Abstract
Culicoides, among the tiniest and most abundant hematophagous insects globally, serve as vectors for a variety of pathogens such as viruses, bacteria, parasites, protozoa, and nematodes. This study aimed to identify Culicoides species and assess their spatial distribution and seasonal occurrence in selected districts of the Central, South, and West Gondar zones, Northwest Ethiopia. A cross-sectional study was conducted between January to July 2023. A total of 44 UV light- onderstepoort traps were deployed in the study districts near specific areas. The traps were operational from dusk (6:00 PM) until dawn (6:00 AM) and were suspended at a height of 1.5 to 2 m above the ground. Poisson regression was used to assess associations, the Shannon diversity index to measure diversity, and QGIS 3.22.6 to create maps. In this study, 8,857 Culicoides were captured across the 44 trapping sites. Of the total flies captured flies, 8,838 were identified as belonging to 12 distinct species, while the classification of the remaining 19 flies remained unclear. Notably, C. kingi (54.01%) was the most prevalent species, followed by C. imicola (44.55%). The abundance of Culicoides observed from January to late April (3505) was significantly lower compared to the wet season (5355), with a marked increase in the capture of C. kingi (2499) from May to late July. A statistically significant association (p < 0.05) was observed between the occurrence of Culicoides and factors such as district, sampling point, and season. Spatial analysis revealed that C. kingi had a broader range of suitability than other Culicoides species, with high suitability observed in East Dembia. The diversity index analysis indicated that Culicoides species diversity was higher in samples from animal pens (H = 0.73) and during the wet season (H = 0.75). Additionally, this study documented the presence of eight Culicoides species namely C. corsicus, C. kibunensis, C. reioxi, C. kiouxi, C. saharienines, C. desertorum, C. reithi, and C. festivipennis, which have not been previously documented in Ethiopia. In conclusion, the study highlighted that the occurrence of Culicoides species was higher in East Dembia, with moderate presence in Wegera and West Armacho. Further research is needed to assess the impact of various Culicoides species on animal and human health, as well as their economic implications, and to develop corresponding control strategies based on these findings.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74524-z.
Keywords: Culicoides, Seasonal occurrence, Identification, Spatial distribution, Northwest, Ethiopia
Subject terms: Ecological epidemiology, Microbial ecology, Infectious diseases
Introduction
The genus Culicoides, comprising over 1,000 species, is highly significant within the family Ceratopogonidae. Commonly known as biting midges, members of the Ceratopogonidae family are dipteran flies belonging to the suborder Nematocera. They are among the smallest and most abundant blood-feeding flies globally, with sizes reaching up to 3 mm1,2. The Ceratopogonidae family includes approximately 78 genera and over 4,000 documented species. This family is divided into four subfamilies: Leptoconopinae, Forcipomyiinae, Dasyheleinae, and Ceratopogoninae. With the exception of Dasyheleinae, all subfamilies include species that feed on vertebrate blood. Only four genera are known to prey on both humans and animals. Culicoidesincludes the majority of globally problematic species and serves as a primary vector for animal-borne disease agents3,4.
Globally, 1,368 species of Culicoideshave been identified. The ability of these vectors to transmit viruses can be influenced by various environmental factors, including climate change5–7. This is further compounded by the necessity for these species to primarily feed on ruminants and be present in large numbers. Consequently, only a few species have been identified as potential or confirmed carriers of the blue-tongue virus (BTV) in different geographical areas. For instance, in Africa, species such as C. imicola and C. bolitinos; in Asia, C. imicola and C. fulvus; C. brevitarsis and C. fulvus in Australia; C. sonorensis in North America; and C. insignis and C. pusillusin Central and South America have been implicated8. In Northern Europe, C. obsoletus and C. pulicariscomplexes are associated with BTV transmission9. Female Culicoidesare hematophagous and serve as vectors for various pathogens. They bite hosts such as amphibians, birds, mammals, and both wild and domestic animals to obtain blood, which is necessary for egg maturation10–12.
Culicoides is both a hematophagous insect and a vector for various viruses, filarial nematodes, and protozoa, making it globally significant in veterinary health1,3. Their role in transmitting viruses, bacteria, protozoa, and nematodes is crucial13,14. Over 50 arboviruses, classified under the Bunyaviridae, Reoviridae, and Rhabdoviridae families, have been identified in various Culicoidesspecies15. After ingesting infected blood from animals, these viruses multiply in the midgut cells of female Culicoides, then migrate to the salivary glands and are transmitted through subsequent biting episodes in animal hosts1. The propagation of BTV infection relies on the presence of Culicoides, with factors such as species, population density, virus strain16, and external conditions like temperature17 influencing infection likelihood. Although reliable data on Culicoidesin Northwest Ethiopia is lacking, existing research on the Ceratopogonidae family has predominantly focused on its role in transmitting viruses such as African horse sickness, Bluetongue virus, and Schmallenberg virus1,3,15. Numerous studies have also explored the presence, species composition, and geographical distribution of Culicoides18–23.
In Ethiopia, several Culicoides species have been documented, including C. milnei, C. zuluensis, C. imicola, C. neavei, C. fulvithorax, and C. isioloensis24, C. fuscicaudae25, and C. kingi, C. imicola, C. milnei, C. schultzeei, C. zuluenesis, and C. pycnostictus26. The country faces recurrent and severe outbreaks of Culicoides-borne diseases. Between 2007 and 2010, 737 outbreaks of African horse sickness (AHS) were reported27. Serological evidence for bluetongue (BT) exist28,29but there is no molecular confirmation or isolation of Schmallenberg virus (SBV), despite a high apparent seroprevalence of 56.6%30. The lack of data on BT and SBV in Northwest Ethiopia may be due to misdiagnoses of other prevalent ruminant illnesses, such as foot and mouth disease (FMD), peste des petits ruminants (PPR), lumpy skin disease, and sheep and goat pox, which present similar clinical symptoms31. Understanding the species composition and population abundance of Culicoides in a given area is crucial for effective risk mitigation. Therefore, this study aimed to identify Culicoides species, map their spatial distribution, and document their seasonal occurrences in selected districts within the Central, South, and West Gondar zones of Northwest Ethiopia.
Materials and methods
Description of sample collection area
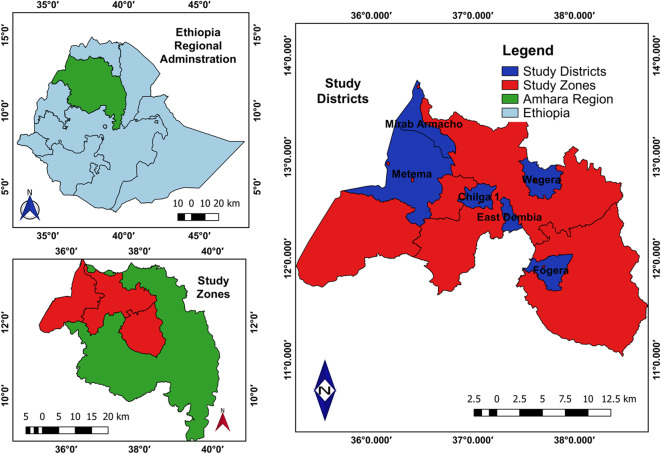
Samples were collected from six selected districts in the three Zones of Northwest Ethiopia: Central Gondar (Chilga, Wegera, and East Dembia), West Gondar (Metema and West Armacho), and South Gondar (Fogera). These districts were chosen for their unique geographical locations bordering neighboring countries and their large animal populations. Chilga District is situated at latitude 12°32’52” N and longitude 37°3’48” E, at an elevation of 2248 meters above sea level (m.a.s.l.). It features a mild climate with temperatures ranging from temperate to warm, averaging 20°C year-round, and receives 1175 mm of annual rainfall32. Wegera District is located at latitude 12°52’5” N and longitude 37°39’47” E, with elevations ranging from 1100 to 3040 m.a.s.l. The district experiences annual rainfall between 1000 to 1200 mm, with temperatures ranging from 14°C to 33°C. The rainy season extends from June to September, with peak rainfall occurring in July and August33. East Dembia District, at latitude 12°40’ N and longitude 37°10’ E, has an average elevation of 2053 m.a.s.l., an average annual temperature of 28 °C, and receives annual rainfall ranging from 995 to 1175 mm32.
Metema is located at latitude 12°58′N and longitude 36°12′E, with an elevation of 712 m above sea level. Bordered by Sudan to the west, Chilga to the east, Tach Armacho to the northeast, and West Armacho to the north, Metema is a key development corridor in the Amhara region. The area is characterized by fertile farms and large-scale cultivation of cash crops such as sesame, maize, cotton, and sorghum. The district has a total population of 115,285, residing in both urban and rural areas34,35. West Armacho is situated at latitude 13°13’33” N and longitude 36°26’21” E, with an elevation of 667 m above sea level. It experiences high temperatures, averaging 38 °C, and high humidity at 78%. Daytime temperatures can soar up to 43 °C from March to May. West Armacho is an agricultural region known for extensive farming, with a population of 34,473. It is bordered by Metema to the south, Sudan to the west, Tigray Region to the north, and Central Gondar to the east35,36.
Fogera District is located at latitude 11°56’59” N and longitude 37°35’9” E. It is bordered by Dera to the south, Lake Tana to the west, Reb to the north, Ebenat to the northeast, and Farta to the east. The district’s altitude ranges from 1774 to 2410 m above sea level, providing favorable conditions for extensive crop production and livestock rearing. Fogera has an average annual temperature of 27 °C37 (Fig. 1).
Fig. 1.
Map of Culicoides collection sites (QGIS 3.22.6 software was used to draw).
Description of laboratory processing areas
Culicoides were identified at the Veterinary Parasitology Laboratory of the University of Gondar. Species identification and enumeration were performed at the Animal Health Institute (AHI) in Sebeta, Ethiopia.
Study design
A cross-sectional study design was employed between January and July 2023 to collect, classify, and ascertain the occurrence and spatial dispersion of Culicoides in the study area.
Culicoides collection and identification
Culicoides collection
C Culicoides trapping was conducted in two distinct phases between January and July 2023. The initial sampling round, from January to April 2023, covered the dry season, while the second round, from May to July 2023, corresponded with the wet season following the rainy period. Ultraviolet (UV) light/suction traps, manufactured by the Onderstepoort Veterinary Institute (OVI) in South Africa and powered by a 12 V car battery, were used. The OVI trap has been recognized as an effective method for capturing a diverse range and large quantity of Culicoides species38. These traps are equipped with UV lights to attract insects, gauze, fans, and collection beakers. Trap deployment sites were carefully chosen to ensure that they were conducive to Culicoides breeding, including locations near animal pens, indoors, outdoors, wetlands, and livestock farms. Culicoides and other insects were collected in 500 ml beakers containing 250 ml of phosphate-buffered saline. After collection, samples were stored at -4 °C for 15 min to immobilize the flies. Subsequently, the identified Culicoides were preserved in cryovial tubes containing cotton and 70% ethanol to maintain their integrity for species identification.
Identification of Culicoides spp
Culicoides species were initially distinguished from other insects by using a stereomicroscope at the Veterinary Parasitology Laboratory of the University of Gondar. The identified Culicoides, preserved in cryovial tubes, were then transferred to the Animal Health Institute (AHI) for species classification and quantification. This was done by examining their morphological characteristics under a stereomicroscope. Key distinguishing features of Culicoides from other fly species include the size and shape of the antennae, wing pigmentation patterns, vein alignment with the wings, and the arrangement of wing macrotrichia at both distal and proximal ends. Additionally, the presence of grey and white spots in the anal region helps in identification. These distinctive patterns are specific to each species and are readily observable using dissection microscopy. Specimens were also mounted on slides and examined under a light microscope to assess morphological attributes such as the shape, size, position, and number of female spermathecae (as male Culicoides lack spermathecae). Male Culicoides exhibit hairy protrusions on the antennae, and particular attention was paid to ocular features, with the compound eye and inter-eye distance serving as crucial identification markers. This included determining whether the eyes were fused or separated by one or two facets. Furthermore, the ratio of antennae length, specifically segment XI divided by segment X, was calculated. The maxillary palps, consisting of five segments, were examined for variations in the shape of the third segment, which can be cylindrical, triangular, swollen, moderately swollen, or slender. Finally, all observed characteristics were compared with images in the Interactive Identification Key for Culicoides (IIKC) database developed by Mathieu et al39.
Spatial distribution of Culicoides
For spatial distribution analysis, geographic coordinates were recorded using a global positioning system (GPS) or smartphones, specifying latitude and longitude. A table joint technique was used to present the collected data on Culicoides species from both the dry and wet seasons. The spatial distribution model was developed using QGIS software, version 3.26.2.
Data management and analysis
The data collected from each location were documented using Microsoft Excel (2019). Descriptive statistics, expressed as percentages, were used to present the number of species obtained at each sampling location and during different seasons. The Shannon diversity index and Shannon equitability index were employed to measure the diversity and evenness of Culicoides species at sampling points and across seasons. Statistical significance was assessed using Poisson regression, with a p-value < 0.05 considered significant. A spatial distribution map was created using QGIS software, version 3.26.2.
Results
Entomological survey
In the present study, 8,857 Culicoides were collected from 44 collection locations. A total of twelve Culicoides species were identified, encompassing 8,838 individuals. Additionally, nine Culicoides specimens were of unknown classification. Ten of the identified Culicoides showed similarity with C. pole and C. festivipennis. Notably, Culicoides kingi was the most prevalent species, accounting for 4,784 (54.01%), followed by C. imicola (3946; 44.55%). The abundances of the remaining species were as follows: C. reithi (60; 0.78%); C. desestrum (23; 0.26%); C. corsicus (14; 0.158%); C. festipennis (5; 0.056%); C. reioxi (3; 0.034%); C. kibunensi (1; 0.011%); C. kiouxi (1; 0.011%); and C. saharienines (1; 0.011%). Additionally, the distribution of Culicoides across different districts revealed that East Dembia yielded the highest number of Culicoides, with 5,968 (67.38%), followed by Fogera with 822 (9.28%), Metema with 687 (7.76%), West Armacho with 592 (6.69%), Wegera with 447 (5.04%), and Chilga with 341 (3.85%). There was a statistically significant association (p < 0.05) between the distribution of Culicoides and districts. The results also indicated that the diversity of Culicoides species was higher and more evenly distributed in samples collected from East Dembia (EH = 0.32) (Table 1).
Table 1.
Culicoides species collected across the study sites.
| Culicoides species | Districts | Total (%) | |||||
|---|---|---|---|---|---|---|---|
| East Dembia | Metema | West Armacho | Chilga | Wegera | Fogera | ||
| C. kingi | 3,096 | 409 | 341 | 177 | 287 | 474 | 4,784 (54.01%) |
| C. imicola | 2,815 | 273 | 238 | 153 | 130 | 337 | 3,946 (44.55%) |
| C. corsicus | 14 | 0 | 0 | 0 | 0 | 0 | 14 (0.158%) |
| C. kibunensis | 1 | 0 | 0 | 0 | 0 | 0 | 1 (0.011%) |
| C. reioxi | 3 | 0 | 0 | 0 | 0 | 0 | 3 (0.034%) |
| C. kiouxi | 1 | 0 | 0 | 0 | 0 | 0 | 1 (0.011%) |
| C. saharienines | 1 | 0 | 0 | 0 | 0 | 0 | 1 (0.011%) |
| C. desertorum | 10 | 0 | 13 | 0 | 0 | 0 | 23 (0.26%) |
| C. reithi | 14 | 5 | 0 | 11 | 25 | 5 | 60 (0.78%) |
| C. festipennis | 0 | 0 | 0 | 0 | 5 | 0 | 5 (0.056%) |
| C. undifiend spp | 3 | 0 | 0 | 0 | 0 | 6 | 9 (0.101%) |
| Similarity with C. paole & C. festivipennis | 10 | 0 | 0 | 0 | 0 | 0 | 10 (0.112%) |
| Total (%) |
5968 # (67.38%) |
687 # (7.76%) |
592 # (6.69%) |
341 (3.85%) |
447 # (5.04%) |
822 # (9.28%) |
8,857 (100%) |
Analysis was performed using Poisson regression; compared with the districts, #p ≤ 0.001
Shannon diversity index value; (East Dembia = 0.76; Metema = 0.71; West Armacho = 0.77; Chilga = 0.81; Wegera = 0.86; Fogera = 0.75)
Shannon equitability index value; (East Dembia = 0.32; Metema = 0.65; West Armacho = 0.70; Chilga = 0.74; Wegera = 0.62; Fogera = 0.54)
The results in Table 2 show that the majority of Culicoides (6,790; 76.7%) were collected in the vicinity of animal pens. A statistically significant association was observed (p < 0.05) between the number of Culicoides collected and the sampling points. The results also indicated that the diversity of Culicoides species was higher in samples collected from animal pens (H = 0.73) (Table 2).
Table 2.
Culicoides species collected at different sampling points.
| Culicoides species | Sampling point | Total count | ||
|---|---|---|---|---|
| Indoor | Animal pen | Outdoor on field | ||
| C. kingi | 287 | 3,570 | 927 | 4,784 |
| C. imicola | 130 | 3,152 | 664 | 3,946 |
| C. corsicus | 0 | 14 | 0 | 14 |
| C. kibunensis | 0 | 1 | 0 | 1 |
| C. reioxi | 0 | 3 | 0 | 3 |
| C. kiouxi | 0 | 1 | 0 | 1 |
| C. saharienines | 0 | 1 | 0 | 1 |
| C. desertorum | 0 | 10 | 13 | 23 |
| C. reithi | 25 | 19 | 16 | 60 |
| C. festipennis | 5 | 0 | 0 | 5 |
| C. undifiend spp | 0 | 9 | 0 | 9 |
| Similarity with C. paole & C. festivipennis | 10 | 0 | 0 | 10 |
| Total | 447 # | 6,790 | 1,620# | 8,857 |
Analysis was performed using Poisson regression; compared with the sampling points, #p ≤ 0.001
Shannon diversity index value; (Indoor = 0.94; Animal pen = 0.75; Outdoor = 0.77)
Shannon equitability index value; (Indoor =0.58; Animal pen = 0.33; Outdoor = 0.56)
Seasonal occurrence and spatial distribution of Culicoides
During this investigation, all 12 Culicoides species were observed during the dry season. However, the quantity of Culicoides collected during the dry season (3,505) was significantly lower than that collected during the wet season (5,355), with a statistically significant association (p < 0.05). C. kingi emerged as the most prevalent species captured during the dry season. In addition, five Culicoides species were identified during the wet season, with C. imicola emerging as the predominant species (52.25%), followed by C. kingi (46.67%). The diversity of Culicoides species was higher in the wet season (H = 0.77) compared to the dry season (H = 0.75) (Table 3).
Table 3.
Culicoides species collected during the different seasons.
| Culicoides species | Dry season | Wet season |
|---|---|---|
| Total number (%) | Total number (%) | |
| C. kingi | 2,284 (65.2%) | 2,499 (46.67%) |
| C. imicola | 1,148 (32.8%) | 2,798 (52.25%) |
| C. corsicus | 14 (0.4%) | 0 |
| C. kibunensis | 1 (0.028%) | 0 |
| C. reioxi | 1 (0.028%) | 2 (0.037%) |
| C. kiouxi | 1 (0.028%) | 0 |
| C. saharienines | 1 (0.028%) | 0 |
| C. desertorum | 19 (0.54%) | 4 (0.074%) |
| C. reithi | 12 (0.34%) | 52 (0.97%) |
| C. festipennis | 5 (0.143%) | 0 |
| C. undifiend spp | 9 (0.26%) | 0 |
| Similarity with C. paole & C. festivipennis | 10 (0.285) | 0 |
| Total | 3,505 (100%) | 5,355 (100%)# |
Analysis was performed using Poisson regression; compared with the season, #p ≤ 0.001
Shannon diversity index value; (Dry = 0.77; Wet = 0.75)
Shannon equitability index value; (Dry = 0.31; Wet = 0.47)
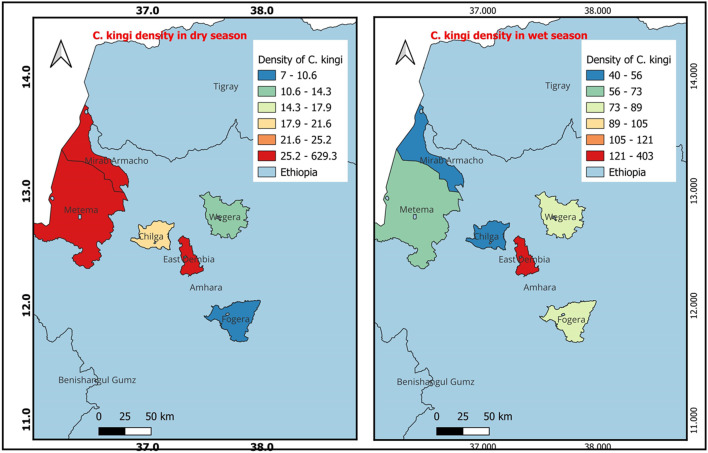
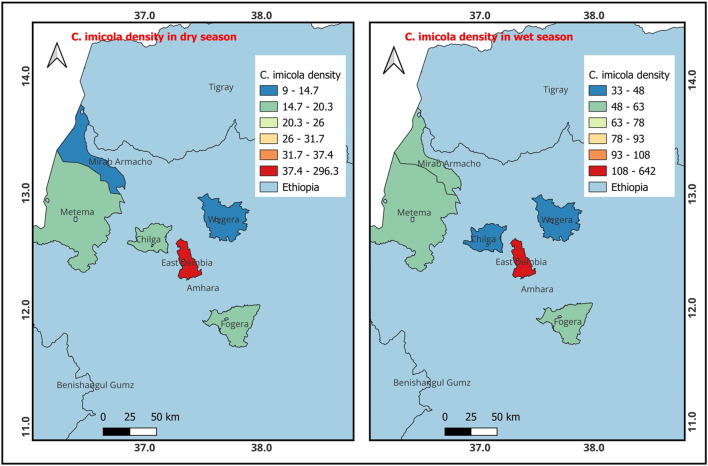
The findings presented in Fig. 2 illustrate the spatial distribution of C. kingi during both the wet and dry seasons. During the dry season, C. kingi was most predominant in East Dembia, followed by Metema and Mirab Armacho. In the wet season, C. kingi showed a preference for East Dembia, followed by Fogera and Wegera. Similarly, Fig. 3 shows that C. imicola had a higher presence in East Dembia during the dry season, followed by Metema and Chilga. However, in the wet season, C. imicola was most prevalent in East Dembia, followed by Fogera and Metema.
Fig. 2.
Density of C. kingi occurrence during the dry and wet seasons (QGIS 3.22.6 software was used).
Fig. 3.
Density of C. imicola occurrence during the dry and wet seasons (QGIS 3.22.6 software was used).
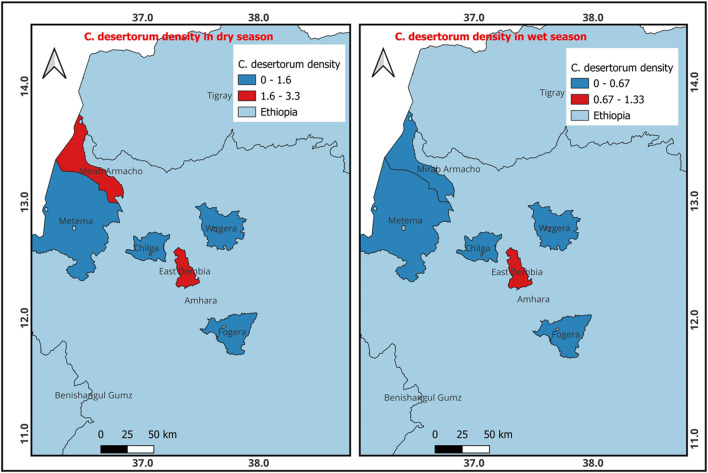
The results presented in Fig. 4 show that during the dry season, C. desertorum was most prevalent in West Armacho, followed by East Dembia. However, during the wet season, the presence of C. desertorum favored East Dembia.
Fig. 4.
Density of C. desertorum occurrence during the dry and wet seasons (QGIS 3.22.6 software was used).
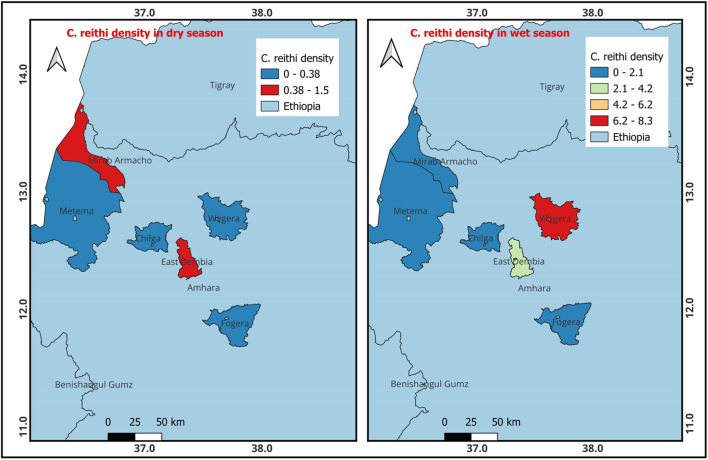
The findings presented in Fig. 5 demonstrate that C. reithi was most prevalent in Mirab Armacho, followed by East Dembia during the dry season. Additionally, the presence of C. reithi was more pronounced in Wegera, followed by East Dembia, during the wet season.
Fig. 5.
Density of C. reithi occurrence during the dry and wet seasons (QGIS 3.22.6 software was used).
Furthermore, the distribution of C. corsicus was most prevalent in East Dembia, as shown in Supplementary Fig. 1. Additionally, C. festivipennis was most prevalent in Wegera (Supplementary Fig. 2). An unknown Culicoides species was most predominant in Fogera during the dry season (Supplementary Fig. 3). Moreover, C. riouxi was exclusively detected in East Dembia during the wet season (Supplementary Fig. 4).
Discussion
During the study period, a total of 8,857 Culicoides were captured across 44 trapping sites. Of these, 8,838 were classified into 12 distinct species, while the remaining nine Culicoides could not be identified to species level. Among the identified species, ten exhibited similarities to C. paole and C. festivipennis, with C. kingibeing the most prevalent at 54.01%. This contrasts with an investigation by Fetene et al26., which found C. imicola to be more abundant than C. kingi. Morphological assessment in the present study confirmed the presence of 12 Culicoides species in the study areas. Notably, C. kingiwas the most frequently collected species, accounting for 4,784 individuals (54.01%). This finding diverges from previous studies in sub-Saharan Africa by Sghaier et al21., Gordon et al22., and Guichard et al40. which identified C. imicolaas the predominant species. Additionally, Mulatu and Hailu24 documented the presence of C. imicola, C. milnei, C. neavei, C. zuluensis, C. fulvithorax, and C. isioloensisin Western Ethiopia, while Khamala and Kettle25 reported C. fuscicaudaein West Africa. Fetene et al26. also identified C. kingi, C. schultzei, and C. pycnostictus, in various Ethiopian regions. However, the current study revealed eight Culicoides species not previously documented in Ethiopia: C. corsicus, C. kibunensis, C. reioxi, C. kiouxi, C. saharienines, C. desertorum, C. reithi, and C. festivipennis.
The current investigation identified East Dembia as the most favorable area for the presence of Culicoides species. This finding of ecological preference aligns with research conducted by Blanda et al10.. in Italy. Our study presents the results of a spatial distribution methodology used to assess the abundance of key Culicoides vector species by analyzing a substantial number of collections across both dry and wet seasons. Culicoides were significantly more abundant in the East Dembia, Fogera, and Metema districts, while lower levels were observed in Mirab Armacho, Wegera, and Chilga. Various factors potentially influence the distribution of Culicoides species, including biotic elements (such as vegetation, human activities, and the presence of other animals) and abiotic factors (such as light, soil composition, water sources, air quality, and climatic conditions), along with other variables that may vary between macrohabitats and microhabitats.
In the current study, during the dry season in East Dembia, C. kingi had an average occurrence ranging from 39.3 to 629.3, followed by C. imicola with an average range of 17.5 to 296.3. During the wet season, C. imicola had an average occurrence ranging from 52.4 to 642, while C. kingi ranged from 87.8 to 402.7. The study found that the occurrence of Culicoides was higher during the wet season compared to the dry season, with the association being statistically significant (p < 0.05). C. kingi was the most prevalent species captured during the dry season, while C. imicola emerged as the predominant species during the wet season. The observed seasonal discrepancies might be attributed to the presence of favorable conditions that support the existence and development of Culicoides. Additionally, soil type appears to be crucial in determining the distribution and abundance of C. imicola, and climatic variables such as mean annual minimum and maximum temperatures and mean annual rainfall were found to influence its occurrence. Similar findings have been reported by Ander et al41., Gordon et al22., Fetene et al26., and Carpenter et al17.. C. imicola has a broad global distribution, extending from South Africa to regions including the Mediterranean, Middle East, and Far East. However, certain areas show low densities or complete absence of C. imicola42, indicating a patchy distribution pattern for this species.
The spatial distribution analysis identified East Dembia as the most favorable area for the presence of Culicoides species. This ecological preference aligns with findings from Blanda et al10.. conducted in Italy, suggesting a correlation with specific biotic or abiotic factors in different districts. The geographical location of East Dembia, particularly its proximity to Lake Tana, likely contributes to its suitability for most Culicoides species. Additionally, the Metema and Mirab Armacho districts were identified as favorable sites for some Culicoides species, potentially due to their high temperatures and proximity to the Sudan border. Other factors influencing the distribution patterns of Culicoides include vegetation, animal presence, and microclimatic conditions such as altitude, temperature, and precipitation. Previous research has shown that the spatial distribution of Culicoides species. can be affected by diverse climatic zones43and local factors like livestock management practices. Additionally, studies have explored the impact of seasonal and meteorological parameters on Culicoides activity41,43–45and generated risk maps based on habitat attributes46–48. For example, a correlation has been identified between the spread of bluetongue disease and specific landscape characteristics such as woodlands and open grasslands49.
Conclusion and recommendations
In the present study, C. kingi and C. imicola emerged as the predominant Culicoides species. The occurrence of Culicoides species was found to be higher during the wet season. There was a statistically significant association between Culicoides occurrence and season, sampling point, and districts. Additionally, East Dembia was identified as the most favorable area for the occurrence of Culicoides species. The study also revealed that the diversity of Culicoides species was higher in samples collected from animal pens and during the wet season. Further research is needed to explore the impact of various Culicoides species on both animal and human health, as well as their economic implications. Corresponding control strategies should be developed based on these findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the University of Gondar, Office of the Vice President for Research and Community Service, for their financial support during this work. The authors also thank the Animal Health Institute, Ethiopia (AHI) for their cooperation during vector collection and identification.
Abbreviations
- AHI
Animal Health Institute
- BT
Bluetongue
- BTV
Bluetongue virus
- GPS
Global positioning system
- IIKC
Interactive Identification Key for Culicoides
- IPM/IVM
Integrated Pest/Vector Management
- OVI
Onderstepoort Veterinary Institute
- QGIS
Quantum Geographic Information System
- UV
ultraviolet
Author contributions
The contribution of the authors to the manuscript as follows, Conceptualization and Methodology: WM, SMI, GGD, ZST, and HD; Data curation and Formal analysis: BAA, ZST and HD; Software, Supervision and Validation: AA, ZST and HD; Writing- Original draft preparation: BAA; and Funding acquisition, Investigation, Project administration, Resources, Visualization and Writing- Review and Editing: WM, ABM, MB, SMI, BD, AK, ATG, MZK, GGD, MDF, TM, HT, MB, ZST, and HD. All authors read and approved the final manuscript.
Funding
This study was funded by the Office of the Vice President for Research and Community Services at the University of Gondar, Gondar, Ethiopia. However, the funding body played no role in the design and execution of the study, including the data collection and analysis.
Data availability
All data generated or analyzed during this study are available upon request from the corresponding author.
Declarations
Ethics statement
This study was reviewed and approved by the Institutional Review Board of the College of Veterinary Medicine and Animal Sciences of the University of Gondar, Ethiopia (reference number: CVMAS/11/127/2022).
Consent for publication
All the authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
6. References
- 1.Mellor, P. S., Boorman, J. & Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 45, 307–40. 10.1146/annurev.ento.45.1.307 (2000). [DOI] [PubMed]
- 2.Mullen, G. R. & Murphree, C. S. Biting midges (ceratopogonidae). In: Medical and Veterinary Entomology. Elsevier; 213–236. (2018). [Google Scholar]
- 3.Carpenter, S., Groschup, M. H., Garros, C., Felippe-Bauer, M. L. & Purse, B. V. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 100, 102–113 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Shults, P., Borkent, A. & Gold, R. The pupa of Culicoides Sonorensis Wirth and Jones (Diptera: Ceratopogonidae) - first detailed description of this stage of the bluetongue virus vector. Ann. Entomol. Soc. Am. 109, 280–318. 10.1093/aesa/sav119 (2016). [Google Scholar]
- 5.Paweska, J. T., Venter, G. J. & Mellor, P. S. Vector competence of South African Culicoides species for bluetongue virus serotype 1 (BTV-1) with special reference to the effect of temperature on the rate of virus replication in C. Imicola and C. Bolitinos. Med. Vet. Entomol. 16, 10–21. 10.1046/j.1365-2915.2002.00334.x (2002). [DOI] [PubMed] [Google Scholar]
- 6.Mellor, P. Environmental influences on arbovirus infections and vectors. Microbe-vector Interact. vector-borne Dis. 181–197. (2004).
- 7.Wilson, A. J. & Mellor, P. S. Bluetongue in Europe: past, present and future. Philosophical Trans. Royal Soc. B: Biol. Sci. 364, 2669–2681. 10.1098/rstb.2009.0091 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldet, T., Mathieu, B., Delecolle, J., Gerbier, G. & Roger, F. Emergence de la fièvre catarrhale ovine dans le Bassin méditerranéen Et surveillance entomologique en France. Rev. d’élevageet médecine vétérinaire des. pays Trop. 58, 125–132 (2005). [Google Scholar]
- 9.Bessell, P. R. et al. Assessing the potential for Bluetongue virus 8 to spread and vaccination strategies in Scotland. Sci. Rep. 6. 10.1038/srep38940 (2016). [DOI] [PMC free article] [PubMed]
- 10.Blanda, V. et al. Geo-statistical analysis of Culicoides spp. distribution and abundance in Sicily, Italy. Parasites Vectors. 11. 10.1186/s13071-018-2658-2 (2018). [DOI] [PMC free article] [PubMed]
- 11.Black, W. et al. S,. Biology of Disease Vectors. 2nd edition. Burlington, USA: Elsevier Academic Press; (2004).
- 12.Meiswinkel, R., Gomulski, L., Delécolle, J., Goffredo, M. & Gasperi, G. The taxonomy of Culicoides vector complexes-unfinished business. Vet. Ital. 40, 151–159 (2004). [PubMed] [Google Scholar]
- 13.Wirth, W., Dyce, A. & Spinelli, G. An atlas of wing photographs, with a summary of the numerical characters of the Neotropical species of Culicoides (Diptera: Ceratopogonidae). Contrib Am Entomol Inst. (1988). https://www.cabidigitallibrary.org/doi/full/10.5555/19920509805. Accessed 9 Aug 2024.
- 14.Atkinson, C. T. Parasitic diseases of Wild Birds. In: (eds Atkinson, C. T., Thomas, N. J. & Hunter, D. B.) Parasitic diseases of Wild Birds. New York, America: John Wiley & Sons, Ltd; 13–35. doi:10.1002/9780813804620. (2009). [Google Scholar]
- 15.Sick, F., Beer, M., Kampen, H. & Wernike, K. Culicoides biting midges—underestimated vectors for arboviruses of public health and veterinary importance. Viruses. 11, 376. 10.3390/v11040376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabachnick, W. J. Culicoides variipennis and bluetongue-virus epidemiology in the United States. Annu. Rev. Entomol. 41, 23–43. 10.1146/annurev.en.41.010196.000323 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Carpenter, S. et al. Temperature dependence of the extrinsic incubation period of Orbiviruses in Culicoides Biting Midges. PLoS ONE. 6, e27987. 10.1371/journal.pone.0027987 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasić, A. et al. Species diversity, host preference and arbovirus detection of Culicoides (Diptera: Ceratopogonidae) in south-eastern Serbia. Parasites Vectors. 12, 1–9. 10.1186/s13071-019-3292-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin, E. et al. Culicoides species community composition and infection status with parasites in an urban environment of east central Texas, USA. Parasit. Vectors. 12, 39. 10.1186/s13071-018-3283-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gusmão, G. M. C., Brito, G. A., Moraes, L. S., Bandeira, M. D. C. A. & Rebêlo, J. M. M. Temporal variation in species abundance and richness of Culicoides (Diptera: Ceratopogonidae) in a Tropical Equatorial Area. J. Med. Entomol. 56, 1013–1018. 10.1093/jme/tjz015 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Sghaier, S. et al. Specie Del genere Culicoides (Diptera Ceratopogonidae) e presenza del virus della Bluetonge in Tunisia. Vet. Ital. 53, 357–366 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Gordon, S. J. G. et al. The occurrence of culicoides species, the vectors of arboviruses, at selected trap sites in Zimbabwe. Onderstepoort J. Vet. Res. 82. 10.4102/ojvr.v82i1.900 (2015). [DOI] [PMC free article] [PubMed]
- 23.Villard, P. et al. Modeling Culicoides abundance in mainland France: implications for surveillance. Parasites Vectors. 12, 391. 10.1186/s13071-019-3642-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulatu, T. & Hailu, A. The occurrence and identification of Culicoides species in the Western Ethiopia. Acad. J. Entomol. 12, 40–43 (2019). [Google Scholar]
- 25.Khamala, C. & Kettle, D. The Culicoides Latreille (Diptera: Ceratopogonidae) of East Africa. (1971). https://www.cabidigitallibrary.org/doi/full/10.5555/19711001710. Accessed 9 Aug 2024.
- 26.Fetene, E. et al. Modeling the spatial distribution of Culicoides species (Diptera: Ceratopogonidae) as vectors of animal diseases in Ethiopia. Sci. Rep. 12, 12904. 10.1038/s41598-022-16911-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aklilu, N. et al. African horse sickness outbreaks caused by multiple virus types in Ethiopia. Transbound. Emerg. Dis. 61, 185–192. 10.1111/tbed.12024 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Gizaw, D., Sibhat, D., Ayalew, B. & Sehal, M. Sero-prevalence study of bluetongue infection in sheep and goats in selected areas of Ethiopia. Ethiop. Vet. J. 20, 105. 10.4314/evj.v20i1.8 (2016). [Google Scholar]
- 29.Abera, T. et al. Bluetongue disease in small ruminants in south western Ethiopia: cross-sectional sero-epidemiological study. BMC Res. Notes. 11, 112. 10.1186/s13104-018-3222-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibhat, B., Ayelet, G., Gebremedhin, E. Z., Skjerve, E. & Asmare, K. Seroprevalence of Schmallenberg virus in dairy cattle in Ethiopia. Acta Trop. 178, 61–67 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Rojas, J. M., Rodríguez-Martín, D., Martín, V. & Sevilla, N. Diagnosing bluetongue virus in domestic ruminants: current perspectives. Vet. Med. Res. Rep. 10, 17–27. 10.2147/vmrr.s163804 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melese, A. T., Ayele, D. T., Aljerf, L., Al-Fekaiki, D. F. & Akele, M. L. Investigating the phytoavailability of metals in roots of Croton macrostachyus and Phytolacca dodecandra: induced rhizosphere processes. BioMetals. 36, 1347–1359. 10.1007/s10534-023-00522-9 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Dessie, A. B., Abtew, A. A. & Koye, A. D. Analysis of Smallholder Farmers’ Cooperation in Eucalyptus Woodlot production in Wegera District, Northern Ethiopia. Small-scale for. 18, 291–308. 10.1007/s11842-019-09418-4 (2019). [Google Scholar]
- 34.Gelaye, K. A. et al. Low knowledge and attitude towards visceral Leishmaniasis among migrants and Seasonal Farm workers in Northwest Ethiopia. Res. Rep. Trop. Med. 11, 159–168. 10.2147/rrtm.s286212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemma, W. Impact of high malaria incidence in seasonal migrant and permanent adult male laborers in mechanized agricultural farms in Metema-Humera lowlands on malaria elimination program in Ethiopia. BMC Public. Health. 20. 10.1186/s12889-020-8415-4 (2020). [DOI] [PMC free article] [PubMed]
- 36.Aschale, Y., Mengist, A., Bitew, A., Kassie, B. & Talie, A. Prevalence of malaria and associated risk factors among asymptomatic migrant laborers in West Armachiho District, Northwest Ethiopia. Res. Rep. Trop. Med. 9, 95–101. 10.2147/rrtm.s165260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fikadu, A. A. & Gebre, G. G. Evidence from Fogera district in Ethiopia on configuration of farmer’s information literacy conditions that explain better productivity performance of the horticultural crops. Agric. Food Secur. 10, 1–13. 10.1186/s40066-021-00299-5 (2021). [Google Scholar]
- 38.Venter, G. J. et al. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet. Parasitol. 166, 299–307 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Mathieu, B. et al. Development and validation of IIKC: an interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasites Vectors. 5, 137. 10.1186/1756-3305-5-137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guichard, S. et al. Worldwide niche and future potential distribution of Culicoides Imicola, a major vector of bluetongue and African horse sickness viruses. PLoS ONE. 9, e112491. 10.1371/journal.pone.0112491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ander, M., Meiswinkel, R. & Chirico, J. Seasonal dynamics of biting midges (Diptera: Ceratopogonidae: Culicoides), the potential vectors of bluetongue virus, in Sweden. Vet. Parasitol. 184, 59–67. 10.1016/j.vetpar.2011.08.009 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Venter, G. J. & Meiswinkel, R. The virtual absence of Culicoides imicola (Diptera: Ceratopogonidae) in a light-trap survey of the colder, high-lying area of the eastern Orange Free State, South Africa, and implications for the transmission of arboviruses. (1994). https://scholar.archive.org/work/xjjpvusiafa3njhwri6zf3ki7e/access/wayback/http://repository.up.ac.za/bitstream/handle/2263/33050/43venter1994.pdf?sequence=1. Accessed 9 Aug 2024. [PubMed]
- 43.Brugger, K. & Rubel, F. Characterizing the species composition of European Culicoides vectors by means of the Köppen-Geiger climate classification. Parasites Vectors. 6, 333. 10.1186/1756-3305-6-333 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders, C. J. et al. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J. Appl. Ecol. 48, 1355–1364. 10.1111/j.1365-2664.2011.02051.x (2011). [Google Scholar]
- 45.Racloz, V., Venter, G., Griot, C. & Stärk, K. D. C. Estimating the temporal and spatial risk of bluetongue related to the incursion of infected vectors into Switzerland. BMC Vet. Res. 4, 42. 10.1186/1746-6148-4-42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conte, A. et al. Culicoides Imicola. Vet. Ital. 40, 311 (2004).20419684 [Google Scholar]
- 47.Takken, W. et al. The phenology and population dynamics of Culicoides spp. in different ecosystems in the Netherlands. Prev. Vet. Med. 87, 41–54 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Caligiuri, V. et al. Bluetongue surveillance in the Campania region of Italy using a geographic information system to create risk maps. Vet. Ital. 40, 385–389 (2004). [PubMed] [Google Scholar]
- 49.Guis, H. et al. Use of high spatial resolution satellite imagery to characterize landscapes at risk for bluetongue. Vet. Res. 38, 669–683. 10.1051/vetres:2007025 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available upon request from the corresponding author.