Abstract
As healthcare workers run a high and constant occupational risk of hepatitis B virus (HBV) infection through exposure to biological material, vaccination is mandatory as well as the monitoring of antibody levels one to two months after complete immunisation. The aim of this descriptive cross-sectional study was to determine HBV vaccine coverage of 200 primary and secondary healthcare workers (100 each) from Šabac, Serbia and their blood anti-HBs titre. We also wanted to identify factors that could predict the titre. Anti-HBV vaccination covered all participants, of whom 89.5 % were fully vaccinated, and 85 % had a protective antibody titre. We found a statistically significant association between antibody titre and the number of received vaccine doses, chronic jaundice, autoimmune disease, and cancer in our participants. The fact that 15 % did not achieve the protective antibody titre confirms the necessity of its control after immunisation, which is not routinely carried out in most countries, Serbia included. It is, therefore, necessary to develop a detailed strategy for monitoring vaccination and serological status of healthcare workers in order to improve their safety at work. An important role should also be given to continuous education of healthcare workers from the beginning of schooling to the end of their professional career.
Keywords: antibodies, anti-HBs titre, coverage, HBV, healthcare workers
Abstract
Budući da su zdravstveni radnici izloženi visokom i stalnom profesionalnom riziku od infekcije virusom hepatitisa B (HBV), zbog izloženosti biološkom materijalu cijepljenje je obvezno, kao i praćenje razine protutijela jedan do dva mjeseca nakon kompletne imunizacije. Cilj ovog deskriptivnog presječnog ispitivanja bio je utvrditi pokrivenost cjepivom protiv HBV-a 200 djelatnika primarne i sekundarne zdravstvene zaštite (po 100) u Šapcu u Srbiji i njihov anti-HBs titar u krvi te identificirati čimbenike koji bi mogli predvidjeti titar. Cijepljenjem protiv HBV-a obuhvaćeni su svi ispitanici, od kojih je 89,5 % bilo potpuno cijepljeno, a 85 % imalo je zaštitni titar protutijela. Pronašli smo statistički značajnu povezanost između titra protutijela i broja primljenih doza cjepiva te povijesti žutice, autoimunih i malignih bolesti naših ispitanika. Činjenica da 15 % nije postiglo zaštitni titar protutijela potvrđuje nužnost njegove kontrole nakon imunizacije, koja se u većini zemalja, pa tako i u Srbiji, ne provodi rutinski. Stoga je potrebno izraditi detaljnu strategiju praćenja cijepljenja i serološkoga statusa zdravstvenih radnika kako bi se poboljšala njihova sigurnost na radu. Veliku ulogu ima i trajna edukacija zdravstvenih djelatnika od početka školovanja do završetka profesionalne karijere.
Ključne Riječi: anti-HBs titar, HBV, pokrivenost, protutijela, zdravstveni radnici
Hepatitis B infection is an inflammation of the liver caused by the hepatitis B virus (HBV), which, due to its high prevalence and serious consequences, represents a threat to public health on the global level (1, 2). According to some estimates, a quarter of the world’s population is infected with HBV, and the World Health Organization (WHO) warns that mortality has reached 1.3 million deaths a year (3, 4).
In the European Union, the legislative framework for the prevention and treatment of HBV infection is identical. However, there are differences in implementation and control. Since its approval in 1982, the use of recombinant HB vaccine (5) had proven safe and so highly effective that the WHO recommended it should become part of the calendar of mandatory immunisation of children, which most countries adopted it in their national immunisation programmes and mandated immunisation for all children in the first year of life (6). The latest WHO guidelines seek to expand eligibility for testing and treatment with the goal to eliminate HBV infection by 2030 by reducing the number of newly infected persons and deaths (7).
Given the nature of their work, healthcare workers are more exposed to blood-borne infections than the general population (8, 9), and the primary method to prevent HBV infection is with hepatitis B vaccine or prophylaxis after exposure to patient’s biological material ( 10,11,12,13).
Even though HBV prevention in healthcare is regulated by law – immunisation in Serbia has been mandatory for healthcare workers since 1989 (14) – cases of both acute and chronic HBV infection are still reported in everyday practice, most often due to poor immune response to the vaccine or inefficient post-exposure prophylaxis. The risk of infection among healthcare workers, doctors and nurses in particular, depends on the achieved immune response measured by antibody titre (anti-HBs), the frequency of exposure to blood and body fluids through skin and mucosa, as well as on the knowledge of and compliance with measures after such exposure (11, 12, 15). Vaccination prevents HBV infection in more than 90 % of cases (16, 17), and the achieved immunity is measured with anti-HBs titre, which should be ≥10 mIU/mL (18, 19).
Considering that immunisation may fail due to issues related to the vaccine and the host, the aim of this study was to determine vaccine coverage of healthcare workers in Šabac, Serbia and their anti-HBs titre. We also aimed to determine the relationship between specific factors that may affect immunisation and antibody titre.
PARTICIPANTS AND METHODS
This descriptive cross-sectional study of 100 physicians and 100 hundred nurses in primary and secondary health care in Šabac, Serbia took place from September 2020 to February 2021. The minimum sample size of 178 participants was based on statistical assessment described by Yanase et al. (20), with defined alpha error of 0.05, and confidence interval of 95 %. Our sample consists of 200 out of 255 healthcare workers from our previous pilot study who agreed to have their blood anti-HBs titre determined. At the time of the study, 100 worked at the Dr Draga Ljočić Healthcare Centre, consisting of nine services, and the other 100 at the Dr Laza K. Lazarević General Hospital, consisting of 18 units (nine surgical and nine internal medicine).
The inclusion criteria were direct contact with and treatment of patients, work experience of at least one year, and voluntary informed consent to participate in this study.
Methods
The participants were asked to complete a questionnaire with socio-demographic data (10 items) and vaccination data (25 items divided into two parts), which aimed to determine anti-HBV vaccination coverage (seven items) and diseases/comorbidities that may affect vaccine effectiveness (18 items).
In addition to completing the questionnaire, the participants were calculated their body mass index (BMI) from self-reported height and weight, considering that obesity weakens the immune system, so that it becomes sensitive to bacterial, viral, and fungal infections, including HBV infection (21).
The participants also gave 9 mL of blood, collected in a vacutainer tube without gel separator to determine the anti-HBs titre and total antibody to hepatitis B core antigen (total anti-HBc) at the University of Novi Sad Faculty of Medicine Centre for Medical and Pharmaceutical Research. Testing was carried out at the Centre’s Laboratory for Virological Testing on a Vidas II device (bioMerieux, Lyon, France) using the enzyme-linked fluorescence assay (ELFA) (22). All procedures and reporting followed the Guidelines for Good Laboratory Practice (23).
The study complied to the Declaration of Helsinki and was approved by the ethics committees of the Šabac General Hospital, Šabac Health Centre, and the University of Novi Sad Faculty of Medicine.
Statistical analysis
All statistics (descriptive and inferential) were run on the IBM SPSS software version 20.0 (Armonk, NY, USA). The Chi-squared test was used to determine the differences in categorical variables, and the Student’s t-test was used to determine the differences in continuous (numerical) variables. The association of anti-HBs titre with categorical and/or numerical variables was determined with Fisher’s exact test and ANOVA test. In all analyses, a p value of less than 0.05 (p<0.05) was considered statistically significant.
RESULTS
Table 1 shows detailed sociodemographic data and significant differences between groups of participants. Their overall mean age was 44.52±0.73 years and mean work experience 19.66±0.69 years. There are significant differences in some categorical variables, namely sex (dominantly female, p<0.001), workplace (dominantly outpatient clinic, p<0.001), and occupation (dominantly nurses p<0.001).
Table 1.
Differences in sociodemographic characteristics between participants
| Variables | N (%) | Chi-squared test | p | |
|---|---|---|---|---|
| Health institution | Primary health centre | 100 (50.0) | 0.000 | 1.000 |
| Hospital | 100 (50.0) | |||
| Sex | Male | 14 (7.0) | 147.920 | 0.000 |
| Female | 186 (93.0) | |||
| Field of medicine | Surgery | 60 (30.0) | 1.030 | 0.598 |
| Internal medicine | 71 (35.5) | |||
| General medicine | 69 (34.5) | |||
| Workplace | Outpatient clinic | 102 (51.0) | 33.970 | 0.000 |
| Hospital ward | 63 (31.5) | |||
| Intensive and semi-intensive care | 35 (17.5) | |||
| Occupation | Specialist | 16 (8.0) | 248.750 | 0.000 |
| General practitioner | 7 (3.5) | |||
| College-educated nurse | 19 (9.5) | |||
| Senior nurse | 30 (15.0) | |||
| Nurse | 128 (64.0) |
As far as continuous variables are concerned, they all significantly differ (Table 2).
Table 2.
Differences in continuous (numerical) variables
| Variables | Mean | SD | Student’s t-test | p |
|---|---|---|---|---|
| Age | 44.52 | 10.450 | 71.330 | 0.003 |
| Body height | 168.18 | 7.046 | 175.020 | 0.000 |
| Body mass | 69.28 | 13.956 | 135.040 | 0.000 |
| BMI | 24.47 | 4.473 | 7.221 | 0.008 |
| Years of service | 4.33 | 1.939 | 44.800 | 0.000 |
All participants reported to have been vaccinated (200 of 200) in the deltoid muscle, which correlates with the data obtained from epidemiological services and the electronic database of both health institutions. One hundred and seventy-nine (89.5 %) received the complete vaccination series of three shots (Figure 1), of whom 41.5 % according to the 0–1–6 schedule (day zero, one month after the first dose, and six months after the first dose). Most participants (65 %) had been immunised more than 10 years before the study.
Figure 1.
Distribution of participants according to the number of received vaccine doses (three doses denote completed vaccination)
Figure 2 shows the distribution of chronic diseases reported by the participants: 16.5 % had a chronic disease, whereas the rest reported none.
Figure 2.
Distribution of participants by self-reported chronic diseases. HBV – hepatitis B virus; COPD – chronic obstructive pulmonary disease
The calculated BMI of our respondents shows that 32 % were overweight (BMI 25–29.9) and 9.5 % obese (BMI >30).
Thirty-five participants reported abnormal lipid status, of whom nine used and 26 did not use therapy. Tobacco smoking was reported by 66 participants.
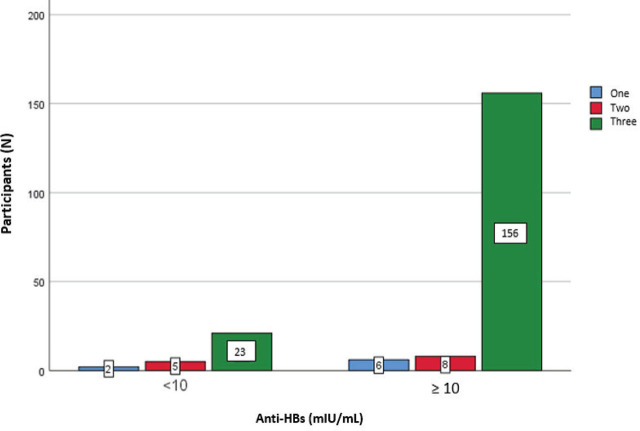
Blood samples showed that 15 % (30 participants) did not develop seroconversion, i.e., did not have the protective antibody titre of over ≥10 mIU/mL (Figure 3).
Figure 3.
Distribution of participants by anti-HBs titre threshold and number of received vaccine doses
Four participants had positive total anti-HBc, which is equal to the number of participants who reported being infected with the hepatitis B virus during their lifetime. In all other participants, total anti-HBc was negative.
Fisher’s test shows a significant association between the anti-HBs titre and the number of vaccine doses received (p=0.015). Participants who were fully vaccinated had a higher antibody titre than those who received one or two doses (Table 3).
Table 3.
Association between anti-HBs titre threshold and the number of vaccine doses received (three or less)
| Anti-HBs <10 mIU/mL | Number of participants | Fisher’s exact test | p | |
|---|---|---|---|---|
| Number of vaccine doses | Number of participants | 30 | 7.212 | 0.015 |
| 1 | 2 | |||
| 2 | 5 | |||
| 3 | 23 | |||
| Anti-HBs ≥10 mIU/mL | ||||
| Number of vaccine doses | Number of participants | 170 | ||
| 1 | 6 | |||
| 2 | 8 | |||
| 3 | 156 | |||
ANOVA has also established a highly significant association between low anti-HBs titre (<10 mIU/mL) and the reported autoimmune disease (p=0.000) and cancer (p=0.015). Previous jaundice is significantly associated with the high titre (≥10 mIU/mL; p=0.034) (Table 4).
Table 4.
Association between anti-HBs titre threshold and chronic, non-infectious conditions and smoking
| Variables | Anti-HBs (mIU/mL) | Mean square | ANOVA test | p |
|---|---|---|---|---|
| Insulin dependent diabetes | <10 | 0.066 | 2.699 | 0.102 |
| ≥10 | ||||
| Insulin independent diabetes | <10 | 0.129 | 0.496 | 0.513 |
| ≥10 | ||||
| Chronic obstructive pulmonary diseases | <10 | 0.003 | 0.124 | 0.725 |
| ≥10 | ||||
| Autoimmune disease | <10 | 0.889 | 22.843 | 0.000 |
| ≥10 | ||||
| Oncological disease | <10 | 0.029 | 6.046 | 0.015 |
| ≥10 | ||||
| Liver disease | <10 | 0.020 | 2.054 | 0.153 |
| ≥10 | ||||
| Prolonged jaundice | <10 | |||
| ≥10 | 0.233 | 4.545 | 0.034 | |
| ≥10 | ||||
| Disturbance of lipid status | <10 | 0.650 | 1.312 | 0.253 |
| ≥10 | ||||
| Tobacco smoking | <10 | 6857.533 | 0.935 | 0.335 |
| ≥10 |
In turn, we found no significant association between the anti-HBs titre and participants’ affiliation, sex, field of medicine, workplace, occupation (ANOVA, Table 5), or any of the continuous variables (year of age, years of service, body mass, body height, and BMI) (Fisher’s test, Table 6).
Table 5.
Association between anti-HBs titre threshold and categorical variables
| Variables | Anti-HBs (mIU/mL) | N (%) | ANOVA test | p |
|---|---|---|---|---|
| Healthcare institution | <10 | 30 | 0.360 | 0.549 |
| ≥10 | 170 | |||
| Sex | <10 | 30 | 0.579 | 0.448 |
| ≥10 | 170 | |||
| Field of medicine | <10 | 30 | 0.179 | 0.673 |
| ≥10 | 170 | |||
| Workplace | <10 | 30 | 0.036 | 0.850 |
| ≥10 | 170 | |||
| Profession | <10 | 30 | 3.298 | 0.071 |
| ≥10 | 170 |
Table 6.
Association between anti-HBs titre threshold and continuous variables
| Variables | Anti-HBs (mIU/mL) | Fisher’s exact test | p |
|---|---|---|---|
| Age | <10 | 31.334 | 0.854 |
| ≥10 | |||
| Body height | <10 | 1.552 | 0.215 |
| ≥10 | |||
| Body mass | <10 | 50.215 | 0.088 |
| ≥10 | |||
| BMI | <10 | 1.148 | 0.284 |
| ≥10 | |||
| Years of service | <10 | 0.954 | 0.330 |
| ≥10 |
DISCUSSION
All our participants received at least one dose of HB vaccine, which translates to 100 % coverage. Studies across the world report healthcare worker coverage ranging from 10–81 % in Africa to over 80 % in the USA and Australia ( 24, 25, 26, 27, 28, 29, 30, 31, 32). In Asia, surveys of healthcare workers providing secondary and tertiary care report 65 % and 67 % coverage, respectively (33, 34). In Serbia, recent data indicate that the coverage ranges between 31 and 96 % ( 35, 36, 37).
As for our findings that 89.5 % of our participants from Šabac completed the immunisation (received three shots), these figures excel reports of 50 % from three university hospitals in Belgrade (38) or 39.2 % from the public sector and 27.8 % from the private sector in the province of Vojvodina (39).
However, immunisation is not the same as having immunity, that is, having the anti-HBs titre >10 mIU/mL (regardless of the dynamic of decline). This is why all healthcare workers are advised to have their titre checked after having received three vaccine doses, especially those who come into contact with patient blood and body fluids (40, 41). Our study shows that 85 % of participants achieved immunity to HBV. These findings should be taken with some reserve, however, as 55 healthcare workers recruited in our previous pilot study refused to take immunity testing during recruitment, and were therefore excluded from this study. Although all of them were vaccinated, they stated that they considered themselves protected by the vaccine (38/55), were not interested in whether they achieved full protection (10/55), or were not exposed to blood-borne infections (7/55). Another report from Serbia speaks of 93 % seroconversion in their sample of 100 healthcare workers (36). Reported immunity among healthcare workers varies widely across the world, from 15–21 % in certain parts of Asia (42), 30.1 % in Bangladesh (43), 36.7 % in Indonesia (44), 47–74 % in Kenya (45, 46), 56.7 % in Congo (47), 77.1 % in Tanzania (48), 83.3 % in Tehran, Iran (49), 83 % in Japan (20) to 90 % in Egypt (50). Variation is particularly visible in India, ranging from 32 % (51), over 60.8 % (52) to 96 % (53). In Europe, a survey conducted in 21 countries of the European Union in 2016 showed that not all healthcare workers were protected after complete vaccination in as many as 18 countries (54). One meta-analysis of 19 Italian studies established that 27 % of healthcare workers did not achieve immunity (55), whereas another Italian study (56) among nurses showed a 85.7 % immunity. In Spanish Catalonia, one study reported 64.4 % (57), while a 2020 study in Turkey showed a 66.7 % protection (58).
As for factors that may prevent achieving immunity to HBV infection, we found a significant association between antibody titre and received vaccine doses or history of jaundice, autoimmune, and cancer. Age, sex, BMI, and smoking, in turn, are not significantly associated with anti-HBs titre. A 1987–1991 study conducted among 595 healthcare workers in Minnesota, USA six months after the third received vaccine dose (59) showed an association between insufficient anti-HBs titre and the type of vaccine, age, sex, BMI, and smoking, depending on which recombinant HB vaccine the participants received. We, however, could not establish which exactly recombinant vaccine our participant received to look deeper into the issue.
Complete vaccination is certainly a significant predictor of immunity to HBV infection, as it allows most recipients to acquire a sufficiently protective antibody titre (60). However, even receiving three vaccination doses may fail. Of the 30 participants (15 %) in our study whose antibody titre was below 10 mIU/mL, as many as 23 were fully vaccinated. Our analysis, however, has not found that age alone could be the reason, despite some indications that the titre drops with age (53, 61, 62). Perhaps the reason lies in the fact that anti-HBs titre remains high enough even 30 years after vaccination (63).
Our findings also fail to confirm earlier reports of association between obesity and non-response to HBV vaccine (21) or between BMI below 25 and seroprotection in healthcare workers (64). Truth be told, only 19 participants in our study were obese (BMI >30), which may not have been a representative sample to support these findings.
In contrast, failure to respond to vaccination is clearly associated with chronic jaundice, autoimmune disease, and cancer. Some studies argue against a link between HBV infection positive status and chronic diseases (65), while others indicate that people with lower kidney function, the elderly with diabetes, and people with inflammatory bowel diseases have a lower likelihood of seroconversion (66, 67).
Our study has certain limitations. Some are inherent to the study design partly relying on self-reported information. Then there is the issue of representation, as significantly more nurses participated than doctors. However, nurses are generally dominating in health care. Furthermore, the study does not include other categories of healthcare workers, such as laboratory and cleaning staff, who also run a risk of exposure to patient blood and other biological material.
CONCLUSION
Despite its limitations, our study shows total vaccination coverage of healthcare workers in Šabac, Serbia and a high rate (89.5 %) of those who completed the series by taking all three shots. The fact that 15 % did not achieve the protective antibody titre confirms the necessity of its control after immunisation, which is not routinely carried out in most countries, Serbia included. It is, therefore, necessary to develop a detailed strategy for monitoring vaccination and serological status of healthcare workers in order to improve their safety at work.
Perhaps even more important is to educate future health professionals about protection against HBV infection. Such education should continue throughout the working life to ensure the highest possible level of protection.
Footnotes
Conflicts of interest
None to declare.
REFERENCES
- 1.Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol. 2023;8:879–907. doi: 10.1016/S2468-1253(23)00197-8. [DOI] [PubMed] [Google Scholar]
- 2.Brouard C, Parenton F, Youssouf H, Chevaliez S, Gordien E, Jean M, Bruyand M, Vaux S, Lot F, Ruello M. Unono Wa Maore group. Hepatitis B, C, and delta in the general population in Mayotte: hepatitis B as a major public health concern. BMC Infect Dis. 2022;22(1):716. doi: 10.1186/s12879-022-07679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesan M, Eikenberry A, Poluektova LY, Kharbanda KK, Osna NA. Role of alcohol in pathogenesis of hepatitis B virus infection. World J Gastroenterol. 2020;26:883–903. doi: 10.3748/wjg.v26.i9.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global hepatitis report 2024: Action for access in low- and middle-income countries [displayed 20 Jun 2024] Available at https://iris.who.int/bitstream/handle/10665/376409/B09024-eng.pdf .
- 5.Gerlich WH. Hepatitis-B-Impfstoffe – Geschichte, Erfolge, Herausforderungen und Perspektiven [Hepatitis B vaccines-history, achievements, challenges, and perspectives, in German] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022;65:170–82. doi: 10.1007/s00103-021-03484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Introduction of hepatitis B vaccine into childhood immunization services. Management guidelines, including information for health workers and parents. Geneva: World Health Organization; 2001. [displayed 15 Jun 2024]. Available at https://iris.who.int/bitstream/handle/10665/66957/WHO_V-B_01.31_eng.pdf?sequence=1 . [Google Scholar]
- 7.World Health Organization. Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection [displayed 15 Jun 2024] Available at https://www.who.int/publications/i/item/9789240090903 . [Google Scholar]
- 8.Nagashima S, Yamamoto C, Ko K, Chuon C, Sugiyama A, Ohisa M, Akita T, Katayama K, Yoshihara M, Tanaka J. Acquisition rate of antibody to hepatitis B surface antigen among medical and dental students in Japan after three-dose hepatitis B vaccination. Vaccine. 2019;37:145–51. doi: 10.1016/j.vaccine.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Trevisan A, Giuliani A, Scapellato ML, Anticoli S, Carsetti R, Zaffina S, Brugaletta R, Vonesch N, Tomao P, Ruggieri A. Sex disparity in response to hepatitis B vaccine related to the age of vaccination. Int J Environ Res Public Health. 2020;17(1):327. doi: 10.3390/ijerph17010327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Đurić P, Brkić S, Ćosić G, Petrović V, Ilić S. Kontrola i prevencija krvnoprenosivih infekcija u zdravstvenim ustanovama [Control and Prevention of Blood-borne Infections in Health Care Facilities, in Serbian] Novi Sad: Institut za javno zdravlje Vojvodine; 2007. [Google Scholar]
- 11.Đurić P, Ilić S. HIV infekcija i zdravstveni radnici [HIV Infections and HealthcareWorkers, in Serbian] Novi Sad: Novosadski humanitarni centar; 2007. [Google Scholar]
- 12.Heininger U, Gambon M, Gruber V, Margelli D. Successful hepatitis B immunization in non- and low responding health care workers. Hum Vaccin. 2010;6:725–8. doi: 10.4161/hv.6.9.12420. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO position papers on Hepatitis B [displayed 12 Jun 2024] Available at https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/hepatitis-b .
- 14.Institut za javno zdravlje Srbije “Dr Milan Jovanović Batut”. Izveštaj o zaraznim bolestima u Republici Srbiji za 2022. godinu [Report on infectious diseases in Serbia in 2022, in Serbian] [displayed 10 Jun 2024] Available at https://www.batut.org.rs/download/izvestaji/GodisnjiIzvestajZarazneBolestiSrbija2022.pdf .
- 15.Čanak G. Infektivne bolesti sa negom zaraznih bolesnika [Infectious diseases and patient care, in Serbian] Novi Sad: Medicinski fakultet Univerziteta u Novom Sadu; 2009. [Google Scholar]
- 16.Thun JM, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D. Cancer Epidemiology and Prevention. 4th ed. New York (NY): Oxford University Press; 2017. [Google Scholar]
- 17.Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Wang F, Zheng H, Sun X, Miao N, Yin Z, Feng Z, Liang X, Wang Y. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23:765–72. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. Geneva: World Health Organization; 2024. [displayed 15 Jun 2024]. Available at https://www.who.int/publications/i/item/9789240090903 . [Google Scholar]
- 19.Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, de Perio MA, Reilly M, Byrd K, Ward JW. Centers for Disease Control and Prevention (CDC) CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep. 2013;62(RR-10):1–19. [displayed 15 Jun 2024]. Available at https://pubmed.ncbi.nlm.nih.gov/24352112/ [PubMed] [Google Scholar]
- 20.Yanase M, Murata K, Mikami S, Nozaki Y, Masaki N, Mizokami M. Hepatitis B virusvaccination-related seroprevalence among health-care personnel in a Japanese tertiary medical center. Hepatol Res. 2016;46:1330–7. doi: 10.1111/hepr.12691. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Guo Z, Dong C. Influences of obesity on the immunogenicity of hepatitis B vaccine. Hum Vaccin Immunother. 2017;13:1014–7. doi: 10.1080/21645515.2016.1274475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.bioMérieux. VIDAS ® Anti-HBc Total II (HBCT) [displayed 23 July 2020] Available at https://www.biomerieux.com/corp/en/our-offer/clinical-products/vidas-hepatitis-panel.html .
- 23.Smernice dobre laboratorijske prakse [Guidelines for good laboratory practice, in Serbian] Službeni glasnik RS 28/2008.
- 24.Galanakis E, Jansen A, Lopalco PL, Giesecke J. Ethics of mandatory vaccination for healthcare workers. Euro Surveill. 2013;18(45):20627. doi: 10.2807/1560-7917.es2013.18.45.20627. [DOI] [PubMed] [Google Scholar]
- 25.Issa A, Ayoola YA, Abdulkadir MB, Ibrahim RO, Oseni TIA, Abdullahi M, Ibraheem RM, Lawal AF, Dele-Ojo BF, Owolabi BI, Echieh CP. Hepatitis B vaccination status among health workers in Nigeria: a nationwide survey between January to June 2021. Arch Public Health. 2023;81(1):123. doi: 10.1186/s13690-023-01142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndunguru B, Wilfred D, Kapesa A, Kilonzo SD, Mirambo M, Hyera F, Massaga F. Low uptake of hepatitis B vaccination among healthcare workers in primary health facilities in Mwanza region, North-Western Tanzania. Front Public Health. 2023;11:1152193. doi: 10.3389/fpubh.2023.1152193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anthony M, Babatunde S. Uptake and completion rates of hepatitis B vaccination among health care workers at a referral hospital in UYO, Akwa Ibom State. EPRA Int J Multidiscip Res. 2023;9:168–77. doi: 10.36713/epra2013. [DOI] [Google Scholar]
- 28.Dayyab FM, Iliyasu G, Ahmad BG, Bako AT, Ngamariju SS, Habib AG. Hepatitis B vaccine knowledge and self-reported vaccination status among healthcare workers in a conflict region in Northeastern Nigeria. Ther Adv Vaccines Immunother. 2020;8 doi: 10.1177/2515135519900743. 2515135519900743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awoke N, Mulgeta H, Lolaso T, Tekalign T, Samuel S, Obsa MS, Olana R. Full-dose hepatitis B virus vaccination coverage and associated factors among health care workers in Ethiopia: A systematic review and meta-analysis. PLoS One. 2020;15(10):0241226. doi: 10.1371/journal.pone.0241226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan Q, Wang F, Zheng H, Zhang G, Miao N, Sun X, Woodring J, Chan PL, Cui F. Hepatitis B vaccination coverage among health care workers in China. PLoS One. 2019;14(5):e0216598. doi: 10.1371/journal.pone.0216598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bookstaver PB, Foster JL, Lu ZK, Mann JR, Ambrose C, Grant A, Burgess S. Hepatitis B virus seroconversion rates among health sciences students in the southeastern United States. J Am Coll Health. 2016;64:69–73. doi: 10.1080/07448481.2015.1042876. [DOI] [PubMed] [Google Scholar]
- 32.Ocan M, Acheng F, Otike C, Beinomugisha J, Katete D, Obua C. Antibody levels and protection after Hepatitis B vaccine in adult vaccinated healthcare workers in northern Uganda. PLoS One. 2022;17(1):e0262126. doi: 10.1371/journal.pone.0262126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harun MGD, Sumon SA, Mohona TM, Rahman A, Abdullah SAHM, Islam MS, Anwar MMU. Hepatitis B Vaccination Coverage among Bangladeshi Healthcare Workers: Findings from Tertiary Care Hospitals. Vaccines (Basel) 2023;11(1):41. doi: 10.3390/vaccines11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salman MS, Amna RS, Syed IA, Mairaj S. Determinants of hepatitis B vaccination status in health care workers of two secondary care hospitals of Sindh, Pakistan: a cross-sectional study. Hum Vaccin Immunother. 2021;17:5579–84. doi: 10.1080/21645515.2021.1986332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanazir M. Ispitivanje prediktora vakcinalnog statusa povezanog sa imunizacijom protiv hepatitisa B kod osoba zaposlenih u zdravstvenim ustanovama [A study of the predictors of vaccination status associated with immunisation against hepatitis B in healthcare staff, in Serbian] [PhD thesis] Beograd: Medicinski fakultet Univerziteta u Beogradu; 2016. [Google Scholar]
- 36.Bogdanović Vasić S, Stojčević Maletić J, Brestovački Svitlica B, Mićunović S, Knežević V, Antonić R, Ružić M. Protection of health workers employed in tertiary health institution from hepatitis B virus infection. Srp Arh Celok Lek. 2020;148:695–700. doi: 10.2298/SARH200419059B. [DOI] [Google Scholar]
- 37.Janićijević I, Perović M, Rančić N, Mitić S. Vakcinacija zdravstvenih radnika protiv virusnog hepatita B [Vaccination of healthcare workers against hepatitis virosa B, in Serbian] Timočki medicinski glasnik. 2011;36:188–91. [displayed 16 September 2023]. Available at http://www.tmg.org.rs/v360402.htm . [Google Scholar]
- 38.Marković-Denić L, Branković M, Maksimović N, Jovanović B, Petrović I, Simić M, Lesić A. Occupational exposures to blood and body fluids among health care workers at university hospitals. Srp Arh Celok Lek. 2013;141:789–93. doi: 10.2298/sarh1312789m. [DOI] [PubMed] [Google Scholar]
- 39.Đurić P. Uticaj programa unapređenja prevencije i kontrole krvnoprenosivih infekcija na smanjenje profesionalnog rizika u zdravstvu [Influence of program of strengthening prevention and control of blood-borne infections to reduction of professional risk of health personnel, in Serbian] [PhD thesis] Novi Sad: Medicinski fakultet Univerziteta u Novom Sadu; 2008. [Google Scholar]
- 40.Romano L, Zanetti AR. Hepatitis B Vaccination: A Historical Overview with a Focus on the Italian Achievements. Viruses. 2022;14(7):1515. doi: 10.3390/v14071515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grazzini M, Arcangeli G, Mucci N, Bonanni P, Bini C, Bechini A, Boccalini S, Tiscione E, Paolini D. High chance to overcome the non-responder status to hepatitis B vaccine after a further full vaccination course: results from the extended study on healthcare students and workers in Florence, Italy. Hum Vaccin Immunother. 2020;16:949–54. doi: 10.1080/21645515.2019.1680082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kashyap B, Tiwari U, Prakash A. Hepatitis B virus transmission and health care workers: Epidemiology, pathogenesis and diagnosis. Indian J Med Spec. 2018;9:30–5. doi: 10.1016/j.injms.2018.01.003. [DOI] [Google Scholar]
- 43.Biswas RSR, Karim MN, Bhattacharjee B. Hepatitis B virus infection and vaccination status among health care workers of a tertiary care hospital in Bangladesh. J Sci Soc. 2015;42:176–9. doi: 10.4103/0974-5009.165561. [DOI] [Google Scholar]
- 44.Muljono HD, Wijayadi T, Sjahril R. Hepatitis B virus infection among health care workers in Indonesia. Euroasian J Hepatogastroenterol. 2018;8:88–92. doi: 10.5005/jp-journals-10018-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kisangau EN, Awour A, Juma B, Odhiambo D, Muasya T, Kiio SN, Too R, Lowther SA. Prevalence of hepatitis B virus infection and uptake of hepatitis B vaccine among healthcare workers, Makueni County, Kenya 2017. J Public Health. 2019;41:765–71. doi: 10.1093/pubmed/fdy186. [DOI] [PubMed] [Google Scholar]
- 46.Mwangi IA, Wesongah JO, Musyoki VM, Omosa-Manyonyi GS, Farah B, Edalia LG, Mbuchi M. Assessment of hepatitis B vaccination status and hepatitis B surface antibody titres among health care workers in selected public health hospitals in Kenya. PLOS Glob Public Health. 2023;3(4):e0001741. doi: 10.1371/journal.pgph.0001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lungosi MB, Muzembo BA, Mbendi NC, Nkodila NA, Ngatu NR, Suzuki T, Wada K, Mbendi NS, Ikeda S. Assessing the prevalence of hepatitis B virus infection among health care workers in a referral hospital in Kisantu, Congo DR: a pilot study. Ind Health. 2019;57:621–6. doi: 10.2486/indhealth.2018-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller A, Stoetter L, Kalluvya S, Stich A, Majinge C, Weissbrich B, Kasang C. Prevalence of hepatitis B virus infection among health care workers in a tertiary hospital in Tanzania. BMC Infect Dis. 2015;15:386. doi: 10.1186/s12879-015-1129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amini M, Ansari I, Yekesadat S, Vaseie M. Response rate to the vaccination with hepatitis B vaccine among cardiovascular health staff in Teheran. Rev Latinoam de Hipertens. 2019;14:561–7. [Google Scholar]
- 50.El Sayed ZM, El Razek HMA. Longitudinal study of hepatitis B immunization in healthcare workers in one Egyptian center. J Immunol Res. 2016;3(1):1026. [Google Scholar]
- 51.Gupta M, Prince H, Dhiman RK. Baseline hepatitis B vaccination and anti HBs level among healthcare workers in a newly commissioned government dedicated oncology hospital. Int J Infect Dis. 2023;134(Suppl 1):S1–19. doi: 10.1016/j.ijid.2023.05.055. [DOI] [Google Scholar]
- 52.Prashant P, Nitin A, Suryasanta D, Kumar D. Status of protection against hepatitis B infection among healthcare workers in a tertiary healthcare center in India: results can’t be ignored! J Hematol Clin Res. 2018;2:001–5. doi: 10.29328/journal.jhcr.1001005. [DOI] [Google Scholar]
- 53.Lall M, Sen S, Patrikar S, Karade S, Gupta RM. Post vaccination antibody titres of hepatitis B surface antigen (anti-HBs) in a mixed cohort of health care workers. Med J Armed Forces India. 2022;78:198–203. doi: 10.1016/j.mjafi.2020.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Schryver A, Lambaerts T, Lammertyn N, François G, Bulterys S, Godderis L. European survey of hepatitis B vaccination policies for healthcare workers: An updated overview. Vaccine. 2020;38(11):2466–72. doi: 10.1016/j.vaccine.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Francesco PB, Pasquale S, Giovanni M, Martinelli A, Vimercati L, Germinario CA, Tafuri S. Prevalence of healthcare workers fully vaccinated against hepatitis B without circulating antibodies in Italy and role of age at baseline cycle vaccination: a systematic review and meta-analysis. Expert Rev Vaccines. 2023;22:139–47. doi: 10.1080/14760584.2023.2162507. [DOI] [PubMed] [Google Scholar]
- 56.Coppeta L, Balbi O, Pietroiusti A, Biondi G, Baldi S, Magrini A. Immune Status for Hepatitis B and Risk for Occupational Exposure Virus Among Italian Nurses. Arch Clin Infect Dis. 2022;17(5):e105876. doi.org/10.5812/archcid-105876. [Google Scholar]
- 57.Domínguez A, Urbiztondo L, Bayas JM, Borrás E, Broner S, Campins M, Costa J, Esteve M. Working Group for the Study of the Immune Status in Healthcare Workers of Catalonia. Serological survey of hepatitis B immunity in healthcare workers in Catalonia (Spain) Hum Vaccin Immunother. 2017;13(2):435–9. doi: 10.1080/21645515.2017.1264791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Başpınar MM. Screening of Hepatitis A and B Seropositivity among Turkish Healthcare Providers Admitted to Occupational Health Services. Int J Clin Pract. 2022;2022:6065335. doi: 10.1155/2022/6065335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood RC, MacDonald KL, White KE, Hedberg CW, Hanson M, Osterholm MT. Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA. 1993;270:2935–9. [PubMed] [Google Scholar]
- 60.Dowran R, Malekzadeh M, Nourollahi T, Sarkari B, Sarvari J. The prevalence of hepatitis B virus markers among students of Shiraz University of Medical Sciences. Adv Biomed Res. 2021;10:7. doi: 10.4103/abr.abr_173_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King H, Xing J, Dean H, Holtzman D. Trends in prevalence of protective levels of hepatitis B surface antibody among adults aged 18–49 years with risk factors for hepatitis B virus infection-United States, 2003–2014. Clinic Infect Dis. 2020;70:1907–15. doi: 10.1093/cid/ciz537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaiear N, Krisorn P, Seehamoke C. A longitudinal study of hepatitis B surface antibody level after the accelerated vaccination protocol applied to health workers in a hospital of Thailand. Clin Epidemiol Glob Health. 2023;21:101271. doi: 10.1016/j.cegh.2023.101271. [DOI] [Google Scholar]
- 63.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, Spradling PR, Baum R, Hennessy T, McMahon BJ. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214:16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 64.Vivian Efua SD, Armah D, Delali AW. Hepatitis B virus vaccination post serological testing and antibody levels of vaccinated health care workers in Accra, Ghana. Vaccine X. 2023;14:100294. doi: 10.1016/j.jvacx.2023.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fabrizi F, Donato FM, Messa P. Association between hepatitis B virus and chronic kidney disease: a systematic review and meta-analysis. Ann Hepatol. 2017;16:21–47. doi: 10.5604/16652681.1226813. [DOI] [PubMed] [Google Scholar]
- 66.DaRoza G, Loewen A, Djurdjev O, Love J, Kempston C, Burnett S, Kiaii M, Taylor PA, Levin A. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis. 2003;42:1184–92. doi: 10.1053/j.ajkd.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 67.Jiang HY, Wang SY, Deng M, Li YC, Ling ZX, Shao L, Ruan B. Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta-analysis. Vaccine. 2017;35:2633–41. doi: 10.1016/j.vaccine.2017.03.080. [DOI] [PubMed] [Google Scholar]