Abstract
Radioembolization is a locoregional transarterial therapy that combines radionuclide and micron-sized beads to deliver radiation internally to the target tumors based on the arterial blood flow. While initially developed as a palliative treatment option, radioembolization is now used for curative intent treatment, neoadjuvant therapy, and method to downstage or bridge for liver transplant. Radioembolization has become increasingly utilized and is an important therapeutic option for the management of hepatocellular carcinoma and liver metastasis. This article provides an overview of the techniques, challenges, and novel developments in radioembolization, including new dosimetry techniques, radionuclides, and new target tumors.
Keywords: radioembolization, selective internal radiation therapy, dosimetry, eye90, holmium
Brief history of radioembolization
Radiation therapy involves the use of particles or electromagnetic waves to induce damage to cancer cells.1,2 Ionizing radiation, which forms sufficient particle energy, can be divided into either photon radiation (e.g., X-rays and gamma rays) or particle radiation (e.g., electron, proton, neutron, carbon ions, alpha particles, and beta particles). 3 Both forms of radiation can be used for imaging and treating cancer. Radiation therapy can be delivered either by external beam radiation therapy (i.e., local treatment by an extracorporeal machine), internal radiation therapy (i.e., intracorporeal radiation source), and radiopharmaceuticals (i.e., systemic treatment with drugs that contain radioisotopes).4,5 When internal radiation therapy is done with a solid source, such as a seed, ribbon, or capsule, it is termed brachytherapy. Brachytherapy with beta particles has revolutionized the treatment of liver cancer.
Transarterial radioembolization (TARE), also known as selective internal radiation therapy (SIRT), is a locoregional therapy that involves transarterial administration of micron-sized beads (microspheres) that carry radioactive isotopes to block a small portion of the blood supply and deliver highly targeted doses of radiation to tumors. 6 It was first developed as a palliative option for patients with unresectable liver tumors, but it is now increasingly utilized with curative intent. TARE is effective for treating liver cancer because of the liver’s dual blood supply. As liver tumors grow, their perfusion becomes dependent on the hepatic artery, while the liver is perfused primarily by the portal vein, enabling tumor-targeted therapy 7 with minimal collateral damage. TARE treatment for hepatocellular carcinoma (HCC) can increase the time to tumor progression, induce hypertrophy of the liver remnant, and/or downstage or bridge patients for transplantation or resection.6,8,9 Yttrium-90 ( 90 Y) is the radioactive isotope most commonly used with TARE. Compared with other techniques, such as surgery or ablation, radioembolization is more flexible with regard to the site and number of tumors treated. 10
Several clinical trials have reported the effective use of microspheres to deliver targeted radiation to liver tumors.11,12 There are also several ongoing clinical trials investigating the role of TARE for cancer patients, especially in the setting of systematic therapy. 13 Two manufactured microspheres are commercially available for clinical use: glass beads (TheraSphere®, BTG International Ltd, London, UK) and resin beads (SIR-Spheres®, SirTex Medical, Sydney, NSW, Australia).10,14,15 TheraSphere was approved by the Food and Drug Administration’s (FDA) under the Humanitarian Device Exemption provision for unresectable HCC in 1999 and was given full premarketing approval in 2021. SIR-Spheres was given approval 2002 to treat colorectal cancer with liver metastasis in conjunction with adjuvant floxuridine chemotherapy. 16 Glass beads (20–30 microns in diameter) are slightly smaller than resin beads (mean of 32 microns in diameter) and produce a lower degree of embolization and in most clinical practice deliver a higher activity per particle (Glass: 2500 Bq per microsphere; Resin: 50 Bq per microsphere 17 ).10,18–21 However, with the advent of flexible daily dosing adjustments, the activity of individual resin microspheres can vary depending on the day of calibration but does not generally exceed activity per particle when compared to glass (4000 Bq max). Similarly, offering different activities of Yttrium-90 ( 90 Y) glass beads within a 2-week range can provide flexibility in treatment planning by varying the number of microspheres per vial and their activity levels. By adjusting the number of microspheres and their activity, clinicians can optimize the radiation dose distribution within the liver, enhancing the effectiveness of the treatment while minimizing side effects.
Radioembolization for HCC
TARE may be considered for solitary HCC ⩽ 8 cm, given the findings from the LEGACY trial. 22 For patients with Barcelona clinic liver cancer (BCLC) stage A or B, radiation lobectomy via TARE may be considered in some patients in order to increase remnant liver volume, especially as a bridge to resection. 23 For patients who are not candidates for liver transplant, resection, or ablation, TARE may be considered if the patient meets the inclusion criteria from the LEGACY trial. However, TARE is not mentioned for patients with BCLC stage B. Finally, despite their use in real-life clinical scenarios, TARE is not included as an evidence-based recommendation for BCLC stage C patients because of negative results from prospective phase III trials.24–27 However, these studies used outdated methodologies.
According to the European Society for Medical Oncology (ESMO), radioembolization is not indicated as first-line therapy in patients with HCC who have intermediate or advanced stages. However, phase III studies have shown that TARE was associated with higher response rates, delayed progression of disease, and fewer adverse events when compared with sorafenib despite being negative trials.28,29 Retrospective cohort studies have demonstrated comparable survival rates to transarterial chemoembolization (TACE) and sorafenib.30–32 ESMO, therefore, recommends TARE for patients in “exceptional circumstances” or patients who have liver-confined disease with preserved liver function for which TACE and systemic therapy are not feasible. 33 ESMO also considers TARE instead of TACE for treating small liver tumors in patients who are waiting for liver transplants in order to avoid drop-out because of disease progression. 33 Moreover, TRACE was a phase II trial of 72 patients with unresectable HCC that showed a significantly higher survival in median overall survival for TARE over drug eluting beads TACE (DEB-TACE) (30.2 vs 15.6 months, p = 0.006). 34 The National Comprehensive Cancer Network (NCCN) acknowledges the use of radioembolization in some patients with advanced HCC with liver-confined or limited extrahepatic disease and recommends a tumor dose of >205 Gy or >400 Gy if selective. 35
There have been several studies comparing TARE to ablation and surgery. A 2022 systematic review and network meta-analysis evaluated 24 randomized controlled trials and propensity score-matched cohort analyses with 5549 patients with HCC to compare radioembolization, ablation, TACE, and radiation. 36 The study found that 1-year overall survival was greater for radioembolization than TACE but no significant difference was seen in the other 1-year overall survival comparisons or in the 2- and 3-year survival comparisons (except radiation over ablation at 3-year). 36 Other studies have suggested that radiation segmentectomy has similar outcomes and may be more cost-efficient than ablation with or without TACE for small <2 cm HCC. 37 The RASER study was a single-center, single-arm study of 29 patients with unresectable ⩽3 cm Child–Pugh score A-B7, ECOG 0, HCC who underwent radiation segmentectomy and were deemed unfavorable to undergo ablation. 38 The study showed a complete response in 24 (83%) patients and a partial response in 5 (17%). 38 With regard to surgery, while it is generally recommended that small HCCs that are resectable undergo surgery, the management of large ⩾5 cm tends to be unclear and associated with poor postsurgical outcomes. 39 In a 2022 retrospective cohort study, 500 patients who underwent resection were compared to 57 patients who underwent TARE for single nodular ≥5 cm HCC at 2 tertiary centers in Korea. 40 The study found comparable results in overall survival, time to progression, and time to intrahepatic progression but a shorter hospital stay and fewer adverse events in the TARE arm. 40
Given that TARE delivers intense radiation to hepatic tumors with small embolic effects, radiologic and pathologic of TARE tend to be unique when compared with chemotherapy or TACE. Interval changes in size, tumor burden, and diffusion restriction are helpful to evaluate.41,42 It can take several months after TARE to see a radiographic response and typically show reduced size and decreased enhancement. Additional post-treatment findings, including peritumoral edema, inflammation, ring enhancement, fibrosis, and capsular retraction, may make it harder to interpret; however, these findings are not usually signs of tumor progression.41,42 Pathologic changes in the tumor bed after TARE include necrosis, mucinous changes, the presence of foamy histiocytes, ectatic vessels, calcification, and fibrosis. 43
Radioembolization for metastatic colorectal cancer
While combination chemotherapy and biologics have improved the survival for patients with metastatic colorectal cancer to the liver, these therapies are very difficult to endure and about a third of patients discontinue them before finishing an entire cycle.44,45 TARE offers a promising alternative that has been shown to be effective and well tolerated, but most of the data are limited to retrospective, single-center studies, and the exact patient population to receive the most benefit with TARE is still being determined. 46
According to the NCCN guidelines for metastatic disease, TARE may be considered for hepatic metastatic colorectal cancer that is not resectable due to insufficient remnant liver volume. It may also be considered in selective patients with colorectal cancer with predominant hepatic metastases and whose disease is chemotherapy-resistant or chemotherapy-refractory. 47 ESMO recommends 90Y radioembolization for patients with metastatic colorectal cancer, who have liver-limited disease that is unresponsive to any available chemotherapeutic options. 48
A recent study from the multicenter and prospective Radiation-Emitting SIR-Spheres in Non-Resectable (RESiN) liver tumor patient registry elucidated the role of TARE in treating liver-dominant metastatic colorectal cancer with regard to the ideal timing and patient characteristics that would lead to improved outcomes and safety. 49 The study, which consisted of 42 centers and 498 patients, investigated TARE as first-line therapy in 17% of patients, second-line therapy in 41% of patients, and third-line therapy or beyond (salvage) in 43% of patients. The study found a median overall survival of 15 months (95% CI: 13.3–16.9) for the entire cohort, and survival was statistically different based on the line of therapy (13.9 months for first-line therapy, 17.4 months for second-line therapy, and 12.5 months for third-line therapy, p = 0.002). Progression-free survival (PFS) was 7.4 months (95% CI: 6.4–9.5) in the entire cohort and significantly differed based on the line of therapy (7.9 months for first-line therapy, 10 months for second-line therapy, and 5.9 months for third-line therapy, p = 0.004). The study also found promising safety profiles with grade 3 or greater hepatic function toxicity rates less than 10%. These findings suggest that TARE may be more beneficial after patients progress on first-line chemotherapy.
Radioembolization technique
Various practice guidelines and recommendations have been published that provide details on the administration and optimization of TARE.14,50–55 This section will summarize a brief overview of the process with illustrative case studies.
Once patients are determined to be eligible candidates for TARE, they typically undergo a series of standard preparatory steps.56–58 Patients first undergo a hepatic angiogram to map out the patient’s hepatic arterial anatomy, identify the vessels supplying the tumor, which can demonstrate anatomic variations in about 45% of patients, 59 and assess for extrahepatic perfusion. A detailed understanding of the patient’s arterial anatomy is important to prevent non-target extrahepatic deposition of radioactive particles and damage to non-target tissues. 56 Furthermore, the angiogram can help select the optimal catheter position to maximize tumor treatment while minimizing non-involved liver damage.58,60 During angiography, collateral and extrahepatic vessels can be embolized if needed. 57 Afterward, 99mTechnetium-macroaggregated albumin (Tc-MAA) imaging and scintigraphy are performed to identify the distribution of the beads and identify potential gastrointestinal, intrahepatic, and/or pulmonary shunts. Generally, elevated lung-shunt fraction (LSF) is considered to be greater than 20% 61 and the maximum tolerated dose to the lung is 30 gray (Gy) for a single treatment or 50 Gy for multiple treatments. 57 In addition to conventional angiography, cone-beam CT is used to more accurately define vascular anatomy and tissue perfusion, which also aids in dosimetry calculations.62–65
After assessment of extrahepatic shunting, TARE procedure is performed with delivery of either resin or glass microsphere to the target hepatic artery. 57 For resin 90Y radioembolization, a catheter is placed in the target hepatic artery supplying tumor distal to the arteries supplying the gastrointestinal system and in similar location of Tc-MAA administration. The microspheres are injected slowly at a rate of <0.3 mL/s to avoid reflux. Rather than blind infusion, intermittent fluoroscopic guidance should be utilized to confirm antegrade flow. In addition, 5% glucose solution reduces stasis and is recommended over sterile water to minimize patient discomfort. Contrast medium should be repeatedly injected through the left-hand port of the delivery set to ensure that the catheter is correctly placed with forward flow. Glass 90Y radioembolization involves the injection of fewer microspheres (approximately 1.2–8 million), and a small volume (approximately 20–60 mL). The actual infusion itself is done with a slow hand injection and free breathing. About 60 mL of normal saline solution is delivered, and the infusion takes about 5 min. Like with resin 90Y radioembolization, blind infusions are not recommended with 166Ho microsphere given the relatively high embolic load, and the catheter is placed in the target hepatic artery distal to the arteries supplying the gastrointestinal system and in similar location of Tc-MAA administration with a slow infusion rate of <0.3 mL/min. Contrast media can be used to ensure good catheter position and forward flow. The use of antireflux catheter can be used to improve tumor deposition and minimize non-target injury to normal liver and extrahepatic organs.
TARE can be delivered in a single treatment session or span multiple sessions, depending on the specific tumor(s) and location. The International Commission on Radiation Units and Measurements provides definitions for planning target volume (PTV), clinical target volume, and anisotropic margin that allows for patient- and procedure-specific geometric uncertainty. 66 The PTV may involve a segment, a lobe, or both lobes of the liver. 40 Targeting several tumors throughout the liver during a single session is defined as whole liver delivery; treating tumors in one lobe during a single session, followed by the other lobe in a separate session, is defined as sequential delivery; and targeting just one lobe is defined as lobar delivery. 58
The type of TARE can be divided by the targeted area(s). Radiation segmentectomy (Figure 1) is the delivery of an ablative dose that is limited to ⩽2 adjacent hepatic segments; radiation lobectomy is the delivery to the entire right or left lobe; and radiation sectorectomy is the delivery to specific parts of the liver that are determined by Couinaud’s divisions, which involves a plane dependent on the portal veins separated by a plane arising from the left, middle, and right hepatic veins.56,63
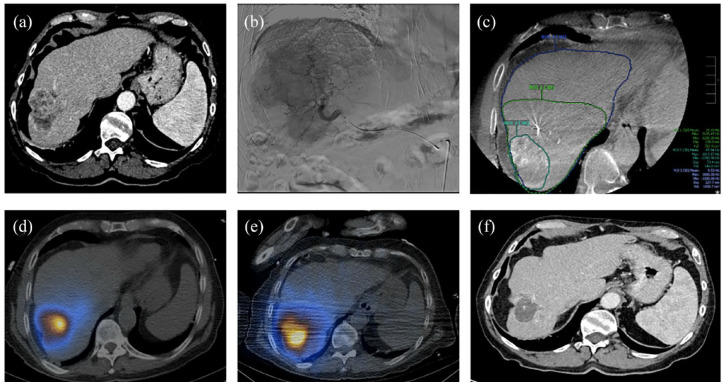
Figure 1.
90Y segmentectomy. (a) Axial CT shows solitary colorectal metastatic disease that is too large for successful ablation. (b) Angiogram reveals a larger disease burden than expected, affecting two segments. (c) CBCT obtained during angiogram and used to accurately calculate volumes for dose calculations. (d) SPECT/CT after Tc99-MAA administration. (e) Post 90Y SPECT; a 200 Gy dose was calculated and delivered to segments 5 and 8 (resin). (f) Axial CT shows interval decrease in size of tumor post 90Y.
CBCT, cone-beam CT; CT, computed tomography; SPECT, single-photon emission computed tomography; Tc-MAA, 99mTechnetium-macroaggregated albumin.
Some patients with lobar disease who are not amendable to radiation segmentectomy or lobectomy because of extensive disease may undergo a palliative-intent lobar dose (Figure 2).
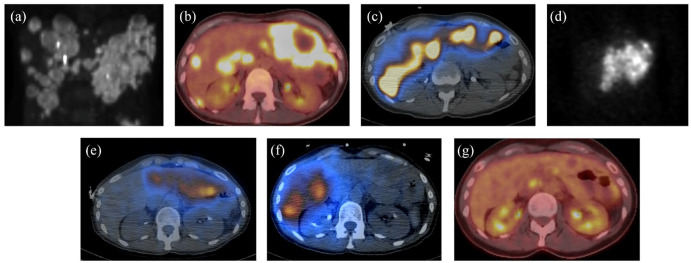
Figure 2.
90Y Lobar radioembolization. (a) Pre 90Y radioembolization planar PET/CT demonstrates the extent of colorectal metastatic disease in the liver. (b) Pre-90Y radioembolization axial fused PET/CT shows active metastasis in both lobes. (c) Axial fused SPECT Tc99-MAA shows delivery of Tc99-MAA particles in both lobes on mapping/shunt study. (d) Coronal planar SPECT study shows 90Y delivery to the left hepatic lobe. (e and f) Axial fused SPECT post 90Y demonstrates 90Y delivery to the left hepatic lobe after the first session (e) and right hepatic lobe on the follow up session (f); 110 Gy dose delivered to the tumor. (g) 10-Month post-treatment PET/CT shows a good response (e.g., no hypermetabolic disease).
CT, computed tomography; PET, positron emission tomography; SPECT, single-photon emission computed tomography; Tc-MAA, 99mTechnetium-macroaggregated albumin.
The aim is to slow down tumor progression with some degree of tolerable doses to functional tissue. 57
Another TARE option is to treat the whole liver, which is typically done for diffuse disease localized across both hepatic lobes. 56 This technique can be done by either treating each lobe separately or treating both lobes simultaneously. The bilobar technique typically requires waiting at least 30 days for liver regeneration after the first lobe is treated and is associated with fewer adverse events.19,56,67
Follow-up
Early imaging follow-up can demonstrate hepatic parenchymal enhancement after TARE, which may mimic the treated malignancy.68,69 As a result, imaging is recommended at around 6–8 weeks after treatment to evaluate the response. The type of post-treatment imaging also depends on the treatment modality. For 90Y radioembolization, 90Y positron emission tomography (PET) and single-photon emission computed tomography/computed tomography (SPECT/CT) are used to approximate 90Y distribution and confirm tumor uptake, while for 166Ho radioembolization, SPECT (ideally with additional low-dose CT) or magnetic resonance imaging (MRI) may be used. 57
Challenges and complications of radioembolization
While shown to be effective and relatively safe, TARE has several limitations, including the heterogeneous distribution of the beads and radiation dose. 56 Radioembolization, especially to infiltrative lesions, is also susceptible to the crossfire phenomenon (i.e., nearby tissues acquire radiation from surrounding targeted regions) or to radiation-induced bystander effects (i.e., signal-mediated effects that arise in unirradiated cells within an irradiated region). 70
Patients can experience various complications, such as post-radioembolization syndrome, which is similar to the post-embolization syndrome observed in patients receiving conventional TACE 71 but generally milder. There can be changes in hepatic volumes after TARE, possibly mediated by fibrosis and hepatic remodeling.72,73 These changes can lead to increases in portal pressures, such as splenomegaly.72,74,75 Hepatic complications, such as hepatic abscesses 76 or radioembolization-induced liver disease, which is characterized by jaundice, and ascites, may also occur within 1–2 months after TARE without progression of disease or occlusion of the biliary ducts. 77 Patients can also develop delayed hepatotoxicity, especially if they have a tumor burden of greater than 50% and cirrhosis. 78 Biliary complications, such as biliary necrosis with biloma formation, may also arise. 79 Furthermore, patients may develop perihepatic fluid and pleural effusions, radiation pneumonitis, lymphopenia, gastrointestinal complications, as well as pulmonary complications via microsphere shunting.75,80,81 The overall incidence of these complications comprises a very small fraction of patients.
Radioembolization in the era of systemic therapies
Integrating TARE with systemic therapies presents numerous potential synergistic benefits for the treatment of primary and metastatic liver tumors, including enhancing the sensitivity of tumor cells to chemotherapy and triggering heightened local and systemic immune responses when combined with immune checkpoint inhibitors (ICIs). 82 In this section, we explore the current evidence of TARE with systemic therapies in HCC, metastatic colorectal cancer to the liver, and intrahepatic cholangiocarcinoma.
Hepatocellular carcinoma
ICIs with a vascular endothelial-derived growth factor inhibitor (atezolizumab plus bevacizumab and tremelimumab plus durvalumab) have now become the new standard of care first-line therapy for unresectable HCC over sorafenib.83–86 Despite limited studies, radioembolization is a promising candidate for synergy with immunotherapy and has shown to recruit and activate intra-tumor effector immune cells and overcome exhaustion.82,87,88 A 2020 retrospective study investigated the safety of TARE with nivolumab or nivolumab plus ipilimumab in 26 patients with HCC and found no 30-day mortality or grades 3–4 hepatobiliary or immunotherapy-related toxicities. The median overall survival from first immunotherapy was 17.2 and 16.5 months from first TARE. 89 More recently, phase II NASIR-HCC was a single-arm trial to evaluate TARE followed by nivolumab in 42 patients who were naïve to immunotherapy and had unresectable HCC. 90 The study found only 8 patients had treatment-related adverse events and 5 had grade 3–4 serious adverse events. The objective response rate was 41.5%, and four patients were able to be downstaged to partial hepatectomy. The time to progression was 8.8 months, and the median overall survival was 20.9 months. 90 In another phase I/IIa trial, TARE followed by durvalumab for locally advanced unresectable HCC in 24 patients demonstrated a median time to progression of 15.2 months, an 18-month overall survival of 58.3%, median PFS of 6.9 months, and an objective response rate of 83.3%. The study found 11 (47.8%) any-grade treatment-related adverse events and 2 (8.7%) grade 3 treatment-related adverse events. 91 Finally, in a. open-label pilot study of 29 patients with poor prognosis HCC, combination pembrolizumab and TARE demonstrated a median PFS of 9.95 months, median overall survival of 27.3 months, objective response rate of 30.8%, disease control rate of 84.6%, and 48.1% had adverse events grade 3 or higher. 92
Other studies investigating the role of TARE in the setting of systemic therapies for HCC have been recently conducted with promising findings. In a 2024 retrospective analysis of 44 patients with HCC who received TARE within 4 weeks of ICI or tyrosine kinase inhibitor (TKI) therapy, propensity score matching analysis showed that those receiving TARE and ICI had significantly greater objective response rates (89.5% vs 36.8%; p < 0.001) and disease control rates (94.7% vs 63.2%; p < 0.001) but no significant difference in median PFS and overall survival (OS). 93 A 2023 retrospective analysis of 19 patients with unresectable HCC who underwent concurrent atezolizumab/bevacizumab or nivolumab combination with TARE (10 with atezolizumab/bevacizumab and 9 with nivolumab) found an objective response rate of 58% (60% in nivolumab vs 56% in atezolizumab/bevacizumab; p = 0.7) and a complete response of 16% (10% vs 22%; p = 0.8). 94 The study also found a median OS of 12.9 months (16.4 months for nivolumab vs 10.7 months for atezolizumab/bevacizumab) and 0% grade ⩾ 3 adverse events in the nivolumab group and 11% in the atezolizumab/bevacizumab group. 94
Intrahepatic cholangiocarcinoma
The landmark phase ABC-02 trial established cisplatin plus gemcitabine as the first-line for unresectable cholangiocarcinoma. 95 Currently, due to the results from the phase III TOPAZ-1 trial, the new first-line standard of care is gemcitabine, cisplatin, and durvalumab. 96 However, this regimen is still associated various Grade 3 and 4 adverse events. 97 The use of TARE has been increasing for intrahepatic cholangiocarcinomas due to its capacity to selectively administer high-dose radiation to the tumor. A single-arm phase II MISPHEC trial of 41 patients demonstrated the effective anti-tumor activity of gemcitabine and cisplatin plus TARE for the treatment of unresectable intrahepatic cholangiocarcinoma. 98 The median PFS was 14 months, the median overall survival was 22 months, and 9 (22%) patients were able to be downstaged to surgical intervention. However, the study had 29 (71%) grade 3–4 toxicities. A prospective, single-arm, open-label feasibility study was conducted on 24 chemotherapy-naïve patients with unresectable intrahepatic cholangiocarcinoma to evaluate the safety and effectiveness of TARE as first-line therapy without chemotherapy. The study found a median hepatic PFS of 5.5 months, median overall survival of 19.4 months (25.9 months in solitary disease and 10.7 months in multifocal disease), and 2 (8%) Grade 3 toxicities. Due to the previous findings of the potential synergism of radiation with capecitabine,99–103 combination of TARE plus cisplatin and gemcitabine was evaluated in a retrospective study of 13 patients with intrahepatic cholangiocarcinoma. The study showed a median overall survival of 29 months, 1-year overall survival of 84.6%, and 2-year survival of 52.9%. Furthermore, seven patients were downstaged to surgery and had a more favorable overall survival. In addition, a prospective, single-arm, open-label feasibility study was conducted on 24 chemotherapy-naïve patients with unresectable intrahepatic cholangiocarcinoma to evaluate the safety and effectiveness of TARE as first-line therapy without chemotherapy. The study found a median hepatic PFS of 5.5 months, a median OS of 19.4 months (25.9 months in solitary disease and 10.7 months in multifocal disease), and 2 (8%) Grade 3 toxicities.
More recently, a study analyzed data from patients with liver-only intrahepatic cholangiocarcinoma treated with chemotherapy alone from the ABC-01, ABC-02, ABC-03, BINGO, and AMEBICA trials and compared that to patients treated with TARE and chemotherapy in MISPHEC. The study found that after weighting, the combination arm had a significantly greater median OS (21.7 vs 15.9 months) and median PFS (14.3 vs 8.4 months) than the chemotherapy alone. 104
Metastatic colorectal cancer
More literature is available for combination of systemic therapy with TARE in metastatic colorectal cancer compared with HCC. A phase III trial of 74 patients with bilobar non-resectable liver metastases from primary adenocarcinoma of the colon evaluated TARE plus floxuridine versus floxuridine alone 105 and found a significantly greater partial and complete response rate as well as median time to disease progression in the liver for patients receiving combination therapy. The combination arm was also associated with an upward trend toward increased survival after 15 months but was not statistically significant. Similar findings were seen in a small phase II trial of 21 patients with previously untreated advanced colorectal liver metastases who were randomized to TARE vs. TARE plus fluorouracil/leucovorin. Median OS was greater in the combination arm (29.4 vs 12.8 months) as well as grade 3–4 toxicity events. 106 Another phase III trial of 44 patients with unresectable, chemotherapy-refractory liver-limited metastatic colorectal cancer investigated TARE plus fluorouracil versus fluorouracil alone and showed a greater median time to liver progression in the combination therapy arm (5.5 vs 2.1 months), greater median time to tumor progression (4.5 vs 2.1 months) 107 but no significant difference in observed in grade 3–4 toxicities. The SIRFLOX study, which combined the findings from three phase III trials24–26—FOXFIRE, SIRFLOX, and FOXFIRE-Global investigated the use of first-line radioembolization plus chemotherapy versus chemotherapy alone in patients with metastatic colorectal cancer to the liver. 27 In this study, 554 patients were randomly assigned to FOLFOX plus TARE and 549 patients to FOLFOX alone. 27 The study found no difference in overall survival but greater odds of a patient having a grade 3+ adverse event in the combination arm. Finally, the phase III EPOCH trial analyzed the combination of TARE with second-line systemic chemotherapy in 428 patients with colorectal liver metastases and found a longer PFS (median PFS 8.0 vs 7.2 months), hepatic PFS in the TARE plus chemotherapy arm when compared to the chemotherapy alone arm (median hepatic PFS 9.1 vs 7.2 months), 108 and higher objective response rates (34.0% vs 21.1%). However, there were more grade 3 adverse events in the TARE arm (68.4% vs 49.3%) and no difference in median OS. 108
Neuroendocrine liver metastases
Neuroendocrine liver metastases are another area where radioembolization has a promising role. Several studies have shown that TARE of neuroendocrine liver metastases in the salvage setting is relatively efficacious and safe.109–115 Several small studies have been conducted to analyze the combination of TARE with systemic treatments in neuroendocrine liver metastases.116–118 Two retrospective studies compared TACE to radioembolization in patients with neuroendocrine liver metastases.119,120 One showed that while TACE had a greater disease control rate, there were no differences in median overall survival or PFS. 119 The other study showed that patients treated with conventional TACE had a significantly greater median overall survival than those treated with DEB-TACE and TARE and a significantly greater hepatic PFS when compared to TARE. 120 Long-term outcomes of TARE in 107 patients with neuroendocrine liver metastasis in the RESiN liver tumor registry were analyzed, and showed 1-, 2-, and 3-year overall survival of 75%, 62%, and 46%, respectively, and the median overall survival was 33 months and highest in patients with pancreatic and hindgut primaries and lowest in foregut primaries. Thirteen (7.6%) patients had grade 3 hepatic toxicity, with ascites being the most common. 114 A recent study of 47 patients with neuroendocrine liver metastases who underwent TARE found that a pre-Tmean/Lmax > 1.9 and a SUVmax > 28 on 68Ga-DOTATATE PET/CT were significant prognostic factors of longer overall survival and hepatic PFS. 121
New developments in radioembolization
Holmium-166
Holmium-166 (166Ho) is a neutron-activated isotope used as an alternative to 90Y for radioembolization. 122 166 Ho microspheres received the Conformitè Europëenne (CE) mark in 2015 as QuiremSpheres™ (Quirem BV, Deventer, The Netherlands) for the management of unresectable liver tumors.123,124 Furthermore, a scout dose of 166Ho, which is used for the assessment of intra- and extrahepatic distribution of the injected microspheres before treatment, was also given CE mark in 2018 as QuiremScout™ (Quirem BV).123,124
Unlike 90Y microspheres, 166Ho microspheres emit both high-energy beta radiation for the treatment of tumors and gamma radiation, which can be utilized for nuclear imaging purposes. In addition, 166Ho microspheres are also paramagnetic and can be visualized by MRI.125–127 Following promising results from animal studies,128–132 several prospective trials of 166Ho in patients with HCC and unresectable and chemorefractory liver metastasis have shown to be safe and feasible.118,127,133–136 Several trials are still ongoing and will further elucidate the safety and clinical application of 166Ho treatment.
Eye90 microspheres
Although 90Y PET and bremsstrahlung SPECT are used for post-treatment imaging, these techniques do not have sufficient spatial resolution to estimate the actual micro-distribution of 90Y activity.56,136,137 CT imaging could potentially provide better visualization of the dose distribution (e.g., reduced partial volume effects and faster scanning time), but one of its main limitations has been the lack of sufficient radiopacity required to be visualized. 137 Recently, a preclinical radiopaque microsphere, Eye90 microspheres™ (ABK Biomedical Inc., Halifax, NS, Canada), has been developed and has the theoretical benefit of improved visibility with X-ray and CT imaging. 138 In a rabbit model, a preclinical study performed precision dosimetry with Eye90137 and demonstrated that the CT-based method of post-TARE dosimetry had better visualization of the dose distribution, less partial volume effects, improved representation of dose heterogeneity, and less respiratory motion uncertainties. A clinical trial evaluating its safety, effectiveness, and impact on quality of life in patients with unresectable HCC or metastatic colorectal cancer is under investigation. 138 A sample case of Eye90 microspheres (Figure 3).
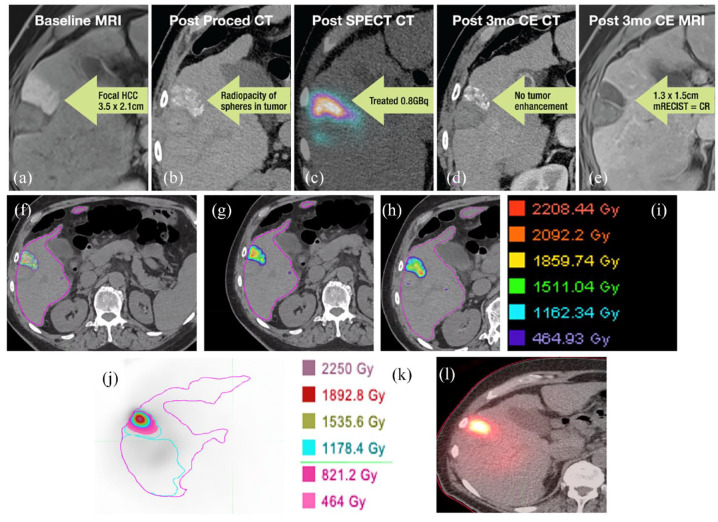
Figure 3.
Radioembolization using Eye90 microsphere. (a) Baseline MRI shows a focal HCC measuring 3.5 × 2.1 cm. (b) Post-procedure CT shows radiopacity of spheres in the tumor. (c) Post-SPECT CT of treated tumor with 0.8 GBq. (d) 3-Month post-treatment CE CT reveals no tumor enhancement. (e) 3-Month post-treatment CE MRI shows 1.3 × 1.5 cm density, corresponding to complete response by mRECIST criteria. (f–h) CT dosimetry with (i) corresponding dose color map legend. (j) SPECT with (k) corresponding dose color map legend. (l) SPECT CT shows proper deposition of Eye90 within the target tumor.
CE, Conformitè Europëenne; CT, computed tomography; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging.
New dosimetry developments
There are three models used for dosimetry for TARE: the single-compartment model, the multi-compartment model, and the voxel-based model.139,140 The single-compartment model treats the tumor and normal liver tissue together, and a mean dose is determined based on the perfused volume. Under this model, classical methods to prescribe radiation doses include the body surface area (BSA)-based and the medical internal radiation dose (MIRD) methods. The BSA-based method, which was historically used with resin microspheres, lacks the individualization of activity administration according to the actual liver and tumor volumes. 57 The single-compartment MIRD method, which is mostly used with glass microsphere, assumes a complete homogenous distribution of the microspheres in the perfused volume. This method determines the absorbed dose to a compartment based on the desired dose to the tumor regardless of tumor burden. 57
The multi-compartment model calculates a mean dose by considering each compartment (the tumor, the normal liver, and the lung), aiming to maximize delivery to the tumor and minimize toxicity to surrounding tissue. 57 Unlike the BSA-based and MIRD single-compartment methods, the partition method follows the multi-compartment model and uses a tumor-to-normal (T/N) uptake ratio, which is considered more individualized and accurate. However, it requires Tc-MAA or similar microspheres that may have inconsistent distributions. 57 A major limitation of the multi-compartment model is the lack of an accurate surrogate and variabilities in perfusion; hence, there is no FDA approval.
Voxel-based dosimetry estimates dose gradients within each compartment instead of averaging over each compartment.57,139 A voxel is a volumetric pixel defining a point in 3D space that is obtained through PET/CT, SPECT, or SPECT/CT. Several software systems have been created to convert the voxel’s value into a radiation dose; therefore, voxel-based dosimetry has been shown to be more accurate than using mean absorbed dose. 141 Voxel-based dosimetry allows for improved personalization by having each voxel be a source and/or target for 3D visualization of absorbed dose distributions. 50 The idea of personalized dosimetry is gaining traction, especially after the DOSISPHERE-01 trial showed its higher response rate with a personalized than standard dosimetry. 142 Voxel-based dosimetry, as the top-tier personalized dosimetry approach, could be utilized for both segmentectomy and lobar treatment (Figure 4).
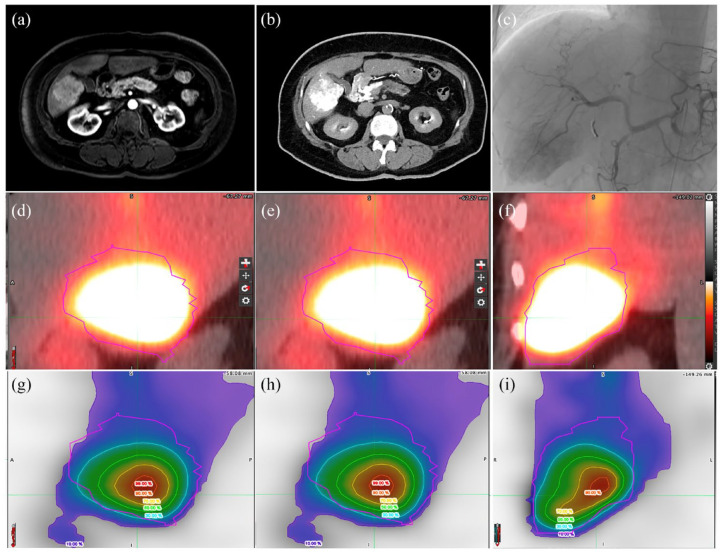
Figure 4.
Voxel base dosimetry. A 65-year-old female with a history of prior malignancy presenting with unresectable newly diagnosed biopsy-proven right liver hepatocellular carcinoma. (a) MRI shows arterially enhancing mass on post-contrast T1 weighted image (panel and (b) in room hybrid CT. (c) Arteriogram shows hypervascular mass supplied by right hepatic artery, followed by radioembolization using Flex-3 resin 90Y microspheres (SIRTEX Medical, Woburn, MA, USA). (d–f) Post-90Y administration SPECT-CT confirmed target embolization of the tumor with high uptake, and dosimetry demonstrated a mean and maximum tumor dose of about 500 and 1300 Gy, respectively. (g–i) Voxel-based dosimetry highlights the differential absorbed dose in the tumor displayed as a percentage of the maximum tumor dose (displayed using rainbow colors) with highest absorbed dose in the tumor center compared to the lowest in the margin.
CT, computed tomography; MRI, magnetic resonance imaging.
In a retrospective study, the different dosimetry approaches on the pre-treatment absorbed dose calculations based on 99mTc-MAA images were evaluated in 14 patients (n = 101 individual tumors). The mean absorbed dose among the various dosimetry methods was higher in tumor volumes than in non-tumor volumes. The study found that differences between the “Multi-tumor Partition Model,” which considers each individual tumor a different compartment and allows for the calculation of mean absorbed doses within each individual tumor, and two 3D voxel dosimetry methods (dose-point kernel convolution and local deposition method (LDM)) was much lower than differences between the partition model and both 3D-voxel based dosimetry methods. Historically, many of the post-radioembolization images and dosimetry calculations were limited by in-house developed algorithms. 143 However, MIM SurePlan™ introduced a commercial software, LiverY90 (MIM Software, Inc., Cleveland, OH, USA), that received FDA approval (FDA 510(K) Number K172218) to convert 90Y PET and/or SPECT images into dose maps to perform dosimetry. 143 This software allows for two algorithms, the Voxel S Value and the LDM, and studies have validated the software for clinical integration.143,144
Scout dose of Y90 for dosimetry
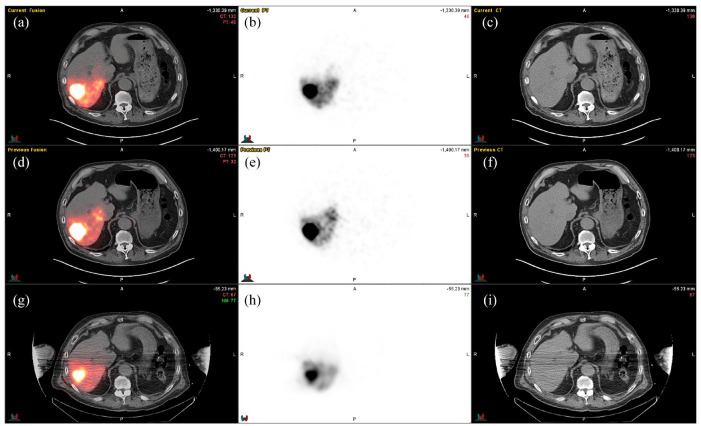
Low-dose/scout 90Y microspheres have been proposed for pre-treatment planning and shunt study as 99mTc-MAA does not accurately predict shunting (Figure 5).
Figure 5.
Scout dosimetry using a small dose of resin rather than Tc-MAA. A patient with a 4.5 cm HCC. (a–c) Therapeutic 90Y administration of 30 mCi with corresponding (a) fusion, (b) PET, and (c) CT. (d–f) Scout 90Y dose of 15 mCi. (g–i) Tc99-MAA administration of 2 mCi.
CT, computed tomography; HCC, hepatocellular carcinoma; PET, positron emission tomography; Tc-MAA, 99mTechnetium-macroaggregated albumin.
A recent prospective, single-arm clinical trial compared the accuracy and safety of scout dose resin 90Y microspheres with 99mTc-MAA SPECT in 30 patients with HCC. 145 Compared to 99mTc-MAA, scout dose 90Y had higher linear correlation with therapeutic 90Y dose with regard to the ratio of tumor:normal tissue (r = 0.53 vs r = 0.904), LSF (r = 0.76 vs r = 0.39), and predicted mean tumor dose (r = 0.900 vs r = 0.74). There was also a stronger agreement between the scout dose 90Y and the therapeutic 90Y dose than with 99mTc-MAA with regard to tumor:normal ratio and LSF via descriptive Bland–Altman analysis. In the non-segmental cohort, 99mTc-MAA had no significant correlation with the predicted mean tumor dose (r = 0.341) and non-tumoral liver dose (r = 0.441) of the therapeutic 90Y dose, but scout dose 90Y was very strongly correlated with predicted mean tumor dose (r = 0.93) and non-tumoral liver dose (r = 0.95). In the segmental cohort, both 99mTc-MAA and scout dose 90Y were significantly correlated with the predicted mean tumor dose and non-tumoral liver dose of the therapeutic 90Y dose. These findings suggest that scout dose 90Y may be superior in estimating the biodistribution of 90Y microspheres, especially for non-segmental treatments. A current major limitation of resin scout dosimetry involves administering close to 40% of a standard BSA dose.
Radioembolization as neoadjuvant therapy
Recently, TARE has been used as a neoadjuvant therapy for otherwise unresectable hepatic tumors. 146 One of the major limitations with resecting hepatic tumors is insufficient future liver remnant, which can lead to post-hepatectomy liver failure. 147 However, since lobar radioembolization can increase future liver remnants, new efforts are starting to use TARE prior surgery. A recent single center retrospective analysis analyzed 26 patients (16 with primary and 10 with metastatic disease) who underwent neoadjuvant lobar TARE followed by hepatectomy. 147 The study did not find grade IV morbidities or 90-day mortalities. Only one (3.8%) patient experienced post hepatectomy liver failure, which is lower than the average rate of 7.6% after hepatectomy. 148 The median survival of the study cohort was 28.9 months from surgery and 37.6 months from TARE. In the multi-center, retrospective LEGACY study that assessed 90Y radioembolization in 162 patients with solitary unresectable HCC ⩽8 cm, 11 (6.8%) patients had TARE prior to resection and 34 (21.0%) prior to transplantation. 22 The study found that the patients who underwent radioembolization prior to resection or liver transplant had an overall survival of 100% (95% confidence interval (CI): 100%−100%) at 24 months and 92.8% (95% CI: 74.2%−98.2%) at 36 months.
90 Y radioembolization is also increasingly used in combination with immunotherapy for patients with HCC. Results from the phase II trial (CA 209-678) examined 90Y radioembolization followed by nivolumab in 36 patients with advanced HCC. 149 The trial found a complete response in 1 (3%) patient, a partial response in 10 (28%) patients, and an overall objective response rate of 30.6% (95% CI: 16.4%−48.1%). Only two (6%) patients had a grade III/IV treatment-related adverse event, and five (14%) patients had a treatment-related serious adverse event. The response rate from this study is comparable with the 41.5% response rate reported from the findings of the phase II NASIR-HCC trial. 90 In a retrospective case series, the safety and efficacy of combined 90Y radioembolization and immunotherapy in 11 patients with unresectable liver metastases from uveal melanoma was assessed. 151 The study found a median hepatic PFS of 15.0 months (95% CI: 5.9–24.1 months) and an overall survival of 17.0 months (95% CI: 1.8–32.2 months) from the start of TARE. The study also found that one (9.1%) patient experienced a complete response, two (18.2%) patients with partial response, four (36.4%) patients with stable disease, and four (36.4%) patients with progressive disease.
New indications for here have been several studies comparing radioembolization
Prostate tumors
Cutting-edge applications of TARE outside the liver are starting to be developed. One such application has been for the treatment of prostate cancer. Prostate cancer is currently the second most common cancer in men in the United States with an estimated 268,490 new cases and about 34,500 deaths in 2022. 151 The benefits of TARE for prostate cancer is radiation’s efficacy for prostatic lesions while minimizing some of its off-target effects and the short penetration of beta radiation compared to standard radiation sources like X-rays that have higher penetrations. While still at a very early stage, TARE to the prostate is currently being tested on animal models. A recent study investigated the use of 90Y radioembolization to the prostatic artery in 14 male castrated beagles who were induced to have prostatic hyperplasia. 152 Each dog had 90Y radioembolization to one of their prostatic hemigland with the other as control. The study found that all dogs underwent successful 90Y radioembolization with localization and coverage only to the treated hemigland, and a dose-dependent decrease in the treated hemigland size was observed at 40 days (25%−60%, p < 0.001). There were no adverse events or radiographic or TARE-related histologic extraprostatic changes. These early findings demonstrate that the technique is safe, feasible, and effective in canine models and sets the stage for future clinical investigations.
Brain tumors
Another potential application of 90Y radioembolization is for the treatment of brain tumors. In a preclinical proof-of-concept and safety analysis, 153 eight dogs (five with spontaneous intra-axial brain masses and three controls) underwent 90Y radioembolization of their cerebral artery. A month after the procedure, there was a 24%−94% reduction in mass volume, and a partial response in three (60%) of the dogs was observed at 6-month follow-up. Six (75%) dogs had developed acute neurologic deficits after the procedure, but these deficits resolved within 7–33 days after the procedure. Another study evaluated a patient-specific pipeline, previously used to estimate 90Y dose distribution and absorption for liver cancer, called CFDose to predict dosimetry for TARE of brain cancer. 154 This novel approach segments the vascular network of patients from imaging, analyzes their blood flow behavior, and calculates the absorbed dose distribution by convolving the predicted distribution with a point kernel. In this proof-of-concept study, CFDose was tested in a head CT angiogram of a patient with no brain cancer. Future investigations in patients with brain cancer will likely provide more insight on the feasibility of CFDose or similar personalized dosimetry models.
Lung tumors
While still at an early stage, lung tumors may be a potential target for TARE. A case report in 2013 describes the use of 90Y radioembolization via bronchial arteries in a patient with metastatic colorectal cancer and in another with metastatic renal cell cancer with promising results (e.g., stable disease or partial remission in the treated lesions). 155 Furthermore, in a recent abstract, eight patients with primary or metastatic lung tumors who were going to get bronchial artery embolization for hemoptysis were administered Tc99m-MAA via the bronchial artery immediately prior to bland embolization to assess the anticipated dose to tumor and organs at risk if they were to have received 90Y radioembolization. 156 Investigators found tumor mean dose and biological effective doses would range from 175 to 2928 Gy with a mean of 813 Gy while satisfying dosimetry constraints to organs at risk.
Artificial intelligence
Artificial intelligence has been transforming the approach to dosimetry by reducing the labor- and time-intensive aspects of segmentation of target and organ at risks. A recent study found that convolutional neural network (CNN)-based algorithms can automatically segment lung, liver, and tumors on 99mTc-MAA SPECT/CT images for 90Y radioembolization planning. 157 The study found that the overall segmentation took approximately 1 min per patient on a consumer-level computer system, and the dosimetry parameters for the three CNN-based segmentation algorithms were comparable to that of reference segmentations by physicians. Artificial intelligence has also been shown to predict treatment response to TARE. In a retrospective single-center radiomics analysis comprising 36 patients with 104 liver metastases who underwent cone-beam computed tomography before TARE, a custom artificial neural network had a sensitivity of 94.2% and specificity of 67.7% (The area under curve = 0.85), after dimension reduction (15 of 104 remained for the analysis), for predicting treatment response. 159 In another retrospective study, a machine learning technique was used to predict individual risk from baseline pre-therapeutic factors in 366 patients with primary (n = 92) or secondary (n = 274) hepatic tumors who underwent 90Y radioembolization. 159 The study identified baseline cholinesterase and bilirubin as the most important factors (forest-averaged lowest minimal depth 1.2 and 1.5, respectively), while sex was the least important (5.5) factor. A retrospective study developed a deep neural network to predict the treatment response in 77 patients with 103 lesions with HCC who underwent 90Y radioembolization. 160 The study found that their model had a higher F1-score, accuracy, and sensitivity at predicting complete treatment response when compared to the voxel-based dosimetry model. Another analysis utilized machine learning and machine vision image analysis to predict modified Response Evaluation Criteria in Solid Tumors treatment response from preprocedural imaging in 30 patients treated with TARE for HCC and found an area under the receiver operating characteristic curve of 0.626 ± 0.17 with an improvement to 0.858 ± 0.114 with support vector machine models. 161
Conclusion
Radioembolization is a valuable and increasingly utilized therapeutic option for the management of patients with primary and metastatic liver tumors. While initially offered as a palliative option for the treatment of hepatic tumors, radioembolization has expanded over the years to its inclusion as an option with curative intent. Nonetheless, a multidisciplinary approach is required to select the appropriate patients to maximize outcomes. Growing advances in radioembolization and its dosimetry continue to expand the treatment options for patients with liver tumors. Novel indications for radioembolization are emerging and offer a promising treatment for future research and clinical applications.
Acknowledgments
None.
Footnotes
ORCID iD: Arian Mansur  https://orcid.org/0000-0003-2406-5127
https://orcid.org/0000-0003-2406-5127
Contributor Information
Arian Mansur, Harvard Medical School, Boston, MA, USA.
Peiman Habibollahi, Division of Diagnostic Imaging, Department of Interventional Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Adam Fang, Division of Vascular and Interventional Radiology, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, USA.
Armeen Mahvash, Division of Diagnostic Imaging, Department of Interventional Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Vahid Etezadi, Division of Vascular and Interventional Radiology, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, USA.
Robert P. Liddell, Division of Vascular and Interventional Radiology, Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Juan C. Camacho, Department of Clinical Sciences, College of Medicine, Florida State University, Tallahassee, FL, USA Vascular and Interventional Radiology, Radiology Associates of Florida, Sarasota, FL, USA.
Emil I. Cohen, Division of Vascular and Interventional Radiology, Department of Radiology, Georgetown University School of Medicine, Washington, DC, USA
Nima Kokabi, Department of Radiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Aravind Arepally, Radiology Associates of Atlanta, Atlanta, GA, USA; ABK Biomedical Inc., Atlanta, GA, USA.
Christos Georgiades, Division of Vascular and Interventional Radiology, Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Nariman Nezami, Division of Vascular and Interventional Radiology, Department of Radiology, Georgetown University School of Medicine, 3800 Reservoir Road, NW, CCC Bldg., Room CG225, Washington, DC 20007, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Arian Mansur: Conceptualization; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Peiman Habibollahi: Investigation; Writing – review & editing.
Adam Fang: Investigation; Writing – review & editing.
Armeen Mahvash: Investigation; Writing – review & editing.
Vahid Etezadi: Investigation; Writing – review & editing.
Robert P. Liddell: Investigation; Writing – review & editing.
Juan C. Camacho: Investigation; Writing – review & editing.
Emil I. Cohen: Investigation; Writing – review & editing.
Nima Kokabi: Investigation; Visualization; Writing – review & editing.
Aravind Arepally: Investigation; Visualization; Writing – review & editing.
Christos Georgiades: Investigation; Writing – review & editing.
Nariman Nezami: Conceptualization; Investigation; Methodology; Visualization; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
N.N. is consultant to CAPS Medical and Boston Scientific. P.H. is a consultant to Sirtex and Varian, as well as a speaker to Sirtex. A.A. is chief medical officer for ABK Biomedical Inc. The other authors report no conflicts of interest. A.M. (4th co-author) is a consultant for Sirtex and ABK and has received research support from Sirtex, Boston Scientific, and Siemens Healthineers.
Availability of data and materials: Not applicable.
References
- 1. Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer 2004; 4: 737–747. [DOI] [PubMed] [Google Scholar]
- 2. Baskar R, Dai J, Wenlong N, et al. Biological response of cancer cells to radiation treatment. Front Mol Biosci 2014; 1: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allison RR, Sibata C, Patel R. Future radiation therapy: photons, protons and particles. Future Oncol 2013; 9: 493–504. [DOI] [PubMed] [Google Scholar]
- 4. National Cancer Institute. Radiation therapy to treat cancer, https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy (2019, accessed 14 August 2022).
- 5. American Cancer Society. The science behind radiation therapy, https://www.cancer.org/content/dam/CRC/PDF/Public/6151.00.pdf (2014, accessed 14 August 2022).
- 6. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699. [DOI] [PubMed] [Google Scholar]
- 7. Tsochatzis EA, Fatourou E, O’Beirne J, et al. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol 2014; 20: 3069–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qadan M, Fong ZV, Delman AM, et al. Review of use of Y90 as a bridge to liver resection and transplantation in hepatocellular carcinoma. J Gastrointest Surg 2021; 25: 2690–2699. [DOI] [PubMed] [Google Scholar]
- 9. Titano J, Voutsinas N, Kim E. The role of radioembolization in bridging and downstaging hepatocellular carcinoma to curative therapy. Semin Nucl Med 2019; 49: 189–196. [DOI] [PubMed] [Google Scholar]
- 10. Lee EJ, Chung HW, Jo JH, et al. Radioembolization for the treatment of primary and metastatic liver cancers. Nucl Med Mol Imaging 2019; 53: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herba MJ, Illescas FF, Thirlwell MP, et al. Hepatic malignancies: improved treatment with intraarterial Y-90. Radiology 1988; 169: 311–314. [DOI] [PubMed] [Google Scholar]
- 12. Wollner I, Knutsen C, Smith P, et al. Effects of hepatic arterial yttrium 90 glass microspheres in dogs. Cancer 1988; 61: 1336–1344. [DOI] [PubMed] [Google Scholar]
- 13. Fabritius MP, Ricke J. Overview of ongoing clinical trials on radioembolization. Cardiovasc Intervent Radiol 2022; 45: 1659–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giammarile F, Bodei L, Chiesa C, et al. Therapy, Oncology and Dosimetry Committees. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging 2011; 38: 1393–1406. [DOI] [PubMed] [Google Scholar]
- 15. Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics 2005; 25(Suppl. 1): S41–S55. [DOI] [PubMed] [Google Scholar]
- 16. Wang EA, Stein JP, Bellavia RJ, et al. Treatment options for unresectable HCC with a focus on SIRT with Yttrium-90 resin microspheres. Int J Clin Pract 2017; 71: 20170730. [DOI] [PubMed] [Google Scholar]
- 17. Maciak M, Konior M, Wawszczak D, et al. Physical properties and biological impact of 90Y microspheres prepared by sol–gel method for liver radioembolization. Radiat Phys Chem 2023; 202: 110506. [Google Scholar]
- 18. Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 2009; 19: 951–959. [DOI] [PubMed] [Google Scholar]
- 19. Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006; 17: 1251–1278. [DOI] [PubMed] [Google Scholar]
- 20. Iñarrairaegui M, Pardo F, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol 2012; 38: 594–601. [DOI] [PubMed] [Google Scholar]
- 21. Cremonesi M, Chiesa C, Strigari L, et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front Oncol 2014; 4: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salem R, Johnson GE, Kim E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology 2021; 74: 2342–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vouche M, Lewandowski RJ, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol 2013; 59: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dutton SJ, Kenealy N, Love SB, et al. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer. BMC Cancer 2014; 14: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibbs P, Gebski V, Van Buskirk M, et al. Selective internal radiation therapy (SIRT) with yttrium-90 resin microspheres plus standard systemic chemotherapy regimen of FOLFOX versus FOLFOX alone as first-line treatment of non-resectable liver metastases from colorectal cancer: the SIRFLOX study. BMC Cancer 2014; 14: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol 2016; 34: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 27. Wasan HS, Gibbs P, Sharma NK, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol 2017; 18: 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018; 36: 1913–1921. [DOI] [PubMed] [Google Scholar]
- 29. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017; 18: 1624–1636. [DOI] [PubMed] [Google Scholar]
- 30. Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016; 151: 1155–1163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140: 497–507.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riaz A, Gabr A, Abouchaleh N, et al. Radioembolization for hepatocellular carcinoma: statistical confirmation of improved survival in responders by landmark analyses. Hepatology 2018; 67: 873–883. [DOI] [PubMed] [Google Scholar]
- 33. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 871–873. [DOI] [PubMed] [Google Scholar]
- 34. Dhondt E, Lambert B, Hermie L, et al. 90Y radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: results from the TRACE phase II randomized controlled trial. Radiology 2022; 303: 699–710. [DOI] [PubMed] [Google Scholar]
- 35. National Comprehensive Cancer Network. Hepatobiliary cancers (Version 1.2022),https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (2022, accessed 12 July 2022).
- 36. Chow R, Simone CB, 2nd, Jairam MP, et al. Radiofrequency ablation vs radiation therapy vs transarterial chemoembolization vs yttrium 90 for local treatment of liver cancer—a systematic review and network meta-analysis of survival data. Acta Oncol 2022; 61: 484–494. [DOI] [PubMed] [Google Scholar]
- 37. Ozen M, Patel RK. Ablation versus radiation segmentectomy for small liver tumors. Semin Intervent Radiol 2023; 40: 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim E, Sher A, Abboud G, et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. Lancet Gastroenterol Hepatol 2022; 7: P843–P850. [DOI] [PubMed] [Google Scholar]
- 39. Hanazaki K, Kajikawa S, Shimozawa N, et al. Hepatic resection for large hepatocellular carcinoma. Am J Surg 2001; 181: 347–353. [DOI] [PubMed] [Google Scholar]
- 40. Kim J, Kim JY, Lee JH, et al. Long-term outcomes of transarterial radioembolization for large single hepatocellular carcinoma: a comparison to resection. J Nucl Med 2022; 63: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 41. Joo I, Kim HC, Kim GM, et al. Imaging evaluation following 90Y radioembolization of liver tumors: what radiologists should know. Korean J Radiol 2018; 19: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spina JC, Hume I, Pelaez A, et al. Expected and unexpected imaging findings after 90Y transarterial radioembolization for liver tumors. Radiographics 2019; 39: 578–595. [DOI] [PubMed] [Google Scholar]
- 43. Wang LM, Jani AR, Hill EJ, et al. Anatomical basis and histopathological changes resulting from selective internal radiotherapy for liver metastases. J Clin Pathol 2013; 66: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ji JH, Park SH, Lee J, et al. Prospective phase II study of neoadjuvant FOLFOX6 plus cetuximab in patients with colorectal cancer and unresectable liver-only metastasis. Cancer Chemother Pharmacol 2013; 72: 223–230. [DOI] [PubMed] [Google Scholar]
- 45. Liddell RP. 90Y transarterial radioembolization for metastatic colorectal cancer. Radiology 2022; 305: 237–238. [DOI] [PubMed] [Google Scholar]
- 46. Briody H, Duong D, Yeoh SW, et al. Radioembolization for hepatocellular carcinoma-current evidence and patterns of utilization. J Vasc Interv Radiol 2023; 34(7): 1200–1213. [DOI] [PubMed] [Google Scholar]
- 47. National Comprehensive Cancer Network. Colon cancer (Version 1.2022), https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (2022, accessed 12 July 2022).
- 48. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 49. Emmons EC, Bishay S, Du L, et al. Survival and toxicities after 90Y transarterial radioembolization of metastatic colorectal cancer in the RESIN Registry. Radiology 2022; 305: 228–236. [DOI] [PubMed] [Google Scholar]
- 50. Levillain H, Bagni O, Deroose CM, et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imaging 2021; 48: 1570–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiesa C, Sjogreen-Gleisner K, Walrand S, et al. EANM dosimetry committee series on standard operational procedures: a unified methodology for 99mTc-MAA pre- and 90Y peri-therapy dosimetry in liver radioembolization with 90Y microspheres. EJNMMI Phys 2021; 8: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arntz PJW, Deroose CM, Marcus C, et al. Joint EANM/SNMMI/IHPBA procedure guideline for [99mTc]Tc-mebrofenin hepatobiliary scintigraphy SPECT/CT in the quantitative assessment of the future liver remnant function. HPB (Oxford) 2023; 25: 1131–1144. [DOI] [PubMed] [Google Scholar]
- 53. Hong K, Akinwande O, Bodei L, et al. ACR-ABS-ACNM-ASTRO-SIR-SNMMI practice parameter for selective internal radiation therapy or radioembolization for treatment of liver malignancies. Brachytherapy 2021; 20: 497–511. [DOI] [PubMed] [Google Scholar]
- 54. Braat AJ, Smits ML, Braat MN, et al. 90Y hepatic radioembolization: an update on current practice and recent developments. J Nucl Med 2015; 56: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 55. Busse NC, Al-Ghazi MSAL, Abi-Jaoudeh N, et al. AAPM medical physics practice guideline 14.a: Yttrium-90 microsphere radioembolization. J Appl Clin Med Phys 2024; 25: e14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim SP, Cohalan C, Kopek N, et al. A guide to 90Y radioembolization and its dosimetry. Phys Med 2019; 68: 132–145. [DOI] [PubMed] [Google Scholar]
- 57. Weber M, Lam M, Chiesa C, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging 2022; 49: 1682–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007; 68: 13–23. [DOI] [PubMed] [Google Scholar]
- 59. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966; 112: 337–347. [DOI] [PubMed] [Google Scholar]
- 60. Dezarn WA, Cessna JT, DeWerd LA, et al. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys 2011; 38: 4824–4845. [DOI] [PubMed] [Google Scholar]
- 61. Downing T, Bennett P, Kouri B. Hepatic metastases therapy. In: Bennett P, Oza UD, Trout AT, et al. (eds) Diagnostic imaging: nuclear medicine. 2nd ed. Philadelphia: Elsevier, 2016, pp. 478–481. [Google Scholar]
- 62. Martin M, Hocquelet A, Debordeaux F, et al. Comparison of perfused volume segmentation between cone-beam CT and 99mTc-MAA SPECT/CT for treatment dosimetry before selective internal radiation therapy using 90Y-glass microspheres. Diagn Interv Imaging 2021; 102: 45–52. [DOI] [PubMed] [Google Scholar]
- 63. Villalobos A, Soliman MM, Majdalany BS, et al. Yttrium-90 radioembolization dosimetry: what trainees need to know. Semin Intervent Radiol 2020; 37: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Knight GM, Gordon AC, Gates V, et al. Evolution of personalized dosimetry for radioembolization of hepatocellular carcinoma. J Vasc Interv Radiol 2023; 34: 1214–1225. [DOI] [PubMed] [Google Scholar]
- 65. Stein SI, Soliman MM, Sparapani J, et al. Conventional hepatic volumetry may lead to inaccurate segmental Yttrium-90 radiation dosimetry. Cardiovasc Intervent Radiol 2021; 44: 1973–1985. [DOI] [PubMed] [Google Scholar]
- 66. Prescribing, recording, and reporting electron beam therapy. J ICRU 2004; 4: 2. [DOI] [PubMed] [Google Scholar]
- 67. Seidensticker R, Seidensticker M, Damm R, et al. Hepatic toxicity after radioembolization of the liver using 90Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol 2012; 35: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 68. Maas M, Beets-Tan R, Gaubert JY, et al. Follow-up after radiological intervention in oncology: ECIO-ESOI evidence and consensus-based recommendations for clinical practice. Insights Imaging 2020; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garg T, Shrigiriwar A, Habibollahi P, et al. Intraarterial therapies for the management of hepatocellular carcinoma. Cancers (Basel) 2022; 14: 3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res 2011; 176: 139–157. [DOI] [PubMed] [Google Scholar]
- 71. Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol 2014; 4: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jakobs TF, Saleem S, Atassi B, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90yttrium microspheres. Dig Dis Sci 2008; 53: 2556–2563. [DOI] [PubMed] [Google Scholar]
- 73. Singh P, Anil G. Yttrium-90 radioembolization of liver tumors: what do the images tell us? Cancer Imaging 2014; 13: 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Son MH, Ha LN, Bang MH, et al. Diagnostic and prognostic value of 99mTc-MAA SPECT/CT for treatment planning of 90Y-resin microsphere radioembolization for hepatocellular carcinoma: comparison with planar image. Sci Rep 2021; 11: 3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alem Z, Murray TE, Egri C, et al. Treatment response assessment following transarterial radioembolization for hepatocellular carcinoma. Abdom Radiol (NY) 2021; 46: 3596–3614. [DOI] [PubMed] [Google Scholar]
- 76. Mascarenhas N, Ryu RK, Salem R. Hepatic radioembolization complicated by abscess. Semin Intervent Radiol 2011; 28: 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013; 57: 1078–1087. [DOI] [PubMed] [Google Scholar]
- 78. Currie BM, Hoteit MA, Ben-Josef E, et al. Radioembolization-induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol 2019; 30: 1915–1923. [DOI] [PubMed] [Google Scholar]
- 79. Atassi B, Bangash AK, Lewandowski RJ, et al. Biliary sequelae following radioembolization with Yttrium-90 microspheres. J Vasc Interv Radiol 2008; 19: 691–697. [DOI] [PubMed] [Google Scholar]
- 80. Carr BI, Metes DM. Peripheral blood lymphocyte depletion after hepatic arterial 90Yttrium microsphere therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012; 82: 1179–1184. [DOI] [PubMed] [Google Scholar]
- 81. Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys 1995; 33: 919–924. [DOI] [PubMed] [Google Scholar]
- 82. Mabud TS, Hickey R. Radioembolization in the setting of systemic therapies. Semin Intervent Radiol 2021; 38: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pinter M, Scheiner B, Pinato DJ. Immune checkpoint inhibitors in hepatocellular carcinoma: emerging challenges in clinical practice. Lancet Gastroenterol Hepatol 2023; 8: 760–770. [DOI] [PubMed] [Google Scholar]
- 84. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022; 76: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 86. Kudo M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr 2022; 11: 592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chew V, Lee YH, Pan L, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019; 68: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Craciun L, de Wind R, Demetter P, et al. Retrospective analysis of the immunogenic effects of intra-arterial locoregional therapies in hepatocellular carcinoma: a rationale for combining selective internal radiation therapy (SIRT) and immunotherapy. BMC Cancer 2020; 20: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhan C, Ruohoniemi D, Shanbhogue KP, et al. Safety of combined Yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vasc Interv Radiol 2020; 31: 25–34. [DOI] [PubMed] [Google Scholar]
- 90. de la Torre-Aláez M, Matilla A, Varela M, et al. Nivolumab after selective internal radiation therapy for the treatment of hepatocellular carcinoma: a phase 2, single-arm study. J Immunother Cancer 2022; 10: e005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee YB, Nam JY, Cho EJ, et al. A phase I/IIa trial of Yttrium-90 radioembolization in combination with durvalumab for locally advanced unresectable hepatocellular carcinoma. Clin Cancer Res 2023; 29: 3650–3658. [DOI] [PubMed] [Google Scholar]
- 92. Yu S, Yu M, Keane B, et al. A pilot study of pembrolizumab in combination with Y90 radioembolization in subjects with poor prognosis hepatocellular carcinoma. Oncologist 2024; 29: 270-e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Garcia-Reyes K, Gottlieb RA, Menon KM, et al. Radioembolization plus immune checkpoint inhibitor therapy compared with radioembolization plus tyrosine kinase inhibitor therapy for the treatment of hepatocellular carcinoma. J Vasc Interv Radiol 2024; 35: 722–730.e1. [DOI] [PubMed] [Google Scholar]
- 94. Villalobos A, Dabbous HH, Little O, et al. Safety and efficacy of concurrent atezolizumab/bevacizumab or nivolumab combination therapy with Yttrium-90 radioembolization of advanced unresectable hepatocellular carcinoma. Curr Oncol 2023; 30: 10100–10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 96. Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 2022; 7: 522–532. [DOI] [PubMed] [Google Scholar]
- 97. Rimini M, Fornaro L, Lonardi S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: an early exploratory analysis of real-world data. Liver Int 2023; 43: 1803–1812. [DOI] [PubMed] [Google Scholar]
- 98. Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 2020; 6: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hickey R, Mulcahy MF, Lewandowski RJ, et al. Chemoradiation of hepatic malignancies: prospective, phase 1 study of full-dose capecitabine with escalating doses of yttrium-90 radioembolization. Int J Radiat Oncol Biol Phys 2014; 88: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 100. Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012; 13: 579–588. [DOI] [PubMed] [Google Scholar]
- 101. Sawada N, Ishikawa T, Sekiguchi F, et al. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res 1999; 5: 2948–2953. [PubMed] [Google Scholar]
- 102. Saelen MG, Ree AH, Kristian A, et al. Radiosensitization by the histone deacetylase inhibitor vorinostat under hypoxia and with capecitabine in experimental colorectal carcinoma. Radiat Oncol 2012; 7: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ahmed O, Yu Q, Patel M, et al. Yttrium-90 radioembolization and concomitant systemic gemcitabine, cisplatin, and capecitabine as the first-line therapy for locally advanced intrahepatic cholangiocarcinoma. J Vasc Interv Radiol 2023; 34: 702–709. [DOI] [PubMed] [Google Scholar]
- 104. Edeline J, Bridgewater J, Campillo-Gimenez B, et al. Chemotherapy with or without selective internal radiation therapy for intrahepatic cholangiocarcinoma: data from clinical trials. Hepatology 2024; 79: 96–106. [DOI] [PubMed] [Google Scholar]
- 105. Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001; 12: 1711–1720. [DOI] [PubMed] [Google Scholar]
- 106. Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004; 88: 78–85. [DOI] [PubMed] [Google Scholar]
- 107. Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010; 28: 3687–3694. [DOI] [PubMed] [Google Scholar]
- 108. Mulcahy MF, Mahvash A, Pracht M, et al. Radioembolization with chemotherapy for colorectal liver metastases: a randomized, open-label, international, multicenter, phase III trial. J Clin Oncol 2021; 39: 3897–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Devcic Z, Rosenberg J, Braat AJ, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med 2014; 55: 1404–1410. [DOI] [PubMed] [Google Scholar]
- 110. Peker A, Çiçek O, Soydal Ç, et al. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn Interv Radiol 2015; 21: 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Barbier CE, Garske-Román U, Sandström M, et al. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur J Nucl Med Mol Imaging 2016; 43: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 112. Braat A, Kappadath SC, Ahmadzadehfar H, et al. Radioembolization with 90Y resin microspheres of neuroendocrine liver metastases: international multicenter study on efficacy and toxicity. Cardiovasc Intervent Radiol 2019; 42: 413–425. [DOI] [PubMed] [Google Scholar]
- 113. Schaarschmidt BM, Wildgruber M, Kloeckner R, et al. 90Y radioembolization in the treatment of neuroendocrine neoplasms: results of an international multicenter retrospective study. J Nucl Med 2022; 63: 679–685. [DOI] [PubMed] [Google Scholar]
- 114. Wong TY, Zhang KS, Gandhi RT, et al. Long-term outcomes following 90Y radioembolization of neuroendocrine liver metastases: evaluation of the radiation-emitting SIR-spheres in non-resectable liver tumor (RESiN) registry. BMC Cancer 2022; 22: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ramdhani K, Braat A. The evolving role of radioembolization in the treatment of neuroendocrine liver metastases. Cancers (Basel) 2022; 14: 3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Soulen MC, van Houten D, Teitelbaum UR, et al. Safety and feasibility of integrating Yttrium-90 radioembolization with capecitabine–temozolomide for grade 2 liver-dominant metastatic neuroendocrine tumors. Pancreas 2018; 47: 980–984. [DOI] [PubMed] [Google Scholar]
- 117. Kim HS, Shaib WL, Zhang C, et al. Phase 1b study of pasireotide, everolimus, and selective internal radioembolization therapy for unresectable neuroendocrine tumors with hepatic metastases. Cancer 2018; 124: 1992–2000. [DOI] [PubMed] [Google Scholar]
- 118. Braat A, Bruijnen RCG, van Rooij R, et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol 2020; 21: 561–570. [DOI] [PubMed] [Google Scholar]
- 119. Egger ME, Armstrong E, Martin RC, 2nd, et al. Transarterial chemoembolization vs radioembolization for neuroendocrine liver metastases: a multi-institutional analysis. J Am Coll Surg 2020; 230: 363–370. [DOI] [PubMed] [Google Scholar]
- 120. Do Minh D, Chapiro J, Gorodetski B, et al. Intra-arterial therapy of neuroendocrine tumour liver metastases: comparing conventional TACE, drug-eluting beads TACE and yttrium-90 radioembolisation as treatment options using a propensity score analysis model. Eur Radiol 2017; 27: 4995–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ingenerf M, Grawe F, Winkelmann M, et al. Neuroendocrine liver metastases treated using transarterial radioembolization: identification of prognostic parameters at 68Ga-DOTATATE PET/CT. Diagn Interv Imaging 2024; 105: 15–25. [DOI] [PubMed] [Google Scholar]
- 122. Mumper RJ, Ryo UY, Jay M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med 1991; 32: 2139–2143. [PubMed] [Google Scholar]
- 123. Reinders MTM, Smits MLJ, van Roekel C, et al. Holmium-166 microsphere radioembolization of hepatic malignancies. Semin Nucl Med 2019; 49: 237–243. [DOI] [PubMed] [Google Scholar]
- 124. Stella M, Braat A, van Rooij R, et al. Holmium-166 radioembolization: current status and future prospective. Cardiovasc Intervent Radiol 2022; 45: 1634–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nijsen JF, Seppenwoolde JH, Havenith T, et al. Liver tumors: MR imaging of radioactive holmium microspheres—phantom and rabbit study. Radiology 2004; 231: 491–499. [DOI] [PubMed] [Google Scholar]
- 126. de Wit TC, Xiao J, Nijsen JF, et al. Hybrid scatter correction applied to quantitative holmium-166 SPECT. Phys Med Biol 2006; 51: 4773–4787. [DOI] [PubMed] [Google Scholar]
- 127. Smits ML, Nijsen JF, van den Bosch MA, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol 2012; 13: 1025–1034. [DOI] [PubMed] [Google Scholar]
- 128. Zielhuis SW, Nijsen JF, Krijger GC, et al. Holmium-loaded poly(L-lactic acid) microspheres: in vitro degradation study. Biomacromolecules 2006; 7: 2217–2223. [DOI] [PubMed] [Google Scholar]
- 129. Nijsen F, Rook D, Brandt C, et al. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: a biodistribution study. Eur J Nucl Med 2001; 28: 743–749. [DOI] [PubMed] [Google Scholar]
- 130. Vente MA, Nijsen JF, de Wit TC, et al. Clinical effects of transcatheter hepatic arterial embolization with holmium-166 poly(L-lactic acid) microspheres in healthy pigs. Eur J Nucl Med Mol Imaging 2008; 35: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zielhuis SW, Nijsen JF, Seppenwoolde JH, et al. Long-term toxicity of holmium-loaded poly(L-lactic acid) microspheres in rats. Biomaterials 2007; 28: 4591–4599. [DOI] [PubMed] [Google Scholar]
- 132. Turner JH, Claringbold PG, Klemp PF, et al. 166Ho-microsphere liver radiotherapy: a preclinical SPECT dosimetry study in the pig. Nucl Med Commun 1994; 15: 545–553. [PubMed] [Google Scholar]
- 133. Prince JF, van den Bosch M, Nijsen JFW, et al. Efficacy of radioembolization with 166Ho-microspheres in salvage patients with liver metastases: a phase 2 study. J Nucl Med 2018; 59: 582–588. [DOI] [PubMed] [Google Scholar]
- 134. van Roekel C, van den Hoven AF, Bastiaannet R, et al. Use of an anti-reflux catheter to improve tumor targeting for holmium-166 radioembolization—a prospective, within-patient randomized study. Eur J Nucl Med Mol Imaging 2021; 48: 1658–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Reinders MTM, van Erpecum KJ, Smits MLJ, et al. Safety and efficacy of holmium-166 radioembolization in hepatocellular carcinoma—the HEPAR primary study. J Nucl Med 2022; 63: 1891–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Roosen J, Westlund Gotby LEL, Arntz MJ, et al. Intraprocedural MRI-based dosimetry during transarterial radioembolization of liver tumours with holmium-166 microspheres (EMERITUS-1): a phase I trial towards adaptive, image-controlled treatment delivery. Eur J Nucl Med Mol Imaging 2022; 49: 4705–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Henry EC, Strugari M, Mawko G, et al. Precision dosimetry in yttrium-90 radioembolization through CT imaging of radiopaque microspheres in a rabbit liver model. EJNMMI Phys 2022; 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. ABK Biomedical. Safety and effectiveness of Eye90 Microspheres™ in the treatment of unresectable HCC and mCRC, https://ctv.veeva.com/study/safety-and-effectiveness-of-eye90-microspheres-in-the-treatment-of-unresectable-hcc-and-mcrc (accessed 14 August 2022).
- 139. Bastiaannet R, Kappadath SC, Kunnen B, et al. The physics of radioembolization. EJNMMI Phys 2018; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Toskich BB, Liu DM. Y90 radioembolization dosimetry: concepts for the interventional radiologist. Tech Vasc Interv Radiol 2019; 22: 100–111. [DOI] [PubMed] [Google Scholar]
- 141. Chiesa C, Bardiès M, Zaidi H. Voxel-based dosimetry is superior to mean absorbed dose approach for establishing dose–effect relationship in targeted radionuclide therapy. Med Phys 2019; 46: 5403–5406. [DOI] [PubMed] [Google Scholar]
- 142. Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021; 6: 17–29. [DOI] [PubMed] [Google Scholar]
- 143. Maughan NM, Garcia-Ramirez J, Arpidone M, et al. Validation of post-treatment PET-based dosimetry software for hepatic radioembolization of Yttrium-90 microspheres. Med Phys 2019; 46: 2394–2402. [DOI] [PubMed] [Google Scholar]
- 144. Guerrero M, Yao W, Lin M, et al. Validation of a commercial software dose calculation for Y-90 microspheres. Brachytherapy 2022; 21: 561–566. [DOI] [PubMed] [Google Scholar]
- 145. Kokabi N, Webster LA, Elsayed M, et al. Accuracy and safety of scout dose resin Yttrium-90 microspheres for radioembolization therapy treatment planning: a prospective single-arm clinical trial. J Vasc Interv Radiol 2022; 33: 1578–1587.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Carvalho VO, Galastri FL, Affonso BB, et al. Transarterial radioembolization for liver tumors as neoadjuvant therapy: three case reports. Einstein (Sao Paulo) 2020; 18: eRC4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ahmed A, Stauffer JA, LeGout JD, et al. The use of neoadjuvant lobar radioembolization prior to major hepatic resection for malignancy results in a low rate of post hepatectomy liver failure. J Gastrointest Oncol 2021; 12: 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vibert E, Pittau G, Gelli M, et al. Actual incidence and long-term consequences of posthepatectomy liver failure after hepatectomy for colorectal liver metastases. Surgery 2014; 155: 94–105. [DOI] [PubMed] [Google Scholar]
- 149. Tai D, Loke K, Gogna A, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol 2021; 6: 1025–1035. [DOI] [PubMed] [Google Scholar]
- 150. Zheng J, Irani Z, Lawrence D, et al. Combined effects of Yttrium-90 transarterial radioembolization around immunotherapy for hepatic metastases from uveal melanoma: a preliminary retrospective case series. J Vasc Interv Radiol 2018; 29: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 151. American Cancer Society. Key statistics for prostate cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html (2022, accessed 29 October 2022).
- 152. Mouli SK, Raiter S, Harris K, et al. Yttrium-90 radioembolization to the prostate gland: proof of concept in a canine model and clinical translation. J Vasc Interv Radiol 2021; 32: 1103–1112.e12. [DOI] [PubMed] [Google Scholar]
- 153. Pasciak AS, Manupipatpong S, Hui FK, et al. Yttrium-90 radioembolization as a possible new treatment for brain cancer: proof of concept and safety analysis in a canine model. EJNMMI Res 2020; 10: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Taebi A, Costa GCA, Roncali E. Personalized dosimetry for brain cancer radioembolization: a feasibility study. J Nucl Med 2021; 62: 1582.33712535 [Google Scholar]
- 155. Ricke J, Großer O, Amthauer H. Y90-radioembolization of lung metastases via the bronchial artery: a report of 2 cases. Cardiovasc Intervent Radiol 2013; 36: 1664–1669. [DOI] [PubMed] [Google Scholar]
- 156. Wehrenberg-Klee E, An T, Heidari P, et al. Abstract No. 19 ▪ FEATURED ABSTRACT bronchial artery Tc99m-MAA injection in patients with pulmonary malignancies demonstrates future treatment potential of bronchial Y90 radioembolization. J Vasc Interv Radiol 2024; 35: S10. [Google Scholar]
- 157. Chaichana A, Frey EC, Teyateeti A, et al. Automated segmentation of lung, liver, and liver tumors from Tc-99m MAA SPECT/CT images for Y-90 radioembolization using convolutional neural networks. Med Phys 2021; 48: 7877–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kobe A, Zgraggen J, Messmer F, et al. Prediction of treatment response to transarterial radioembolization of liver metastases: radiomics analysis of pre-treatment cone-beam CT: a proof of concept study. Eur J Radiol Open 2021; 8: 100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ingrisch M, Schöppe F, Paprottka K, et al. Prediction of 90Y radioembolization outcome from pretherapeutic factors with random survival forests. J Nucl Med 2018; 59: 769–773. [DOI] [PubMed] [Google Scholar]
- 160. Wagstaff WV, Villalobos A, Gichoya J, et al. Using deep learning to predict treatment response in patients with hepatocellular carcinoma treated with Y90 radiation segmentectomy. J Digit Imaging 2023; 36: 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Arndt S, Sandow T, Milburn J, et al. 3:00 PM Abstract No. 181 Machine learning and machine vision image analysis can predict treatment response from preprocedural imaging alone for Y90 radioembolization and DEB-TACE in hepatocellular carcinoma. J Vasc Interv Radiol 2018; 29: S79–S80. [Google Scholar]