Abstract
Background
The Anopheles Hyrcanus group, which transmits Plasmodium vivax, consists of six confirmed species in South Korea. An epidemiological study revealed differences in the seasonal occurrence patterns of each species. Pyrethroid resistance in An. sinensis dates back to the early 2000s, whereas information on pyrethroid resistance in other species is lacking despite their greater significance in malaria epidemiology.
Methods
Anopheles mosquitoes were collected from two malaria-endemic regions in South Korea for 2 years and their knockdown resistance (kdr) mutations were genotyped. The larval susceptibility to λ-cyhalothrin was compared in six Anopheles species and its seasonal changes in three species were investigated. The full-length sequences of the voltage-sensitive sodium channel (VSSC) were compared across six species to evaluate potential target-site insensitivity. The contribution of the kdr mutation to phenotypic resistance was confirmed by comparing median lethal time (LT50) to λ-cyhalothrin between populations of Anopheles belenrae with distinct genotypes.
Results
The composition and seasonal occurrence of rare species (Anopheles kleini, Anopheles lestri, and Anopheles sineroides) varied considerably, whereas An. sinensis occurs continuously throughout the season. A kdr mutation in the form of heterozygous allele was newly identified in An. belenrae, An. lesteri, An. pullus, and An. sineroides. The baseline susceptibility to λ-cyhalothrin was the highest in An. belenrae, followed by An. lesteri, An. sineroides, An. kleini, An. pullus, and An. sinensis, with median lethal concentration (LC50) values ranging from 6.0- to 73.5-fold higher than that of An. belenrae. The susceptibility of An. sinensis and An. pullus varied by season, whereas that of An. belenrae remained stable. The kdr-heterozygous An. belenare population exhibited 5.1 times higher LT50 than that of the susceptible population. Species-specific VSSC sequence differences were observed among the six species.
Conclusions
Our findings suggest that the status and extent of pyrethroid resistance vary among Anopheles Hyrcanus group species. While An. sinensis, the predominant species, developed a considerable level of pyrethroid resistance through kdr mutation, the resistance levels of other species appeared to be less pronounced. Large-scale monitoring is crucial to fully understand species-specific seasonal occurrence and resistance status for effective management strategies, considering the ongoing impact of climate change on their vectorial capacity.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-024-06462-8.
Keywords: Anopheles Hyrcanus group, Species composition, Seasonal dynamics, Pyrethroid resistance, λ-cyhalothrin, Kdr mutation, Baseline susceptibility, Voltage-sensitive sodium channel
Background
The Anopheles Hyrcanus group, a vector of Plasmodium vivax in East Asia, comprises six species (Anopheles belenrae, Anopheles kleini, Anopheles lesteri, Anopheles pullus, Anopheles sinensis, and Anopheles sineroides). All these species are present in the northern Gyeonggi province of South Korea [1, 2]. Epidemiological studies in northern Gyeonggi province have revealed that An. pullus was prevalent in early summer, An. kleini in mid-summer, and An. sinensis from mid-summer to late summer [1]. Comparative studies of the vector competence of An. sinensis, An. kleini, An. pullus, and An. belenrae indicated that An. kleini had the strongest vector competence, whereas An. sinensis exhibited the lowest susceptibility to P. vivax [3, 4]. Therefore, obtaining information on the seasonal abundance of Anopheles Hyrcanus group member species in malaria-endemic areas is crucial for understanding the population dynamics of vector populations and the epidemiology of malaria.
Pyrethroid resistance in the field population of An. sinensis, the predominant species, has been consistently reported in South Korea since 2001 [5]. Resistance to permethrin and deltamethrin in An. sinensis increased significantly over time, with resistance ratios of 15.6 and 22.6, respectively [6]. Pyrethroid resistance mutations were detected in South Korea before the early 2000s, with frequencies ranging from 25% to 97% [7]. Despite their distinct physiological and ecological characteristics related to vector competence, information on the differences in insecticide resistance traits among minor species of the Anopheles Hyrcanus group is scarce. This scarcity is primarily due to the challenges in distinguishing individual species from overlapping habitats. No susceptibility tests have been conducted on Anopheles species other than An. sinensis. However, they have a lower population density than An. sinensis, and assessing the pyrethroid resistance status of other species with higher vector competence is crucial for establishing an effective management strategy and preparing for potential outbreaks caused by climate change.
The development of pyrethroid resistance in mosquitoes primarily occurs through two major mechanisms: target alterations and metabolic detoxifications [8]. One of the most common target site alterations observed in Anopheles mosquitoes involves mutations in the voltage-sensitive sodium channel (vssc) gene, collectively known as knockdown resistance (kdr) mutations (L1014F/C/S and N1013S, N1575Y, and V1010L mutations) [9]. The L1014F mutation has been extensively reported in An. sinensis. Its absence in the other five species was documented in a single genotyping study a decade ago [10]. In addition to the major kdr mutation (L1014F) found in most insects, additional mutations such as M918T in Musca domestica and V1016I and F1534C in Aedes aegypti have been reported to be potentially associated with resistance [11]. Therefore, more comprehensive mutation detection in current mosquito populations is necessary to assess the recent pyrethroid resistance status across individual species in the Anopheles Hyrcanus group.
To address this gap in our understanding, the present study aimed to monitor the seasonal occurrence of Anopheles species in malaria-endemic Paju and Gimpo areas over 2 years using conventional species identification methods and to determine their kdr mutation frequencies. Additionally, we compared the larval susceptibilities of the six species in the Anopheles Hyrcanus group with λ-cyhalothrin through bioassays. Finally, we conducted an extensive search for VSSC mutations potentially associated with pyrethroid resistance across six species.
Methods
Mosquito collection and species identification
Blood-fed Anopheles mosquitoes were collected from Gyeonggi province, South Korea (Paju-1: 37.871385, 126.772513; Paju-2: 37.819755, 126.694960; and Gimpo: 37.708802, 126.643029), from May 2022 to September 2023 using black light traps (BT Global, South Korea) with an attractant overnight in a cowshed. Blood-fed mosquitoes were moved into 30 cm3 cages and provided with 10% sucrose for one day. Oviposition was induced by isolating individual Anopheles mosquitoes using a single wing cut in a paper cup containing 50 ml of water. Molecular identification was performed using DNA released from each wing by 10 µl of DNA-releasing buffer (5% of DMSO, 5% of PEG 200, 20 mM of NaOH, and 1 mM of EDTA). A multiplex polymerase chain reaction (PCR) method [12] with modifications to four primer sequences (see Additional file 1: Tables S.1) was used to identify six Anopheles Hyrcanus group species included in this study. To examine the seasonal distribution of these species, all individual mosquitoes collected at each site were identified using the multiplex PCR method during weeks 21–37 (Paju 2022), weeks 28–39 (Paju 2023), weeks 21–40 (Gimpo 2022), and weeks 28–40 (Gimpo 2023).
Genotyping of kdr mutation in six Anopheles Hyrcanus group species
Randomly selected mosquitoes collected from the Paju area in 2022 and 2023 were individually genotyped using the primer sets from a previous study (An_vssc-F: GACTTCATGCACTCCTTCATG; An_vssc-R: GTTGGTGCAGAGAGCGATGA) [13]. The 20 µl reaction mixture composed of 0.8 µl of released DNA template, 0.5 mM of each primer, 0.2 mM of dNTP, 2 µl of 10X buffer, and 0.1 µl of EX taq polymerase (Takara, Seoul, Korea). After 35 cycles of 95 ℃ for 30 s, 55 ℃ for 30 s, and 72 ℃ for 50 s, amplicons were purified and then sequenced using ABI3730xl. For mutation frequency calculation, individuals with homozygous susceptible and resistant alleles were recorded as 0 and 1, respectively, whereas heterozygous individuals were recorded as 0.5.
λ-cyhalothrin susceptibility of six Anopheles species larvae
Λ-cyhalothrin is a type II pyrethroid insecticide that has been widely used for mosquito control in South Korea. It was selected in this study to estimate pyrethroid resistance level in Anopheles larvae, as previous research found it to be the most effective against An. sinensis larvae among eight different pyrethroids tested [6]. Mosquito larvae hatched from eggs were reared under controlled conditions at 25 ℃, 14 h light and 10 h dark cycle (14L:10D), and 60% humidity. The filter paper was placed in the breeding dish, and tetramin flakes were provided daily to the F1 generation larvae. λ-cyhalothrin susceptibility tests were conducted on F1 larvae of mosquitoes collected from the Paju area from August to September, where all six species were confirmed to be present. Larval bioassays were conducted with 10–15 third instar larvae following World Health Organization (WHO) guidelines [14]. Four concentrations (0, 10, 100, and 1000 ppb) of λ-cyhalothrin were treated for 24 h and the mortality was observed. Mosquito species (An. sinensis and An. pullus), showing less than 50% mortality at 1000 ppb, were additionally treated with a 10,000 ppb concentration. To prepare these working solutions, 0.5 ml of the serially diluted 1–1000 ppm stock solutions of λ-cyhalothrin (analytical standard, Sigma-Aldrich 31058) in acetone were diluted 100-fold with 49.5 ml of distilled water using a 5-oz. paper cup. Mortality was observed after 24 h and the larvae were counted as “dead” if they could not escape from the forceps when held. To evaluate seasonal changes in susceptibility, bioassays will be performed on the collection groups at weeks 23, 24, 35, and 36 in 2022.
Cloning of vssc gene
The full-length vssc gene, which encodes the target of pyrethroid insecticides, was amplified and cloned to identify point mutations or transcript variants potentially causing insensitivity to pyrethroids across the six Anopheles species. A 6.4 kb-sized fragment (using primer An_vE1-0F and An_vE32-6399R in Table 1) was amplified from the cDNA of each species using Advantage 2 Taq polymerase (Takara, Seoul, Korea) in a 10 µl reaction for 30 cycles. Subsequently, nested PCRs were conducted to obtain four overlapping cDNA fragments (1.5–1.8 kb) covering the entire sequences using four different primer sets (Table 1). Amplified fragments were ligated into the pGEM-T easy vector (Promega, WI, USA) using T4 DNA ligase (Promega) and transformed into DH5a competent cells at 42 ℃ for 45 s. Plasmids were extracted from overnight cultured colonies using a mini prep kit (Bioneer, Daejeon, South Korea) and sequenced. The sequences were aligned using CLC Main Workbench 8.1.2 (QIAGEN) with the reference sequence of An. sinensis (ASIS024373) obtained from VectorBase.
Table 1.
Primer sets used for cloning the vssc gene
| Primer name | F/R | Sequence (5′-3′) | Position (nt) |
|---|---|---|---|
| An_vE1-0F | F | ATGACCGAAGACTCCGATTCG | 0 |
| An_vE1-19F | F | CGATATCTGAGGAAGAACGTAG | 19 |
| An_vE13-1781R | R | GGCAGGTGTTCTTGTGCATC | 1781 |
| An_vE13-1702F | F | GGACGTTTCGTTGGTGTACC | 1702 |
| An_vE22-3290R | R | AGCTCATTTTCACCATGCTCTG | 3290 |
| An_vE22-3266F | F | CTGCAGAGCATGGTGAAAATG | 3266 |
| An_vE29-4886R | R | GCATRTTAAATCCGATGAACAACAT | 4886 |
| An_vE29-4712F | F | GTTCATGACAGAAGATCAGAAGA | 4712 |
| An_vE32-6377R | R | ATACTGGGCGATCGTGAGTG | 6377 |
| An_vE32-6399R | R | GACATCTGCCGTGCGTGATGT | 6399 |
Copy number validation of vssc gene
Given that duplication of the VSSC target protein may affect pyrethroid sensitivity, the copy number of vssc loci was assessed by quantitative real-time PCR (qPCR) using the LightCycler 96 (Roche, Basel, Switzerland). Single-copy genes such as ribosomal protein S7 and ribosomal protein S3 were used as references. gDNA was extracted separately from four to seven individuals of each of the six species. The reaction mixture (10 µl) contained 10 ng of gDNA template, 0.5 µM of each primer, and 5 µl of TB Green™ Premix Ex Taq™ II (Takara Biotechnology, Japan). qPCR for each species was conducted with technical replicates using the amplification condition of 95 °C denaturation for 1 min followed by 35 cycles of 95 °C for 15 s, 57 °C for 20 s, and 72 °C for 30 s.
Median lethal time (LT50) of susceptible and heterozygous resistant Anopheles belenrae
To demonstrate that the kdr trait (L1014F mutation) confers pyrethroid resistance to An. belenrae, fourth instar larvae produced from females with the heterozygous kdr genotype were treated with 1 ppm λ-cyhalothrin. Mortality to λ-cyhalothrin was observed at 15, 20, 40, 60, 120, and 180 min after treatment and dead larvae were immediately frozen to release DNA individually. After mortality evaluation, the kdr genotype of all tested individual mosquitoes was investigated using the method described above (Mosquito collection and species identification). Individual larvae were sorted on the basis of their genotype and mortality responses. Mortality time curves were then generated for the larvae of each genotype, distinguishing between the homozygous susceptible and heterozygous groups. Finally, LT50 values were predicted and compared between the two groups.
Data analysis
The median lethal concentration (LC50) of six Anopheles species and the LT50 of An. belenrae was calculated using logit analysis and a log-likelihood test was performed using POLO ver 2.0 (LeOra Software, Parma, Italy). To determine the statistical significance of seasonal changes in susceptibility among the three Anopheles species, an unpaired t-test was conducted using the Sidak-Bonferroni method. The data were visualized using SigmaPlot 10.0 (Systat Software Inc., CA, USA) and GraphPad Prism 6.0 (GraphPad Software, CA, USA). Sequence alignment was conducted using the CLC Main Workbench 8 (Qiagen, MD, USA).
Results
Seasonal distribution of Anopheles Hyrcanus group species
A total of five species were collected in 2022 from the Paju-1 site in Paju, excluding An. lesteri. Anopheles sinensis, An. pullus, and An. belenrae were consistently observed throughout the season (Fig. 1). Anopheles pullus dominated until June 4, after which An. sinensis became predominant, accounting for over 80% of the population from July, maintaining this trend until September 15. Anopheles pullus was the second most prevalent species, ranging from 1.3% (on July 26) to 80% (on May 25). Anopheles belenrae was observed at very low densities, ranging from 1.3% (on July 26) to 9.3% (on June 11). Moreover, An. sineroides was observed in both the early and late seasons, peaking at 4.3% (on June 4), whereas An. kleini was observed only from July to August, peaking at 5.6% (on July 10).
Fig. 1.
Species composition ratio of the Anopheles Hyrcanus group collected from Paju (A and B) and Gimpo (C and D) areas during 2022–2023
In 2023, collections were conducted at both Paju-1 and Paju-2, approximately 8 km apart. Similar to 2022, An. sinensis was the most abundant species, accounting for more than 50% of the total population from July to September, followed by An. pullus, peaking at 21.3% (on July 17). Unlike in 2022, An. lesteri was observed, with the simultaneous presence of all six species confirmed in July. The composition of An. lesteri reached 12.9% during the late season.
At Gimpo, collections were conducted in 2022 and 2023. Four species were collected during the 2022 collection, with An. lesteri and An. sineroides not observed. Anopheles sinensis was the only species observed throughout the season. The composition ratios of An. pullus and An. belenrae were the highest in the early season (40% and 16.3%, respectively), decreased in mid-summer, and then increased again in the late season (10.5% and 15.8%, respectively). In 2023, only three species (An. sinensis, An. pullus, and An. belenrae) were observed at Gimpo since the collection began in July.
Frequency of kdr mutation (L1014F/C) in six Anopheles Hyrcanus group species
The kdr mutation allele was genotyped in 186 individuals from 5 species collected in 2022 (Table 2 and Fig. 2A) and 381 individuals from all 6 Anopheles species collected in 2023 in Paju (Table 3 and Fig. 2B). Anopheles sinensis was the only species exhibiting a homozygous resistance allele (FF/CF/CC) on all collection dates, which is consistent with previous findings [10]. Notably, the L1014F mutation was identified in An. belenrae and An. pullus for the first time, although at relatively low frequencies (8.7% and 4.3%, respectively) in the heterozygous form, compared to An. sinensis (58.2%) by 2022. Furthermore, the L1014F mutation was newly identified in An. lesteri and An. sineroides by 2023 and the L1014C mutation was detected in An. belenrae and An. lesteri. The frequency of the heterozygous genotype was the highest in An. sinensis (58.2% in 2022 and 45.6% in 2023), followed by An. belenrae (8.7% in 2022 and 21.2% in 2023) (Tables 2 and 3). Despite the larger population size of An. pullus compared with An. belenrae and An. lesteri, it showed relatively low frequencies of kdr mutation. Interestingly, An. kleini exhibited an absence of kdr mutations, which could be attributed to either the limited sample size examined or lower prevalence within the population.
Table 2.
Number of mosquitoes with L1014F/C mutation in six Anopheles Hyrcanus group species collected from Paju at eight different timepoints in 2022
| 1014 allele | 04-JUN | 11-JUN | 10-JUL | 26-JUL | 18-AUG | 26-AUG | 01-SEP | 15-SEP | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| An. blelnrae | LL | 3 | 5 | 1 | 5 | 4 | 2 | 1 | 21 (91.3%) | |
| LF | 1 | 1 | 2 (8.7%) | |||||||
| An. kleini | LL | – | – | 2 | – | 4 | – | – | – | 6 (100%) |
| An. pullus | LL | 14 | 16 | 3 | 1 | 3 | 5 | 2 | 44 (95.7%) | |
| LF | 2 | 2 (4.3%) | ||||||||
| An. sinensis | LL | 1 | 2 | 0 | 1 | 1 | 1 | 2 | 1 | 9 (8.7%) |
| LF | 8 | 6 | 3 | 6 | 8 | 1 | 9 | 3 | 44 (42.7%) | |
| LC | 0 | 2 | 1 | 2 | 1 | 1 | 5 | 4 | 16 (15.5%) | |
| CF/FF | 2 | 7 | 4 | 0 | 8 | 7 | 4 | 2 | 34 (33.0%) | |
| An. sineroides | LL | 2 | 2 | 1 | 3 | 8 (100%) |
Fig. 2.
Percentage of mosquitoes with homozygous susceptible (SS), heterozygous (RS), and homozygous resistant (RR) alleles. Mosquitoes were collected from Paju in 2022 (A) and 2023 (B)
Table 3.
Number of mosquitoes with L1014F/C mutation in six Anopheles Hyrcanus group species collected from Paju at six different timepoints in 2023
| 1014 allele | 24-JUL | 16-AUG | 27-AUG | 03-SEP | 13-SEP | 20-SEP | Total | |
|---|---|---|---|---|---|---|---|---|
| An. belenrae | LL | – | 3 | 2 | 20 | 6 | 25 | 56 (78.9%) |
| LF | – | 0 | 2 | 4 | 1 | 2 | 9 (12.7%) | |
| LC | – | 1 | 4 | 1 | 0 | 0 | 6 (8.5%) | |
| An. kleini | LL | 2 | 2 | 2 | 2 | – | – | 8 (100%) |
| An. lesteri | LL | 4 | – | 1 | 2 | 8 | 16 | 31 (88.6%) |
| LF | 0 | – | 2 | 0 | 0 | 0 | 2 (5.7%) | |
| LC | 0 | – | 1 | 0 | 0 | 1 | 2 (5.7%) | |
| An. pullus | LL | 12 | 1 | 9 | 25 | 16 | 21 | 84 (94.4%) |
| LF | 0 | 0 | 2 | 2 | 1 | 0 | 5 (5.6%) | |
| An. sinensis | LL | 3 | 1 | 2 | 9 | 8 | 4 | 27 (16.0%) |
| LF | 10 | 0 | 6 | 11 | 15 | 10 | 52 (30.8%) | |
| LC | 4 | 0 | 7 | 5 | 5 | 4 | 25 (14.8%) | |
| CF/FF/CC | 8 | 3 | 14 | 13 | 8 | 19 | 65 (38.5%) | |
| An. sineroides | LL | 1 | – | 3 | 1 | 0 | 3 | 9 (88.9%) |
| LF | 0 | – | 0 | 0 | 1 | 0 | 1 (11.1%) |
Relative susceptibility to λ-cyhalothrin in six Anopheles Hyrcanus group species
The LC50 values of six field-collected Anopheles species were determined using bioassays conducted over 2 years. Subsequently, the baseline susceptibility ratio (BSR) was estimated for each species relative to the most susceptible species in each group (Table 4). Anopheles belenrae exhibited the highest susceptibility (LC50 = 36.3 ppb), followed by An. lesteri (LC50 = 218 ppb; BSR = 6.0). Anopheles sineroides exhibited an LC50 of 366 ppb (BSR = 10.1), whereas An. kleini and An. pullus showed similar levels of baseline susceptibility, with LC50 values of 464 ppb (BSR = 12.8) and 469 ppb (BSR = 12.9). Anopheles sinensis was the most resistant species, with a 75.3-fold higher LC50 value (2736 ppb) than An. belenrae. Given the averages of 91% and 84%, An. sinensis exhibited kdr mutations in the 2022 and 2023 Paju collections (Tables 2 and 3), which was likely a result of the resistance induced by the kdr mutation.
Table 4.
LC50 and baseline susceptibility ratio (BSR) of Anopheles Hyrcanus group larvae at 24 h on λ-cyhalothrin treatment
| spp. | Number of parental groups | Number of tested larvae | LC50 (ppb) | 95% confidence limits | slope | df | χ2 | BSR |
|---|---|---|---|---|---|---|---|---|
| An. belenrae | > 12 | 331 | 36.3 | 22.7–52.0 | 2.44 ± 0.34 | 8 | 27.6 | 1.0 |
| An. kleini | 4 | 102 | 464 | 155–1995 | 1.34 ± 0.51 | 5 | 10.3 | 12.8 |
| An. lesteri | 4 | 72 | 218 | 95.7–483 | 2.05 ± 0.48 | 8 | 8.23 | 6.0 |
| An. pullus | 12 | 184 | 469 | 250–909 | 1.57 ± 0.26 | 12 | 30.8 | 12.9 |
| An. sinensis | > 12 | 317 | 2736 | 1424–8622 | 1.17 ± 0.23 | 8 | 22.3 | 75.3 |
| An. sineroides | 3 | 55 | 366 | 115–1609 | 1.67 ± 0.55 | 5 | 11.36 | 10.1 |
Seasonal changes of susceptibility to λ-cyhalothrin in three Anopheles species
To track seasonal change in mosquito susceptibility, bioassays using 100 and 1000 ppb of λ-cyhalothrin (Fig. 3A) were conducted for three commonly occurring Anopheles species (An. sinensis, An. pullus, and An. belenrae) during various seasons. At the start point (week 23), the mortality of F1 larvae was similar across the three species, ranging from 54% to 61% at 100 ppb and 86% to 100% at 1000 ppb. Throughout the season, the mortality rate of An. sinensis and An. pullus decreased significantly, whereas An. belenrae maintained a constant mortality rate of > 50%. In addition, when comparing the kdr mutation frequency of the Paju 2022 collections with the susceptibility data (Fig. 3B), fluctuations with a double peak in mid-July and late August were observed. These fluctuations suggest the presence of selection pressure in the study area, which drives the development of pyrethroid resistance.
Fig. 3.
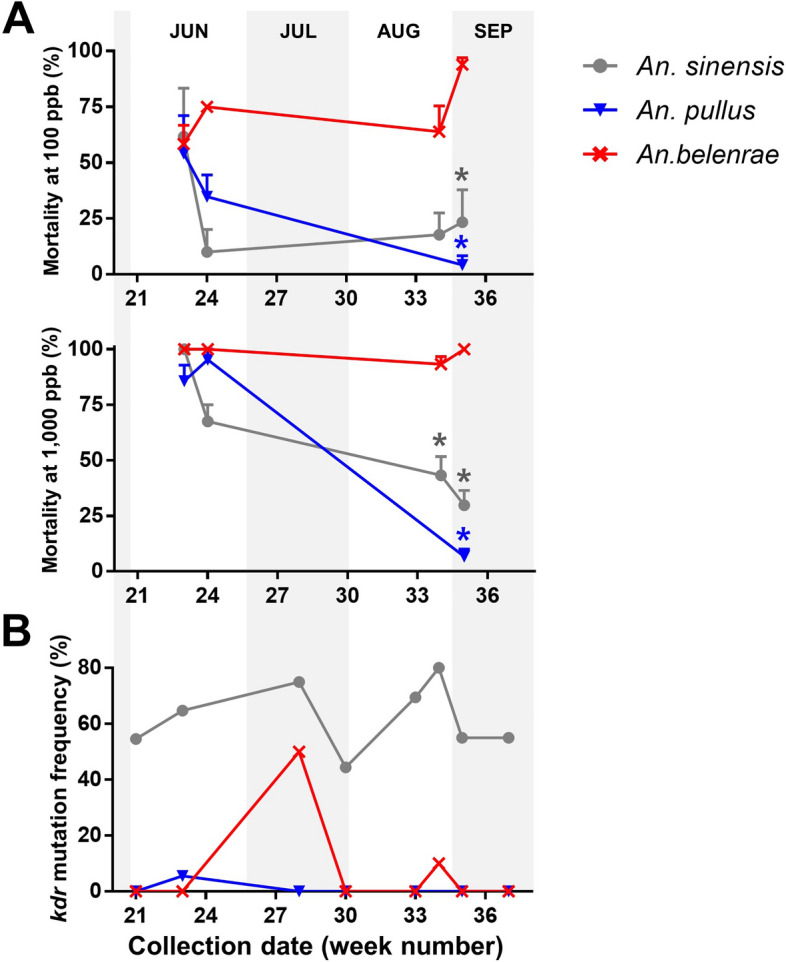
Dynamics of λ-cyhalothrin susceptibility and kdr mutation frequency in three Anopheles species in 2022, Paju. Mortality at 24 h after λ-cyhalothrin treatment at 100 and 1000 ppb (A) on third instar larvae was investigated. F1 generations of An. belenrae, An. pullus, and An. sinensis collected from Paju in 2022 were used for bioassays to synchronize the conditions. X-axis indicates the collection date of the parental generation
Differences in VSSC protein sequences and gene copy numbers among Anopheles Hyrcanus group species
The full-length sequences of VSSC, obtained from 2–4 clones of each species, were submitted to GenBank (Additional file 2: Table S.2) and aligned (Additional file 3: Figs. S1–4). Although several polymorphisms were detected in some clones, no exact matches were found between any of the six Anopheles species and the previously reported point mutations associated with pyrethroid resistance in mosquitoes and other insects [11]. However, cross-species comparison revealed various single amino acid substitutions, the insertion of 2–40 amino acids including exon k, and the deletion of 8–19 amino acids including exon h (Table 5, [15]). Additionally, we identified nine species-specific substitutions. Among these, five were conservative replacements, suggesting that they may not affect VSSC function. The remaining four radical substitutions included A812S and K1479T, which were exclusively found in An. pullus, whereas H315D and N2046I were found solely in An. lesteri. Considering that H315D and K1479T are located in the pore regions of domains I and III, respectively, and that A812S is positioned at the beginning of S1 of domain II, they may influence the overall channel kinetics, potentially altering pyrethroid sensitivity. As duplication of the vssc gene can affect pyrethroid sensitivity, the copy number in individual mosquitoes was estimated (Additional file 4: Fig. S5). No significant differences were found in the average copy number among the six Anopheles species (analysis of variance, ANOVA, F = 2.258, P = 0.075), indicating that duplication of the vssc gene does not commonly occur in these populations. However, one An. sineroides individual showed 1.45 copies, which may suggest the presence of a heterozygous form of vssc duplication.
Table 5.
Sequence variants of VSSC among Anopheles Hyrcanus group species
| Species | Sequence variation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid substitution (position) | Deletion | Insertion | ||||||||||
| 39 | 315 | 812 | 1076 | 1236 | 1348 | 1479 | 2042 | 2045 | D3-4 linker | D3-s3 | C-terminus | |
| An. belenrae | R | H | A | M | E | L | K | T | N | – | – | – |
| An. kleini | R | H | A | M | E | L | K | T | N | – | – | – |
| An. lesteri | R | D | A | M | D* | I* | K | T | I | – | – | – |
| An. pullus | R | H | S | L* | E | I* | T | T | N | exon h 1 | – | – |
| An. sinensis | R | H | A | M | E | L | K | T | N | exon h | exon k 2 | – |
| An. sineroides | K* | H | A | M | E | L | K | S* | N | – | – | GG |
* This indicates the conservative substitution of amino acid
1, 2 The exon names were referred to Lee et al., 2002
Positive association of kdr genotype with phenotypic λ-cyhalothrin resistance in An. belenrae
Excluding An. sinensis, An. belenrae showed the highest level of kdr mutation, reaching a maximum of 75%, although none of the individuals displayed a homozygous resistance allele. To assess the role of the kdr allele in pyrethroid resistance, the LT50 values of the two genotypes (homozygous susceptible and heterozygous) were estimated using An. belenrae as a model species (Fig. 4). Heterozygous individuals displayed a slower response (LT50 = 162 min) than susceptible homozygous individuals (LT50 = 31.5 min), resulting in a 5.1-fold difference. The hypothesis of equality was rejected at P < 0.001 (chi-squared test, χ2 = 36.6, df = 2). This result suggests that even the heterozygous allele confers phenotypic resistance and the population with a heterozygous resistance allele can survive when exposed to pyrethroid insecticides.
Fig. 4.
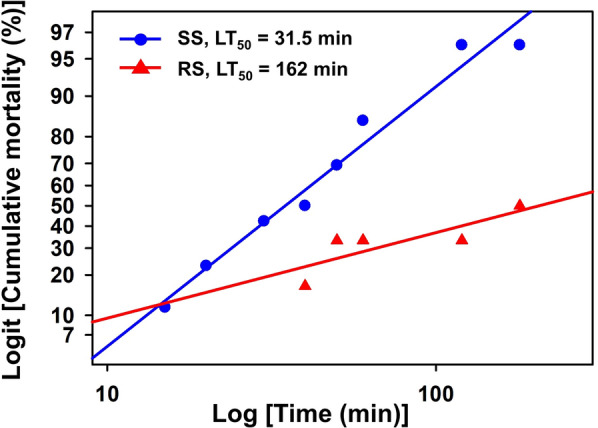
Time-mortality responses of An. belenrae larvae with either homozygous susceptible (SS) or heterozygous (RS) genotype. Mortality was recorded at specified time intervals following treatment with 1 ppm of λ-cyhalothrin. Scored mosquito larvae were immediately collected and their genotypes were analyzed
Discussion
Compared with previous studies investigating mosquito species composition in the northern Gyeonggi region, significant annual variations were evident. Even after collecting data for 2 years in the same area, substantial differences persisted. Notably, there were significant changes in An. lesteri, from 0 individuals in 2022 to 56 individuals in 2023. Anopheles kleini, which is hypothesized to exhibit the highest vector competence [16], was observed only in the Paju area among the study locations, albeit at a relatively low level of up to 5.5%. Nevertheless, considering its high density of 61.8% in the 2020 survey [17], the continuous monitoring of this mosquito species is crucial.
In this study, the presence of kdr mutations in some Anopheles species, such as An. belenrae, An. pullus, An. lesteri, and An. sineroides, was newly reported through genotyping. It is important to note, however, that the small sample size in low-density species could lead to biased interpretations. For example, in week 28 of 2022 in Paju, kdr mutation was observed in 50% of An. belenrae individuals tested (Fig. 3B). Yet, when considering the entire year of 2022, only 8.7% of An. belenrae carried the heterozygous resistance allele. The higher percentage in week 28 was due to the fact that only one individual collected during that week carried the LF allele. The significant differences in kdr mutation frequency among species may be attributed to the population sizes or fitness of resistant subpopulations compared with that of susceptible subpopulations [18, 19]. Large population size and wide distribution are essential for generating a multitude of individual variations within a species. This enables sufficient genetic diversity and the spread of traits associated with insecticide resistance within the population, thereby overcoming the limitations of genetic drift [10]. Lower frequencies of kdr mutations in the minor Anopheles species than in the predominant An. sinensis may be attributed to their relatively low population densities, which could constrain the gene pool available for the proliferation of resistance traits. Ecological differences, such as larval habitats or adult flight ranges, may also contribute to variations in insecticide exposure. Anopheles sinensis mosquitoes, owing to their wide distribution, are more likely to be exposed to pyrethroids, resulting in an increased kdr frequency compared with other species with a more limited distribution. The absence of homozygous kdr allele in minor species, coupled with their relatively higher λ-cyhalothrin susceptibility, suggests that these mosquitoes are in the early stages of pyrethroid resistance development. Nevertheless, even the heterozygous genotype conferred phenotypic resistance, as determined by the LT50 comparison in An. belenrae, heterozygous individuals would have a survival advantage over susceptible homozygous individuals under pyrethroid selection pressure. Therefore, the ongoing use of pyrethroids in the field may eventually lead to the development of high levels of resistance even in minor species of Anopheles mosquitoes. Given that Anopheles mosquitoes are believed to be indirectly selected by insecticides used in agriculture or industry rather than being directly targeted by public health pyrethroids [20], continuous monitoring of agricultural and industrial pyrethroids, along with effective management of mosquito habitats, is crucial for mitigating selection pressure against Anopheles mosquitoes. Because kdr mutations are known to incur high fitness costs in many insects [21, 22], including outdoor mosquitoes [13], understanding the fitness cost associated with kdr mutations in individual species of the Anopheles Hyrcanus group is imperative for efficient resistance management.
This study represents the first comparison of baseline susceptibility to pyrethroid insecticides among coexisting species. Despite continuous exposure to pyrethroid insecticides, significant differences in baseline susceptibility were observed among species. Anopheles sinensis exhibited a 75-fold reduction in λ-cyhalothrin susceptibility compared with An. belenrae. This remarkably low baseline susceptibility of An. sinensis appears to be primarily attributable to its high kdr mutation frequency (62%). Additionally, the presence of a transcription variant with a 41-bp insertion in transmembrane segment 3 of An. sinensis VSSC homology domain III, known as exon k [23], likely contributed to the further reduction in pyrethroid sensitivity, as supported by functional expression studies [24].
Anopheles pullus and An. kleini exhibited an approximately 13-fold reduction in susceptibility compared with An. belenrae; however, the kdr mutation frequencies were significantly lower than those of An. sinensis. Species-specific substitutions in the An. pullus VSSC may reduce its λ-cyhalothrin susceptibility. Nevertheless, the VSSC amino acid sequence of An. kleini was not significantly different from that of An. belenrae, and the observed difference in λ-cyhalothrin susceptibility between the two species was not due to any differential target site sensitivity. Taken together, these findings suggest that other resistance mechanisms, such as metabolic or cuticular penetration factors, may be involved in the cross-species differences in susceptibility, rather than the target site insensitivity mechanism, including the kdr mutation. Therefore, investigating the intrinsic differences in the genetic and physiological properties that govern insecticide susceptibility and their correlation with vector competence among species is essential to gaining a comprehensive understanding of the species-specific susceptibility status.
Due to the difficulty in obtaining synchronized adult specimens, baseline susceptibility to pyrethroids in this study was assessed by measuring larval susceptibility to λ-cyhalothrin. However, because pyrethroid insecticides are not typically used as larvicides, larval assays may not perfectly reflect the pyrethroid susceptibility levels of adult mosquitoes.
Insecticide resistance in vector mosquitoes significantly affects the transmission of vector-borne diseases by influencing their longevity and reproductive capacity. Numerous studies have demonstrated the correlation between the prevalence and transmission rates of vector-borne diseases and insecticide resistance in An. gambiae, a tropical malaria vector [25]. Interestingly, among domestic vector species, An. kleini, which exhibits relatively high vector competence [16], showed considerable resistance to pyrethroids despite the absence of kdr mutation. This finding prompted speculation that the potentially enhanced metabolic detoxification by An. kleini may be associated with increased vector competence. This association suggests a trade-off between its capacity for insecticide detoxification and the immune response, potentially leading to reduced immunity against parasitic infection. Further comprehensive research is required to understand the relationship between vector competence and insecticide resistance.
Conclusions
We investigated the distribution of the Anopheles Hyrcanus group, a vector of vivax malaria in South Korea, along with their pyrethroid susceptibility status. Anopheles sinensis, the most prevalent species, developed resistance most likely through target-site mutations. Our research also revealed diverse kdr mutation frequencies and notable variations in susceptibility to pyrethroid insecticides among previously overlooked minor species. Specifically, low frequencies of kdr mutations were identified in An. belenrae, An. lesteri, An. pullus, and An. sineroides for the first time, providing evidence for the development of initial resistance. Given that pyrethroid insecticides remain the primary choice for mosquito control, further studies on the resistance mechanisms, particularly those focusing on metabolic resistance, are crucial for these species. Furthermore, the effective management of mosquito vector populations is contingent on various factors, including seasonal density and resistance to insecticides. Thus, enhancing surveillance systems on the basis of species-specific phenology and seasonal dynamics of resistance is crucial for better responsiveness to ongoing climate change and effectively curtailing malaria transmission.
Supplementary Information
Additional file 1: Tables S.1 Multiplex PCR primer sets used for species identification of six Anopheles Hyrcanus group species.
Additional file 2: Tables S.2 GenBank accession numbers of cloned voltage-sensitive sodium channel sequences of six Anopheles Hyrcanus group.
Additional file 3: Figures S.1–4 The deduced protein sequence alignment of the vssc gene encompassing domains I–IV of the six species belonging to the Anopheles Hyrcanus group with An. sinensis obtained from VectorBase. The start and end points of each domain are indicated by red arrows and the blue boxes indicate segments 1–6 within each domain. Sequence variations are denoted by a yellow background, with species-specific variations highlighted by red boxes.
Additional file 4: Figure S.5 The copy number of vssc gene estimated using the single copy genes (RPS3 and RPS7) in six Anopheles Hyrcanus group species.
Acknowledgement
We are grateful to Se-in Kang for his assistance with mosquito collection in 2023.
Abbreviations
- kdr
Knockdown resistance
- VSSC
Voltage-sensitive sodium channel
- pPCR
Quantitative real-time polymerase chain reaction
- LC50
Median lethal concentration
- LT50
Median lethal time
- BSR
Baseline susceptibility ratio
Author contributions
D.E.L. collected the mosquitoes, conducted the overall experiments, and drafted the manuscript. J.H.H., G.C.L., J.C., and W.K. participated in mosquito collection, conducted molecular identification, and calculated species composition ratios. S.H.L. and J.H.K. coordinated the project, contributed to the study design, and provided critical revisions to the manuscript. All authors have reviewed and approved the final version of the manuscript.
Funding
This work was supported by Research Resettlement Fund for the new faculty of Seoul National University.
Availability of data and materials
The nucleotide and amino acid sequences generated in this study have been deposited in the GenBank database under the accession numbers (PQ136754-PQ136840). These sequences include the partial voltage-sensitive sodium channel (vssc) genes from six Anopheles Hyrcanus Group species.
Declarations
Ethics approval and consent to participate
No specific permits were required for this study. The study did not involve endangered or protected species. Therefore, the local ethics committee deemed that approval was unnecessary.
Consent for publication
All authors provided their consent for the publication of this report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim HC, Rueda LM, Wilkerson RC, Foley DH, Sames WJ, Chong ST et al. Distribution and larval habitats of Anopheles species in northern Gyeonggi Province, Republic of Korea. J Vector Ecol. 2011;36(1):124–34. [DOI] [PubMed] [Google Scholar]
- 2.Rueda LM, Kim HC, Klein TA, Pecor JE, Li C, Sithiprasasna R et al. Distribution and larval habitat characteristics of Anopheles Hyrcanus group and related mosquito species (Diptera: Culicidae) in South Korea. J Vector Ecol. 2006;31:198–205. 10.3376/1081-1710(2006)31[198:dalhco]2.0.co;2 & https://www.ncbi.nlm.nih.gov/pubmed/16859110. [DOI] [PubMed] [Google Scholar]
- 3.Lee WJ, Klein TA, Kim HC, Choi YM, Yoon SH, Chang KS et al. Anopheles kleini, Anopheles pullus, and Anopheles sinensis: potential vectors of Plasmodium vivax in the Republic of Korea. J Med Entomol. 2007;44:1086–90. 10.1603/0022-2585(2007)44[1086:akapaa]2.0.co;2 & https://www.ncbi.nlm.nih.gov/pubmed/18047210. [DOI] [PubMed] [Google Scholar]
- 4.Ubalee R, Kim HC, Phasomkusolsil S, Tawong J, Takhampunya R, Kayha A et al. Vector competence and the Susceptibility of Anopheles pullus and Anopheles belenrae to Plasmodium vivax-Infected Blood From Thai Patients. J Med Entomol. 2022;59:1047–52. 10.1093/jme/tjab226 & https://www.ncbi.nlm.nih.gov/pubmed/35043209. [DOI] [PubMed] [Google Scholar]
- 5.Shin EH, Park YI, Lee HI, Lee WJ, Shin YH, Shim JC. Insecticide Susceptibilities of Anopheles sinensis (Diptera: Culicidae) Larvae from Paju-shi. Korea Entomol Res. 2003;33:33–7. [Google Scholar]
- 6.Chang KS, Yoo DH, Shin EH, Lee WG, Roh JY, Park MY. Susceptibility and Resistance of Field Populations of Anopheles sinensis (Diptera: Culicidae) Collected from Paju to 13 Insecticides. Osong Public Health Res Perspect. 2013;4:76–80. 10.1016/j.phrp.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Baek JH, Lee W-J, Lee SH. Frequency detection of pyrethroid resistance allele in Anopheles sinensis populations by real-time PCR amplification of specific allele (rtPASA). Pestic Biochem Phys. 2007;87:54–61. 10.1016/j.pestbp.2006.06.009 [Google Scholar]
- 8.Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–59. 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- 9.Silva AP, Santos JM, Martins AJ. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids—a review. Parasit Vectors. 2014;7:450. 10.1186/1756-3305-7-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang S, Jung J, Lee S, Hwang H, Kim W. The polymorphism and the geographical distribution of the knockdown resistance (kdr) of Anopheles sinensis in the Republic of Korea. Malar J. 2012;11:151. 10.1186/1475-2875-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17. 10.1016/j.ibmb.2014.03.012 & https://www.ncbi.nlm.nih.gov/pubmed/24704279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Lee JS, Groebner JL, Kim HC, Klein TA, O’Guinn ML et al. A newly recognized species in the Anopheles Hyrcanus Group and molecular identification of related species from the Republic of South Korea (Diptera: Culicidae). Zootaxa. 2005;939:1–8. [Google Scholar]
- 13.Lee DE, Kim HC, Chong ST, Klein TA, Choi KS, Kim YH et al. Regional and seasonal detection of resistance mutation frequencies in field populations of Anopheles Hyrcanus Group and Culex pipiens complex in Korea. Pestic Biochem Physiol. 2020;164:33–9. 10.1016/j.pestbp.2019.12.005 & https://www.ncbi.nlm.nih.gov/pubmed/32284134. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Guidelines for laboratory and field testing of mosquito larvicides. Control of Neglected Tropical Diseases (NTD). 2005.
- 15.Lee SH, Ingles PJ, Knipple DC, Soderlund DM. Developmental regulation of alternative exon usage in the house fly Vssc1 sodium channel gene. Invert Neurosci. 2002;4:125–33. 10.1007/s10158-001-0014-1. [DOI] [PubMed] [Google Scholar]
- 16.Ubalee R, Kim HC, Schuster AL, McCardle PW, Phasomkusolsil S, Takhampunya R et al. Vector competence of Anopheles kleini and Anopheles sinensis (Diptera: Culicidae) from the Republic of Korea to Vivax malaria-infected blood from patients from Thailand. J Med Entomol. 2016;53:1425–32. 10.1093/jme/tjw109 & https://www.ncbi.nlm.nih.gov/pubmed/27493248. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Kim HC, Klein TA, Ryu J, Won MH, Choi JW et al. Species diversity of Anopheles mosquitoes and Plasmodium vivax infection rates, Gyeonggi Province, Republic of Korea during 2020. J Med Entomol. 2022;59:1778–86. 10.1093/jme/tjac086. [DOI] [PubMed] [Google Scholar]
- 18.Levick B, South A, Hastings IM. A two-locus model of the evolution of insecticide resistance to inform and optimise public health insecticide deployment strategies. PLoS Comput Biol. 2017;13:e1005327. 10.1371/journal.pcbi.1005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Zhang G. Some dynamic models for development of insecticide resistance in insect population. Comput Ecol Softw. 2018;8:1–6. [Google Scholar]
- 20.Lee DE, Shin J, Kim YH, Choi KS, Choe H, Lee KP, Lee SH et al. Inference of selection pressures that drive insecticide resistance in Anopheles and Culex mosquitoes in Korea. Pestic Biochem Physiol. 2023;194:105520. 10.1016/j.pestbp.2023.105520 & https://www.ncbi.nlm.nih.gov/pubmed/37532334. [DOI] [PubMed] [Google Scholar]
- 21.Kliot A, Ghanim M. Fitness costs associated with insecticide resistance. Pest Manag Sci. 2012;68:1431–7. 10.1002/ps.3395. [DOI] [PubMed] [Google Scholar]
- 22.Hanai D, Hardstone Yoshimizu M, Scott JG. The insecticide resistance allele kdr-his has a fitness cost in the absence of insecticide exposure. J Econ Entomol. 2018;111:2992–5. [DOI] [PubMed] [Google Scholar]
- 23.Olson RO, Liu Z, Nomura Y, Song W, Dong K. Molecular and functional characterization of voltage-gated sodium channel variants from Drosophila melanogaster. Insect Biochem Mol Biol. 2008;38:604–10. 10.1016/j.ibmb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Du Y, Liu Z, Dong K. Distinct functional properties of sodium channel variants are associated with usage of alternative exons in Nilaparvata lugens. Insect Biochem Mol Biol. 2020;118:103292. 10.1016/j.ibmb.2019.103292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minetti C, Ingham VA, Ranson H. Effects of insecticide resistance and exposure on Plasmodium development in Anopheles mosquitoes. Curr Opin Insect Sci. 2020;39:42–9. 10.1016/j.cois.2019.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Tables S.1 Multiplex PCR primer sets used for species identification of six Anopheles Hyrcanus group species.
Additional file 2: Tables S.2 GenBank accession numbers of cloned voltage-sensitive sodium channel sequences of six Anopheles Hyrcanus group.
Additional file 3: Figures S.1–4 The deduced protein sequence alignment of the vssc gene encompassing domains I–IV of the six species belonging to the Anopheles Hyrcanus group with An. sinensis obtained from VectorBase. The start and end points of each domain are indicated by red arrows and the blue boxes indicate segments 1–6 within each domain. Sequence variations are denoted by a yellow background, with species-specific variations highlighted by red boxes.
Additional file 4: Figure S.5 The copy number of vssc gene estimated using the single copy genes (RPS3 and RPS7) in six Anopheles Hyrcanus group species.
Data Availability Statement
The nucleotide and amino acid sequences generated in this study have been deposited in the GenBank database under the accession numbers (PQ136754-PQ136840). These sequences include the partial voltage-sensitive sodium channel (vssc) genes from six Anopheles Hyrcanus Group species.





