Abstract
Virus-specific cytotoxic T lymphocytes are key effectors for the clearance of virus-infected cells and are required for the normal clearance of respiratory syncytial virus (RSV) in mice. Although perforin/granzyme-mediated lysis of infected cells is thought to be the major molecular mechanism used by CD8+ cytotoxic T lymphocytes for elimination of virus, its role in RSV has not been reported. Here, we show that viral clearance in perforin knockout (PKO) mice is slightly delayed but that both PKO and wild-type mice clear virus by day 10, suggesting an alternative mechanism of RSV clearance. Effector T cells from the lungs of both groups of mice were shown to lyse Fas (CD95)-overexpressing target cells in greater numbers than target cells expressing low levels of Fas, suggesting that Fas ligand (CD95L)-mediated target cell lysis was occurring in vivo. This cell lysis was associated with a delay in RSV-induced disease in PKO mice compared to the time of disease onset for wild-type controls, which correlated with increased and prolonged production of gamma interferon and tumor necrosis factor alpha levels in PKO mice. We conclude that while perforin is not necessary for the clearance of primary RSV infection, the use of alternative CTL target cell killing mechanisms is less efficient and can lead to enhanced disease.
Human respiratory syncytial virus (RSV) is a Pneumovirus in the family Paramyxoviridae (7). Infants and toddlers afflicted with RSV usually experience only a mild upper respiratory tract infection. However, lower respiratory tract infections and bronchiolitis are seen in 20 to 30% of infected children, which results in >120,000 hospitalizations annually in the United States alone (37). In addition, a study has uncovered that RSV is a serious problem among the institutionalized elderly, causing severe lower respiratory tract disease and high rates of mortality (9).
In RSV infection, antiviral cytotoxic T lymphocytes (CTL) have been described for both humans and mice. RSV-specific CTL have been isolated from adult peripheral blood mononuclear cells (2) and from children following acute RSV infection (6, 17). In mice, depletion of B cells in primary RSV infection had no impact on viral clearance (12) but depletion of CD4+ and CD8+ T lymphocytes led to an extended period of virus replication and a lack of visible illness (13). Depletion of CD8+ T lymphocytes alone led to a delay in viral clearance and an abolition of illness, thus demonstrating that T lymphocytes are the major effectors of RSV clearance during primary infection (11, 13, 15). Transfer of RSV-specific CTL clones promoted clearance of RSV in mice (4, 29), but transfer of excessive numbers of RSV-specific CD8+ CTL exacerbated disease (5). A recent study has shown that Fas mRNA and protein levels are significantly increased following RSV infection in a human respiratory epithelial cell line (A549) (32). The occurrence of apoptotic cells increased when Fas was cross-linked using anti-Fas antibody, suggesting that RSV-infected cells may be particularly susceptible to FasL-mediated lysis.
Direct T-cell-mediated target cell lysis can occur by one of two processes (3). The first is by the perforin/granzyme lytic pathway, while the other is mediated by the interaction of Fas (CD95) and Fas ligand (CD95L). Although both pathways function to induce apoptosis of target cells by cell-to-cell contact, they differ mechanistically (38). Perforin is stored within cytoplasmic granules in CTL (25). Upon major histocompatibility complex (MHC):peptide–T-cell receptor recognition, the perforin molecules are released from the T cell, insert into the plasma membrane of the target cell, and undergo Ca2+-dependent polymerization to form pores (25). Pore formation results in osmotic lysis and granzyme-induced apoptosis of the target cell (16). The second mechanism for target cell lysis is the interaction of Fas on target cells with FasL expressed on activated effector CTL. Fas is ubiquitously expressed in humans and mice and has been shown to be upregulated upon RSV infection in vitro, followed by apoptosis (32). In contrast to the perforin pathway, the interaction of Fas and FasL induces apoptosis in a Ca2+-independent manner (35). Like the perforin/granzyme pathway, cytolysis mediated via Fas-FasL interactions is also antigen specific (26), although demonstrations of FasL-mediated nonspecific bystander killing have also been documented (35, 40).
A sizeable body of work has implicated perforin-dependent target cell lysis as the major mechanism of clearance in virus-infected cells (3, 18). FasL-mediated apoptosis, on the other hand, has been reported to be more important in maintaining homeostasis of the peripheral T-lymphocyte population (8, 24, 27, 30, 36). However, newer evidence is beginning to advocate that elimination of virus-infected cells is not restricted to perforin/granzyme-mediated lysis. FasL-dependent lysis of cells infected with lymphocytic choriomeningitis virus (LCMV) has been shown to occur by CD4+ CTL (43). Similarly, both the perforin and FasL pathways have been shown to contribute to influenza virus clearance (39).
The following report defines the role of perforin in RSV disease and viral clearance using perforin knockout (PKO) mice bred onto an H-2Kd BALB/c background (42). RSV disease, as measured by physical signs of illness and weight loss, was delayed in PKO mice and prolonged compared to that of wild-type (WT) mice. Virus clearance was slightly delayed on days 6 and 8 but was accomplished by day 10 in both PKO and WT mice, suggesting that perforin-mediated lysis was not necessary for viral clearance. Cytolytic assays were performed on day 8 after infection using Fas-overexpressing target cells (L1210Fas+) (35) and Fas-deficient target cells (L1210Fas−) (41). These data indicate that antiviral CTL from PKO mice are capable of lysing target cells by the Fas/FasL pathway, as the activity was completely inhibited by anti-FasL antibody. These data show that perforin is not the only mechanism of CTL-mediated RSV clearance and further suggest that the FasL pathway may compensate for the absence of perforin and alter disease expression.
MATERIALS AND METHODS
Mice.
Pathogen-free BALB/c mice, 8 to 10 weeks of age, were purchased from Harlan Laboratories (Indianapolis, Ind.). PKO mice backcrossed onto a BALB/c (H-2Kd) background (42) were a kind gift from John Harty (University of Iowa, Iowa City). Mice were housed and cared for in accordance with the Guide for the Care and Use of Laboratory Animals as previously described (14). Experiments were performed with age-matched groups.
Cell lines.
HEp-2 cells, used to determine the titer of RSV in lungs, were maintained in Eagle's minimal essential medium containing 10% fetal bovine serum (10% EMEM). The L1210Fas+ (35) and L1210Fas− (41) cell lines are derived from the murine lymphocytic leukemia cell line L1210 (American Type Culture Collection CCL-219) and are transfected with Fas sense and antisense cDNAs, respectively. Both cell lines were a gift from Michail Sitkovksy (National Institutes of Health, Bethesda, Md.). The L1210 cell lines were maintained in 10% EMEM. All media were supplemented with 2 mM glutamine, 10 U of penicillin G per ml, and 10 μg of streptomycin sulfate per ml.
Virus infection.
The RSV challenge stock was derived from the A2 strain of RSV by sonication of HEp-2 monolayers as previously described (14). Mice were anesthetized intramuscularly with ketamine (40 μg/g of body weight) and xylazine (6 μg/g of body weight) prior to intranasal inoculation with 107 PFU of RSV in 100 μl of 10% EMEM. Mice were weighed daily after infection for 14 days. Illness was graded daily by a blinded observer, i.e., someone unaware of the identity of each experimental group of mice. Clinical illness was scored as follows: 0, no apparent illness; 1, slightly ruffled fur; 2, ruffled fur but active; 3, ruffled fur and inactive; 4, ruffled fur, inactive, hunched posture, and gaunt; and 5, dead.
Synthetic peptides.
Peptides synthesized by Biosynthesis (Lewisville, Tex.) included RSV 82–90 (SYIGSINNI), derived from M2 protein of the RSV A2 strain, and influenza virus nucleoprotein (NP) 147-155 (TYQRTRALV) (22), derived from influenza virus A/Puerto Rico/8/34 nucleoprotein. Both peptides are H-2Kd restricted.
Plaque assays.
Animals were sacrificed, and lung tissues were removed and quick frozen in 10% EMEM. Thawed tissues were kept chilled while they were being individually ground. Dilutions of clarified supernatant were inoculated on 80%-confluent HEp-2 cell monolayers and overlaid with 0.75% methylcellulose in 10% EMEM. After incubation for 4 days at 37°C, the monolayers were fixed with 10% buffered formalin and stained with hematoxylin-eosin. PFU were counted and expressed as log10 PFU per gram of tissue.
Quantitation of IFN-γ and TNF-α.
The same supernatant used to measure virus titer in the lung was used to measure gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) cytokines using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Endogen, Woburn, Mass.). Briefly, 50 μl of the supernatant from ground lungs of RSV-infected mice was thawed and added to precoated 96-well microtiter plates. Peroxidase-labeled anticytokine antibody was added to detect bound cytokine, and the plates were developed by addition of a tetramethylbenzidene substrate.
Cytotoxic T-cell assays.
Lungs were harvested on day 8 postinfection. Lymphocytes were manually isolated by mashing lung tissue between the frosted ends of two sterile glass microscope slides in RPMI 1640 containing 10% fetal bovine serum. Lymphocytes were isolated by centrifugation (1,000 × g) on a cushion of Ficoll-Hypaque (1.09 specific gravity) at room temperature, washed twice, and resuspended in 10% RPMI 1640. L1210 target cells were incubated with 50 μl of relevant peptides (0.1 mg/ml) and 51Cr (100 mCi/107 cells) for 60 min at 37°C, washed three times in 10% EMEM, and distributed in V-bottom 96-well plates (Costar, Cambridge, Mass.) at 2 × 104 cells per 100 μl per well. Lung effector cells (2 × 106/100 μl) were added at a ratio of 100:1 (effector to target cells) and serially diluted down to 25:1 in triplicate. The plate was centrifuged at 150 × g for 30 s before incubation at 37°C for 4 h. The cells were gently pelleted, and the cells in 50 μl of the supernatant were counted in a 96-well TopCount NXT gamma counter (Packard, Meriden, Conn.). Spontaneous and total release were measured by treating the targets cells with 10% RPMI 1640 or with 5% Triton X-100 detergent, respectively. Specific release of 51Cr from target cells is defined as 100 × (sample cpm − background cpm)/(total cpm − background cpm), where cpm is counts per minute.
Surface staining and flow cytometry.
Lung lymphocytes (5 × 105) were washed once in staining buffer (phosphate-buffered saline–0.1% sodium azide–2% fetal calf serum) and surfaced stained with Cy-Chrome-conjugated monoclonal rat anti-mouse CD8α (clone 53-6.7) antibody, fluorescein isothiocyanate-conjugated monoclonal rat anti-mouse CD4 (clone GK1.5), and phycoerythrin (PE)-conjugated monoclonal hamster anti-mouse FasL (clone MFL3) (PharMingen, San Diego, Calif.). Cells were washed twice in staining buffer, and three-color analysis was performed on a FACSCaliber (Becton Dickinson, San Jose, Calif.) argon-ion laser at 15 mW and 488 nm. Forty thousand events were collected at an average of 1,000 events/s. Data were analyzed using CellQuest version 3.1 (Becton Dickinson).
Enumeration of M2-specific CD8+ T lymphocytes.
Enumeration of RSV M2-specific CD8+ T cells was performed by H-2Kd peptide tetramer staining as previously reported (1). PE-conjugated influenza virus NP and RSV M2 peptide-specific tetramers were gifts from John Altman (Emory University, Atlanta, Ga.).
Statistical analysis.
Data from individual mouse experiments were maintained in a Paradox database. Statistical analysis was performed by transferring data from the database into SAS (Chapel Hill, N.C.) statistical software to perform analysis of variance using Kruskal-Wallis and Wilcoxon rank sum tests. Comparisons were made between individual experiments by statistical modeling and trend analysis calculated by the general linear model method in the SAS program. P values of less than 0.05 were considered statistically significant. Further statistical analysis was done using Corel QuattroPro version 6.0 for Windows. Two-tailed Student's t test was used for comparison of means, and values of P less than 0.05 were considered statistically significant.
RESULTS
RSV-infected mice can clear virus without perforin.
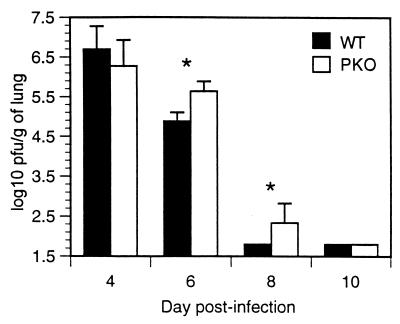
Lung supernatants were assayed for virus titer on days 4, 6, 8, and 10 after RSV infection. As shown in Fig. 1, both groups of mice cleared virus, regardless of the presence of perforin protein. However, virus clearance was delayed in PKO mice. PKO mice retained a significantly higher titer of virus at days 6 and 8, yet by day 10, virus had been cleared in both groups of mice. These data show that RSV clearance from the lungs does not require perforin, suggesting that there are alternate mechanisms for viral clearance in RSV infection.
FIG. 1.
Kinetics of viral replication in the lungs. The limit of detection for virus replication is 1.8 log10 PFU/g of lung. Data are a combination of results of two independent experiments with 10 WT mice and 9 PKO mice (P < 0.05 for days 6 and 8).
RSV-infected PKO mice lyse target cells using the Fas/FasL pathway.
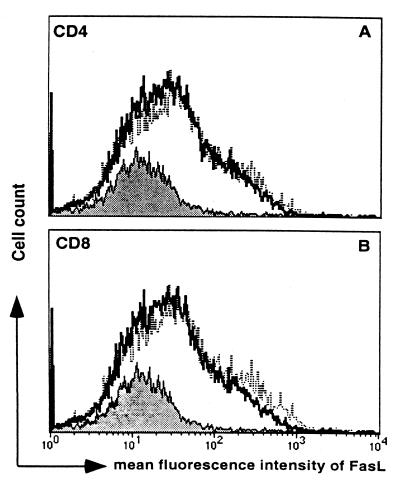
The observation that PKO mice were able to clear virus nearly as rapidly as WT mice suggested that other non-perforin-mediated mechanisms of virus clearance may have been involved. Therefore, we assessed the level of FasL expression on T cells of RSV-infected WT and PKO mice using multiparameter flow cytometry analysis. Surface FasL was increased on CD4+ and CD8+ T cells from the lungs in both WT and PKO mice compared to levels in uninfected mice (Fig. 2). FasL expression was measured on days 4, 6, 8, and 10, and peaked on day 8 in both WT and PKO mice (data not shown).
FIG. 2.
Mean flourescence intensity of FasL expression on CD4+ (A) and CD8+ (B) T cells from lungs of day 8 RSV-infected mice. Results for uninfected mice are indicated by the solid lines, for WT mice by the bold lines, and for PKO mice by the dotted lines. Data are representative of the results of three independent experiments with five mice per group.
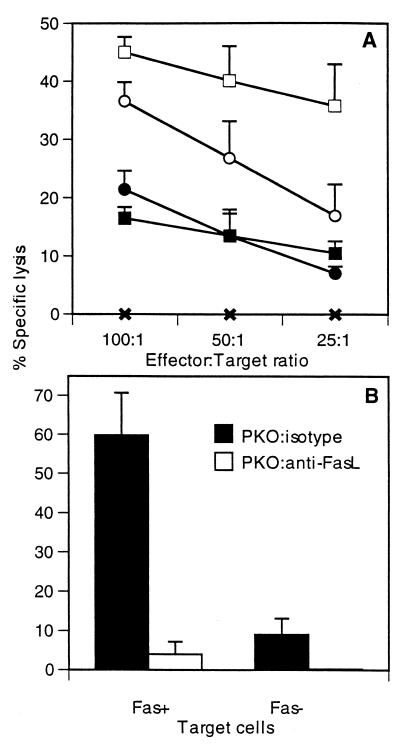
We next analyzed the contribution of FasL-mediated killing using target cells overexpressing Fas (L1210Fas+) and target cells deficient in Fas (L1210Fas−). Since both WT and PKO mice express high levels of FasL on T cells, we expected increased cytolytic activity against L1210Fas+ target cells. Lung lymphocytes were assayed without in vitro stimulation on day 8 after RSV infection. Effectors from WT and PKO mice were incubated with either L1210Fas− target cells or L1210Fas+ target cells at different effector-to-target cell ratios, and 51Cr release was measured. As seen in Fig. 3A, at an effector/target cell ratio of 100:1, 37% ± 3.0% (mean ± standard deviation) of L1210Fas+ target cells from WT mice were lysed, and 22% ± 3.0% (P < 0.05) of L1210Fas− target cells were lysed. Similarly, effectors from PKO mice show 45% ± 3.0% lysis in L1210Fas+ target cells, whereas L1210Fas− target cells show only 16% ± 2.0% (P < 0.05) lysis. These data show that the overexpression of Fas significantly enhances target cell lysis.
FIG. 3.
Direct CTL assay of lungs of RSV-infected mice day 8 after infection. (A) L1210Fas+ and L1210Fas− target cells sensitized with RSV M2 peptide were used to measure the relative contributions of perforin- and FasL-mediated killing in WT and PKO mice. ✖, uninfected mice: ●, WT effectors with L1210Fas− targets; ○, WT effectors with L1210Fas+ targets: ■, PKO effectors with L1210Fas− targets; □, PKO effectors with L1210Fas+ targets. (B) Anti-FasL antibody was used to show FasL involvement in target cell lysis using effectors from perforin-deficient mice. The effector-to-target ratio for panel B is 100:1. Data are representative of results of three independent experiments with five mice per group.
To directly show that FasL-mediated killing was responsible for the increased killing of L1210Fas+ target cells, we used anti-FasL antibody to block FasL-mediated killing. Treatment of effectors from PKO mice with anti-FasL antibody completely inhibited killing in L1210Fas+ target cells. This result demonstrates that the observed 60% ± 11.0% lysis of L1210Fas+ target cells was entirely mediated by FasL (Fig. 3B). In contrast, anti-FasL treatment of cytolytic effectors from WT mice only partially diminished cytolytic activity. L1210Fas− target cells have low levels of Fas expression, accounting for 9% ± 4.0% of the lysis observed in these target cells using effectors from PKO mice. This lysis was completely inhibited with anti-FasL antibody (Fig. 3B). Background killing from uninfected mice was less than 2% (data not shown). These data show that RSV-specific effectors from the lungs of both WT and PKO mice are able to lyse target cells by the Fas/FasL pathway.
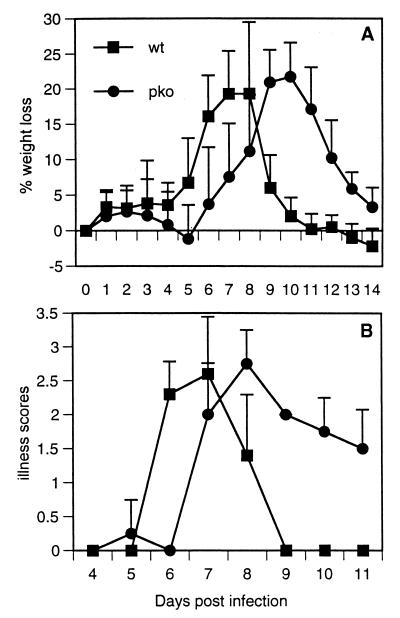
Weight loss and illness are delayed in PKO mice.
Mice were weighed and scored for illness daily after infection for 14 and 11 days. WT mice experienced peak weight loss on day 8 after RSV infection, whereas PKO mice experienced peak weight loss on day 10 (Fig. 4A). Illness was graded daily by a blinded observer, with increasing scores denoting increasing illness. WT mice had a peak illness score of 2.6 ± 0.8 (mean ± standard deviation) on day 7. PKO mice had a peak illness score of 2.8 ± 0.5 on day 8. Although weight loss and illness were delayed in PKO mice and recovery was more prolonged, peak magnitudes of disease were equal in the two groups (Fig. 4B).
FIG. 4.
Percentages of weight lost (A) and illness scores (B) for mice infected intranasally with live RSV. Illness scores range from 0 (no illness) to 5 (death). Data are representative of results of three independent experiments with five mice per group.
Kinetics of IFN-γ and TNF-α cytokine production in lungs.
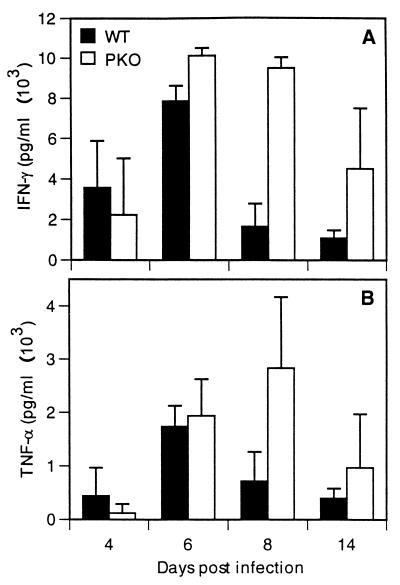
The cytokines IFN-γ and TNF-α possess antiviral activity (21, 23), are known to be produced by CD8+ T cells, and can cause illness when present in excess. We therefore asked whether the levels of IFN-γ and TNF-α correlated with virus clearance or illness. Lung supernatants were assayed for IFN-γ and TNF-α by ELISA on days 4, 6, 8, and 10 after infection. As shown in Fig. 5A, PKO mice produced five fold more IFN-γ on day 8 and threefold more on day 10 than WT controls. Similarly, TNF-α production was threefold higher on day 8 than that of the WT controls (Fig. 5B). These data indicate that the levels of IFN-γ and TNF-α were increased and prolonged in PKO mice and may have contributed to viral clearance and influenced the kinetics of illness.
FIG. 5.
Kinetics of cytokine IFN-γ (A) and TNF-α (B) expression in the lungs. Cytokine concentrations were measured using ELISA. Averages and standard deviations were calculated from results for five mice per group.
Histopathological examination of lung sections.
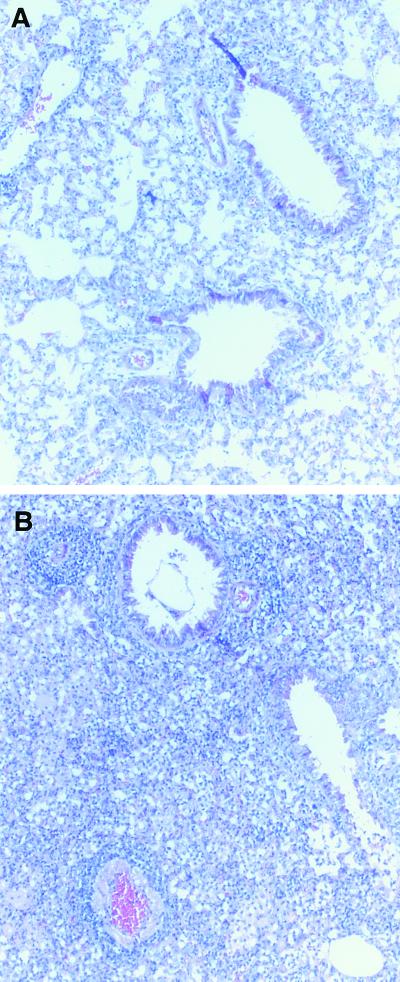
Lungs harvested on day 8 from RSV-infected WT and PKO mice were fixed and stained with hematoxylin-eosin. In typical RSV infection, lung pathology on day 8 from infected mice showed increased cellularity in the interstitium and alveoli and around the bronchovascular bundles (Fig. 6A). In PKO mice the mononuclear infiltration was increased, particularly in the interstitial spaces (Fig. 6B). The increased cellularity in PKO mice was confirmed by the total lymphocyte counts derived from whole lungs and indicated that the non-perforin-mediated virus clearance was achieved at the expense of enhanced lung pathology.
FIG. 6.
Hematoxylin-eosin staining of day 8 lung sections from RSV-infected mice. (A) Section of lung from a WT mouse infected with RSV. (B) Section of lung from a PKO mouse infected with RSV. The images are representative of lung sections taken from five different mice per group.
Frequency of RSV M2-specific CTL.
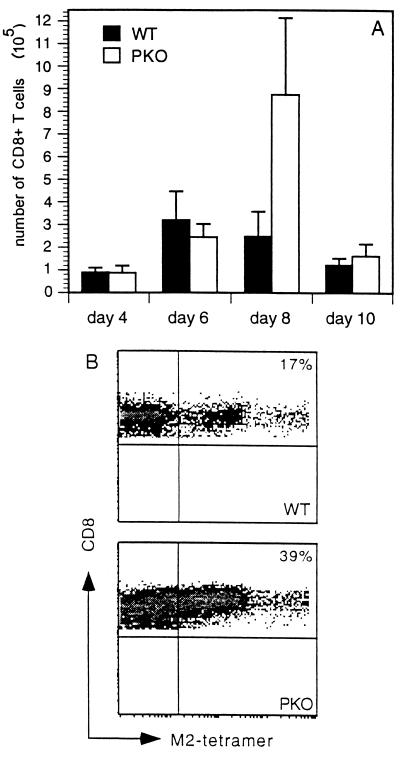
Next, we asked whether the composition of the cells in the lungs varied in the PKO mice, especially with respect to CD8+ T cells. Total cell counts from lungs of WT and PKO mice showed that PKO mice had more cells than WT mice, except on day 10, when there was a sharp decline (data not shown). No significant differences were found in the total numbers of CD8+ T cells in the lungs on days 4, 6, and 10. However, PKO mice had a significantly higher percentage of CD8+ T cells on day 8 (Fig. 7A). This large increase in CD8+ T cells may be compensation for the lack of perforin and indicate that non-perforin-mediated virus clearance is less efficient.
FIG. 7.
Kinetics of CD8+ T cells in the lungs of RSV-infected mice. (A) The number of CD8+ T cells was determined by multiplying the percentage of CD8+ T cells, as determined by FACS analysis, by the total number of cells isolated from the right lung of RSV-infected mice. Means and standard deviations were calculated from results for five WT mice and four PKO mice per group. (B) RSV M2-specific CD8+ T cells on day 8 postinfection were detected by FACS analysis using major histocompatibility complex class I tetramers. A total of 106 cells were stained with PE-labeled influenza virus or RSV M2 tetramers and allophycocyanin-labeled CD8, and 100,000 events were collected for flow cytometric analysis. Cells were also stained with the vital dye 7-AAD and fluorescein isothiocyanate–Annexin-V and gated on live cell populations. Data are representative of two independent experiments with four mice per group.
We then performed four-color flow cytometry to quantitate RSV-specific CD8+ T cells in WT and PKO mice. We used a PE-labeled, M2-specific H-2Kd tetramer, an allophycocyanin labeled anti-CD8 antibody, the vital dye 7-amino-actinomycin D (7-AAD), and Annexin-V. The vital dye 7-AAD and Annexin-V were used to exclude dead cells and include live and early apoptotic cells. An influenza virus NP peptide bound to the H-2Kd tetramer was used as a negative control. Analysis of RSV-specific CTL frequency on day 8 after infection revealed that PKO mice had a >2-fold-greater number of antigen-specific CTL than WT mice (Fig. 7B). The influenza virus NP-specific H-2Kd tetramer and uninfected mice exhibited a frequency of tetramer-positive cell staining of less than 0.4% (data not shown).
DISCUSSION
The mechanism by which virus-specific CTL lyse target cells may have profound effects on the pathogenesis of disease. The findings in this study highlight the importance of alternative mechanisms of viral clearance in PKO mice. This study shows for the first time that lung T lymphocytes express high levels of surface FasL after primary RSV infection. More importantly, antiviral CTL can lyse target cells by a perforin-independent, FasL-dependent mechanism that can be inhibited by anti-FasL antibody (Fig. 3B). This complete inhibition of target cell lysis by anti-FasL in our 51Cr release assays strongly suggests that FasL-mediated lysis of RSV-infected cells can act in a compensatory manner in the absence of perforin.
While our study focuses on the CD8+ CTL response to primary RSV infection, it is important to realize that natural killer (NK) cells possess the same cytolytic machinery as CD8+ CTL. Therefore, perforin deficiency may also affect the NK cell response to RSV, which may have importance in initiating clearance and promoting the adaptive immune response. Thus, we cannot exclude the possibility that the delayed illness and exaggerated response are related to an altered NK cell response. Ongoing studies are under way in our lab to address this possibility.
On the other hand, viral clearance can also be mediated in the absence of cell-to-cell contact by the antiviral cytokines IFN-γ and TNF-α and -β (20, 21, 23, 33, 34). Influenza virus-specific CTL stimulated with peptide produce IFN-γ and TNF-α, which enhance the lysis of influenza virus-infected cells in vitro. Even though anti-FasL antibodies completely inhibited target cell lysis in our 51Cr release assays, these are short (4- to 8-h) assays. Zheng et al. have shown that TNF-α can mediate mature T-cell receptor-induced apoptosis (44). In their experiments, apoptosis observed at 24 h could be inhibited by treatment of cells with the Fas-Fc fusion protein but not by TNFR-Fc. However, administration of Fas-Fc or TNFR-Fc at 48 h led to a decrease in apoptosis. Our data show that on day 8 postinfection, IFN-γ and TNF-α levels were significantly elevated in PKO mice (Fig. 5). These results suggest the possibility that the antiviral cytokines IFN-γ and TNF-α contributed to the delayed clearance observed in our experiments (Fig. 4A). Nevertheless, our lab has shown that anti-TNF-α treatment of WT mice during primary RSV infection, despite diminishing illness, has no impact on viral clearance (31). For this reason, we hypothesize that the elevated and persistent IFN-γ and TNF-α levels in PKO mice are more likely to be responsible for the late illness that was observed (Fig. 4B). These cytokine levels may have been increased because RSV clearance required a larger T-cell infiltrate in the lungs of PKO mice, which implies that T lymphocytes from PKO mice are probably less efficient at RSV clearance on a per cell basis than WT mice.
Interestingly, this late illness was still seen in PKO mice on and after day 10 postinfection (Fig. 4), by which time the virus had been cleared and a dramatic reduction in the number of CD8+ CTL was observed (Fig. 7A). Additionally, the total number of cells in WT mice fell from an average of 1.88 × 106 cells on day 8 to 0.54 × 106 cells on day 10. In PKO mice, the total cell number declined from 2.65 × 106 on day 8 to 0.45 × 106 on day 10 (data not shown). Therefore, the total number of inflammatory cells in the lungs does not explain the kinetics of illness, supporting the hypothesis that the altered cellular composition and prolonged production of IFN-γ and TNF-α are responsible for the delayed and prolonged illness.
As has been mentioned, the current state of the literature suggests that the Fas/FasL pathway is more important for T-cell homeostasis (8, 24, 27, 30, 36) and that the perforin/granzyme pathway is more important for clearance of viruses (3, 18). However, recent data suggest that the perforin pathway may also contribute to T-cell homeostasis (19, 28). PKO mice infected with LCMV have significantly increased numbers of LCMV-specific CD8+ T cells, to which the inability of PKO mice to downregulate the T-cell response was attributed (28). Our findings are consistent with this conclusion, as we found increased numbers of lymphocytes in infected lungs by fluorescence-activated cell sorter (FACS) analysis and histopathology on day 8 after RSV infection. PKO mice had greater cellularity focused primarily around the interstitial spaces than did WT controls (Fig. 6). In addition, we found a significantly higher percentage of M2-specific CD8+ T cells in PKO mice than in WT controls by use of tetramer analysis (Fig. 7B).
Overproduction of interleukin 4 (IL-4) is one setting in which RSV infection is known to result in severe or enhanced disease. This has been demonstrated for IL-4-overexpressing mice and by immunization with formulations that promote Th2 responses (10). It has been postulated that severe primary RSV disease or vaccine-enhanced RSV disease following administration of formalin-inactivated RSV in humans is also related to IL-4 overproduction. The basis for severe disease when IL-4 is overproduced has been assumed to be an exaggerated Th2 CD4 T-cell response with the attendant induction of immunoglobulin E and recruitment of eosinophils. However, our previous work has shown that IL-4 can induce a shift to a more FasL-mediated CTL killing mechanism (1). We propose that this shift may be a factor in severe RSV disease associated with overproduction of IL-4. In the present study, we have demonstrated that mice deficient in perforin suffer a larger cellular infiltrate in their lungs, possibly with an augmented amount of bystander killing, and increased production of IFN-γ and TNF-α. These events evoke more serious pathology and may potentially explain the FI-RSV vaccine-enhanced disease as a condition of IL-4 overproduction causing a shift to a FasL-dominant CTL killing mechanism. In summary, this study shows that, while perforin is important in the clearance of primary RSV infection, CTL possess alternative mechanisms for the elimination of RSV. Modulating the mechanism of CTL-mediated lysis of RSV-infected cells may be an important factor in the balance of viral clearance and lung immunopathology.
ACKNOWLEDGMENTS
S. Aung and J. A. Rutigliano contributed equally to the performance of this study and the authorship of this report.
We thank John Harty for providing the PKO mice, Joyce Johnson for the pathology photographs, Robert A. Parker for producing software to assist with statistical analysis, Michail V. Sitkovsky for the L1210Fas+ and L1210Fas− cell lines, John Altman and Dale Long for supplying the influenza virus NP and RSV M2 tetramers, Bo Li for technical assistance, and David McFarland for assistance with flow cytometry analysis.
This work was supported by NIH grant RO1-AI-33933.
REFERENCES
- 1.Aung S, Graham B S. IL-4 diminishes perforin-mediated and increases fas ligand-mediated cytotoxicity in vivo. J Immunol. 2000;164:3487–3493. doi: 10.4049/jimmunol.164.7.3487. [DOI] [PubMed] [Google Scholar]
- 2.Bangham C R, Openshaw P J, Ball L A, King A M, Wertz G W, Askonas B A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. [PubMed] [Google Scholar]
- 3.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 4.Cannon M J, Stott E J, Taylor G, Askonas B A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987;62:133–138. [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon M J, Openshaw P J, Askonas B A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba Y, Higashidate Y, Suga K, Honjo K, Tsutsumi H, Ogra P L. Development of cell-mediated cytotoxic immunity to respiratory syncytial virus in human infants following naturally acquired infection. J Med Virol. 1989;28:133–139. doi: 10.1002/jmv.1890280304. [DOI] [PubMed] [Google Scholar]
- 7.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P W, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 8.Depraetere V, Golstein P. Fas and other cell death signaling pathways. Semin Immunol. 1997;9:93–107. doi: 10.1006/smim.1997.0062. [DOI] [PubMed] [Google Scholar]
- 9.Falsey A R, Walsh E E. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer J E, Johnson J E, Kuli-Zade R K, Johnson T R, Aung S, Parker R A, Graham B S. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J Virol. 1997;71:8672–8677. doi: 10.1128/jvi.71.11.8672-8677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham B S. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4:290–293. doi: 10.1016/0966-842x(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 12.Graham B S, Bunton L A, Rowland J, Wright P F, Karzon D T. Respiratory syncytial virus infection in anti-mu-treated mice. J Virol. 1991;65:4936–4942. doi: 10.1128/jvi.65.9.4936-4942.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham B S, Bunton L A, Wright P F, Karzon D T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Investig. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham B S, Perkins M D, Wright P F, Karzon D T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 15.Graham B S, Johnson T R, Peebles R S. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology. 2000;48:237–247. doi: 10.1016/s0162-3109(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 16.Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs D, Bangham C R, McMichael A J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987;2:769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 18.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 19.Kagi D, Odermatt B, Mak T W. Homeostatic regulation of CD8+ T cells by perforin. Eur J Immunol. 1999;29:3262–3272. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Karupiah G, Xie Q W, Buller R M, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 21.Klavinskis L S, Geckeler R, Oldstone M B. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumor necrosis factor alpha, displays antiviral activity in vivo. J Gen Virol. 1989;70:3317–3325. doi: 10.1099/0022-1317-70-12-3317. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni A B, Morse III H C, Bennink J R, Yewdell J W, Murphy B R. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ T cells. J Virol. 1993;67:4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwano K, Kawashima T T, Arai S. Antiviral effect of TNF-alpha and IFN-gamma secreted from a CD8+ influenza virus-specific CTL clone. Viral Immunol. 1993;6:1–11. doi: 10.1089/vim.1993.6.1. [DOI] [PubMed] [Google Scholar]
- 24.Lenardo M J. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C C, Walsh C M, Young J D. Perforin: structure and function. Immunol Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 26.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 27.Lynch D H, Ramsdell F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 28.Matloubian M, Suresh M, Glass A, Galvan M, Chow K, Whitmire J K, Walsh C M, Clark W R, Ahmed R. A role for perforin in downregulating T-cell responses during chronic viral infection. J Virol. 1999;73:2527–2536. doi: 10.1128/jvi.73.3.2527-2536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz J L, McCarthy C A, Clark M E, Hall C B. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cells. J Virol. 1991;65:4494–4497. doi: 10.1128/jvi.65.8.4494-4497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 31.Neuzil K M, Tang Y W, Graham B S. Protective role of TNF-alpha in respiratory syncytial virus infection in vitro and in vivo. Am J Med Sci. 1996;311:201–204. doi: 10.1097/00000441-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 32.O'donnell D R, Milligan L, Stark J M. Induction of CD95 (Fas) and apoptosis in respiratory epithelial cell cultures following respiratory syncytial virus infection. Virology. 1999;257:198–207. doi: 10.1006/viro.1999.9650. [DOI] [PubMed] [Google Scholar]
- 33.Orange J S, Wang B, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orange J S, Biron C A. Characterization of early IL-12, IFN-alpha/beta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. Immunology. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 35.Rouvier E, Luciani M F, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell J H. Activation-induced death of mature T cells in the regulation of immune responses. Curr Opin Immunol. 1995;7:382–388. doi: 10.1016/0952-7915(95)80114-6. [DOI] [PubMed] [Google Scholar]
- 37.Shay D K, Holman R C, Newman R D, Liu L L, Stout J W, Anderson L J. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 38.Shresta S, Pham C T, Thomas D A, Graubert T A, Ley T J. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10:581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 39.Topham D J, Tripp R A, Doherty P C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 40.Vignaux F, Golstein P. Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? Eur J Immunol. 1994;24:923–927. doi: 10.1002/eji.1830240421. [DOI] [PubMed] [Google Scholar]
- 41.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White D W, MacNell A, Busch D H, Pilip I M, Pamer E G, Harty J T. Perforin-deficient CD8+ T cells: in vivo priming and antigen-specific immunity against Listeria monocytogenes. J Immunol. 1999;162:980–988. [PubMed] [Google Scholar]
- 43.Zajac A J, Quinn D G, Cohen P L, Frelinger J A. Fas-dependent CD4+ cytotoxic T-cell-mediated pathogenesis during virus infection. Proc Natl Acad Sci USA. 1996;93:14730–14735. doi: 10.1073/pnas.93.25.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]