Abstract
Peripheral nerve injuries within the upper extremities can lead to impaired function and reduced quality of life. Although autografts have traditionally served as the primary therapeutic approach to bridge nerve gaps, these present challenges related to donor site morbidity. This review delves into the realm of biomaterials tailored for addressing nerve gaps. Biomaterials, whether natural or synthetically derived, offer the potential not only to act as scaffolds for nerve regeneration but also to be enhanced with growth factors and agents that promote nerve recovery. The historical progression of these biomaterials as well as their current applications, advantages, inherent challenges, and future impact in the arena of regenerative medicine are discussed. By providing a comprehensive overview, we aim to shed light on the transformative potential of biomaterials in peripheral nerve repair and the path toward refining their efficacy in clinical settings.
Key words: Biomaterials development, Nerve regeneration, Peripheral nerve, Tissue engineering, Upper extremities
Historically, peripheral nerve gaps caused by trauma or disease were addressed using autografts.1 However, the use of nerve autografts is associated with donor site morbidity, including numbness and potential for neuropathic pain secondary to neuroma formation.2 Advances in material science and biomedical engineering offer biomaterials as a promising alternative.
These substances, either derived from natural sources or synthesized in laboratories, can be tailored to encourage nerve growth, regeneration, and repair. Biomaterials not only provide a scaffold for nerve regeneration but can also be imbued with growth factors and other agents that stimulate and guide nerve recovery. This review aims to comprehensively examine the emerging field of biomaterials tailored for addressing nerve gaps in the upper extremities. We will focus on the evolution of these materials as well as a critical appraisal of their efficacy, advantages, and challenges, which will aid in forecasting their potential impact on future medical interventions.
Overview and Current Treatment Options for Peripheral Nerve Gaps
Epidemiology and classification of peripheral nerve injury
Peripheral nerve injury (PNI) is more prevalent in young men and is seen mostly in the upper extremities.1 Trauma is the most common cause.2 PNI is estimated to affect 3% of patients admitted to Level 1 trauma centers.3,4 Repetitive motion or overuse can also result in nerve compression and injury. Certain medical conditions, including diabetes, autoimmune disorders, and infections, can lead to nerve dysfunction and damage over time.5 Seddon’s 1943 classification remains relevant today, categorizing injuries into neurapraxia, axonotmesis, and neurotmesis.6 Sunderland later proposed a more detailed classification in 1951, which divided peripheral nerve injuries into five degrees based on severity, ranging from neurapraxia to neurotmesis.7
Current treatment for peripheral nerve gaps
The focus in the treatment of peripheral nerve gaps is minimizing tension and achieving a secure coaptation. Where tension on the nerve ends is a concern, nerve grafting should be considered with autografts or allografts.6,8
Acellular nerve allografts (ANA) are nonimmunogenic human peripheral nerve tissues that undergo a chemical decellularization process to effectively eliminate cellular debris and axon-growing inhibitors, while preserving the inherent neuronal structure required for nerve regeneration.7,9,10 Advantages include availability in various lengths, the lack of donor site complications, a lower rate of host rejection, and no need for immunosuppressive therapy.11,12 Animal trials have demonstrated encouraging nerve regeneration capacity for ANAs.7,13
The initial reports from human clinical trials showed that ANAs are associated with substantial functional recovery, with lower extremity nerve injuries and nerve gaps exceeding 15 mm being risk factors for unfavorable functional recovery.14,15 A few studies have reported failures directly related to the acellular nerve grafts. The most common cause of ANA-specific failure was postoperative degradation or absorption, without signs of functional improvement.16, 17, 18
Autografts remain the gold standard for long nerve gaps. Cable sural nerve autografts are the most used reconstructive modality for reconstruction of proximal long nerve gaps.19 Vascularized nerve grafts provide another option for reconstruction of long nerve gaps.20 Nerve transfer may be an option in some cases when nearby healthy, redundant functioning nerves are available.21 Nerve guidance conduits are another option currently used for nerve gaps. Tubular structures made of various biomaterials serve as a scaffold for nerve regeneration and also protect and guide regenerating nerve fibers across the gap. These products are designed to be wrapped around nerves after neurolysis or nerve coaptation, providing a protective and biocompatible environment that encourages axonal regrowth. Their biodegradable nature facilitates resorption, leaving behind a healed and functional nerve.
Some popular products in this category include small intestinal submucosa wraps like AxoGuard (Axogen Inc., Alachua, FL).22 Other products include collagen-based devices (NeuraGen®, NeuraWrap™).23 The initial animal studies for NeuraGen showed comparative results with direct nerve suturing and autografts.24,25
The efficacy of nerve conduits has been shown through studies involving human subjects to assess the efficacy of collagen-based devices.26, 27, 28 The main limitation of collagen-based nerve conduits is the extended time required for degradation, which may predispose to compression neuropathy.29 Incorporating stem cells into nerve conduits was also found to be a safe option with promising potential.30,31
Choice of Biomaterials
Synthetic materials
The evolution of synthetic materials, especially polymers, offers new options for addressing nerve injuries and gaps. One of the standout polymers in this domain is poly (lactic-co-glycolic acid) (PLGA), which offers a plethora of advantages tailored to meet therapeutic requirements. One of the chief strengths of PLGA and similar synthetic materials is their customizable properties. This ability allows design and modification of these polymers according to the specific therapeutic needs of a case. For instance, the degradation rates of these polymers can be controlled. By doing so, they can create an optimal environment that is conducive to nerve regeneration over a defined period, ensuring that the material degrades at a pace that complements the nerve healing process.
This controlled degradation is pivotal because it prevents potential complications that could arise from materials persisting longer than necessary or breaking down too quickly, which could be detrimental to the healing process. Another significant advantage is the adaptability of these materials. The ability to mold and shape PLGA to fit specific physiological requirements is paramount. Different nerve injuries present varied topographical challenges, and having a material that can be crafted to seamlessly integrate into these diverse landscapes augments its clinical utility. This flexibility eliminates the “one-size-fits-all” approach and brings about a more personalized medical intervention strategy.
However, although these synthetic materials come with numerous benefits, challenges remain. The integration of synthetic materials with other emerging technologies, such as nanotechnology or bioactive agents, might potentiate their efficacy, paving the way for groundbreaking medical solutions that could revolutionize treatment for nerve injuries.
In the context of the article, tissue-engineered nerve grafts have been identified as a promising solution for repairing long-distance peripheral nerve defects.32, 33, 34, 35 The core element of tissue engineering, biomaterials, is rapidly evolving, with synthetic materials like PLGA offering superior plasticity compared to natural materials.36, 37, 38, 39 Chitosan, a natural polymer, has been combined with PLGA to create a balanced microenvironment conducive for nerve regeneration.40, 41, 42, 43, 44 The PLGA scaffolds, in particular, neutralize the degradation rates of other polymers, ensuring an optimal environment for nerve regeneration (Fig. 1).45, 46, 47, 48, 49 This combination of natural and synthetic materials, along with the understanding of the neural regeneration microenvironment, represents a significant step toward innovative medical interventions for nerve injuries.
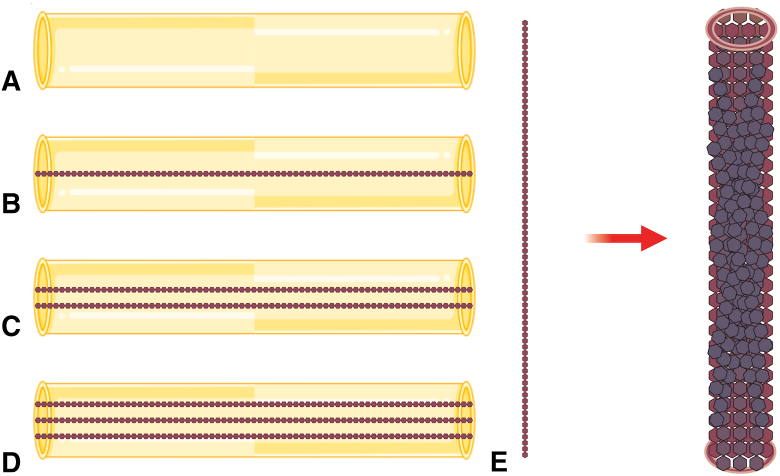
Figure 1.
Schematics of tissue-engineered nerve grafts with chitosan nerve conduits and different amounts of PLGA in scaffolds. A Chitosan nerve conduit. B Chitosan nerve conduit with one PLGA scaffold. C Chitosan nerve conduit with two PLGA scaffolds. D Chitosan nerve conduit with three PLGA scaffolds. E PLGA scaffold zoomed in.
Natural materials
Natural materials, including substances like collagen and chitosan, are renowned for their exceptional biocompatibility. Stemming from organic sources, collagen and chitosan have been rigorously researched and used given their high biocompatibility. This biocompatibility leads to reduced foreign body reactions upon implantation, diminishing inflammation, and potential side effects. A salient feature of these materials is their intrinsic ability to promote cellular adhesion and growth.50
Being components native to living organisms, they foster a conducive environment for cell development and nerve regeneration. Their organic origin ensures harmonious integration with host tissues, reducing the chances of adverse reactions or rejection. However, accompanying these advantages are certain challenges. In scenarios demanding durability and resilience, these materials may not meet the required standards of mechanical robustness and structural consistency.51 The tunability of mechanical properties for natural materials is poor, and they may be brittle and not hold sutures well. Potential options include material refinement or merging natural substrates with synthetic counterparts to achieve the necessary robustness.
In a study by Spearman et al,52 hyaluronic acid (HA), a key component of the neural extracellular matrix that supports neural cell function and modulates inflammatory responses, emerged as a promising scaffold for tissue regeneration because of its modifiable mechanical traits and controllable degradation.52 Notably, two HA methacrylation techniques, glycidyl methacrylated HA (GMHA) and methacrylic anhydride HA (MAHA), were examined, showcasing their capability to mimic the mechanical attributes of a range of rat-derived tissues. For neural tissue engineering, these hydrogels, when combined with proteins and either GMHA or MAHA, align well with the mechanical characteristics of the rat sciatic nerve, promoting axonal growth.
Such advancements in HA-based scaffolds hint at potential clinical applications, especially in nerve conduits or other nerve-related scaffolds, which can serve as bridge solutions for nerve injury or resection. Methacrylated HA stands out as a versatile platform for soft tissue engineering. However, certain limitations have been identified, particularly with GMHA’s degradation and cell adhesion properties. Addressing these challenges, a strategy that combines HA with fibrinogen was developed, enhancing both cell adhesion and hydrogel degradation properties.53 Preliminary results show promise for this modified approach, though in-depth in vivo assessments are yet to be conducted. As with naturally derived materials, although their inherent properties encourage cell growth and minimize rejection risks, they can occasionally lack in providing the required durability, suggesting the need for material enhancement or integration with synthetic substrates.
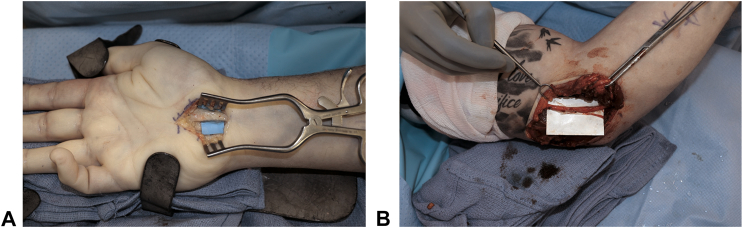
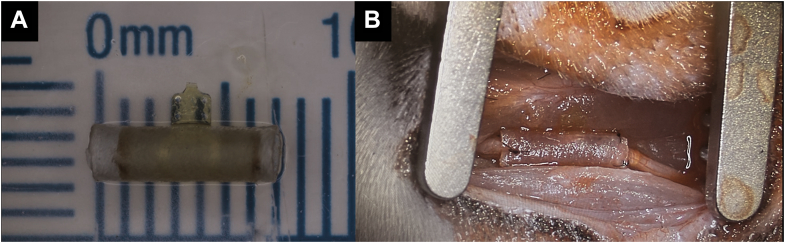
VersaWrap (Alafair Biosciences, Austin, TX) is a commercially available HA-based scaffold that can be used as a nerve wrap (Fig. 2). As research progresses, we can expect to see hybrid materials, combinations of natural and synthetic materials, that merge the best of both worlds.54 An example could include leveraging the biocompatibility of natural materials like collagen and chitosan while bolstering their mechanical strength through synthetic augmentation. This evolution could redefine the paradigm of medical interventions, presenting more effective, efficient, and patient-friendly solutions.
Figure 2.
VersaWrap application. A VersaWrap applied as a nerve wrap in a patient undergoing surgery for recurrent carpal tunnel syndrome. B VersaWrap applied in a patient with recurrent cubital tunnel syndrome.
Conductive polymers
Conductive polymers offer a potential groundbreaking approach to nerve gap repair. Incorporating elements that foster nerve growth through electrical conductivity, these polymers have ushered in a new era of innovative solutions in the field.55 Using polymers like polypyrrole and polyaniline, researchers have endeavored to create environments conducive to cell proliferation and neurite extension, thereby offering hope in the nerve gap repair landscape.55 Nonetheless, concerns linger, including the long-term stability and potential cytotoxicity associated with these polymers, highlighting a need for further research to ensure their safe and effective deployment in clinical settings.56
A recent study sheds light on the challenges faced by diabetic patients in peripheral nerve repair.57 Although autologous nerve transplantation remains the gold standard for PNI repair, it has its limitations, such as size mismatch and limited donor sources.58 Hydrogels have potential as nerve conduits given their ability to mimic the extracellular matrix and load stem cells.59,60 However, traditional hydrogels lack topographical cues for directional alignment, which is crucial for nerve repair.61 To address this, a graphene-based conduit can be used, leveraging the unique properties of graphene for tissue engineering.62 This conduit combines the toughness of natural silk with the bioactivity of gelatin methacryloyl, forming a double network hydrogel loaded with the growth factor Netrin-1.63 This design ensures rapid setting, mechanical support, biocompatibility, and sustained growth factor delivery. The inclusion of graphene not only enhances the conduit’s mechanical properties but also fosters cell proliferation.63
Soluble Factors
Soluble factors, including growth factors, hold a cardinal position in the endeavor for nerve gap repair. According to Daly et al,64 soluble factors dictate cellular behaviors integral for nerve regeneration, forming a fundamental part of the biomaterial strategies in the nerve repair landscape. Growth factors, such as nerve growth factor and brain-derived neurotrophic factor, have regenerative capacities, fostering an environment conducive to nerve growth.56 Advances in delivery systems enabling controlled release of these growth factors have been highlighted as a cornerstone in modern nerve repair strategies.65
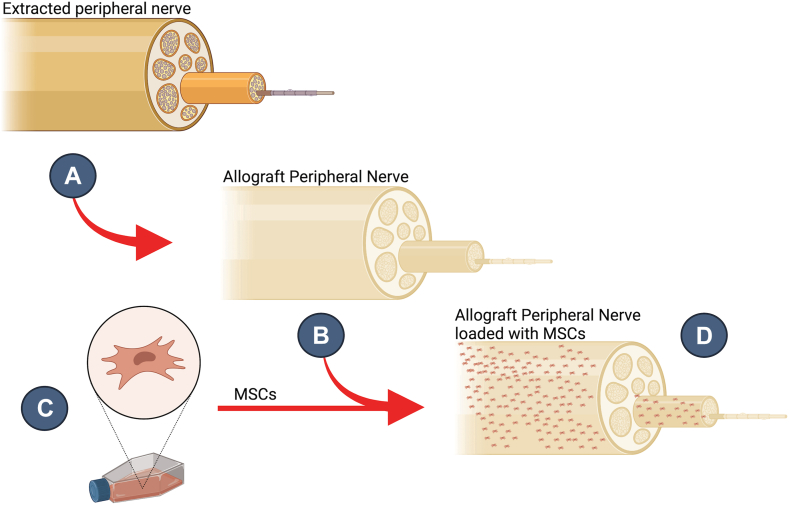
In the context of the evolution of materials used, seeding decellularized nerve allografts with adipose-derived mesenchymal stromal cells (MSCs) offers a promising avenue. Processed nerve allografts, when combined with the trophic functions of MSCs, particularly those from adipose tissue, have shown potential in stimulating nerve regeneration. These MSCs secrete a plethora of growth factors, including vascular endothelial growth factor, nerve growth factor, and brain-derived neurotrophic factor, which are pivotal for nerve repair (Fig. 3). The interaction between MSCs and the decellularized nerve allografts not only augments the mRNA and protein expression of these growth factors but also provides a conducive microenvironment for nerve regeneration.66 The intricate balance of spatial and temporal distribution requires meticulous planning to avert unwanted proliferative responses.67 Furthermore, although the combination of a patient’s own adipose-derived stem cells with processed nerve allografts presents an attractive individualized peripheral nerve repair strategy, the actual mechanisms underlying the neurotrophic potential of MSCs remain elusive. By harnessing the natural capabilities of MSCs and the structural benefits of allografts, this combined approach might pave the way for more effective, economical, and accessible treatments for nerve injuries.
Figure 3.
Allograft loaded with mesenchymal stromal cells. Steps of the process are shown. A Peripheral nerve extracted. B Decellularized extracted peripheral nerve creating the allograft, preserving microarchitecture and biochemical cues. C MSCs are grown inside a flask. D Allograft loaded with MSCs.
Mechanical and Structural Design Considerations
Advances such as multi-channel conduits and three-dimensional printed anatomical pathways present promising avenues in guiding nerve growth more effectively.68 These conduits, through a representation of a semblance to the natural environment, offer a pathway to foster nerve regeneration and functional recovery. However, these technologies, despite their prospective benefits, confront substantial barriers, including design intricacies and reproducibility issues, which hinder their large-scale adoption in the clinical landscape.69
The endeavor to mirror the natural physiological environment while maintaining mechanical stability remains a pertinent research area, urging a deeper exploration into innovative design approaches that balance both aspects to foster a successful nerve regeneration pathway. One compelling technology, magnetic templating developed by the Rinaldi lab at the University of Florida, relies on the incorporation of microarchitecture within hydrogels.70 This study aimed to engineer a hydrogel scaffold embedded with porous microchannels that emulate intricate tissue microstructures with the intent to direct cell growth in a scalable and cost-efficient manner for tissue restoration.
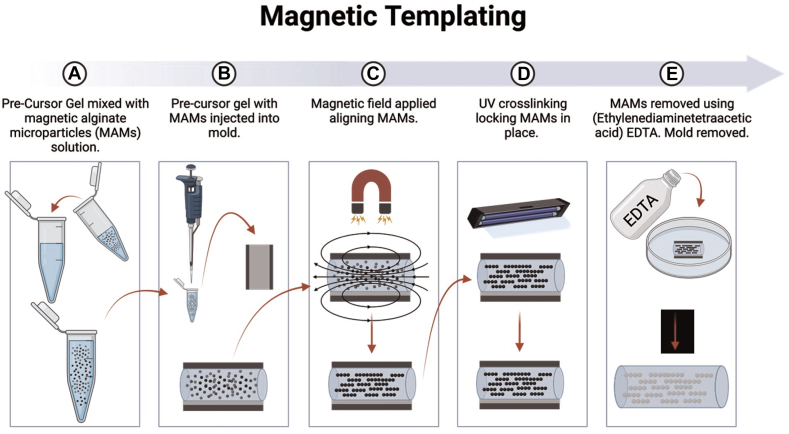
In this approach, termed magnetic templating, magnetic alginate microparticles (MAMs) are interspersed within a hydrogel precursor (Fig. 4). They are then oriented using a magnetic field and eventually degraded post-hydrogel crosslinking, resulting in a precisely aligned porous structure.70 A systematic protocol, leveraging microfluidics, was formulated to produce consistent MAMs, optimizing the replicability and adjustability of the templated microdesign.70 Iron content analysis revealed the technique’s capacity to regulate the magnetic iron oxide concentration within the MAMs. Both Brownian dynamic simulations and nanocomputed tomography corroborated the alignment between the projected and observed areal densities of the MAM chains, attesting to the method’s precision in generating aligned microchannels.70
Figure 4.
Magnetic templating protocol. A Precursor gel is mixed with MAMs solution. B Precursor gel mixed with MAMs is injected into mold. C External magnetic field is applied to gel containing MAMs, aligning them parallel to the magnetic field. D Gel containing MAMs is crosslinked, locking aligned MAMs into place. E MAMs are removed using EDTA, forming a templated gel. EDTA, ethylenediaminetetraacetic acid; MAM, magnetic alginate microparticle.
Mechanical evaluations, encompassing oscillatory rheology and stress relaxation tests, have shown that magnetically templated microchannels markedly affect the overarching attributes of the hydrogel. In vitro experiments incorporating the cultivation of rat Schwann cells on these patterned hydrogels represent potential applications in peripheral nerve gap repair. The outcomes showcased the hydrogels’ capability to direct cell growth along the channels.70 This research underscores the promise in crafting microstructured biomaterials using the magnetic templating technique, exhibiting versatility for diverse tissue restoration endeavors. Nonetheless, advancements in channel length and density are requisite to effectively stimulate peripheral nerve regrowth.70
Electrical Stimulation
It has been demonstrated that electrical stimulation can enhance nerve regeneration, improve functional recovery, and modulate neural plasticity.71,72 Furthermore, the application of electrical stimulation has been shown to be effective in reducing muscle atrophy, promoting sensory and motor axon regeneration, and improving the reinnervation process.73,74 In their work, Brushart et al73 explored how electrical stimulation contributes to motoneuron regeneration. The study found that although stimulation promotes regeneration, it does not necessarily increase its speed or condition the neuron, providing valuable insights into the nuanced impacts of electrical stimulation on nerve regeneration.73 Geremia et al74 conducted a study highlighting the beneficial effects of electrical stimulation in promoting sensory neuron regeneration and the expression of growth-associated genes, underscoring its significance in peripheral nerve repair. In light of this, the development and application of advanced devices like the Magnetically Aligned Regenerative Tissue-Engineered Electronic Nerve Interface (MARTEENI) hold great promise.75
The MARTEENI device (Fig. 5), which consists of a flexible and scalable regenerative peripheral nerve interface within a microchannel-embedded hydrogel (GMHA-collagen), has shown significant results in in vivo studies involving Lewis rats.75 The device has demonstrated potential in channel-isolated activity and electrophysiology and fostered the formation of minifascicles by regenerating axons within the templated hydrogel. These promising findings suggest that combining such innovative devices with electrical stimulation strategies can potentially optimize therapeutic outcomes for peripheral nerve repair and regeneration. Although advancements in hydrogel chemistry have been notable, it was observed that residue GMHA potentially blocked regeneration sites. Additionally, foreign body encapsulation was evident around electrode sites. Nonetheless, the integration of electrical stimulation with cutting-edge technologies such as MARTEENI in peripheral nerve repair strategies holds significant promise.
Figure 5.
MARTEENI device. A Nonfunctional MARTEENI device with a triple stack electrode thread forming a three-dimensional microelectrode cloud embedded in a templated 10 mg/mL GMHA and 3 mg/mL Collagen with AxoGuard®. B MARTEENI Device implanted within an ∼250 g female Lewis rat using the sciatic nerve transection model.
Clinical Translation and Recent Advances
Nonresorbable devices consist of a polyvinyl alcohol backbone with SalubriaTM hydrogel, a nondegradable biomaterial containing a similar water proportion to human tissue, which was first described by Ku et al.29 However, there is very little clinical evidence on its utilization.
The potential of nerve conduits infused with stem cells has been explored and found to be a safe option.30,31 Allogenic Schwann cell transplantation has been introduced as an alternative option for axonal growth for long gap nerve repair. Its efficacy for enhanced nerve regeneration has been proven in animal models.76,77
Furthermore, nerve implants embedded with stem cell and growth factors represent a novel and highly promising alternative. Although its use has not been tested in humans, an in vivo study by Wang et al78 showed promising regenerative capacity of nerve implants seeded with umbilical cord-derived stem cells and dihydroxyphenylalanine-insulin-like growth factor 1. Artificial nerve conduits represent other emerging options that have been studied widely recently. The main issue with artificial nerve conduits is the need for an extracellular matrix-like matrix required for nerve regeneration. Ikegami et al79 showed that in vivo application of heparin/growth factor to an artificial nerve conduit can be accompanied with sustained stability and improved axonal regeneration.
Conclusion
In conclusion, the landscape of PNI repair has witnessed significant advancements, with the introduction of diverse innovative technologies and methodologies. Magnetic templating is an example of one such innovation, leveraging microarchitecture within hydrogels to improve nerve regeneration by mimicking complex tissue microarchitecture, presenting a scalable and cost-effective approach to tissue repair. Moreover, devices like MARTEENI, combining magnetic alignment with regenerative peripheral nerve interface technology, showcase the potential of integrating mechanical design with electrical stimulation to optimize therapeutic outcomes. These technologies, along with growth factor integration, advanced mechanical design, various grafting materials, and electrical stimulation, contribute to a broadened and enriched therapeutic repertoire for PNI. However, challenges such as economic barriers, design complexities, reproducibility issues, and clinical translation still loom, highlighting the need for continued exploration, innovation, and refinement. The progression in this field is promising, fostering hope for the development of more effective, scalable, and cost-efficient solutions for individuals afflicted with peripheral nerve gaps.
Conflicts of Interest
No benefits in any form have been received or will be received related directly to this article.
Footnotes
B.M.S. and R.S. contributed equally to this manuscript
References
- 1.Li N.Y., Onor G.I., Lemme N.J., Gil J.A. Epidemiology of peripheral nerve injuries in sports, exercise, and recreation in the United States, 2009–2018. Phys Sportsmed. 2021;49(3):355–362. doi: 10.1080/00913847.2020.1850151. [DOI] [PubMed] [Google Scholar]
- 2.McAllister R.M., Gilbert S.E., Calder J.S., Smith P.J. The epidemiology and management of upper limb peripheral nerve injuries in modern practice. J Hand Surg Br. 1996;21(1):4–13. doi: 10.1016/s0266-7681(96)80004-0. [DOI] [PubMed] [Google Scholar]
- 3.Huckhagel T., Nüchtern J., Regelsberger J., Lefering R., Dgu T. Nerve injury in severe trauma with upper extremity involvement: evaluation of 49,382 patients from the TraumaRegister DGU® between 2002 and 2015. Scand J Trauma Resusc Emerg Med. 2018;26:1–8. doi: 10.1186/s13049-018-0546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padovano W.M., Dengler J., Patterson M.M., et al. Incidence of nerve injury after extremity trauma in the United States. Hand. 2022;17(4):615–623. doi: 10.1177/1558944720963895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin C., Karagoz H., Yuksel F., et al. The effect of perineurotomy on nerve regeneration in diabetic rats. Plast Reconstr Surg. 2012;130(5):651e–661e. doi: 10.1097/PRS.0b013e318267d3bd. [DOI] [PubMed] [Google Scholar]
- 6.Scholz T., Krichevsky A., Sumarto A., et al. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009;25(6):339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- 7.Whitlock E.L., Tuffaha S.H., Luciano J.P., et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39(6):787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 8.Gaudin R., Knipfer C., Henningsen A., Smeets R., Heiland M., Hadlock T. Approaches to peripheral nerve repair: generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res Int. 2016;2016 doi: 10.1155/2016/3856262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir D. The potentiation of peripheral nerve sheaths in regeneration and repair. Exp Neurol. 2010;223(1):102–111. doi: 10.1016/j.expneurol.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Hudson T.W., Liu S.Y., Schmidt C.E. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10(9-10):1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 11.Berger A., Hierner R., Walter G. Springer; 2007. The Allogenic Nerve Graft. [DOI] [PubMed] [Google Scholar]
- 12.Rbia N., Shin A.Y. The role of nerve graft substitutes in motor and mixed motor/sensory peripheral nerve injuries. J Hand Surg Br. 2017;42(5):367–377. doi: 10.1016/j.jhsa.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Neubauer D., Graham J.B., Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207(1):163–170. doi: 10.1016/j.expneurol.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karabekmez F.E., Duymaz A., Moran S.L. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y) 2009;4(3):245–249. doi: 10.1007/s11552-009-9195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safa B., Jain S., Desai M.J., et al. Peripheral nerve repair throughout the body with processed nerve allografts: results from a large multicenter study. Microsurgery. 2020;40(5):527–537. doi: 10.1002/micr.30574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson C., Schneider J.M., Pohl U., Power D.M. Failed acellular nerve allografts: a critical review. Ann Plast Surg. 2022;89(1):63–71. doi: 10.1097/SAP.0000000000003055. [DOI] [PubMed] [Google Scholar]
- 17.Berrocal Y.A., Almeida V.W., Levi A.D. Limitations of nerve repair of segmental defects using acellular conduits: case report. J Neurosurg. 2013;119(3):733–738. doi: 10.3171/2013.4.JNS121938. [DOI] [PubMed] [Google Scholar]
- 18.Nietosvaara Y., Grahn P., Sommarhem A. Failed peripheral nerve reconstruction with processed nerve allografts in three patients. J Hand Surg Eur Vol. 2019;44(3):318–320. doi: 10.1177/1753193418817968. [DOI] [PubMed] [Google Scholar]
- 19.Norkus T., Norkus M., Ramanauskas T. Donor, recipient and nerve grafts in brachial plexus reconstruction: anatomical and technical features for facilitating the exposure. Surg Radiol Anat. 2005;27:524–530. doi: 10.1007/s00276-005-0024-5. [DOI] [PubMed] [Google Scholar]
- 20.Singh V.K., Haq A., Tiwari M., Saxena A.K. Approach to management of nerve gaps in peripheral nerve injuries. Injury. 2022;53(4):1308–1318. doi: 10.1016/j.injury.2022.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Hussain G., Wang J., Rasul A., et al. Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery. Int J Biol Sci. 2020;16(1):116. doi: 10.7150/ijbs.35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S., Shen W., Ge X., et al. Advances in large gap peripheral nerve injury repair and regeneration with bridging nerve guidance conduits. Macromol Biosci. 2023 doi: 10.1002/mabi.202300078. [DOI] [PubMed] [Google Scholar]
- 23.Stang F., Fansa H., Wolf G., Keilhoff G. Collagen nerve conduits–assessment of biocompatibility and axonal regeneration. Biomed Mater Eng. 2005;15(1–2):3–12. [PubMed] [Google Scholar]
- 24.Archibald S.J., Krarup C., Shefner J., Li S.T., Madison R.D. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991;306(4):685–696. doi: 10.1002/cne.903060410. [DOI] [PubMed] [Google Scholar]
- 25.Mackinnon S.E., Hudson A.R., Bojanowski V., Hunter D.A., Maraghi E. Peripheral nerve injection injury with purified bovine collagen—an experimental model in the rat. Ann Plast Surg. 1985;14(5):428–436. doi: 10.1097/00000637-198505000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Ashley W.W., Weatherly T., Park T.S. Collagen nerve guides for surgical repair of brachial plexus birth injury. J Neurosurg. 2006;105(6):452–456. doi: 10.3171/ped.2006.105.6.452. [DOI] [PubMed] [Google Scholar]
- 27.Farole A., Jamal B.T. A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: a case series. J Oral Maxillofac Surg. 2008;66(10):2058–2062. doi: 10.1016/j.joms.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Bushnell B.D., McWilliams A.D., Whitener G.B., Messer T.M. Early clinical experience with collagen nerve tubes in digital nerve repair. J Hand Surg Br. 2008;33(7):1081–1087. doi: 10.1016/j.jhsa.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Kehoe S., Zhang X., Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Bedar M., Van Wijnen A.J., Shin A.Y. Safety of allogeneic mesenchymal stem cell seeding of neuragen nerve guides in a rabbit model. Tissue Eng. 2023;29(2):43–53. doi: 10.1089/ten.tec.2022.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathot F., Rbia N., Thaler R., Bishop A.T., Van Wijnen A.J., Shin A.Y. Introducing human adipose-derived mesenchymal stem cells to Avanceⓡ nerve grafts and NeuraGenⓡ nerve guides. J Plast Reconstr Aesthet Surg. 2020;73(8):1473–1481. doi: 10.1016/j.bjps.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu P., Wang G., Qian T., et al. The balanced microenvironment regulated by the degradants of appropriate PLGA scaffolds and chitosan conduit promotes peripheral nerve regeneration. Mater Today Bio. 2021;12 doi: 10.1016/j.mtbio.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chooi W.H., Chew S.Y. Modulation of cell-cell interactions for neural tissue engineering: potential therapeutic applications of cell adhesion molecules in nerve regeneration. Biomaterials. 2019;197:327–344. doi: 10.1016/j.biomaterials.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Jahromi M., Razavi S., Bakhtiari A. The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J Tissue Eng Regen Med. 2019;13(11):2077–2100. doi: 10.1002/term.2945. [DOI] [PubMed] [Google Scholar]
- 35.Onode E., Uemura T., Takamatsu K., et al. Bioabsorbable nerve conduits three-dimensionally coated with human induced pluripotent stem cell-derived neural stem/progenitor cells promote peripheral nerve regeneration in rats. Sci Rep. 2021;11(1):4204. doi: 10.1038/s41598-021-83385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang W., Begum R., Barber T., et al. Regenerative potential of silk conduits in repair of peripheral nerve injury in adult rats. Biomaterials. 2012;33(1):59–71. doi: 10.1016/j.biomaterials.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Zaszczynska A., Sajkiewicz P., Gradys A. Piezoelectric scaffolds as smart materials for neural tissue engineering. Polymers. 2020;12(1):161. doi: 10.3390/polym12010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S.G. Immunomodulation and smart materials for maxillofacial tissue engineering. Maxillofac Plast Reconstr Surg. 2020;42:1–2. doi: 10.1186/s40902-020-0247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghane N., Khalili S., Nouri Khorasani S., Esmaeely Neisiany R., Das O., Ramakrishna S. Regeneration of the peripheral nerve via multifunctional electrospun scaffolds. J Biomed Mater Res A. 2021;109(4):437–452. doi: 10.1002/jbm.a.37092. [DOI] [PubMed] [Google Scholar]
- 40.Chang S.H., Lin Y.Y., Wu G.J., Huang C.H., Tsai G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int J Biol Macromol s. 2019;131:167–175. doi: 10.1016/j.ijbiomac.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 41.Islam M.M., Shahruzzaman M., Biswas S., Sakib M.N., Rashid T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact Mater. 2020;5(1):164–183. doi: 10.1016/j.bioactmat.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdulghani S., Mitchell G.R. Biomaterials for in situ tissue regeneration: a review. Biomolecules. 2019;9(11):750. doi: 10.3390/biom9110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater. 2020;5(9):686–705. [Google Scholar]
- 44.Alvites R.D., Branquinho M.V., Sousa A.C., et al. Combined use of chitosan and olfactory mucosa mesenchymal stem/stromal cells to promote peripheral nerve regeneration in vivo. Stem Cells Int. 2021;2021 doi: 10.1155/2021/6613029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang X., Xue C., Wang Y., Ding F., Yang Y., Gu X. Bridging peripheral nerve defects with a tissue engineered nerve graft composed of an in vitro cultured nerve equivalent and a silk fibroin-based scaffold. Biomaterials. 2012;33(15):3860–3867. doi: 10.1016/j.biomaterials.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Williams D. Cambridge University Press; 2014. Essential Biomaterials Science. [Google Scholar]
- 47.Xi K., Gu Y., Tang J., et al. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat Commun. 2020;11(1):4504. doi: 10.1038/s41467-020-18265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bucan V., Vaslaitis D., Peck C.T., Strauß S., Vogt P.M., Radtke C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol Neurobiol. 2019;56:1812–1824. doi: 10.1007/s12035-018-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clements M.P., Byrne E., Guerrero L.F.C., et al. The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron. 2017;96(1):98–114.e7. doi: 10.1016/j.neuron.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Y., Wang A., Patel S., et al. Engineering bi-layer nanofibrous conduits for peripheral nerve regeneration. Tissue Eng Part C Methods. 2011;17(7):705–715. doi: 10.1089/ten.tec.2010.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalamagkas K., Tsintou M., Seifalian A. Advances in peripheral nervous system regenerative therapeutic strategies: a biomaterials approach. Mater Sci Eng C Mater Biol Appl. 2016;65:425–432. doi: 10.1016/j.msec.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 52.Spearman B.S., Agrawal N.K., Rubiano A., Simmons C.S., Mobini S., Schmidt C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J Biomed Mater Res A. 2020;108(2):279–291. doi: 10.1002/jbm.a.36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasper M., Ellenbogen B., Hardy R., et al. Development of a magnetically aligned regenerative tissue-engineered electronic nerve interface for peripheral nerve applications. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hones K.M., Nichols D.S., Barker H., Cox E., Hones J.A., Chim H. Outcomes following use of VersaWrap nerve protector in treatment of patients with recurrent compressive neuropathies. Front Surg. 2023;10 doi: 10.3389/fsurg.2023.1123375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson M., Shelke N.B., Manoukian O.S., Yu X., McCullough L.D., Kumbar S.G. Peripheral nerve regeneration strategies: electrically stimulating polymer based nerve growth conduits. Crit Rev Biomed Eng. 2015;43(2-3):131–159. doi: 10.1615/CritRevBiomedEng.2015014015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez Rezza A., Kulahci Y., Gorantla V.S., Zor F., Drzeniek N.M. Implantable biomaterials for peripheral nerve regeneration–technology trends and translational tribulations. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.863969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Y., Huang Q., Wang P., et al. Conductive hydrogel conduits with growth factor gradients for peripheral nerve repair in diabetics with non-suture tape. Adv Healthc Mater. 2022;11(16) doi: 10.1002/adhm.202200755. [DOI] [PubMed] [Google Scholar]
- 58.Bergmeister K.D., Aman M., Muceli S., et al. Peripheral nerve transfers change target muscle structure and function. Sci Adv. 2019;5(1) doi: 10.1126/sciadv.aau2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jo H., Yoon M., Gajendiran M., Kim K. Recent strategies in fabrication of gradient hydrogels for tissue engineering applications. Macromol Biosci. 2020;20(3) doi: 10.1002/mabi.201900300. [DOI] [PubMed] [Google Scholar]
- 60.Chang Y.C., Chen M.H., Liao S.Y., et al. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl Mater Interfaces. 2017;9(43):37623–37636. doi: 10.1021/acsami.7b12567. [DOI] [PubMed] [Google Scholar]
- 61.Shellard A., Szabó A., Trepat X., Mayor R. Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis. Science. 2018;362(6412):339–343. doi: 10.1126/science.aau3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munir K.S., Wen C., Li Y. Carbon nanotubes and graphene as nanoreinforcements in metallic biomaterials: a review. Adv Biosyst. 2019;3(3) doi: 10.1002/adbi.201800212. [DOI] [PubMed] [Google Scholar]
- 63.Sun Y., Liu X., George M.N., et al. Enhanced nerve cell proliferation and differentiation on electrically conductive scaffolds embedded with graphene and carbon nanotubes. J Biomed Mater Res A. 2021;109(2):193–206. doi: 10.1002/jbm.a.37016. [DOI] [PubMed] [Google Scholar]
- 64.Daly W., Yao L., Zeugolis D., Windebank A., Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9(67):202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madduri S., Gander B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J Control Release. 2012;161(2):274–282. doi: 10.1016/j.jconrel.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 66.Rbia N., Bulstra L.F., Lewallen E.A., Hovius S.E.R., van Wijnen A.J., Shin A.Y. Seeding decellularized nerve allografts with adipose-derived mesenchymal stromal cells: an in vitro analysis of the gene expression and growth factors produced. J Plast Reconstr Aesthet Surg. 2019;72(8):1316–1325. doi: 10.1016/j.bjps.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Li R., Li D.H., Zhang H.Y., Wang J., Li X.K., Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin. 2020;41(10):1289–1300. doi: 10.1038/s41401-019-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson B.N., Lancaster K.Z., Zhen G., et al. 3D printed anatomical nerve regeneration pathways. Adv Funct Mater. 2015;25(39):6205–6217. doi: 10.1002/adfm.201501760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan B., Levenberg S. The role of biomaterials in peripheral nerve and spinal cord injury: a review. Int J Mol Sci. 2022;23(3):1244. doi: 10.3390/ijms23031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh I., Lacko C.S., Zhao Z., Schmidt C.E., Rinaldi C. Preparation and evaluation of microfluidic magnetic alginate microparticles for magnetically templated hydrogels. J Colloid Interface Sci. 2020;561:647–658. doi: 10.1016/j.jcis.2019.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Majed A.A., Neumann C.M., Brushart T.M., Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20(7):2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13(2):295–310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brushart T.M., Hoffman P.N., Royall R.M., Murinson B.B., Witzel C., Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22(15):6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geremia N.M., Gordon T., Brushart T.M., Al-Majed A.A., Verge V.M. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205(2):347–359. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 75.Atkinson E.W., Kuliasha C.A., Kasper M., et al. Examining the in vivo functionality of the magnetically aligned regenerative tissue-engineered electronic nerve interface (MARTEENI) J Neural Eng. 2022;19(5) doi: 10.1088/1741-2552/ac8bfe. [DOI] [PubMed] [Google Scholar]
- 76.Guenard V., Kleitman N., Morrissey T.K., Bunge R.P., Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12(9):3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mosahebi A., Fuller P., Wiberg M., Terenghi G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173(2):213–223. doi: 10.1006/exnr.2001.7846. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z., Zhang Y., Wang L., Ito Y., Li G., Zhang P. Nerve implants with bioactive interfaces enhance neurite outgrowth and nerve regeneration in vivo. Colloids Surf B Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112731. [DOI] [PubMed] [Google Scholar]
- 79.Ikegami Y., Shafiq M., Aishima S., Ijima H. Heparin/growth factors-immobilized aligned electrospun nanofibers promote nerve regeneration in polycaprolactone/gelatin-based nerve guidance conduits. Adv Fiber Mater. 2023;5(2):554–573. [Google Scholar]