Abstract
Opioids remain the mainstay of post-surgical pain management; however, concerns regarding addiction and side effects necessitate the exploration of alternatives. This narrative review highlights the potential of nerve blocks as a safe and effective strategy for post-surgical pain control. This review explores the use of various nerve block techniques tailored to specific surgical procedures. These include nerve blocks for abdominal surgeries; fascial plane blocks for chest surgeries; nerve blocks for arm surgeries; and nerve blocks for lower limb surgery including; femoral, hip, and knee surgeries. By targeting specific nerves, these blocks can provide targeted pain relief without the negative side effects associated with opioids. Emerging evidence suggests that nerve blocks can be as effective as opioids in managing pain, while potentially offering additional benefits such as faster recovery, improved patient satisfaction, and reduced reliance on opioids. However, the effectiveness of nerve blocks varies depending on type of surgery, and in individual patients. Rebound pain, which temporary increase in pain after a block wears off, can occur. In addition, some techniques require specialized guidance for accurate placement. In conclusion, nerve blocks show great promise as effective alternatives for managing post-surgical pain. They can reduce the need for opioids and their side effects, leading to better patient outcomes and satisfaction. Future studies should assess the long-term impacts of specific nerve blocks on mortality rates, cost-effectiveness, and their incorporation into multimodal pain management approaches to further enhance post-surgical care.
Keywords: postoperative pain, nerve blocks, opioid-sparing, pain management, surgical procedures, complications
Introduction
Annually, the US witnesses 100 million surgical procedures, over 60% in outpatient settings, and 80% of patients experience postoperative pain. Better control of postoperative discomfort has become a priority over the past 20 years because of its positive impact on clinical and economic outcomes.1 Postoperative pain poses a crucial obstacle to effective rehabilitation following surgery including hip surgery. Despite extensive research, the optimal analgesic approach remains controversial. Opioids, which are commonly employed for pain management, have significant side effects including nausea, vomiting, profound dizziness, and urinary retention.2 Although frequently integrated into multimodal analgesia, parenteral opioid analgesics pose notable risks, notably respiratory depression, making their use challenging, particularly among the elderly.3 Elderly individuals, characterized by physiological changes and numerous comorbidities, pose challenges in assessing and treating pain.4 Acetaminophen is effective in managing osteoarthritis and low back pain symptoms and, offering safety advantages over traditional NSAIDs. However, its long-term use at high doses may pose a risk of renal toxicity. Adjusting the dosage should be considered before using stronger medications.5 Pain can be triggered by harmful sensory inputs or neuropathological processes, and memories, expectations, and emotions can shape and alter the pain experience.5 Despite the pain management guidelines, their impact in clinical practices and pain control remains limited.
A national survey revealed only that 46% of hospitals had formal pain management programs. Of the surveyed adults who underwent surgery, 77% reported postoperative pain, with 61% experiencing moderate-to-extreme pain.1 Chronic pain is widely acknowledged as a significant public health concern with substantial psychological, physical, and socio-economic impacts. Owing to the diverse study parameters, prevalence estimates vary (2–55%). The absence of standardized criteria necessitates consideration of proposed alternatives to address this complex concern.6 Researchers are looking at ways to prevent this pain, including the use of different medications before, during, and after surgery.7
The research findings provide moderately compelling evidence that regional anesthesia could potentially decrease the likelihood of developing persistent postoperative pain (PPP) within 3–18 months post-thoracotomy, and 3–12 months after post-cesarean section. Similarly, there is limited evidence to suggest that regional anesthesia may lower the risk of postmastectomy pain syndrome within 3–12 months of breast cancer surgery.8 Another study involving 50 patients undergoing primary direct anterior approach THA, assigned to receive either preoperative FICB or intraoperative surgeon-delivered surgeon-delivered (PCB), found no significant differences in average VAS pain scores in the PACU or in pain scores and patient-reported pain satisfaction at the 3-week postoperative visit between the two groups. The authors found that the PCB and FICB were equally effective in managing postoperative pain, as evidenced by similar patient-reported pain levels and in-hospital opioid use.9 However, other studies have reported different findings, one study found no significant difference in overall discomfort scores after surgery compared to traditional methods, it suggests that femoral nerve block (FNB) may provide better pain relief at rest.10 A meta-analysis of RCT indicated that FIB did not provide substantial pain relief after THA, possibly due to incomplete coverage of the surgical area.11 This narrative review investigates the effectiveness of nerve blocks in management of both acute and chronic postoperative pain. It analyzes clinical trials, reviews, and meta-analyses to assess how various nerve block techniques can decrease pain, and opioid reliance, and prevent the development of long-term pain following surgery. Additionally, this review aimed to evaluate the potential effects of local anesthetics after the nerve block analgesia, with a specific focus on minimizing the risk of persistent pain after surgery.
Persistent Surgical Pain
Despite progress in the surgical results, the weight of the postoperative pain persists. Each year, more than 300 million surgeries worldwide subject patients to this notable, yet frequently disregarded, outcome.12 A substantial proportion of patients undergoing surgery experience difficulty continuing moderate-to-severe pain (Table 1). For some, this discomfort may escalate in intensity, posing a significant challenge to recovery.13 Failure to effectively manage postoperative pain can have significant negative consequences, both for patients’ well-being and healthcare systems.12 Research dating back to 1998 established surgery as a significant contributor to chronic pain. Incidence rates, which depend on definitions and types of surgical procedures, range from 5% to 50%.14
Table 1.
Potential for Moderate to Long-Lasting Pain Following Surgical Procedures
| Type of Surgery | Moderate to Persistent Pain (%) | References |
|---|---|---|
| Hernia repair | 15.8 – 16.2% | 20 |
| Hysterectomy | 28% | 21 |
| Amputation | 50 – 85% | 22 |
| Knee replacement | 20% – 40% | 23 |
| Hip replacement | 23% | 24 |
| Cesarean section | 25.5% | 25 |
| Cholecystectomy | 5 to 32% | 7 |
| Mastectomy | 13 to 69% | 7 |
| Lumbar surgery | 20.8% to 61.8% | 26 |
| Thoracotomy | 5 – 65% | 27 |
| Cardiac surgery | 27 – 41% | 28 |
Notes: The data in this table demonstrate the variability in the incidence of moderate to persistent pain across different surgical procedures, underscoring the need for personalized pain management strategies to alleviate long-term discomfort.
In study conducted by Gan et al1 involving 300 participants, a large majority 80–86% reported experiencing acute postoperative pain. Additionally, the study highlighted a high prevalence of moderate to extreme pain; staggering 70–75% of surgical patients experience pain immediately after surgery, and this discomfort persists in a significant portion (60–74%) even after discharge, impacting their recovery during the crucial first two weeks at home. A recent survey in Norway revealed that 40.4% of patients experienced Persistent Postoperative Pain (PPP), with 18.3% reporting persistent moderate to severe postoperative pain.15 Studies have shown that pre-existing chronic pain before surgery can significantly increase the risk of developing postoperative cognitive dysfunction within one week of surgery. This risk appears to be particularly high in elderly patients undergoing major surgeries like hip arthroplasty.14 A study of 175 patients revealed that 28% had Persistent Postoperative Pain (PPP), which affects Quality of Life (QoL) and daily activities. Risk factors included preoperative pain, medication use, and knee/hip replacements which showed a higher association with PPP. Treatment yielded a 69% symptomatic relief in affected patients.15
Challenges and New Approaches to Persistent Surgical Pain
Patient with chronic pain patients find it harder to treat post-surgery pain and require more opioids. Uncontrolled pain leads to longer hospital stays and other health problems. Effective pain control methods are crucial for the effective planning of surgery. Pre-existing pain, chronic opioid use, surgery type, and inflammation all worsen post-surgical pain.16 Total knee arthroplasty (TKA) is a major surgery identified as a significant risk factor for developing chronic opioid dependence after surgery and hospital stays exceeding three days, with age having a mixed influence on predicting changes in opioid use.17 A study involving 11,986 adult surgical patients found that complications were associated with enduring post-surgical pain. Insights into this connection could inform strategies to mitigate persistent pain after surgery.18 Current pain medications often cause problems and do not work well for long-term pain management. Studying how nerves contribute to inflammation offers a new target for pain relief that might avoid the side effects associate with other medications.19
A review of 162 studies revealed opioids might slightly help with short-term pain, but have side effects that worsen over time. There were no advantages over other pain drugs. Long-term use may lead to serious health problems, and combining them with other drugs may be risky.20 In a longitudinal study, 840 (11.4%) and 726 (11.0%) participants had complete baseline data and were followed up at one-year and two-year intervals to assess outcomes reported acute and severe discomforts, respectively. The increased likelihood of discomfort at both time points was linked to pre-fracture hip pain and opioid use. Female sex, younger age, and significant pre-fracture functional status were also associated with persistent pain. Approximately 10% of hip fracture patients undergoing arthroplasty may experience significant pain for up two years post-surgery.21 PPP is a frequently encountered complication that has often not receive sufficient attention. Although it cannot be completely avoided, recognizing potential risk factors and implementing preventive measures such as enhancing pain management could potentially decrease its occurrence. Effective management by a multidisciplinary team can enhance pain control and the quality of life of patients.22
Preoperative Factors of Postoperative Persistent Pain
Chronic pain after surgery is a complex issue with no easy solution. It can vary depending on the patient and type of surgery (Table 1). Pain significantly affects the patients’ lives.7 High levels of pain before surgery, significant pain catastrophizing, increased neuropathic pain symptoms, dysfunctional pain modulation, and elevated clinical pain intensity one year after total knee arthroplasty are all interconnected (Figure 1).23 The amalgamation of heightened preoperative clinical pain scores, elevated pain catastrophizing levels, and dysfunctional pain modulation mechanisms anticipated approximate 20.5% of the variability in resting pain levels at 12 months post-total knee arthroplasty. Both preoperative clinical pain intensity and pain catastrophizing individually contributed to the predictive model.23
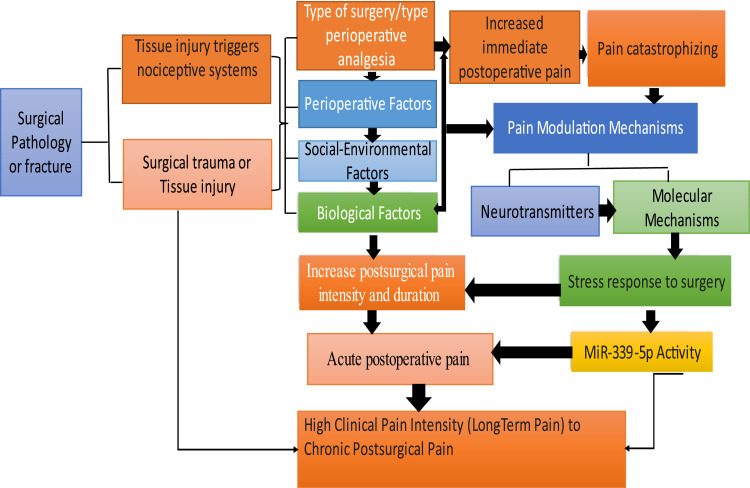
Figure 1.
The transition from acute pain to chronic pain. This figure illustrates the complex process through which acute postoperative pain can progress to chronic pain: Surgical trauma or tissue injury activate nociceptive systems. Perioperative factors, such as the type of surgery, analgesic approach, and pain catastrophizing, interact with social-environmental and biological factors to influence pain modulation mechanisms. Increased postsurgical pain intensity and duration, driven by neurotransmitter activity, molecular mechanisms, stress responses, and MiR-339-5p activity, contribute to the development of high clinical pain intensity and chronic postsurgical pain.
Preoperative risk factors can increased the postoperative discomfort after THA. It is important to address modifiable factors before surgery and utilize non-modifiable factors for patient education, shared decision-making, and personalized pain control.24 Preoperative psychological factors such as anxiety, depression, and low sexual satisfaction, along with pre-surgery pain, increased pain after movement, and female sex, all contribute to the heightened risk of chronic pain in Chinese women after surgery. These findings underscore the importance of providing preoperative psychosocial support and ensuring effective postoperative pain management a preventive measures.25
Understanding Postoperative Pain Transition
Multiple biological, psychological, and social factors interact to create a complex transition from acute to chronic post-surgical pain (Figure 1).26 Researchers have examined response of rats to pain after surgery. They identified distinct nerve cells responsible for various types of pain, such as pain while at rest or during movement and emotional shifts. This discovery indicates potential new drug targets for post-surgical pain management with fewer side effects. Exploring these mechanisms could potentially improve pain treatment for patients.27 The feeling of stressed around the surgery may extend the incision pain recovery time. Researchers have identified a molecule (miR-339-5p) in the brain that appears to play a role in the brain. This molecule can turn a pain-blocking receptor (MOR) by latching onto a specific part of its gene (oprm1). This pathway may cause chronic post-surgical pain (CPSP).28 Chronic postoperative discomfort may be reduced by aggressive perioperative multimodal treatment; however, some studies have not supported this.26
Multimodal Approach to Post-Surgery Pain
Although opioids traditionally play a crucial role in controlling postoperative pain, their negative effects can hinder patient recovery. The opioid crisis, fueled in part by excessive medical opioid use, has led surgeons to adopt Enhanced Recovery After Surgery. These techniques incorporate various non-opioid pain relief techniques to reduce opioid use during and after a patient’s hospitalization (Tables 2- 6).29 Despite these acknowledged disadvantages, opioids continue to be used in managing perioperative pain, even though studies have highlighted the effectiveness of techniques that are free of or use fewer opioids.30 Non-opioid and adjuvant analgesics are essential for managing perioperative pain by modifying changes in nociceptive systems caused by tissue injury. Commonly used non-opioid analgesics include acetaminophen, nonsteroidal anti-inflammatory drugs NSAIDs, and coxibs.31 Although non-opioid analgesics are effective for certain types of pain, they may not be as effective for dynamic pain, and the use of opioid analgesics is linked to potential adverse effects.32
Table 2.
Nerve Block Techniques for Upper Limb Surgeries
| Nerve Block Technique | Indications | Advantages | Limitations |
|---|---|---|---|
| Brachial Plexus Block | Upper limb surgeries (shoulder, arm, hand) | Effective pain relief for the entire arm | Requires specialized guidance for placement; risks include bleeding and nerve injury |
| Supraclavicular Brachial Plexus Block | Upper limb surgeries (shoulder, arm, hand) | Reliable and rapid onset anesthesia | Requires specialized guidance; complications include vessel and nerve injury, bleeding, and infections |
| Interscalene Brachial Plexus Block | Upper limb surgeries (shoulder, arm, hand) | Effective anesthesia for procedures above the elbow | Potential for complications, including phrenic nerve block |
| Axillary Brachial Plexus Block | Upper limb surgeries (arm, hand) | Effective anesthesia for procedures below the elbow | Risk of nerve injury, pain at the injection site, and bleeding |
Notes: These Brachial plexus nerve block techniques are widely used in shoulder and arm surgeries to provide effective pain relief and regional anesthesia, but require precise placement to minimize the risk of complications such as nerve damage and bleeding.
Table 6.
Nerve Block Techniques Used in Various Surgeries
| Nerve Block Technique | Indications | Advantages | Limitations |
|---|---|---|---|
| Quadratus Lumborum Block (QLB) | Flank surgeries, lower abdominal surgeries, hip surgery | Pain relief after flank, lower abdominal, and hip surgeries | Adverse effects of local anesthetics, including muscle weakness, toxicity, and infection |
| Paravertebral Block | Major abdominal or thoracic surgeries, chronic pain management | Effective pain relief | Higher risk of complications compared to other techniques; risks include pulmonary hemorrhage |
| Epidural Analgesia | Major abdominal or thoracic surgeries, Cesarean sections | Continuous pain relief | Higher risk of infections compared to other techniques; typically placed before surgery |
| Erector Spinae Plane Block (ESPB) | Upper abdominal surgeries, spinal surgeries, hip surgery | Effective pain management | Risks include bleeding, infection, pain at the injection site, and local anesthetic toxicity |
Notes: Nerve block techniques like QLB, Paravertebral Block, Epidural Analgesia, and ESPB are used to provide effective pain relief for various surgical procedures, but each method carries specific risks that require careful monitoring during and after implementation.
NSAIDs can offer pain relief; however, caution is advised when treating patients with renal impairment. Anticonvulsant medications such as gabapentin and pregabalin, offer an alternative approach to pain management, especially for neuropathic pain, through a combination of therapeutic strategies.33 The relationship between pain and depression is bidirectional, involving neurotransmitters such as serotonin and norepinephrine, as well as brain regions responsible for pain perception and mood regulation. Antidepressants, particularly tricyclic antidepressants (TCAs), are beneficial for the management of chronic pain.34 Moreover, the use of intraoperative dexmedetomidine administration at a rate of 0.5 μg/kg/h is linked to a reduced occurrence of long-term post-hysterectomy pain.25 Perioperative peripheral nerve blocks reduce opioid use both during and after surgery, with the most significant decrease in the first twenty-four to seventy-two hours postoperatively patients report lower pain score. Its effectiveness is high, but the use of nerve blocks varies (Tables 2–6), – and the reasons for this are being investigated.35 Anna Unneby et al,36 conducted an RCT that showed femoral nerve blocks reduced pain scores and opioid use in hip fracture patients, including those with dementia, compared to conventional pain relief.
Table 3.
Nerve Block Techniques for Chest Wall Surgeries
| Nerve Block Technique | Indications | Advantages |
|---|---|---|
| Serratus Anterior Plane Block (SAPB) | Chest wall and breast surgeries | - Effective pain relief in the front and side of the chest - Promotes faster recovery - Reduces reliance on opioids |
| Deep Parasternal Intercostal Plane Block (DPIPB) | Cardiac and thoracic surgeries | Effective pain relief during and after surgery |
| Pectoralis Nerve Block | Chest wall and breast surgeries | Effective pain relief for chest wall and breast procedures |
Notes: Nerve block techniques such as SAPB, DPIPB, and the Pectoralis Nerve Block can effectively manage postoperative pain, reduce the need for opioids, and promote faster recovery in various chest wall and breast surgeries.
Table 4.
Nerve Block Techniques for Abdominal Surgeries
| Nerve Block Technique | Indications | Advantages |
|---|---|---|
| TAP Block (Transversus Abdominis Plane Block) | Abdominal surgeries, laparoscopic procedures | Pain relief after abdominal surgeries |
| Rectus Sheath Block (RSB) | Abdominal surgeries | Pain relief after abdominal surgeries |
| Ilioinguinal and Iliohypogastric Nerve Block | Lower abdominal wall surgeries (eg, inguinal hernia repair, lower flank incisions) | Pain relief for lower abdominal wall surgeries |
Notes: These nerve block techniques are effective and widely used to enhance pain management after surgery, diminish the reliance on opioid analgesics, and facilitate quicker recovery following abdominal procedures.
Table 5.
Nerve Block Techniques for Hip and Knee Surgeries
| Nerve Block Technique | Indications | Advantages | Limitations |
|---|---|---|---|
| Lumbar and Sacral Plexus Block | Lower limb surgeries such as hip and femoral surgeries | Stable blood pressure, postoperative comfort, reduced hospital stays, lower costs | Adverse effects of local anesthetic, including muscle weakness |
| Psoas Compartment Block (PCB) | Hip, femoral, or knee surgery | Alleviates postoperative pain, effective for 48 hours (continuous) | Several complications, such as organ injury when not well-monitored |
| Femoral Nerve Block | Hip or knee surgery, ACL reconstruction (combined with sciatic nerve block) | Better pain relief than local infiltration | May not provide effective analgesia for hip surgery, fall risks, allergy to local anesthetic |
| PENG Block | Hip replacement surgery | Reduced pain during movement, lower opioid use, faster recovery | Conflicting research on postoperative analgesia effectiveness, pain at injection site, infection |
| Local Anesthesia Infiltration (LAI) | Various surgeries (including hip and knee) | Comparable pain relief to single nerve block | Pain at injection site, infection, bleeding |
| Adductor Canal Block (ACB) | TKA | Improved early pain control, reduced opioid use, improved sleep quality (combined with LIA) | Adverse effects of local anesthetic, discomfort at injection site |
| Fascia Iliaca Compartment Block (FICB) | Hip, femur, and knee surgeries | Reduced pain scores, reduced need for rescue analgesia and postoperative complications | Adverse effects of local anesthetic, reduced muscle strength, infection, and allergy |
| Saphenous Nerve Block | Knee surgery (especially for high-risk patients) | Improved pain relief | Local anesthetic toxicity, pain at injection site, infection, bleeding, quadriceps weakness |
| Sciatic Nerve Block | Leg surgeries | Effective pain management | Adverse effects of local anesthetic, including toxicity, discomfort at injection site, infection, and bleeding |
| Popliteal Nerve Block (PSNB) | Leg surgeries | Effective pain management, improved patient comfort | Adverse effects of local anesthetic, including toxicity, discomfort at injection site, infection, and bleeding |
Notes: These nerve block techniques are widely used in various lower limb surgeries to provide effective regional pain relief, although each approach has its unique risks and limitations that require careful consideration during implementation.
Regional Anesthesia
Postoperative pain persisted after cardiac surgery. Regional anesthesia or local anesthetic infusions reduce pain during thoracotomy, cesarean section, and breast cancer surgery. Notably, regional anesthesia in cardiac surgery, for both children and adults, significantly lowers postoperative discomfort scores and decreases opioid need (Tables 3–6).37 Perioperative regional anesthesia has benefits beyond pain relief. Studies have shown that neuraxial anesthesia in hip and knee arthroplasty reduces patient deaths, major morbidities, and time of hospital stay compared to general anesthesia.38 A study focusing on optimal pain control strategies after an open liver procedure proposes a combination of anti-inflammatory medications with either a specific spinal blocks or a targeted nerve blocks. Opioid medications should be reserved for cases in which alternative treatment methods are ineffective.39 Considering the promising outcomes of recent trials, the adoption of regional anesthesia should be strongly considered for patients undergoing cancer surgery. This approach offers several benefits, including improved pain management, faster postoperative recovery, and reduced reliance on opioids.40
Local Anesthetics as New Weapon Against Pain
Procaine and tetracaine fall under the ester category, whereas lidocaine, mepivacaine, bupivacaine, ropivacaine, and levobupivacaine are classified as amides. These anesthetics are administered locally and undergo liver elimination, whereas the ester-bonded undergo hydrolysis. Residual drug molecules penetrate nerve cells, inhibiting action potential generation and transmission. Local anesthetics exist as ionic (BH+) and nonionic (B) types, with the lipid-soluble nonionic type passing through nerve cell membranes. Once within the cell, the ionic type increases, binding to Na+ channels on the cell membrane, blocking Na+ influx, and inhibiting action potential generation.41 Local anesthetics function through various mechanisms to inhibit pain transmission. Their primary focus is sodium channels in nerves; however, they also affect calcium channels, receptors, and other molecules (Figure 2). This comprehensive approach interferes with pain transmission across the entire nervous system from the peripheral nerves to the spinal cord.42 They also suppress inflammation and release of neurotransmitters. They further inhibit pain transmission and inflammation of the spinal cord.
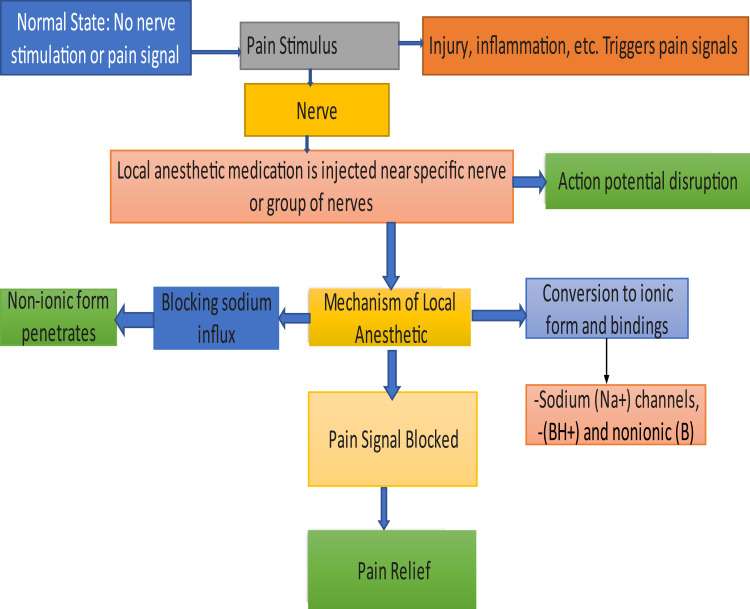
Figure 2.
Mechanism of local anesthetics. This figure illustrates how local anesthetics block pain signals: Normally, no pain signals are transmitted, but injury or inflammation triggers nerve pain. When local anesthetic is injected near the targeted nerves, it disrupts action potentials by blocking sodium (Na+) channels. The anesthetic penetrates in its non-ionic form, converts to an ionic form, and binds to sodium channels, ultimately blocking the transmission of pain signals and providing pain relief.
These diverse actions have powerful analgesic effect.42 Liposomal bupivacaine could be a valuable option for managing post-surgical pain as opioid drugs become increasingly limited.43
Adjuvant Prolonging Local Anesthetic Effects
Adjuvant analgesics, including corticosteroids, bisphosphonates, antidepressants, anticonvulsants, and topical analgesics, complement or replace opioids for enhanced pain management.44 These include NMDA receptor antagonists, such as ketamine and magnesium, anticonvulsants such as gabapentinoids, membrane stabilizers such as lidocaine, and alpha-2-agonists such as clonidine and dexmedetomidine.31 Several agents, when combined with local anesthetics, prolong the duration of action. However, they also cause adverse effects. While adrenaline minimally extends analgesia, buprenorphine significantly prolongs it at the expense of an increased likelihood of nausea and vomiting. Alpha-2-agonists such as clonidine and dexmedetomidine moderately extend analgesia but are linked to sedation, low blood pressure, and bradycardia.38 The administration of perineural dexamethasone demonstrates a dose-response pattern, showing that doses of up to 4 mg can prolong the average duration of pain relief by an additional 8 h. However, it is necessary to consider the compatibility of dexamethasone with ropivacaine because of possible crystallization, unlike bupivacaine.38
In a study conducted by Felipe Muñoz-Leyva,45 incorporating supplementary interventions such as iPACK block, IV dexmedetomidine, IV ketamine, additional IV dexamethasone doses, and repeated injections of adductor canal block alongside existing methods did not result in any further decrease in opioid consumption or pain scores, nor did it lead to enhanced functional outcomes following total knee arthroplasty. Similarly, a recent investigation on botulinum toxin A (BoNT/A) of phantom limb pain yielded inconclusive results, maintaining the uncertainty surrounding the efficacy of various medications such as opioids, anticonvulsants, NMDA receptor antagonists, antidepressants, calcitonin, and local anesthetics for pain relief.46 Among the various substances investigated for local anesthesia infiltration, alpha-2 agonists present the strongest evidence, while others such as fentanyl, ketorolac, dexamethasone, and magnesium exhibit potential but require additional validation.47 While most studies have confirmed their safety, well-designed randomized controlled trials are indispensable for comprehensive comparisons and substantiated conclusions regarding the benefits of additional substances in local infiltrative anesthesia for postoperative pain relief.47
Postoperative Pain Management with Peripheral Nerve Blocks
Nerve Block in the Upper Limb Surgeries
Interscalene Block
Reverse total shoulder arthroplasty (RTSA) is commonly performed for patient with advanced shoulder arthritis. The use of a combined suprascapular and axillary nerve block has been demonstrated to produce effective postoperative comfort, thereby reducing the need for opioid medications and mitigating respiratory risk. A case study highlighted the successful management of pain without opioids in a patient with chronic obstructive pulmonary disease (COPD), contributing to a smooth recovery process.48 While the interscalene block is a standard approach for shoulder surgery (Table 2), it carries the risk of phrenic nerve palsy. Given that the suprascapular and axillary(SSAX) nerves innervate the shoulder region, the combination of these blocks using the SSAX method has been shown to offer efficient analgesia without compromising diaphragmatic function. A study involving four cases demonstrated successful pain control without any associated complications.49 Additionally, preoperative patient factors have been identified as predictive indicators of increased respiratory complications in elective shoulder arthroplasty procedures when utilizing continuous interscalene brachial plexus blocks.50
Supraclavicular Block
In an analysis involving 15 studies with 1065 participants, suprascapular nerve block (SCB) demonstrated non-inferiority to interscalene block (ISB) in controlling postoperative pain during shoulder surgery. SCB was found to decrease the odds of respiratory dysfunction and minor complications, positioning it as a viable alternative to ISB with comparable efficacy in opioid usage and pain relief outcomes.48 In a per-protocol cohort of 103 patients, suprascapular nerve block (52SCB) was shown to be non-inferior to interscalene block (51ISB) in providing postoperative analgesia for ambulatory arthroscopic rotator cuff repair.
SCB exhibited similar levels of pain control and patient satisfaction (98%) but resulted in lower morphine consumption (9.4mg versus 14.7mg) when compared to ISB.51
Brachial Plexus Block
Ultrasound-guided selective nerve blockades at the upper and lower arm levels have been shown to preserve elbow motor function, offer comparable postoperative analgesia, and improve patient satisfaction after hand or forearm surgery (Table 3).52 In a study conducted by Patel et al,53 researchers investigated a one-injection nerve block technique using a specific medication (liposomal bupivacaine) in patients undergoing shoulder surgery. This approach proved successful in controlling pain up to 48 h after surgery and led to a decreased requirement for opioid medications compared With patients who did not undergo this nerve block. Furthermore, Farladansky et al compared pregabalin with brachial plexus nerve blockade for pain management after shoulder surgery. The results indicated that both interventions provided similar pain relief for 10 days post-surgery.54
Techniques for Chest Wall Surgeries
Serratus Anterior Plane Block (SAPB) and Deep Parasternal Intercostal Plane Block
Opioid use following surgery is a concern; however, new techniques are emerging that offer effective pain relief with fewer side effects. Fascial plane blocks are promising alternatives to traditional methods and, show particular promise in cardiac and thoracic surgery.55 One such block, SAPB, effectively targets the areas of discomfort that patients typically experience after thoracic surgery, including incision site and chest tube placement (Table 3). This safe and easy-to-perform block can promote faster recovery, reduce reliance on opioids, and prevent other complications.56 SAPB is a highly efficient regional block method that focuses on the nerve network supplying the chest wall and breast, offering effective pain relief in the front and side of the chest. It is anticipated to be utilized as a standalone technique in minimally invasive breast surgery, decreasing the need for opioids, alleviating post-surgery acute pain, and facilitating rapid patient recovery.57 However, tailoring this approach to the specific needs of patient is crucial. For coronary artery bypass grafting (CABG) surgery, a multimodal approach plan, including parasternal nerve blocks performed during surgery, produces effective postoperative pain-reduction and opioid use.58 Additionally, a preemptive Deep Parasternal Intercostal Plane Block (DPIPB) performed on both sides effectively relieved pain in patients undergoing CABG, both during the surgical procedure and in the initial phase of recovery. Notably, the block achieved similar pain relief when administered at either the T3-4 or T4-5 intercostal spaces.59 Bilateral deep PIP blocks have the potential to create a broad sensory block across the skin with varying distributions along the mid-sternum, lasting for a significant period.60 Ultrasound-assisted DPIP block proved to be a safe and efficient method for providing pain relief while maintaining stable hemodynamic function during sternotomy incision. Its ability to reduce the need for intraoperative opioids makes it a valuable component of a comprehensive pain management approach for cardiac surgery.61
Pectoralis Nerve Block
Chest wall nerve blocks offer a promising avenue for enhanced pain management after minimally invasive cardiac surgery (Table 3). These blocks involve the administration a local anesthetic near specific chest wall muscles, potentially diminishing the need for pain medications and expediting the recovery process.62 The utilization of Pectoralis Nerve Block II has shown potential as a valuable adjunct in pain management for patients undergoing robot-assisted trans axillary thyroidectomy (RATT), contributing to improved recovery by alleviating flap dissection-related pain.63 Moreover, PECS and intercostal nerve blocks have demonstrated efficacy in providing pain relief during heart device implantation in patients with heart failure; with comparable postoperative pain levels and opioid utilization between PECS block and control groups, alongside low complication rates.64 The application of PECTS II during breast reconstruction surgery has been shown to alleviate postoperative pain in the initial hours following the procedure, leading to reduced opioid needs within the first twenty-four hours and improved immediate postoperative pain relief.65 Severe pain following chest surgery can transition to chronic pain and escalate opioid consumption. Regional anesthesia techniques, including chest wall nerve blocks (Table 3), may serve as a viable approach to mitigate both short-term and long-term postoperative pain, thereby aiding patients in minimizing their reliance on opioids and enhancing their overall recovery experience.62
Techniques for Abdominal Wall Surgeries
Transversus Abdominis Plane Block (TAP B)
TAPB is a regional anesthesia method that involves administering a local anesthetic around the transversus abdominis muscle, a significant component of the abdominal wall. TAPB are frequently used to alleviate pain following abdominal surgeries such as major abdominal surgeries (Table 4). Utilizing ultrasound guidance, TAPB is effective in decreasing postoperative opioid utilization in patients undergoing Periacetabular Osteotomy (PAO), potentially improving pain control during the initial 48 h postoperatively.66 A meta-analysis involving 13 studies (600 patients with TAP block, 762 without) on laparoscopic colorectal surgery indicated that TAP block reduced postoperative opioid utilization and shorted the time to bowel movement without increasing the incidence of complications.67 In the context of cesarean delivery, subarachnoid morphine (SAM) was found to produce more pain relief and prolong the duration before additional pain medication is required, compared to TAPB. However, SAM is associated with a higher incidence of nausea and itching, suggesting that while it may offer enhanced pain control, it also carries a higher likelihood of side effects.68
Rectus Sheath Block (RSB)
A study involving 100 robot-assisted laparoscopic prostatectomy (RALP) patients divided into RSB and non-RSB groups showed that preoperative RSB led to notably decreased pain scores and diminished opioid use after surgery, which consequently improved early pain management. However, further research is warranted to assess the long-term analgesic effectiveness of RSB.69 A meta-analysis found that post-surgery pain intensity did not decrease with RSB compared with that in the control group. RSB did not delay the initial request for opioids/analgesics. The heterogeneity of the studies indicates that there is no significant evidence to support RSB over non-RSB treatment alternatives. Sensitivity analysis confirmed the consistency of the results.70
However, the rectus sheath block was found to be reliable and efficient method for relieving postoperative pain in abdominal surgeries, particularly within the first 2 hours after the procedure. Moreover, it is associated with reduced opioid usage for up to twenty- four hours after surgery, as indicated by the equivalent dose of intravenous morphine.71
Ilioinguinal and Iliohypogastric Nerve Block
Research comparing ultrasound-guided (USG) bilateral ilioinguinal and iliohypogastric nerve blocks to standard post-cesarean section care has demonstrated reduced pain levels, decreased morphine consumption, longer intervals without analgesics, and fewer complications in the nerve block group. This approach has proven to be effective in managing both acute and chronic postoperative pain, highlighting its safety and efficacy.72 An analysis conducted by Yue Xiang Wang et al suggests that USG II/IH nerve blocks and TAPBs are more effective for pain management following open inguinal surgery compared to traditional techniques, indicating their superiority in pain control (Table 4).73 The combination of genitofemoral nerve block with ilioinguinal and iliohypogastric nerve blocks (Table 4) has been shown to enhance anesthesia in elderly patients undergoing inguinal hernia repair, leading to reduced pain levels, decreased opioid usage, and fewer complications when performed under precise USG.74 In the case in an elderly patient with severe heart failure undergoing complex inguinal hernia repair, a single paravertebral block at the T11 level with ropivacaine effectively provided anesthesia. This technique not only facilitates successful surgery but also enables efficient postoperative pain management for the patient.75
Techniques for Hip and Knee Surgeries
Lumbar and Sacral Plexus Block
Providing anesthesia for surgery should be aligned with the surgical requirements, patient comfort, and proficiency of anesthetists. Utilizing a combined lumbar and sacral plexus block is a straightforward, secure, and efficient anesthetic approach. Its advantages include consistent hemodynamics, extended postoperative pain management, prompt mobilization, decreased hospitalization duration, and lowered expenses.76 Simultaneously blocking the sacral and lumbar plexuses offers a reliable and optimal anesthetic solution for high-risk patients undergoing single-leg surgery. This approach guarantees stable blood pressure, postoperative comfort, cost savings, and a reduced hospitalization duration.76
During THA or TKA, psoas compartment block (PCB) can be used to block the 2nd-to-4th lumbar nerve roots located within the psoas muscle. It is considered superior to other techniques in terms of anesthesia and analgesia because it blocks the obturator nerve and anesthetizes the other lumbar plexus nerves.77 In hip or knee surgery, PCB is used to alleviate postoperative pain. Continuous PCB treatment was effective for 48 h. A single-shot PCB reduces the need for morphine after THA and results in zero verbal rating.78 Continuous psoas compartment block effectively manages post-THA pain, reducing pain levels and the need for rescue analgesia, demonstrating superior pain relief compared to systemic analgesic injections.79
Pericapsular Nerve Group Block (PENGB)
Researchers assessed the effectiveness of the PENGB in controlling postoperative pain after hip replacement surgery. Almost 500 patients were given either a block or placebo injection. The group that received the block experienced less pain during movement, used less opioid; pain medication, and recovered function more quickly.80 Furthermore, patients who received additional PENGB during THA reported lower immediate postoperative pain and maintained quadriceps muscle strength.81 However, a review of nine studies found that the PENGB did not significantly differ from the control groups in terms of morphine usage, or time to need rescue analgesia after surgery. Additionally, static and dynamic pain scores did not show significant differences compared with the other nerve block methods. Therefore, the PENGB is a feasible alternative for hip surgery.82 The PENGB was found to be an effective method for reducing hip surgery pain in elderly patients. It helps diminish the use of opioids and the occurrence of complications while also improving early movement and rehabilitation. This technique is a dependable and efficient method for relieving pain.83 A preliminary study involving 74 patients suggested that the PENGB could be an effective option for managing pain during hip surgery with minimal side effects. However, further research is required to confirm its effectiveness and safety.84 A meta-analysis indicated that PENGB successfully reduced opioid use following hip fracture surgery for up to 24 hours, providing a potential alternative for pain management with a lower risk of addiction. Nonetheless, more extensive and prolonged studies are required to confirm this benefit.85
Fascia Iliaca Compartment Block (FICB)
Research has found that utilizing 0.5 mL/kg of local anesthetic (LA) under ultrasound guidance for infra-inguinal FICB results in the most effective relief after femoral and knee surgeries. Pain levels and use of pain medication were reduced, particularly in the first 6–8h, indicating that FICB is a safe and adequate approach for managing post-surgery discomfort.86 Continuous FICB improved recovery quality and reduced pain scores in elderly THA patients at 24h without causing complications related to the regional block. This finding that continuous FICB is a safe and adequate pain control technique for THA.87 In contrast, a large study showed that FICB did not provide better results than systemic analgesics for delirium, opioid use, or analgesic-related complications. However, it did significantly improve pain scores, indicating uncertainty in the clinical effectiveness of FICB and the need for a well-designed randomized controlled trial to further investigate its impact on delirium and opioid use.88 However, another research suggests that FICB leads to better pain control than intravenous fentanyl for positioning hip fracture patients for spinal anesthesia, with improved positioning quality, reduced procedure time, and reduced use of opioid pain medication on the first day after surgery. Additionally, individuals who underwent this nerve block reported higher levels.89 Elderly individuals with hip fractures may experience extended pain relief and improved recovery quality over 24 hours with Fascia Iliaca Compartment Block (FICB). However, it is important to note that this method can reduce postoperative muscle strength.90 S-FICB provides pain relief similar to Epidural Analgesia (EA) in the early period following hip procedure. Nevertheless, individuals in the EA group showed reduced pain levels on the Visual Analog Scale (VAS) compared to those in the S-FICB group after the 18th hour. Furthermore, patients undergoing EA have a higher likelihood of experiencing hypotension.91
Femoral Nerve Block (FNB)
The study compared various nerve block techniques for ACL reconstructions. All blocks reduced opioid use compared to not using a block; however, combining FNB and sciatic nerve blockades produced the most effective pain relief. However, it was noted that this combination may limit the function more than other techniques. Adductor canal block, local anesthesia infiltration, and no block were found to be ineffective for pain management.92 In a comparison between FNB and local anesthetic infiltration for post-ACL reconstruction pain management, FNB was found to offer better pain relief. However, existing literature has inadequately reported functional outcomes.93 The five-nerve genicular nerve block presents a viable alternative to local anesthetic infiltration in fast-track total knee arthroplasty, achieving effective pain management with lower opioid consumption.94 Ultrasound-guided femoral, obturator, and sciatic nerve blocks are adequate for ACL reconstruction, providing sufficient pain relief, reduced opioid use, and excellent safety.95
Local Anesthesia Infiltration
Injection of local anesthetics through has been demonstrated to be an effective means of pain relief. The comfort provided by injecting a local anesthetic was comparable to that provided by a single-shot nerve blockade. Despite the wealth of existing research on this subject, additional studies are necessary to enhance the efficacy of postoperative pain management; to reduce both pain levels and opioid consumption.96 A review of 27 trials indicated that Local Infiltration Analgesia (LIA) is sufficient to manage postoperative pain after Total Knee Arthroplasty (TKA), leading to reduced pain and opioid use. However, hip Arthroplasty (THA) was limited. Wound catheters did not show any benefits and pain relief did not affect the length of hospital stay. LIA is effective in TKA but less so in THA.97 LIA effectively alleviates acute pain, reduces postoperative nausea and vomiting, shortens the hospital stay, and improves range of motion after TKA. Peri-articular injection provides 24-hour pain relief, while catheter placement extends the analgesic effects to 48 hours but comes with a risk of infection. The efficacy of LIA with regional anesthetic techniques remains unproven.98
Combining an Adductor Canal Block with LIA improves early pain management after TKA by reducing the need for opioids, improving sleep quality, and maintaining a low complication rate.99 LIA or periarticular analgesia (PAI) during joint replacement surgery offers pain relief at rest, similar to that of peripheral nerve blocks or epidurals. However, this method may have limitations in terms of pain management during movement. Meta-analyses presented divergent outcomes for knee and hip surgeries, highlighting the need for additional research on hospital stay and time to discharge.100
Adductor Canal Block (ACB)
A comparison was made between total knee arthroplasty patients who received ACB with periarticular infiltration (PI), ACB alone, or PI alone. The group that received ACB + PI reported less walking pain in the first and second 24 hours after surgery.101 A meta-analysis of 11 trials involving 1185 patients concluded that continuous catheter-based ACB does not provide additional pain relief compared to single-shot ACB within the first two days after TKA.102 However, ACB combined with LIA offered better early pain relief, reduced opioid use, improved sleep quality, and no increase in TKA. Although the marginal statistical advantage may lack clinical significance, combined approaches still show promise for effective pain management and are recommended for improved knee pain control.99
Another meta-analysis by Wei Zuo et al,103 revealed that the ACB + LIA group had lower VAS scores at rest on the day of surgery and the following day, as well as reduced morphine consumption on those days compared to the ACB alone group. Additionally, the ACB + LIA group showed significantly better postoperative range of motion, with no significant differences in adverse event rates between the two groups. In a study involving 565 patients, those who received ACB were less likely to require anti-nausea medication after surgery. However, other outcomes, such as the length of hospital stay and narcotic consumption, did not show significant differences between the groups. After adjusting for risk factors, the only notable outcome was the reduced likelihood of experiencing nausea in patients who received ACB.104
Saphenous Nerve Block (SNB)
Utilizing SNB as a supplementary analgesic approach holds promise for effectively managing postoperative pain in patients undergoing knee surgery, particularly those undergoing TKA, with the aid of a continuous nerve catheter.105 The saphenous nerve is responsible for intense and rapid pain, and blocking this nerve improves the pain relief. These findings indicate that it may be beneficial to routinely include a saphenous nerve block in major ankle surgeries.106 Researchers suggest utilizing a combination of popliteal and SNB for knee surgeries, especially for high-risk patients with other medical conditions who are unsuitable for general or spinal anesthesia. This minimally invasive method provides effective pain control after surgery and presents a beneficial option for general anesthesia for procedures below the knee. The block can be performed with or without a nerve stimulator, and experience with 60 cases using this technique has demonstrated few adverse effects and positive outcomes.107 The study found that adding dexamethasone to a single-injection SNB prolonged pain relief in the ankle region and reduced opioid use on the first postoperative day. It suggests that this approach can improve pain management after major ankle surgery.108 However, Understanding the anatomy can be beneficial in identifying a protected area to avoid motor block during saphenous nerve block procedures.109 The combination of a Crosswise Approach to Popliteal Sciatic block (CAPSB) with saphenous nerve block may offer comprehensive pain relief for surgeries below the knee.110 Administering continuous popliteal and SNB in the adductor canal demonstrated decreased opioid requirements and lower pain levels, resulting in increased patient satisfaction upon discharge from the surgery center compared to employing only a continuous popliteal block alongside a single-injection SNB. Considering both popliteal and Saphenous nerve catheters together are crucial for enhancing postoperative pain management and streamlining outpatient procedures.111
Sciatic Nerve Block and Popliteal Nerve Block (PSNB)
Ultrasound-guided PSNB is a reliable and efficient technique for managing resting pain and enhancing patient comfort and adherence during endovascular procedures for chronic limb-threatening ischemia. This method is especially beneficial for frail patients with numerous comorbidities who are not suitable candidates for deep sedation or general anesthesia.112 Research has demonstrated that the addition of an SNB to a sciatic nerve block provides adequate comfort following extensive ankle surgery.106 Regional anesthesia proved to be a secure and dependable addition to managing perioperative pain, showing great effectiveness in patients undergoing elective orthopedic forefoot procedures (Tables 5). Nonetheless, those who underwent PFB experienced notably improved pain control and reduced need for opioids in the early perioperative period compared with those who were treated with an ankle block.113
Techniques Used in Various Surgeries
Quadratus Lumborum Block (QLB)
In a study involving 82 patients undergoing laparotomy, postoperative analgesia was provided through either a QLB or a pre-peritoneal catheter block with ropivacaine infusion for 48h. Pain scores during coughing were similar between the groups; however, the QLB group exhibited lower resting pain scores and higher satisfaction levels. The administered block levels ranged from T4 to L1.114 Nerve block targeting the QLB has been identified as an effective method for controlling pain after TKA. This approach has been proven to reduce pain, opioid usage, and nausea, while enhancing patient satisfaction.115 Another study investigated the use of a QLB to enhance pain relief after cesarean sections performed under spinal anesthesia and morphine administration. While the block resulted in reduced pain scores 6h after surgery, it did not significantly impact the overall morphine need within one day.116 However, a systematic examination and analysis revealed that QLB notably decreased morphine consumption and discomfort ratings among individuals undergoing hip surgery.2 Although the quality of evidence is moderate and statistically significant outcomes have been observed, the practical significance of QLB pain relief is questionable of its minimal effect size.2
Epidural Analgesia and Paravertebral Block
Effective pain management is crucial in patients undergoing thoracic surgery. Employing both medications and regional anesthesia, such as nerve blocks, in tandem is deemed the optimal strategy to decrease opioid consumption and mitigate associated adverse effects. The selection of a nerve block depends on the patient’s health condition and the proficiency of the healthcare provider (Tables 2–6).117 For thoracotomy, regional anesthesia is typically recommended, with Thoracic Epidural Analgesia (TEA) or Paravertebral Block (PVB) is the preferred options. If these methods are not feasible, alternatives such as an Intercostal Nerve Block (ICNB) can be considered (Table 3). Judicious use of opioids is essential to mitigate the risk of addiction, although it may still be necessary in certain situations. Nevertheless, healthcare providers strive to limit opioid administration to reduce adverse effects and potential addiction.117
Traditional methods of thoracic anesthesia, including epidural, spinal, and paravertebral blocks, carry inherent risks, particularly in patients undergoing cardiac surgery. Modern techniques that incorporate ultrasound guidance and regional nerve blocks offer safer alternatives, minimizing complications associated with conventional approaches.118 Although epidural analgesia initially provides superior pain control compared to systemic opioids, it has limitations, notably a declining efficacy within 18–24 hours.119,120 Studies exploring the effects of postoperative epidural analgesia on pain control and rehabilitation in patients with hip fracture have produced diverse findings, highlighting the necessity for a thorough assessment before its applications.121 However, pairing peripheral nerve blocks with epidural anesthesia offers supplementary benefits, particularly in elderly patients with cardiac comorbidities, thereby improving the overall perioperative care.122
Erector Spinae Plane Block (ESPB)
Surgical treatment of thoracic outlet syndrome (TOS) often results in significant postoperative pain. A successful multimodal analgesic approach utilizing ESPB after supraclavicular surgery has been reported to enhance postoperative pain management and promote recovery in patients.123 The application of ESPB with continuous infusion provides effective analgesia during trans-axillary first rib resection for venous thoracic outlet syndrome, facilitating efficient pain control; and enabling unrestricted postoperative physiotherapy without complications.124 In the context of post-video-assisted thoracic surgery (VATS), the combination of rhomboid intercostal block (RIB) and ESPB has demonstrated notable benefits, including reduced fentanyl consumption, lower pain scores within the initial 24h, and distinct inflammatory factor concentrations compared to serratus anterior block (SAB). Ultrasound-guided RIB and ESP have been shown effectiveness to enhance pain management and reduce opioid use after surgery.125 Additionally, ESPB has shown efficacy as an efficient option in adult cardiac surgery for perioperative pain control and facilitating a fast-tracking process.126 Research suggests that bilateral USG ESPB provides superior and prolonged postoperative pain management for total abdominal hysterectomy, resulting in decreased morphine consumption compared to patients without the block.127
A meta-analysis evaluating ESPB for lumbar surgery indicated reduced opioid utilization and improved pain management and postoperative outcomes, leading to enhanced patient satisfaction and potentially shorter hospital stay. However, low-quality evidence from the study necessitates further research to validate this findings.128 Studies involving 171 participants across various anesthetic planes have assessed ESPB for lumbar spine surgery, noting benefits in pain relief and decreased analgesic requirements. Nevertheless, the efficacy and safety of ESPB remain uncertain, underscoring the need for high-quality randomized controlled trials for confirmation.129 However, a series of cases documented in the literature demonstrated that patients who underwent hip and proximal femoral surgery experienced successful management of postoperative pain after receiving an ESPB at the 4th lumbar vertebra level.130 Additionally, the report also highlights the success of the ESPB block in alleviating severe thoracic neuropathic pain in two cases. This technique is safe and straightforward for managing both chronic and acute thoracic pain.131
Complications Associated with Nerve Blocks
Although peripheral nerve blocks and continuous PNBs are valuable pain management tools, the potential adverse events warrant consideration. PNBs present potential hazards such as local nerve damage, anesthetic systemic toxicity, infection, and motor block (Tables 2–6). The psoas component block introduces additional concerns, including retroperitoneal hematoma, kidney injury, and subarachnoid puncture. Implementing meticulous monitoring and adherence to optimal catheter placement strategies significantly reduces the complication rates. However, a comprehensive risk-benefit analysis considering both potential advantages and adverse effects remains crucial before implementing PNBs or CPNBs in clinical practice (Table 6).77,132 Additionally, research suggests that certain pain management techniques used during lower limb surgery may weaken muscles, potentially leading to a higher risk of falls after patients are discharged from the hospital (Table 5).90,133 Nevertheless, pulmonary bleeding has been reported in individuals who underwent a paravertebral block for thoracic surgery (Table 6).134
Nerve Injury
Nerve injury in regional anesthesia is uncommon (8–10% of temporary symptoms). Permanent damage is rare (<0.1%), and catheters slightly increase this risk (0.21%). The main culprit: is direct injection into the nerve, especially at high pressures.122 Although rare, nerve injuries are a risk factor for regional anesthesia. The update from the American Society of Regional Anesthesia and Pain Medicine offers improved prevention methods such as ultrasound guidance and monitoring. It also addresses new considerations, such as spinal issues, blood pressure management, and procedures on sedated patients or those with pre-existing nerve conditions.135
Infection Risk
Single-shot nerve blocks have minimal infection risk, while catheters carry a 0–3.2% chance. The risk increases in vulnerable patients (critical illness, trauma, immune issues, male sex, and no antibiotics). Removing catheters within 48–72 hours helps reduce the risk infection.122
Local Anesthetic Toxicity
High-dose FICB (75mL) raised toxicity concerns, whereas large FICB volumes (40–60mL) caused issues and weakness-related falls. Combining fascia iliaca and sacral plexus blocks offers advantages such as reduced toxicity, effective pain control, and potentially faster healing than traditional methods.71,136
Personalized Pain Control: Minimizing Opioid Reliance for Patients
General anesthesia allows pain signals to reach the brain, causing issues such as blood pressure swings. It can also affect heart and lung function. Peripheral nerve blocks target specific areas, prevent pain signals, and has a minimal impact on rest (Tables 2–6). They offer good pain relief and are suitable for elderly patients who might not be candidates for spinal anesthesia.137 Impaired cognitive status in hip fracture leads to poorer postoperative outcomes, including extended recovery periods and increased mortality rates, thus impacting daily functionality.138 Those with fractures and cognitive impairment often endure heightened pain levels due to delayed pain management and reduced analgesic access compared to their cognitively intact counterparts.138 Chronic pain management typically involves NSAIDs, antidepressants, and opioids; however, concerns about the opioid crisis and medication efficacy have prompted personalized treatment plans.139 Despite the historical efficacy of opioids in post-surgical pain relief, concerns still persist regarding their immediate complications and potential for long-term addiction.140 Specifically, elderly patients are at an increased risk of opioid-related adverse effects.141 A comprehensive approach to postoperative pain management entails considering various analgesic modalities to optimize pain control and minimize complications such as fear, anxiety, and cognitive disturbances.142 Severe pain following hip fractures may contribute to delirium, exacerbated by opioid use.143,144 Moreover, inadequate pain relief may result in issues such as apprehension, unease, hostile conduct, impairment of thinking, and bodily alterations, necessitating individualized therapies.145
Multimodal analgesia, which integrates diverse pain medications and techniques, has emerged as a preferred approach, particularly following knee replacement surgery (TKA), to improve pain relief while reducing reliance on opioids and mitigating their adverse effects.146 Regular assessment of pain levels and functional status is crucial for effective pain management and optimal recovery, particularly in elderly patients.147 Tailored analgesia, considering age-related changes and cognitive status, along with a multimodal; opioid-sparing approach, is recommended for efficient pain management and improved postoperative outcomes.148 Although opioids remain essential for intraoperative pain control, remifentanil is a favorable option for elderly patients because of its rapid onset and minimal side effects.148
A multimodal pain program after total hip replacement that avoided parenteral narcotics, resulted in positive outcomes. Only 15% of patients required parenteral narcotics, with low pain scores and minimal complications such as respiratory depression and ileus. The program effectively managed the pain, reduced emesis, and accelerated recovery.149 Treatment options for acute thoracic surgery pain include opioids, regional anesthesia, and new blocks. The best approach considers patient, surgical, and institutional variables, with a trend towards tailored multimodal therapy (Table 6).150 Combining various pain medications that address different pain mechanisms is effective in post-surgical pain management. This approach reduces the reliance on opioids and their side effects. While the ideal combination is still under investigation, certain medications such as NSAIDs and acetaminophen are commonly used. Opioids can still be used for additional pain relief.27
Strategies and Considerations in Peripheral Nerve Blocks
Regional anesthesia techniques have the potential to decrease the need for opioids (Tables 2–6); however, there is a risk of rebound pain. Physicians have developed various strategies to mitigate this risk, including prescribing medications and educating patients. These measures assist patients in effectively controlling pain and avoiding prolonged use of opioids.151 The study examined whether nerve blocks could predict long-term surgical outcomes for lower back pain. Sixty patients were followed up for 2 years, and short-term improvement after the block was associated with improvement in lower back pain at 1 year; it did not predict the overall function or quality of life after surgery. In other words, the pain relief effect of a block is not a reliable indicator of long-term success.152 A study of 21 patients found that peripheral nerve blocks effectively controlled postoperative pain; however, stopping the blocks caused intense increases in pain, especially in younger patients. Rebound pain was less severe in older patients; but still problematic. High morphine consumption during rebound pain suggests that rebound pain may outweigh the benefits of nerve blocks, especially in younger patients.153
The research discovered that PNB therapy resulted in an average decrease in pain of 3.3 with a standard deviation of 2.8. Furthermore, 68% of patients reported long-term pain relief lasting ≥ 1 month. More than half of the patients experienced a pain reduction of 50% or more, with an average pain reduction rate of 43.6%. Importantly, the majority of patients (13 of 22) were open to receiving a second PNB treatment, and all nine who did not experienced adverse effects. These results indicated that PNB may be an effective and viable approach for managing CPSP.154 Surgical pain usually subsides within 24–72 hours. When peripheral nerve blocks (PNBs) are successful, pain signals are entirely blocked (Figure 1). However, after the PNB wears off, there may be a sudden increase in pain intensity, commonly known as ‘rebound pain’.155 Rebound pain (RP) is a common issue. Recognizing and managing it can help to prevent short-term pain, patient frustration, unnecessary complications, and resource use. Doctors can anticipate, treat, and potentially avoid RP altogether.156
Rebound pain is a common occurrence after peripheral nerve blocks, affecting 35–50% of patients.157 Additionally, rebound pain was observed in 61.7% of the patient with post-peripheral nerve block, and 50.6% experienced severe rebound pain.158 Preoperative dexamethasone use, preoperative pain, type of surgery, postoperative NSAID use, and opioid use were independent risk factors. Healthcare providers should employ preventive measures for patients who are at increased risk of experiencing rebound pain.158 Current evidence supports the use of dexamethasone, either intravenously or perineurally, to mitigate rebound pain in patients who have undergone a peripheral nerve block following surgery. The consistent and noteworthy positive outcomes render this approach robust; therefore, dexamethasone should be used whenever possible to prevent rebound pain with peripheral nerve blocks.157 While continuous infraclavicular BPB did not result in a decrease in overall opioid usage compared to BPB alone, it proved to successfully manage rebound pain at 9 and 12h after surgery for distal radius fracture fixation under BPB.159 The double-blind prospective study found that one dose of intravenious ketamine (0.3 mg/kg) did not diminish the incidence or intensity of rebound pain following surgery. Predictive risk factors for rebound pain included bone surgery, severe preoperative pain, and high pain catastrophizing. By Day 30, patients with rebound pain reported increased daily pain with neuropathic features, but central sensitization was not involved. The findings indicate that despite adequate postoperative analgesia, rebound pain is common, highlighting the need for individualized pain management strategies based on preoperative pain intensity and patient pain perception.160
It is often underreported in clinical trials but is prevalent in routine practice. Younger patients, females, and those undergoing bone surgeries are at higher risk for this challenging postoperative pain response.157 The use of adjuvants in peripheral nerve blocks was associated with a lower likelihood of developing rebound pain compared to nerve blocks without adjuvants. This effect may be due to the addition of adjuvants, which prolong the duration of analgesia and reduce the overall dose of local anesthetics required, thereby decreasing the incidence of rebound pain when the nerve block wears off.158 A RCT found that the combination of pre-operative dexamethasone and post-operative FICB resulted in significantly lower VAS scores, patient-controlled intravenous analgesia (PCIA) pressing times, serum levels of inflammatory factors (IL-1β, IL-6, CRP), and Pittsburgh sleep quality index (PSQI) scores compared to the control group and the FICB-only group.161
Although nerve blocks offer a promising alternative, some limitations must be addressed. The effectiveness can vary depending on the type of surgery and individual patient factors (Tables 2–6). Additionally, rebound pain, which is a temporary increase in pain after the block is worn off, can occur as a side effect. Furthermore, some techniques require specialized guidance for accurate placement, potentially limiting their wider application. Future research should optimize nerve block techniques for different surgical procedures and patient populations. Further studies should investigate methods to minimize the risk of rebound pain and explore ways to make the most effective techniques more accessible through advancements in guidance technology. Additionally, a cost-effectiveness analysis comparing nerve blocks to traditional opioid-based pain management strategies would be valuable for healthcare decision- making.
Conclusion
This review underscores the potential of nerve blocks as a valuable alternative for managing post-surgical pain, offering significant advantages over traditional opioid-based methods. Techniques such as peripheral nerve blocks (PNB) and fascial plane blocks provide targeted analgesia across various surgical procedures, effectively reducing the need for opioids and minimizing associated risks. While these nerve block techniques demonstrate substantial short-term pain relief and enhance patient satisfaction, challenges such as rebound pain particularly in younger patients following the cessation of blocks necessitate careful management and patient education. Furthermore, the long-term effectiveness of nerve blocks in predicting surgical outcomes can vary, highlighting the importance of selecting the appropriate technique based on individual patient characteristics and the specific surgical context. Future research should focus on optimizing nerve block protocols, exploring their long-term effects, and conducting cost-effectiveness analyses compared to traditional opioid-based pain management strategies. By integrating nerve blocks into multimodal analgesia approaches, healthcare providers can improve recovery trajectories, enhance patient outcomes, and contribute to efforts aimed at mitigating the post-surgical pain and opioid crisis.
Funding Statement
This research was funded by the Science and Technology Planning Social Development Project of Zhenjiang City: SH2022083.
Author Contributions
All authors contributed significantly to the reported work, including conceptualizing the study, designing the study, executing the study, drafting, revising or critically reviewing the article, approving the final version for publication, agreeing on the journal to which the article has been submitted, and taking responsibility for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160. doi: 10.1185/03007995.2013.860019 [DOI] [PubMed] [Google Scholar]
- 2.Li J, Wei C, Huang J, et al. Efficacy of quadratus lumborum block for pain control in patients undergoing hip surgeries: a systematic review and meta-analysis. Front Med. 2022;8. doi: 10.3389/fmed.2021.771859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Kaushal M, Malviya A, Kumar S, Diwan S. Current concepts in postoperative pain management surgeries of hip joint: a narrative review. Int J Regional Anaesth. 2022;3:49–55. doi: 10.13107/ijra.2022.v03i02.056 [DOI] [Google Scholar]
- 4.Unneby A, Gustafson Y, Olofsson B, Lindgren BM. Between Heaven and Hell: experiences of Preoperative Pain and Pain Management among Older Patients with Hip Fracture. SAGE Open Nurs. 2022;8. doi: 10.1177/23779608221097450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ickowicz E. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x [DOI] [PubMed] [Google Scholar]
- 6.Azevedo LF, Costa-Pereira A, Mendonça L, Dias CC, Castro-Lopes JM. Epidemiology of chronic pain: a population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J Pain. 2012;13(8):773–783. doi: 10.1016/j.jpain.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 7.Cregg R, Anwar S, Farquhar-Smith P. Persistent postsurgical pain. Curr Opin Support Palliat Care. 2013;7(2):144–152. doi: 10.1097/SPC.0b013e328360b09e [DOI] [PubMed] [Google Scholar]
- 8.Weinstein E, Levene J, Cohen M, et al. Local anaesthetics and regional anaesthesia for preventing persistent postoperative pain. Cochrane Database Syst Rev. 2017. in press. doi: 10.1002/14651858.CD007105.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry CR, Fahs AM, Kurdziel MD, Koueiter DM, Fayne RJ, Verner JJ. Intraoperative psoas compartment block vs preoperative fascia iliaca block for pain control after direct anterior total hip arthroplasty: a randomized controlled trial. J Arthroplasty. 2018;33(6):1770–1774. doi: 10.1016/j.arth.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 10.Rowlands M, Van De Walt G, Bradley J, et al. Femoral Nerve Block Intervention in Neck of Femur Fracture (FINOF): a randomised controlled trial. BMJ Open. 2018;8(4):1–8. doi: 10.1136/bmjopen-2017-019650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai W, Leng X, Hu X, Cheng J, Ao Y. The effect of fascia iliaca block on postoperative pain and analgesic consumption for patients undergoing primary total Hip arthroplasty: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2021;16(1):1–11. doi: 10.1186/s13018-021-02585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyland SJ, Brockhaus KK, Vincent WR, et al. Perioperative pain management and opioid stewardship: a practical guide. Healthcare. 2021;9(3):1–56. doi: 10.3390/healthcare9030333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suksompong S, von Bormann S, von Bormann B. Regional catheters for postoperative pain control: review and observational data. Anesthesiol Pain Med. 2020;10(1):1–10. doi: 10.5812/aapm.99745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huai X, Jiao Y, Gu X, et al. Preoperative chronic pain as a risk factor for early postoperative cognitive dysfunction in elderly patients undergoing hip joint replacement surgery: a prospective observational cohort study. Front Neurosci. 2021;15. doi: 10.3389/fnins.2021.747362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimaraes-pereira L, Valdoleiros I, Reis P, Abelha F. Evaluating persistent postoperative pain in one tertiary hospital: incidence, quality of life. Associated Factors Treatment. 2016;6(2). doi: 10.5812/aapm.36461.Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin LF, Cheng K, Washington SM, et al. Green light exposure elicits anti-inflammation, endogenous opioid release and dampens synaptic potentiation to relieve post-surgical pain. J Pain. 2023;24(3):509–529. doi: 10.1016/j.jpain.2022.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen EY, Lasky R, Dotterweich WA, Niu R, Tybor DJ, Smith EL. Chronic prescription opioid use before and after total hip and knee arthroplasty in patients younger than 65 years. J Arthroplasty. 2019;34(10):2319–2323. doi: 10.1016/j.arth.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 18.Willingham M, Rangrass G, Curcuru C, et al. Association between postoperative complications and lingering post-surgical pain: an observational cohort study. Br J Anaesth. 2020;124(2):214–221. doi: 10.1016/j.bja.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Ren Y, Mo Y, et al. Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects. Cell Res. 2022;32(5):461–476. doi: 10.1038/s41422-022-00616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou R, Hartung D, Turner J, et al. Opioid treatments for chronic pain. comparative effectiveness review no. 229 (Prepared by the Pacific Northwest evidence-based practice center under contract no. 290-2015-00009-I.). AHRQ Publ No 20-EHC011. 2020; 229. doi: 10.23970/AHRQEPCCER229. [DOI]
- 21.Noori A, Sprague S, Bzovsky S, et al. Predictors of long-term pain after hip arthroplasty in patients with femoral neck fractures: a cohort study. J Orthop Trauma. 2020;34(11):S55–S63. doi: 10.1097/BOT.0000000000001929 [DOI] [PubMed] [Google Scholar]
- 22.Thapa P, Euasobhon P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. 2018;31(3):155–173. doi: 10.3344/kjp.2018.31.3.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen DB, Laursen M, Edwards RR, Simonsen O, Arendt-Nielsen L, Petersen KK. The combination of preoperative pain, conditioned pain modulation, and pain catastrophizing predicts postoperative pain 12 months after total knee arthroplasty. Pain Med. 2021;22(7):1583–1590. doi: 10.1093/pm/pnaa402 [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Rao S, Mekkawy KL, et al. Risk factors for pain after total hip arthroplasty: a systematic review. Arthroplasty. 2023;5(1). doi: 10.1186/s42836-023-00172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han C, Ge Z, Jiang W, Zhao H, Ma T. Incidence and risk factors of chronic pain following hysterectomy among Southern Jiangsu Chinese women. BMC Anesthesiol. 2017;17(1):1–11. doi: 10.1186/s12871-017-0394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective fa. Expert Rev Neurother. 2009;9(5):723–744. doi: 10.1586/ern.09.20 [DOI] [PubMed] [Google Scholar]
- 27.Pogatzki-zahn EM, Segelcke D, Schug SA. Postoperative pain — from mechanisms to treatment. Pain Rep. 2017;2(2):e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Sun M, Liu P, Shao W, Xiong M, Xu B. Perioperative stress prolong post-surgical pain via miR-339-5p targeting oprm1 in the amygdala. Korean J Pain. 2022;35(4):423–432. doi: 10.3344/kjp.2022.35.4.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques a review. JAMA Surg. 2017;152(7):691–697. doi: 10.1001/jamasurg.2017.0898 [DOI] [PubMed] [Google Scholar]
- 30.Adams TJ, Aljohani DM, Forget P. Perioperative opioids: a narrative review contextualising new avenues to improve prescribing. Br J Anaesth. 2023;130(6):709–718. doi: 10.1016/j.bja.2023.02.037 [DOI] [PubMed] [Google Scholar]
- 31.Ghai B, Jafra A, Bhatia N, Chanana N, Bansal D, Mehta V. Opioid sparing strategies for perioperative pain management other than regional anaesthesia: a narrative review. J Anaesth Clin Pharmacol. 2022;38(1):3–10. doi: 10.4103/joacp.JOACP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TKT N, Lin JA, Sasaki S. A preliminary analysis of a modified anterior approach to hip pericapsular neurolysis for inoperable hip fracture using the ideal framework. Healthcare. 2022;10(6):1–16. doi: 10.3390/healthcare10061002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edinoff AN, Askins D, Bobo E, et al. The emerging role of ketamine in acute postoperative pain management. Curr Pain Headache Rep. 2023;27(9):387–397. doi: 10.1007/s11916-023-01134-1 [DOI] [PubMed] [Google Scholar]
- 34.Bonilla-Jaime H, Sánchez-Salcedo JA, Estevez-Cabrera MM, Molina-Jiménez T, Cortes-Altamirano JL, Alfaro-Rodríguez A. Depression and Pain: use of Antidepressants. Curr Neuropharmacol. 2021;20(2):384–402. doi: 10.2174/1570159x19666210609161447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardwell TW, Zabala V, Mineo J, Ochner CN. The effects of perioperative peripheral nerve blocks on peri- and postoperative opioid use and pain management. Am Surg. 2022;88(12):2842–2850. doi: 10.1177/00031348211023395 [DOI] [PubMed] [Google Scholar]
- 36.Unneby A, Svensson O, Gustafson Y, Olofsson B. Femoral nerve block in a representative sample of elderly people with hip fracture: a randomised controlled trial. Injury. 2017;48(7):1542–1549. doi: 10.1016/j.injury.2017.04.043 [DOI] [PubMed] [Google Scholar]
- 37.Guay J, Kopp SL. Postoperative pain management for cardiac surgery: do we need new blocks? J Cardiothorac Vasc Anesth. 2020;34(11):2994–2995. doi: 10.1053/j.jvca.2020.06.039 [DOI] [PubMed] [Google Scholar]
- 38.Albrecht E, Chin KJ. Advances in regional anaesthesia and acute pain management: a narrative review. Anaesthesia. 2020;75(S1):e101–e110. doi: 10.1111/anae.14868 [DOI] [PubMed] [Google Scholar]
- 39.Dieu A, Huynen P, Lavand’homme P, et al. Pain management after open liver resection: procedure-specific postoperative pain management (PROSPECT) recommendations. Reg Anesth Pain Med. 2021;46(5):433–435. doi: 10.1136/rapm-2020-101933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cata JP. Outcomes of regional anesthesia in cancer patients. Curr Opin Anaesthesiol. 2018;31(5):593–600. doi: 10.1097/ACO.0000000000000636 [DOI] [PubMed] [Google Scholar]
- 41.Ohseto K, Uchino H, Iida H. Nerve Blockade and Interventional Therapy. Springer; 2019. doi: 10.1007/978-4-431-54660-3 [DOI] [Google Scholar]
- 42.Yanagidate F, Strichartz GR. Local anesthetics. Handb Exp Pharmacol. 2007;177:95–127. doi: 10.1007/978-3-540-33823-9_4 [DOI] [PubMed] [Google Scholar]
- 43.Barletta M, Reed R. Local anesthetics: pharmacology and special preparations. Vet Clin North Am Small Anim Pract. 2019;49(6):1109–1125. doi: 10.1016/j.cvsm.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 44.Curseen KA, Taj J, Grant Q. Pain management in patients with serious illness. Med Clin North Am. 2020;104(3):415–438. doi: 10.1016/j.mcna.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 45.Muñoz-Leyva F, Jack JM, Bhatia A, et al. No benefits of adding dexmedetomidine, ketamine, dexamethasone, and nerve blocks to an established multimodal analgesic regimen after total knee arthroplasty. Anesthesiology. 2022;137(4):459–470. doi: 10.1097/ALN.0000000000004326 [DOI] [PubMed] [Google Scholar]
- 46.Alviar MJM, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;2016(10). doi: 10.1002/14651858.CD006380.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai JW, An D, Perlas A, Chan V. Adjuncts to local anesthetic wound infiltration for postoperative analgesia: a systematic review. Reg Anesth Pain Med. 2020;45(8):645–655. doi: 10.1136/rapm-2020-101593 [DOI] [PubMed] [Google Scholar]
- 48.Toubasi A, Irvine DS, Jandali K, Sweeney D, Monasterio SM. Continuous suprascapular catheter and axillary nerve block for analgesia for reverse total shoulder arthroplasty: a case report. Cureus. 2023;15(cc):4. doi: 10.7759/cureus.49670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saravanan R, Nivedita K, Karthik K. Selective suprascapular and axillary nerve (SSAX) block - A diaphragm sparing regional anesthetic technique for shoulder surgeries: a case series. Saudi J Anaesth. 2022;16(4):457–459. doi: 10.4103/sja.sja-782-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L, Gessner D, Kou A, Kasimova K, Memtsoudis SG, Mariano ER. Rate of occurrence of respiratory complications in patients who undergo shoulder arthroplasty with a continuous interscalene brachial plexus block and associated risk factors. Reg Anesth Pain Med. 2023;48(11):540–546. doi: 10.1136/rapm-2022-104264 [DOI] [PubMed] [Google Scholar]
- 51.Cabaton J, Nové-Josserand L, Mercadal L, Vaudelin T. Analgesic efficacy of ultrasound-guided interscalene block vs. supraclavicular block for ambulatory arthroscopic rotator cuff repair: a randomised noninferiority study. Eur J Anaesthesiol. 2019;36(10):778–786. doi: 10.1097/EJA.0000000000001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu W, Zhou R, Chen L, et al. The ultrasound-guided selective nerve block in the upper arm: an approach of retaining the motor function in elbow. BMC Anesthesiol. 2018;18(1):1–7. doi: 10.1186/s12871-018-0584-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel MA, Gadsden JC, Nedeljkovic SS, et al. Brachial plexus block with liposomal bupivacaine for shoulder surgery improves analgesia and reduces opioid consumption: results from a multicenter, randomized, double-blind, controlled trial. Pain Med. 2020;21(2):387–400. doi: 10.1093/pm/pnz103 [DOI] [PubMed] [Google Scholar]
- 54.Farladansky E, Hazan S, Maman E, et al. Perioperative oral pregabalin results in postoperative pain scores equivalent to those of interscalene brachial plexus block after arthroscopic rotator cuff repair: a randomized clinical trial. Arthrosc J Arthrosc Relat Surg. 2022;38(1):31–37. doi: 10.1016/j.arthro.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 55.Anesth J, Crit A, Capuano P, et al. Fascial plane blocks for cardiothoracic surgery: a narrative review. J Anesth Analg Crit Care. 2024;4(1):20. doi: 10.1186/s44158-024-00155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Yang X, Gu H, Chai X, Wang D. The role of serratus anterior plane block during in video-assisted thoracoscopic surgery. Pain Ther. 2021;10(2):1051–1066. doi: 10.1007/s40122-021-00322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chai B, Wang Q, Du J, et al. Research progress on serratus anterior plane block in breast surgery: a narrative review. Pain Ther. 2023;12(2):323–337. doi: 10.1007/s40122-022-00456-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abadi A, Cohen R. Evaluation of an enhanced recovery after surgery protocol including parasternal intercostal nerve block in cardiac surgery requiring sternotomy. Am Surg. 2021;87(10):1561–1564. doi: 10.1177/00031348211024638 [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Li Q, Liao Y, et al. Preemptive deep parasternal intercostal plane block for perioperative analgesia in coronary artery bypass grafting with sternotomy: a randomized, observer-blind, controlled study. Ann Med. 2023;55(2). doi: 10.1080/07853890.2024.2302983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Min J, Chen S. Sensory assessment and block duration of deep parasternal intercostal plane block in patients undergoing cardiac surgery: a prospective observational study. Pain Ther. 2022;11(3):951–958. doi: 10.1007/s40122-022-00403-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong HMK, Chen PY, Tang GCC, et al. Deep parasternal intercostal plane block for intraoperative pain control in cardiac surgical patients for sternotomy: a prospective randomized controlled trial. J Cardiothorac Vasc Anesth. 2024;38(3):683–690. doi: 10.1053/j.jvca.2023.11.038 [DOI] [PubMed] [Google Scholar]
- 62.Kelava M, Alfirevic A, Bustamante S, Hargrave J, Marciniak D. Regional anesthesia in cardiac surgery: an overview of fascial plane chest wall blocks. Anesth Analg. 2020;131(1):127–135. doi: 10.1213/ANE.0000000000004682 [DOI] [PubMed] [Google Scholar]
- 63.Chae MS, Park Y, Shim JW, et al. Clinical application of pectoralis nerve block II for flap dissection-related pain control after robot-assisted transaxillary thyroidectomy: a preliminary retrospective cohort study. Cancers. 2022;14(17):1–11. doi: 10.3390/cancers14174097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujiwara A, Komasawa N, Minami T. Pectoral nerves (PECS) and intercostal nerve block for cardiac resynchronization therapy device implantation. Springerplus. 2014;3(1):1–2. doi: 10.1186/2193-1801-3-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moon J, Park HS, Kim JY, et al. Analgesic efficacies of intraoperative pectoralis nerve II block under direct vision in patients undergoing robotic nipple-sparing mastectomy with immediate breast reconstruction: a prospective, randomized controlled study. J Pers Med. 2022;12(8):1309. doi: 10.3390/jpm12081309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Löchel J, Janz V, Leopold VJ, Krämer M, Wassilew GI. Transversus abdominis plane block for improved early postoperative pain management after periacetabular osteotomy: a randomized clinical trial. J Clin Med. 2021;10(3):1–7. doi: 10.3390/jcm10030394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palareti G, Legnani C, Cosmi B, et al. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: analysis of results obtained in the DULCIS study. Int J Lab Hematol. 2016;38(1):42–49. doi: 10.1111/ijlh.12426 [DOI] [PubMed] [Google Scholar]
- 68.Kanazi GE, Aouad MT, Abdallah FW, et al. The analgesic efficacy of subarachnoid morphine in comparison with ultrasound-guided transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2010;111(2):475–481. doi: 10.1213/ANE.0b013e3181e30b9f [DOI] [PubMed] [Google Scholar]
- 69.Shim JW, Jung S, Moon HW, et al. Rectus sheath block for acute pain management after robot-assisted prostatectomy. Asian J Surg. 2022;45(10):1843–1848. doi: 10.1016/j.asjsur.2021.10.035 [DOI] [PubMed] [Google Scholar]
- 70.Abdildin Y, Tapinova K, Salamat A, Shaimakhanov R, Aitbayev A, Viderman D. Rectus sheath block in abdominal surgery: a systematic review with meta-analysis. Rom J Anaesth Intensive Care. 2023;30(1):43–50. doi: 10.2478/rjaic-2023-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali S, Athar M, Ahmed SM. Basics of CPB. Indian J Anaesth. 2019;49(4):257–262. doi: 10.4103/ija.IJA [DOI] [Google Scholar]
- 72.Elahwal L, Elrahwan S, Elbadry AA. Ilioinguinal and iliohypogastric nerve block for acute and chronic pain relief after caesarean section: a randomized controlled trial. Anesthesiol Pain Med. 2022;12(2). doi: 10.5812/aapm.121837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Wu T, Terry MJ, et al. Improved perioperative analgesia with ultrasound-guided ilioinguinal/iliohypogastric nerve or transversus abdominis plane block for open inguinal surgery: a systematic review and meta-analysis of randomized controlled trials. J Phys Ther Sci. 2016;28(3):1055–1060. doi: 10.1589/jpts.28.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Z, Xia W, Peng X, Ke J, Wang W. Evaluation of ultrasound-guided genitofemoral nerve block combined with ilioinguinal/iliohypogastric nerve block during inguinal hernia repair in the elderly. Curr Med Sci. 2019;39(5):794–799. doi: 10.1007/s11596-019-2107-2 [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Gu F, Liu Y, Wang C, Xu C. Heliyon the analgesic efficacy of paravertebral block at T11 level as a single anaesthetic technique in an older adult with severe cardiac insufficiency undergoing open complex inguinal hernia repair: a case report. Heliyon. 2023;9(4):e14962. doi: 10.1016/j.heliyon.2023.e14962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah RB, Desai B, Gajjar VA, Soni D, Prajapati V. Combined lumbar-sacral plexus block for surgical anesthesia in high risk patients (ASA grade III/IV) undergoing lower limb surgeries 1 MedDocs Publishers Annals of Anesthesia and Pain Medicine. Ann Anesth Pain Med. 2020;3(1):1–5. [Google Scholar]
- 77.Green C, Byrne AM, O’Loughlin P, Molony D, Harmon D, Masterson E. Surgeon delivered psoas compartment block in total hip arthroplasty. J Arthroplasty. 2014;29(2):393–396. doi: 10.1016/j.arth.2013.06.028 [DOI] [PubMed] [Google Scholar]
- 78.Awad IT, Duggan EM. Posterior lumbar plexus block: anatomy, approaches, and techniques. Reg Anesth Pain Med. 2005;30(2):143–149. doi: 10.1016/j.rapm.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 79.Becchi C, Al Malyan M, Coppini R, Campolo M, Magherini M, Boncinelli S. Opioid-free analgesia by continuous psoas compartment block after total hip arthroplasty. A randomized study. Eur J Anaesthesiol. 2008;25(5):418–423. doi: 10.1017/S026502150700302X [DOI] [PubMed] [Google Scholar]
- 80.Domagalska M, Ciftci B, Reysner T, Kolasiński J, Wieczorowska-Tobis K, Kowalski G. Pain management and functional recovery after pericapsular nerve group (PENG) block for total hip arthroplasty: a prospective, randomized, double-blinded clinical trial. J Clin Med. 2023;12(15). doi: 10.3390/jcm12154931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin DY, Brown B, Morrison C, et al. The pericapsular nerve group (PENG) block combined with local infiltration analgesia (LIA) compared to placebo and LIA in hip arthroplasty surgery: a multi-center double-blinded randomized-controlled trial. BMC Anesthesiol. 2022;22(1):1–9. doi: 10.1186/s12871-022-01787-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu L, Shen X, Liu H. The efficacy of pericapsular nerve group block for postoperative analgesia in patients undergoing hip surgery: a systematic review and meta-analysis of randomized controlled trials. Front Med. 2023;10. doi: 10.3389/fmed.2023.1084532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng J, Du L, Chen G, Zhang L, Deng X, Zhang W. Efficacy of pericapsular nerve group (PENG) block on perioperative pain management in elderly patients undergoing hip surgical procedures: a protocol for a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2023;13(1):e065304. doi: 10.1136/bmjopen-2022-065304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teles AS, Altınpulluk EY, Sahoo RK, et al. Beyond the pericapsular nerve group (PENG) block: a narrative review. Turkish J Anaesthesiol Reanim. 2022;50(3):167–172. doi: 10.5152/TJAR.2021.21230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miao Y, Wang X. Comparison of pericapsular nerve group (PENG) block with fascia iliaca compartment block (FICB) in hip fractures: a systematic review and meta-analysis of randomized controlled trials. Published online 2023. 1–14.
- 86.Gül R, Kılınç M, Şahin L. Fascia iliaka kompartman bloğunda kullanılan bupivakainin farklı volümlerinin karşılaştırılması. Ulus Travma Acil Cerrahi Derg. 2023;29(3):337–343. doi: 10.14744/tjtes.2023.51268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao Y, Li H, Hu H, Xu Y, Zhou J, Liu Y. Effects of continuous fascia iliaca compartment block on early quality of recovery after total hip arthroplasty in elderly patients: a randomized controlled trial. J Pain Res. 2022;15:1837–1844. doi: 10.2147/JPR.S368285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salottolo K, Meinig R, Fine L, et al. A multi-institutional prospective observational study to evaluate fascia iliaca compartment block (FICB) for preventing delirium in adults with hip fracture. Trauma Surg Acute Care Open. 2022;7(1):1–7. doi: 10.1136/tsaco-2022-000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diakomi M, Papaioannou M, Mela A, Kouskouni E, Makris A. Preoperative fascia iliaca compartment block for positioning patients with hip fractures for central nervous blockade: a randomized trial. Reg Anesth Pain Med. 2014;39(5):394–398. doi: 10.1097/AAP.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Liu S, Cao Y, Yan L, Shen Y. Effect of perioperative ultrasound guided fascia iliaca compartment block in elderly adults with hip fractures undergoing arthroplasty in spinal anesthesia—a randomized controlled trial. BMC Geriatr. 2023;23(1):1–7. doi: 10.1186/s12877-023-03786-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azizoğlu M, Rumeli Ş. Comparison of the suprainguinal fascia iliaca compartment block with continuous epidural analgesia in patients undergoing hip surgeries: a retrospective study. Brazilian J Anesthesiol. 2022;72(3):342–349. doi: 10.1016/j.bjane.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hussain N, Brull R, Vannabouathong C, et al. Network meta-analysis of the analgesic effectiveness of regional anaesthesia techniques for anterior cruciate ligament reconstruction. Anaesthesia. 2023;78(2):207–224. doi: 10.1111/anae.15873 [DOI] [PubMed] [Google Scholar]
- 93.Kirkham KR, Grape S, Martin R, Albrecht E. Analgesic efficacy of local infiltration analgesia vs. femoral nerve block after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Anaesthesia. 2017;72(12):1542–1553. doi: 10.1111/anae.14032 [DOI] [PubMed] [Google Scholar]
- 94.Cuñat T, Mejía J, Tatjer I, et al. Ultrasound-guided genicular nerves block vs. local infiltration analgesia for total knee arthroplasty: a randomised controlled non-inferiority trial. Anaesthesia. 2023;78(2):188–196. doi: 10.1111/anae.15909 [DOI] [PubMed] [Google Scholar]
- 95.Bareka M, Ntalouka MP, Angelis F, et al. Femoral–obturator–sciatic (FOS) nerve block as an anesthetic triad for arthroscopic ACL reconstruction: is this the magic trick we were missing? J Clin Med. 2024;13(4):1054. doi: 10.3390/jcm13041054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lv Y, Qiu Y, Wang C, Tuo Y. The effect of local anesthetic injection for post-operative pain management after anterior cruciate ligament surgery. Asian J Surg. 2022;45(1):671–674. doi: 10.1016/j.asjsur.2021.11.020 [DOI] [PubMed] [Google Scholar]
- 97.Andersen L, Kehlet H. Analgesic efficacy of local infiltration analgesia in hip and knee arthroplasty: a systematic review. Br J Anaesth. 2014;113(3):360–374. doi: 10.1093/bja/aeu155 [DOI] [PubMed] [Google Scholar]
- 98.Seangleulur A, Vanasbodeekul P, Prapaitrakool S, et al. The efficacy of local infiltration analgesia in the early postoperative period after total knee arthroplasty: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33(11):816–831. doi: 10.1097/EJA.0000000000000516 [DOI] [PubMed] [Google Scholar]
- 99.Luo ZY, Yu QP, Zeng WN, et al. Adductor canal block combined with local infiltration analgesia with morphine and betamethasone show superior analgesic effect than local infiltration analgesia alone for total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2022;23(1):1–11. doi: 10.1186/s12891-022-05388-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X, Zhang H, Zhang H, Guo M, Gao Y, Du C. Local infiltration vs epidural analgesia for postoperative pain control after total knee or hip arthroplasty: a meta-analysis of randomized controlled trials. Med. 2020;99(44):E22674. doi: 10.1097/MD.0000000000022674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sawhney M, Mehdian H, Kashin B, et al. Pain after unilateral total knee arthroplasty: a prospective randomized controlled trial examining the analgesic effectiveness of a combined adductor canal peripheral nerve block with periarticular infiltration versus adductor canal nerve block alone vers. Anesth Analg. 2016;122(6):2040–2046. doi: 10.1213/ANE.0000000000001210 [DOI] [PubMed] [Google Scholar]
- 102.Hussain N, Brull R, Zhou S, et al. Analgesic benefits of single-shot versus continuous adductor canal block for total knee arthroplasty: a systemic review and meta-analysis of randomized trials. Reg Anesth Pain Med. 2022;48(2):49–60. doi: 10.1136/rapm-2022-103756 [DOI] [PubMed] [Google Scholar]
- 103.Zuo W, Guo W, Ma J, Cui W. Dose adductor canal block combined with local infiltration analgesia has a synergistic effect than adductor canal block alone in total knee arthroplasty: a meta-analysis and systematic review. J Orthop Surg Res. 2019;14(1):1–8. doi: 10.1186/s13018-019-1138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holbert SE, Baxter SN, Brennan JC, et al. Adductor canal blocks are not associated with improved early postoperative outcomes in patients undergoing total knee arthroplasty. Ochsner J. 2023;23(1):9–15. doi: 10.31486/toj.22.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin SQ, Ding XB, Tong Y, et al. Effect of saphenous nerve block for postoperative pain on knee surgery: a meta-analysis. Int J Clin Exp Med. 2015;8(1):368–376. [PMC free article] [PubMed] [Google Scholar]
- 106.Bjørn S, Wong WY, Baas J, et al. The importance of the saphenous nerve block for analgesia following major ankle surgery: a randomized, controlled, double-blind study. Reg Anesth Pain Med. 2018;43(5):474–479. doi: 10.1097/AAP.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 107.Donohue CM, Goss LR, Metz S, Weingarten MS, Dyal LB. Combined popliteal and saphenous nerve blocks at the knee: an underused alternative to general or spinal anesthesia for foot and ankle surgery. J Am Podiatr Med Assoc. 2004;94(4):368–374. doi: 10.7547/0940368 [DOI] [PubMed] [Google Scholar]
- 108.Bjorn S, Linde F, Nielsen KK, Borglum J, Hauritz RW, Bendtsen TF. Effect of perineural dexamethasone on the duration of single injection saphenous nerve block for analgesia after major ankle surgery: a randomized, controlled study. Reg Anesth Pain Med. 2017;42(2):210–216. doi: 10.1097/AAP.0000000000000538 [DOI] [PubMed] [Google Scholar]
- 109.Kapoor R, Adhikary SD, Siefring C, McQuillan PM. The saphenous nerve and its relationship to the nerve to the vastus medialis in and around the adductor canal: an anatomical study. Acta Anaesthesiol Scand. 2012;56(3):365–367. doi: 10.1111/j.1399-6576.2011.02645.x [DOI] [PubMed] [Google Scholar]
- 110.Mistry T, Sonawane K, Keshri V, Balavenkatasubramanian J, Sekar C. Ultrasound-guided CAPS (crosswise approach to popliteal sciatic) block: a novel technique for supine popliteal fossa block. Cureus. 2022;14(1):1–7. doi: 10.7759/cureus.20894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jarrell K, McDonald E, Shakked R, Nicholson K, Kasper V, Raikin SM. Combined popliteal catheter with single-injection vs continuous-infusion saphenous nerve block for foot and ankle surgery. Foot Ankle Int. 2018;39(3):332–337. doi: 10.1177/1071100717744331 [DOI] [PubMed] [Google Scholar]
- 112.Discalzi A, Maglia C, Nardelli F, et al. Endovascular revascularization of critical limb ischemia: the role of ultrasound-guided popliteal sciatic nerve block for the procedural pain management. Eur Radiol. 2024;34(1):287–293. doi: 10.1007/s00330-023-09988-0 [DOI] [PubMed] [Google Scholar]
- 113.Schipper ON, Hunt KJ, Anderson RB, Davis WH, Jones CP, Cohen BE. Ankle block vs single-shot popliteal fossa block as primary anesthesia for forefoot operative procedures: prospective, randomized comparison. Foot Ankle Int. 2017;38(11):1188–1191. doi: 10.1177/1071100717723132 [DOI] [PubMed] [Google Scholar]
- 114.Rao Kadam V, Ludbrook G, van Wijk RM, et al. Comparison of ultrasound-guided transmuscular quadratus lumborum block catheter technique with surgical pre-peritoneal catheter for postoperative analgesia in abdominal surgery: a randomised controlled trial. Anaesthesia. 2019;74(11):1381–1388. doi: 10.1111/anae.14794 [DOI] [PubMed] [Google Scholar]
- 115.Huda AU, Minhas R. Quadratus lumborum block reduces postoperative pain scores and opioids consumption in total hip arthroplasty: a meta-analysis. Cureus. 2022;2021. doi: 10.7759/cureus.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Irwin R, Stanescu S, Buzaianu C, et al. Quadratus lumborum block for analgesia after caesarean section: a randomised controlled trial. Anaesthesia. 2020;75(1):89–95. doi: 10.1111/anae.14852 [DOI] [PubMed] [Google Scholar]
- 117.Marshall K, McLaughlin K. Pain management in thoracic surgery. Thorac Surg Clin. 2020;30(3):339–346. doi: 10.1016/j.thorsurg.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 118.Barr LF, Boss MJ, Mazzeffi MA, Taylor BS, Salenger R. Postoperative multimodal analgesia in cardiac surgery. Crit Care Clin. 2020;36(4):631–651. doi: 10.1016/j.ccc.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 119.Soffin EM, Wu CL. Regional and multimodal analgesia to reduce opioid use after total joint arthroplasty: a narrative review. HSS J. 2019;15(1):57–65. doi: 10.1007/s11420-018-9652-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raja M, Rajaprabu R, Ialambeitlang H, Kharpran R. Comparison of epidural with iliopsoas plane catheter insertion for providing post-operative analgesia in Hip surgeries. Cancer Discovery. 2018;8:632–637. doi: 10.47009/jamp.2023.5.2.13329500295 [DOI] [Google Scholar]
- 121.Foss NB, Kristensen MT, Kristensen BB, Jensen PS, Kehlet H. Effect of postoperative epidural analgesia on rehabilitation and pain after hip fracture surgery. Anesthesiology. 2005;102(6):1197–1204. doi: 10.1097/00000542-200506000-00020 [DOI] [PubMed] [Google Scholar]
- 122.Kamel I, Ahmed M, Sethi A. Regional anesthesia for orthopedic procedures: what orthopedic surgeons need to know. World J Orthop. 2022;13(1):11–35. doi: 10.5312/wjo.v13.i1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barros M, Carvalho T, Pires AC, Teixeira G, Cardoso H. Effective postoperative pain management in thoracic outlet syndrome surgery: the role of the erector spinae plane block. Cureus. 2023;15(11):1–4. doi: 10.7759/cureus.48944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McCance L, Smith EC, Moore J, Forget P. Erector spinae plane block with catheter infusion for analgesia in a patient undergoing transaxillary first rib resection. Anaesth Rep. 2022;10(2):2–5. doi: 10.1002/anr3.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang JG, Jiang CW, Deng W, Liu F, Wu XP. Comparison of rhomboid intercostal block, erector spinae plane block, and serratus plane block on analgesia for video-assisted thoracic surgery: a prospective, randomized, controlled trial. Int J Clin Pract. 2022;2022:1–9. doi: 10.1155/2022/6924489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagaraja P, Ragavendran S, Singh N, et al. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21(3):323. doi: 10.4103/aca.aca_16_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamel AAF, Amin OAI, Ibrahem MAM. Bilateral ultrasound-guided erector spinae plane block versus transversus abdominis plane block on postoperative analgesia after total abdominal hysterectomy. Pain Physician. 2020;23(4):375–382. doi: 10.36076/ppj.2020/23/375 [DOI] [PubMed] [Google Scholar]
- 128.Oh SK, Lim BG, Won YJ, Lee DK, Kim SS. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: a systematic review and meta-analysis. J Clin Anesth. 2022;78:110647. doi: 10.1016/j.jclinane.2022.110647 [DOI] [PubMed] [Google Scholar]
- 129.Liang X, Zhou W, Fan Y. Erector spinae plane block for spinal surgery: a systematic review and meta-analysis. Korean J Pain. 2021;34(4):487–500. doi: 10.3344/KJP.2021.34.4.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tulgar S, Selvi O, Senturk O, Ermis MN, Cubuk R, Ozer Z. Clinical experiences of ultrasound-guided lumbar erector spinae plane block for hip joint and proximal femur surgeries. J Clin Anesth. 2018;47:5–6. doi: 10.1016/j.jclinane.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 131.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi: 10.1097/AAP.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 132.Mannion S. Psoas compartment block. Contin Educ Anaesth Crit Care Pain. 2007;7(5):162–166. doi: 10.1093/bjaceaccp/mkm029 [DOI] [Google Scholar]
- 133.Xing JG, Abdallah FW, Brull R, et al. Preoperative femoral nerve block for hip arthroscopy. Am J Sports Med. 2015;43(11):2680–2687. doi: 10.1177/0363546515602468 [DOI] [PubMed] [Google Scholar]
- 134.Hill RP, Greengrass R. Pulmonary haemorrhage after percutaneous paravertebral block (multiple letters). Br J Anaesth. 2000;84(3):423–424. doi: 10.1093/oxfordjournals.bja.a013461 [DOI] [PubMed] [Google Scholar]
- 135.Neal JM, Barrington MJ, Brull R, et al. The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine: executive summary 2015. Reg Anesth Pain Med. 2015;40(5):401–430. doi: 10.1097/AAP.0000000000000286 [DOI] [PubMed] [Google Scholar]
- 136.Jørgensen CC, Petersen PB, Daugberg LO, et al. Peripheral nerve-blocks and associations with length of stay and readmissions in fast-track total hip and knee arthroplasty. Acta Anaesthesiol Scand. 2023;67(2):169–176. doi: 10.1111/aas.14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.ChiCTR2100043422. A randomized controlled study of anterior lumbar plexus combined with parasacral sciatic nerve block under the guidance of ultrasound and nerve stimulator in elderly patients undergoing hip arthroplasty. Published online 2021. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02279974/full. Accessed September 26, 2024.
- 138.Li XY, Zhang L, Ding YM, Wang CX, Qiu Y. Effects of fascia iliaca compartment block as an adjunctive management to parecoxib for pain control after total Hip arthroplasty. Med. 2022;101(30):E29688. doi: 10.1097/MD.0000000000029688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Edwards RR, Schreiber KL, Dworkin RH, et al. Optimizing and accelerating the development of precision pain treatments for chronic pain: IMMPACT review and recommendations. J Pain. 2023;24(2):204–225. doi: 10.1016/j.jpain.2022.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Young A, Robinson R, Nguyen E, et al. Impact of local anesthesia block on pain medication use and length of hospital stay in elderly indigenous patients in Alaska hospitalized for fragility fracture. OTA Int Open Access J Orthop Trauma. 2022;5(4):e207. doi: 10.1097/oi9.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wainwright TW, Gill M, McDonald DA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: enhanced recovery after surgery (ERAS®) society recommendations. Acta Orthop. 2020;91(1):3–19. doi: 10.1080/17453674.2019.1683790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dangle J, Kukreja P, Kalagara H. Review of current practices of peripheral nerve blocks for hip fracture and surgery. Curr Anesthesiol Rep. 2020;10(3):259–266. doi: 10.1007/s40140-020-00393-7 [DOI] [Google Scholar]
- 143.Morisson L, Laferrière-Langlois P, Carrier FM, et al. Effect of electroencephalography-guided anesthesia on neurocognitive disorders in elderly patients undergoing major non-cardiac surgery: a trial protocol the POEGEA trial (POncd Elderly GEneral Anesthesia). PLoS One. 2021;16:1–16. doi: 10.1371/journal.pone.0255852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simić A, Adam VN, Rošić D, et al. Peripheral nerve blocks for hip fractures in emergency medicine. Acta Clin Croat. 2022;61:78–83. doi: 10.20471/acc.2022.61.s1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sakic L, Tonkovicc D, Hrgovic Z, Klasan A. Spinal dexamethasone effect on cognitive disorders after hip surgery. Med Arch. 2023;77(1):18–23. doi: 10.5455/medarh.2023.77.18-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li J, Ma Y, Xiao L. Postoperative pain management in total knee arthroplasty. Orthop Surg. 2019;11(5):755–761. doi: 10.1111/os.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ljungqvist O, Hubner M. Enhanced recovery after surgery—ERAS—principles, practice and feasibility in the elderly. Aging Clin Exp Res. 2018;30(3):249–252. doi: 10.1007/s40520-018-0905-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Del Tedesco F, Sessa F, Xhemalaj R, Sollazzi L, Dello Russo C, Aceto P. Perioperative analgesia in the elderly. Saudi J Anaesth. 2023;17(4):491–499. doi: 10.4103/sja.sja_643_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maheshwari AV, Boutary M, Yun AG, Sirianni LE, Dorr LD. Multimodal analgesia without routine parenteral narcotics for total hip arthroplasty. Clin Orthop Relat Res. 2006;453(453):231–238. doi: 10.1097/01.blo.0000246545.72445.c4 [DOI] [PubMed] [Google Scholar]
- 150.Hamilton C, Alfille P, Mountjoy J, Bao X. Regional anesthesia and acute perioperative pain management in thoracic surgery: a narrative review. J Thorac Dis. 2022;14(6):2276–2296. doi: 10.21037/jtd-21-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dada O, Zacarias AG, Ongaigui C, et al. Does rebound pain after peripheral nerve block for orthopedic surgery impact postoperative analgesia and opioid consumption? A narrative review. Int J Environ Res Public Health. 2019;16(18):1–12. doi: 10.3390/ijerph16183257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ko S, Jun CM, Min WK, et al. Pain relief after selective nerve root block as a predictor of postoperative functional outcome in patients with degenerative lumbar spinal stenosis patients undergoing decompressive surgery. Spine. 2022;47(9):666–671. doi: 10.1097/BRS.0000000000004216 [DOI] [PubMed] [Google Scholar]
- 153.Sort R, Brorson S, Gögenur I, Nielsen JK, Møller AM. Rebound pain following peripheral nerve block anaesthesia in acute ankle fracture surgery: an exploratory pilot study. Acta Anaesthesiol Scand. 2019;63(3):396–402. doi: 10.1111/aas.13290 [DOI] [PubMed] [Google Scholar]
- 154.Choi YH, Kim DH, Paik NJ, Park J. Long-term analgesic effects of peripheral nerve block in patients with central post-stroke pain: a retrospective series. Pain Pract. 2021;21(8):843–849. doi: 10.1111/papr.13031 [DOI] [PubMed] [Google Scholar]
- 155.Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. 2021;126(4):862–871. doi: 10.1016/j.bja.2020.10.035 [DOI] [PubMed] [Google Scholar]
- 156.Luebbert E, Rosenblatt MA. Postoperative rebound pain: our current understanding about the role of regional anesthesia and multimodal approaches in prevention and treatment. Curr Pain Headache Rep. 2023;27(9):449–454. doi: 10.1007/s11916-023-01136-z [DOI] [PubMed] [Google Scholar]
- 157.Singh NP, Makkar JK, Chawla JK, Sondekoppam RV, Singh PM. Prophylactic dexamethasone for rebound pain after peripheral nerve block in adult surgical patients: systematic review, meta-analysis, and trial sequential analysis of randomised controlled trials. Br J Anaesth. 2023;1–10. doi: 10.1016/j.bja.2023.09.022 [DOI] [PubMed] [Google Scholar]
- 158.Admassie BM, Tegegne BA, Alemu WM, Getahun AB. Magnitude and severity of rebound pain after resolution of peripheral nerve block and associated factors among patients undergoes surgery at university of gondar comprehensive specialized hospital northwest, Ethiopia, 2022. Longitudinal cross-sectional study. Ann Med Surg. 2022;84:104915. doi: 10.1016/j.amsu.2022.104915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee JH, Kim HJ, Kim JK, Cheon S, Shin YH. Does intravenous patient-controlled analgesia or continuous block prevent rebound pain following infraclavicular brachial plexus block after distal radius fracture fixation? A prospective randomized controlled trial. Korean J Anesthesiol. 2023;76(6):559–566. doi: 10.4097/kja.23076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Touil N, Pavlopoulou A, Barbier O, Libouton X, Lavand’homme P. Evaluation of intraoperative ketamine on the prevention of severe rebound pain upon cessation of peripheral nerve block: a prospective randomised, double-blind, placebo-controlled study. Br J Anaesth. 2022;128(4):734–741. doi: 10.1016/j.bja.2021.11.043 [DOI] [PubMed] [Google Scholar]
- 161.Liu X, Hu X, Li R, Zhang Y. Combination of post-fascia iliaca compartment block and dexmedetomidine in pain and inflammation control after total hip arthroplasty for elder patients: a randomized control study. J Orthop Surg Res. 2020;15(1):1–6. doi: 10.1186/s13018-020-1562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]