Abstract
The present study examined genetic, prenatal, and postnatal environmental pathways in the intergenerational transmission of anxiety and depressive symptoms from parents to early adolescents (when these symptoms start to increase), while considering timing effects of exposure to parent anxiety and depressive symptoms postnatally. The sample was from the Early Growth and Development Study, including 561 adopted children (57% male, 55% White, 13% Black/African American, 11% Hispanic/Latine, 20% multiracial, 1% other; 407 provided data in early adolescence) and their birth (BP) and adoptive parents (AP). Using a trait-state-occasion model with eight assessments from child ages 9 months to 11 years, we partitioned trait-like AP anxiety and depressive symptoms from time-specific fluctuations of AP anxiety and depressive symptoms. Offspring anxiety and depressive symptoms were assessed at 11 years (while controlling for similar symptoms at 4.5 years). Results suggested that time-specific fluctuations of AP1 (mostly mothers) anxiety/depressive symptoms in infancy (9 months) were indirectly associated with offspring anxiety/depressive symptoms at 11 years via offspring anxiety/depressive symptoms at 4.5 years; time-specific fluctuations of AP1 anxiety/depressive symptoms at child age 11 years were concurrently associated with offspring anxiety/depressive symptoms at 11 years. AP2 (mostly fathers) anxiety/depressive symptoms were not associated with offspring symptoms. Genetic and prenatal influences measured by BP internalizing problems were not associated with offspring symptoms. Results suggested infancy and early adolescence as developmental periods when children are susceptible to influences of parent anxiety and depressive symptoms. Preventive interventions should consider time-specific fluctuations in parent anxiety and depressive symptoms during these developmental periods.
Keywords: Anxiety and depressive symptoms, timing effects, trait-state-occasion model, behavioral genetics, adoption
Anxiety and depressive symptoms can be transmitted within families (Goodman et al., 2011; Murray et al., 2009). Goodman and Gotlib (1999) proposed an integrative model suggesting genetic, prenatal, and social environmental pathways that offspring of mothers with depression could be affected. Decades of research have suggested multiple possible mechanisms (e.g., physiological, emotional, cognitive) through which parent anxiety and depressive symptoms may be associated with similar symptoms in children (Gotlib et al., 2020; Murray et al., 2009). However, it remains unclear whether these associations truly reflect causal influences from parents to children (Gotlib et al., 2020). Specifically, genetically informed studies including both parents and children (e.g., children-of-twins, children born through assisted reproduction technology, parent-offspring adoption) are useful for testing whether associations between parent and child anxiety and depressive symptoms are confounded by shared genes. Such family-based genetically informed studies have consistently found that associations between parent and child anxiety and depressive symptoms are mainly explained by environmental, rather than genetic pathways (Ahmadzadeh et al., 2019; Eley et al., 2015; Lewis et al., 2011; Silberg et al., 2010), although studies with a larger sample size and more family types/members may have more power of detecting the genetic transmission pathway (Gjerde et al., 2021). One exception from these findings is that associations between prenatal maternal anxiety and depressive symptoms and child internalizing problems may be mainly explained by shared genetic factors or shared family environmental confounding (Gjerde et al., 2020; Gjerde et al., 2017; Hannigan et al., 2018).
Although the distinction between prenatal and postnatal exposure to parent anxiety and depressive symptoms has received particular attention, the effect of developmental timing has seldomly been systematically examined postnatally, especially when using genetically informed designs. In their integrative framework for the intergenerational transmission of depression, Goodman and Gotlib (1999) considered timing of exposure as an important moderator for several reasons. First, children might have different levels of vulnerability to the impact of maternal depression at different developmental stages, and that younger children may be more susceptible to negative influences because of developmental needs and plasticity. Second, disruption in early developmental tasks might alter later developmental trajectories even when maternal depression is no longer present. An updated review suggested that more longitudinal studies are still needed to understand the best timing to intervene on parent or child anxiety/depressive symptoms (Gotlib et al., 2020). To examine timing effects of exposure to parental anxiety and depressive symptoms, we focused on early adolescent anxiety and depressive symptoms as the main outcome (measured at age 11 years), predicted by parent anxiety and depressive symptoms from infancy to early adolescence across eight waves. Adolescence is a critical onset period for anxiety and depressive symptoms (Nivard et al., 2015; Petersen et al., 2018; Twenge & Nolen-Hoeksema, 2002), and a subgroup of adolescents may show persistent high levels of symptoms into adulthood (Dekker et al., 2007; Musliner et al., 2016; Pine et al., 1998). Previous studies have suggested that parent anxiety and depressive symptoms (mostly maternal depressive symptoms) during early infancy (Bureau et al., 2009; Korhonen et al., 2014; Sweeney & MacBeth, 2016), early childhood (Côté et al., 2018; Naicker et al., 2012; Spence et al., 2002), and adolescence (Griffith et al., 2021; Reeb et al., 2010; Spence et al., 2002) were associated with adolescent anxiety and depressive symptoms.
Although these studies provide valuable information for whether exposure to parent anxiety and depressive symptoms during specific developmental periods is associated with adolescent anxiety and depressive symptoms, the majority of studies did not use genetically informed designs. This limitation is important to address because controlling for genetic confound allows a more accurate estimation of environmental influences from parents to adolescents, which is particularly useful for evaluating whether intervening on parent anxiety and depressive symptoms can indeed lead to decreases in similar symptoms of adolescents. To address this limitation, the present study used a parent-offspring adoption design to partition genetic, prenatal, and postnatal environmental pathways from one another in the intergenerational transmission of anxiety and depressive symptoms from parents to adolescents. The parent-offspring adoption design includes birth parents, adoptive parents, and the adopted child. Because children were adopted shortly after birth (with no evidence of selective placement, see Leve et al., 2019), associations between birth parent and child anxiety and depressive symptoms suggest genetic and prenatal transmission; associations between adoptive parent and child symptoms suggest postnatal environmental transmission. It is worth mentioning here that we focused solely on parent (both birth and adoptive) and adolescent anxiety and depressive symptoms. Therefore, our findings are specific to genetic, prenatal, and postnatal environmental mechanisms for the intergenerational transmission of anxiety and depressive symptoms from parents to offspring without accounting for genetic, prenatal, or postnatal environmental influences from other parental characteristics.
To advance our understanding of postnatal environmental timing effects, adoptive parent anxiety and depressive symptoms were measured repeatedly from infancy to early adolescence. Although our main outcome was early adolescent anxiety and depressive symptoms measured at age 11, we controlled for anxiety and depressive symptoms in early childhood (measured at age 4.5 years) to account for continuity in symptoms, which may be confounded with influences from parent symptoms. However, adoptive parent anxiety and depressive symptoms prior to early childhood may also contribute to early adolescent anxiety and depressive symptoms indirectly through early childhood anxiety and depressive symptoms. Therefore, mediation pathways were allowed from adoptive parent symptoms before early childhood to early adolescent symptoms (at age 11 years) through early childhood symptoms (at age 4.5 years). In both adoptive parents and offspring, anxiety and depressive symptoms were grouped together to capture the broader dimension representing general distress that is shared between anxiety and depressive symptoms (Watson, Levin‐Aspenson, et al., 2022). This decision was made because anxiety and depression are known to be highly correlated (i.e., r = .50–.78, p < .05 in the current sample) and there has been no clear evidence suggesting that anxiety and depressive symptoms are transmitted differently from parents to offspring.
When examining timing effects of exposure to adoptive parent anxiety and depressive symptoms, it is also important to consider the fact that both anxiety and depressive symptoms are relatively stable during adulthood (Nivard et al., 2015). To truly understand whether there are timing effects, we need longitudinal studies that measure parent symptoms repeatedly across multiple child developmental stages. Such designs provide the opportunity to partition trait-like symptom levels that are relatively stable across time, from time-specific fluctuations in symptom levels. In longitudinal models, trait-like symptom levels capture between-person variance, while time-specific fluctuations of symptom levels capture within-person variance. Separating trait-like (between-person) and time-specific (within-person) symptom levels is ideal for testing timing effects, as we can examine whether the time-specific fluctuation of parent symptoms during a specific child developmental stage is associated with early adolescent symptoms, while controlling for the effect of parents’ trait-like symptoms. The present study applied the trait-state-occasion model (TSO; Cole et al., 2005) to partition trait-like (trait factor) and time-specific (occasion factors) anxiety and depressive symptoms of adoptive parents at child ages 9 months to 11 years, and then used trait and occasion factors to predict early adolescent anxiety and depressive symptoms at age 11 years. The TSO model was chosen over other models that can separate between-person and within-person variances because 1) the TSO model was found to be more stable than other similar models such as the latent trait–state–error model (TSE) and the latent state–trait model with autoregression (LST-AR) (Cole et al., 2005); 2) unlike multilevel models, the TSO model allowed the within-person components (the occasion factors) of parent anxiety and depressive symptoms measured at different times to have different effects on child outcomes, thus fits our aim to examine timing effects of exposure to parent anxiety and depressive symptoms; 3) we did not hypothesize systematic patterns of change in parent anxiety and depressive symptoms across the waves, therefore, the TSO model rather than the latent curve model with structured residuals was selected.
One similar study has used the TSO model to examine the effects of maternal anxiety and depressive symptoms (measured from child ages 3 months to 12.5 years) on adolescent internalizing problems, and reported that maternal trait-like symptoms, but not time-specific fluctuations in symptoms, were associated with adolescent internalizing problems (Missler et al., 2021). However, time-specific influences of parent anxiety and depressive symptoms on child and adolescent internalizing problems have been reported elsewhere (Cioffi et al., 2021; Xerxa et al., 2021). Moreover, Missler et al. (2021) examined biological parents rearing their biological children, thus the association between parent and child anxiety and depressive symptoms may be confounded by shared genetics factors, especially for the association between maternal trait-like symptoms and adolescent symptoms, as stability of anxiety and depressive symptoms are mostly explained by genetic influences (Nivard et al., 2015).
Finally, most extant research has focused on the influences of maternal anxiety and depressive symptoms on adolescents, with little attention to the influences from fathers. Several large studies have shown that paternal mental health problems were associated with higher levels of offspring psychopathology across childhood and adolescence (Flouri et al., 2019; Lewis et al., 2017; Ramchandani et al., 2005), which suggests the importance of taking paternal anxiety and depressive symptoms into account. Two reports using the same sample as our study have reported different timing effects of maternal and paternal depressive symptoms on child behavioral problems. Pemberton et al. (2010) found that paternal depressive symptoms at 9 months were directly associated with toddler externalizing problems, while a time-specific effect was not evident for the influence of maternal depressive symptoms. In a different report, Cioffi et al. (2021) found time-specific associations among maternal depressive symptoms and child internalizing problems from early to middle childhood, but not for paternal depressive symptoms.
Using the same longitudinal parent-offspring adoption sample as Cioffi et al. (2021) and Pemberton et al. (2010), the present study used the TSO model to study timing effects of exposure to adoptive parent (both mothers and fathers) anxiety and depressive symptoms that influence early adolescent anxiety and depressive symptoms, by examining the associations among time-specific fluctuations of parent symptoms at multiple child developmental stages and child symptoms in early adolescence. We hypothesized that exposure to adoptive parent anxiety and depressive symptoms during infancy, early childhood, and early adolescence would be associated with early adolescent anxiety and depressive symptoms at age 11 years. However, these associations may be attenuated when controlling for trait-like adoptive parent symptoms, and genetic and prenatal influences measured by birth parent internalizing problems. We did not hypothesize that there would be a significant direct pathway of genetic transmission, but did expect to find a mediated pathway through birth mother internalizing problems during pregnancy. We examined the influences of both adoptive mother and father anxiety and depressive symptoms. As no research has studied timing effects of paternal anxiety and depressive symptoms on adolescent internalizing problems, no specific hypothesis was postulated regarding the different effects of paternal and maternal symptoms.
Methods
Transparency and openness
We report how we determined our sample size, data exclusions, and measures. Data and analysis code are available upon request from the first author. Measures used in this study should be requested from the original authors. This study’s design and its analyses were not pre-registered.
Participants
The Early Growth and Development Study (EGDS; Leve et al., 2019) consists of 561 linked families, including birth parents (BPs), adoptive parents (APs), and adopted children. The EGDS sample has two cohorts (n = 361 for Cohort I, n = 200 for Cohort II), the recruitment began in 2003 for Cohort I and in 2008 for Cohort II through 45 US adoption agencies in 15 states. Eligibility for inclusion included: domestic adoption placement; placement occurred within 3 months postpartum; the adoptive family was not biologically related to the child; the child had no known major medical conditions; BPs and APs were able to understand English at the 8th-grade level. More details about the sample are described in Leve et al. (2019). Informed consent was obtained from all participants. Ethical approval was received from the institutional review boards at all universities participating in recruitment and data collection.
Fifty-seven percent of the children were male. The median age of the child at adoption placement was 2 days (SD = 11.32 days, range = 0–91 days). Fifty-five percent of the children were White, 13% were Black or African American, 11% were Hispanic or Latine, 20% were multiracial, and 1% were Asian, American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, unknown, or not reported.
For APs, the median level of educational attainment was four-year college. The median household income at the start of the study was from $100,001 to $125,000. The mean age at adoption for AP1s (mostly mothers) was 37.43 years (SD = 5.59 years, range = 23.73–55.08 years), for AP2s (mostly fathers) was 38.30 years (SD = 5.83 years, range = 24.39–59.79 years). For AP1s, 92% were White, 4% were African American, 2% were Latine, 1% were multiracial, and 2% were Asian, American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, unknown, or not reported. For AP2s, 90% were White, 5% were African American, 2% were Latine, 1% were multiracial, and 2% were Asian, American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, unknown, or not reported. At the start of the study, there were 41 same-sex APs (18 male-male couples, 23 female-female couples) and they were included in all analyses.
For BPs, the median level of educational attainment was high school. The median household income at the start of the study was lower than $15,000 for birth mothers (BMs) and was from $15,001 to $25,000 for birth fathers (BFs). The mean age at childbirth for BMs was 24.35 years (SD = 6.03 years, range = 13.63–43.39 years), for BFs was 26.08 years (SD = 7.77 years, range = 15.47–58.91 years). For BMs, 70% were White, 13% were African American, 7% were Latine, 5% were multiracial, and 5% were Asian, American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, unknown, or not reported. For BFs, 70% were White, 12% were African American, 10% were Latine, 5% were multiracial, and 4% were Asian, American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, unknown, or not reported.
The present study used data from BM interviews at 5, 18 and 56 months (only Cohort I at 56 months), BF interviews at 18 and 56 months (only Cohort I at 56 months), AP interviews at 9, 18 and 27 months and 4.5, 6, 7, 8 (Cohort I only) and 11 years, adopted child interviews at 11 years.
Measures
Offspring anxiety and depressive symptoms
Adolescent anxiety and depressive symptoms at age 11 years (Mean age = 11.38, SD = .54) were assessed using AP1 and AP2 reports on the anxious/depressed subscale from the 6–18 years version of the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001). APs completed 13-items on this subscale on a three-point scale. The mean of AP1 (α = .83) and AP2 (α = .82) reports was used to reduce common-method bias in analytic models examining associations between maternal/paternal and adolescent anxiety and depressive symptoms (r = .55). To control for earlier anxiety and depressive symptoms levels, we included child anxiety and depressive symptoms at age 4.5 years (Mean age = 4.74, SD = .27) in all models, measured using the anxious/depressed subscale from the 1/2–5 years version of the Child Behavior Checklist (Achenbach & Rescorla, 2000). APs completed the eight-item questionnaire on a three-point scale. The mean of AP1 (α = .68) and AP2 (α = .67) reports was used (r = .36).
Adoptive parent (AP) anxiety and depressive symptoms
APs reported their anxiety and depressive symptoms at child ages 9, 18, and 27 months and 4.5, 6, 7, 8 (Cohort I only) and 11 years. At child ages 9, 18, 27 months for both cohorts and 4.5 years for Cohort I, AP anxiety symptoms were measured using the Beck Anxiety Inventory (BAI; Beck & Steer, 1993a). This measure consisted of 21 items rated on a four-point scale and a sum score was created for each participant at each assessment (AP1, α = .76–.82; AP2, α = .74–.82). AP depressive symptoms were measured using the Beck Depression Inventory (BDI; Beck & Steer, 1993b). This measure consisted of 20 items rated on a four-point scale and a sum score was created for each participant at each assessment (AP1, α = .73–.83; AP2, α = .73–.86). The study team made the decision to not include the item measuring suicidal ideation (which was part of the original BDI) when Cohort I was assessed because clinical supervision was needed but was not available at the time. For consistency, we used the 20-item score for both cohorts.
At child age 4.5 years for Cohort II, 8 years for Cohort I, and 6, 7 and 11 years for both cohorts, AP anxiety symptoms were measured using the Trait Anxiety Scale from the State Trait Anxiety Inventory for Adults (STAI; Spielberger et al., 1983). This measure consisted of 20 items rated on a four-point scale and a sum score was created for each participant at each assessment after reversing several items (AP1, α = .91–.92; AP2, α = .91–.93). AP depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). This measure consisted of 20 items rated on a four-point scale and a sum score was created for each participant at each assessment after reversing several items (AP1, α = .80 −.90; AP2, α = .80 −.85).
Because different measures of AP anxiety and depressive symptoms were used at child age 4.5 years for Cohorts I and II, scores on each scale were standardized within cohorts and then combined. Parent anxiety and depressive symptom levels were comparable between the two cohorts when same measures were used. For all other waves, scores were standardized within the entire sample. These standardized scores of anxiety and depressive symptoms were used in all analyses.
Birth mother (BM) internalizing problems in pregnancy (prenatal transmission)
BM anxiety and depressive symptoms during pregnancy were measured retrospectively at ~5 months postpartum administered using a life history calendar method covering the 9 months of the pregnancy (Caspi et al., 1996; Freedman et al., 1988). The number of diagnosed internalizing disorders during pregnancy was calculated based on information from the Composite International Diagnostic Interview (CIDI; Kessler & Üstün, 2004), administered at child ages 18 months and 56 months (only Cohort I at 56 months). A composite score of BM anxiety symptoms, depressive symptoms, and the number of internalizing disorders diagnosed during pregnancy was computed using principal component analyses (PCA). PCA was chosen because the first component derived from PCA can explain as much of the total variance in the indicators as possible, while other similar approaches (e.g., factor analysis) focus on best describing associations among multiple indicators. Therefore, in our study, PCA was chosen for data reduction to capture the most comprehensive score indicating prenatal exposure to BM internalizing problems.
Birth parent (BP) lifetime internalizing problems (genetic transmission)
A composite score of lifetime internalizing problems in BPs was used to indicate the genetic transmission pathway from BPs to offspring (Marceau et al., 2019), using information from the CIDI assessed at child ages 18 months and 56 months (only Cohort I at 56 months). Information on the number of symptoms, number of diagnoses, and age of onset of each internalizing disorder was obtained from CIDI. The proportion of first-degree relatives with internalizing problems was included as the fourth indicator. PCA was used to create the composite score using the four indicators based on similar logic mentioned above for prenatal exposure to BM internalizing problems. Diagnoses/symptoms occurring only during pregnancy were not included and were considered as prenatal influences rather than genetic influences.
Covariates.
Additional variables that may be associated with child (age 4.5 years) and adolescent (age 11 years) anxiety and depressive symptoms were tested as covariates, including obstetric complications (Marceau et al., 2016), average adoption openness from child ages 9 months to 11 years (Ge et al., 2008), child sex, pubertal development (Petersen et al., 1988), BM and AP socio-economic status (SES, including household income, educational attainment, and neighborhood safety).
Missing data
Percentages of missing data are presented in Table 1. Missing data analysis suggested that obstetric complications, adoption openness, child sex, BM SES, or AP SES were not associated with the pattern of missing in child (4.5 years) or adolescent (11 years) anxiety and depressive symptoms (ps > .05). Lower AP1 (mostly mothers) anxiety symptoms at child ages 9 months and 4.5 years were associated with missing data on adolescent anxiety and depressive symptoms at age 11 years; 9 months: t(294.40) = 2.01, p = .05; 4.5 years: t(137.51) = 2.06, p = .04; so did lower AP2 (mostly fathers) depressive symptoms at child age 7 years, t(113.95) = 2.31, p = .02. As AP anxiety and depressive symptoms were already included as predictors of adolescent anxiety and depressive symptoms, full information maximum likelihood (FIML) was used to address missing data under the assumption of missing at random.
Table 1.
Descriptive statistics
| Range | ||||||
|---|---|---|---|---|---|---|
| n | Missing (%) | M | SD | Min | Max | |
| Child anxiety and depressive symptoms | ||||||
| 4.5 years | 427 | 24% | 1.91 | 1.60 | 0 | 10.00 |
| 11 years | 407 | 27% | 3.18 | 3.21 | 0 | 15.00 |
| AP1 (mostly mothers) anxiety symptoms | ||||||
| 9 months | 546 | 3% | 3.74 | 3.58 | 0 | 21.00 |
| 18 months | 513 | 9% | 3.43 | 3.59 | 0 | 28.00 |
| 27 months | 522 | 7% | 3.46 | 4.02 | 0 | 31.00 |
| 4.5 years (BAI-Cohort I) | 297 | 18% | 5.01 | 4.72 | 0 | 27.00 |
| 4.5 years (STAI-Cohort II) | 147 | 26% | 34.64 | 9.09 | 21.00 | 69.00 |
| 6 years | 444 | 21% | 34.79 | 8.94 | 20.00 | 71.00 |
| 7 years | 344 | 39% | 34.59 | 8.39 | 20.00 | 72.00 |
| 8 years (Cohort I only) | 237 | 58% | 34.34 | 8.68 | 20.00 | 68.00 |
| 11 years | 392 | 30% | 33.39 | 8.62 | 20.00 | 64.00 |
| AP1 (mostly mothers) depressive symptoms | ||||||
| 9 months | 546 | 3% | 3.63 | 3.30 | 0 | 17.00 |
| 18 months | 512 | 9% | 3.86 | 3.92 | 0 | 25.00 |
| 27 months | 522 | 7% | 3.89 | 4.19 | 0 | 30.00 |
| 4.5 years (BDI-Cohort I) | 297 | 18% | 4.48 | 4.38 | 0 | 28.00 |
| 4.5 years (CES-D-Cohort II) | 147 | 26% | 6.58 | 6.30 | 0 | 35.00 |
| 6 years | 444 | 21% | 7.21 | 7.58 | 0 | 52.00 |
| 7 years | 447 | 20% | 7.30 | 7.29 | 0 | 50.00 |
| 8 years (Cohort I only) | 240 | 57% | 7.55 | 7.22 | 0 | 54.00 |
| 11 years | 390 | 30% | 7.12 | 6.66 | 0 | 38.00 |
| AP2 (mostly fathers) anxiety symptoms | ||||||
| 9 months | 519 | 7% | 3.02 | 3.14 | 0 | 19.00 |
| 18 months | 491 | 12% | 2.31 | 3.13 | 0 | 23.00 |
| 27 months | 492 | 12% | 2.28 | 3.20 | 0 | 30.00 |
| 4.5 years (BAI-Cohort I) | 283 | 22% | 3.25 | 4.04 | 0 | 28.00 |
| 4.5 years (STAI-Cohort II) | 133 | 33% | 34.03 | 8.32 | 20.00 | 58.00 |
| 6 years | 405 | 28% | 32.90 | 8.47 | 20.00 | 59.00 |
| 7 years | 299 | 47% | 33.17 | 8.56 | 20.00 | 57.00 |
| 8 years (Cohort I only) | 189 | 66% | 32.33 | 9.18 | 20.00 | 62.00 |
| 11 years | 336 | 40% | 31.25 | 8.37 | 20.00 | 62.00 |
| AP2 (mostly fathers) depressive symptoms | ||||||
| 9 months | 519 | 7% | 3.02 | 3.22 | 0 | 27.37 |
| 18 months | 487 | 13% | 2.96 | 3.44 | 0 | 22.00 |
| 27 months | 491 | 12% | 2.88 | 3.82 | 0 | 41.00 |
| 4.5 years (BDI-Cohort I) | 284 | 21% | 3.67 | 4.67 | 0 | 39.00 |
| 4.5 years (CES-D-Cohort II) | 133 | 33% | 6.50 | 6.06 | 0 | 29.00 |
| 6 years | 408 | 27% | 6.44 | 5.97 | 0 | 29.00 |
| 7 years | 391 | 30% | 6.17 | 6.05 | 0 | 28.00 |
| 8 years (Cohort I only) | 193 | 66% | 6.46 | 6.33 | 0 | 38.00 |
| 11 years | 335 | 40% | 6.16 | 5.89 | 0 | 30.00 |
| BP internalizing (genetic influences) | 551 | 2% | 0 | 1.64 | −4.06 | 9.14 |
| BM internalizing during pregnancy | 539 | 4 % | 0 | 1.00 | −1.23 | 3.41 |
Note. BP = birth parents, BM = birth mother, AP = adoptive parents. BAI = Beck Anxiety Inventory, STAI = State Trait Anxiety Inventory, BDI = Beck Depression Inventory, CES-D = Center for Epidemiologic Studies Depression Scale. BP internalizing (genetic influences) and BM internalizing during pregnancy are standardized scores. AP and child anxiety and depressive symptoms are raw scores, although standardized scores for AP symptoms are used in analyses. AP anxiety symptoms are measured by BAI (possible range from 0–63) prior to 4.5 years, measured by STAI (possible range from 20–80) after 4.5 years. AP depressive symptoms are measured by BDI (possible range from 0–60) prior to 4.5 years, measured by CES-D (possible range from 0–60) after 4.5 years.
Less obstetric complications was associated with missing data on AP1 (mostly mothers) anxiety and depressive symptoms at child age 9 months; anxiety: t(14.99) = 2.97, p = .01; depression: t(14.99) = 2.97, p = .01. Lower adoption openness was associated with missing data on AP2 (mostly fathers) anxiety and depressive symptoms at child ages 9, 18, and 27 months(anxiety 9 months: t[47.59] = 2.89, p = .01; anxiety 18 months: t[87.19] = 2.58, p = .01; anxiety 27 months: t[87.15] = 3.01, p = .003; depression 9 months: t[47.59] = 2.89, p = .01; depression 18 months: t[94.10] = 2.88, p = .005; depression 27 months: t[88.88] = 2.93, p = .004). Lower BM SES was associated with missing data on AP1 (mostly mothers) anxiety symptoms at child age 7 years (t[463.37] = 2.38, p = .02), AP1 (mostly mothers) anxiety and depressive symptoms at child age 8 years (anxiety: t[498.86] = 2.21, p = .03; depression: t[507.89] = 2.14, p = .03), AP2 (mostly fathers) anxiety and depressive symptoms at child ages 4.5, 6, and 8 years (anxiety 4.5 years: t[239.47] = 1.99, p = .05; anxiety 6 years: t[290.29] = 2.75, p = .01; anxiety 8 years: t[365.22] = 2.01, p = .05; depression 4.5 years: t[235.26] = 1.99, p = .05; depression 6 years: t[280.41] = 2.58, p = .01; depression 8 years: t[378.38] = 2.13, p = .03), and AP2 (mostly fathers) anxiety symptoms at child age 7 years (t[548.77] = 3.12, p = .002). Higher AP SES was associated with missing data on AP1 (mostly mothers) anxiety symptoms at child age 7 years (t[4981.62] = −2.08, p = .04).
Analyses
Lavaan package 0.6–11 (Rosseel, 2012) in R (R Core Team, 2022) was used to fit all structural equation models. Robust maximum likelihood estimation (MLR) was used due to the violation of multivariate normality in study variables. All models were fitted separately for AP1 (mostly mothers) and AP2 (mostly fathers) anxiety and depressive symptoms because our sample size did not allow sufficient power to examine the influences of both parents in a single model.
Before fitting the TSO models, longitudinal confirmatory factor analysis (CFA) models were used to examine the factor structure and measurement equivalence of anxiety and depressive symptoms across time. Residual variances of manifest variables were correlated if they were assessed with the same measure. If the model assuming measurement equivalence fit well, the loadings of anxiety and depressive symptoms were tested for whether they could be constrained to be equal. If the loadings of anxiety and depressive symptoms could be constrained to be equal, the residual variances of anxiety and depressive symptoms at the same time (e.g., at 9 months) were tested if they could also be equal, because the total variance of all the anxiety and depressive symptoms variables were the same and was approximately one (they were all standardized variables).
Next, the TSO models for AP anxiety and depressive symptoms from child ages 9 months to 11 years were fitted separately for AP1s and AP2s (Figure 1). In the TSO models, St is a state factor representing the relative standing of anxiety and depressive symptoms levels at time t, which is a latent factor indicated by anxiety (AXt) and depressive symptoms (DPt) at time t. The total variance of the state factor at time t is completely explained by the occasion factor at time t (Ot) and the trait factor (AP Trait). The trait factor is a higher-order factor derived from the state factors that reflects the stable relative standing of anxiety and depressive symptoms levels across time (between-person variance). The occasion factor at time t explains the remaining variance of the state factor at time t that is unexplained by the trait factor and represents time-specific fluctuations in symptoms (within-person variance). It is worth noting that the TSO model used in the present study does not consider means because standardized scores across several different measures were used (so that the means were all approximately zero); therefore, the phrase “relative standing” is used to reflect rank order instead of absolute mean values. Meanwhile, autoregressive pathways are considered between occasion factors at time t-1 and time t, accounting for consistency in time-specific variances between two adjacent times.
Figure 1.
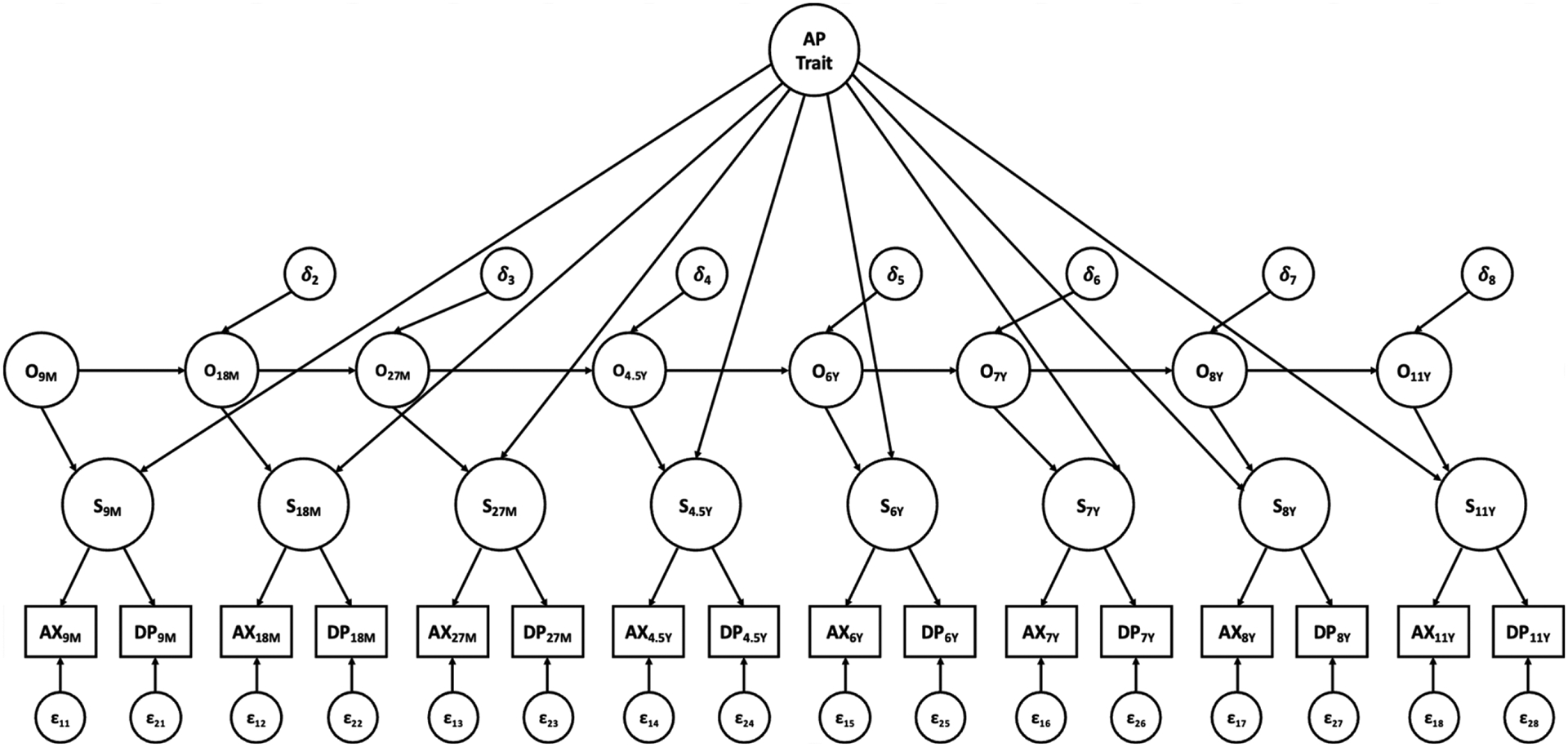
Trait-state-occasion model for adoptive parent anxiety and depressive symptoms from child ages 9 months to 11 years. Adoptive mother and father symptoms modeled separately. AP Trait = adoptive parent trait factor, O = occasion factor, S = state factor, AX = anxiety symptoms, DP = depressive symptoms. 9M to 27M = child age 9 months to 27 months, 4.5Y to 11Y = child age 4.5 years to 11 years. Correlated residuals in manifest variables with the same measure not shown for clarity.
The following simplifying assumptions suggested in Cole et al. (2005) were tested to determine the final TSO model: 1) equality of the factor loadings of St on the trait factor; 2) equality of the coefficients of autoregressive pathways. However, adapting to the unequal time distances across occasions (ranging from 9 to 36 months), the autoregressive coefficients were not constrained to be exactly the same. Instead, if the coefficient from O9M to O18M is β, then the coefficient from O27M to O4.5Y is β3, because the distance between 27 months and 4.5 years is three times the distance between 9 months and 18 months. The same rule was applied to all the autoregressive coefficients when the equality constraint was tested.
After fitting the TSO models, child (age 4.5 years) and adolescent (age 11 years) anxiety and depressive symptoms were regressed on all of the occasion factors and the trait factor, along with genetic BP lifetime internalizing problems (indicating genetic transmission) and BM internalizing problems in pregnancy (indicating prenatal transmission) (Figure 2). The models were run separately for AP1 and AP2 symptoms. To reduce the number of additional paths in the models, covariates were included in the model only if they were significantly correlated with child anxiety and depressive symptoms at age 4.5 years or 11 years. Hence, only BM SES was retained. Mediation pathways were tested using the MODEL INDIRECT command in Mplus 8.5 (Muthén & Muthén, 1998–2017), and 95% confidence intervals (CI) were computed using the bias-corrected bootstrapping approach.
Figure 2.

Full structural equation model. Genetic influences, prenatal influences, occasion factors and the trait factor predicting early adolescent anxiety and depressive symptoms at age 11. Model was run separately for adoptive mothers and fathers. State factors and observed variables in the trait-state-occasion model are not shown for clarity. BM = birth mother, BP = birth parent, AP Trait = adoptive parent trait factor, O = occasion factor, Sx = symptoms.
Results
Descriptive statistics for study variables are shown in Table 1 and correlations among study variables are in Table 2.
Table 2.
Correlations among study variables
| (i) Correlations among AP1 (mostly mothers) anxiety and depressive symptoms and other study variables | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| 1 ANX-9M | 1 | |||||||||||||||||||
| 2 ANX-18M | .59 | 1 | ||||||||||||||||||
| 3 ANX-27M | .55 | .56 | 1 | |||||||||||||||||
| 4 ANX-4.5Y | .53 | .46 | .49 | 1 | ||||||||||||||||
| 5 ANX-6Y | .44 | .39 | .41 | .59 | 1 | |||||||||||||||
| 6 ANX-7Y | .43 | .32 | .43 | .50 | .81 | 1 | ||||||||||||||
| 7 ANX-8Y | .40 | .42 | .41 | .38 | .74 | .76 | 1 | |||||||||||||
| 8 ANX-11Y | .40 | .37 | .41 | .40 | .69 | .73 | .76 | 1 | ||||||||||||
| 9 DEP-9M | .50 | .40 | .43 | .41 | .48 | .43 | .43 | .35 | 1 | |||||||||||
| 10 DEP-18M | .41 | .53 | .47 | .41 | .43 | .42 | .46 | .35 | .63 | 1 | ||||||||||
| 11 DEP-27M | .40 | .38 | .61 | .44 | .48 | .53 | .44 | .41 | .56 | .62 | 1 | |||||||||
| 12 DEP-4.5Y | .40 | .32 | .34 | .66 | .57 | .57 | .53 | .42 | .42 | .37 | .48 | 1 | ||||||||
| 13 DEP-6Y | .38 | .32 | .31 | .47 | .75 | .66 | .59 | .50 | .34 | .32 | .39 | .51 | 1 | |||||||
| 14 DEP-7Y | .35 | .28 | .36 | .43 | .60 | .76 | .62 | .48 | .32 | .31 | .43 | .48 | .63 | 1 | ||||||
| 15 DEP-8Y | .34 | .44 | .35 | .34 | .57 | .56 | .73 | .58 | .39 | .42 | .41 | .49 | .58 | .56 | 1 | |||||
| 16 DEP-11Y | .32 | .30 | .34 | .33 | .53 | .55 | .64 | .75 | .30 | .27 | .31 | .34 | .50 | .48 | .60 | 1 | ||||
| 17 BP INT (Lifetime) | .04 | .06 | .03 | −.04 | −.01 | −.02 | .02 | −.01 | .07 | .05 | .08 | .01 | −.06 | .01 | .01 | −.07 | 1 | |||
| 18 BM INT (Pregnancy) | −.01 | −.06 | −.01 | −.07 | −.02 | −.13 | −.03 | −.02 | .01 | −.03 | −.03 | −.04 | −.03 | −.07 | −.05 | 0 | .44 | 1 | ||
| 19 Child AXDP-4.5Y | .16 | .07 | .08 | .13 | .13 | .08 | .12 | .09 | .12 | .05 | .06 | .11 | .11 | .04 | .07 | .02 | .01 | −.01 | 1 | |
| 20 Child AXDP-11Y | .13 | .05 | .11 | .10 | .14 | .15 | .16 | .20 | .03 | .01 | .08 | .04 | .11 | .10 | .03 | .16 | 0 | −.05 | .38 | 1 |
| (ii) Correlations among AP2 (mostly fathers) anxiety and depressive symptoms and other study variables | ||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| 1 ANX-9M | 1 | |||||||||||||||||||
| 2 ANX-18M | .57 | 1 | ||||||||||||||||||
| 3 ANX-27M | .53 | .56 | 1 | |||||||||||||||||
| 4 ANX-4.5Y | .43 | .51 | .49 | 1 | ||||||||||||||||
| 5 ANX-6Y | .33 | .35 | .37 | .47 | 1 | |||||||||||||||
| 6 ANX-7Y | .31 | .37 | .41 | .40 | .81 | 1 | ||||||||||||||
| 7 ANX-8Y | .37 | .49 | .41 | .42 | .78 | .75 | 1 | |||||||||||||
| 8 ANX-11Y | .29 | .40 | .36 | .41 | .74 | .77 | .75 | 1 | ||||||||||||
| 9 DEP-9M | .53 | .44 | .45 | .40 | .39 | .41 | .42 | .37 | 1 | |||||||||||
| 10 DEP-18M | .44 | .59 | .56 | .42 | .44 | .46 | .46 | .41 | .65 | 1 | ||||||||||
| 11 DEP-27M | .33 | .40 | .68 | .35 | .44 | .48 | .43 | .43 | .58 | .73 | 1 | |||||||||
| 12 DEP-4.5Y | .38 | .44 | .42 | .69 | .50 | .42 | .45 | .40 | .51 | .54 | .45 | 1 | ||||||||
| 13 DEP-6Y | .38 | .43 | .40 | .47 | .77 | .69 | .66 | .57 | .43 | .52 | .50 | .57 | 1 | |||||||
| 14 DEP-7Y | .22 | .37 | .32 | .42 | .63 | .76 | .65 | .66 | .36 | .38 | .35 | .45 | .64 | 1 | ||||||
| 15 DEP-8Y | .30 | .40 | .30 | .24 | .56 | .54 | .72 | .61 | .33 | .36 | .37 | .23 | .59 | .61 | 1 | |||||
| 16 DEP-11Y | .26 | .32 | .37 | .36 | .60 | .59 | .60 | .78 | .34 | .39 | .42 | .40 | .55 | .62 | .60 | 1 | ||||
| 17 BP INT (Lifetime) | 0 | .06 | .03 | .06 | .02 | −.04 | −.21 | −.02 | .03 | .09 | 0.05 | .03 | .01 | .03 | −.18 | −.02 | 1 | |||
| 18 BM INT (Pregnancy) | .02 | .03 | −.01 | .09 | .04 | −.05 | −.11 | 0 | 0 | .04 | −.02 | .08 | .08 | .09 | −.12 | 0 | .44 | 1 | ||
| 19 Child AXDP-4.5Y | .02 | .06 | .07 | .06 | .05 | .07 | .15 | .09 | .12 | .13 | .06 | .04 | .03 | .04 | .12 | 0 | .01 | −.01 | 1 | |
| 20 Child AXDP-11Y | .09 | .16 | .19 | .07 | .08 | .15 | .16 | .17 | .03 | .12 | .11 | .06 | .06 | .18 | .16 | .14 | 0 | −.05 | .38 | 1 |
Note. AP = adoptive parent, BP = birth parents, BM = birth mother, ANX = anxiety symptoms, DEP = depressive symptoms, AXDP = anxiety and depressive symptoms, INT = internalizing problems, M = months, Y = years. Bolded number indicates that the correlation is significant at the level of p < .05.
Longitudinal CFA and TSO models
For AP1s (mostly mothers), the longitudinal CFA model freely estimating all factor loadings did not fit the data well with the warning that “the covariance matrix of the residuals of the observed variables (theta) is not positive definite”. The model assuming measurement equivalence fit the data well (see Table 3 for all model fit indices and chi-square difference test results). Constraining the loadings of anxiety and depressive symptoms to equality worsened the model fit. However, the p-value was only significant at .05 but not .01 level, and other fit indices suggested that the alternative model also fit the data well. With the aim of understanding the influences of both parental depressive and anxiety symptoms, rather than prioritizing either the effect of depressive or anxiety symptoms, we accepted the model constraining the loadings of anxiety and depressive symptoms to equality. The model constraining the residual variances of anxiety and depressive symptoms measured at the same time (e.g., AX9M and DP9M) to equality fit well. Therefore, the final longitudinal CFA model for AP1s (mostly mothers) had the loadings of anxiety and depressive symptoms both fixed to one, and the residual variances of anxiety and depressive symptoms measured at the same time constrained to equality. The same final longitudinal CFA model was obtained for AP2s (mostly fathers), none of the chi-square difference tests were significant.
Table 3.
Model fit indices for longitudinal confirmatory factor analysis models
| Model | χ 2 | df | p | Δχ 2 | Δdf | p | RMSEA | CFI | TLI | SRMR |
|---|---|---|---|---|---|---|---|---|---|---|
| AP1 (mostly mothers) | ||||||||||
| Base model (free AX, DP loadings) | / | / | / | / | / | / | / | / | / | / |
| Equal AX loadings, equal DP loadings | 69.91 | 51 | .04 | / | / | / | .03 | .99 | .99 | .04 |
| All AX, DP loadings equal 1 | 75.04 | 52 | .02 | 6.27 | 1 | .01 | .03 | .99 | .98 | .04 |
| All AX, DP loadings equal 1 & Equal residual variances for AX t , DP t | 75.83 | 60 | .08 | 4.71 | 8 | .79 | .02 | 1.00 | .99 | .04 |
| AP2 (mostly fathers) | ||||||||||
| Base model (free AX, DP loadings) | 72.79 | 44 | .004 | / | / | / | .04 | .99 | .97 | .02 |
| Equal AX loadings, equal DP loadings | 82.35 | 51 | .004 | 10.07 | 7 | .18 | .03 | .99 | .97 | .04 |
| All AX, DP loadings equal 1 | 82.40 | 52 | .005 | .66 | 1 | .42 | .03 | .99 | .98 | .04 |
| All AX, DP loadings equal 1 & Equal residual variances for AX t , DP t | 79.40 | 60 | .05 | 2.70 | 8 | .95 | .02 | .99 | .99 | .04 |
Note. AP = adoptive parent, AX = anxiety symptoms, DP = depressive symptoms. RMSEA = root mean square error of approximation, CFI = comparative fit index, TLI = Tucker-Lewis index, SRMR = standardized root mean residual. The final models selected are in bold. χ 2 represents robust model test statistics, Δχ2 is a function of standard model test statistics in Satorra-Bentler scaled chi-square difference tests.
As discussed previously, factor structures obtained from the longitudinal CFA models were used to construct the TSO models, and additional constraints for the TSO models were tested. See Table 4 for all model fit indices and chi-square difference test results. For AP1s (mostly mothers), the TSO model with the factor loadings of St on the trait factor constrained to equality fit well, chi-square difference test comparing this model to the unconstrained model was not significant. The model that further constrained the autoregressive pathways resulted in very bad fit. Therefore, the final TSO model obtained for AP1s (mostly mothers) were the one with only factor loadings of St on the trait factor constrained to equality. For AP2s (mostly fathers), the same final TSO model was obtained, with factor loadings of St on the trait factor constrained to equality, which fit the data well. The model that constrained the autoregressive pathways significantly worsened the model fit comparing to the model that only constrained the loadings of St on trait but not the autoregressive pathways. In the final TSO model for AP1s (mostly mothers), from 48.01% to 76.50% of the state factors’ variances were explained by the trait factor, and from 23.49% to 51.99% of the state factors’ variances were explained by the occasion factors. Standardized coefficients for autoregressive pathways ranged from .04 to .67 (significant coefficients ranged from .45 to .67). In the final TSO model for AP2s (mostly fathers), from 46.38% to 68.66% of the state factors’ variances were explained by the trait factor, and from 31.34% to 53.62% of the state factors’ variances were explained by the occasion factors. Standardized coefficients for autoregressive pathways ranged from −.11 to .79 (significant coefficients ranged from .26 to .79). Detailed model fitting results for the TSO models are presented in Figure 3.
Table 4.
Model fit indices for trait-state-occasion models
| Model | χ 2 | df | p | Δχ 2 | Δdf | p | RMSEA | CFI | TLI | SRMR |
|---|---|---|---|---|---|---|---|---|---|---|
| AP1 (mostly mothers) | ||||||||||
| Base model (free St loadings on trait) | 107.49 | 73 | .005 | / | / | / | .02 | .99 | .98 | .04 |
| Equal St loadings on trait | 105.83 | 80 | .03 | 1.93 | 7 | .96 | .02 | .99 | .99 | .04 |
| Equal St loadings on trait & Constrained autoregressive coefficients | 1580.21 | 86 | < .001 | 395.08 | 6 | < .001 | .18 | .53 | .34 | .41 |
| AP2 (mostly fathers) | ||||||||||
| Base model (free St loadings on trait) | 94.81 | 73 | .04 | / | / | / | .02 | .99 | .99 | .04 |
| Equal St loadings on trait | 97.45 | 80 | .09 | 3.27 | 7 | .876 | .02 | .99 | .99 | .05 |
| Equal St loadings on trait & Constrained autoregressive coefficients | 141.93 | 86 | < .001 | 32.88 | 6 | < .001 | .03 | .98 | .97 | .08 |
Note. AP = adoptive parent, S = state factor. RMSEA = root mean square error of approximation, CFI = comparative fit index, TLI = Tucker-Lewis index, SRMR = standardized root mean residual. The final models selected are in bold. χ 2 represents robust model test statistics, Δχ2 is a function of standard model test statistics in Satorra-Bentler scaled chi-square difference tests.
Figure 3.
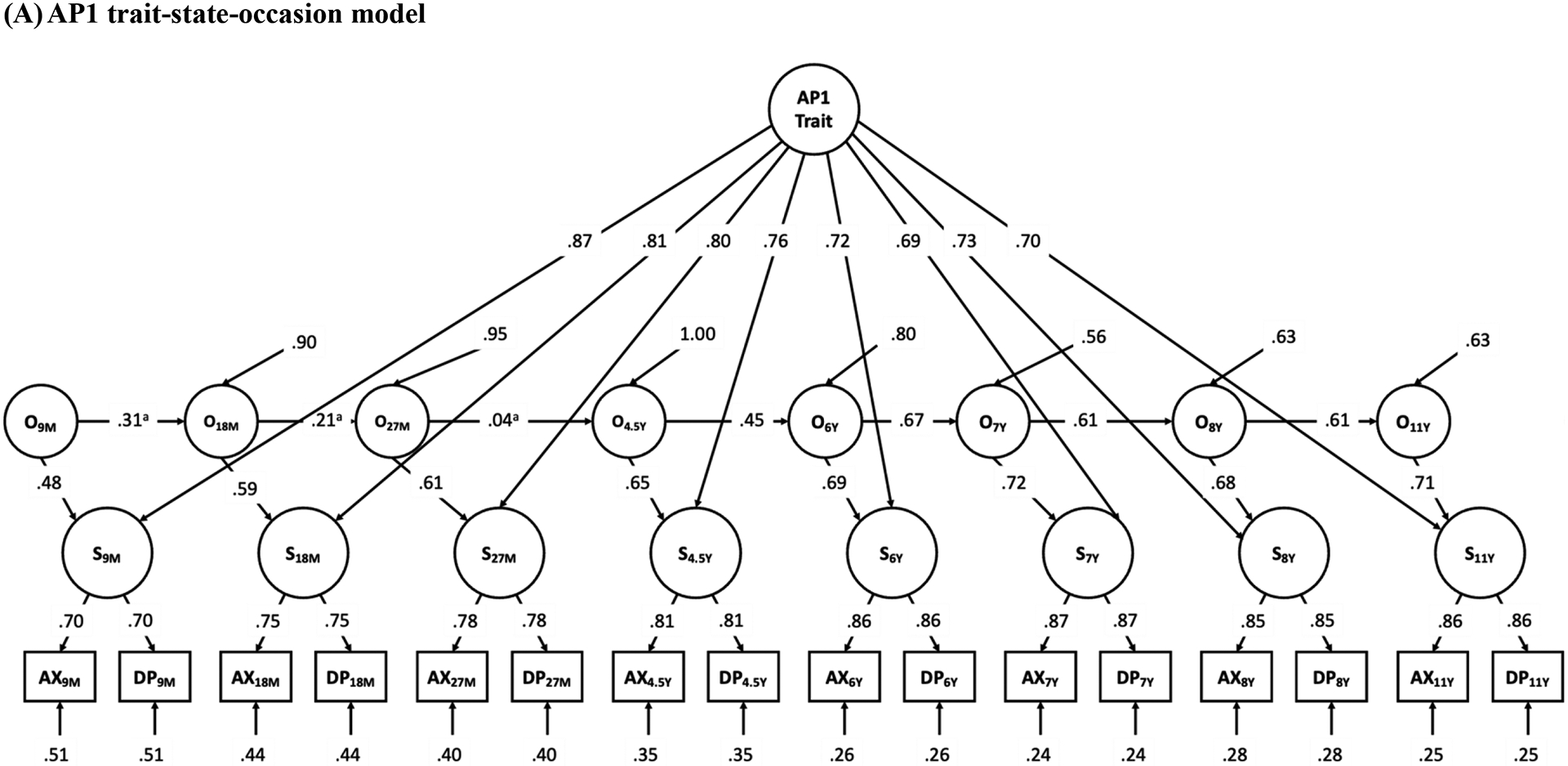

Results of trait-state-occasion models for adoptive parent 1 (mostly mothers, Panel A) and adoptive parent 2 (mostly fathers, Panel B) anxiety and depressive symptoms from child ages 9 months to 11 years. AP Trait = adoptive parent trait factor, O = occasion factor, S = state factor, AX = anxiety symptoms, DP = depressive symptoms. 9M to 27M = child age 9 months to 27 months, 4.5Y to 11Y = child age 4.5 years to 11 years. Correlated residuals in manifest variables with the same measure not shown for clarity. Parameter estimates are standardized (anon-significant autoregressive pathways, all other coefficients are significant at the p < .05 level). Unstandardized pathways constrained to equality may have different standardized parameter estimates.
Predicting offspring anxiety and depressive symptoms
We then used the trait and occasion factors of AP symptoms, along with BP lifetime internalizing problems (indicating genetic transmission) and BM internalizing problems during pregnancy (indicating prenatal transmission) to predict child (age 4.5 years) and adolescent (age 11 years) anxiety and depressive symptoms. The models showed multicollinearity problems, which have been reported in previous studies using the TSO model to predict child outcomes (Missler et al., 2021; Prenoveau et al., 2017). Previous studies have addressed this issue by reducing the number of occasion factors predicting child outcomes. In the present study, we combined theory- and data-driven approaches to determine which occasion factors should be retained to predict child and adolescent symptoms. We retained the paths from the occasion factor in early infancy (O9M), as early infancy is one of the most widely studied developmental periods during which parent anxiety and depressive symptoms were found to impact child and adolescent symptoms (Bureau et al., 2009; Korhonen et al., 2014; Sweeney & MacBeth, 2016). We also included paths from the concurrent occasion (O4.5Y to child symptoms at 4.5 years, O11Y to adolescent symptoms at 11 years). This decision was not based on timing effects, but based on the reports that concurrent maternal anxiety and depressive symptoms were stronger predictors of child internalizing problems, as compared to maternal symptoms in early infancy (Gjerde et al., 2020; Gjerde et al., 2017). We then adopted a data-driven approach by adding regression paths from other occasion factors to either child or adolescent symptoms one by one, as suggested by the highest modification index. The final model was obtained when a new path did not significantly improve model fit.
Following the above procedures, we obtained final models for AP1 (mostly mothers) and AP2 (mostly fathers) symptoms separately. Results are presented in Figure 4. In the AP1 model, the occasion factor at child age 9 months was positively associated with child anxiety and depressive symptoms at 4.5 years, and occasion factor at child age 11 years was positively associated with early adolescent anxiety and depressive symptoms at 11 years. The indirect effect from the occasion factor at 9 months to child anxiety and depressive symptoms at 4.5 years to early adolescent symptoms at 11 years was significant (β = .09, 95% CI [.01, .25]). The trait factor was not associated with child or adolescent symptoms. For AP2s, none of the occasion factors or the trait factor was significantly associated with child or adolescent anxiety and depressive symptoms. The association between the AP2 trait factor and child anxiety and depressive symptoms at age 4.5 years approached significance (p = .10). Genetic and prenatal influences measured by BP internalizing problems were positively associated with each other, but neither was associated with child or adolescent anxiety and depressive symptoms. To ensure that missing data did not influence our results, we re-ran the final models for AP1 and AP2 with auxiliary variables that were associated with AP anxiety and depressive symptoms’ patterns of missingness (but were not already included in the models). For the AP1 model, obstetric complications and AP SES were added as auxiliary variables; for the AP2 model, adoption openness was added as the auxiliary variable. All significant results remained the same.
Figure 4.


Results of full structural equation models. Genetic influences, prenatal influences, adoptive parent 1 (mostly mothers, Panel A) and adoptive parent 2 (mostly fathers, Panel B) occasion and trait factors predicting early adolescent anxiety and depressive symptoms at age 11. State factors and observed variables in the trait-state-occasion model are not shown for clarity. Birth mother socio-economic status was included as a covariate. BM = birth mother, BP = birth parent, AP Trait = adoptive parent trait factor, O = occasion factor, Sx = symptoms. Parameter estimates are standardized (†p < .10, *p < .05, **p < .01). Solid lines are significant pathways at the p < .05 level, dashed lines are non-significant pathways. Model fit indices for AP1 model: χ 2 (150) = 185.75; p = .01; RMSEA = .02; CFI = .99; TLI = .99; SRMR = .04; AP2 model: χ 2 (150) = 169.73; p = .13; RMSEA = .02; CFI = .99; TLI = .99; SRMR = .05.
Discussion
The present study examined genetic, prenatal, and postnatal environmental pathways of the intergenerational transmission of anxiety and depressive symptoms from parents to early adolescents using a longitudinal parent-offspring adoption design. We focused on the timing effects of exposure to postnatal parent symptoms while controlling for genetic and prenatal influences measured by BP internalizing problems. When distinguished from trait-like parent anxiety and depressive symptoms, within-person fluctuations of AP1 (mostly mothers) anxiety and depressive symptoms in early infancy had long-term influences on anxiety and depressive symptoms in early adolescence. We also showed that this effect was mediated through children’s anxiety and depressive symptoms at age 4.5 years. This indirect effect suggests a developmental cascade model in which disruption in early developmental tasks could lead to maladaptive developmental trajectories in later ages (Goodman & Gotlib, 1999; Masten & Cicchetti, 2010). During infancy, adult caregivers are the primary environmental influences for children’s regulation of emotional displays (Zeman et al., 2006). Children also start to develop separation anxiety during infancy, which indicates the solidification of infant-caregiver attachment (Ainsworth et al., 2015; Zeman et al., 2006). All of these developmental features in infancy may contribute to susceptibility to negative parenting behaviors that are associated with parent anxiety and depressive symptoms (Lovejoy et al., 2000). Future research should examine whether negative parenting behaviors associated with parent anxiety and depressive symptoms mediate this time-specific effect we observed.
Our results suggested another time-specific influence from AP1 (mostly mothers) anxiety and depressive symptoms at child age 11 years to concurrent symptoms of anxiety and depression in the adolescents. The interpretation of this association is less clear because it is impossible to separate parent-to-child and child-to-parent effects from one another in our cross-sectional analyses (Ahmadzadeh et al., 2019; Brooker et al., 2015). It is also unclear whether this association suggests a “recency effect” or “time-specific effect”. A recency effect would suggest that the influence of parent anxiety and depressive symptoms is greater when it is more proximal to the adolescent outcome (Shanahan et al., 2011). This type of effect is consistent with the literature suggesting the association between recent/concurrent maternal depression and child/adolescent internalizing problems (Fihrer et al., 2009; Gjerde et al., 2020; Gjerde et al., 2017; Mars et al., 2012; Turney, 2011; van der Waerden et al., 2015). In particular, Griffith et al. (2021) found that during a 2-year period in adolescence, parent and adolescent depressive symptoms were concurrently, but not prospectively associated. Such a finding is consistent with a “recency effect” rather than adolescence being a susceptible developmental period to influences of parent depressive and anxiety symptoms (i.e., time-specific effect). However, we also observed that the association between concurrent AP1 (mostly mothers) and child symptoms was significant at age 11 years but not at age 4.5 years, which suggests that the association between concurrent parent and offspring anxiety and depressive symptoms is stronger in early adolescence than in early childhood. We speculate that during adolescence, potential stress associated with transitions across physical, cognitive, and psychosocial domains (e.g., puberty, transition to middle school) may impact both parents and adolescents (Susman & Dorn, 2009; Windle et al., 2008), thus elevating their anxiety and depressive symptoms at the same time. Previous findings also suggested that the environmental influences on childhood anxiety and depressive symptoms do not have as much impact on anxiety and depressive symptoms across adolescence and early adulthood (Kendler et al., 2008). Therefore, childhood anxiety and depressive symptoms may have very different etiology with parent anxiety and depressive symptoms, while adolescent anxiety and depressive symptoms share more etiological influences with parent symptoms.
The trait factor of adoptive parent anxiety and depressive symptoms was not associated with early adolescent anxiety and depressive symptoms, which seems contradictory to previous findings (e.g., Missler et al., 2021; Prenoveau et al., 2017; van der Waerden et al., 2015). However, some of the studies have only included parent symptoms in a shorter period until toddlerhood or early childhood (Prenoveau et al., 2017; van der Waerden et al., 2015), while the trait factor in the present study represents the stable component of parent anxiety and depressive symptoms over 11 years. A trait factor captured by parent anxiety and depressive symptoms that were measured only across a short period of time may actually reflect the timing effects we observed in the present study, instead of truly representing the long-term stability of symptom levels. The study conducted by Missler et al. (2021) used the TSO model to examine a similar range of child ages, however, a major difference between the present study and Missler et al. (2021) is the procedure used to decide which occasion factors were used to predict child and adolescent symptoms. To solve the similar issue of multicollinearity, Missler et al. (2021) estimated the model including only nonadjacent occasion factors, while we started by including the most salient occasion factors suggested by previous literature. Different model selection processes could contribute to different findings in the timing effects, mainly due to what other time-specific influences (i.e., occasion factors) are being controlled for. In addition, we accounted for genetic and prenatal transmission of anxiety and depressive symptoms via birth parents when examining postnatal environment influences from adoptive parent anxiety and depressive symptoms, while Missler et al. (2021) studied biological children raised by their biological parents.
Our results did not support influences of paternal anxiety and depressive symptoms on child/adolescent anxiety and depressive symptoms, which is different from previous work (Ahmadzadeh et al., 2019; Lewis et al., 2017). The lack of association from fathers could be due to the fact that fathers spend less time with children than mothers (Yeung et al., 2001). However, it is worth mentioning that the association between AP2 (mostly fathers) trait factor and child symptoms at age 4.5 years was marginally significant, which potentially suggests a lack of statistical power to detect possible effects. Another limitation of the current report was examining AP1 (mostly mothers) and AP2 (mostly fathers) symptoms in separate models due to lack of power, which limited the opportunity of examining whether father symptoms could indirectly influence the child via maternal symptoms, as has been reported in previous studies with this sample (Pemberton et al., 2010).
Our results did not find a significant genetic transmission pathway of anxiety and depressive symptoms from birth parents, which is consistent with other family-based genetically informed studies (Eley et al., 2015; Silberg et al., 2010). Several possible explanations exist for the null findings. First, the genetic transmission of psychopathology may not be specific. Studies examining genetic and environmental structures on different types of psychopathology have reported a general genetic factor contributing to all psychiatric disorders beyond internalizing- and externalizing-specific genetic influences (Lahey et al., 2011). The non-specific genetic transmission would, therefore, yield weak/null findings of a genetic transmission pathway from parents to offspring if focusing on internalizing problems specifically. Second, adult and child internalizing problems may not be influenced by the same genes, as new genetic influences continue to emerge (and earlier genetic influences attenuate) across childhood and early adulthood (Kendler et al., 2008). Finally, the null finding in the current study may be due to a lack of power. Gjerde et al. (2021) found a genetic pathway from maternal depressive symptoms to early childhood internalizing problems, using a multiple-children-of-twins-and-siblings design with a large sample size, although the environmental pathway started to play an increasingly important role when children grow older. Therefore, it is possible that such studies may yield similar findings when offspring is in adolescence.
We did not find a significant effect of birth mother internalizing problems during pregnancy on adolescent anxiety and depressive symptoms. This finding does not, however, indicate null effects of all prenatal risk factors on child and adolescent anxiety and depressive symptoms, since we did not include other prenatal risk factors in our analyses. Instead, our findings only suggests that for the intergenerational transmission of anxiety and depressive symptoms, the prenatal pathway was not significant (when controlling for genetic transmission). One of the biggest advantages of using a parent-offspring adoption design is the ability to separate prenatal and postnatal influences, because prenatal environment is provided by BMs while postnatal environment is provided by APs. Our results suggest that without confounding each other, postnatal, but not prenatal maternal anxiety and depressive symptoms, had an impact on child and early adolescent anxiety and depressive symptoms. This finding is consistent with some studies that found no unique effects of prenatal parental depressive symptoms on child internalizing problems, after partialling out variance explained by postnatal parental depressive symptoms (Fredriksen et al., 2019), but is contradictory to other studies that still found significant prenatal influences after controlling for postnatal influences (Leis et al., 2014; O’Donnell et al., 2014; Pearson et al., 2013). However, the use of an adoption design allowed a clearer separation of prenatal and postnatal effects, which may yield different results as compared to studies in which both prenatal and postnatal influences were contributed by biological parents.
Finally, our findings are most informative for the intergenerational transmission process of general internalizing problems rather than anxiety or depressive symptoms individually. In particular, we focused on capturing general distress shared between anxiety and depressive symptoms. The traditional categorical framework of psychopathology (e.g., DSM-5) has been controversial, with recent research suggesting that psychopathology may be better described by multiple dimensions that consist of different underlying components (Haslam et al., 2020; Krueger et al., 2018). For example, preliminary psychometric findings suggested that internalizing problems can be measured by four underlying components: distress, fear, body dysmorphia, and mania; most of the commonly measured anxiety and depressive symptoms fell under the component of distress (Watson, Forbes, et al., 2022). Therefore, we examined continuous levels of symptoms (rather than diagnoses); our approach of combining anxiety and depressive symptoms is consistent with a dimensional framework of psychopathology, and our findings have implications for how the distress component of internalizing problems is transmitted from parents to offspring.
Several methodological limitations of this study should be noted. First, we used a combined theory-driven and data-driven approach to determine the final models predicting child outcomes. Ideally, this approach and findings should be replicated using other samples. Second, different measures of anxiety and depressive symptoms were used across waves, which could influence the structure of TSO models. In our TSO models, the correlations among the latter five occasion factors (from child ages 4.5 years to 11 years) were stronger than the correlations among the first four occasion factors (from child ages 9 months to 4.5 years), which could simply reflect different levels of test-retest reliability of different measures. This pattern may contribute to why our findings differ from studies using similar approaches (Missler et al., 2021; Prenoveau et al., 2017). Third, anxiety and depressive symptoms were skewed in APs and children, with the majority having low levels of symptoms. To address this, we used the robust maximum likelihood estimation to make the findings more generalizable to non-normal data. However, the generalizability of our findings to samples with more severe symptoms should be considered with this limitation. Fourth, we only accounted for parent-to-child influences in the intergenerational transmission of anxiety and depressive symptoms, while child-to-parent influences have also been reported (Ahmadzadeh et al., 2019). The possibility of child-driven effects should be considered especially for the concurrent association between maternal and adolescent anxiety and depressive symptoms at age 11 years. Furthermore, including child symptoms at more timepoints and simultaneously modeling parent-to-child and child-to-parent effects would allow a more thorough understanding of timing effects in the intergenerational transmission of anxiety and depressive symptoms. Finally, to understand the intergenerational transmission of anxiety and depressive symptoms, we focused solely on birth and adoptive parent internalizing problems, whereas other genetic, prenatal, and postnatal environmental influences from parents (e.g., externalizing behaviors, substance use) and other sources (e.g., peers, neighborhood) could also contribute to anxiety and depressive symptoms in children and adolescents.
Conclusions
Using a parent-offspring adoption design, the present study partitioned genetic, prenatal, and postnatal environmental pathways from one another in the intergenerational transmission of anxiety and depressive symptoms. For postnatal environmental transmission, we examined time-specific effects of adoptive parent anxiety and depressive symptoms on early adolescent anxiety and depressive symptoms, suggesting early infancy and early adolescence as two developmental periods during which children may be particularly susceptible to influences from parent (mostly mothers) anxiety and depressive symptoms. These time-specific associations were found when being partitioned from the effects of trait-like parent anxiety and depressive symptoms over 11 years. These findings suggest that prevention programs should utilize longitudinal data and attend to within-person changes in maternal anxiety and depressive symptoms during certain developmental periods (e.g., early infancy, early adolescence). To obtain information about “trait-level” anxiety and depressive symptoms for each individual, prevention programs could collaborate with general practitioners or community health providers to access individuals’ anxiety and depressive symptoms levels before they were involved in the program, although such information would benefit from reliance on more routine screening of anxiety and depressive symptoms in general healthcare settings—a practice recommended by the US Preventive Services Task Force (https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/anxiety-adults-screening). Prevention programs should also consider following parents longitudinally, where feasible, to monitor fluctuations in symptom levels. Future genetically informed studies should utilize similar models to see if results in the present study can be replicated. More research examining mediators for the associations between parent and adolescent anxiety and depressive symptoms could help clarify developmental mechanisms of these time-specific effects.
Public significance statements:
Anxiety and depressive symptoms run in families mainly through environmental influences. We found that time-specific fluctuations of adoptive parent (mostly mothers) anxiety and depressive symptoms during infancy and early adolescence were associated with early adolescent anxiety and depressive symptoms. Programs aimed at preventing anxiety and depressive symptoms during early adolescence could screen for time-specific fluctuations of parent (mainly mothers) anxiety and depressive symptoms during infancy and early adolescence.
Acknowledgement:
This project was supported by R01 HD042608 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse and OBSSR, NIH, U.S. PHS (PI Years 1-5: Reiss; PI Years 6-10: Leve), R01 DA020585 from the National Institute on Drug Abuse, the National Institute of Mental Health and OBSSR, NIH, U.S. PHS (PI: Neiderhiser), R01 MH092118 from the National Institute of Mental Health, NIH, U.S. PHS (MPIs: Neiderhiser, Leve), R01 DK090264 from the National Institute of Diabetes and Digestive and Kidney Disease, NIH, U.S. PHS (PI: Ganiban), R01 DA035062 from the National Institute on Drug Abuse, NIH, U.S. PHS (PI: Leve), R56 HD042608 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, U.S. PHS (PI: Leve), R01 DA045108 from the National Institute on Drug Abuse (PI: Neiderhiser), and the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), and UH3OD023389 (MPIs: Leve, Neiderhiser, Ganiban). The authors wish to thank our ECHO colleagues, the medical, nursing, and program staff, as well as the children and families participating in the ECHO cohorts. Tong Chen was supported by the Prevention and Methodology Training Program (T32 DA017629; MPIs: Maggs, Lanza) with funding from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the Office of the Director, or the National Institutes of Health. The authors wish to thank David Reiss for his feedback on this project.
References
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms & profiles. University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Ahmadzadeh YI, Eley TC, Leve LD, Shaw DS, Natsuaki MN, Reiss D, … McAdams TA (2019). Anxiety in the family: A genetically informed analysis of transactional associations between mother, father and child anxiety symptoms. Journal of Child Psychology and Psychiatry, 60(12), 1269–1277. 10.1111/jcpp.13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, & Wall SN (2015). Patterns of attachment: A psychological study of the strange situation. Psychology Press. [Google Scholar]
- Beck AT, & Steer RA (1993a). Beck Anxiety Inventory manual. Psychological Corporation. [Google Scholar]
- Beck AT, & Steer RA (1993b). Beck depression inventory manual.
- Brooker RJ, Neiderhiser JM, Leve LD, Shaw DS, Scaramella LV, & Reiss D (2015). Associations between infant negative affect and parent anxiety symptoms are bidirectional: Evidence from mothers and fathers. Frontiers in Psychology, 6, 1875. 10.3389/fpsyg.2015.01875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau J-F, Easterbrooks MA, & Lyons-Ruth K (2009). Maternal depressive symptoms in infancy: Unique contribution to children’s depressive symptoms in childhood and adolescence? Development and Psychopathology, 21(2), 519–537. 10.1017/S0954579409000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, … Silva PA (1996). The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. International Journal of Methods in Psychiatric Research, 6(2), 101–114. [DOI] [Google Scholar]
- Cioffi CC, Leve LD, Natsuaki MN, Shaw DS, Reiss D, Ganiban JM, & Neiderhiser JM (2021). Examining reciprocal associations between parent depressive symptoms and child internalizing symptoms on subsequent psychiatric disorders: An adoption study. Depression and Anxiety, 38(12), 1211–1224. 10.1002/da.23190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, Martin NC, & Steiger JH (2005). Empirical and conceptual problems with longitudinal trait-state models: Introducing a trait-state-occasion model. Psychological Methods, 10(1), 3–20. 10.1037/1082-989X.10.1.3 [DOI] [PubMed] [Google Scholar]
- Côté SM, Ahun MN, Herba CM, Brendgen M, Geoffroy M-C, Orri M, … Boivin M (2018). Why is maternal depression related to adolescent internalizing problems? A 15-year population-based study. Journal of the American Academy of Child and Adolescent Psychiatry, 57(12), 916–924. 10.1016/j.jaac.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Dekker MC, Ferdinand RF, Van Lang ND, Bongers IL, Van Der Ende J, & Verhulst FC (2007). Developmental trajectories of depressive symptoms from early childhood to late adolescence: Gender differences and adult outcome. Journal of Child Psychology and Psychiatry, 48(7), 657–666. 10.1111/j.1469-7610.2007.01742.x [DOI] [PubMed] [Google Scholar]
- Eley TC, McAdams TA, Rijsdijk FV, Lichtenstein P, Narusyte J, Reiss D, … Neiderhiser JM (2015). The intergenerational transmission of anxiety: A children-of-twins study. American Journal of Psychiatry, 172(7), 630–637. 10.1176/appi.ajp.2015.14070818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fihrer I, McMahon CA, & Taylor AJ (2009). The impact of postnatal and concurrent maternal depression on child behaviour during the early school years. Journal of Affective Disorders, 119(1–3), 116–123. 10.1016/j.jad.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Flouri E, Sarmadi Z, & Francesconi M (2019). Paternal psychological distress and child problem behavior from early childhood to middle adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 58(4), 453–458. 10.1016/j.jaac.2018.06.041 [DOI] [PubMed] [Google Scholar]
- Fredriksen E, von Soest T, Smith L, & Moe V (2019). Parenting stress plays a mediating role in the prediction of early child development from both parents’ perinatal depressive symptoms. Journal of Abnormal Child Psychology, 47(1), 149–164. 10.1007/s10802-018-0428-4 [DOI] [PubMed] [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, & Young-DeMarco L (1988). The life history calendar: A technique for collecting retrospective data. Sociological Methodology, 18, 37–68. 10.2307/271044 [DOI] [PubMed] [Google Scholar]
- Ge X, Natsuaki MN, Martin DM, Leve LD, Neiderhiser JM, Shaw DS, … Reiss D (2008). Bridging the divide: Openness in adoption and postadoption psychosocial adjustment among birth and adoptive parents. Journal of Family Psychology, 22(4), 529–540. 10.1037/a0012817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde LC, Eilertsen EM, Eley TC, McAdams TA, Reichborn-Kjennerud T, Roysamb E, & Ystrom E (2020). Maternal perinatal and concurrent anxiety and mental health problems in early childhood: A sibling-comparison study. Child Development, 91, 456–470. 10.1111/cdev.13192 [DOI] [PubMed] [Google Scholar]
- Gjerde LC, Eilertsen EM, Hannigan LJ, Eley T, Røysamb E, Reichborn-Kjennerud T, … Ystrom E (2021). Associations between maternal depressive symptoms and risk for offspring early-life psychopathology: The role of genetic and non-genetic mechanisms. Psychological Medicine, 51(3), 441–449. 10.1017/S0033291719003301 [DOI] [PubMed] [Google Scholar]
- Gjerde LC, Eilertsen EM, Reichborn-Kjennerud T, McAdams TA, Zachrisson HD, Zambrana IM, … Ystrom E (2017). Maternal perinatal and concurrent depressive symptoms and child behavior problems: A sibling comparison study. Journal of Child Psychology and Psychiatry, 58(7), 779–786. 10.1111/jcpp.12704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, & Gotlib IH (1999). Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review, 106(3), 458–490. 10.1037/0033-295x.106.3.458 [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, & Heyward D (2011). Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review, 14(1), 1–27. 10.1007/s10567-010-0080-1 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Goodman SH, & Humphreys KL (2020). Studying the intergenerational transmission of risk for depression: Current status and future directions. Current Directions in Psychological Science, 29(2), 174–179. 10.1177/0963721420901590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JM, Young JF, & Hankin BL (2021). Longitudinal coupling of depression in parent–adolescent dyads: Within-and between-dyads effects over time. Clinical Psychological Science, 9(6), 1059–1079. 10.1177/2167702621998313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan LJ, Eilertsen EM, Gjerde LC, Reichborn-Kjennerud T, Eley TC, Rijsdijk FV, … McAdams TA (2018). Maternal prenatal depressive symptoms and risk for early-life psychopathology in offspring: Genetic analyses in the norwegian mother and child birth cohort study. Lancet Psychiatry, 5(10), 808–815. 10.1016/S2215-0366(18)30225-6 [DOI] [PubMed] [Google Scholar]
- Haslam N, McGrath MJ, Viechtbauer W, & Kuppens P (2020). Dimensions over categories: A meta-analysis of taxometric research. Psychological Medicine, 50(9), 1418–1432. 10.1017/S003329172000183X [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, & Lichtenstein P (2008). A developmental twin study of symptoms of anxiety and depression: Evidence for genetic innovation and attenuation. Psychological Medicine, 38(11), 1567–1575. 10.1017/S003329170800384X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, & Üstün TB (2004). The world mental health (wmh) survey initiative version of the world health organization (WHO) composite international diagnostic interview (cidi). International Journal of Methods in Psychiatric Research, 13(2), 93–121. 10.1002/mpr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen M, Luoma I, Salmelin R, & Tamminen T (2014). Maternal depressive symptoms: Associations with adolescents’ internalizing and externalizing problems and social competence. Nordic Journal of Psychiatry, 68(5), 323–332. 10.3109/08039488.2013.838804 [DOI] [PubMed] [Google Scholar]
- Krueger RF, Kotov R, Watson D, Forbes MK, Eaton NR, Ruggero CJ, … Zimmermann J (2018). Progress in achieving quantitative classification of psychopathology. World Psychiatry, 17(3), 282–293. 10.1002/wps.20566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, & Rathouz PJ (2011). Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Archives of General Psychiatry, 68(2), 181–189. 10.1001/archgenpsychiatry.2010.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis JA, Heron J, Stuart EA, & Mendelson T (2014). Associations between maternal mental health and child emotional and behavioral problems: Does prenatal mental health matter? Journal of Abnormal Child Psychology, 42(1), 161–171. 10.1007/s10802-013-9766-4 [DOI] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Ganiban J, Natsuaki MN, Shaw DS, & Reiss D (2019). The early growth and development study: A dual-family adoption study from birth through adolescence. Twin Research and Human Genetics, 22(6), 716–727. 10.1017/thg.2019.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G, Neary M, Polek E, Flouri E, & Lewis G (2017). The association between paternal and adolescent depressive symptoms: Evidence from two population-based cohorts. Lancet Psychiatry, 4(12), 920–926. 10.1016/S2215-0366(17)30408-X [DOI] [PubMed] [Google Scholar]
- Lewis G, Rice F, Harold GT, Collishaw S, & Thapar A (2011). Investigating environmental links between parent depression and child depressive/anxiety symptoms using an assisted conception design. Journal of the American Academy of Child and Adolescent Psychiatry, 50(5), 451–459. 10.1016/j.jaac.2011.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, & Neuman G (2000). Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review, 20(5), 561–592. 10.1016/s0272-7358(98)00100-7 [DOI] [PubMed] [Google Scholar]
- Marceau K, De Araujo-Greecher M, Miller ES, Massey SH, Mayes LC, Ganiban JM, … Neiderhiser JM (2016). The perinatal risk index: Early risks experienced by domestic adoptees in the United States. PLoS ONE, 11(3), e0150486. 10.1371/journal.pone.0150486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Rolan E, Leve LD, Ganiban JM, Reiss D, Shaw DS, … Neiderhiser JM (2019). Parenting and prenatal risk as moderators of genetic influences on conduct problems during middle childhood. Developmental Psychology, 55(6), 1164–1181. 10.1037/dev0000701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars B, Collishaw S, Smith D, Thapar A, Potter R, Sellers R, … Thapar A (2012). Offspring of parents with recurrent depression: Which features of parent depression index risk for offspring psychopathology? Journal of Affective Disorders, 136(1–2), 44–53. 10.1016/j.jad.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Development and Psychopathology, 22(3), 491–495. 10.1017/S0954579410000222 [DOI] [PubMed] [Google Scholar]
- Missler MA, van Straten A, Denissen JJ, Donker T, de Weerth C, & Beijers R (2021). The first 12.5 years of parenthood: A latent trait-state occasion model of the longitudinal association between maternal distress and child internalizing and externalizing problems. Developmental Psychology, 57(7), 1124–1135. 10.1037/dev0001203 [DOI] [PubMed] [Google Scholar]
- Murray L, Creswell C, & Cooper P (2009). The development of anxiety disorders in childhood: An integrative review. Psychological Medicine, 39(9), 1413–1423. 10.1017/S0033291709005157 [DOI] [PubMed] [Google Scholar]
- Musliner KL, Munk-Olsen T, Eaton WW, & Zandi PP (2016). Heterogeneity in long-term trajectories of depressive symptoms: Patterns, predictors and outcomes. Journal of Affective Disorders, 192, 199–211. 10.1016/j.jad.2015.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus user’s guide (Eighth ed.). Muthén & Muthén. [Google Scholar]
- Naicker K, Wickham M, & Colman I (2012). Timing of first exposure to maternal depression and adolescent emotional disorder in a national canadian cohort. PLoS ONE, 7(3), e33422. 10.1371/journal.pone.0033422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivard MG, Dolan CV, Kendler KS, Kan KJ, Willemsen G, van Beijsterveldt CE, … Boomsma DI (2015). Stability in symptoms of anxiety and depression as a function of genotype and environment: A longitudinal twin study from ages 3 to 63 years. Psychological Medicine, 45(5), 1039–1049. 10.1017/S003329171400213X [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Barker ED, & O’Connor TG (2014). The persisting effect of maternal mood in pregnancy on childhood psychopathology. Development and Psychopathology, 26(2), 393–403. 10.1017/S0954579414000029 [DOI] [PubMed] [Google Scholar]
- Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, … Stein A (2013). Maternal depression during pregnancy and the postnatal period: Risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry, 70(12), 1312–1319. 10.1001/jamapsychiatry.2013.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton CK, Neiderhiser JM, Leve LD, Natsuaki MN, Shaw DS, Reiss D, & Ge X (2010). Influence of parental depressive symptoms on adopted toddler behaviors: An emerging developmental cascade of genetic and environmental effects. Development and Psychopathology, 22(4), 803–818. 10.1017/S0954579410000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Petersen IT, Lindhiem O, LeBeau B, Bates JE, Pettit GS, Lansford JE, & Dodge KA (2018). Development of internalizing problems from adolescence to emerging adulthood: Accounting for heterotypic continuity with vertical scaling. Developmental Psychology, 54(3), 586. 10.1037/dev0000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, & Ma Y (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry, 55(1), 56–64. 10.1001/archpsyc.55.1.56 [DOI] [PubMed] [Google Scholar]
- Prenoveau JM, Craske MG, West V, Giannakakis A, Zioga M, Lehtonen A, … Cooper P (2017). Maternal postnatal depression and anxiety and their association with child emotional negativity and behavior problems at two years. Developmental Psychology, 53(1), 50–62. 10.1037/dev0000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A language and environment for statistical computing. In R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Ramchandani P, Stein A, Evans J, O’Connor TG, & Team AS (2005). Paternal depression in the postnatal period and child development: A prospective population study. The Lancet, 365(9478), 2201–2205. 10.1016/S0140-6736(05)66778-5 [DOI] [PubMed] [Google Scholar]
- Reeb BT, Conger KJ, & Wu Y (2010). Paternal depressive symptoms and adolescent functioning: The moderating effect of gender and father hostility. Fathering, 8(1), 131–142. 10.3149/fth.0801.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y (2012). Lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48(2), 1–36. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Shanahan L, Copeland WE, Costello EJ, & Angold A (2011). Child-, adolescent-and young adult-onset depressions: Differential risk factors in development? Psychological Medicine, 41(11), 2265–2274. 10.1017/S0033291711000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg JL, Maes H, & Eaves LJ (2010). Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: An extended children of twins study. Journal of Child Psychology and Psychiatry, 51(6), 734–744. 10.1111/j.1469-7610.2010.02205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH, Najman JM, Bor W, O’Callaghan MJ, & Williams GM (2002). Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry, 43(4), 457–469. 10.1111/1469-7610.00037 [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, & Jacobs AG (1983). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press. [Google Scholar]
- Susman EJ, & Dorn LD (2009). Puberty: Its role in development. In Lerner RM & Steinberg L (Eds.), Handbook of adolescent psychology (Vol. 1, pp. 116–151). John Wiley & Sons, Inc. 10.1002/9780470479193.adlpsy001006 [DOI] [Google Scholar]
- Sweeney S, & MacBeth A (2016). The effects of paternal depression on child and adolescent outcomes: A systematic review. Journal of Affective Disorders, 205, 44–59. 10.1016/j.jad.2016.05.073 [DOI] [PubMed] [Google Scholar]
- Turney K (2011). Chronic and proximate depression among mothers: Implications for child well‐being. Journal of Marriage and Family, 73(1), 149–163. 10.1111/j.1741-3737.2010.00795.x [DOI] [Google Scholar]
- Twenge JM, & Nolen-Hoeksema S (2002). Age, gender, race, socioeconomic status, and birth cohort difference on the children’s depression inventory: A meta-analysis. Journal of Abnormal Psychology, 111(4), 578–588. 10.1037//0021-843x.111.4.578 [DOI] [PubMed] [Google Scholar]
- van der Waerden J, Galéra C, Larroque B, Saurel-Cubizolles M-J, Sutter-Dallay A-L, Melchior M, & EDEN Mother–Child Cohort Study Group. (2015). Maternal depression trajectories and children’s behavior at age 5 years. The Journal of Pediatrics, 166(6), 1440–1448. 10.1016/j.jpeds.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Watson D, Forbes MK, Levin-Aspenson HF, Ruggero CJ, Kotelnikova Y, Khoo S, … Kotov R (2022). The development of preliminary HiTOP internalizing spectrum scales. Assessment, 29(1), 17–33. 10.1177/10731911211003976 [DOI] [PubMed] [Google Scholar]
- Watson D, Levin‐Aspenson HF, Waszczuk MA, Conway CC, Dalgleish T, Dretsch MN, … Hobbs KA (2022). Validity and utility of hierarchical taxonomy of psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry, 21(1), 26–54. 10.1002/wps.20943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, … Dahl RE (2008). Transitions into underage and problem drinking: Developmental processes and mechanisms between 10 and 15 years of age. Pediatrics, 121(Supplement_4), S273–S289. 10.1542/peds.2007-2243C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerxa Y, Rescorla LA, van der Ende J, Hillegers MH, Verhulst FC, & Tiemeier H (2021). From parent to child to parent: Associations between parent and offspring psychopathology. Child Development, 92(1), 291–307. 10.1111/cdev.13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung WJ, Sandberg JF, Davis‐Kean PE, & Hofferth SL (2001). Children’s time with fathers in intact families. Journal of Marriage and Family, 63(1), 136–154. 10.1111/j.1741-3737.2001.00136.x [DOI] [Google Scholar]
- Zeman J, Cassano M, Perry-Parrish C, & Stegall S (2006). Emotion regulation in children and adolescents. Journal of Developmental and Behavioral Pediatrics, 27(2), 155–168. 10.1097/00004703-200604000-00014 [DOI] [PubMed] [Google Scholar]


