Abstract
Objective
To investigate the efficacy of elagolix plus add-back therapy (estradiol [1 mg] and norethindrone acetate [0.5 mg] once daily) on patient-reported nonbleeding symptoms and menstrual bleeding associated with uterine fibroids (UFs) across different subpopulations.
Design
Post hoc analysis of two phase 3 clinical trials—Elaris UF-1 and UF-2.
Setting
A total of 76 (UF-1) and 77 (UF-2) US clinical sites.
Patient(s)
Women (N = 591) with UFs and heavy menstrual bleeding.
Intervention(s)
Elagolix (300 mg) twice daily with add-back therapy (the indicated dose for UF-associated heavy menstrual bleeding) vs. placebo for 6 months.
Main Outcome Measure(s)
“Very much improved” or “much improved” change in nonbleeding symptoms (abdominal/pelvic pain, abdominal/pelvic pressure/cramping, back pain, and abdominal bloating) and menstrual bleeding measured using a Patient Global Impression of Change scale. Improvements were assessed in subpopulations stratified using baseline characteristics (age, race [self-reported], body mass index, and International Federation of Gynecology and Obstetrics fibroid classification).
Result(s)
Across subpopulations, differences favored elagolix plus add-back therapy (vs. placebo) for most symptoms at month 1 and all symptoms at months 3 as well as 6. In patients with characteristics commonly associated with high disease burden (age >40 years, Black/African American), those treated with elagolix plus add-back therapy reported significantly greater improvements vs. placebo at months 1–6 (P<.05) for all nonbleeding and bleeding symptoms (P≤.05).
Conclusion(s)
Premenopausal women with heavy menstrual bleeding and UFs receiving elagolix plus add-back therapy experienced significant improvements in nonbleeding as well as bleeding symptoms from months 1–6, regardless of baseline characteristics.
Clinical Trial Registration Number
NCT02654054 and NCT02691494.
Key Words: Heavy menstrual bleeding, leiomyomas, oral GnRH antagonists, Patient Global Impression of Change, uterine fibroids
Uterine fibroids (UFs), also known as leiomyomas, are nonmalignant tumors that develop in the smooth muscle of the uterus (1, 2, 3). Many women with UFs have significant symptoms that negatively impact their quality of life (1, 4, 5, 6). The most common symptom associated with UFs is prolonged or excessively heavy menstrual bleeding, which can result in iron-deficiency anemia (2, 3, 7). Other frequently reported symptoms associated with UFs include pelvic pressure and pain, urinary and gastrointestinal symptoms, as well as reproductive dysfunction (2, 3, 7).
Elagolix is an oral, nonpeptide, gonadotropin-releasing hormone competitive antagonist that rapidly and reversibly suppresses ovarian sex hormones in women in a dose-dependent manner. In two phase 3 double-blind, randomized, placebo-controlled trials (Elaris UF-1 and UF-2), elagolix (300 mg) twice daily plus hormonal add-back therapy (estradiol [1 mg] and norethindrone acetate [0.5 mg] once daily) significantly reduced menstrual blood loss compared with placebo in premenopausal women with UFs and heavy menstrual bleeding (8). On the basis of the results from these trials, elagolix was approved for use in combination with add-back therapy in the United States for the management of heavy menstrual bleeding associated with UFs in premenopausal women (9). Secondary analyses of data from the Elaris UF-1 and UF-2 studies have subsequently found that elagolix plus add-back therapy reduces heavy menstrual bleeding in women with UFs, regardless of age, body mass index (BMI), race, ethnicity, baseline menstrual blood loss, fibroid location, uterine and primary fibroid volume, as well as in those with coexisting adenomyosis (10, 11). However, to date, little is known about the efficacy of elagolix plus add-back therapy on other nonbleeding symptoms associated with UFs, especially from a patient’s perspective.
Therefore, in this post hoc analysis of data from the Elaris UF-1 and UF-2 trials, we investigated the effect of elagolix (300 mg) twice daily plus add-back therapy (the indicated dose for uterine-fibroid-associated heavy menstrual bleeding) (9) compared with placebo on nonbleeding and bleeding symptoms as reported by patients using the Patient Global Impression of Change (PGIC) scale throughout the studies. We also explored whether the baseline demographic and clinical characteristics of the women in the studies had any impact on their response to treatment.
Materials and methods
Study design, patients, and treatment
Full details of the Elaris UF-1 and UF-2 trials (NCT02654054 and NCT02691494) as well as primary endpoints have been reported previously (8). Briefly, these studies were identically designed, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials. Elaris UF-1 was conducted at 76 sites across the United States, including Puerto Rico, between December 2015 and December 2018; Elaris UF-2 was conducted at 77 sites across the United States and Canada between February 2016 and January 2019. Patients included in the studies were premenopausal women, aged 18–51 years, with an ultrasound-confirmed diagnosis of UFs and heavy menstrual bleeding (>80 mL blood loss per menstrual cycle for at least two separate cycles). Patients were excluded at screening if they were pregnant, had persistent or complex ovarian cysts, had cancer, had pelvic inflammatory disease, or had a history of osteoporosis or a bone mineral density T score of −1.5 or less at the lumbar spine, total hip, or femoral neck.
Both trials started with a hormonal washout and a screening period of 2–3 menstrual cycles (equivalent to approximately 2.5–3.5 months). This was followed by a 6-month treatment period, during which patients were randomized within 10 days of the start of their menses to receive elagolix (300 mg) twice daily plus add-back therapy, elagolix (300 mg) twice daily monotherapy, or placebo (Supplemental Fig. 1, available online). This post hoc analysis compared patients receiving elagolix plus add-back therapy vs. placebo.
Both Elaris trials were conducted in accordance with the Good Clinical Practice Guidelines as defined by the International Conference on Harmonization, the Declaration of Helsinki, and/or all applicable federal as well as local regulations and were approved by the institutional review boards of the investigational centers, as appropriate. All patients provided written informed consent.
Endpoints and assessments
Responses to the PGIC scale were evaluated to assess the five nonbleeding symptoms (abdominal or pelvic pain, abdominal or pelvic pressure, abdominal or pelvic cramping, back pain, and abdominal bloating) as well as menstrual bleeding. The 7-point PGIC scale is used to depict a patient’s rating of their symptom improvement from baseline. Patients were asked to rate their change in symptoms at months 1, 3, and 6 as follows: “very much improved” = 1, “much improved” = 2, “minimally improved” = 3, “no change” = 4, “minimally worse” = 5, “much worse” = 6, or “very much worse” = 7.
Data on patients who reported a “very much improved” or “much improved” response for each of the six symptoms contained in the PGIC scale were summarized by subpopulations according to baseline patient characteristics. The subpopulations used included: age (<35, 35–39, and ≥40 years), BMI (<30, 30 to <35, and ≥35 kg/m2), lowest International Federation of Gynecology and Obstetrics (FIGO) fibroid classification (0–3, 4, and 5–8), highest FIGO fibroid classification (0–3, 4, and 5–8), primary FIGO fibroid classification (0–3, 4, and 5–8), and race (self-reported on patient enrollment forms; White or Black/African American).
Statistical analysis
Data from the Elaris UF-1 and UF-2 studies were pooled for this exploratory analysis. Descriptive P values for differences between elagolix plus add-back therapy and placebo in patients who reported “very much improved” or “much improved” symptoms at months 1, 3, and 6 were based on an exploratory analysis of the covariance model with treatment as the main effect. However, no formal hypotheses were tested in this post hoc analysis, and, as such, no adjustments were made for multiplicity, and P values were only exploratory and descriptive.
Results
Patients
Patient demographics and baseline characteristics of women (N = 591) included in this analysis of data from the Elaris UF-1 as well as UF-2 trials were similar between the elagolix plus add-back therapy (n = 395) and placebo groups (n = 196; Table 1 and Supplemental Fig. 2) and representative of women with UFs. The mean ages were 42.0–42.6 years, 67.3%–67.9% were Black/African Americans, the mean BMIs were 33.3–33.8 kg/m2, 49.1%–50.0% had a FIGO primary classification of 4 indicating a primary intramural fibroid, and the mean menstrual blood loss per cycle was 233.4–254.8 mL.
Table 1.
Patient baseline demographics and clinical characteristics.
| Characteristic | Elagolix + add-back therapya (n = 395) | Placebo (n = 196) |
|---|---|---|
| Age, y, mean (±SD) | 42.6 (5.3) | 42.0 (5.6) |
| Race, n (%) | n = 394b | n = 196 |
| White | 118 (29.9) | 60 (30.6) |
| Black/African American | 265 (67.3) | 133 (67.9) |
| Other | 7 (1.8) | 2 (1.0) |
| Multiple | 4 (1.0) | 1 (0.5) |
| BMI, kg/m2, mean (±SD) | 33.3 (6.9) | 33.8 (7.5) |
| Primary fibroid volume, cm3, mean (±SD) | 75.6 (112.8) | 87.7 (129.1) |
| MBL volume, mL, mean (±SD) | 233.4 (149.4) | 254.8 (175.7) |
| Uterine volume, cm3, mean (±SD) | 485.1 (384.7) | 512.4 (405.9) |
| FIGO primary fibroid classification cohort, n (%) | n = 389 | n = 192 |
| 0–3 (submucous) | 78 (20.1) | 38 (19.8) |
| 4 (intramural) | 191 (49.1) | 96 (50.0) |
| 5–8 (subserosal) | 120 (30.8) | 58 (30.2) |
Note: BMI = body mass index; FIGO = International Federation of Gynecology and Obstetrics; MBL = menstrual blood loss; SD = standard deviation.
Add-back therapy consisted of estradiol (1 mg) and norethindrone acetate (0.5 mg).
One patient in the elagolix + add-back therapy cohort was missing data on race.
PGIC scale response in all patient subpopulations
Overall, more patients in the elagolix plus add-back therapy group than the placebo group reported a “very much improved” or “much improved” response to the six nonbleeding and bleeding symptoms assessed, and these reports were consistent across all subpopulations studied (Table 2 and Supplemental Tables 1–3, available online). Significantly more patients reported nonbleeding symptom improvements (“very much improved” or “much improved”) with elagolix plus add-back therapy vs. patients receiving placebo for most symptoms as early as month 1 and for all symptoms by months 3 as well as 6 (P<.05; Table 2 and Supplemental Tables 1–3). Significantly more patients reported improvements (“very much improved” or “much improved”) in menstrual bleeding with elagolix plus add-back therapy vs. patients receiving a placebo for months 1 through 6 (P<.006; Table 2 and Supplemental Tables 1–3).
Table 2.
Meaningful improvementa at month 6 in nonbleeding symptoms and menstrual bleeding across different patient subpopulations as evaluated using the Patient Global Impression of Change scale.
| Subpopulationb | Treatment | Patients with meaningful improvementa, n/N (%) |
|||||
|---|---|---|---|---|---|---|---|
| Abdominal or pelvic pain | Abdominal or pelvic pressure | Abdominal or pelvic cramping | Back pain | Abdominal bloating | Menstrual bleeding | ||
| Overall | |||||||
| ELA + AB | 222/304 (73.0)∗∗∗ | 212/304 (69.7)∗∗∗ | 221/304 (72.7)∗∗∗ | 174/304 (57.2)∗∗∗ | 174/304 (57.2)∗∗∗ | 266/305 (87.2)∗∗∗ | |
| Placebo | 33/153 (21.6) | 26/153 (17.0) | 38/153 (24.8) | 32/153 (20.9) | 23/153 (15.0) | 35/153 (22.9) | |
| BMI, kg/m2 | |||||||
| <30 | ELA + AB | 67/101 (66.3)∗∗∗ | 63/101 (62.4)∗∗∗ | 68/101 (67.3)∗∗∗ | 56/101 (55.4)∗∗∗ | 62/101 (61.4)∗∗∗ | 86/101 (85.1)∗∗∗ |
| Placebo | 10/54 (18.5) | 7/54 (13.0) | 11/54 (20.4) | 9/54 (16.7) | 6/54 (11.1) | 9/54 (16.7) | |
| 30–<35 | ELA + AB | 61/85 (71.8)∗∗∗ | 58/85 (68.2)∗∗∗ | 61/85 (71.8)∗∗∗ | 45/85 (52.9)∗∗∗ | 44/85 (51.8)∗∗∗ | 75/85 (88.2)∗∗∗ |
| Placebo | 8/35 (22.9) | 6/35 (17.1) | 9/35 (25.7) | 7/35 (20.0) | 6/35 (17.1) | 8/35 (22.9) | |
| ≥35 | ELA+AB | 93/117 (79.5)∗∗∗ | 90/117 (76.9)∗∗∗ | 92/117 (78.6)∗∗∗ | 73/117 (62.4)∗∗∗ | 68/117 (58.1)∗∗∗ | 104/118 (88.1)∗∗∗ |
| Placebo | 15/64 (23.4) | 13/64 (20.3) | 18/64 (28.1) | 16/64 (25.0) | 11/64 (17.2) | 18/64 (28.1) | |
| Primary FIGO fibroid classification | |||||||
| 0–3 | ELA + AB | 45/65 (69.2)∗∗∗ | 48/65 (73.8)∗∗∗ | 44/65 (67.7)∗∗∗ | 33/65 (50.8)∗∗∗ | 39/65 (60.0)∗∗∗ | 55/65 (84.6)∗∗∗ |
| Placebo | 1/28 (3.6) | 1/28 (3.6) | 2/28 (7.1) | 2/28 (7.1) | 2/28 (7.1) | 1/28 (3.6) | |
| 4 | ELA + AB | 101/140 (72.1)∗∗∗ | 92/140 (65.7)∗∗∗ | 97/140 (69.3)∗∗∗ | 81/140 (57.9)∗∗∗ | 79/140 (56.4)∗∗∗ | 122/141 (86.5)∗∗∗ |
| Placebo | 21/71 (29.6) | 16/71 (22.5) | 22/71 (31.0) | 16/71 (22.5) | 15/71 (21.1) | 18/71 (25.4) | |
| 5–8 | ELA + AB | 73/94 (77.7)∗∗∗ | 69/94 (73.4)∗∗∗ | 78/94 (83.0)∗∗∗ | 59/94 (62.8)∗∗∗ | 55/94 (58.5)∗∗∗ | 85/94 (90.4)∗∗∗ |
| Placebo | 9/50 (18.0) | 8/50 (16.0) | 12/50 (24.0) | 12/50 (24.0) | 5/50 (10.0) | 14/50 (28.0) | |
Note: BMI = body mass index; ELA + AB = elagolix plus add-back therapy (estradiol [1 mg] and norethindrone acetate [0.5 mg]); FIGO = International Federation of Gynecology and Obstetrics; PGIC = Patient Global Impression of Change.
∗P<.05; ∗∗P<.01; ∗∗∗P<.001. P values were descriptive only, and no adjustments were made for multiplicity.
Meaningful improvement is defined using PGIC responses of “very much improved” or “much improved.”
Subpopulations on the basis of baseline demographic or clinical characteristics.
PGIC scale response in patients aged older than 40 years
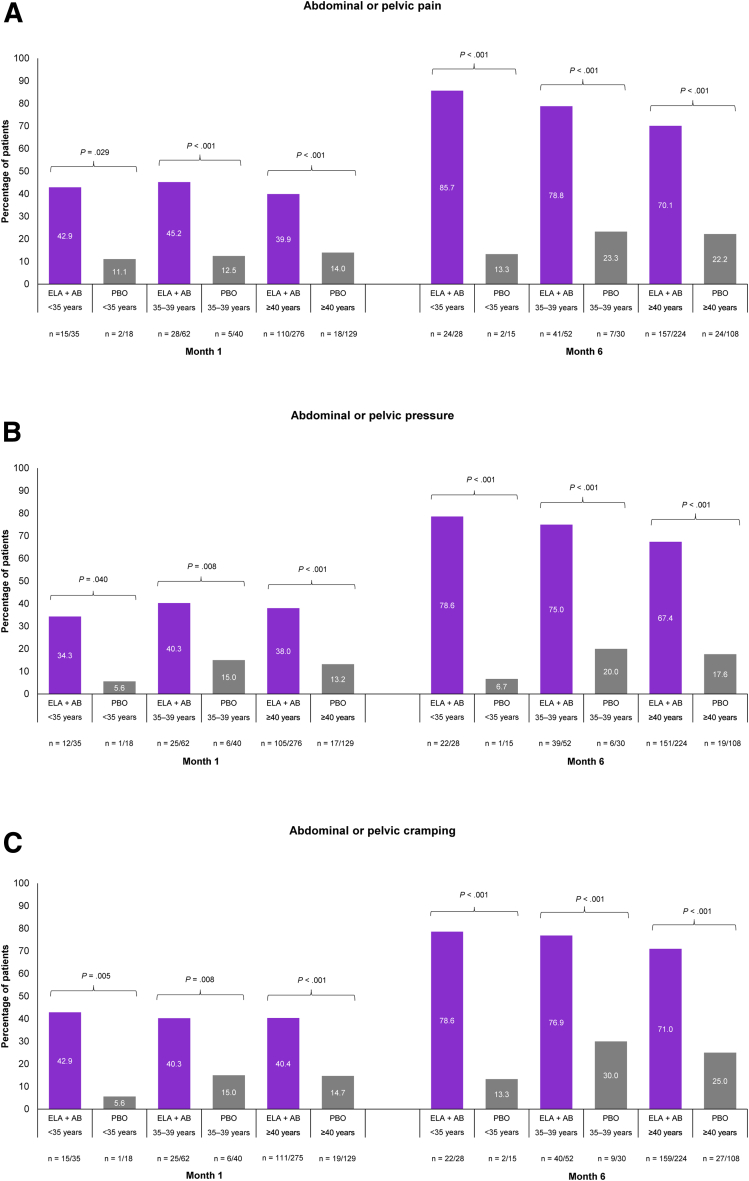
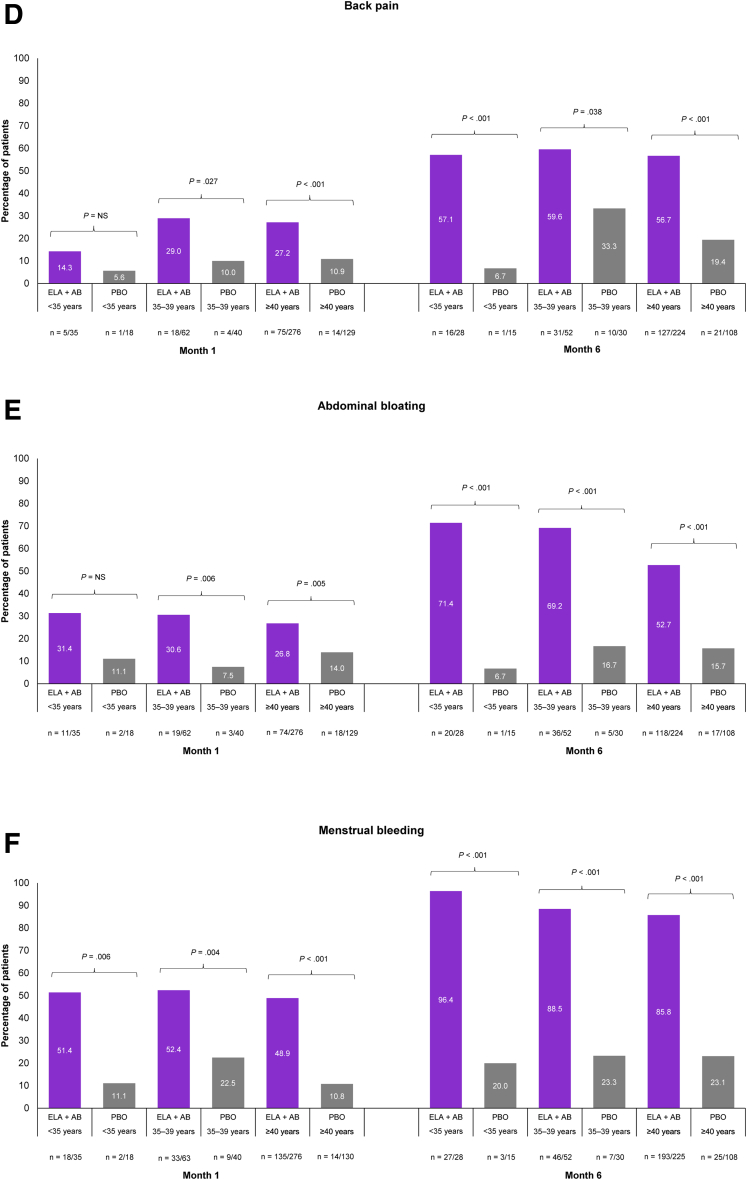
Increasing age, especially in patients aged ≥40 years, is a recognized risk factor for UFs (12, 13). In those patients aged ≥40 years, elagolix plus add-back therapy was significantly better than placebo at achieving PGIC scale responses of “very much improved” or “much improved” across all nonbleeding and menstrual bleeding symptoms assessed (Fig. 1 and Supplemental Fig. 3). Differences between treatment groups were seen as early as month 1 and increased through months 3 as well as 6. At month 6, a significantly higher proportion of patients treated with elagolix plus add-back therapy vs. placebo reported “very much improved” or “much improved” nonbleeding symptoms (Fig. 1A–E): abdominal or pelvic pain (70.1% vs. 22.2%, P<.001), abdominal or pelvic pressure (67.4% vs. 17.6%, P<.001), abdominal or pelvic cramping (71.0% vs. 25.0%, P<.001), back pain (56.7% vs. 19.4%, P<.001), and abdominal bloating (52.7% vs. 15.7%, P<.001). At month 1, 48.9% of patients receiving elagolix plus add-back therapy reported “very much improved” or “much improved” menstrual bleeding compared with 10.8% of patients receiving placebo (P<.001), which increased to 85.8% vs. 23.1% of patients, respectively, by month 6 (P<.001; Fig. 1F).
Figure 1.
Meaningful improvements in nonbleeding symptoms and menstrual bleeding across age groups were evaluated using the Patient Global Impression of Change (PGIC) scale at months 1 and 6. Abdominal or pelvic pain (A), abdominal or pelvic pressure (B), abdominal or pelvic cramping (C), back pain (D), abdominal bloating (E), and menstrual bleeding (F). Meaningful improvement is defined using PGIC scale responses of “very much improved” or “much improved.” P values were descriptive only, and no adjustments were made for multiplicity. ELA + AB = elagolix plus add-back therapy (estradiol [1 mg] and norethindrone acetate [0.5 mg]); NS = not significant; PBO = placebo.
PGIC scale response in Black/African American patients
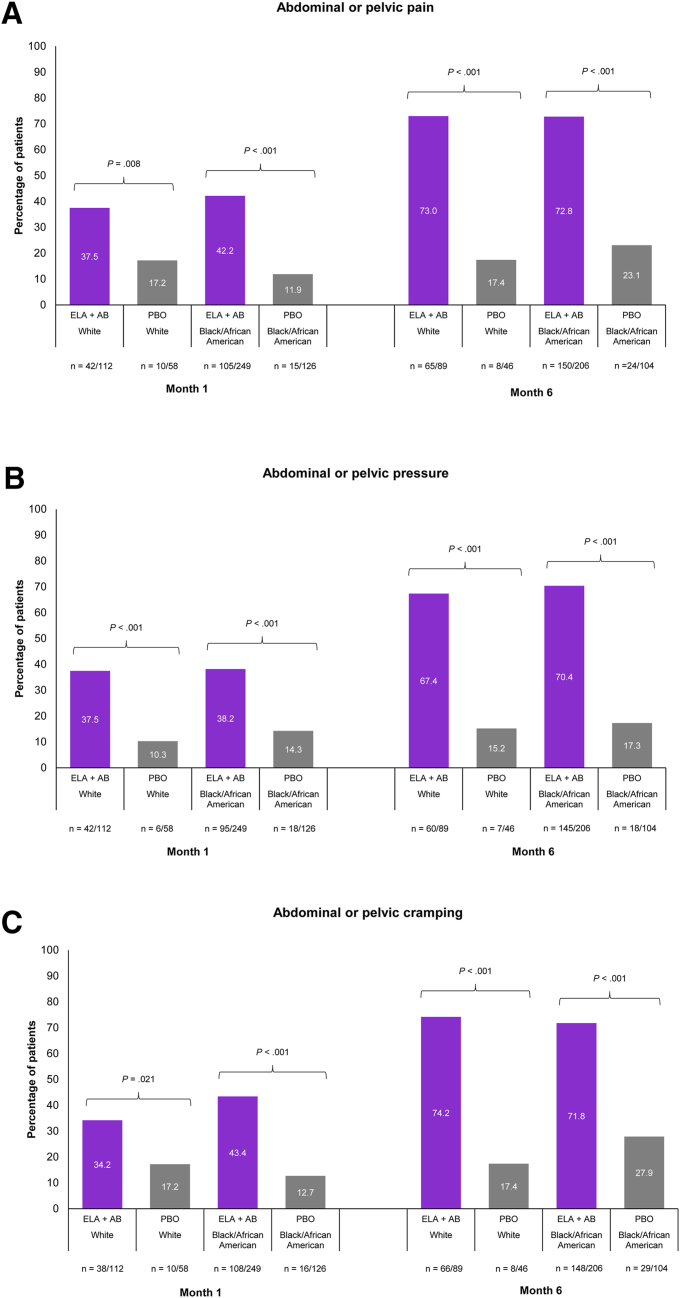
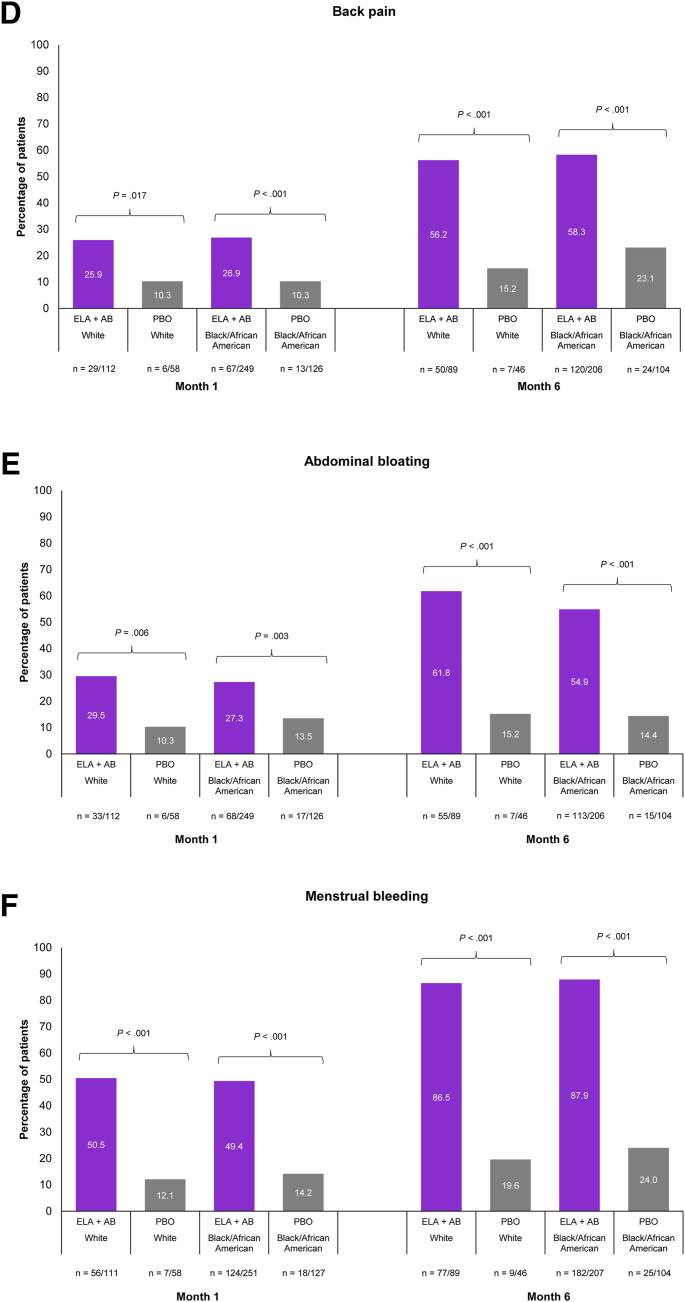
Evidence suggests that Black/African American women are disproportionally affected with regard to UF frequency as well as severity (12, 13, 14). In Black/African American patients, PGIC scale responses of “very much improved” or “much improved” were also consistently as well as significantly higher in patients receiving elagolix plus add-back therapy vs. patients receiving placebo across the nonbleeding and bleeding symptoms studied (Fig. 2 and Supplemental Fig. 4). “Very much improved” or “much improved” nonbleeding symptoms were reported as early as month 1 in significantly larger proportions of patients treated with elagolix plus add-back therapy than in those treated with placebo, with the proportion of patients increasing at months 3 and 6 (Fig. 2A–E and Supplemental Fig. 4A–E). By month 6 (Fig. 2A–E), a significantly higher proportion of patients treated with elagolix plus add-back therapy vs. placebo reported “very much improved” or “much improved” abdominal or pelvic pain (72.8% vs. 23.1%, P<.001), abdominal or pelvic pressure (70.4% vs. 17.3%, P<.001), abdominal or pelvic cramping (71.8% vs. 27.9%, P<.001), back pain (58.3% vs. 23.1%, P<.001), and abdominal bloating (54.9% vs. 14.4%, P<.001). In Black/African American patients receiving elagolix plus add-back therapy, 49.4% reported “very much” or “much improved” menstrual bleeding at month 1 vs. 14.2% of patients receiving placebo (P<.001; Fig. 2F). This increased to 87.9% vs. 24.0% of patients, respectively, by month 6 (P<.001; Fig. 2F).
Figure 2.
Meaningful improvements in nonbleeding symptoms and menstrual bleeding by race were evaluated using the Patient Global Impression of Change (PGIC) scale at months 1 and 6. Abdominal or pelvic pain (A), abdominal or pelvic pressure (B), abdominal or pelvic cramping (C), back pain (D), abdominal bloating (E), and menstrual bleeding (F). Meaningful improvement is defined using PGIC responses of “very much improved” or “much improved.” Only White and Black/African American races were included in this analysis because of the small patient numbers of other races. P values were descriptive only, and no adjustments were made for multiplicity. ELA + AB = elagolix plus add-back therapy (estradiol [1 mg] and norethindrone acetate [0.5 mg]); PBO = placebo.
Discussion
In this post hoc analysis of data from the Elaris UF-1 and UF-2 studies, we investigated the impact of treatment with elagolix plus add-back therapy on patient-reported outcomes of nonbleeding symptoms and menstrual bleeding using the PGIC scale across subpopulations. This analysis represented the first time that patient-reported improvements in nonbleeding symptoms have been shown with elagolix plus add-back therapy. Another novel aspect of this analysis was the evaluation of the potential impacts of elagolix plus add-back therapy on symptom improvement on the basis of differences in demographic and clinical characteristics. We found that elagolix plus add-back therapy significantly improved all nonbleeding symptoms assessed compared with placebo. These improvements were seen as early as month 1 for abdominal or pelvic pain, abdominal or pelvic pressure, and abdominal or pelvic cramping in all subpopulations stratified by baseline demographics as well as clinical characteristics. Improvements in back pain and abdominal bloating were also seen in most subpopulations by month 1, with the exception of the patients aged <35 years as well as <30 kg/m2 BMI subpopulations, in which back pain and abdominal bloating were significantly improved by month 3. All improvements were sustained through the end of the study period at month 6. The efficacy of elagolix plus add-back therapy for improving nonbleeding symptoms and menstrual bleeding regardless of the different subpopulation characteristics further supports this medication as a treatment for premenopausal women with UFs across races, age groups, body compositions (as assessed using BMI), as well as fibroid classifications.
In addition, in women with UFs and heavy menstrual bleeding at baseline, elagolix plus add-back therapy significantly improved bleeding by the first posttreatment evaluation at 1 month. The improvements in all the assessments were sustained through months 3 and 6. These results confirm the original findings reported by Schlaff et al. (8).
Although data are limited on the clinical burden of symptomatic UFs, evidence suggests that Black/African American women are disproportionally affected with respect to a greater risk of fibroid disease, a higher symptom burden, and an earlier age of onset compared with women of other races (12, 13, 14). Therefore, we examined closely the impact of race on the efficacy of elagolix plus add-back therapy. In the Elaris UF-1 and UF-2 studies, greater than two-thirds of the study patients were Black/African American women (8). In this group of women in our current post hoc analysis, the percentage who reported a “very much improved” or “much improved” status on the PGIC scale for all nonbleeding symptoms and menstrual bleeding was significantly greater for those receiving elagolix plus add-back therapy vs. those receiving placebo for months 1 through 6. Furthermore, the results were similar to those observed in White patients across all symptoms and time points assessed, supporting the position that elagolix plus add-back therapy is efficacious at improving the symptoms of UFs, regardless of race.
Uterine fibroids typically become symptomatic in women aged 30–49 years (1), and increasing age, especially in women aged ≥40 years, has been found to be a risk factor for UFs (12, 13). In our post hoc analysis, we therefore also closely examined the impact of age on the efficacy of elagolix plus add-back therapy on improving symptoms. As was observed in other subpopulations, significantly more patients receiving elagolix plus add-back therapy than patients receiving placebo reported a “very much improved” or “much improved” status in all six symptoms, regardless of age group (<35, 35–39, and ≥40 years) from months 1 through 6. The only exception was back pain and abdominal bloating in the group with patients <35 years at month 1; however, both groups achieved significant treatment responses at months 3 and 6.
Clinical endpoints, such as the volume of menstrual bleeding, are useful and necessary in clinical trials to accurately measure the efficacy of treatment. However, the perceived impact of treatment by patients is equally important, and the US Food and Drug Administration recommends the inclusion of patient-reported outcome results from clinical trials to provide valuable information representing the effect of disease on health and functioning from a patient’s perspective (15). There is limited literature reporting patient-reported outcomes in women with UFs. This analysis helps to fill this gap in the literature with the use of the PGIC scale, a widely used patient-reported outcomes tool, to assess patients’ evaluation of common UF-associated symptoms.
Numerous reports have associated nonbleeding and bleeding symptoms with reduced quality of life (1, 4, 5, 6). Soliman et al. (6) found that mean UF Symptom and Quality of Life (UFS-QoL) questionnaire scores were significantly worse among women with nonbleeding as well as bleeding symptoms, and women with severe symptoms reported the worst UFS-QoL scores. In the Elaris UF-1 and UF-2 trials, elagolix plus add-back therapy resulted in the least square mean changes in the UFS-QoL score of 38.0–42.0, although placebo was associated with changes of 6.5–10.9 (8). Although quality of life was not assessed in this specific analysis of subpopulations from the Elaris UF-1 and UF-2 trials, significant symptom improvements would be expected to have a positive impact on quality of life in patients receiving elagolix plus add-back therapy.
This exploratory analysis has a few limitations. As a post hoc assessment, this study stratified patients into subgroups across a variety of baseline demographic and clinical characteristics. The purpose of our descriptive analysis was to explore possible differential effects of elagolix plus add-back therapy across subpopulations; it was not designed, statistically powered, or prespecified to detect a treatment effect. As an exploratory analysis with no formal hypothesis testing, there was no need for adjustment, and P values were provided as a descriptive measure of strength only. In addition, patient numbers for some patient subgroups were small, which may have limited the ability of this analysis to detect significant differences between the treatment groups. For example, the number of patients who responded as having “much worse” or “very much worse” nonbleeding and bleeding symptoms in each subgroup was too small to provide interpretable data on whether there would be any meaningful difference. Thus, we were only able to perform an exploratory assessment of symptom improvement. Further, because of the racial composition of enrolled patients in the Elaris UF-1 and UF-2 trials, patient numbers were sufficient to only evaluate Black/African American or White races in our analyses. However, we still found significant differences between elagolix plus add-back therapy and placebo at months 1, 3, and 6 in most of the patient subgroups for the six symptoms evaluated. Finally, the Elaris UF-1 and UF-2 trials had a double-blind, placebo-controlled treatment period with a duration of 6 months, and PGIC scale assessments were not collected during the extension trial that followed it. Thus, future studies would be required to evaluate differences in nonbleeding and bleeding symptoms beyond 6 months.
To conclude, in this analysis, premenopausal women with baseline heavy menstrual bleeding associated with UFs experienced improvements in nonbleeding and bleeding symptoms as early as month 1, which were sustained for up to 6 months of treatment with elagolix plus add-back therapy compared with placebo, regardless of subpopulation by baseline patient characteristics. These results support the clinical usefulness of elagolix (300 mg) twice daily plus add-back therapy to manage nonbleeding and bleeding symptoms in women with heavy menstrual bleeding associated with UFs across a wide spectrum of patients with varying baseline characteristics.
CRediT Authorship Contribution Statement
James A. Simon: Data curation, Investigation, Writing – review & editing. Elizabeth A. Stewart: Conceptualization, Writing – review & editing. Susan Jewell: Conceptualization, Methodology, Data curation, and Writing – review and editing. Moming Li: Data curation, Formal analysis, Writing – reviewing and editing. Michael C. Snabes: Writing – review & editing.
Declaration of Interests
J.A.S. has served as a consultant, speaker, and/or scientific advisor for Bayer (current), Besins (ended), California Institute of Integral Studies (CIIS; current), Carmargo (ended), Covance (current), Dare (current), DEKA M.E.L.A S.r.l. (current), Femasys (current), KaNDy/NeRRe (ended), Madorra (current), Mayne (current), Mitsubishi Tanabe (current), Myovant (current), Pharmavite (current), QUE Oncology (current), Sebela (ended), Sprout Pharmaceuticals (ended), and Vella Bioscience (current); and has received research support from AbbVie (current), Bayer (current), Dare (current), Ipsen, (current), Myovant (current), ObsEva (current), Sebela (ended), and Viveve (current). E.A.S. has received grant/research financial support from National Institutes for Health-related to uterine fibroids (R01HD60503, R01HD109127-01A1, and P50HS023418); served as a consultant for AbbVie, Alnylam Pharmaceuticals, Aska Pharmaceuticals, and Myovant; holds a patent for Methods and Compounds for Treatment of Abnormal Uterine Bleeding (US Patent 6440445), which has no commercial activity; and has received royalties from UpToDate and payments for the development of educational content MED-IQ, Omnia, Omnicuris, Physicians Educational Resources, and Web-MD; serves as an unpaid advisor to the Fibroid Foundation and unpaid treasurer of the International Gynecologic Society. S.J. has reported serving as a full-time employee of AbbVie Inc. and may hold AbbVie Inc. stock and/or options. M.L. has reported serving as a full-time employee of AbbVie Inc. and may hold AbbVie Inc. stock and/or options. M.C.S. has reported serving as a full-time employee of AbbVie Inc. and may hold AbbVie Inc. stock and/or options.
Acknowledgments
AbbVie Inc. and the authors thank all the trial investigators and the patients who participated in this clinical trial. Medical writing support, funded by AbbVie Inc., was provided by Kerren Davenport, B.Sc., and Ray Beck, Jr., Ph.D., of JB Ashtin.
Footnotes
Supported by AbbVie Inc., North Chicago, Illinois, and participated in the study design, research, data collection, analysis, interpretation of the data, reviewing, and approval of publication.
AbbVie Inc., North Chicago, Illinois, is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/, then select “Home.”
Supplemental data
References
- 1.Stewart E.A. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 2.Aninye I.O., Laitner M.H. Uterine fibroids: assessing unmet needs from bench to bedside. J Womens Health (Larchmt) 2021;30:1060–1067. doi: 10.1089/jwh.2021.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uimari O., Subramaniam K.S., Vollenhoven B., Tapmeier T.T. Uterine fibroids (leiomyomata) and heavy menstrual bleeding. Front Reprod Health. 2022;4 doi: 10.3389/frph.2022.818243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peuranpää P., Heliövaara-Peippo S., Fraser I., Paavonen J., Hurskainen R. Effects of anemia and iron deficiency on quality of life in women with heavy menstrual bleeding. Acta Obstet Gynecol Scand. 2014;93:654–660. doi: 10.1111/aogs.12394. [DOI] [PubMed] [Google Scholar]
- 5.Kocaoz S., Cirpan R., Degirmencioglu A.Z. The prevalence and impacts heavy menstrual bleeding on anemia, fatigue and quality of life in women of reproductive age. Pak J Med Sci. 2019;35:365–370. doi: 10.12669/pjms.35.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soliman A.M., Margolis M.K., Castelli-Haley J., Fuldeore M.J., Owens C.D., Coyne K.S. Impact of uterine fibroid symptoms on health-related quality of life of US women: evidence from a cross-sectional survey. Curr Med Res Opin. 2017;33:1971–1978. doi: 10.1080/03007995.2017.1372107. [DOI] [PubMed] [Google Scholar]
- 7.Stewart E.A., Nowak R.A. Uterine fibroids: hiding in plain sight. Physiology (Bethesda) 2022;37:16–27. doi: 10.1152/physiol.00013.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaff W.D., Ackerman R.T., Al-Hendy A., Archer D.F., Barnhart K.T., Bradley L.D., et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328–340. doi: 10.1056/NEJMoa1904351. [DOI] [PubMed] [Google Scholar]
- 9.Oriahnn (elagolix, estradiol, and norethindrone acetate capsules; elagolix capsules) [package insert] AbbVie Inc.; 2023. https://www.rxabbvie.com/pdf/oriahnn_pi.pdf?_ga=2.133552776.70762558.1701285691-1478887843.1701285691 Available at: [Google Scholar]
- 10.Muneyyirci-Delale O., Archer D.F., Owens C.D., Barnhart K.T., Bradley L.D., Feinberg E., et al. Efficacy and safety of elagolix with add-back therapy in women with uterine fibroids and coexisting adenomyosis. F S Rep. 2021;2:338–346. doi: 10.1016/j.xfre.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hendy A., Bradley L., Owens C.D., Wang H., Barnhart K.T., Feinberg E., et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021;224(72):e1–e50. doi: 10.1016/j.ajog.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q., Ciebiera M., Bariani M.V., Ali M., Elkafas H., Boyer T.G., et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr Rev. 2022;43:678–719. doi: 10.1210/endrev/bnab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart E.A., Cookson C.L., Gandolfo R.A., Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124:1501–1512. doi: 10.1111/1471-0528.14640. [DOI] [PubMed] [Google Scholar]
- 14.Stewart E.A., Nicholson W.K., Bradley L., Borah B.J. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 2013;22:807–816. doi: 10.1089/jwh.2013.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Drug Administration . Rockville, MD; 2009. Patient-reported outcome measures: use in medical product development to support labeling claims. Guidance for industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.