Abstract
The earliest events within the peripheral mammalian nervous system that cause herpes simplex virus type 1 (HSV-1) to reactivate from latency are unknown but are highly likely to include altered regulation of cellular transcription factors. Using gene array analysis, we have examined the changes that occur in cellular mRNA levels in mouse trigeminal ganglia following explantation, a stimulus that results in HSV-1 reactivation from latency. We have detected both increased and decreased expression levels of particular cellular transcripts, which include RNAs encoding neuronal factors, transcription factors, and factors involved in the cell cycle. Among the transcription factors that are upregulated is Bcl-3, a coactivator for NFκB. We have confirmed these increases in Bcl-3 transcription levels using reverse transcription-PCR and S1 nuclease protection assays. In addition, we have shown Bcl-3 upregulation at the protein level. Importantly, Bcl-3 RNA levels were found to increase specifically in neuronal cells within the trigeminal ganglia. We discuss a potential role for this factor in upregulating ICP0 transcription, which is an important viral event for initiation of HSV-1 reactivation.
Following primary infection, herpes simplex virus (HSV) establishes a latent state within neurons of sensory ganglia. During latency, episomal HSV genomes are generally inactive, with viral transcription mainly from the latency-associated transcripts, although low levels of certain immediate-early (IE) and early (E) transcripts have been detected (18, 37). The virus can undergo periodic reactivation leading to recurrent disease in response to certain stimuli, such as stress, tissue damage, or the presence of immune system modulators, neurotransmitters, hormones, or growth factors (19, 20, 55). Similar to primary infection, the reactivation process includes several steps leading to the release of complete viral particles, including transcription of viral (IE and E) genes and replication of the viral DNA.
The temporal pattern of HSV gene expression during primary infection in tissue culture is well documented (28, 29). The initial event is IE transcription, mediated by the cellular transcription factors Oct-1 and HCF functioning in a complex with the viral transactivator VP16 (a virion protein) through binding to TAATGARAT upstream sequences (52, 61). The IE gene products are transcription factors that promote E and late (L) gene expression. It has been shown that this cascade of viral gene expression is not seen during the reactivation process. Rather, IE and E transcripts are both detected at the earliest times of reactivation and the onset of their expression is simultaneous (63). Since VP16 is not expressed in latently infected neurons and moreover is not required for reactivation from ganglionic explants (60), it is likely that viral transcription is initiated during reactivation by endogenous factors expressed from these tissues. Candidates for this function are cellular factors that regulate the HSV type 1 (HSV-1) genome, either by direct binding such as DNA-bound activators or by coactivators that associate with and alter activator function. Activators or coactivators would be either induced by the mRNA expression level or posttranslationally modified after the reactivation stimuli or both. There is a second possibility that certain cellular factors repress HSV-1 transcription during latency and that their modification or lowered expression at the onset of reactivation alleviates HSV-1 transcriptional inhibition.
The murine trigeminal ganglion (TG) explant model has been extensively used to study latency and reactivation of HSV-1 and in particular to examine altered expression patterns of specific mRNAs during reactivation (16, 63, 64). In this model system, explanted latently infected TG are incubated in culture medium for several hours, which is sufficient for detection of IE and E transcripts. The first viral transcripts appear at approximately 4 to 8 h postexplantation (p.e.), implying that the initiating molecular events in the cell responsible for transcriptional reactivation occur before that time (16, 63, 64). Based on these observations, an approach to studying the earliest cellular events preceding and required for viral activation is to examine altered expression in uninfected rather than HSV-1-infected ganglion explants. For example, among several cellular transcription factors whose expression increased in explanted TG (c-fos, c-jun, c-myc, and oct-1), each was shown by reverse transcription (RT)-PCR and in situ hybridization to exhibit induced expression in both infected and uninfected TG explants (63, 68).
Thus, certain transcription factors have been shown to be changed in expression or cellular localization under these conditions, such as the Oct-1-related brain-specific Brn-3 (39) and Oct-1-associated HCF (38). However, the overall pattern and seminal molecular events triggering the transition from latency to reactivation remain poorly understood. In order to take a broad view of changes underlying reactivation, we have used large-scale screening of mRNA changes. One such method, differential display RT-PCR, led to the identification of a murine interferon-related gene, TIS7, as a potential causative agent of reactivation (64). In order to expand our screening to as large a set of genes as possible, in this report we describe the use of gene array technology, which has become an increasingly valuable tool for evaluating changes in gene expression. Recent studies have used gene array filters to assess differences in gene expression among normal, invasive, and metastatic breast cell populations and to monitor gene expression patterns in prostate cancer specimens (6, 59). Gene arrays have also been used to evaluate differential cellular gene regulation during human immunodeficiency virus type 1 (HIV-1) infection (24). However, this is a novel approach to studying differential cellular expression after explantation of TG, our model system for the study of HSV-1 reactivation.
We have used gene array filters representing one-third of the mouse genome to examine changes in RNA expression in TG populations immediately following and several hours after explantation, a time frame that we have previously established is sufficient for HSV-1 reactivation. Using this system we have detected induced expression of the transcription factor Bcl-3 among other genes exhibiting altered expression. This factor has a potential role in the regulation of the ICP0 promoter, suggesting that Bcl-3 is involved in viral transcriptional reactivation after latency.
MATERIALS AND METHODS
TG explantation.
Four- to six-week-old female BALB/c mice were obtained from Jackson Laboratory. Mice were sacrificed by cervical dislocation, and TG were excised. Groups of 6 to 10 explanted TG were incubated in Dulbecco's modified Eagle medium supplemented with 5% fetal bovine serum at 37°C for 4 h p.e. In a single experiment, TG were explanted in the absense of serum.
RNA extraction.
Ganglia used for RNA preparation were snap-frozen in liquid nitrogen. RNA was extracted from TG by using TRIzol as described by the manufacturer (Gibco BRL). RNA was subjected to digestion with RNase-free DNase I (Boehringer Mannheim) and ethanol precipitation. RNA concentrations were measured by spectrophotometry. RNA integrity was determined by agarose gel electrophoresis (45).
Gene array cDNA filters.
The gene array used in this study was the Gene Discovery Array Mouse I from Genome Systems. Each gene array is a 22- by 22-cm nylon filter spotted with 18,378 mouse cDNA clones retrieved from the IMAGE Consortium collection. Thirty control spots were included on the filters for normalization of the intensities. The normalization was done using internal control DNA spots that were derived from specific Arabidopsis and Drosophila genes, and corresponding RNAs were provided with the gene filters to be mixed with the test RNAs for cDNA preparation and labeling. cDNA clones in bacterial host cells were grown on the membrane and processed to release the plasmid DNA. There are three classifications of clones on the membranes. The first type of clone is a cluster representative, found to have a 40-bp minimal overlap with a 95% sequence identity with other cDNA clones in the IMAGE Consortium clone set. The 5′-most clone (expression sequence tag) of each cluster was chosen as the representative for the filter. The second type of clone is a singleton. These are clones that did not cluster with other image clones in the IMAGE Consortium clone set. The third type of clone is a gene index cluster representative (produced by The Institute for Genomic Research [http://www.tigr.org]). One set of RNAs was analyzed in duplicate, and multiple RNAs were prepared for the RT-PCR confirmation of many of the RNAs that were changed in expression on the gene filters.
cDNA probe preparation, hybridization, and image analysis.
mRNA was isolated from total RNA using the mRNA purification kit from Pharmacia Biotech. cDNA was generated from 2.5 μg of mRNA by using Moloney murine leukemia virus reverse transcriptase (Gibco BRL), oligo(dT) priming, and [α-33P]dCTP (3,000 Ci/mmol; 10 μCi/μl; NEN) for labeling. Unincorporated nucleotides were removed with G-50 MicroColumns (Pharmacia). The radioactivity of the probe was measured with a scintillation counter, and the same amount of labeled cDNA was used for each filter. Gene array filters were prehybridized overnight at 42°C with hybridization solution (5× Denhardt, 2% sodium dodecyl sulfate [SDS], 100 μg of sheared salmon sperm/μl [SIGMA]). After overnight hybridization at 42°C, the filters were washed at 68°C with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 1% SDS, as described by Genome Systems. The membranes were then exposed overnight to a PhosphorScreen (Molecular Dynamics) and scanned at 176-μm resolution in a phosphorimager instrument (PhosphorImager 445 SI; Molecular Dymamics). The software used by the company (GDS software) identified the spots on the membranes and measured the hybridization intensities for each clone, using 30 control spots for normalization.
RT-PCR.
cDNA was generated from 1 μg of total RNA by using a first-strand cDNA synthesis kit for RT-PCR, priming with oligo(dT) (Boehringer Mannheim). Reactions contained 30 to 50 ng of cDNA, 200 μM (each) nucleotide triphosphates, 1 μM each primer, and 2 U of Taq polymerase in PCR buffer (Boehringer Mannheim). The primers used in this study are as follows: for β-actin, ATAGCACAGCTTCCCTTTGAT and AACATGCATTGTTACCAACT (452-bp product); for TIS7, CTCTTATCTCGGCATTTG and GGACAAGAGAAAGCAGCG (342-bp product); for Bcl-3, CTCCTCACCCTCGCTGTCTC and CTGGCTGTCCTTTGGTTCCT (447-bp product); for LZIP, GATCCTGGTGGTCAGGATCT and CTAGATCTATGGAGACGTGC (300-bp product); for Sp1, GGATGGTTCTGGTCAAATAC and GTCTGGTTCTGCTGGATGTT (531-bp product); for C/EBP-β, CGCCAAGCCGAGCAAGAAGC and CACCTTGTGCTGCGTCTCCA (475-bp product); and for HCF, GGTTCAAGCAAGACATGAAG and ATGGCGGCGCCCAGGATGCC (500-bp product). Primers were designed based on known sequences retrieved from the GenBank database. Cycling reactions were performed with a Perkin-Elmer Cetus Gene Amp PCR System thermocycler. After one cycle of denaturation for 10 min at 94°C, the cycles were as follows: (i) denaturation at 94°C for 1 min, (ii) annealing at 59°C for 1 min, and (iii) extension for 1 min at 72°C. The final cycle was terminated with a final extension for 10 min at 72°C. Amplification was initially carried out for 25 to 35 cycles. Twenty-five cycles was determined to be the most appropriate number for obtaining quantitative results, since the amplification was still in the linear range (21). RT reactions were included in each set of experiments as negative controls. In every case, the size of the PCR product bands corresponded to the predicted size. Aliquots of 10% of the PCR products were analyzed by agarose gel electrophoresis and stained with ethidium bromide. Gels were subsequently scanned using a Fluorimager (Molecular Dynamics), and the intensity of the bands was quantified with ImageQuant. The intensities of bands corresponding to 0- and 4-h samples were normalized to the intensity of β-actin bands corresponding to the same time points. Signal intensities, as detected by the Fluorimager, were compared to known amounts of DNA loaded on the gel in order to determine how the signal reflected actual changes.
S1 nuclease protection assay.
Total RNA was isolated as described above. Fifty micrograms of total RNA was used per reaction. RNAs from 0 and 4 h p.e. were incubated and hybridized to either radioactively labeled β-actin probe 5′-CACCATCACACCTTGGTGCCTAGAGCGGCCCACGATGGAGGGGA ATACAGGGGGGG-3′, which was used to demonstrate equal RNA amounts per reaction, or radioactively labeled Bcl-3 probe 5′TGGCAGCGCGGCGCCCGGGGTGCCCTTGGGCGGGTGCGCAGGTCCACGGGGGGGG-3′ (45). Each probe includes seven extra guanine bases at its 3′ end to demonstrate probe protection. After incubation with S1 nuclease, the reactions were loaded onto a denaturing polyacrylamide gel, and the intensity of the bands corresponding to the undigested probe was measured using PhosphorImager and ImageQuant.
In situ hybridization.
Ganglia used for in situ hybridization were fixed with 4% paraformaldehyde for 5 h and then embedded in paraffin wax. Six-micrometer serial sections were cut and processed as described previously (64). Nonisotopic labeling was performed using the digoxigenin (DIG) DNA labeling and detection kit (Boehringer Mannheim). The DNA template used for the labeling reaction was a 447-bp PCR product. The quantity of the labeled product was determined by membrane dot blotting. After deparaffination with xylene and serial ethanol washes, tissue on the slides was digested with 1 μg of proteinase K/μl, hybridized to the probe at 37°C overnight, and washed as described previously (42, 46). Hybridized probe was detected with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (BCIP) alkaline phosphatase substrate, giving a purple color. Slides were counterstained with mild methyl green stain. The slides were checked by a neuropathologist with experience (Ehud Levi, University of Pennsylvania) for identification of the Bcl-3-positive cells as neurons.
Western blotting.
Protein extracts were prepared for three time points postexplantation (0, 4, and 8 h [uninfected TG]). One milliliter of T-PER tissue protein extraction reagent (Pierce) was added to eight ganglia per time point, and the tissue was homogenized. Fifty microliters of protein extract for each time point was used for SDS-polyacrylamide gel electrophoresis and Western blotting. All samples had an equal amount of protein, as examined by Coomassie staining. Anti-Bcl-3 antibody (200 μg/ml; Santa Cruz) was used for Western blots at a 1:200 dilution. A second Western blot was performed using the same dilution of antibody and blocking peptide (Santa Cruz) in an antibody/peptide ratio of 1:5.
RESULTS
Altered gene expression revealed by mouse gene array hybridization.
Trigeminal ganglia were explanted from uninfected mice, and total RNA was purified immediately (0 h) or after 4 h in culture. The RNA was reverse transcribed using oligo(dT) primers to produce 33P-labeled cDNA. Hybridization of mouse gene arrays (Genome Systems) was performed using the radiolabeled cDNA probe. One gene array filter was hybridized with a cDNA probe corresponding to 0 h p.e., and another filter was hybridized with a probe corresponding to 4 h p.e. Gene array filters were visualized by phosphorimaging (Fig. 1), and scanned images were sent to Genome Systems for quantitative analysis (Table 1).
FIG. 1.
Hybridized gene array filters. A representative small area of the filters is shown, hybridized with a cDNA probe derived from TG explanted for 0 (left) or 4 (right) h. RNAs that are decreased in abundance after the 4-h incubation are boxed in the 0-h sample (left), while RNAs that are increased in abundance following incubation are boxed in the 4-h sample (right). As indicated within each box, the expressed sequence tags are spotted in duplicate on the filters.
TABLE 1.
Genes altered in expression following TG explantation
| Gene | GenBank accession no. | Change in expression level (fold) |
|---|---|---|
| Induced genes | ||
| Cell surface glucoprotein MUC18 | AA088962 | 45 |
| Cystathione gamma-lyase | AA245993 | 24 |
| Cytochrome P450 | AA266005 | 23 |
| Similar to human acidic ribosomal protein P1 | AA067662 | 20 |
| Retinoid X receptor interacting protein | AA242625 | 19 |
| Aldehyde dehydrogenase | AA469589 | 17 |
| Similar to calcineurin B subunit isoform 1 | AA537502 | 14 |
| Pseudo-prolactin receptor | AA244758 | 11 |
| Metallothionein-I | AA051654 | 10 |
| Transcription factor S-II | AA237509 | 9 |
| B-cell lymphoma 3-encoded protein (Bcl-3) | AA266002 | 8 |
| Zinc finger protein | AA414512 | 8 |
| Prostaglandin endoperoxide | AA245992 | 7 |
| Cyclin-dependent kinase homolog | AA117324 | 7 |
| Aldose 1-epimerase precursor | AA245672 | 7 |
| Uricase | AA244961 | 7 |
| Similar to human Ras-related protein RAB-4 | AA445222 | 6 |
| Glutathione S-transferase | AA445233 | 6 |
| Alpha-N-acetylglucosaminidase | AA268207 | 6 |
| Ubiquitin-conjugating enzyme E2-18 KD | AA238200 | 6 |
| Protein phosphatase type 1 | AA518429 | 5 |
| MHC class I Qa-Tla | AA051194 | 5 |
| Q7(b) cell surface antigen | AA174486 | 5 |
| GTPase-activating protein GAPIII | W33527 | 5 |
| Primase large subunit | AA237894 | 4 |
| Similar to human gamma interferon inducible protein | AA403965 | 4 |
| RNA polymerase II elongation factor SIII, p15 | AA461967 | 4 |
| R-ras | AA522428 | 4 |
| Similar to human RAB-3A | AA445184 | 4 |
| Neural retina-specific leucine zipper protein | AA517895 | 4 |
| LZIP-1 and LZIP-2 | AA266568 | 3 |
| Similar to human GC-rich sequence DNA-binding factor | AA184767 | 3 |
| Cyclin F | AA240462 | 3 |
| Suppressed genes | ||
| Similar to human putative Ser/Thr protein kinase p78 | AA221667 | 84 |
| Plasminogen activator inhibitor 2 | AA124868 | 61 |
| P1 protein, DNA replication factor | W97481 | 47 |
| Dihydroxyphenylacetate oxidase | AA137764 | 30 |
| Carboxylesterase | AA237640 | 30 |
| Similar to human GTPase RHOC | AA221997 | 28 |
| ATP-dependent RNA helicase | AA212347 | 26 |
| Similar to human zinc finger protein 32 | AA462760 | 23 |
| Semaphorin B | AA222348 | 21 |
| Myelin proteolipid protein | AA067291 | 20 |
| Semaphorin D | W98303 | 19 |
| B-myb | AA432951 | 12 |
| Synaptogyrin | AA275862 | 12 |
| Kinesin-related protein | AA261010 | 11 |
| Similar to human transaldolase | AA543582 | 10 |
| Hsp70-related NST-1 | AA119654 | 10 |
| Short-chain acyl-coenzyme A dehydrogenase | AA414061 | 10 |
| Transforming growth factor beta-3 | AA103431 | 9 |
| Similar to human dihydrolipoamide | ||
| Succinyltransferase | AA220084 | 7 |
| Myelin gene expression factor | AA210564 | 7 |
| Ras-related YPT1 | AA290385 | 6 |
| Malonyl-coenzyme A decarboxylase precursor | AA238355 | 6 |
| IGF-II | AA103939 | 6 |
| PML isoform 1 | AA138191 | 5 |
| Metalloproteinase inhibitor 3 precursor | AA423441 | 5 |
| Serine/threonine protein kinase | AA511351 | 5 |
| Interferon regulatory factor 3 | AA103471 | 5 |
| Retinoic acid-responsive protein | AA222497 | 5 |
| NCAM-140 and NCAM-180 isoforms | AA039178 | 5 |
| Ubiquitin-activating enzyme | AA221449 | 4 |
| Glutamate dehydrogenase | AA511913 | 4 |
| TEIIIC2 subunit | AA445571 | 4 |
| Transforming growth factor beta-inducible protein | AA034808 | 4 |
| Similar to human serum response factor | AA154152 | 4 |
| Similar to human synaptobrevin 2 | AA036389 | 4 |
| Chromatin nonhistone HMG | AA538243 | 4 |
| Neurofilament triplet M | W64752 | 3 |
| Tumor necrosis factor I receptor | AA003594 | 3 |
The gene arrays used represent 18,000 mouse expression sequence tags retrieved from the IMAGE consortium clone selection and arrayed on nitrocellulose filters in duplicate. The detection limit of this method, as described by the company, is 1 in 10,000 (about five copies per cell).
A representative area of the gene filter is displayed in Fig. 1, and several RNAs are pointed out that either increased or decreased in abundance following the 4-h incubation in culture. The quantitative analysis of the hybridization results showed that approximately 300 mRNAs were induced and 500 mRNAs were repressed more than threefold. Among the changes detected at 4 h, more than two-thirds corresponded to unknown genes. The known genes can be classified into several groups as follows: (i) neuron-specific genes, (ii) transcription and replication factors, (iii) cell cycle-related genes, (iv) signal transduction genes, and (v) genes related to metabolism. The analysis of results, including representatives from all groups of genes, is summarized in Table 1.
HSV-1 infects neurons within the TG, where latent infection is established. However, TG are complex tissues composed of many different types of cells, such as neurons, glia, and connective tissue cells. Thus, detection of neuronal gene expression in the gene array filters is of great importance, since this is evidence that mRNA derived from neurons is strongly represented in the total RNA from TG. We did detect neuronal gene expression in considerable abundance; for example, neurofilament M displayed a hybridization intensity that was 10- to 40-fold higher than signals of moderate to low abundance. This result suggests that neuronal RNA is significantly represented in the total TG RNA, although neurons are only 10% of the ganglion cell population (62).
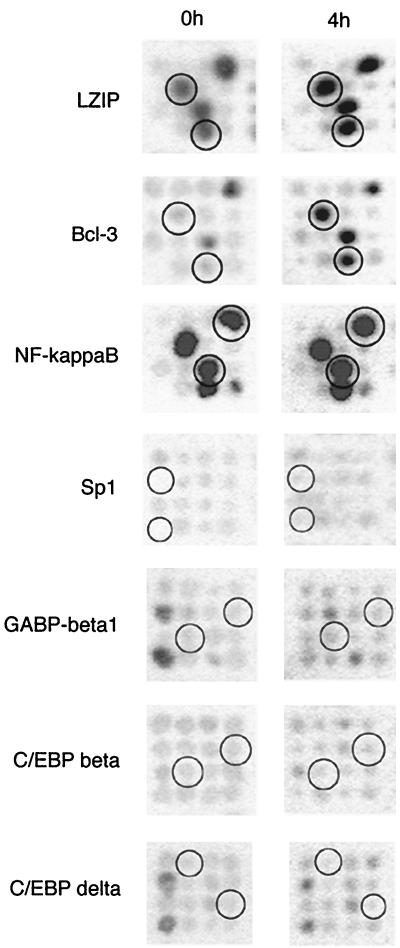
Within genes of the other categories, the one that initially gained our attention was Bcl-3, a transcription coactivator and member of the IκB family known to associate with NF-κB (p50) homodimers. RNA corresponding to the Bcl-3 gene increased in abundance (eightfold) in the 4-h sample (Fig. 2). Bcl-3 may associate with the ICP0 promoter (or enhancer) (see below), reactivation occurs with low efficiency in the absence of ICP0 (9, 10), and we located genes encoding other ICP0-relevant transcription factors (see Fig. 7) on the filters. Sp1, C/EBP-β and -δ, and GA binding protein β were present on the gene arrays, but their expression was both of low abundance and stable between the 0- and 4-h samples (Fig. 2). In contrast, the NF-κB p50 precursor (p105) was slightly upregulated 4 h p.e. (<threefold increase) and was moderately to highly abundant (20 to 50 times higher intensity than that of signals of low expression) (Fig. 2). Expressed sequence tags for Oct-1 and HCF-1 were not present on the filters. LZIP, a transcription factor similar to VP16 with respect to its association with HCF (22, 41), was also present on the gene arrays with a highly abundant message that showed moderate upregulation in the 4-h sample. It has been hypothesized that LZIP may bind a CRE element in the ICP0 promoter (40).
FIG. 2.
Expression levels of certain transcription factors in TG at 0 and 4 h p.e. The duplicate expressed sequence tag spots are circled for the indicated transcription factors.
FIG. 7.
Regulatory elements of the ICP0 promoter located upstream of the transcriptional start site. These elements are the Oct-1 binding site (TAATGARAT), the GABP binding site (CGGAAR), the Sp1 binding site (GGGCGG), the C/EBP binding site (CCAAT), the CRE element (AATCGTCA), the NF-κB binding site (GGGCTTCCC), and the putative NF-κB (p50) binding site (CCCCTTTGGGG).
Confirmation of changes in gene expression by RT-PCR and S1 nuclease protection assay.
RT-PCR was performed for several genes of interest in order to confirm the hybridization results. Total RNA prepared from 0- and 4-h explanted TG was DNase digested and subsequently reverse transcribed using oligo(dT) primers. The cDNAs were then used as template for the PCRs, one corresponding to the 0-h TG and the other to the 4-h TG, for each set of primers. The sequences of the specific primers used for each set of reactions, as well as the size of each PCR product, are described in Materials and Methods. All parameters of the reaction, such as template concentration and number of cycles, were determined for each set of primers so that the reaction was within the linear range of the PCR (21). Controls used in this experiment were β-actin and TIS7. It has previously been shown that β-actin is a housekeeping gene with constant expression in both 0- and 4-h explants (63) and therefore was used to confirm that the cDNAs used for each reaction were of the same quantity (Fig. 3). TIS7 was upregulated in the 4-h sample (Fig. 3), as observed previously (64), and it thus served as a positive control. Bcl-3 was also induced in the 4-h TG explants (Fig. 3), and quantitation of PCR products showed a 4.5-fold difference in expression of Bcl-3 in the two samples. Reproducibility was tested by performing the reaction several times from independently prepared sets of cDNA (data not shown).
FIG. 3.
RT-PCR detection of mRNA expression levels in uninfected and infected TG. (A) RT-PCR of RNA from uninfected TG. Detection of LZIP, HCF-1, and Bcl-3 in TG at 0 and 4 h p.e. Controls include β-actin (as a cDNA loading control) and TIS7, which were previously shown to be stable (β-actin) or to increase (TIS7) under these conditions (61, 63). The increased expression of Bcl-3 was 4.5-fold (with negligible deviation from the mean value), as determined from three experiments that included two separate samples from explanted TG. (B) RT-PCR of Bcl-3 RNA from infected TG.
To further confirm the upregulation of Bcl-3 at the mRNA level, an S1 nuclease protection assay was performed (Fig. 4), using probes for Bcl-3 and β-actin. β-actin again served as a control to verify that the same amount of mRNA was used at each time point. Quantitation of the gel bands confirmed that Bcl-3 mRNA increased fivefold in the 4-h p.e. sample relative to the 0-h sample. This level of increase was comparable to that detected with RT-PCR.
FIG. 4.
S1 nuclease protection analysis of Bcl-3 RNA. RNA was isolated from 0- and 4-h explanted TG and analyzed with Bcl-3- or β-actin-specific probes. Each time point was analyzed in duplicate.
Again, to confirm and extend the gene array results, we used RT-PCR to determine the level of LZIP, HCF-1, Sp1, and C/EBP-β RNAs. HCF-1 showed stable levels of expression between the 0- and 4-h samples (Fig. 3A), while RT-PCR for Sp1 and C/EBP-β did not yield any products, consistent with the low abundance on the gene array filter. LZIP showed a slight increase in expression, also similar to the gene array filters.
All of the above experiments were performed using material from uninfected TG, because our focus has been to identify critical cellular factors that are candidates to initiate reactivation. However, it is clearly important to determine whether Bcl-3 is increasing under reactivation conditions when virus is present. Thus, we tested latently infected TG for Bcl-3 mRNA after explantation. RT-PCR analysis for infected TG showed upregulation of the Bcl-3 message in the 4-h sample (Fig. 3B), at similar levels to those of uninfected RNA.
Bcl-3 is induced in neuronal cells.
Since HSV-1 establishes latency and reactivates in neurons, it is critical to demonstrate neuron-specific localization of the increased Bcl-3 gene expression. TG tissue sections from 0- and 4-h uninfected explants were analyzed by in situ hybridization. Nonisotopic detection with DIG-labeled DNA probes has been used successfully in several studies to identify neuronal mRNAs and appears to provide great sensitivity (42, 46). Therefore, a DIG-labeled DNA probe specific for Bcl-3 was used for hybridization to tissue. Bcl-3 expression was not detected at 0 h p.e. but was induced at 4 h p.e (Fig. 5). Neuronal cells expressing Bcl-3 are identified by dark purple staining (Fig. 5, right panel). Their identity as neurons was confirmed by comparing with hematoxylin-eosin-stained slides. Thus, the results suggest that Bcl-3 RNA levels are increasing specifically in neuronal cells, which are the sites of HSV-1 latent infection.
FIG. 5.
Detection of Bcl-3 mRNA in TG explants at 0 and 4 h p.e. by in situ hybridization. The tissue sections were stained with nitroblue tetrazolium-BCIP alkaline phosphatase substrate to detect the Bcl-3 DNA DIG probe (dark purple). The counter staining was done with methyl green and appears as a light blue-green color.
Protein levels of Bcl-3 are increased after explantation.
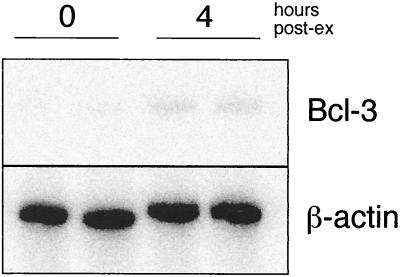
Western blot analysis of TG protein extracts was performed in order to examine whether the increased mRNA levels of Bcl-3 correspond to a similar increase in protein levels. For this purpose, an additional 8-h time point was also included in the analysis. Blotting with anti-Bcl-3 antibody showed a specific band at approximately 55 kDa present in the 4- and 8-h p.e. samples, whereas the signal was significantly lower in the 0-h sample (Fig. 6). Furthermore, the 55-kDa bands specifically disappeared when the hybridization was done in the presence of a Bcl-3 blocking peptide, confirming their identity as Bcl-3. As described by other authors, Bcl-3, a protein with a calculated molecular mass of 48 kDa, migrates at a position corresponding to 55 to 60 kDa when it is posttranslationally modified. The active form of Bcl-3 is extensively phosphorylated (5, 7, 23), suggesting that Bcl-3 protein is not only upregulated in the 4- and 8-h samples but may be present in its active form.
FIG. 6.
Western blot analysis of Bcl-3 from TG explants. Protein extracts were prepared from samples explanted for 0, 4, or 8 h. The Bcl-3 peptide that was used as antigen to generate antisera was included during incubation of the filter on the right. Molecular size markers are indicated.
DISCUSSION
The wide screening results presented in this study yield important information about the cellular environment which is present after TG explantation. Among the differentially expressed genes, there are several cellular factors involved in regulatory pathways, such as Ras-related proteins (R-ras, RAB, RHOC), immune system modulators (major histocompatibility complex and interferon-related proteins), growth factors (transforming growth factor and insulin-like growth factor), cell cycle-related proteins (cyclin-dependent kinase [CDK] homolog, cyclin F), and transcription factors (Bcl-3).
Cellular CDKs have previously been shown to have a role in transcription of HSV-1 IE and E genes in cell culture (57). The mechanism of CDK action on HSV-1 transcription is unclear, but it may involve phosphorylation of the VP16-HCF-Oct1 complex (33), which is not present in the earliest, initiating stages of viral reactivation.
Decreased expression of cellular transcriptional repressors that may maintain HSV-1 in the latent state would be expected to result in reactivation of the virus. However, we did not detect an increase in expression of any transcriptional repressors in the gene array.
With respect to the other factors, it is unknown whether these molecules influence HSV-1 reactivation; however, one of the identified proteins, Bcl-3, may be directly linked to HSV-1 transcription mechanisms as a possible DNA-binding activator or coactivator of viral promoters.
Bcl-3 is induced by the stress of explantation.
Bcl-3 is present among the genes whose expression was induced after the stress of explantation. The induction in expression was confirmed by RT-PCR and S1 nuclease protection assay. In addition, in situ hybridization showed that at 4 h p.e. upregulated Bcl-3 mRNA was located within neurons of the ganglia tissue. This mRNA upregulation corresponds to an increase in Bcl-3 protein levels. The bcl-3 gene was first identified as a proto-oncogene aberrantly expressed in human B-cell chronic lymphocytic leukemias (51). The Bcl-3 protein contains seven ankyrin repeats and shares structural features with members of the IκB family (35, 49). It also exhibits transactivation domains at the amino terminus as well as potential phosphorylation sites at the carboxyl-terminal part (5, 31). IκB proteins are known for their inhibitory effect on NF-κB activity by sequestering it into the cytoplasm (2, 3). Bcl-3 has a different function from IκB proteins, since it localizes predominantly to the nucleus and promotes nuclear translocation of the p50 subunit of NF-κB (48, 69, 71). Furthermore, it is associated with p50 or p52 homodimers bound to κB DNA sites and activates transcription both in vivo and in vitro (5, 8, 23). This interaction is dependent on the phosphorylation state of Bcl-3 (5, 7, 23). Since Bcl-3 from explanted TG migrates around 55 kDa in SDS-polyacrylamide gels, we conclude that Bcl-3 is present in the 4- and 8-h samples in its phosphorylated form.
Another interesting aspect of Bcl-3 function is its physical interaction with the histone acetyltransferase Tip60, as revealed by a yeast two-hybrid study (14). Tip60 was discovered as an HIV-1 Tat interacting protein (34) and is capable of acetylating several lysine residues in the amino-terminal tails of core histones H2A, H3, and H4 (36). There has been abundant evidence in recent years that recruitment of chromatin remodeling factors, including histone acetyltransferases, is required to reactivate quiescent genomic regions, and our results suggest that this mechanism may be essential for HSV-1 reactivation as well.
Little is known about the signaling pathways that lead to transcriptional activation of Bcl-3 itself, although interleukins may be involved through a STAT-dependent mechanism (53). There is also a putative NF-κB binding site in the Bcl-3 promoter region, raising the possibility of NF-κB–Bcl-3-dependent transcription and/or autoregulation (48, 49).
NF-κB is expressed in trigeminal ganglia.
In this study, we also show that the mRNA of the NF-κB p50 precursor (p105) is expressed in relatively large amounts in TG and is slightly induced after explantation. The cellular transcription factor NF-κB was first described as essential for immunoglobulin light chain κ gene expression in B cells (58). It is sequestered into the cytoplasm by the IκB proteins and has access to the nucleus only after certain signals that induce phosphorylation and subsequent ubiquitination and degradation of the IκB inhibitor (1). NF-κB is a dimer of subunits, including p50, p52, c-Rel, p65 (RelA), and RelB (4). The major form of NF-κB is the heterodimer p50-p65 that acts as a transcriptional activator through binding to κB promoter sequences. A role in promoting transcription has recently emerged for p50 homodimers as well. It has been shown that they can activate transcription by recruiting Bcl-3 to certain promoter sites. In addition to its significance in regulating gene expression of cells in the immune system, NF-κB is a crucial transcription factor for other cell types, including neurons of the central and peripheral nervous system (50). Diverse stimuli, such as nerve growth factor (11, 44, 70), neurotransmitters (glutamate) (50), oxidative stress, interleukin-1, tumor necrosis factor alpha, phorbol esters, and virus infection, activate NF-κB in neurons. Tumor necrosis factor alpha is induced in TG explants (64), and nerve growth factor has been previously shown to be involved in the reactivation process. Moreover, there is evidence that the activated intranuclear state of NF-κB is constitutive in many neurons in vivo (50) and that this factor is further activated by ischemic or excision injury (43, 65). As previously mentioned, Bcl-3 promotes NF-κB p50 nuclear translocation. The contribution of NF-κB to reactivation is unknown; however, HSV-1 primary infection causes NF-κB nuclear translocation which then increases the virus's efficiency to replicate (51).
Cellular transcription factors and transcription mechanisms during HSV-1 reactivation.
The promoter sequences that play a predominant role during acute infection are the TAATGARAT motifs, present in a number of copies in the IE promoters. Oct-1 recognizes these sites after interacting with the VP16–HCF-1 complex and mediates transcription of IE genes. In contrast, IE and E transcription occurs simultaneously during reactivation, without the presence of VP16. Among the several IE and E genes that are expressed at the onset of reactivation, ICP0 has been shown to be important for efficient reactivation (although ICP0 is not essential in normal primary infection) (9, 10, 27). A close inspection of the ICP0 promoter reveals binding sites for Sp1, C/EBP, CREB (or ATF), NF-κB, GABP, and several TAATGARAT motifs (Fig. 7). Sp1 is able to bind the G-rich regions of viral promoters (32), but we have found that Sp1 mRNA is absent or expressed in low levels in TG. Sp1 binding sites may still be important for viral transcription, since there are many transcription factors with binding capabilities comparable to those of Sp1 (12), some of which may show considerable levels of expression in sensory neurons. GABP binds GA-rich sequence flanking TAATGARAT motifs (17). As in the case of Sp1, GABP is expressed in limited amounts in TG. This low level of GABP mRNA reflects the low protein levels detected in a previous study (26).
Our results indicate that Bcl-3 and NF-κB (p50) are expressed in TG and show different degrees of induction after explantation. Bcl-3 transcript levels are significantly increased in the 4-h TG explants and are localized in neurons. Previous observations that NF-κB is present in neurons in its activated form constitutively or after induction suggest an NF-κB neuronal localization as well (50). Since each is present in neurons, these two factors, Bcl-3 and NFκB, possibly contribute to viral transcription in a coordinated way by upregulation of the HSV-1 ICP0 promoter. NF-κB binds to a κB motif at position −273 of the ICP0 promoter (56). There is also one putative κB site at −51, similar to palindromic sequences known to bind p50 homodimers (25). Importantly, this putative NF-κB binding site is not included in the region from −420 to −70 that was shown to be dispensable for reactivation (13).
During latency, the viral genome is packaged in a compact chromatin structure which is likely to be refractory to replication and transcription (15, 47, 54). Changes in chromatin configuration, such as acetylation of histone tails, allow regulatory factors and the replication and transcription enzymatic machinery to access DNA. An indication that chromatin remodeling is possibly crucial for viral transcriptional activation comes from the study of VP16 function. Several histone acetyltransferases can be recruited by VP16, leading to localized histone acetylation which is accompanied by induction of transcription (30, 66, 67). In a possible reactivation scenario, NF-κB p50 may already be present in the nucleus or enters after explantation, with the contribution of the increased amounts of Bcl-3. p50 homodimers may bind DNA and recruit Bcl-3 to the ICP0 promoter. Then, in a manner similar to VP16-mediated transcription, Bcl-3 recruits histone acetyltransferases, possibly Tip60, resulting in chromatin remodeling. This would then lead to promoter access by other transcription factors. The overall result would be stimulation of the ICP0 promoter, which may be the critical first step in the reactivation process.
ACKNOWLEDGMENTS
We thank Nigel Fraser for providing latently infected cell RNA. We thank Nigel Fraser and Tim Block for helpful discussions and Alex Seitz for help in preparing the in situ hybridization pictures.
This work was supported by NIH grant NS33768 to S.L.B.
REFERENCES
- 1.Baeuerle P A, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1998;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 5.Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 6.Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y, Mahlamaki E, Schraml P, Moch H, Willi N, Elkahloun A G, Pretlow T G, Gasser T C, Mihatsch M J, Sauter G, Kallioniemi O P. Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst. 1999;91:1758–1764. doi: 10.1093/jnci/91.20.1758. [DOI] [PubMed] [Google Scholar]
- 7.Bundy D L, McKeithan T W. Diverse effects of BCL3 phosphorylation on its modulation of NF-kappaB p52 homodimer binding to DNA. J Biol Chem. 1997;272:33132–33139. doi: 10.1074/jbc.272.52.33132. [DOI] [PubMed] [Google Scholar]
- 8.Caamano J H, Perez P, Lira S A, Bravo R. Constitutive expression of Bcl-3 in thymocytes increases the DNA binding of NF-kappaB1 (p50) homodimers in vivo. Mol Cell Biol. 1996;16:1342–1348. doi: 10.1128/mcb.16.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai W, Astor T L, Liptak L M, Cho C, Coen D M, Schaffer P A. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J Virol. 1993;67:7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai W Z, Schaffer P A. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989;63:4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter B D, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle P A, Barde Y A. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 12.Cook T, Gebelein B, Urrutia R. Sp1 and its likes: biochemical and functional predictions for a growing family of zinc finger transcription factors. Ann N Y Acad Sci. 1999;880:94–102. doi: 10.1111/j.1749-6632.1999.tb09513.x. [DOI] [PubMed] [Google Scholar]
- 13.Davido D J, Leib D A. Role of cis-acting sequences of the ICP0 promoter of herpes simplex virus type 1 in viral pathogenesis, latency and reactivation. J Gen Virol. 1996;77:1853–1863. doi: 10.1099/0022-1317-77-8-1853. [DOI] [PubMed] [Google Scholar]
- 14.Dechend R, Hirano F, Lehmann K, Heissmeyer V, Ansieau S, Wulczyn F G, Scheidereit C, Leutz A. The Bcl-3 oncoprotein acts as a bridging factor between NF-kappaB/Rel and nuclear co-regulators. Oncogene. 1999;18:3316–3323. doi: 10.1038/sj.onc.1202717. [DOI] [PubMed] [Google Scholar]
- 15.Deshmane S L, Fraser N W. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989;63:943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devi-Rao G B, Bloom D C, Stevens J G, Wagner E K. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douville P, Hagmann M, Georgiev O, Schaffner W. Positive and negative regulation at the herpes simplex virus ICP4 and ICP0 TAATGARAT motifs. Virology. 1995;207:107–116. doi: 10.1006/viro.1995.1056. [DOI] [PubMed] [Google Scholar]
- 18.Fraser N W, Block T M, Spivack J G. The latency-associated transcripts of herpes simplex virus: RNA in search of function. Virology. 1992;191:1–8. doi: 10.1016/0042-6822(92)90160-q. [DOI] [PubMed] [Google Scholar]
- 19.Fraser N W, Spivack J G, Wroblewska Z, Block T, Deshmane S L, Valyi-Nagy T, Natarajan R, Gesser R M. A review of the molecular mechanism of HSV-1 latency. Curr Eye Res. 1991;10:1–13. doi: 10.3109/02713689109020352. [DOI] [PubMed] [Google Scholar]
- 20.Fraser N W, Valyi-Nagy T. Viral, neuronal and immune factors which may influence herpes simplex virus (HSV) latency and reactivation. Microb Pathog. 1993;15:83–91. doi: 10.1006/mpat.1993.1059. [DOI] [PubMed] [Google Scholar]
- 21.Freeman W M, Walker S J, Vrana K E. Quantitative RT-PCR: pitfalls and potential. BioTechniques. 1999;26:112–122. doi: 10.2144/99261rv01. , 124–125. [DOI] [PubMed] [Google Scholar]
- 22.Freiman R N, Herr W. Viral mimicry: common mode of association with HCF by VP16 and the cellular protein LZIP. Genes Dev. 1997;11:3122–3127. doi: 10.1101/gad.11.23.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita T, Nolan G P, Liou H C, Scott M L, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 24.Geiss G K, Bumgarner R E, An M C, Agy M B, van't Wout A B, Hammersmark E, Carter V S, Upchurch D, Mullins J I, Katze M G. Large-scale monitoring of host cell gene expression during HIV-1 infection using cDNA microarrays. Virology. 2000;266:8–16. doi: 10.1006/viro.1999.0044. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh G, van Duyne G, Ghosh S, Sigler P B. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature. 1995;373:303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- 26.Hagmann M, Georgiev O, Schaffner W, Douville P. Transcription factors interacting with herpes simplex virus alpha gene promoters in sensory neurons. Nucleic Acids Res. 1995;23:4978–4985. doi: 10.1093/nar/23.24.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halford W P, Schaffer P A. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J Virol. 2001;75:3240–3249. doi: 10.1128/JVI.75.7.3240-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda K, Steger D J, Eberharter A, Workman J L. Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes. Mol Cell Biol. 1999;19:855–863. doi: 10.1128/mcb.19.1.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue J, Takahara T, Akizawa T, Hino O. Bcl-3, a member of the I kappa B proteins, has distinct specificity towards the Rel family of proteins. Oncogene. 1993;8:2067–2073. [PubMed] [Google Scholar]
- 32.Jones K A, Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus “immediate-early” gene transcription in vitro. Nature. 1985;317:179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- 33.Jordan R, Schang L, Schaffer P A. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J Virol. 1999;73:8843–8847. doi: 10.1128/jvi.73.10.8843-8847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamine J, Elangovan B, Subramanian T, Coleman D, Chinnadurai G. Identification of a cellular protein that specifically interacts with the essential cysteine region of the HIV-1 Tat transactivator. Virology. 1996;216:357–366. doi: 10.1006/viro.1996.0071. [DOI] [PubMed] [Google Scholar]
- 35.Kerr L D, Duckett C S, Wamsley P, Zhang Q, Chiao P, Nabel G, McKeithan T W, Baeuerle P A, Verma I M. The proto-oncogene bcl-3 encodes an I kappa B protein. Genes Dev. 1992;6:2352–2363. doi: 10.1101/gad.6.12a.2352. [DOI] [PubMed] [Google Scholar]
- 36.Kimura A, Horikoshi M. Tip60 acetylates six lysines of a specific class in core histones in vitro. Genes Cells. 1998;3:789–800. doi: 10.1046/j.1365-2443.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 37.Kramer M F, Coen D M. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristie T M, Vogel J L, Sears A E. Nuclear localization of the C1 factor (host cell factor) in sensory neurons correlates with reactivation of herpes simplex virus from latency. Proc Natl Acad Sci USA. 1999;96:1229–1233. doi: 10.1073/pnas.96.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillycrop K A, Liu Y Z, Theil T, Moroy T, Latchman D S. Activation of the herpes simplex virus immediate-early gene promoters by neuronally expressed POU family transcription factors. Biochem J. 1995;307:581–584. doi: 10.1042/bj3070581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu R, Misra V. Potential role for luman, the cellular homologue of herpes simplex virus VP16 (alpha gene trans-inducing factor), in herpesvirus latency. J Virol. 2000;74:934–943. doi: 10.1128/jvi.74.2.934-943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu R, Yang P, O'Hare P, Misra V. Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol. 1997;17:5117–5126. doi: 10.1128/mcb.17.9.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo L G, Jackson I M. Advantage of double labeled in situ hybridization for detecting the effects of glucocorticoids on the mRNAs of protooncogenes and neural peptides (TRH) in cultured hypothalamic neurons. Brain Res Protoc. 1999;4:201–208. doi: 10.1016/s1385-299x(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 43.Ma W, Bisby M A. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res. 1998;797:243–254. doi: 10.1016/s0006-8993(98)00380-1. [DOI] [PubMed] [Google Scholar]
- 44.Maggirwar S B, Sarmiere P D, Dewhurst S, Freeman R S. Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons. J Neurosci. 1998;18:10356–10365. doi: 10.1523/JNEUROSCI.18-24-10356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 46.Melloni R H, Jr, Aronin N, DeGennaro L J, Ferris C F, Harrison R J. Dde-I restriction endonuclease fragmentation: a novel method of generating cDNA probes for in situ hybridization in brain. J Histochem Cytochem. 1997;45:755–763. doi: 10.1177/002215549704500514. [DOI] [PubMed] [Google Scholar]
- 47.Muggeridge M I, Fraser N W. Chromosomal organization of the herpes simplex virus genome during acute infection of the mouse central nervous system. J Virol. 1986;59:764–767. doi: 10.1128/jvi.59.3.764-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nolan G P, Fujita T, Bhatia K, Huppi C, Liou H C, Scott M L, Baltimore D. The bcl-3 proto-oncogene encodes a nuclear I kappa B-like molecule that preferentially interacts with NF-kappa B p50 and p52 in a phosphorylation-dependent manner. Mol Cell Biol. 1993;13:3557–3566. doi: 10.1128/mcb.13.6.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohno H, Takimoto G, McKeithan T W. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- 50.O'Neill L A, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 51.Patel A, Hanson J, McLean T I, Olgiate J, Hilton M, Miller W E, Bachenheimer S L. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 52.Preston C M, Frame M C, Campbell M E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1998;52:425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 53.Richard M, Louahed J, Demoulin J B, Renauld J C. Interleukin-9 regulates NF-kappaB activity through BCL3 gene induction. Blood. 1999;93:4318–4327. [PubMed] [Google Scholar]
- 54.Rock D L, Fraser N W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983;302:523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- 55.Roizman B, Sears A E. Fundamental virology. Philadelphia, Pa: Lippincott; 1996. [Google Scholar]
- 56.Rong B L, Libermann T A, Kogawa K, Ghosh S, Cao L X, Pavan-Langston D, Dunkel E C. HSV-1 inducible proteins bind to NF-kappa B-like sites in the HSV-1 genome. Virology. 1992;189:750–756. doi: 10.1016/0042-6822(92)90599-k. [DOI] [PubMed] [Google Scholar]
- 57.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein NF-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 59.Sgroi D C, Teng S, Robinson G, LeVangie R, Hudson J R, Elkahloun A G. In vivo gene expression profile analysis of human breast cancer progression. Cancer Res. 1999;59:5656–5661. [PubMed] [Google Scholar]
- 60.Steiner I, Spivack J G, Deshmane S L, Ace C I, Preston C M, Fraser N W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol. 1990;64:1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stern S, Tanaka M, Herr W. The Oct-1 homeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989;341:624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- 62.Stevens J G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tal-Singer R, Lasner T M, Podrzucki W, Skokotas A, Leary J J, Berger S L, Fraser N W. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J Virol. 1997;71:5268–5276. doi: 10.1128/jvi.71.7.5268-5276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tal-Singer R, Podrzucki W, Lasner T M, Skokotas A, Leary J J, Fraser N W, Berger S L. Use of differential display reverse transcription-PCR to reveal cellular changes during stimuli that result in herpes simplex virus type 1 reactivation from latency: upregulation of immediate-early cellular response genes TIS7, interferon, and interferon regulatory factor-1. J Virol. 1998;72:1252–1261. doi: 10.1128/jvi.72.2.1252-1261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tong L, Toliver-Kinsky T, Taglialatela G, Werrbach-Perez K, Wood T, Perez-Polo J R. Signal transduction in neuronal death. J Neurochem. 1997;71:447–459. doi: 10.1046/j.1471-4159.1998.71020447.x. [DOI] [PubMed] [Google Scholar]
- 66.Tumbar T, Sudlow G, Belmont A S. Large-scale chromatin unfolding and remodeling induced by VP16 acidic activation domain. J Cell Biol. 1999;145:1341–1354. doi: 10.1083/jcb.145.7.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Utley R T, Ikeda K, Grant P A, Cote J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 68.Valyi-Nagy T, Deshmane S, Dillner A, Fraser N W. Induction of cellular transcription factors in trigeminal ganglia of mice by corneal scarification, herpes simplex virus type 1 infection, and explantation of trigeminal ganglia. J Virol. 1991;65:4142–4152. doi: 10.1128/jvi.65.8.4142-4152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe N, Iwamura T, Shinoda T, Fujita T. Regulation of NFKB1 proteins by the candidate oncoprotein BCL-3: generation of NF-kappaB homodimers from the cytoplasmic pool of p50–p105 and nuclear translocation. EMBO J. 1997;16:3609–3620. doi: 10.1093/emboj/16.12.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood J N. Regulation of NF-kappa B activity in rat dorsal root ganglia and PC12 cells by tumour necrosis factor and nerve growth factor. Neurosci Lett. 1995;192:41–44. doi: 10.1016/0304-3940(95)11603-t. [DOI] [PubMed] [Google Scholar]
- 71.Xiao P, Capone J P. A cellular factor binds to the herpes simplex virus type 1 transactivator Vmw65 and is required for Vmw65-dependent protein-DNA complex assembly with Oct-1. Mol Cell Biol. 1990;10:4974–4977. doi: 10.1128/mcb.10.9.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]