Abstract
Purpose
A systematic review to identify treatment approaches for the management of pain following peripheral nerve injury.
Methods
A published literature search was performed for the concepts of peripheral nerve injury and pain management with related synonyms. The strategies were created using a combination of controlled vocabulary terms and keywords and were executed in Embase.com, Ovid-Medline All, and Scopus from database inception. Database searches were completed on August 22, 2023.
Results
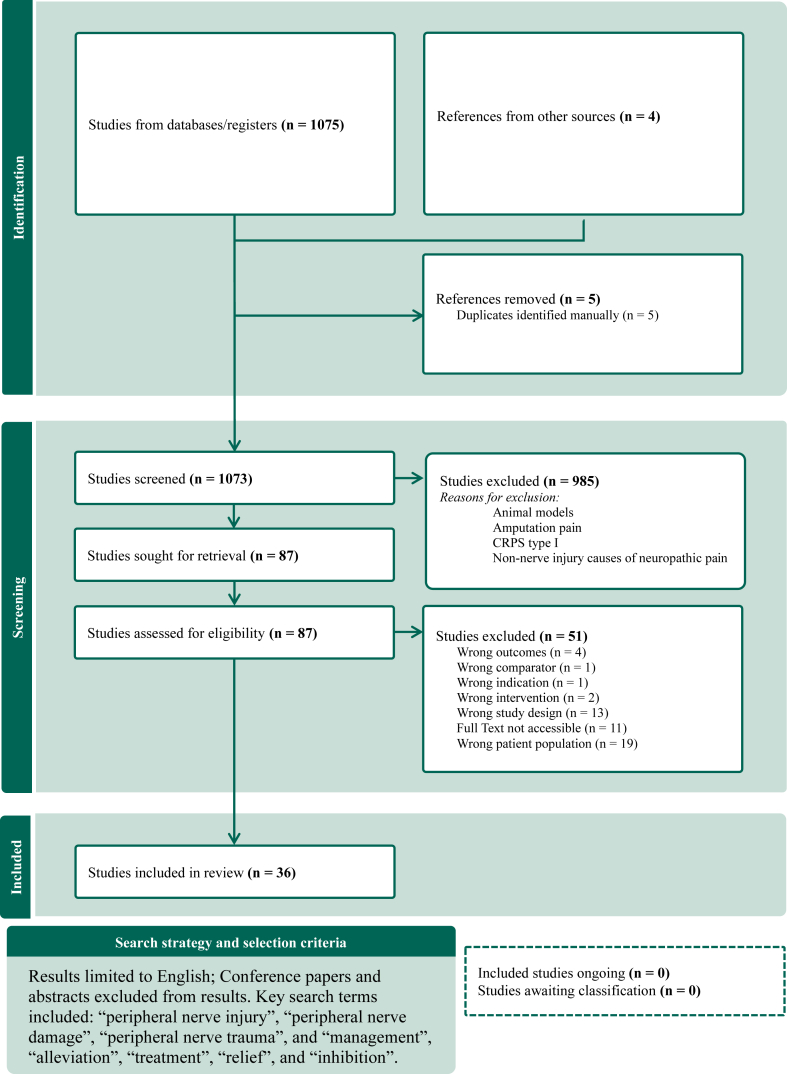
The initial search resulted in a total of 1,793 citations. In total, 724 duplicates were removed, leaving 1,069 unique citations remaining for analysis. This review excluded all papers that were not specific to pain following peripheral nerve injury. Case and cohort studies (n < 5) were also excluded. Thirty-two articles on pain management strategies after peripheral nerve injury remained, with years of publication ranging from 1981 to 2023. An additional four articles were identified by manual search. Of the 36 articles reviewed, 15 articles reported on the approach to the treatment of pain after a peripheral nerve injury, and the other 22 articles consisted of cohort and case series studies.
Conclusions
There is a lack of literature describing efficacy of various treatment strategies for pain following peripheral nerve injuries. Few studies provide clear, stepwise clinical guidance for practicing physicians and other health care providers on the treatment of these complicated patients.
Key words: Nerve grafting, Nerve injury, Nerve reconstruction, Nerve repair, Neuropathic pain
Peripheral nerve injury results in a variety of symptoms, including motor deficits, sensory disturbances, and pain. Of these, pain is the most poorly understood by clinicians and researchers. Pain and suffering following peripheral nerve injury poses a challenge to many health care members, including surgeons, psychologists, pain management specialists, and physical and occupational therapists.1 Analysis of patients with chronic pain through a biopsychosocial lens found that nerve-related pain had a pervasive impact on patients’ work, family, social network, psychologic state, and health-related quality of life.2 Pain from a peripheral nerve injury affects physical and emotional functioning, sleep, and global quality of life.3 Beyond the scope of patient mental health and quality of life, pain symptoms in patients contribute to a significant health care burden, including cost of health care and resource utilization.4 Given the prevalence of peripheral nerve injury and the influence pain has on patients’ lives, we performed a systematic review to assess the currently available treatment approaches and options to address pain after peripheral nerve injury.
Materials and Methods
Literature survey and results
We searched the published literature using strategies designed by a medical librarian for the concepts of peripheral nerve injury and pain management with related synonyms (Fig. 1). The strategies were created using a combination of controlled vocabulary terms and keywords and were executed in Embase.com, Ovid-Medline All, and Scopus from database inception. Database searches were completed on August 22, 2023. A total of 1,793 results were retrieved from the database literature search and imported to Endnote. In total, 724 duplicate citations were identified and removed. Case and cohort studies (n < 5) were excluded during screening. Thirty-two of the 1,037 remaining articles were included in the analysis. An additional four citations were included by manual search. The citations were uploaded to Covidence screening software for further review and composition of study selection diagram (Fig. 1). This rapid review was performed and abides by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) methodology.5
Figure 1.
Study selection.
Inclusion and exclusion
All peer-reviewed studies reporting specifically on the treatment of pain after nerve injury were included. Our study excluded articles that addressed the management of chronic regional pain syndrome type I, chronic postsurgical pain without an identifiable nerve lesion, and entrapment neuropathies. Case reports and small cohort studies (n < 5 participants) were excluded.
Study screening
Title, abstract, and full-text screening was conducted by two reviewers independently. Conflicts between the reviewers were resolved after discussion in both the title-abstract phase and during full-text review. Data extraction was performed by one of the two original reviewers and another author.
Search strategy
Our search terms included the following phrases: “(exp Peripheral Nerve Injuries/ OR (("peripheral nerve∗" adj2 injur∗) OR ("peripheral nerve∗" adj2 damage∗) OR ("peripheral nerve∗" adj2 trauma∗)).ti,ab,kf.) AND (exp Pain Management/ OR ((pain adj2 management∗) OR (pain adj2 alleviat∗) OR (pain adj2 treatment∗) OR (pain adj2 prevention∗) OR (pain adj2 relief∗) OR (pain adj2 inhibition∗)).ti,ab,kf.)”. Peripheral nerve damage results in a variety of potential sequelae with unique identifiers such as “neuromas” and “post-traumatic peripheral neuropathic pain.” These phrases were not specifically included in our search and therefore may not capture all treatment methods used for nerve injuries.
Results
For our review, we classified studies into two groups: 1) articles that addressed general treatment concepts and approaches and 2) articles that used cohorts and case series to demonstrate effectiveness of a given treatment.
Treatment concepts and approaches
Surgery
Surgical intervention was brought up as a cornerstone of treatment for many instances of neuropathic pain, but was consistently considered after other less invasive techniques were attempted. The most common approach described was operative neurolysis, with dorsal root entry zone (DREZ) lesioning the most frequently discussed approach to neurolysis.6, 7, 8, 9, 10, 11, 12 Neuroma and nerve resection with and subsequent transposition or nerve graft repair was also commonly mentioned, although the literature varied in whether muscle or bone was a preferable site for nerve transposition.6, 7, 8, 9, 10, 11,13,14 Less frequently referenced operative procedures included decompression of the nerve, sympathectomy, and nerve capping.6,9, 10, 11,15
Stimulation
Peripheral nerve stimulation, spinal cord stimulation, deep brain stimulation, and motor cortex stimulation were the most frequently mentioned invasive methods of stimulation.6, 7, 8, 9,11,12,14, 15, 16 Transcutaneous electrical neuromuscular stimulation was found to be a noninvasive method of stimulation for pain after nerve injury.12,14,16,17 One paper mentioned a stimulatory procedure termed “electroacupuncture,” although the authors noted it had a larger role in treatment of acute pain.16
Injections
Injections of a variety of mediums were included in the conservative approach of 10 different papers. Nerve blocks with local anesthetic proximal to the lesion were most frequently done with either lidocaine or bupivacaine, although Jaeger et al10 and Neumann16 both note a reported improvement when the anesthetic is coadministered with steroids.7,8,11,12,14 When less aggressive injections failed to provide relief or if there was concern for sympathetic activation of the injured nerves, sympathetic blocks were used.9,11,15,16,18 Epidural injections, local anesthetic injections, and chemical neurolysis at the site of injury were mentioned with the same frequency as sympathetic nerve blocks (Table 1). Local Botox injections were mentioned in one paper and described as an evolving tool in the treatment of pain.15
Table 1.
Treatment Concepts
| Treatment approach | # of papers | |
|---|---|---|
| Surgery, n = 11 | ||
| Operative neurolysis | 8 | |
| Resection + transposition | 6 | |
| Resection + repair | 6 | |
| Decompression | 4 | |
| Sympathectomy | 3 | |
| Nerve capping | 2 | |
| Resection alone | 1 | |
| Pharmacologic, n = 11 | ||
| Antidepressants | 11 | |
| Steroids | 11 | |
| Antiepileptics | 11 | |
| Opioids | 10 | |
| NSAIDs | 5 | |
| Topical analgesics | 5 | |
| Calcium channel blockers | 3 | |
| Sympatholytics/alpha antagonists | 2 | |
| Phenothiazine | 2 | |
| Bisphosphonates | 1 | |
| Ketamine | 3 | |
| Reserpine | 1 | |
| Barbiturates | 1 | |
| Injections, n = 10 | ||
| Nerve blocks | 5 | |
| Stellate nerve block | 3 | |
| Epidural block | 3 | |
| Neurolytic injections | 3 | |
| Anesthetic injections | 3 | |
| Botox | 1 | |
| Stimulation, n = 10 | ||
| Peripheral nerve stimulation | 5 | |
| TEN stimulation | 4 | |
| Spinal cord stimulation | 8 | |
| Deep brain stimulation | 3 | |
| Motor cortex stimulation | 2 | |
| Physical therapy, n = 12 | ||
| Exercise therapy | 8 | |
| Desensitization | 5 | |
| Occupational therapy | 4 | |
| Splinting | 2 | |
| Physiotherapy | 2 | |
| Psychiatric, n = 6 | ||
| Psychotherapy, general | 6 | |
| Cognitive behavioral therapy | 2 | |
| Biofeedback | 2 |
n, number of articles that reported on the treatment category; TEN, transcutaneous electrical nerve.
Pharmacy
Conservative treatment of neuropathic pain with pharmacologic intervention as a first-line approach was a uniting theme among papers regarding treatment of nerve lesions. The most common mentioned drug classes were antidepressants, antiepileptics, and steroids (Table 1). Although varied, most of these papers specified tricyclic antidepressants as the antidepressant of choice and gabapentin or carbamazepine as the antiepileptic of choice.6, 7, 8, 9,13,15,16 Opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) were also common analgesics, with multiple papers encouraging limited narcotic implementation given the risk for addiction or reliance.7,9,10,16 Although opioids were frequently mentioned as an option, there is a discrepancy between literature on the long-term efficacy of opioids in treating neuropathic pain.6,7 Topical analgesics, such as lidocaine and capsaicin, were suggested in two studies.7,15 Less common pharmaceutical interventions included calcium channel blockers, alpha antagonists, phenothiazines, ketamine, reserpine, and bisphosphonates, and barbiturates.8,9,11,15,16
Physical therapy
Physical therapy (PT) as part of the pain management regimen was mentioned repeatedly, but few papers went into depth on the actual treatment prescribed. Exercise therapy, including range of motion movements and resistance activities, was the most common protocol recommended, although many papers simply acknowledged the role of consulting PT without providing more insight.6,9,12, 13, 14,16,17,19 In a similar vein, many authors noted the need for occupational therapy to be engaged with patient care, but did not mention therapy goals or specifics of collaboration.6,8,16,19 The role of static and dynamic splinting and physiotherapy, or manual therapy, were each noted by two of the 12 papers that discussed PT.8,9,14,17
Psychological and psychiatric care
Although the correlation between psychological factors and the onset and maintenance of neuropathic pain was heavily acknowledged, mental health treatment was the least commonly addressed intervention, with only six of the 15 papers mentioning psychotherapy.6,12,14,16,19,20 Of the papers that explored mental health treatment, the general term “psychotherapy” was often referred to without specific recommendations on the type of psychotherapy. The specific therapeutic approaches that were noted in a few of the papers included cognitive behavioral therapy, biofeedback, and positive mindfulness through body scanning and meditation.12,14,16,20 Of note, some papers acknowledged the dual effect of the antidepressants used in analgesia and in treating associated psychiatric illness, although the dose for treating pain is often lower than the therapeutic threshold for treating depression or anxiety.7,15
Treatment sequence
Although almost every paper indicated that PT, pharmaceutical management, and stimulation therapies should be attempted before advancing to surgery for most cases of neuropathic pain, only five of the 15 reported a sequence of interventions to follow in treating pain.12, 13, 14, 15, 16 The treatment algorithm for managing pain after nerve injury started with the use of NSAIDs and transitioned to anticonvulsants or antidepressants next. If pain remains, opioids can be considered. Two papers specified attempting nerve blocks or injected anesthesia before moving from pharmaceuticals to surgical management.15,16 Notably, four papers discussed the role of preventative measures that can be taken by the surgical team to reduce incidence and recurrence of nerve lesions and postoperative neuromas, including appropriate surgical site analgesia preoperatively and ensuring nerves injured during the procedure are repaired or transposed resected.6,10,13,15
Cohort and case series studies (number of articles, n = 22)
Treatment types
For the cohort and case series studies, we have listed the chosen pain measurement variable (visual analog scale (VAS), numerical rating scale (NRS), clinical evaluation, etc.) used by the study along with the portion of the participants that experienced complete pain resolution, pain reduction, no change, and worsening of pain after treatment (Table 2). Most of the studies that used numerical and ordinal measures of pain defined their own thresholds for what made treatment a “success” or “failure.” For example some reported a >30% reduction in VAS at the final follow-up was considered a “success.” For the purpose of our study, the data extracted represent the portion of the cohort that experienced complete pain resolution, overall reduction in pain, no change in pain, or worsening of pre-existing pain.
Table 2.
Surgical Techniques and Outcomes
| Surgical | Final Follow-up | n | Pain outcome measure | Resolution | Reduction | No change | Worsening | Discontinued | Lost to Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Decrouy-Duruz 201822 | Neurolysis, neurectomy, translocation | 68 months (mean) | 231 | VAS | 0 | 111 | 14 | 2 | 0 | 104 |
| Henriques 202321 | Neurolysis, neurectomy | 12 months | 20 | VAS | 5 | 15 | 0 | 0 | 0 | 0 |
| Barrett 199923 | Neurolysis, neurectomy | 28 months (mean) | 13 | Binary pain rating | 9 | 0 | 0 | 4 | 0 | 0 |
| Yamashita 199824 | Nerve resection | 91.2 months (mean) | 20 | Clinical evaluation | 5 | 15 | 0 | 0 | 0 | 0 |
| Guo 202025 | Reconstruction | 12.45 months (mean) | 38 | VAS, pain symptom type, pain location | 0 | 20 | 0 | 10 | 0 | 8 |
| Rodriguez-Collazo 202144 | Conduit assisted allograft neurorrhaphy | 30.86 months (mean) | 36 | VAS | 0 | 36 | 0 | 0 | 0 | 0 |
| Ruiz-Juretschke 201126 | DREZ lesioning for BP avulsion | Not specified for subgroup | 8 | VAS | 2 | 6 | 0 | 0 | 0 | 0 |
| 366 | Total | 21 | 203 | 0 | 16 | 0 | 112 |
| Stimulation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nashold Jr. 198227 | Intraoperatively place direct nerve stimulator | 4 to 9 years | 35 | Report of pain reduction | 0 | 15 | 20 | 0 | 0 | 0 |
| Novak 200045 | Intraoperatively placed direct nerve stimulator | 21 months (mean) | 17 | VAS (0–100) | 1 | 16 | 0 | 0 | 0 | 0 |
| Kretzschmar 202128 | Dorsal root ganglion stimulator | 36 months | 27 | VAS (0–100) | 0 | 23 | 0 | 0 | 4† | 0 |
| Bouche 201746 | Percutaneous electrical nerve stimulation | 27.5 months (mean) | 26 | Percent of pain improvement | 0 | 20 | 0 | 0 | 4 | 2 |
| Sierakowski 201629 | Transcutaneous electrical nerve stimulation | 19 months (mean) | 72 | VAS (0–100) | 8 | 27 | 31 | 6 | 0 | 0 |
| Johnson 201530 | Transcutaneous electrical nerve stimulation | 6 weeks | 8 | NRS (0–10) | 0 | 4 | 1 | 0 | 3 | 0 |
| 185 | Total | 9 | 105 | 52 | 6 | 11 | 2 |
| Injection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meyer-Friesem 201931 | Perineural injection of botulinum-A toxin | Up to 7 days after injection | 60 | NRS (0-10) | 0 | 41 | 9 | 10 | 0 | 0 |
| 60 | Total | 0 | 41 | 9 | 10 | 0 | 0 |
| Pharmaceutical | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gordh 200833 | Gabapentin vs placebo | 5 weeks | 120 | VAS and pain relief score | 0 | 44 | 54 | 0 | 11 | 0 |
| Jenkins 201237 | Pregabalin vs placebo∗ | 2 weeks | 23 | NRS (0–10) | - | - | - | - | - | - |
| van Seventer 201035 | Pregabalin vs placebo | 8 weeks | 127 | NRS (0–10) | - | - | - | - | - | - |
| Markman 201836 | Pregabalin vs placebo∗ | 3 months | 274 | NRS (0–10) | - | 191 | - | - | - | - |
| Kelle 201232 | Gabapentin vs pregabalin∗ | 3 months | 30 | VAS and LANSS pain scale | - | - | - | - | - | - |
| 150 | Total | 0 | 235 | 54 | 0 | 11 | 0 |
| Other | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Barrett 199923 | Multiapproach conservative | 28 months (mean) | 14 | Binary pain rating | 8 | 0 | 6 | 0 | 0 | 0 |
| Correa-Illanes 201238 | 5% Lidocaine medicated plaster | 19.5 weeks (mean) | 19 | NRS, painful area size | 0 | 19 | 0 | 0 | 0 | 0 |
| 33 | 8 | 19 | 6 | 0 | 0 | 0 | ||||
| Total n = | 810 | Total | 38 | 603 | 121 | 32 | 22 | 114 | ||
| Valid percent‡ | 5% | 64% | 16% | 4% | 3% | 15% |
BP, brachial plexus; LANSS, Leeds assessment of neuropathic symptoms and signs.
Studies reported mean reduction in VAS. No data reported on what percent of cohort experienced changes in their pain before and after treatment.
Two patients never had the stimulator device placed; two had the device explanted at 1 year.
Valid percent reported.
Surgery (number of articles, n = 7)
Cohort studies evaluating surgery as a treatment choice for chronic pain after nerve injury included a total of 366 participants. Of the seven cohort studies that reported postsurgical outcomes, three of them studied neurectomy with neurolysis.21, 22, 23 The total number of participants treated with this approach was 264, making it the most reported upon surgical technique. Similar to that of neurectomy, complete nerve resection was studied by Yamashita et al,24 who reported that all patients treated with this technique experienced pain reduction with the mean final follow-up being 91.2 months ranging from 24 to 216 months. Comparatively, a study of brachial plexus reconstruction by Guo et al25 demonstrated that 10 out of 20 patients reported worsening of pain after their operation. This study’s mean final follow-up was only 12.45 months ranging from 10 to 14 months. This exhibits the importance of preoperative expectation management with patients as the recovery time after nerve surgery is lengthy and can even be more painful than the original injury. One study found that the use of DREZ lesioning in patients with brachial plexus avulsions reduced pain in all eight patients but did not specify this group’s follow-up period.26 The study also reported the outcome of DREZ lesioning for treatment of other causes of chronic pain, such as Complex Regional Pain Syndrome type I, neoplastic nerve invasion, and spinal cord trauma. Out of the entire studied cohort (n = 18), 22% of patients experienced complications, such as temporary alteration of proprioception and vibratory sense, wound drainage issues, and one permanent spinal cord injury.
Stimulation (n = 6)
Studies on the use of nerve stimulation techniques composed the next biggest composite cohort of 185 patients. Three studies reviewed the effectiveness of surgical implantation of a peripheral nerve stimulator directly beneath or next to the injured nerve. A study from 1982 reported mixed results with 20 out of 35 patients noting no change in pain after implantation.27 Two studies have been reported since 2000, with the most recent demonstrating pain relief of at least 40% for up to 2 years in 20 of the 26 patients (76%).28,29 Of the remaining three articles assessing stimulation techniques, one investigated dorsal root ganglion (DRG) stimulation, whereas the other two evaluated external, noninvasive stimulation.28, 29, 30 The 36-month study using DRG stimulation included a cohort of patients with upper or lower extremity nerve injuries.28 In this study, DRG leads were placed in the dermatome corresponding to the nerve injury in the cervical, thoracic and/or lumbar region.28 Placing DRG leads above T10 is not United States Food and Drug Administration approved and not routinely practiced, thus limiting the scope of this treatment to lower extremity peripheral nerve injuries. It should be mentioned that both peripheral nerve stimulation and spinal cord stimulation technology is rapidly advancing in recent years, and these articles do not reflect the technology in current use.
Injections (n = 1)
One study evaluated Botulinum-A toxin for painful peripheral nerve injury. Test nerve blocks using local anesthetics were performed to identify the affected nerve. Successful nerve identification was confirmed by 100% relief of pain and diminished pinprick sensation in the nerve’s distribution. Once the affected nerves’ territory was successfully identified, Botulinum-A toxin was injected at the same site deemed successful by the test blocks. In painful peripheral nerve injury patients, Botulinum-A toxin provided >50% pain relief in 14 (23%), but provided no change to 9 (15%) and worsening pain in 10 (16%) patients.31
Pharmacologic treatment (n = 6)
Our search identified two randomized clinical trials and an additional manual search resulted in the identification of a meta-analysis analyzing three additional pharmaceutical trials for our patient population32, 33, 34, 35, 36, 37. All trials shared the same primary endpoint of mean pain score on either the VAS or the NRS. Pregabalin was compared with placebo in three trials, and gabapentin was compared with placebo in one trial.33,35, 36, 37 Two of the three trials reported “significant” pain improvement in the pregabalin arms.35,37 The mean treatment difference noted in these trials were both less than −1 point on the 11-point NRS and had confidence intervals maximally ranging from −1.45 to −0.17. The third pregabalin study found no significant improvement in the treatment arm versus placebo (treatment difference of −0.22, 95% confidence interval −0.54 to 0.10)36. The same study reported the percent of patients achieving 30% or 50% pain relief at various time intervals with inconsistent significance throughout; however, at final follow-up, there were no differences in percent of patients achieving relief to either degree (30% or 50%) compared with placebo. Gabapentin was compared with placebo and pregabalin.32,33 Gabapentin did not show a significant change in mean VAS pain score compared with placebo. However, gabapentin had a higher response to treatment rate if the response was defined by 30% relief. This difference in response to treatment diminished if “response to treatment” was defined as 50% pain relief. Gabapentin was subsequently compared with pregabalin, and no clear superiority in the reduction of pain was found between the treatments.32 The most common adverse effects noted was somnolence and dizziness.
Other and multiple intervention comparison (n = 2)
A treatment method for pain attributed to nerve injury that was not otherwise mentioned else where was the use of 5% lidocaine medicated plaster.38 At the end of 19 weeks, participants using the 5% lidocaine medicated plaster reported a reduction in pain, and 11 participants were using the plaster as monotherapy. The other study included in this section is a retrospective cohort study by Barrett et al23 comparing patients treated with conservative and surgical management for their peripheral nerve injuries. All patients in the study had received some type of conservative treatment with 14 managed nonsurgically and 13 with surgery.23 Of the nonsurgical patients, eight had complete resolution of their pain, and six reported no change at a mean follow-up time of 28 months. Conservative methods for treatment included the following 1) NSAIDs, topical capsaicin, local anesthetics, and/or steroid injections with physical therapy; 2) a period of immobilization; and 3) orthotics, braces, or splints with PT.
Discussion
The treatment of pain after peripheral nerve injury can involve both invasive and noninvasive methods. In our review, we found that no studies have been done to compare the efficacy of anticonvulsants and antidepressants in treating pain after nerve lesions despite these being considered first-line therapies. Gabapentin and pregabalin were the only two drugs to have undergone a clinical trial for assessment of their effectiveness at treating pain after nerve injury.32,33 Our prior qualitative work suggests there may be an underlying negative sentiment toward these medications in this patient population attributed to their demonstrated and anecdotal lack of efficacy and high rates of adverse effects.39 All trials comparing pregabalin to placebo demonstrated negligible clinical differences in mean pain score with treatment difference being less than −1 in all trials regardless of statistical significance for their primary endpoints.35, 36, 37 Given that the minimal clinically important difference for pain reduction on the NRS is generally accepted as a 2-point difference in musculoskeletal care, it is important to evaluate the clinical efficacy of gabapentinoids in this population.40 The use of gabapentinoids for neuropathic pain after traumatic peripheral nerve injury is not approved by the United States Food and Drug Administration and considered an off-label use.41 The use of opioids was mentioned as a last line pharmacologic treatment method given concern for reliance and addiction, a concern shared by many patients.39 Future research efforts for treating pain after nerve injury should include larger clinical trials comparing the efficacy of different drug classes and the development of drugs that more adequately target the molecular mechanisms behind nerve pain.
One of the articles included in this review was a survey of 59 peripheral nerve surgeons. Although the surgeons identified psychosocial factors as being most important in the development of pain after nerve injury, it was the least addressed comorbidity within the publications included in this review. Of the studies that did not mention specific methods for managing mental health, many still emphasized the importance of mental health but did not go on to recommend any specific solutions. There were no cohort studies assessing the effects of receiving adequate mental health care and how this may influence the experience of pain after peripheral nerve injury. Additionally, two cohort studies made comorbid psychiatric illness an exclusion criteria without clarification of what illnesses would be excluded.28,42
This review demonstrates that although surgery can be used as a method for the treatment of pain after nerve injury, it is usually recommended after failure of other more conservative treatment strategies. Surgical studies demonstrated the largest cohort of participants (n = 366), despite this being the last line of treatment. Conservative methods for treating pain after nerve injury frequently include a multidisciplinary approach that utilizes some combination of pharmaceuticals, injections, nerve stimulation, and physical and occupational therapy, a strategy that mirrors the common approach to care of patients with severe nerve injuries.43 However, our study was not able to compose a treatment algorithm to guide the approach to pain after nerve injury because of the lack of literature focused on pain in this specific population. Overall, there is an emphasis on the importance of mental health as it relates to pain but little guidance on how this should be approached specifically in patients with peripheral nerve injuries. Psychologic interventions are well-established as a method of treatment for chronic pain, but these types of interventions are not well-integrated into care guidelines for patients with pain from a peripheral nerve injury.1 Given patients with nerve injuries frequently go on to develop chronic pain, we should aim to incorporate a biopsychosocial treatment approach early on in the care of these patients by using some of the treatment strategies deployed in chronic pain management like cognitive behavior therapy, acceptance and commitment therapy, and biofeedback.
In light of the findings in this review, we believe that future investigations should be focused on how we can (1) provide better psychosocial support for these patients and (2) recommend more appropriate pharmacologic therapies. The journey of understanding what these patients need most from us as clinicians starts with a patient-centered and patient-driven assessment of individual needs and expectations. After gaining a better understanding of how these patients face and live with their pain, we can begin efforts to develop outcome measures and treatment regimens that address their values and priorities pertaining to pain after nerve injury.
Conflicts of Interest
No benefits in any form have been received or will be received related directly to this article.
References
- 1.Cohen S.P., Vase L., Hooten W.M. Chronic pain: an update on burden, best practices, and new advances. The Lancet. 2021;397(10289):2082–2097. doi: 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 2.Dueñas M., Ojeda B., Salazar A., Mico J.A., Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457–467. doi: 10.2147/JPR.S105892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen M.P., Chodroff M.J., Dworkin R.H. The impact of neuropathic pain on health-related quality of life: Review and implications. Neurology. 2007;68(15):1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- 4.Pérez C., Navarro A., Saldaña M.T., Wilson K., Rejas J. Modeling the predictive value of pain intensity on costs and resources utilization in patients with peripheral neuropathic pain. Clin J Pain. 2015;31(3):273–279. doi: 10.1097/AJP.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 5.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roganovic Z., Mandic-Gajic G. Pain syndromes after missile-caused peripheral nerve lesions: part 2—treatment. Neurosurgery. 2006;59(6):1238–1251. doi: 10.1227/01.NEU.0000245618.16979.32. [DOI] [PubMed] [Google Scholar]
- 7.Davis G., Curtin C.M. Management of pain in complex nerve injuries. Hand Clinics. 2016;32(2):257–262. doi: 10.1016/j.hcl.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Gebreyohanes A.M.H., Ahmed A.I., Choi D. Dorsal root entry zone lesioning for brachial plexus avulsion: a comprehensive literature review. Operative Surg. 2021;20(4):324–333. doi: 10.1093/ons/opaa447. [DOI] [PubMed] [Google Scholar]
- 9.Wilson R.L. Management of pain following peripheral nerve injuries. Orthop Clin North Am. 1981;12(2):343–359. [PubMed] [Google Scholar]
- 10.Jaeger S.H., Singer D.I., Whitenack S.H. Nerve injury complications. Hand Clinics. 1986;2(1):217–234. [PubMed] [Google Scholar]
- 11.Rajput K., Reddy S., Shankar H. Painful neuromas. Clin J Pain. 2012;28(7):639–645. doi: 10.1097/AJP.0b013e31823d30a2. [DOI] [PubMed] [Google Scholar]
- 12.Rothemeyer S.J., Enslin J.M.N. Surgical management of pain. S Afr Med J. 2016;106(9):858. [Google Scholar]
- 13.Nath R.K., Mackinnon S.E. Management of neuromas in the hand. Hand Clinics. 1996;12(4):745–756. [PubMed] [Google Scholar]
- 14.Li H., Chen J., Wang J., Zhang T., Chen Z. Review of rehabilitation protocols for brachial plexus injury. Front Neurol. 2023;14 doi: 10.3389/fneur.2023.1084223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll I., Curtin C.M. Management of chronic pain following nerve injuries/CRPS type II. Hand Clinics. 2013;29(3):401–408. doi: 10.1016/j.hcl.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Neumann M.M. Nonsurgical management of pain secondary to peripheral nerve injuries. Orthop Clin North Am. 1988;19(1):165–174. [PubMed] [Google Scholar]
- 17.Bond T.J., Lundy J. Physical therapy following peripheral nerve surgeries. Clin Podiat Med Surg. 2006;23(3):651–666. doi: 10.1016/j.cpm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Choi E.J., Choi Y.M., Jang E.J., Kim J.Y., Kim T.K., Kim K.H. Neural ablation and regeneration in pain practice. Korean J Pain. 2016;29(1):3–11. doi: 10.3344/kjp.2016.29.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak C.B., Anastakis D.J., Beaton D.E., Katz J. Evaluation of pain measurement practices and opinions of peripheral nerve surgeons. Hand (New York, N,Y) 2009;4(4):344–349. doi: 10.1007/s11552-009-9177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covington T.J. Evaluation and treatment of pain. J Hand Ther. 1993;6(2):161–167. doi: 10.1016/S0894-1130(12)80298-5. [DOI] [PubMed] [Google Scholar]
- 21.Henriques V.M., Torrão F.J.L., Rosa L.A.N., Sanches G.E., Guedes F. Surgery as an effective therapy for ulnar nerve neuropathic pain caused by gunshot wounds: a retrospective case series. World Neurosurgery. 2023;173:e207–e217. doi: 10.1016/j.wneu.2023.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Decrouy-Duruz V., Christen T., Raffoul W. Evaluation of surgical treatment for neuropathic pain from neuroma in patients with injured peripheral nerves. J Neurosurgery. 2018;128(4):1235–1240. doi: 10.3171/2017.1.JNS161778. [DOI] [PubMed] [Google Scholar]
- 23.Barrett J.P., Downey M.S., Hillstrom H.J. Retrospective analysis of neurapraxia and axonotmesis injuries of select peripheral nerves of the foot and ankle and their conservative and surgical treatment (external neurolysis and neurectomy) J Foot Ankle Surg. 1999;38(3):185–193. doi: 10.1016/s1067-2516(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita T., Ishii S., Usui M. Pain relief after nerve resection for post-traumatic neuralgia. J Bone Joint Surg Br. 1998;80(3):499–503. doi: 10.1302/0301-620x.80b3.8370. [DOI] [PubMed] [Google Scholar]
- 25.Guo J., Gao K., Zhou Y., Zhao X., Lao J. Comparison of neuropathic pain characteristics associated with total brachial plexus injury before and after surgical repair: A retrospective study. Clin Neurol Neurosurg. 2020;191 doi: 10.1016/j.clineuro.2020.105692. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Juretschke F., García-Salazar F., García-Leal R., et al. Treatment of neuropathic deafferentation pain using DREZ lesions; long-term results. Neurología (English Edition) 2011;26(1):26–31. doi: 10.1016/j.nrl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Nashold B.S. Long-term pain control by direct peripheral-nerve stimulation. JBJS. 1982;64(1):1010. [PubMed] [Google Scholar]
- 28.Kretzschmar M., Reining M., Schwarz M.A. Three-year outcomes after dorsal root ganglion stimulation in the treatment of neuropathic pain after peripheral nerve injury of upper and lower extremities. Neuromodulation. 2021;24(4):700–707. doi: 10.1111/ner.13222. [DOI] [PubMed] [Google Scholar]
- 29.Sierakowski A., Jing S.S., Poel J., Elliot D. Transcutaneous peripheral nerve stimulation for the treatment of neuropathic pain in the upper limb. J Hand Surg Asian-Pac Vol. 2016;21(1):37–43. doi: 10.1142/S2424835516500041. [DOI] [PubMed] [Google Scholar]
- 30.Johnson S., Ayling H., Sharma M., Goebel A. External noninvasive peripheral nerve stimulation treatment of neuropathic pain: a prospective audit. Neuromodulation. 2015;18(5):384–391. doi: 10.1111/ner.12244. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Frießem C.H., Eitner L.B., Kaisler M., et al. Perineural injection of botulinum toxin-A in painful peripheral nerve injury – a case series: pain relief, safety, sensory profile and sample size recommendation. Curr Med Res Opin. 2019;35(10):1793–1803. doi: 10.1080/03007995.2019.1626228. [DOI] [PubMed] [Google Scholar]
- 32.Kelle B., Yavuz F., Yasar E., Goktepe A.S. The efficacy of gabapentin and pregabalin in the treatment of neuropathic pain due to peripheral nerve injury. J Musculoskeletal Pain. 2012;20(4):300–305. [Google Scholar]
- 33.Gordh T.E., Stubhaug A., Jensen T.S., et al. Gabapentin in traumatic nerve injury pain: a randomized, double-blind, placebo-controlled, cross-over, multi-center study. Pain. 2008;138(2):255–266. doi: 10.1016/j.pain.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Maleki M.S., Zamani Z., Amiri R., et al. Pregabalin in patients with post-traumatic peripheral neuropathic pain: a meta-analysis of randomized controlled trials. Pain Practice. 2023;23(6):595–602. doi: 10.1111/papr.13221. [DOI] [PubMed] [Google Scholar]
- 35.Van Seventer R., Bach F.W., Toth C.C., et al. Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol. 2010;17(8):1082–1089. doi: 10.1111/j.1468-1331.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- 36.Markman J., Resnick M., Greenberg S., et al. Efficacy of pregabalin in post-traumatic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 trial. J Neurol. 2018;265(12):2815–2824. doi: 10.1007/s00415-018-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins T.M., Smart T.S., Hackman F., Cooke C., Tan K.K. Efficient assessment of efficacy in post-traumatic peripheral neuropathic pain patients: pregabalin in a randomized, placebo-controlled, crossover study. J Pain Res. 2012;5:243–250. doi: 10.2147/JPR.S34098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correa-Illanes G. Use of 5% lidocaine medicated plaster to treat localized neuropathic pain secondary to traumatic injury of peripheral nerves. Local Reg Anesth. 2012;5:47–53. doi: 10.2147/LRA.S31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smolev E.T., Rolf L., Zhu E., et al. “Pill pushers and CBD oil”—a thematic analysis of social media interactions about pain after traumatic brachial plexus injury. J Hand Surg Glob Online. 2020;3(1):36–40. doi: 10.1016/j.jhsg.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salaffi F., Stancati A., Alberto Silvestri C., Ciapetti A., Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Goodman C.W., Brett A.S. A clinical overview of off-label use of gabapentinoid drugs. JAMA Internal Medicine. 2019;179(5):695–701. doi: 10.1001/jamainternmed.2019.0086. [DOI] [PubMed] [Google Scholar]
- 42.Miclescu A., Schmelz M., Gordh T. Differential analgesic effects of subanesthetic concentrations of lidocaine on spontaneous and evoked pain in human painful neuroma: A randomized, double blind study. Scandinavian J Pain. 2015;8(1):37–44. doi: 10.1016/j.sjpain.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 43.Brogan D.M., Osei D.A., Colorado B.S., Sneag D.B., Van Voorhis A., Dy C.J. Team approach: management of brachial plexus injuries. JBJS Reviews. 2022;10(4):e21.00222. doi: 10.2106/JBJS.RVW.21.00222. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Collazo E., Laube Ward K. Conduit-assisted Allograft Neurorrhaphy for the Treatment of Intractable Lower Extremity Pain Due to Neuromas-in-continuity. Plast Reconstr Surg Glob Open. 2021;9(11) doi: 10.1097/GOX.0000000000003867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novak C.B., Mackinnon S.E. Outcome following implantation of a peripheral nerve stimulator in patients with chronic nerve pain. Plast Reconstr Surg. 2000;105(6):1967–1972. doi: 10.1097/00006534-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Bouche B., Manfiotto M., Rigoard P., Lemarie J., Dix-Neuf V., Lanteri-Minet M., Fontaine D. Peripheral nerve stimulation of brachial plexus nerve roots and supra-scapular nerve for chronic refractory neuropathic pain of the upper limb. Neuromodulation. 2017;20(7):684–689. doi: 10.1111/ner.12573. [DOI] [PubMed] [Google Scholar]