Abstract
Objective
To evaluate whether transvaginal radiofrequency (RF) ablation of fibroids is a technique that can be offered to women with reproductive desires.
Design
Unicentric, prospective, observational study.
Setting
University Hospital.
Patient(s)
Twenty-seven individuals who desired to become mothers after undergoing RF ablation for symptomatic fibroids.
Intervention(s)
Transvaginal RF ablation for symptomatic fibroids with a maximum total volume of 145 cm3.
Main Outcome Measure(s)
The reduction in the size of the fibroids, improvement of symptoms, and reproductive outcomes during the 24 months after the ablation. For patients who achieved pregnancy, we assessed the type of conception, course of gestation, type of delivery, neonatal outcomes, and occurrence of both maternal and fetal complications.
Result(s)
A statistically significant reduction in symptoms related to the fibroids 6 months after the ablation was demonstrated through the implementation of the Symptom Severity Scale. No patient required hospitalization after the procedure, and on average from the third day after intervention, they resumed their work activities without the need for analgesics. Among those patients who attempted pregnancy during the 24-month follow-up period, 73.68 % (14/19) achieved motherhood. There were no cases of uterine rupture, premature birth, or intrauterine fetal death.
Conclusion(s)
Radiofrequency ablation for fibroids seems to be a promising, safe, and low-complexity alternative that does not appear to interfere with the development of a normal term gestation.
Key Words: Fibroids, radiofrequency ablation, fertility, pregnancy
Fibroids or leiomyomas are the most frequent benign solid tumors in women of reproductive age, with a cumulative incidence at 50 years of between 70% in Caucasian women and 80% in African American women (1). Despite this high prevalence, their etiologic and prognostic factors remain unclear. Most women with fibroids remain asymptomatic; however, up to 25% of them experience symptoms that include menorrhagia, dyspareunia, secondary dysmenorrhea, pelvic pain, compressive symptoms, or infertility, originating an important public health problem (2).
Different treatments are described to improve their symptoms—from hormonal therapies to the use of interventional radiology or conservative surgeries, such as hysteroscopy or myomectomy. The definitive treatment is the hysterectomy, which is the second most frequent surgery in women after cesarean section and is associated with a significant morbidity and mortality and high economic expense (3, 4, 5, 6, 7).
The classic approaches, medical or surgical, seem insufficient to meet the needs of today’s society where more women demand conservative options that allow the preservation of the uterus (8, 9, 10, 11). The chronological delay of motherhood represents a significant number of patients with fertility problems who have uterine fibroids. These patients often have limited timeframes for achieving pregnancy because of their age, which further complicates the surgical approaches that require extended waiting times before allowing pregnancy (12). For this reason, in recent years, the development of less invasive alternatives has been promoted, such as radiofrequency (RF) ablation, which has already demonstrated its efficacy for the treatment of other solid tumors in the liver and other organs (13, 14).
The RF ablation technique is based on the application of a high frequency electrical current (400 kHz) that produces heat (>65°C) inside the fibroid, achieving coagulative necrosis or irreversible cellular death of the tissue and blood vessels (15). The necrotic tissue is subsequently reabsorbed, achieving reductions of 60%–80% of the fibroid’s volume in a period of 6–12 months after treatment (16). Radiofrequency ablation pursues 2 main objectives: to reduce the fibroid’s size and to improve the symptoms associated with the fibroids. Among the different approaches described for the application of RF, transvaginal access makes it possible to treat fibroids on an outpatient basis, with a minimum discomfort and a shorter recovery period.
The current scientific evidence published on the treatment of fibroids with RF ablation is scarce and heterogeneous (17, 18, 19, 20, 21, 22, 23, 24, 25, 26), and there are few studies that have assessed the safety of the technique in terms of reproductive results. Therefore, the aim of this study was to evaluate the symptomatic control and reproductive outcomes of patients who had previously undergone transvaginal RF ablation.
Materials and methods
Twenty-seven of the 78 women who underwent RF treatment for symptomatic fibroids at our center between July 2018 and September 2021 desired to become mothers. They were identified and included in our study. We analyzed the procedure outcomes and reproductive results of these patients for the subsequent 24 months after the ablation.
All patients who participated in this study were informed beforehand and provided their written consent for the anonymous processing of their data. We obtained approval from the ethics committee of our hospital for the processing of this data. All patients signed an informed consent form for the RF ablation technique, and in all cases, they were informed about the limited published studies supporting its use in women with reproductive desires.
Before the procedure, our patient’s symptoms were recorded according to the Symptom Severity Scale (SSS) (27, 28), a questionnaire used to evaluate the clinical impact associated with fibroids, and they all underwent complete gynecological examination and 2-dimensional Doppler ultrasound. Its purpose was to accurately classify the fibroids, specifying the number and their classification according to the International Federation of Gynecology and Obstetrics (FIGO) (29). Its type of vascularization was assessed following the classification of the Morphological Uterus Sonographic Assessment (MUSA) (30), and any other relevant findings of the genital tract were described. The measurements of the 3 largest orthogonal diameters of each fibroid were recorded to calculate their volume using the ellipsoid volume equation (4π/3 × a × b × c), expressed in cubic centimeters, being the variables a, b, and c, the measurements in centimeters of the 3 radii of the fibroid.
The criteria for indicating the RF ablation technique were established on the basis of previous studies (18, 19, 20) and our experience in clinical practice to date. The inclusion criteria considered were adult women with reproductive desires and with FIGO type 3, 4, 5, and 6 fibroids, with a maximum volume of ≤145 cm3, or multiple fibroids whose total volume did not exceed that value and FIGO type 0, 1, and 2 fibroids identified as high-complexity fibroids through hysteroscopy (Lasmar score, >5). In some cases, RF ablation served as a definitive treatment, whereas in others, it was used as a preliminary step to hysteroscopy to simplify and enhance the procedure.
The exclusion criteria were pelvic inflammatory disease within a period of <3 months, current pregnancy, abnormal results in endometrial biopsy, FIGO type 7 fibroids, fibroids of uncertain locations or with volumes of >145 cm3, and any suspicious uterine tumor lesion that did not meet criteria for benign behavior.
The surgical technique was performed under general anesthesia; thus, patients were requested to undergo a complete preoperative study beforehand. The surgical scheduling was conducted independently of the menstrual cycle and did not require any specific previous preparation. As a prophylactic measure, 2 g of intravenous cefazolin was administered before the procedure, along with dexamethasone (8 mg) for its anti-inflammatory effect. During the procedure, the patient was placed in a lithotomy position. As safety measures, 2 dispersive electrodes were placed on the inner side of both thighs, and the generator was connected to a bag of cold saline solution, which allowed for continuous cooling of the electrode system.
The RF generator used in our center was the STARmed Co. from JJP Hospitalaria S.L. (Sevilla, Spain), programmed at a power of 100 W for all ablations. The needle electrode used was 35 cm long and had a thickness of 17 gauges, with an active tip of 10 mm (REF 17-35s10F) (Supplemental Fig. 1, available online), and the ultrasound system used was a Philips Affiniti 70W. A metal guide of approximately 15–16 gauges was placed on the vaginal probe to allow the needle to slide without damaging its protective coating.
Because of the tissue destruction caused by the technique, ultrasound-guided biopsy was performed using a semiautomatic needle (M-Biopsy REF N 30118030 18 gauges, 30 cm in length) before starting the application of electrical current in all treated patients until January 2021. However, since then, the biopsy has been discontinued because it was considered to provide a low representative sample of the tumor and the ultrasound diagnosis was considered sufficient (assuming that all treated tumors were benign because RF is not indicated for fibroids that do not meet benign criteria).
The objective of the RF was to produce small areas of necrosis of 1 cm3 in the fibroid tissue, with a mean ablation time of approximately 10 seconds. We maintained a distance from the uterus serosa of at least 0.5 cm because of thermal dispersion effect. The number of ablations required to complete the treatment was determined by the fibroid’s volume.
The procedure was considered completed when the ultrasound confirmed an increase in the echogenicity of the fibroid by >80% or the absence of vascularization in the treated area. For this purpose, we administered intravenous contrast (SonoVue [sulfur hexafluoride microbubbles]) before and after the procedure because it allows for on-site verification of fibroid vascularization destruction (Supplemental Fig. 2).
The technique was performed on an outpatient basis, and all procedures were conducted by the same gynecologist. Written care recommendations were provided on discharge. For pain management, mild oral analgesics were prescribed as needed. No maintenance antibiotics were prescribed. Patients were advised to avoid high-impact exercises during a week after the surgery.
Postoperative follow-up visits were conducted at 1, 6, and 12 months after the procedure. During the first postoperative visit, the patient’s clinical progress was assessed, as well as the duration of analgesic use and work leave. At the 6-month follow-up, patients were asked to complete the SSS questionnaire once again, and at the 6- and 12-month visits, Doppler ultrasound was performed to assess the size, location, and vascularization of the treated fibroids (Fig. 1).
Figure 1.
(A) Fibroid types 2–4 before radiofrequency ablation. (B) Fibroid type 1 at 6 months after radiofrequency ablation.
Active attempts to conceive were not advised until 6 months after the treatment to achieve the best results in terms of fibroid size reduction and symptom control. The follow-up time of all these patients was 24 months after the RF ablation.
The variables that were collected included patient’s age at the time of ablation and conception, the number of treated fibroids, and their location, volume, vascularization, and FIGO classification. Obstetric history, symptoms (using the SSS before ablation and at 6 months after), ablation time in seconds, the need for analgesia and work leave, reduction in fibroid volume at 6 months, time to conception, course of pregnancy, gestational age, birth weight, type and mode of delivery, and presence of obstetric complications or uterine rupture were also recorded.
Descriptive data are presented as medians (ranges) for quantitative variables or numbers (percentage) for categorical variables. The SSS values before ablation were compared with those recorded 6 months after the procedure using the Wilcoxon signed rank test (after having checked the absence of normality in these variables using the Shapiro-Wilk test). A P value of <.05 was considered statistically significant. Data were analyzed using SPSS version 20 (IBM Inc., Chicago, IL).
Results
Among the 27 patients with reproductive desires who underwent RF treatment for their fibroids, the 2 main reasons for seeking medical attention were heavy menstrual bleeding unresponsive to other treatments in 66.67% (n = 18) of the cases and referrals from fertility units before starting assisted reproductive techniques in the other 33.33% (n = 9). Notably, all 9 of these patients also presented with heavy menstrual bleeding. The median age of the patients at the time of surgery was 36 years; 77.78% (21) were nulliparous, and 22.22% (6) were multiparous.
A total of 40 fibroids were treated. The baseline characteristics of the patients and fibroids treated are shown in Table 1.
Table 1.
Fibroid and baseline characteristics of 27 patients and transvaginal radiofrequency ablation outcomes.
| Patient characteristics | |
|---|---|
| Median age (and range) of the patients at the time of surgery | 36 y (29–44 y) |
| Obstetric history | |
| Nulliparous | 21 (77.78%) |
| Parous | 6 (22.22%) |
| Main symptom | |
| Heavy menstrual bleeding | 18 (66.67%) |
| Infertility | 9 (33.33%) |
| Fibroid characteristics | |
| Median number (and range) of fibroids treated per patient | 1 (1–5) |
| Total number of fibroids treated in 27 patients | 40 |
| FIGO classification | |
| 1 | 1/40 (2.5%) |
| 2 | 2/40 (5%) |
| 3 | 13/40 (32.5%) |
| 4 | 6/40 (15%) |
| 2–4 | 1/40 (2.5%) |
| 2–5 | 11/40 (27.5%) |
| 5 | 4/40 (10%) |
| 6 | 2/40 (5%) |
| Median maximum diameter (and range) of fibroids | 3 cm (0.8–7.4 cm) |
| Median volume (and range) of fibroids | 10.08 cm3 (0.3–133.83 cm3) |
| MUSA vascularization | |
| Type 2 | 35/40 (87.5%) |
| Type 3 | 5/40 (12.5%) |
| Transvaginal RF ablation outcomes | |
| Median duration (and range) of RF ablation per fibroid | 240 s (10–820 s) |
| Need for admission | 0 |
| Median duration (and range) of analgesic use | 3 d (2–4 d) |
| Median duration (and range) of sick leave | 3 d (3–5 d) |
| Major complications | 0 |
| Median reduction (and range) in fibroid volume at 6 mo | 62.17% (6.28%–100%) |
| Symptom Severity Scalea (median value and range) | |
| Before RF ablation | 30 (18–38) |
| 6 mo after | 13 (11–23) |
Note: FIGO = International Federation of Gynecology and Obstetrics; MUSA = Morphological Uterus Sonographic Assessment; RF = radiofrequency.
Scale from 0 (no symptoms) to 40.
The variables directly related to the transvaginal RF ablation procedure and the 6-month results concerning the size of the fibroids and the patient’s symptoms are also shown in Table 1. All procedures were performed on an outpatient basis; no major or minor complications were reported, and no patients required hospitalization. The most common minor symptoms reported were low-grade fever, fatigue, dysmenorrhea-like pain, scant but continuous vaginal bleeding, or the expulsion of necrotic tissue through the vagina. All patients were able to return to work between the third and fifth days after surgery. At 6 months after the procedure, the decreased in the fibroid size (62.17%) and symptom scale scores (from a median score of 30 before RF ablation—in a scale ranging from 0 [no symptoms] to a maximum score of 40—to 13 afterward; P<.001) were remarkable (Table 1). All patients reported improvement in the reduction in bleeding starting from the third month.
Three cases required hysteroscopy at the 6-month follow-up after reductions in fibroid volumes of 58.53%, 90.60%, and 99.67% (pretreatment volumes of 10.08, 15.38, and 48.95 cm3, respectively). This possibility had been considered before the procedure with the aim of reducing the complexity of hysteroscopy.
In the end, 25 of these 27 patients responded correctly to RF treatment, whereas 2 cases of nonresponse were recorded. One case involved a 32-year-old patient who had previously undergone a laparotomic myomectomy. She presented with a uterine fibroid lesion in the fundus, classified as FIGO type 1, measuring 95.9 cm3 in volume, with a maximum diameter of 7.4 cm and MUSA type 3 vascularization. After the RF treatment, myoma growth was suspected at the first month of follow-up. Therefore, an additional control was performed at 3 months, in which an increase in the myoma volume of up to 125.85 cm3 was verified. Consequently, new laparotomic myomectomy was performed (with a definitive pathological diagnosis of adenomyoma). Nine months later, she achieved a full-term pregnancy with a normal course, which was concluded by a cesarean section. The other patient, with a posterior uterine fibroid classified as FIGO type 3, with a volume of 133.83 cm3, a maximum diameter of 7.2 cm, and MUSA type 2 vascularization, experienced a 28.38% reduction in fibroid volume at 6 months after the RF treatment with a significant improvement in the SSS the questionnaire (from 32/40 before treatment to 18/40 afterward). However, at 21 months, because of the reappearance of heavy menstrual bleeding and dysmenorrhea and with the fibroid growing back to its original size, she had to undergo a laparotomic myomectomy.
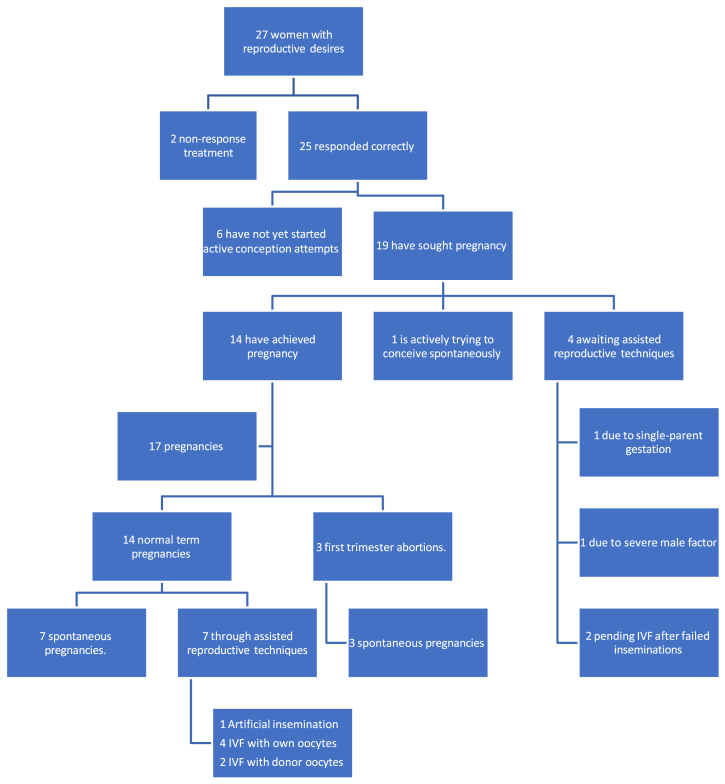
Among these 25 women with reproductive desires and where RF ablation was successful, 19 have sought conception, and 6 have not yet started active conception attempts. From the 19 patients who sought conception, 14 (73.68%) have achieved motherhood, one is actively trying to conceive spontaneously, and 4 are awaiting assisted reproductive techniques (specific reasons are detailed in Fig. 2).
Figure 2.
Flowchart of conception results after radiofrequency ablation for symptomatic fibroids. IVF = in vitro fertilization.
The variables related to fertility, pregnancies, gestational age at delivery, onset of labor, type of delivery, and neonatal outcomes are shown in Table 2. Although the treated fibroids grew during pregnancy, at 6 months postpartum, they reached back the postoperative volume. Regarding the pregnancy outcomes (17 pregnancies in 14 patients), 17.65% were first-trimester spontaneous abortions, and 82.35% were full-term pregnancies, with normal course without requiring obstetric hospitalization and normal newborn weights. It should be noted that the 3 first-trimester spontaneous abortions were recorded in women who later achieved a full-term pregnancy.
Table 2.
Obstetric and neonatal outcomes in 14 patients who achieved pregnancy after a successful radiofrequency ablation.
| No. of patients who attempted pregnancy after RF ablationa | 19 |
|---|---|
| Median time (and range) from RF ablation to conception | 12 mo (5–24 mo) |
| Median age (and range) at the time of conception | 37 y (32–45 y) |
| Total number of pregnancies | 17 |
| Pregnancies | |
| Spontaneous | 10/17 (58.82%) |
| Fertility technique | 7/17 (41.18%) |
| Artificial insemination | 1/7 (14.29%) |
| IVF with own oocytes | 4/7 (57.14%) |
| IVF with donor oocytes | 2/7 (28.57%) |
| First-trimester spontaneous abortions (among total pregnancies) | 3/17 (17.65%) |
| Term pregnancies (among total pregnancies) | 14/17 (82.35%) |
| Term pregnancies among patients who attempted pregnancy | 14/19 (73.68%) |
| Median gestational age at birth (and range) | 40 wk and 2 d (37 wk and 6 d to 41 wk and 4 d) |
| Onset of labor | |
| Spontaneous | 5/14 (35.71%) |
| Induced | 7/14 (50%) |
| Scheduled cesarean section | 2/14 (14.29%) |
| Type of delivery | |
| Normal | 7/14 (50%) |
| Instrumental | 5/14 (35.71%) |
| Cesarean section | 2/14 (14.29%) |
| Median neonatal birth weight (and range) | 3,330 g (3,060–3,880 g) |
| Complication | |
| Postpartum hemorrhage associated with highly attached placenta | 2/14 (14.29%) |
| Placental polyp | 1/14 (7.14%) |
| Retained placenta | 1/14 (7.14%) |
Note: IVF = in vitro fertilization; RF = radiofrequency.
During the 24-month follow-up period.
There were no cases of uterine rupture or intrauterine fetal death. One case of postpartum hemorrhage associated with highly attached placenta was described in a patient who had undergone 5 hysteroscopies before the ablation. Another case involved a placental polyp, which was resolved through hysteroscopic resection 1 month after delivery, and there was 1 case of retained placenta, which was managed with manual placental removal and postpartum curettage 45 minutes after delivery.
Of the 9 women referred from fertility units (women who had already sought assistance because of fertility issues), 6 achieved pregnancies during the 24-month follow-up period, whereas 3 did not: 1 due to failure of the RF treatment and 2 were awaiting assisted reproductive techniques—1 due to severe male factor and the other because the woman did not have a male partner. The characteristics of these 9 patients (main causes of infertility and obstetric outcomes after the RF ablation) are detailed in Supplemental Table 1 (available online).
Discussion
The delay in the age at which women complete their reproductive desires is leading to an increasing number of patients with uterine fibroids and fertility problems. Fibroids are a known cause of infertility, and the need for their treatment before attempting pregnancy when they distort the uterine cavity is globally accepted. In the case of intramural fibroids that do not distort the uterine wall, the indication for treatment is more controversial; however, we are increasingly gathering evidence that supports better reproductive outcomes when treating this type of fibroids (31, 32).
Conventional surgical treatment through myomectomy, whether it is performed abdominally or laparoscopically, results in damage to the integrity of the myometrial fibers and the subsequent need for anatomical reconstruction of the uterus. This delays the possibility of actively pursuing pregnancy and influences the mode of delivery due to the increased risk of uterine rupture (33, 34, 35, 36). Additionally, we should not overlook the inherent risk of myomectomy converting to hysterectomy in 3%–5% of cases, which would prevent the fulfillment of reproductive desires (36).
Hence, new techniques such as RF ablation can provide an alternative for the treatment of uterine fibroids. Among the different approaches described for RF ablation, the transvaginal ultrasound-guided approach offers several advantages over the laparoscopic approach: it has shorter surgical times, reduced hospitalization, and shorter periods of absenteeism from work because of its lower complexity. Moreover, it provides comparable results in terms of reducing the volume of the fibroids and associated symptoms (25, 37, 38, 39, 40, 41).
The obstetric outcomes described in our center are consistent with other published studies (42, 43, 44); however, it is important to note that this was a small case series with heterogeneous variables, which limits the generalization of the findings. Nevertheless, these results suggest that achieving a safe and full-term pregnancy is possible after RF ablation. Further research with larger sample sizes and more controlled variables is needed to confirm and expand on these findings. Our series shows that in most cases, women who undergo RF treatment, after waiting a minimum of 6 months after treatment, achieve spontaneous pregnancy. The rates of first-trimester miscarriage were similar to those of the general population, there were no admissions of any kind during the gestational follow-up of our patients, and gestational ages at delivery and neonatal weights were also within the normal range. Importantly, we have not encountered any cases of uterine rupture or neonatal death. These findings highlight the positive reproductive outcomes associated with RF treatment in our patient population.
Postpartum complications appear to be associated with the process of placental attachment and may be related to tissue thermal damage in cases where the fibroid extends into the endometrial cavity. However, we need broader studies to compare it with the prevalence in the general population. It is also important to consider that, in our case series, patients with these findings had previously undergone hysteroscopic procedures, making it difficult to determine the impact of each procedure on the complications.
This study provides additional support to effectiveness data of RF and provides further examples of positive reproductive outcomes after the use of this treatment for patients who desire pregnancy; RF ablation avoids incisions that distort the uterine anatomy and affect subsequent mode of delivery, and it also reduces intraoperative bleeding and the need for transfusions, as well as hospitalizations or long work absences.
Indeed, larger multicenter studies with longer follow-up periods are needed to evaluate the efficacy and safety of RF ablation as a treatment option for fibroids in women desiring fertility. However, on the basis of the available evidence, RF ablation appears to be a promising, low-complexity, safe, and effective alternative for managing fibroids in women who wish to preserve their reproductive potential. It offers a promising approach to address symptomatic fibroids while preserving fertility and minimizing surgical complications; further research and clinical experience will help refine and establish its role in the management of symptomatic fibroids and fertility preservation.
Conclusion
Radiofrequency ablation demonstrated safety among our 27 patients with reproductive desires because none of them required hospitalization and all returned to normal activities within 3 days. Moreover, the technique proved effective in 25 of 27 patients, resulting in a reduction in fibroid volume and associated symptoms on the basis of the SSS. Among those patients who attempted pregnancy during the 24-month follow-up period, 73.68% (14 of 19) achieved motherhood. Notably, there were no cases of uterine rupture, premature birth, or intrauterine fetal death.
CRediT Authorship Contribution Statement
Ma Eugenia Marín Martínez: Conceptualization, Supervision, Methodology, Investigation, Data curation, Project administration. Writing – original draft. Sara Cruz-Melguizo: Methodology, Formal analysis. Writing – review & editing. Gema Vaquero Argüello: Investigation, Data curation, Conceptualization. Virginia Engels Calvo: Methodology, Writing – review & editing. Ma Luisa De la Cruz Conty: Formal analysis, Writing – review & editing. Tirso Pérez Medina: Resources, Funding acquisition.
Declaration of Interests
M.E.M.M. has nothing to disclose. S.C.-M. has nothing to disclose. G.V.A. has nothing to disclose. V.E.C. has nothing to disclose. M.L.D.l.C.C. has nothing to disclose. T.P.M. reports funding from Fundación para la Investigación Biomédica del Puera de Hierro University Hospital G83726968, for the submitted work.
Acknowledgment
The investigators thank “Fundación para la Investigación Biomédica de Hospital Universitario Puerta de Hierro” for covering the publication costs of the article, and Victoria Rey (Clinica Victoria Rey, Seville, Spain) for her teaching abilities and her perseverance in demonstrating the effectiveness of new techniques.
Supplementary Data
A. Biopsy needle. B. Radiofrequency electrode. C. RF generator JJP. D. Metallic guide.A. Changes in the echogenicity of the fibroid before and after ablation.B. Assessment of the vascularity of the fibroid before and after ablation.
References
- 1.Baird D.D., Dunson D.B., Hill M.C., Cousins D., Schectman J.M. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Styer A.K., Rueda B.R. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3–12. doi: 10.1016/j.bpobgyn.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann A., Bernuit D., Gerlinger C., Schaefers M., Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12:6. doi: 10.1186/1472-6874-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan A.T., Shehmar M., Gupta J.K. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95–114. doi: 10.2147/IJWH.S51083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta J.K., Sinha A., Lumsden M.A., Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD005073.pub4. CD005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart E.A. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646–1655. doi: 10.1056/NEJMcp1411029. [DOI] [PubMed] [Google Scholar]
- 7.Vilos G.A., Allaire C., Laberge P.Y., Leyland N., SPECIAL CONTRIBUTORS The management of uterine leiomyomas. J Obstet Gynaecol Can. 2015;37:157–178. doi: 10.1016/S1701-2163(15)30338-8. [DOI] [PubMed] [Google Scholar]
- 8.Dolmans M.M., Cacciottola L., Donnez J. Conservative management of uterine fibroid-related heavy menstrual bleeding and infertility: time for a deeper mechanistic understanding and an individualized approach. J Clin Med. 2021;10:4389. doi: 10.3390/jcm10194389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercorio A., Della Corte L., Boccia D., Palumbo M., Reppuccia S., Buonfantino C., et al. Myomectomy in infertile women: more harm than good? Front Surg. 2023;10 doi: 10.3389/fsurg.2023.1151901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks E., Mihalov L., Delvadia D., Hudgens J., Mama S., Makai G.E., et al. The INSPIRE Comparative Cost Study: 12-month health economic and clinical outcomes associated with hysterectomy, myomectomy, and treatment with the Sonata System. Clinicoecon Outcomes Res. 2020;12:1–11. doi: 10.2147/CEOR.S214755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freytag D., Günther V., Maass N., Alkatout I. Uterine fibroids and infertility. Diagnostics (Basel) 2021;11:1455. doi: 10.3390/diagnostics11081455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margueritte F., Adam C., Fauconnier A., Gauthier T. Time to conceive after myomectomy: should we advise a minimum time interval? A systematic review. Reprod Biomed Online. 2021;43:543–552. doi: 10.1016/j.rbmo.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Gao J., Ji J.S., Ding X.M., Ke S., Xin Z.H., Ning C.M., et al. Laparoscopic radiofrequency ablation for large subcapsular hepatic hemangiomas: technical and clinical outcomes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziemlewicz T.J., Wells S.A., Lubner M.G., Brace C.L., Lee Jr FT., Hinshaw J.L. Hepatic tumor ablation. Surg Clin North Am. 2016;96:315–339. doi: 10.1016/j.suc.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Luo X., Shen Y., Song W.X., Chen P.W., Xie X.M., Wang X.Y. Pathologic evaluation of uterine leiomyoma treated with radiofrequency ablation. Int J Gynaecol Obstet. 2007;99:9–13. doi: 10.1016/j.ijgo.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Bradley L.D., Pasic R.P., Miller L.E. Clinical performance of radiofrequency ablation for treatment of uterine fibroids: systematic review and meta-analysis of prospective studies. J Laparoendosc Adv Surg Tech A. 2019;29:1507–1517. doi: 10.1089/lap.2019.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santalla-Hernández Á., Naveiro-Fuentes M., Benito-Villena R., López-Criado M.S., González-Paredes A., Fernández-Parra J. Efficacy, complications, and factors predictive of response to treatment with transvaginal radiofrequency ablation for symptomatic uterine myomas. J Minim Invasive Gynecol. 2022;29:743–752. doi: 10.1016/j.jmig.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Rey V.E., Labrador R., Falcon M., Garcia-Benitez J.L. Transvaginal radiofrequency ablation of myomas: technique, outcomes, and complications. J Laparoendosc Adv Surg Tech A. 2019;29:24–28. doi: 10.1089/lap.2018.0293. [DOI] [PubMed] [Google Scholar]
- 19.Kim C.H., Kim S.R., Lee H.A., Kim S.H., Chae H.D., Kang B.M. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563. doi: 10.1093/humrep/deq366. [DOI] [PubMed] [Google Scholar]
- 20.Cho H.H., Kim J.H., Kim M.R. Transvaginal radiofrequency thermal ablation: a day-care approach to symptomatic uterine myomas. Aust N Z J Obstet Gynaecol. 2008;48:296–301. doi: 10.1111/j.1479-828X.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- 21.Cho H.H., Kim M.R., Kim J.H. Outpatient multimodality management of large submucosal myomas using transvaginal radiofrequency myolysis. J Minim Invasive Gynecol. 2014;21:1049–1054. doi: 10.1016/j.jmig.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X., Thapa A., Lu J., Bhujohory V.S., Liu Y., Qiao S. Ultrasound-guided transvaginal radiofrequency myolysis for symptomatic uterine myomas. Eur J Obstet Gynecol Reprod Biol. 2014;177:38–43. doi: 10.1016/j.ejogrb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Brölmann H., Bongers M., Garza-Leal J.G., Gupta J., Veersema S., Quartero R., et al. The FAST-EU trial: 12-month clinical outcomes of women after intrauterine sonography-guided transcervical radiofrequency ablation of uterine fibroids. Gynecol Surg. 2016;13:27–35. doi: 10.1007/s10397-015-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnreiter C., Oppelt P. A systematic review of the treatment of uterine myomas using transcervical ultrasound-guided radiofrequency ablation with the Sonata System. J Minim Invasive Gynecol. 2021;28:1462–1469. doi: 10.1016/j.jmig.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Santalla-Hernández A., Naveiro-Fuentes M., Benito-Villena R., Villegas-Alcazar J., López-Criado M.S., Lara-Serrano A., et al. Complications of transvaginal radiofrequency ablation of fibroids: a 5-year experience. Eur J Obstet Gynecol Reprod Biol X. 2023;20 doi: 10.1016/j.eurox.2023.100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calaf J., Palacios S., Cristóbal I., Cañete M.L., Monleón J., Fernández J., et al. Validation of the Spanish version of the Uterine Fibroid Symptom and Quality of Life (UFS-QoL) questionnaire in women with uterine myomatosis. Med Clin (Barc) 2020;154:207–213. doi: 10.1016/j.medcli.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Coyne K.S., Margolis M.K., Murphy J., Spies J. Validation of the UFS-QOL-hysterectomy questionnaire: modifying an existing measure for comparative effectiveness research. Value Health. 2012;15:674–679. doi: 10.1016/j.jval.2012.03.1387. [DOI] [PubMed] [Google Scholar]
- 28.Laughlin-Tommaso S.K., Hesley G.K., Hopkins M.R., Brandt K.R., Zhu Y., Stewart E.A. Clinical limitations of the International Federation of Gynecology and Obstetrics (FIGO) classification of uterine fibroids. Int J Gynaecol Obstet. 2017;139:143–148. doi: 10.1002/ijgo.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Bosch T., Dueholm M., Leone F.P., Valentin L., Rasmussen C.K., Votino A., et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284–298. doi: 10.1002/uog.14806. [DOI] [PubMed] [Google Scholar]
- 30.Seracchioli R., Rossi S., Govoni F., Rossi E., Venturoli S., Bulletti C., et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663–2668. doi: 10.1093/humrep/15.12.2663. [DOI] [PubMed] [Google Scholar]
- 31.Hurst B.S., Matthews M.L., Marshburn P.B. Laparoscopic myomectomy for symptomatic uterine myomas. Fertil Steril. 2005;83:1–23. doi: 10.1016/j.fertnstert.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Gil Y., Badeghiesh A., Suarthana E., Mansour F., Capmas P., Volodarsky-Perel A., et al. Risk of uterine rupture after myomectomy by laparoscopy or laparotomy. J Gynecol Obstet Hum Reprod. 2020;49 doi: 10.1016/j.jogoh.2020.101843. [DOI] [PubMed] [Google Scholar]
- 33.Kim H.S., Oh S.Y., Choi S.J., Park H.S., Cho G.J., Chung J.H., et al. Uterine rupture in pregnancies following myomectomy: a multicenter case series. Obstet Gynecol Sci. 2016;59:454–462. doi: 10.5468/ogs.2016.59.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanos V., Toney Z.A. Uterine scar rupture - prediction, prevention, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2019;59:115–131. doi: 10.1016/j.bpobgyn.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Gambacorti-Passerini Z.M., Penati C., Carli A., Accordino F., Ferrari L., Berghella V., et al. Vaginal birth after prior myomectomy. Eur J Obstet Gynecol Reprod Biol. 2018;231:198–203. doi: 10.1016/j.ejogrb.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Bernardi T.S., Radosa M.P., Weisheit A., Diebolder H., Schneider U., Schleussner E., et al. Laparoscopic myomectomy: a 6-year follow-up single-center cohort analysis of fertility and obstetric outcome measures. Arch Gynecol Obstet. 2014;290:87–91. doi: 10.1007/s00404-014-3155-2. [DOI] [PubMed] [Google Scholar]
- 37.Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(Suppl):S125–S130. doi: 10.1016/j.fertnstert.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Robles R., Aguirre V.A., Argueta A.I., Guerrero M.R. Laparoscopic radiofrequency volumetric thermal ablation of uterine myomas with 12 months of follow-up. Int J Gynaecol Obstet. 2013;120:65–69. doi: 10.1016/j.ijgo.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Keltz J., Levie M., Chudnoff S. Pregnancy outcomes after direct uterine myoma thermal ablation: review of the literature. J Minim Invasive Gynecol. 2017;24:538–545. doi: 10.1016/j.jmig.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Coyne K., Purdy M.P., Bews K.A., Habermann E.B., Khan Z. Risk of hysterectomy at the time of myomectomy: an underestimated surgical risk. Fertil Steril. 2024;121:107–116. doi: 10.1016/j.fertnstert.2023.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Allen A., Schembri M., Parvataneni R., Waetjen L.E., Varon S., Salamat-Saberi N., et al. Pregnancy outcomes after laparoscopic radiofrequency ablation of uterine leiomyomas compared with myomectomy. Obstet Gynecol. 2024;143:612–618. doi: 10.1097/AOG.0000000000005548. [DOI] [PubMed] [Google Scholar]
- 42.Christoffel L., Bends R., Toub D., Schiermeier S., Pschadka G., Engelhardt M., et al. Pregnancy outcomes after transcervical radiofrequency ablation of uterine fibroids with the Sonata System. J Gynecol Surg. 2022;38:207–213. doi: 10.1089/gyn.2021.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nam J.H. Pregnancy and symptomatic relief following ultrasound-guided transvaginal radiofrequency ablation in patients with adenomyosis. J Obstet Gynaecol Res. 2020;46:124–132. doi: 10.1111/jog.14145. [DOI] [PubMed] [Google Scholar]
- 44.Polin M., Hur H.C. Radiofrequency ablation of uterine myomas and pregnancy outcomes: an updated review of the literature. J Minim Invasive Gynecol. 2022;29:709–715. doi: 10.1016/j.jmig.2022.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Biopsy needle. B. Radiofrequency electrode. C. RF generator JJP. D. Metallic guide.A. Changes in the echogenicity of the fibroid before and after ablation.B. Assessment of the vascularity of the fibroid before and after ablation.