman 2012). Several inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-6, and interleukin 1-β (IL-1β), stop the growth of neurons, especially in the hippocampal region (Monje et al. 2003; Iosif et al. 2006; Raison et al. 2006). Anlar et al. (1999) reported that specific regions of the brain produce insulin-like growth factor 1, which plays a significant role in neurogenesis and cell differentiation. The neurotransmitter gamma-aminobutyric acid (GABA), the dopaminergic and glutamatergic systems, the hypothalamus–pituitary–adrenal (HPA) axis, brain-derived neurotrophic factor (BDNF), and its intracellular signaling pathways are other factors involved in the pathophysiology of depression. Figure 2 shows two pharmacophores that are linked together to generate new molecular templates as anti-depressants agents. Recent research suggests that these factors may contribute to a delayed response to antidepressant therapy (Palazidou 2012).
Fig. 2.

General structure of proposed scaffold as antidepressant agents
During the synapse, the neurotransmitter has the ability to interact with the postsynaptic neuron to provide its intended action. Alternatively, it can undergo metabolism or be taken back up by the presynaptic neuron. In addition, within the synapse, the neurotransmitter has the ability to engage with certain receptors located in the presynaptic neuron, referred to as autoreceptors, which suppress its activity (Kennedy et al. 2013). Thus, potential strategies for enhancing the monamine level in the brain could include as follows: (Celada et al. 2004).
To inhibit the presynaptic autoreceptors, particularly the α2-adrenoceptors (α2-AR), and some 5-HT subtype receptors (1A or 1B);
To inhibit the re-uptake of monoamine neurotransmitters such as NA or 5-HT at the synapse, using selective norepinephrine re-uptake inhibitors (SNRIs), selective serotonin re-uptake inhibitors (SSRIs), or norepinephrine re-uptake inhibitors;
To suppress the metabolic pathways (monoamine oxidase inhibitors, MAOIs);
To directly influence the activity of neurons that produce monoamines.
An effective remedy for this difficulty arose in the early 21st Century with the discovery of ketamine, a glutamatergic drug, which demonstrated rapid and long-lasting antidepressant effects (Berman et al. 2000). Extensive data has been accumulated, demonstrating the potential of glutamatergic medicines, such as ketamine, as novel and more efficacious antidepressant medications (Zarate and Machado-Vieira 2017; Abdallah et al. 2016). In this review, we discussed the pivotal role that ketamine played in the advancement of antidepressants, and its profound impact on enhancing this domain.
Search strategy or methodology
We used a search strategy approach to ensure a comprehensive assessment of current developments in the synthesis of antidepressant derivatives. We conducted a search using a mix of keywords, including “antidepressant derivatives,” “synthesis,” “mood disorders,” and “pharmacologic insights.” We searched PubMed, Scopus, and Google Scholar databases. The review considered studies that provided insights into the molecular elements of antidepressant activity, emphasized pharmacologic assessments, and outlined innovative synthetic approaches.
Epidemiology of depression
Based on the most recent data from the WHO in 2024, the prevalence of depression remains a significant global health concern, affecting approximately 4.2% of the global population. Among adults, the prevalence is 5.4%, and it increases to 6.1% among individuals aged 60 and above. This condition now affects an estimated 300 million people worldwide, highlighting its significant contribution to the global burden of disease and its role as a leading cause of disability (Kaggwa et al. 2022).
Depression continues to disproportionately affect women (8.1%) compared to men (5.1%). This gender disparity persists across almost all countries, with few exceptions. Recent data show that in Croatia and Finland, this gender gap is less pronounced, though still present. The incidence of depression varies significantly across Europe, with Iceland reporting the highest rate at 11.2% and the Czech Republic the lowest at 2.9% (Arias-de la Torre et al. 2021). In Poland, approximately 1.7 million people are currently living with depression. Among the working-age population, around 3.5% (equivalent to roughly 810,000 adult Poles) have experienced at least one depressive episode in their lifetime (Zalewska et al. 2022).
The incidence of 1-month major depression in the Asia–Pacific area varied between 1.3% and 5.5%, whereas prevalence of MDD ranged from 1.7 to 6.7% (Chiu 2004). Ogbo et al. determined that the prevalence of MDD in South Asia was 3.9%. Specifically, the prevalence rates are 3.9% in India, 4.4% in Bangladesh, 4.0% in Nepal, 3.0% in Pakistan, and 3.7% in Bhutan (Ogbo et al. 2018). Depression impacts 5% of the adult population in the Caribbean and Latin America. Furthermore, a majority of 60% of individuals do not undergo any form of medical intervention. Approximately 9.8% of the adult population in South Africa suffer from significant (clinical) depression at some stage in their lifetime (Dobrek and Glowacka 2023; Cuadros et al 2019).
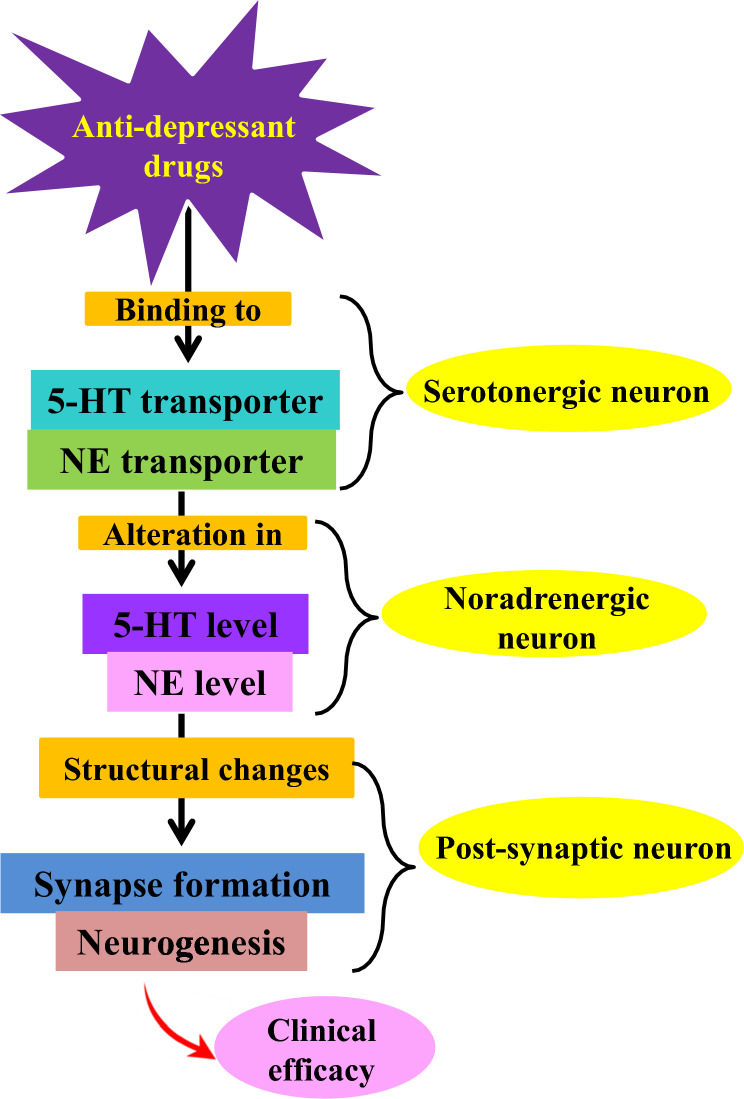
Mechanism of action of antidepressants
The primary mechanism of action of antidepressants is the modulation of neurotransmitter systems in the brain, especially those involving monoamines like dopamine (DA), norepinephrine (NE), and serotonin (5-HT) as shown in Fig. 3 (Hillhouse et al. 2015). The most commonly prescribed antidepressants include serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), MAOIs, and selective serotonin reuptake inhibitors (SSRIs). SSRIs like sertraline and fluoxetine block serotonin reuptake in the synaptic cleft (Cui et al. 2024).
Fig. 3.
General mechanism of action of antidepressants
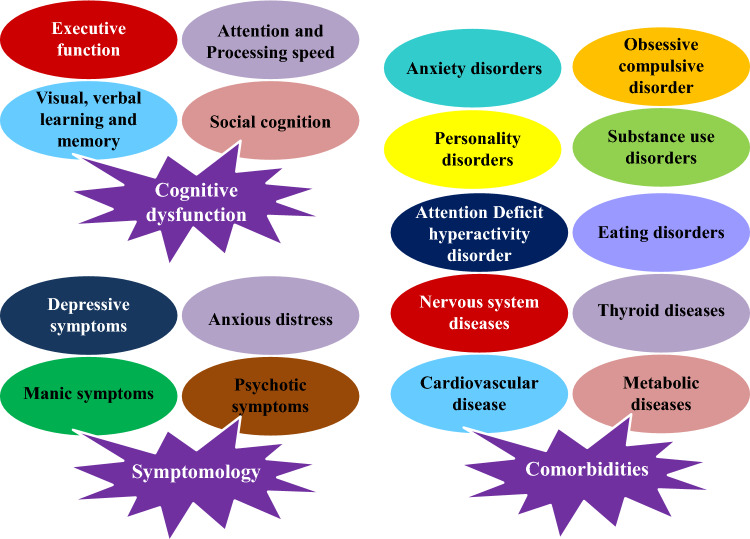
More serotonin is therefore accessible at postsynaptic receptors, which is believed to elevate mood and lessen symptoms of depression (Fig. 4). Similar to venlafaxine and duloxetine, SNRIs also block the reuptake of norepinephrine and serotonin, offering a more comprehensive range of effects that may be advantageous for certain individuals (Cui et al. 2024). Despite their adverse effect profile, TCAs continue to block norepinephrine and serotonin reuptake, but they also interact with other receptors, potentially leading to additional side effects (Sheffler et al 2023). On the other hand, MAOIs increase the amount of these neurotransmitters in the brain by blocking the monoamine oxidase enzyme, which breaks them down. Recent studies have shown that inflammation, the gut microbiome, and neurotrophic factors, including BDNF, are all involved in the pathophysiology of depression (Sansone et al. 2014). This implies that maintaining neuronal health and neuroplasticity may be crucial for the effective use of antidepressants. All things considered, the fundamental objective of anti-depressants is to restore the balance of neurotransmitters and encourage neuroplasticity, which will eventually enhance mood and cognitive performance in those who are depressed, even if the exact processes are still unclear and complicated (Behl et al. 2021).
Fig. 4.
Major warning signs and symptoms of depression
Current treatments of MDD
Recent progress in the field of antidepressant therapies involves the use of TCAs, like Elavil and MAOIs, like Nardil®. They enhance the synaptic levels of either two (NE and 5-HT) or all three (NE, 5-HT, and dopamine (DA) neurotransmitters (Gillman et al. 2007). The concurrent use of a SSRI such as Prozac® and a serotonin reuptake transporter (SERT) inhibitor leads to an elevation in the synaptic concentration of 5-HT and prolongs its effects (Arias et al. 2021). Recent research indicates that the use of both bupropion, a substance that inhibits the Dopamine transporter (DAT), and either an SNRI or SSRI, which are substances that inhibit the absorption of serotonin and norepinephrine, respectively, is more effective in treating depression compared to using only one of these substances (Stahl et al. 2004). Three examples of drugs currently in Phase II clinical trials are SEP-225289 by Sepracor, DOV-21947 by DOV pharmaceutical, and NS-2359 by NeuroSearch. Cymbalta®, a new class of antidepressants known as SNRIs, has been approved for the treatment of anxiety and depression in about 60% of patients (Wenga and Li 2010; Lucas et al. 2010).
The primary issues associated with current antidepressant medications encompass a delayed onset of therapeutic effects, significant adverse reactions, and a suboptimal response rate of less than 50% among patients (McIntyre et al. 2023). The etiology of depression and the mechanism of action of many antidepressant medications are not completely understood. Therefore, there remains a significant demand for expedited, secure, and more efficient remedies for depression (Blackburn et al. 2019). Recent efforts in pharmaceutical development have aimed to go beyond traditional monoamine-based approaches. These efforts have concentrated on exploring glutamatergic systems, neurotrophic factors, and the hypothalamic-pituitary axis (HPA), along with several other less well-understood targets (Fishback et al. 2010; Aboukhatwa and Undieh 2010).
Non-pharmacologictherapies are equally crucial in complementing the comprehensive treatment of depression. The procedures largely consist of psychotherapy methods such as naturopathic therapy, cognitive behavioral therapy, acupuncture, or physical activity interventions. Several studies have shown the positive effects of several dietary supplements in alleviating feelings of depression (Gautam et al. 2020; Farah et al. 2016). These products are formulated with polyunsaturated fatty acids (PUFAs), specifically docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), as well as probiotics. This is based on the belief that inflammation and disruption of the gut-brain axis have a role in causing depression (Thurfah et al. 2022). Several dietary interventions have been studied for their potential use in treating depression. These interventions include specific nutrients like polyphenols, vitamins, and caffeine, as well as certain foods like nuts, fish, vegetables, fruit seeds, fermented products, and coffee/tea. In addition, dietary supplements like acetylcarnitine, S-adenosylmethionine (SAMe), amino acids, and creatine have also been investigated (Ortega et al. 2022).
In cases of severe depression that do not respond to traditional medication, advanced non-pharmacologicmethods like electroconvulsive therapy (ECT) or repetitive transcranial magnetic stimulation (rTMS) are employed (Leblhuber et al. 2021). Various literatures have shown that both treatments are effective in treating depression, with ECT being superior to rTMS. While ECT was shown to be the most effective treatment for MDD, it was also the treatment that was least well tolerated. On the other hand, rTMS was found to be the treatment that was most tolerated for MDD (Rizvi and Khan 2019; Chen et al 2019a, b).
Newer antidepressants for the treatment of MDD
Lanicemine
Lanicemine (AR-R 15896AR or AZD6765), developed by AstraZeneca, exhibits a pharmacologicprofile that closely resembles ketamine (Downey et al. 2016). However, it does not cause the same level of perceptual or cognitive side effects as ketamine when administered at dosages that generate equivalent effects on prefrontal cortex (PFC) activity (Guo et al. 2015; Bui et al. 2015). Preliminary phase Ib and 2a trials demonstrated indications of prompt onset antidepressant effects. A randomized controlled trial (RCT) was conducted with around 150 patients who were divided into three groups (Santi et al. 2024). These groups received either 100 mg lanicemine, 150-mg lanicemine, or a placebo three times a week for duration of 3 weeks. The results of the study demonstrated that both doses of lanicemine were more effective than the placebo in achieving the primary outcome that was predetermined (Sanacora et al. 2017). Curiously, there were indications suggesting the 100-mg dosage outperformed the 150-mg dosage. The 100-mg dosage of lanicemine exhibited substantial differences compared to the placebo at all visits. These differences in scores became apparent starting from week 2 and remained consistent until week 5 (Sanacora et al. 2014). In addition, it improved anxiety symptoms and exhibited significant alterations in clinical global impression (CGI) scores.
In contrast to ketamine, that produced instantaneous effects, lanicemine shown notable distinctions starting from the second week. However, this may be partially attributed to the higher placebo response observed in the lanicemine study (Downey et al. 2016). In a subsequent phase 2b trial, 302 participants were randomly assigned to one of three groups: a group that received 50 mg of lanicemine, a group that received 100 mg of lanicemine, and a placebo group (Sanacora et al. 2017). The treatment was administered three times a week for 3 weeks, followed by once a week for 3 weeks and then once every other week for 6 weeks. The trial did not demonstrate a higher effectiveness for either dosage of the medication in comparison to the placebo at the primary endpoint that was predefined, nor at any of the secondary endpoints (Lee et al. 2017a, b). Nevertheless, the analysis of this study is intricate because to the remarkably high percentage of placebo response, which stands at 39%, among individuals who had been previously treated but did not show any improvement (Jairath et al. 2016). The case of lanicemine serves as a reminder that compounds with innovative mechanisms of action may not necessarily demonstrate its efficacy in clinical trials (Qu et al. 2017).
Desvenlafaxine
Desvenlafaxine is a SNRI that was licensed by the Food and Drug Administration (FDA) in 2009 for the treatment of MDD in adults. It is an active metabolite of venlafaxine. Desvenlafaxine succinate is the form in which it is administered (Liebowitz and Tourian 2010). Desvenlafaxine induces selective inhibition of reuptake at the NE and 5-HT transporters, leading to an elevated extracellular concentration of NE and 5-HT. There is a greater preference for 5-HT transporters compared to NE transporters, with a roughly 10 times stronger affinity (Bruno et al. 2016). However, the affinity for dopamine transporters (DATs) is substantially weaker. Nevertheless, the antidepressant effect does not have any practical consequences on dopamine levels at the amounts needed to block the transporters for 5-HT and NE (Deecher et al. 2006).
Desvenlafaxine has been proven to be effective for treating MDD in multiple Randomized Controlled Trials (RCTs) and open-label trials with adult participants (Boyer et al. 2008; Clayton et al. 2013; Findling et al. 2016). It enhances the overall well-being, as assessed by improvements in emotional state, interpersonal connections, everyday activities, recreational pursuits, financial situation, and physical movement. It relieved depression symptoms in people with liver disease (Feinberg et al. 2010; Fabregas et al. 2014). A case report documented the remission of symptoms in a patient with social phobia who was administered a daily dose of 100 mg. A study without blinding was performed to evaluate the effectiveness of desvenlafaxine in children and teenagers. A dosage range of 50–200 mg was administered to children and adolescents with a positive outcome. Higher doses were linked to a greater occurrence of treatment-emergent adverse events (Findling et al. 2014).
Traxoprodil/ifenprodil
Traxoprodil (CP-101,606) is a compound that functions as an NMDA antagonist that specifically targets the NR2B subunit. An experiment conducted on rats shown that a solitary administration of CP-101606 improved the ability of the hippocampus to change and adapt in response to high-frequency stimulation 24 h after the administration of the drug 9 Yurkewicz et al. 2005). Preskorn et al. presented compelling clinical evidence about the antidepressant properties of traxoprodil in patients with treatment-resistant depression (TRD). The study included 30 patients, with 15 individuals in each treatment group. The results of this trial, which used a placebo control and a double-blind design, clearly demonstrated that patients who were administered CP-101606 experienced a more significant reduction in both MADRS and Hamilton depression rating scale (HDRS) ratings compared to the participants who received the placebo. Moreover, 78% and 32% of patients who received CP-101606 treatment maintained their responder status for 1 week and 30 days following the infusion, respectively (Poleszak et al. 2016).
Traxoprodil is a derivative of ifenprodil, which is classified as a phenylethanolamine chemical. Ifenprodil functions as an allosteric modulator of NMDA receptors, effectively inhibiting glutamate excitotoxicity in cell cultures (Ritter et al. 2023). It blocks NR2B NMDA receptors by enhancing the receptor’s responsiveness to negative regulation by protons. Traxoprodil does not have the psychotomimetic adverse effects that Ifenprodil has, despite both drugs having serotonergic and alpha-adrenergic activities (Hashimoto 2009; Mott et al. 1998). Preskorn et al. (2008) performed a study in which 6 patients in the treatment group experienced dissociative reactions. However, it is worth noting that two patients from the placebo group also had dissociative symptoms.
Levomilnacipran ER
Levomilnacipran ER, also known as 1S, 2R-milnacipran, is a SNRI that has been licensed by the FDA for treating MDD in adults. SNRIs, or SNRIs, all work by blocking the reabsorption of both 5-HT and NE, but they differ in how strongly they affect each transporter (Chen et al. 2015a, b). This has important consequences for their therapeutic use. Levomilnacipran ER is a more potent enantiomer of racemic milnacipran. It exhibits a potency that is twice as strong for inhibiting the reuptake of norepinephrine compared to serotonin. In addition, it selectively inhibits the reuptake of norepinephrine more than venlafaxine, duloxetine, and desvenlafaxine (Bakish et al. 2014).
The effectiveness of levomilnacipran for MDD and overall functioning has been demonstrated in five RCTs including adults, using dosages ranging from 40 to 120 mg per day. In a RCT conducted by Gommoll et al., (2014) there was a noticeable reduction in depression symptoms, although it did not achieve statistical significance. According to Asnis et al. (2013) there was a better response observed for doses of 80 mg and 120 mg compared to a dose of 40 mg.
D-cycloserine (DCS)
The study demonstrated that augmentation with a daily dose of 1 g of DCS (5) for TRD revealed that DCS acts as a partial agonist at the glycine modulatory site associated with the N-methyl-D-aspartate receptor (NMDAR) (Chen et al. 2019a, b). At elevated concentrations, it functions as an NMDAR antagonist. A total of twenty-six patients with treatment-resistant MDD took part in a 6-week parallel group experiment. The trial was double-blind and placebo-controlled, and involved the addition of a high dose (1000 mg/day) of DCS to the patients’ existing antidepressant prescription (Heresco-Levy et al. 2013). The amount of DCS was steadily increased over time. The DCS treatment was well-tolerated and did not cause any psychotomimetic effects. It effectively alleviated depressive symptoms, as indicated by the HDRS and Beck Depression Inventory (Frank et al. 2022). Out of the 13 individuals who had DCS treatment, 54% experienced a 50% drop in their HAMD score, compared to only 15% of the 13 patients who were randomly assigned to receive a placebo (p = 0.039). An important (p = 0.043) relationship was observed between the response to treatment and the levels of glycine in the serum before treatment (Heresco-Levy et al. 2013). In a previous trial conducted by the same group, the use of a lower dosage of DCS (250 mg/day) as an additional treatment to the existing antidepressant therapy did not result in any significant differences in outcome measures (Heresco-Levy et al. 2006).
Coumarin derivatives
Coumarins are a class of non-flavonoid polyphenols, namely o-hydroxycinnamic acid lactones, often referred to as “2H-chromen-2-one1,2-benzopyrone”. They belong to a category of aromatic phytochemicals that have a pleasant odor (El-Sawy et al. 2021). Coumarins and their derivatives have unique structures and are being employed for treating mental health conditions like anxiety, schizophrenia, and depression (Sashishara et al. 2015; Sahni et al. 2021; Rehuman et al. 2020; Delogu et al. 2014). The diverse pharmacologiceffects of coumarins and their derivatives are associated with their capacity to impede the formation of the mitotic spindle leading to cell death, inhibit myosin light chain kinase, and decrease tyrosine phosphorylation (Flores-Morales et al. 2023). In addition, their antidepressant properties may be attributed to their anti-inflammatory and antioxidant characteristics (Nabeel et al. 2021; Kilic 2022; Sinha 2022). Table 1 summarizes various coumarins derivatives and their inhibitory effects against monoamine oxidases.
Table 1.
The IC50/PIC50 values of various coumarins and their derivatives against monoamine oxidases and their inhibitory effects
| Compound | Class/type | Mechanism of action | IC50/pIC50 | Model (Human/animal) | Refs. |
|---|---|---|---|---|---|
| Auraptene | Natural coumarin | Inhibits monoamine oxidase, anti-inflammatory | 34.60 μM | Animal (Rodent) | Jeong et al. (2006); Chang et al. (2021) |
| Umbelliferone | Natural coumarin | Antioxidant, neuroprotective | 87.50 μM | Animal (rodent) | |
| Isopsoralen | Furanocoumarin | Modulates neurotransmitters, neuroprotective | 12.80 μM | Animal (rodent) | Kong et al. (2001) |
| Psoralen | Furanocoumarin | Inhibits monoamine oxidase | 15.20 μM | Animal (rodent) | Kong et al. (2021) |
| Xanthotoxin | Furanocoumarin | Anti-inflammatory, modulates neurotransmitters | 64.60 μM | Animal (rodent) | Carotti et al. (2002) |
| 3,4-dihydro-2(1H)-quinolinone derivatives | Synthetic compound | Inhibits serotonin reuptake, antidepressant-like | 6.89% | Animal (rodent) | Oshiro et al. (2000) |
| Lacinartin | Natural flavonoid | Modulates serotonin and dopamine receptors | 9.20 μM | Animal (rodent) | Jo et al. (2002) |
| Scopoletin | Natural coumarin | Antioxidant, anti-inflammatory, neuroprotective | 19.40 μg/mL | Animal (rodent) | Yung et al. (2001) |
| Decursin | Natural coumarin | Inhibits monoamine oxidase, neuroprotective | 1.89 μM | Animal (rodent) | Lee et al. (2017a, b) |
Arketamine
The NMDAR antagonist (R,S)-ketamine, a dissociative anesthetic, has garnered significant interest in the realm of psychiatric illnesses. Conclusive data has shown that (R,S)-ketamine has both long-lasting and fast-acting antidepressant effects in depressed individuals, post-traumatic stress disorder (PTSD), and bipolar disorder (BD) who do not respond to conventional treatments (Hashimoto 2022; Feder et al. 2021; Fava et al. 2020). (R,S)-ketamine is a racemic combination comprising of (S)-ketamine (also known as esketamine) and (R)-ketamine (also known as arketamine (6)). Despite its lesser affinity for the NMDAR, arketamine exhibits stronger and more enduring antidepressant-like effects compared to esketamine in mouse models of depression (Ebert et al. 1997; Yang et al. 2015).
In addition, it should be observed that the adverse reactions associated with arketamine are fewer in comparison to esketamine and (R,S)-ketamine (Chang et al. 2019). Collectively, arketamine would serve as an innovative, fast-acting antidepressant devoid of the adverse effects associated with esketamine and (R,S)-ketamine (Wei et al. 2022). Arketamine is a novel antidepressant that lacks the adverse effects associated with esketamine and (R,S)-ketamine. Arketamine not only exhibits antidepressant-like effects but it also has positive benefits on many animal models of neurologic illnesses, including Parkinson’s disease and multiple sclerosis (Wang et al. 2022).
Given that depression frequently manifests as a psychiatric symptom in individuals with neurologic illnesses, the administration of arketamine may potentially ameliorate depressed symptoms in these patients (Wei et al. 2022). The exact chemical and cellular mechanisms responsible for the antidepressant effects of (R,S)-ketamine and its enantiomers are still not fully understood. The communication between the brain and the body, specifically through the gut–microbiota–brain axis and the brain-spleen axis, may contribute to the positive effects of arketamine (Jelen et al. 2021; Zhang et al. 2021a, b; Scotton et al. 2022; Yang et al. 2017). However, additional research is required to fully understand this relationship (see Table 2).
Table 2.
List of antidepressant drugs under different phases of clinical trials, along with their mechanism of action, therapeutic indication, and their current trial number
| Drug name | Mechanism of action | Therapeutic indication | Current status | Clinical trial number | Refs. |
|---|---|---|---|---|---|
| Psilocybin | Serotonin (5-HT2A) receptor agonist | Major depressive disorder | Phase II/III | NCT04670081 | Husain et al. (2023) |
| Esketamine | NMDA receptor antagonist | Treatment-resistant depression | Approved/phase IV | NCT03434041 | Zaki et al. (2023) |
| Zuranolone (SAGE-217) | GABA-A receptor positive allosteric modulator | Major depressive disorder | Phase III | NCT04442503 | Hoffmann et al. (2020) |
| Rapastinel | NMDA receptor partial agonist | Major depressive disorder | Phase III (discontinued) | NCT03668600 | Donello et al. (2019) |
| Amitifadine | Triple reuptake inhibitor (SERT, NET, DAT) | Major depressive disorder | Phase II | NCT02781315 | Sharma et al. (2015) |
| BHV-5000 | NMDA receptor modulator | Treatment-resistant depression | Phase II | NCT03080740 | Moore et al. (2022) |
| Agomelatine | Melatonergic agonist and 5-HT2C antagonist | Major depressive disorder | Phase IV (Post-marketing) | NCT04355650 | Olié et al. (2007) |
| JZP-386 | GABA-A receptor modulator | Major depressive disorder | Phase I | NCT04691675 | Luscher et al. (2011) |
| BTRX-246040 | κ-opioid receptor antagonist | Major depressive disorder | Phase I/II | NCT04225155 | Witkin et al. (2019) |
| MIN-117 | Serotonin (5-HT1A) and dopamine (D2) receptor modulator | Major depressive disorder | Phase II | NCT02417188 | Kaufman et al. (2016) |
| AXS-05 | NMDA receptor antagonist and serotonin-norepinephrine reuptake inhibitor | Treatment-resistant depression | Phase III | NCT04408705 | Iosifescu et al. (2022) |
| Seltorexant (JNJ-42847922) | Orexin-2 receptor antagonist | Major depressive disorder | Phase III | NCT04513922 | Recourt et al. (2019) |
| Relamorelin | Ghrelin receptor agonist | Depression with gastroparesis | Phase II | NCT03751620 | Patel et al. (2023) |
| Vilazodone | Serotonin reuptake inhibitor and 5-HT1A partial agonist | Major depressive disorder | Phase IV (approved) | NCT03030180 | Sinha et al. (2021) |
| Vortioxetine | Serotonin modulator and stimulator | Major depressive disorder | Phase IV (approved) | NCT03829100 | Connolly et al. (2016) |
Newer heterocyclic derivatives
Piperazine derivatives
Piperazine is a hexagonal heterocyclic compound containing two nitrogen atoms positioned diagonally from each other. The Piperazine core is recognized for its significant significance in maintaining the pharmacokinetic (PK) profile of designer drugs in balance (Zhang et al. 2021a, b). Currently, there are multiple compounds containing piperazine that have been approved for the treatment of clinical depression. From a mechanistic standpoint, nearly all of them function as either partial or complete agonists of the 5-HT1A (Huang et al. 2017). Upon careful examination of their structural framework, it is noteworthy that the majority of FDA-approved antidepressants containing piperazine exhibit the existence of piperazine as a connecting element between the two aromatic/heteroaromatic sections (Patel and Won 2013; Lacivita et al. 2012).
Zareba et al. synthesized a collection of novels, large-sized Fananserin compounds and assessed their efficacy as antidepressants. The literature and virtual screening both indicate that the intended hits were obtained via SAR experiments for LCAP (longchain arylpiperazine derivatives). A total of eighteen new compounds were created and tested for their antidepressant effectiveness against the 5-HT7, 5-HT1A, and D2 isoforms of dopamine and serotonin receptors. The SAR (Structure–Activity Relationship) investigations demonstrated that as the alkyl chain length is increased up to six carbons and a fluorine substituent is introduced at the 2nd or 3rd position in the arylpiperazine, there is an enhanced affinity for the D2 and 5-HT1A receptors. The docking research revealed that these compounds can establish a hydrogen bond between the nitrogen atom in the carboxylic group of Asp106 and arylpiperazine ring (Zareba et al. 2019).
Gu et al. (2017) developed and created a range of aralkyl and arylalkanol piperazine derivatives with the intention of exploring their potential as antidepressant drugs. A total of 16 compounds were synthesized and assessed for their inhibitory effects on serotonin reuptake, as well as their affinities for the 5-HT7 and 5-HT1A receptors. Among all the compounds, compound 7 exhibited the greatest affinity for 5-HT7 and 5-HT1A receptors.
Ostrowska et al. (2020) created new compounds including piperazine and 6-acetyl-5-hydroxy-4,7-dimethylcoumarin. These compounds have a strong attraction to 5-HT1A, 5-HT2A, and D2 receptors. Compound 8 and compound 9 were found to have a strong attraction to 5-HT1A receptors in in vitro tests, having a Ki value of 1 nM. Both the synthesised compounds exhibited a strong binding ability to 5-HT2A receptors, with respective Ki values of 17.5 and 8 nM. Compounds 8 and 9 were observed to exhibit lower affinity towards D2 receptors, indicating their preference for serotonin receptors. In addition, both of the powerful substances underwent in vivo TST and FST experiments and were determined to be unsuccessful in mice, indicating that these substances lack any antidepressant potential. These adverse outcomes hindered their progress as a potential treatment for depression (Kumar et al. 2021a, b).
Indole derivatives
Indole derivatives are known to be serotoninergic modulators due to their structural similarity. The indole moiety is referred to as bioisosteres due to its resemblance in physical and chemical properties to other biological compounds (Munir et al. 2020). Similarity has been utilized in prototype drug development to enhance both the pharmacologicefficacy and the PK profile. The indole nucleus exhibits strong antidepressant effect. Different derivatives of indole and 5-HT are used as a framework for creating new chemical compounds that target the 5-HT1A receptor and the SERT (Ben-Daniel et al. 2008).
In their study, Cerda-Cavieres et al. (2020a, b) created and tested a collection of 27 compounds with indole structure. These compounds were designed to act as multitarget ligands, targeting the SERT, dopamine D2 receptor, and MAO-A. The goal of this research was to create new treatments for the treatment of MDD. All of the tested drugs exhibited a strong attraction to SERT in the nanomolar range, with five of them demonstrating Ki values ranging from 5 to 10 nM. Compound 10 exhibited similar affinities for both the D2 and SERT receptor, with a D2/SERT ratio of 3.6. This compound has the potential to be a multitarget lead for further improvement (Cerda-Cavieres et al. 2020a, b).
Kumar et al., (2021a, b) developed and created a range of piperazinyl derivatives that were modified with indole functional groups. The majority of the compounds exhibited strong inhibitory activity against MAO-A and demonstrated significant selectivity over MAO-B. The compounds showed high potential as reversible inhibitors of MAO-A, with IC50 values ranging from 0.11 ± 0.03 μM to 0.14 ± 0.02 μM.
Kaczor et al., (2023) conducted a study where they synthesized and extensively examined the structure and function of three indole derivatives (11–13) as ligands for serotonin 5-HT1A and 5-HT2A receptors. The protonatable nitrogen atom of the synthesised compounds and the conserved Asp (3.32) of the receptors created a salt bridge, which mediates the majority of the interaction between the ligands and the receptors. Molecular dynamics (MD) simulations showed that the ligands examined exhibit a high degree of stability in their respective binding sites, except for compound 12 when interacting with the serotonin 5-HT1A receptor (Kaczor et al. 2020).
The 5-HT system is a primary focus for antidepressant medications; the human serotonin transporter (hSERT) plays an important role in regulating the amounts of 5-HT in the synapses. Mattson et al., (2005) conducted the synthesis of a range of indole cyclopropylmethylamines. A subset of these produced compounds was then assessed for their antidepressant effects. Based on the structure–activity relationship, the presence of nitrile substituents at the 5th and 7th positions of the indole ring resulted in a strong affinity for hSERT. However, when the indole ring was substituted at the N-1 position with a methyl or ethyl group, the affinity for hSERT decreased by 10–30 times (Siddiqui et al. 2011).
Piperidine derivatives
The piperidine ring is a widely distributed molecular structure. Piperidine-containing chemicals play an important role in biology and medicine. The biological characteristics of piperidines are greatly influenced by the nature and position of substituents on the heterocyclic ring. Clinical and preclinical investigations have referenced several N-heterocyclic piperidine molecules (Takeuchi et al. 2003; Kallstrom and Leino 2008). Kaya et al., (2022) developed new compounds derived from piperidine and examined their effects on depression-like symptoms. The compounds were assessed for their antidepressant-like effects using TST and FST at a dosage of 50 mg/kg. Furthermore, potential changes in the locomotor behaviors of mice were assessed using activity cage measures. The results demonstrated that compounds 14a-14d effectively decreased the immobility time of mice in both antidepressant activity-screening tests, suggesting that these derivatives had antidepressant-like properties (Bogdanova et al. 2013).
In their study, Yuan et al. (2022) synthesized a range of innovative derivatives of 1-(1-benzoylpiperidin-4-yl) methanamine and assessed their ability to block serotonin reuptake and their affinities for binding to the 5-HT1A receptor. The findings demonstrated that the synthesized compounds displayed potent suppression of serotonin reuptake and significant binding affinity for 5-HT1A receptor. In vitro, the compounds demonstrated favorable metabolic stability and displayed possible antidepressant-like effects in mice when tested in the FST and TST (Marcinkowska et al. 2021).
Pyrimidine derivatives
Pyrimidine and its derivatives have garnered significant attention due to its diverse range of key pharmacologiceffects and favorable pharmacokinetic features (Arias-Gómez et al. 2021). Krol et al., (2021) synthesized two sets of new pyrido-pyrimidine (15a-i) and tetrahydropyrido-pyrimidine (16a-i) compounds. In vitro radioligand binding experiments were used to evaluate the affinities of synthesised compounds for the 5-HT1A receptor and SERT. All derivatives in the series exhibited exceptionally strong binding affinities for the 5-HT1A receptor, while generally displaying weak binding affinities for the SERT protein.
A study evaluated the anticonvulsant and antidepressant effects of a series of derivatives (17) of “5-alkoxytetrazolo [1, 5-c] thieno [2, 3-e] pyrimidine”. The compound 17 was the most effective at a dosage of 100 mg/kg. It shortened the immobility period by 51.62% (Wang et al. 2012). Kim et al., (2010) produced a collection of pyrimidine 4-carboxamide derivatives that include arylpiperazine. An assessment was conducted on several manufactured compounds to determine their level of affinity for serotonin receptors and transporters. The researchers conducted in vitro and structure–activity relationship (SAR) tests and concluded that compound (18) had the highest potency in this series, with an IC50 value greater than 10 μM.
Thiadiazole derivatives
The thiadiazole system is a cyclic compound that is similar to thiosemicarbazide and has the N=C=S linkage, which is responsible for its toxic properties (Serban, et al. 2018). Thiadiazole is a compound that is formed from thiophene by substituting two –CH= groups with pyridine-type nitrogen (–N=). It exists in four different isomeric forms, which are determined by the locations of the nitrogen atoms (Sah et al. 2014). Several synthetic products have been developed using the thiadiazole nucleus, which exhibit a wide range of pharmacologicactivities. For instance, KC12291 has demonstrated cardioprotective effects, while small heterocyclic thiadiazolidinones (TDZD) are the first inhibitors of glycogen synthase kinase 3b that do not compete with ATP. The thiadiazole derivative compounds exhibited strong antidepressant and anxiolytic effects (Castro et al. 2006; Lotfi et al. 2020).
In their study, Saglik et al. (2020) developed a range of 1,3,4-thiadiazole derivatives that contain different alkyl/arylamine groups. These compounds were specifically designed and produced as inhibitors of MAO. The compounds exhibited greater selectivity against hMAO-A compared to hMAO-B. The majority of the compounds had half maximum inhibitory concentration (IC50) values that were lower than the IC50 value of the commonly used medicine moclobemide (IC50 = 4.664 μM). Compound 19 had the highest level of activity, with an IC50 value of 0.060 μM and it also exhibited a comparable inhibitory profile to clorgyline. Compound 19 exhibited a reversible and competitive inhibitory profile, showing selectivity towards MAO-A (Osmaniye et al. 2021).
Sharma et al., (2011) synthesised a novel series of derivatives of “2-amino-5-sulfanyl-1,3,4-thiadiazole”. The recently produced chemicals were identified using analytical and spectroscopic techniques. The compounds underwent screening to assess their activity on the central nervous system. Each of the synthesized compounds demonstrated substantial antidepressant, anxiolytic, and anticonvulsant effects when compared to the reference medications. Jatav et al., (2008) produced a range of “3-[5-substituted phenyl-1, 3, 4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones”. Among the 18 synthesised compounds, compound 20 had significant antidepressant efficacy when tested using the forced swim test method.
Pyrazoline derivatives
Pyrazoline exhibits a distinctive structure consisting of a ring of five atoms, including at least one heteroatom. Pyrazolines are a significant group of heterocycles known for their strong biological and pharmacologiceffects (Barsoum and Girgis 2009). Aggarwal et al. (2021) conducted a synthesis of novel pyrazoline derivatives and assessed their effectiveness as anti-depressants through In-silico and In-vivo experiments. The cyclization of chalcones with phenylhydrazine in glacial acetic acid resulted in the production of pyrazoline derivatives.
A study on the effectiveness of antidepressants was conducted using the TST and FST in live subjects. The confirmation of the likely method by which the chemicals demonstrate neuropharmacological interactions arose from their contact with the residues of the binding sites, specifically ASP 46. This demonstrates that the chemicals possess a strong attraction to the MAO-A target protein (Finberg et al. 2016). Siddiqui et al. (2009) developed and created a series of “N,3-(substituted diphenyl)-5-phenyl-1H-pyrazoline2-carbothioamide derivatives”. Compound (21a) and compound (21b) both shown a substantial reduction in the immobility time at a dose of 25 mg/kg, when compared to the control group. All the chemical derivatives are shown in Figs. 5 and 6.
Fig. 5.
Chemical structures of designed derivatives. (1) Lanicemine (2) Desvenlafaxine (3) Traxoprodil (4) Levomilnacipran ER (5) D-cycloserine (DCS) (6) Arketamine (7–9) Piperazine derivatives (10–13) Indole derivatives (14) Piperidine Derivatives
Fig. 6.
Chemical structures of designed derivatives. (15–18) Pyrimidine derivatives (19–20) Thiadiazole derivatives (21) Pyrazoline derivatives
Miscellaneous agents
Over the past 2 decades, several new drugs with different ways of working have been studied for treating MDD. MK0869, a substance P antagonist, was first welcomed with high anticipation as a ground breaking approach in the management of depression (Humes et al. 2024). Proof-of-concept research provided evidence of its effectiveness and favourable tolerability in treating MDD. However, in later trials, the medicine did not demonstrate substantial effects when compared to a placebo, leading to the discontinuation of its development as a therapy for MDD (Al-Harbi et al. 2012). In addition, the effectiveness of SSR149415, a medication that blocks the vasopressin 1b receptor, in treating depression has been assessed in three clinical trials, yielding varied outcomes (Argyropoulos and Nutt 2000; Griebel et al. 2012).
Moreover, some nutraceuticals have been examined for their potential involvement in the management of depression. Many research and systematic reviews have examined the effectiveness of omega-3 PUFAs in treating and preventing depressive illnesses (Wani et al. 2015). The results of a recent meta-analysis provide evidence for the beneficial effects of omega-3 polyunsaturated fatty acid supplementation in treating MDD. However, the benefits appear to be limited to formulations that contain more than 60% EPA, as opposed to formulations that only contain DHA or a higher proportion of DHA (Liao et al. 2019).
SAMe is an amino acid metabolite found inside cells. It acts as a co-substrate for enzymes involved in the production of hormones and neurotransmitters. SAMe has been investigated as a potential treatment for individuals with depression (Mato et al. 2013). In addition, the use of methylfolate as a supplement to antidepressants has shown possible benefits in treating MDD according to various meta-analyses (Sarris et al. 2020; Firth et al. 2019). However, other writers, like Roberts et al (2018), have highlighted the limited methodological quality of the studies available.
Future perspective
Research is continuously revealing new therapeutic agents and innovative methodologies, indicating promising prospects for the treatment of major depressive disorder (MDD). Future therapies may increasingly concentrate on personalized medicine, which involves customizing therapy to specific patient profiles based on genetic, biochemical, and psychologic characteristics. Once we achieve a fuller understanding of the neurobiological pathways underlying depression, this will become possible. In addition, the use of cutting-edge technology, such as artificial intelligence and machine learning, has the potential to improve drug development procedures, which would ultimately result in the discovery of compounds that are more effective and have fewer adverse effects. As the discipline develops, there is also the possibility of combining pharmaceutical therapies with psychotherapy tactics and lifestyle adjustments, which would result in a more comprehensive approach to the management of depression. The end objective is to enhance patient outcomes and quality of life by creating treatments that not only ease symptoms but also address the fundamental causes of MDD. All those suffering from this terrible ailment will have access to effective therapy in future.
Conclusion
The development of depression is influenced not just by serotonin, but also by glutamate, histamine, noradrenaline, and other receptors, including their subtypes. Monotherapeutic medicines are insufficient for the treatment and management of this intricate disease. In addition, these drugs that target a particular specific area are also associated with diverse adverse effects and toxicities. The significant progress in the healthcare industry has resulted in the creation of multiple therapeutic medicines that operate through various mechanisms and mitigate depression to varying degrees. An extensive examination of referenced literature has uncovered that compounds containing two or more active pharmacophores linked by an optimal spacer are likely to have an effect on many biological targets. Specifically, combinations of substituted piperazine with indole or purine, connected by an ideal spacer, provide powerful compounds with a diverse range of target-binding abilities. These molecules show potential for significant biological effects, improved effectiveness, and little toxicity. These compounds have exhibited exceptional binding affinities to various receptors implicated in the disease’s etiology. In vivo and in silico investigations have further corroborated these findings. This compilation may be useful for medicinal chemists and drug developers seeking insights into the key structural characteristics that contribute to the biological activity associated with depression. These insights can be applied to the rational design of drugs targeting depression. These discoveries can be used by researchers as supporting evidence for the development of more effective, powerful, and safe chemicals to address complex illnesses similar to depression.
Abbreviations
- MDD
Major depressive disorder
- IC50
Half maximal inhibitory concentration
- NMDA
N-methyl-D-aspartate
- 5-HT
5-Hydroxytryptamine (serotonin)
Funding
No funding applicable.
Declarations
Conflict of interest
Authors are required to disclose financial or non-financial interests that are directly or indirectly related to the work submitted for publication. The authors declare no conflicts of interest.
Contributor Information
Kuldeep Singh, Email: kuldeep.singh_mph20@gla.ac.in.
Divya Jain, Email: divyajain31011996@gmail.com.
Sachchida Nand Rai, Email: raibiochem@gmail.com.
References
- Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH (2016) Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety 33:689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboukhatwa MA, Undieh AS (2010) Antidepressant stimulation of CDP diacylglycerol synthesis does not require monoamine reuptake inhibition. BMC Neurosci 11:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal NN, Gatphoh BF, Kumar MV, Ghetia S, Revanasiddappa BC (2021) Synthesis, in silico analysis and antidepressant activity of pyrazoline analogs. Thai J Pharm Sci 45:24–31 [Google Scholar]
- Al-Harbi KS (2012) Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 6:369–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlar B, Sullivan KA, Feldman EL (1999) Insulin-like growth factor-I and central nervous system development. Horm Metab Res 31:120–125 [DOI] [PubMed] [Google Scholar]
- Argyropoulos SV, Nutt DJ (2000) Substance P antagonists: novel agents in the treatment of depression. Expert Opin Investig Drugs 9:1871–1875 [DOI] [PubMed] [Google Scholar]
- Arias HR, Targowska-Duda KM, García-Colunga J, Ortells MO (2021) Is the antidepressant activity of selective serotonin reuptake inhibitors mediated by nicotinic acetylcholine receptors? Molecules (Basel, Switzerland) 26(8):2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-de la Torre J, Vilagut G, Ronaldson A, Serrano-Blanco A, Martín V, Peters M, Valderas JM, Dregan A, Alonso J (2021) Prevalence and variability of current depressive disorder in 27 European countries: a population-based study. The Lancet Public Health 6(10):e729–e738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Gómez A, Godoy A, Portilla J (2021) Functional pyrazolo[1,5-a]pyrimidines: current approaches in synthetic transformations and uses as an antitumor scaffold. Molecules 26(9):2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnis GM, Bose A, Gommoll CP, Chen G (2013) Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebocontrolled study. J Clin Psychiatry 74:242–248 [DOI] [PubMed] [Google Scholar]
- Bakish D, Bose A, Gommoll C, Chen C, Nunez R, Greenberg WM, Khan A (2014) Levomilnacipran ER 40 mg and 80 mg in patients with major depressive disorder: a phase III, randomized, double-blind, fixed-dose, placebocontrolled study. J Psychiatry Neurosci 39:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum FF, Girgis AS (2009) Facile synthesis of bis(4,5-dihydro-1Hpyrazole-1-carboxamides) and their thio-analogues of potential PGE2 inhibitory properties. Eur J Med Chem 44:2172–2177 [DOI] [PubMed] [Google Scholar]
- Behl T, Kaur D, Sehgal A, Singh S, Sharma N, Zengin G, Andronie-Cioara FL, Toma MM, Bungau S, Bumbu AG (2021) Role of monoamine oxidase activity in Alzheimer’s disease: an insight into the therapeutic potential of inhibitors. Molecules 26(12):3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Daniel R, Deuther-Conrad W, Scheunemann M, Steinbach J, Brust P, Mishani E (2008) Carbon-11 labeled indolylpropylamine analog as a new potential PET agent for imaging of the serotonin transporter. Bioorg Med Chem 16:6364–6370 [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354 [DOI] [PubMed] [Google Scholar]
- Blackburn TP (2019) Depressive disorders: treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect 7(3):e00472. 10.1002/prp2.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF (2013) Factors influencing behavior in the forced swim test. Physiol Behav 118:227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P, Montgomery S, Lepola U, Germain JM, Brisard C, Ganguly R, Tourian KA (2008) Efficacy, safety, and tolerability of fixed-dose desvenlafaxine 50 and 100 mg/day for major depressive disorder in a placebocontrolled trial. Int Clin Psychopharmacol 23:243–253 [DOI] [PubMed] [Google Scholar]
- Bruno A, Morabito P, Spina E, Muscatello MR (2016) The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuropharmacol 14(2):191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui KH, Zhou D, Agbo F, Guo J (2015) Effect of multiple intravenous doses of lanicemine (AZD6765) on the pharmacokinetics of midazolam in healthy subjects. J Clin Pharmacol 55:1024–1030 [DOI] [PubMed] [Google Scholar]
- Carotti A, Carrieri A, Chimichi S, Boccalini M, Cosimelli B, Gnerre C, Carotti A, Carrupt PA, Testa B (2002) Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. synthesis and SAR studies. Bioorg Med Chem Lett 16:3551–3555 [DOI] [PubMed] [Google Scholar]
- Castro A, Castaño T, Encinas A, Porcaland W, Gil C (2006) Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles. Bioorg Med Chem 14:1644–1652 [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F (2004) The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci 4:252–265 [PMC free article] [PubMed] [Google Scholar]
- Cerda-Cavieres C, Quiroz G, Iturriaga-Vásquez P, Rodríguez-Lavado J, Alarcón-Espósito J, Saitz C, Pessoa-Mahana CD, Chung H, Araya-Maturana R, Mella-Raipán J, Cabezas D, Ojeda-Gómez C, Reyes-Parada M, Pessoa-Mahana H (2020a) Synthesis, docking, 3-D-qsar, and biological assays of novel indole derivatives targeting serotonin transporter, dopamine D2 receptor, and mao-a enzyme: in the pursuit for potential multitarget directed ligands. Molecules (Basel, Switzerland) 25(20):4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda-Cavieres C, Quiroz G, Iturriaga-Vásquez P, Rodríguez-Lavado J, Alarcón-Espósito J, Saitz C, Pessoa-Mahana CD, Chung H, Araya-Maturana R, Mella-Raipán J, Cabezas D (2020b) Synthesis, docking, 3-D-Qsar, and biological assays of novel indole derivatives targeting serotonin transporter, dopamine D2 receptor, and Mao-A enzyme: In the pursuit for potential multitarget directed ligands. Molecules 25:4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, Ren Q, Dong C, Fujita Y, Hashimoto K (2019) Comparison of antidepressant and side effects in mice after intranasal administration of (R, S)-ketamine,(R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav 181:53–59 [DOI] [PubMed] [Google Scholar]
- Chen L, Boinpally R, Gad N, Greenberg WM, Wangsa J, Periclou A, Ghahramani P (2015a) Evaluation of cytochrome P450 (CYP) 3A4-based interactions of levomilnacipran with ketoconazole, carbamazepine or alprazolam in healthy subjects. Clin Drug Investig 35:601–612 [DOI] [PubMed] [Google Scholar]
- Chen L, Greenberg WM, Gommoll C, O’Connor J, Zukin SR, Periclou A, Ghahramani P (2015b) Levomilnacipran pharmacokinetics in healthy volunteers versus patients with major depressive disorder and implications for norepinephrine and serotonin reuptake inhibition. Clin Ther 37:2059–2070 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Zhao LB, Liu YY, Fan SH, Xie P (2019a) Comparative efficacy and acceptability of electroconvulsive therapy versus repetitive transcranial magnetic stimulation for major depression: a systematic review and multiple-treatments meta-analysis. Behav Brain Res 1:30–36 [DOI] [PubMed] [Google Scholar]
- Chen MH, Cheng CM, Gueorguieva R, Lin WC, Li CT, Hong CJ, Tu PC, Bai YM, Tsai SJ, Krystal JH, Su TP (2019b) Maintenance of antidepressant and antisuicidal effects by D-cycloserine among patients with treatment-resistant depression who responded to low-dose ketamine infusion: a double-blind randomized placebo-control study. Neuropsychopharmacology 44(12):2112–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu E (2004) Epidemiology of depression in the Asia Pacific region. Australas Psychiatry 12:S4–S10 [DOI] [PubMed] [Google Scholar]
- Clack S, Ward T (2019) The classification and explanation of depression. Behav Chang 36:41–55 [Google Scholar]
- Clayton AH, Kornstein SG, Dunlop BW, Focht K, Musgnung J, Ramey T, Ninan PT (2013) Efficacy and safety of desvenlafaxine 50 mg/d in a randomized, placebo-controlled study of perimenopausal and postmenopausal women with major depressive disorder. J Clin Psychiatry 26:909–921 [DOI] [PubMed] [Google Scholar]
- Connolly KR, Thase ME (2016) Vortioxetine: a new treatment for major depressive disorder. Expert Opin Pharmacother 17(3):421–431 [DOI] [PubMed] [Google Scholar]
- Cuadros DF, Tomita A, Vandormael A, Slotow R, Burns JK, Tanser F (2019) Spatial structure of depression in South Africa: a longitudinal panel survey of a nationally representative sample of households. Sci Rep 9:979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Li S, Wang S (2024) Major depressive disorder: hypothesis, mechanism, prevention and treatment. Sig Transduct Target Ther 9:30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deecher DC, Beyer CE, Johnston G, Bray J, Shah S, Abou-Gharbia M, Andree TH (2006) Desvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther 318:657–665 [DOI] [PubMed] [Google Scholar]
- Delogu GL, Serra S, Quezada E, Uriarte E, Vilar S, Tatonetti NP, Viña D (2014) Monoamine oxidase (MAO) inhibitory activity: 3-phenylcoumarins versus 4-hydroxy-3-phenylcoumarins. Chem Med Chem 9(8):1672–1676 [DOI] [PubMed] [Google Scholar]
- Dobrek L, Głowacka K (2023) Depression and Its phytopharmacotherapy-a narrative review. Int J Mol Sci 24:4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donello JE, Banerjee P, Li YX, Guo YX, Yoshitake T, Zhang XL, Miry O, Kehr J, Stanton PK, Gross AL, Burgdorf JS, Kroes RA, Moskal JR (2019) Positive N-methyl-D-aspartate receptor modulation by rapastinel promotes rapid and sustained antidepressant-like effects. Int J Neuropsychopharmacol 22(3):247–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K, Smith MA, McCarthy DJ, Harmer CJ, Goodwin GM, Williams S, Deakin JF (2016) Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol 26(6):994–1003 [DOI] [PubMed] [Google Scholar]
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM (1997) Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol 333:99–104 [DOI] [PubMed] [Google Scholar]
- Elhwuegi AS (2004) Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry 28:435–445 [DOI] [PubMed] [Google Scholar]
- El-Sawy ER, Abdelwahab AB, Kirsch G (2021) Synthetic routes to coumarin(benzopyrone)-fused five-membered aromatic heterocycles built on the α-pyrone moiety. Part II: five-membered aromatic rings with multi heteroatoms. Molecules. 26(11):3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fábregas B, Moura A, Ávila R, Carmo R, Teixeira AL (2014) Serotonin-norepinephrine reuptake inhibitor desvenlafaxine for the treatment of interferon alfa-associated depression in patients with hepatitis C. Braz J Psychiatry 36:183 [DOI] [PubMed] [Google Scholar]
- Farah WH, Alsawas M, Mainou M, Alahdab F, Farah MH, Ahmed AT, Mohamed EA, Almasri J, Gionfriddo MR, Castaneda-Guarderas A, Mohammed K (2016) Non-pharmacological treatment of depression: a systematic review and evidence map. BMJ Evidence-Based Medicine 1:214–221 [DOI] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J (2020) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 1:1592–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK, Horn SR, Kautz M, Corniquel M, Collins KA, Bevilacqua L (2021) A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry 178:193–202 [DOI] [PubMed] [Google Scholar]
- Feinberg S (2010) Correction of venlafaxine-and duloxetine-induced transaminase elevations with desvenlafaxine in a patient with Gilbert’s syndrome. CNS Spectr 15:53–55 [DOI] [PubMed] [Google Scholar]
- Finberg JPM, Rabey JM (2016) Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front Pharmacol 7:340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Groark J, Chiles D, Ramaker S, Yang L, Tourian KA (2014) Safety and tolerability of desvenlafaxine in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Groark J, Tourian KA, Ramaker SA, Chiles D, Yang L, Nichols AI (2016) Pharmacokinetics and tolerability of single-ascending doses of desvenlafaxine administered to children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 26:909–921 [DOI] [PubMed] [Google Scholar]
- Firth J, Teasdale SB, Allott K, Siskind D, Marx W, Cotter J, Veronese N, Schuch F, Smith L, Solmi M, Carvalho AF (2019) The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 18:308–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishback JA, Robson MJ, Xu YT, Matsumoto RR (2010) Sigma receptors: potential targets for a new class of antidepressant drug. Pharmacol Ther 127:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Morales V, Villasana-Ruíz AP, Garza-Veloz I, González-Delgado S, Martinez-Fierro ML (2023) Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules (Basel, Switzerland) 28(5):2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier NM, Duman RS (2012) Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression. Behav Brain Res 227:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Gruenbaum BF, Zlotnik A, Semyonov M, Frenkel A, Boyko M (2022) Pathophysiology and current drug treatments for post-stroke depression: a review. Int J Mol Sci 23(23):15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Tripathi A, Deshmukh D, Gaur M (2020) Cognitive behavioral therapy for depression. Indian J Psychiatry 1:S223–S229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman PK (2007) Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol 151(6):737–748. 10.1038/sj.bjp.0707253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommoll CP, Greenberg WM, Chen C (2014) A randomized, double-blind, placebo-controlled study of flexible doses of levomilnacipran ER (40–120 mg/day) in patients with major depressive disorder. J Drug Assess 16:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Beeské S, Stahl SM (2012) The vasopressin V1b receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind Placebo-controlled studiese. J Clin Psychiatry 73:1403 [DOI] [PubMed] [Google Scholar]
- Gu ZS, Xiao Y, Zhang QW, Li JQ (2017) Synthesis and antidepressant activity of a series of arylalkanol and aralkyl piperazine derivatives targeting SSRI/5-HT1A/5-HT7. Bioorg Med Chem Lett 27:5420–5423 [DOI] [PubMed] [Google Scholar]
- Guo J, Zhou D, Grimm SW, Bui KH (2015) Pharmacokinetics, metabolism and excretion of [(14)C]-lanicemine (AZD6765), a novel low-trapping N-methyl-d-aspartic acid receptor channel blocker, in healthy subjects. Xenobiotica 45:244–255 [DOI] [PubMed] [Google Scholar]
- Hashimoto K (2009) Comments on an innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101606 in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 29:411–412 [DOI] [PubMed] [Google Scholar]
- Hashimoto K (2022) Ketamine: anesthetic, psychotomimetic, antidepressant, or anthelmintic? Mol Psychiatry 27:3116–3118 [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, Kremer I (2006) Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord 93:239–243 [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, Kremer I (2013a) A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol 16(3):501–506 [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH (2015) A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol 23(1):1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I (2002) Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur Psychiatry 17(S3):294s–299s [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Nomikos GG, Kaul I, Raines S, Wald J, Bullock A, Sankoh AJ, Doherty J, Kanes SJ, Colquhoun H (2020) SAGE-217, a novel GABAA receptor positive allosteric modulator: clinical pharmacology and tolerability in randomized phase I dose-finding studies. Clin Pharmacokinet 59(1):111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yang J, Yang S, Cao S, Qin D, Zhou Y, Li X, Ye Y, Wu J (2017) Role of tandospirone, a 5-HT1A receptor partial agonist, in the treatment of central nervous system disorders and the underlying mechanisms. Oncotarget 8(60):102705–102720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes C, Sic A, Knezevic NN (2024) Substance P’s impact on chronic pain and psychiatric conditions—a narrative review. Int J Mol Sci 25(11):5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MI, Blumberger DM, Castle DJ, Ledwos N, Fellows E, Jones BDM, Ortiz A, Kloiber S, Wang W, Rosenblat JD, Mulsant BH (2023) Psilocybin for treatment-resistant depression without psychedelic effects: study protocol for a 4-week, double-blind, proof-of-concept randomised controlled trial. Bjpsych Open 9(4):e134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O (2006) Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci 26:9703–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosifescu DV, Jones A, O’Gorman C, Streicher C, Feliz S, Fava M, Tabuteau H (2022) Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin psychiatry 83(4):2114345 [DOI] [PubMed] [Google Scholar]
- Jairath V, Zou G, Parker CE, Macdonald JK, Mosli MH, Khanna R, Shackelton LM, Vandervoort MK, AlAmeel T, Al Beshir M, AlMadi M, Al-Taweel T, Atkinson NS, Biswas S, Chapman TP, Dulai PS, Glaire MA, Hoekman D, Koutsoumpas A, Minas E, Feagan BG (2016) Systematic review and meta-analysis: placebo rates in induction and maintenance trials of ulcerative colitis. J Crohn’s Colitis. 10(5):607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatav V, Mishra P, Kashaw S, Stables JP (2008) CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur J Med Chem 43:1945–1954 [DOI] [PubMed] [Google Scholar]
- Jelen LA, Young AH, Stone JM (2021) Ketamine: a tale of two enantiomers. J Psychopharmacol 35:109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SH, Han XH, Hong SS, Hwang JS, Hwang JH, Lee D, Lee MK, Ro JS, Hwang BY (2006) Monoamine oxidase inhibitory coumarins from the aerial parts of Dictamnus albus. Arch Pharmacal Res 29:1119–1124 [DOI] [PubMed] [Google Scholar]
- Jo YS, Huong DT, Bae KH, Lee MK, Kim YH (2002) Monoamine oxidase inhibitory coumarin from Zanthoxylum schinifolium. Planta Med 68:84–85 [DOI] [PubMed] [Google Scholar]
- Kaczor AA, Targowska-Duda KM, Silva AG, Kondej M, Biała G, Castro M (2020) N-(2-hydroxyphenyl)-1-[3-(2-oxo-2,3-dihydro-1H- benzimidazol-1-yl)propyl]piperidine-4-carboxamide (D2AAK4), a multi-target ligand of aminergic GPCRs, as a potential antipsychotic. Biomolecules 10(2):349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczor AA, Kędzierska E, Wróbel TM, Grudzińska A, Pawlak A, Laitinen T, Bartyzel A (2023) Synthesis, structural and behavioral studies of indole derivatives D2AAK5, D2AAK6 and D2AAK7 as serotonin 5-HT1A and 5-HT2A receptor ligands. Molecules 28:383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaggwa MM, Najjuka SM, Bongomin F, Mamun MA, Griffiths MD (2022) Prevalence of depression in Uganda: a systematic review and meta-analysis. PLoS One. 17(10):e0276552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källström S, Leino R (2008) Synthesis of pharmaceutically active compounds containing a disubstituted piperidine framework. Bioorg Med Chem 16:601–635 [DOI] [PubMed] [Google Scholar]
- Kaufman J, DeLorenzo C, Choudhury S, Parsey RV (2016) The 5-HT1A receptor in major depressive disorder. Eur Neuropsychopharmacol 26(3):397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C, Turan Yücel NA, Kandemir U, Osmaniye D, Can Ö, Demir Özkay ÜM (2022) Synthesis and antidepressant-like activities of some piperidine derivatives: involvements of monoaminergic and opioidergic systems. Acta Pol Pharm 79:4 [Google Scholar]
- Kennedy MB (2013) Synaptic signaling in learning and memory. Cold Spring Harb Perspect Biol 8:a016824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kılıç CS (2022) Herbal coumarins in healthcare. Herbal biomolecules in healthcare applications. Academic Press, New York, pp 363–380 [Google Scholar]
- Kim JY, Kim D, Kang SY, Park WK, Kim HJ, Jung ME, Son EJ, Pae AN, Kim J, Lee J (2010) Arylpiperazinecontaining pyrimidine 4-carboxamide derivatives targeting serotonin 5-HT(2A), 5-HT(2C), and the serotonin transporter as a potential antidepressant. Bioorg Med Chem Lett 20:6439–6442 [DOI] [PubMed] [Google Scholar]
- Kong LD, Tan RX, Woo AY, Cheng CH (2001) Inhibition of rat brain monoamine oxidase activities by psoralen and isopsoralen: implications for the treatment of affective disorders. Pharmacol Toxicol 88:75–80 [DOI] [PubMed] [Google Scholar]
- Krol M, Ślifirski G, Kleps J, Ulenberg S, Belka M, Bączek T, Siwek A, Stachowicz K, Szewczyk B, Nowak G, Duszyńska B (2021) Synthesis of novel pyrido [1, 2-c] pyrimidine derivatives with 6-Fluoro-3-(4-piperidynyl)-1, 2-benzisoxazole moiety as potential SSRI and 5-ht1a receptor ligands. Int J Mol Sci 22:2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RR, Kumar V, Kaur D, Nandi NK, Dwivedi AR, Kumar V, Kumar B (2021a) Investigation of indole-3-piperazinyl derivatives as potential antidepressants: design, synthesis, in-vitro, in-vivo and in-silico analysis. ChemistrySelect 6:11276–11284 [Google Scholar]
- Kumar RR, Sahu B, Pathania S, Singh PK, Akhtar MJ, Kumar B (2021b) Piperazine, a key substructure for antidepressants: its role in developments and structure-activity relationships. ChemMedChem 16:1878–1901 [DOI] [PubMed] [Google Scholar]
- Kupferberg A, Hasler G (2023) The social cost of depression: Investigating the impact of impaired social emotion regulation, social cognition, and interpersonal behavior on social functioning. J Affect Disorders Rep. 14:100631 [Google Scholar]
- Lacivita E, Di Pilato P, De Giorgio P, Colabufo NA, Berardi F, Perrone R, Leopoldo M (2012) The therapeutic potential of 5-HT1A receptors: a patent review. Expert Opin Ther Pat 22:887–902 [DOI] [PubMed] [Google Scholar]
- Leblhuber F, Geisler S, Ehrlich D, Steiner K, Reibnegger G, Fuchs D, Kurz K (2021) Repetitive transcranial magnetic stimulation in the treatment of resistant depression: changes of specific neurotransmitter precursor amino acids (Vienna, Austria : 1996). J Neural Trans. 128(8):1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Ryu HW, Kang MG, Park D, Lee H, Shin HM, Oh SR, Kim H (2017a) Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. Int J Biol Macromol 1:598–605 [DOI] [PubMed] [Google Scholar]
- Lee M, Yoo J, Kim JG, Kyung HS, Bin SI, Kang SB, Choi CH, Moon YW, Kim YM, Han SB, In Y, Choi CH, Kim J, Lee BK, Cho S (2017b) A randomized, multicenter, phase III trial to evaluate the efficacy and safety of polmacoxib compared with celecoxib and placebo for patients with osteoarthritis. Clin Orthop Surg 9(4):439–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramanieapillai M, Fan B, Lu C, McIntyre RS (2019) Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry 9:190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR, Tourian KA (2010) Efficacy, safety, and tolerability of Desvenlafaxine 50 mg/d for the treatment of major depressive disorder:a systematic review of clinical trials prim care companion. J Clin Psychiatry. 12:PCC.09r00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi S, Rahmani T, Hatami M, Pouramiri B, Kermani ET, Rezvannejad E, Mortazavi M, Hafshejani SF, Askari N, Pourjamali N, Zahedifar M (2020) Design, synthesis and biological assessment of acridine derivatives containing 1, 3, 4-thiadiazole moiety as novel selective acetylcholinesterase inhibitors. Bioorg Chem 105:104457 [DOI] [PubMed] [Google Scholar]
- Lucas MC, Weikert RJ, Carter DS, Cai HY, Greenhouse R, Lyer PS, Lin CJ, Lee EK, Madera AM, Moore A, Ozboya K, Schoenfeld RC, Steiner S, Zhai Y, Lynch SM (2010) Design, synthesis and biological evaluation of new monoamine reuptake inhibitors with potential therapeutic utility in depression and pain. Bioorg Med Chem Lett 20:5559–5566 [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N (2011) The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16(4):383–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkowska M, Bucki A, Sniecikowska J, Zagórska A, Fajkis-Zajączkowska N, Siwek A, Gluch-Lutwin M, Żmudzki P, Jastrzebska-Wiesek M, Partyka A, Wesołowska A, Abram M, Przejczowska-Pomierny K, Cios A, Wyska E, Mika K, Kotańska M, Mierzejewski P, Kolaczkowski M (2021) Multifunctional arylsulfone and arylsulfonamide-based ligands with prominent mood-modulating activity and benign safety profile, targeting neuropsychiatric symptoms of dementia. J Med Chem 64(17):12603–12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato JM, Martínez-Chantar ML, Lu SC (2013) S-adenosylmethionine metabolism and liver disease. Ann Hepatol 12(2):183–189 [PMC free article] [PubMed] [Google Scholar]
- Mattson RJ, Catt JD, Denhart DJ, Deskus JA, Ditta JL, Higgins MA, Marcin LR, Sloan CP, Beno BR, Gao Q, Cunningham MA, Mattson GK, Molski TF, Taber MT, Lodge NJ (2005) Conformationally restricted homotryptamines. 2. Indole cyclopropylmethylamines as selective serotonin reuptake inhibitors. J Med Chem 48:6023–6034 [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Alsuwaidan M, Baune BT, Berk M, Demyttenaere K, Goldberg JF, Gorwood P, Ho R, Kasper S, Kennedy SH, Ly-Uson J, Mansur RB, McAllister-Williams RH, Murrough JW, Nemeroff CB, Nierenberg AA, Rosenblat JD, Sanacora G, Schatzberg AF, Shelton R, Maj M (2023) Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. 22(3):394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmar TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765 [DOI] [PubMed] [Google Scholar]
- Moore TJ, Alami A, Alexander GC, Mattison DR (2022) Safety and effectiveness of NMDA receptor antagonists for depression: a multidisciplinary review. Pharmacotherapy 42(7):567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R (1998) Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci 1:659–667 [DOI] [PubMed] [Google Scholar]
- Munir S, Shahid A, Aslam B, Ashfaq UA, Akash MSH, Ali MA, Almatroudi A, Allemailem KS, Rajoka MSR, Khurshid M (2020) The therapeutic prospects of naturally occurring and synthetic indole alkaloids for depression and anxiety disorders. Evid-Based Complement Alternative Med 2020:8836983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeel Z, Jaber QA, Abdul-Rida NA (2021) Novel benzo [f] coumarin derivatives as probable acetylcholinesterase inhibitors: synthesis, in vitro, and in silico studies for evaluation of their anti-AChE activity. Indonesian J Chem 22:35–46 [Google Scholar]
- Ogbo FA, Mathsyaraja S, Koti RK, Perz J, Page A (2018) The burden of depressive disorders in South Asia, 1990–2016: findings from the global burden of disease study. BMC Psychiatry 18:1–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olié JP, Kasper S (2007) Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol 10(5):661–673 [DOI] [PubMed] [Google Scholar]
- Ortega MA, Fraile-Martínez Ó, García-Montero C, Alvarez-Mon MA, Lahera G, Monserrat J, Llavero-Valero M, Gutiérrez-Rojas L, Molina R, Rodríguez-Jimenez R, Quintero J (2022) Biological role of nutrients, food and dietary patterns in the prevention and clinical management of major depressive disorder. Nutrients 28:3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro Y, Sakurai Y, Sato S, Kurahashi N, Tanaka T, Kikuchi T, Tottori K, Uwahodo Y, Miwa T, Nishi T (2000) 3,4-dihydro-2(1H)-quinolinone as a novel antidepressant drug: synthesis and pharmacology of 1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-3,4- dihydro-5-methoxy-2(1H)-quinolinone and its derivatives. J Med Chem 43(2):177–189 [DOI] [PubMed] [Google Scholar]
- Osmaniye D, Kurban B, Sağlık BN, Levent S, Özkay Y, Kaplancıklı ZA (2021) Novel thiosemicarbazone derivatives in vitro and in silico evaluation as potential MAO-B inhibitors. Molecules (Basel, Switzerland). 26(21):6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowska K, Leśniak A, Karczyńska U, Jeleniewicz P, Głuch-Lutwin M, Mordyl B, Siwek A, Trzaskowski B, Sacharczuk M, Bujalska-Zadrożny M (2020) 6-Acetyl-5-hydroxy-4, 7-dimethylcoumarin derivatives: Design, synthesis, modeling studies, 5-HT1A, 5-HT2A and D2 receptors affinity. Bioorg Chem 100:103912 [DOI] [PubMed] [Google Scholar]
- Palazidou E (2012) The neurobiology of depression. Br Med Bull 101:127–145 [DOI] [PubMed] [Google Scholar]
- Patel VR, Won Park S (2013) An evolving role of piperazine moieties in drug design and discovery. Mini Rev Med Chem 13:1579–1601 [DOI] [PubMed] [Google Scholar]
- Patel A, Arora GS, Roknsharifi M, Javed H, Kaur P (2023) Relamorelin in gastroparesis and diabetic gastroparesis: a meta-analysis on its efficacy and safety. Cureus 15(11):e48303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak E, Stasiuk W, Szopa A, Wyska E, Serefko A, Oniszczuk A, Wośko S, Świąder K, Wlaź P (2016) Traxoprodil, a selective antagonist of the NR2B subunit of the NMDA receptor, potentiates the antidepressant-like effects of certain antidepressant drugs in the forced swim test in mice. Metab Brain Dis 31:803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP101606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637 [DOI] [PubMed] [Google Scholar]
- Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K (2017) Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci Rep 7:15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recourt K, de Boer P, Zuiker R, Luthringer R, Kent J, van der Ark P, Van Hove I, van Gerven J, Jacobs G, van Nueten L, Drevets W (2019) The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl Psychiatry 9(1):216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GM, First MB, Kogan CS, Hyman SE, Gureje O, Gaebel W, Maj M, Stein DJ, Maercker A, Tyrer P, Claudino A, Garralda E, Salvador-Carulla L, Ray R, Saunders JB, Dua T, Poznyak V, Medina-Mora ME, Pike KM, Ayuso-Mateos JL, Kanba S, Keeley JW, Khoury B, Krasnov VN, Kulygina M, Lovell AM, de Jesus MJ, Maruta T, Matsumoto C, Rebello TJ, Roberts MC, Robles R, Sharan P, Zhao M, Jablensky A, Udomratn P, Rahimi-Movaghar A, Rydelius PA, Bährer-Kohler S, Watts AD, Saxena S (2019) Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 1:3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehuman NA, Mathew B, Jat RK, Nicolotti O, Kim H (2020) A comprehensive review of monoamine oxidase-a inhibitors in their syntheses and potencies. Comb Chem High Throughput Screening 1:898–914 [DOI] [PubMed] [Google Scholar]
- Ritter N, Disse P, Wünsch B, Seebohm G, Strutz-Seebohm N (2023) Pharmacological potential of 3-benzazepines in NMDAR-linked pathophysiological processes. Biomedicines 11(5):1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S, Khan AM (2019) Use of transcranial magnetic stimulation for depression. Cureus 23:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Carter B, Young AH (2018) Caveat emptor: folate in unipolar depressive illness, a systematic review and meta-analysis. J Psychopharmacol 32:377–384 [DOI] [PubMed] [Google Scholar]
- Sağlık BN, Çavuşoğlu BK, Çevik UA, Osmaniye D, Levent S, Özkay Y, Kaplancıklı ZA (2020) Novel 1, 3, 4-thiadiazole compounds as potential MAO-A inhibitors–design, synthesis, biological evaluation and molecular modelling. RSC Med Chem 11:1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Bidawat P, Seth M, Gharu CP (2014) Synthesis of formazans from Mannich base of 5-(4-chlorophenyl amino)-2-mercapto-1, 3, 4-thiadiazole as antimicrobial agents. Arab J Chem 7:181–187 [Google Scholar]
- Sahni T, Sharma S, Verma D, Kaur P (2021) Overview of coumarins and its derivatives: synthesis and biological activity. Lett Org Chem 1:880–902 [Google Scholar]
- Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, Quirk MC (2014) Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry 19:978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, Burke MA, Zajecka JM, Barra L, Su HL, Posener JA, Bui KH, Quirk MC, Piser TM, Mathew SJ, Pathak S (2017) Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology 42(4):844–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone RA, Sansone LA (2014) Serotonin norepinephrine reuptake inhibitors: a pharmacological comparison. Innov Clin Neurosci 11(3–4):37–42 [PMC free article] [PubMed] [Google Scholar]
- Santi NS, Biswal SB, Naik BN, Sahoo JP, Rath B (2024) A Randomized controlled trial comparing efficacy and safety of antidepressant monotherapy. Cureus 16(4):e59074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris J, Murphy J, Stough C, Mischoulon D, Bousman C, MacDonald P, Adams L, Nazareth S, Oliver G, Cribb L, Savage K (2020) S-Adenosylmethionine (SAMe) monotherapy for depression: an 8-week double-blind, randomised, controlled trial. Psychopharmacology 237:209–218 [DOI] [PubMed] [Google Scholar]
- Sashidhara KV, Modukuri RK, Singh S, Rao KB, Teja GA, Gupta S, Shukla S (2015) Design and synthesis of new series of coumarin–aminopyran derivatives possessing potential anti-depressant-like activity. Bioorg Med Chem Lett 15:337–341 [DOI] [PubMed] [Google Scholar]
- Scotton E, Antqueviezc B, de Vasconcelos MF, Dalpiaz G, Géa LP, Goularte JF, Colombo R, Rosa AR (2022) Is (R)-ketamine a potential therapeutic agent for treatment-resistant depression with less detrimental side effects? A review of molecular mechanisms underlying ketamine and its enantiomers. Biochem Pharmacol 198:114963 [DOI] [PubMed] [Google Scholar]
- Serban G, Stanasel O, Serban E, Bota S (2018) 2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents. Drug Des Dev Ther 12:1545–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Misra GP, Sainy J, Chaturvedi SC (2011) Synthesis and biological evaluation of 2-amino-5-sulfanyl-1, 3, 4-thiadiazole derivatives as antidepressant, anxiolytics and anticonvulsant agents. Med Chem Res 20:245–253 [Google Scholar]
- Sharma H, Santra S, Dutta A (2015) Triple reuptake inhibitors as potential next-generation antidepressants: a new hope? Future Med Chem 7(17):2385–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler ZM, Patel P, Abdijadid S (2023) Antidepressants. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK538182/. Accessed 25 Dec 2023
- Siddiqui N, Alam P, Ahsan W (2009) Design, synthesis, and in-vivo pharmacological screening of N,3-(substituted diphenyl)-5-phenyl1H-pyrazoline-1-carbothioamides derivatives. Arch Pharm (Weinheim) 342:173–181 [DOI] [PubMed] [Google Scholar]
- Siddiqui N et al (2011) Antidepressant potential of nitrogen-containing heterocyclic moieties: an updated review. J Pharm Bioallied Sci 3(2):194–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Chary S, Thakur P, Talluri L, Reddy M, Verma KK, Saha P, Gupta VB, Ramaiah KA, Khanum SZ (2021) A phase III prospective active and placebo-controlled randomized trial of vilazodone in the treatment of major depressive disorder. Cureus 13(7):e16689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Singh K, Ved A, Hasan SM, Mujeeb S (2022) Therapeutic journey and recent advances in the synthesis of coumarin derivatives. Mini Rev Med Chem 1:1314–1330 [DOI] [PubMed] [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004) A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Primary Care Companion J Clin Psychiatry 6(4):159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Kohn TJ, Honigschmidt NA, Rocco VP, Spinazze PG, Koch DJ, Nelson DL, Wainscott DB, Ahmad LJ, Shaw J, Threlkeld PG, Wong DT (2003) Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities. Part 1. Bioorg Med Chem Lett 13:1903–1905 [DOI] [PubMed] [Google Scholar]
- Thurfah JN, Christine BPP, Alfian SD, Puspitasari IM (2022) Dietary supplementations and depression. J Multidiscip Healthc 17:1121–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SB, Deng XQ, Zheng Y, Yuan YP, Quan ZS, Guan LP (2012) Synthesis and evaluation of anticonvulsant and antidepressant activities of 5-alkoxytetrazolo [1, 5-c] thieno [2, 3-e] pyrimidine derivatives. Eur J Med Chem 56:139–144 [DOI] [PubMed] [Google Scholar]
- Wang X, Yang J, Hashimoto K (2022) (R)-ketamine as prophylactic and therapeutic drug for neurological disorders: beyond depression. Neurosci Biobehav Rev 139:104762 [DOI] [PubMed] [Google Scholar]
- Wani AL, Bhat SA, Ara A (2015) Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integr Med Res 4(3):132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Chang L, Hashimoto K (2022) Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry 27:559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenga Z, Li J (2010) Synthesis and antidepressant activity of optical isomers of 2-(4-benzylpiperazin-1-yl)-1-(5-chloro-6-methoxynaphthalen-2-yl) propan-1-ol (SIPI5056). Bioorg Med Chem Lett 20:1256–1259 [DOI] [PubMed] [Google Scholar]
- Witkin JM, Wallace TL, Martin WJ (2019) Therapeutic approaches for NOP receptor antagonists in neurobehavioral disorders: clinical studies in major depressive disorder and alcohol use disorder with BTRX-246040 (LY2940094). Handb Exp Pharmacol 254:399–415 [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017) Possible role of the gut microbiota–brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry 18:1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan RX, Jiang KY, Wu JW, Zhang ZX, Li MS, Li JQ, Ni F (2022) Synthesis and antidepressant activity of novel 1-(1-benzoylpiperidin-4-yl) methanamine derivatives selectively targeting SSRI/5-HT1A. Bioorg Med Chem Lett 76:129006 [DOI] [PubMed] [Google Scholar]
- Yun BS, Lee IK, Ryoo IJ, Yoo ID (2001) Coumarins with monoamine oxidase inhibitory activity and antioxidative coumarino-lignans from hibiscus s yriacus. J Nat Prod 28:1238–1240 [DOI] [PubMed] [Google Scholar]
- Yurkewicz L, Weaver J, Bullock MR, Marshall LF (2005) The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma 22(12):1428–1443 [DOI] [PubMed] [Google Scholar]
- Zagaja M, Zagaja A, Szala-Rycaj J, Szewczyk A, Lemieszek MK, Raszewski G, Andres-Mach M (2022) Influence of umbelliferone on the anticonvulsant and neuroprotective activity of selected antiepileptic drugs: an in vivo and in vitro study. Int J Mol Sci 23(7):3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki N, Chen LN, Lane R, Doherty T, Drevets WC, Morrison RL, Sanacora G, Wilkinson ST, Popova V, Fu DJ (2023) Long-term safety and maintenance of response with esketamine nasal spray in participants with treatment-resistant depression: interim results of the SUSTAIN-3 study. Neuropsychopharmacology 48(8):1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewska A, Gałczyk M, Van Damme-Ostapowicz K (2022) Level of depression during the COVID-19 pandemic in Poland-A cross-sectional study. Healthcare (Basel, Switzerland) 10(6):1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CAJR, Machado-Vieira R (2017) Ketamine: translating mechanistic discoveries into the next generation of glutamate modulators for mood disorders. Mol Psychiatry 22:324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaręba P, Jaśkowska J, Czekaj I, Satała G (2019) Design, synthesis and molecular modelling of new bulky Fananserin derivatives with altered pharmacological profile as potential antidepressants. Bioorg Med Chem 27:3396–3407 [DOI] [PubMed] [Google Scholar]
- Zhang RH, Guo HY, Deng H, Li J, Quan ZS (2021a) Piperazine skeleton in the structural modification of natural products: a review. J Enzyme Inhib Med Chem 36(1):1165–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sakamoto A, Chang L, Qu Y, Wang S, Pu Y, Tan Y, Wang X, Fujita Y, Ishima T, Hatano M (2021b) Splenic NKG2D confers resilience versus susceptibility in mice after chronic social defeat stress: beneficial effects of (R)-ketamine. Eur Arch Psychiatry Clin Neurosci 271:447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]