Abstract
Severe cytokine release syndrome (sCRS) and immune effector cell‐associated neurotoxicity syndrome (ICANS) have limited the widespread use of chimeric antigen receptor T (CAR T)‐cell therapy. We designed a novel anti‐CD19 CAR (ssCART‐19) with a small hairpin RNA (shRNA) element to silence the interleukin‐6 (IL‐6) gene, hypothesizing it could reduce sCRS and ICANS by alleviating monocyte activation and proinflammatory cytokine release. In a post hoc analysis of two clinical trials, we compared ssCART‐19 with common CAR T‐cells (cCART‐19) in relapsed/refractory B‐cell acute lymphoblastic leukemia (r/r B‐ALL). Among 87 patients, 47 received ssCART‐19 and 40 received cCART‐19. Grade ≥3 CRS occurred in 14.89% (7/47) of the ssCART‐19 group versus 37.5% (15/40) in the cCART‐19 group (p = 0.036). ICANS occurred in 4.26% (2/47) of the ssCART‐19 group (all grade 1) compared to 15% (2/40) of the cCART‐19 group. Patients in the ssCART‐19 group showed comparable rates of treatment response (calculated with rates of complete remission and incomplete hematological recovery) were 91.49% (43/47) for ssCART‐19 and 85% (34/40) for cCART‐19 (p = 0.999). With a median follow‐up of 21.9 months, cumulative nonrelapse mortality was 10.4% for ssCART‐19 and 13.6% for cCART‐19 (p = 0.33). Median overall survival was 37.17 months for ssCART‐19 and 32.93 months for cCART‐19 (p = 0.40). Median progression‐free survival was 24.17 months for ssCART‐19 and 9.33 months for cCART‐19 (p = 0.23). These data support the safety and efficacy of ssCART‐19 for r/r B‐ALL, suggesting its potential as a promising therapy.
INTRODUCTION
Chimeric antigen receptor T (CAR T)‐cell therapy has revolutionized the treatment of hematological malignancies. 1 , 2 However, the occurrence of cytokine release syndrome (CRS) and immune effector cell‐associated neurotoxicity syndrome (ICANS) cannot be ignored in CART therapy. 3 , 4 , 5 , 6 It has been reported that CRS occurred in 54%–91% of relapsed or refractory B‐cell acute lymphoblastic leukemia (r/r B‐ALL) patients after infusion of CAR T‐cells, and grade ≥3 CRS and ICANS could be fatal. 7 , 8 , 9 CRS is a systemic inflammatory response caused by the elevated proinflammatory cytokines secreted from activated CAR T‐cells and/or mononuclear macrophages. IL‐6 secreted by activated CAR T‐cells was found to activate monocytes to secret IL‐6 and therefore was considered the most pivotal cytokine in initiating CRS. High levels of serum IL‐6 were frequently observed in patients with severe cytokine release syndrome (sCRS). 9 , 10 , 11 , 12 , 13 On the other side, IL‐6 can enhance the function of CAR T‐cells; blocking IL‐6 signaling may negatively impact the proliferation and anti‐tumor effects of CAR T‐cells. 14 , 15 IL‐6 receptor monoclonal antibody, tocilizumab, and corticosteroids are recommended to treat sCRS. 16 , 17 , 18 However, tocilizumab was less effective in treating ICANS, probably due to its limited penetration of the blood–brain barrier. 15 As for corticosteroids, they not only inhibit the function of CAR T‐cells but also suppress the systemic immune system, which may lead to severe infections. 19 , 20 , 21 , 22 Therefore, there is an urgent need to reduce sCRS through ways other than drugs.
In our previous study, we developed a novel anti‐CD19 CAR T‐cell product (ssCART‐19) which utilized the small hairpin RNA (shRNA) technology to silence the IL‐6 gene of the anti‐CD19 CAR T‐cells. Preclinical studies demonstrated that IL‐6 knockdown in CAR T‐cells effectively reduced the release of IL‐6 by CAR T‐cells and monocytes. ssCART‐19 also showed improved safety without compromising the efficacy of CAR T‐cells in vitro studies. 23 A phase 1 study (NCT03919240) employing common CART‐19 (cCART‐19) for r/r B‐ALL has also been initiated. However, the safety and efficacy of ssCART‐19 compared with cCART‐19 in r/r B‐ALL remained unknown. During our clinical practice, we also successfully used ssCART‐19 for the treatment of B‐ALL patients with central nervous system infiltration (CNSL). 24 The results implied that ssCART‐19 also significantly reduced leukemic infiltration in the CNS with acceptable toxicity. Also, the safety and efficacy of ssCART‐19 and cCART‐19 in treating r/r B‐ALL with CNSL remained unelucidated.
In this study, we performed a post hoc analysis of 87 patients with r/r B‐ALL who were treated with cCART‐19 in the clinical trial NCT03919240 or with ssCART‐19 in the clinical trial NCT03275493. Data regarding the safety and efficacy of both CART products were directly compared.
PATIENTS AND METHODS
Patients
Inclusion and exclusion criteria for the two trials were presented in https://clinicaltrials.gov/. Patients in both trials who met the following criteria were included in this study: 1. Dose of the infused CAR T‐cells was 5 × 106/kg. 2. The date of infusion of CART‐19 cells was between December 2015 and February 2019. 3. Had bone marrow evaluation results on Day 28 after CAR T‐cells infusion. This post hoc analysis was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. All participants and their guardians provided informed consent for this study.
CAR constructs and CAR T‐cell production
Chimeric antigen receptor (CAR) constructs were expressed in the lentivirus vector backbone. CAR constructs and peripheral blood mononuclear cell transductions were performed as previously described. 23 , 25 Except for an anti‐CD19 murine single‐chain variable fragment (scFv), a 4‐1BB costimulatory moiety was encoded. The feature lies in the CD3 zeta activation domain with an IL‐6 shRNA element against IL‐6 (Supporting Information S1: Figure 1A).
CAR T‐cells were manufactured by the Shanghai Unicar‐Therapy Bio‐Medicine Technology Co., Ltd. A clinical‐grade culture system was employed to expand the CAR T‐cells to reach the required clinical infusion dose. The evaluation of CAR T‐cell product functionality mainly involved assessing the transduction efficiency of CAR T‐cells, CD4+/CD8+ T‐cell ratio, and anti‐tumor effects in vitro. Quality control studies were routinely conducted to ensure that CAR T‐cell products were sterile, mycoplasma‐negative, and endotoxin‐free. Afterward, the CAR T‐cells were washed, frozen, and stored in liquid nitrogen.
CAR T‐cell treatments and response assessments
Autologous leukapheresis was performed. Then patients were allowed to receive bridge chemotherapy prior to CAR T‐cell infusion. The bridge chemotherapy included regimens based on idarubicin, vincristine, prednisone, high‐dose methotrexate, or Hyper‐CVAD. Lymphodepletion chemotherapy with fludarabine (30 mg/m2/day) and cyclophosphamide (300 mg/m2/day) on days −5, −4, and −3 before CAR T‐cells infusion. All patients received CAR T‐cells at a dose of 5 × 106 cells/kg which were infused at escalated doses of 5 × 105, 1.5 × 106, and 3 × 106 cells/kg for 3 consecutive days.
Endpoints
The primary endpoint focused on safety, with CRS and ICANS graded according to the ASTCT Consensus. 26 Other adverse events (AEs) were graded based on CTCAE, Version 5.0. 27 Secondary endpoints included efficacy and survival, evaluated following the NCCN Guidelines. 28 Minimal residual disease (MRD) was assessed through flow cytometry (FCM), with a sensitivity threshold of 1 × 10−4. The extramedullary and intramedullary disease was evaluated on Day 28 after CAR T‐cells infusion, followed by subsequent evaluations every 3 months. Progression‐free survival (PFS) and overall survival (OS) were also secondary endpoints.
The proliferation and persistence of CAR T‐cells in peripheral blood samples collected from patients at predetermined time points were detected using quantitative real‐time PCR (qPCR) and FCM. Calculating the CAR copy number depends on the standard curve of qPCR established with the plasmid encoding the transgene. Cytometric bead array‐based FCM was employed to detect variations in cytokines, including IL‐4, IL‐6, IL‐10, IFN‐γ, TNF‐α, and IL‐17A.
Statistical analysis
The t‐test (paired or unpaired), Chi‐square test, Mann–Whitney nonparametric test, and Fisher's exact test were used for comparing data in both groups. Kaplan–Meier log‐rank test and Fisher's exact test were used for analyzing OS, PFS, complete response (CR)/CR with incomplete hematological recovery (CRi), AEs, CRS, and ICANS. p < 0.05 were considered statistically significant.
RESULTS
Patients and treatment characteristics
A total of 65 patients were enrolled in the ssCART‐19 group and 56 patients in the cCART‐19 group. In the ssCART‐19 group, eight patients did not receive CAR T‐cell therapy due to rapid disease progression (n = 3), production failure (n = 3), or personal withdrawal (n = 2). Additionally, 10 patients received CAR T‐cell infusions other than 5 × 106/kg. In the cCART‐19 group, seven patients did not receive CAR T‐cell therapy due to rapid disease progression (n = 3), production failure (n = 2), or personal withdrawal (n = 2), and nine patients received CAR T‐cell infusions other than 5 × 106/kg. Ultimately, 87 patients were enrolled: 47 patients received an infusion of ssCART‐19, while 40 patients received an infusion of cCART‐19. The baseline characteristics of patients who received CAR T‐cell therapy are presented in Table 1. No significant differences were observed between the baseline data of the two groups.
Table 1.
Baseline characteristics of all 87 treated patients and subgroups.
| Characteristic | ssCART‐19 (n = 47) | cCART‐19 (n = 40) | p Value |
|---|---|---|---|
| Male, No. (%) | 24 (51.06) | 25 (62.5) | 0.579 |
| Median age (range), years | 33 (9–64) | 24 (2–68) | 0.079 |
| Median lines of therapy (range) | 3 (2–8) | 4 (2–9) | 0.453 |
| Allogeneic SCT, No. (%) | 13 (27.66) | 6 (15) | 0.787 |
| Bone marrow blasts, % | |||
| ≥50% | 6 (12.77) | 7 (17.5) | 0.820 |
| ≥5% and <50% | 14 (29.79) | 13 (32.5) | |
| ≥0.01% and <5% | 13 (27.66) | 11 (27.5) | |
| <0.01% | 14 (29.79) | 9 (22.5) | |
| Bone marrow blasts (range) | 4.0 (0.01–86.0) | 4.0 (0.02–98.0) | 0.340 |
| High‐risk cytogenetic factors, No. (%) | |||
| BCR/ABL (Ph+) | 12 (25.53) | 9 (22.5) | 0.164 |
| TP53 | 1 (2.13) | 3 (7.5) | 0.910 |
Abbreviations: Ph+, Philadelphia chromosome‐positive; SCT, stem cell transplantation; TP53, TP53 gene mutation.
CAR T‐cell manufacturing data
The CAR T manufacturing process was identical in both groups. The expression of IL‐6 in ssCART‐19 was significantly decreased at both the mRNA and protein levels (Supporting Information S1: Figure 1B,C). To evaluate the impact of IL‐6 knockdown on efficacy, production data from ssCART‐19 cells and cCART‐19 cells were analyzed. The transfection efficiency was 39.98% (range: 14.0%–65.0%) for ssCART‐19 and 44.66% (range: 13.70%–84.66%) for cCART‐19 (p = 0.313) (Figure 1A). The CD4+/CD8+ T‐cell ratio was similar in both groups (1.931 vs. 1.531, p = 0.440) (Figure 1B), and the target cell killing efficacy of ssCART‐19 cells was comparable to that of cCART‐19 cells (40.22% vs. 44.96%, p = 0.364) (Figure 1C).
Figure 1.
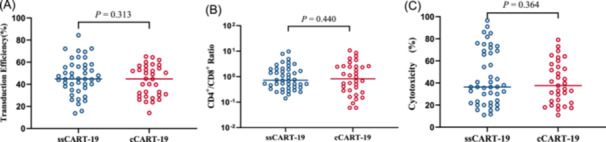
CAR T manufacturing data. (A) Transduction efficiency of ssCART‐19 group and cCART‐19 group (p = 0.313; t‐test). (B) CD4+/CD8+ T‐cell ratio of ssCART‐19 and cCART‐19 group (p = 0.440; t‐test). (C) Cytotoxicity of ssCART‐19 and cCART‐19 groups against target cells (p = 0.364; t‐test).
Safety
AEs within 28 days of CAR T‐cell infusion in both groups are presented in Table 2. There was no difference in the frequency of overall AEs between the ssCART‐19 group and cCART‐19 group (p = 0.204) (Figure 2A). The incidence of grade 3 or higher AEs and CRS were significantly lower in the ssCART‐19 group than in the cCART‐19 group (p = 0.026) (Figure 2B). The ssCART‐19 group showed significantly lower incidence and any grade CRS compared to the cCART‐19 group (p < 0.05, Figure 2C,D). However, the incidence of grade 3–4 CRS (14.89% vs. 37.5%) and any grade ICANS (4.26% vs. 15%) in the ssCART‐19 group was lower than that in the cCART‐19 group (Table 2). The ssCART‐19 group showed a significantly lower incidence of ICANS compared to the cCART‐19 group (p < 0.05, Figure 2E,F). With regard to hematologic toxicities, the incidence of grade 3–4 neutropenia was 29.79% versus 50%, and grade 3–4 thrombocytopenia was 36.17% versus 45% in the two groups, respectively, which were both significantly lower in the ssCART‐19 group than in the cCART‐19 group.
Table 2.
AEs among all 87 treated patients.
| ssCART‐19 (n = 47) | cCART‐19 (n = 40) | ||||
|---|---|---|---|---|---|
| Adverse events | Any grade | Grade ≥3 | Any grade | Grade ≥3 | p* Value |
| Hematologic event | |||||
| Anemia | 32 (68.09) | 15 (31.92) | 28 (70) | 15 (37.5) | 0.654 |
| Febrile neutropenia | 8 (17.02) | 6 (12.77) | 6 (15) | 6 (15) | 0.763 |
| Neutropenia | 26 (55.32) | 14 (29.79) | 25 (62.5) | 20 (50) | 0.078 |
| Thrombocytopenia | 25 (53.20) | 17 (36.17) | 21 (52.5) | 18 (45) | 0.511 |
| Cardiac disorders | |||||
| Sinus tachycardia | 4 (8.51) | 0 (0) | 2 (5) | 0 (0) | NE. |
| Heart failure | 4 (8.51) | 0 (0) | 2 (5) | 1 (2.5) | 1.000 |
| Gastrointestinal event | |||||
| Abdominal distension | 5 (10.64) | 0 (0) | 4 (10) | 0 (0) | NE. |
| Abdominal pain | 1 (2.13) | 0 (0) | 6 (15) | 0 (0) | NE. |
| Nausea | 11 (23.40) | 0 (0) | 10 (25) | 0 (0) | NE. |
| Vomiting | 7 (14.89) | 0 (0) | 9 (22.5) | 0 (0) | NE. |
| General disorders | |||||
| Chill | 11 (23.40) | 0 (0) | 7 (17.5) | 0 (0) | NE. |
| Fatigue | 20 (42.55) | 0 (0) | 16 (40) | 0 (0) | NE. |
| Immune system disorders | |||||
| CRS | 32 (68.09) | 7 (14.89) | 34 (85) | 15 (37.5) | 0.036 |
| ICANS | 2 (4.26) | 0 (0) | 6 (15) | 2 (5) | 0.044 |
| Infections and infestations | |||||
| Unknown type infection | 20 (42.55) | 3 (6.38) | 17 (42.5) | 5 (12.5) | 0.462 |
| Lung infection | 9 (19.15) | 1 (2.13) | 9 (22.5) | 4 (10) | 0.176 |
| Laboratory tests | |||||
| Alanine aminotransferase increased | 5 (10.64) | 2 (4.26) | 4 (10) | 2 (5) | 1.000 |
| Aspartate aminotransferase increased | 5 (10.64) | 1 (2.13) | 6 (15) | 1 (2.5) | 1.000 |
| Hypokalemia | 12 (25.53) | 0 (0) | 5 (12.5) | 0 (0) | NE. |
| Nervous system disorders | |||||
| Epilepsy | 2 (4.26) | 0 (0) | 6 (15) | 2 (5) | 0.209 |
| Cognitive disturbance | 1 (2.13) | 0 (0) | 5 (12.5) | 1 (2.5) | 0.460 |
| Respiratory, thoracic, and mediastinal disorders | |||||
| Cough | 10 (21.28) | 0 (0) | 6 (15) | 1 (2.5) | 0.460 |
| Dyspnea | 5 (10.64) | 0 (0) | 5 (12.5) | 1 (2.5) | 0.460 |
| Hypoxia | 6 (12.77) | 1 (2.13) | 15 (37.5) | 1 (2.5) | 1.000 |
| Skin and subcutaneous tissue disorders | |||||
| Rash | 4 (8.51) | 0 (0) | 3 (7.5) | 0 (0) | NE. |
| Vascular disorders | |||||
| Hypotension | 18 (38.30) | 6 (12.77) | 25 (62.5) | 7 (17.5) | 0.562 |
Note: Data are No. (%). Bone marrow blasts were measured by flow cytometry after bridging chemotherapy and FC lymphodepletion. Bold values indicate statistical significant at p < 0.05.
Abbreviations: NE., not estimated; P*, Comparison of ≥3 grade AEs between two groups (Chi‐squared test).
Figure 2.
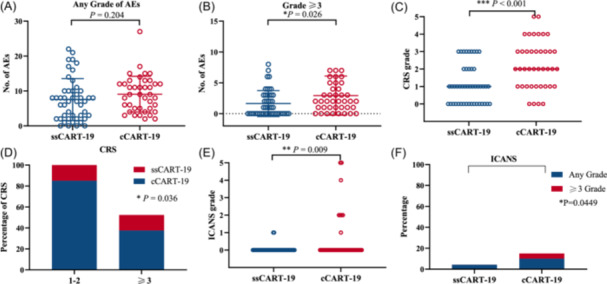
AEs, CRS, and ICANS in patients with 28 days after CAR T‐cell infusion. (A) Number of AEs at any grade for ssCART‐19 and cCART‐19 groups (p = 0.204; t test). (B) Number of grade ≥3 AEs in the ssCART‐19 group and cCART‐19 group (p = 0.026; t test). (C) Number of CRS at any grade for ssCART‐19 and cCART‐19 groups (p < 0.001; Mann–Whitney nonparametric test). (D) Percentage of different grades of CRS for ssCART‐19 and cCART‐19 groups (p = 0.036; Fisher's exact test). (E) Grade of ICANS for ssCART‐19 and cCART‐19 groups (p = 0.009; Mann–Whitney nonparametric test). Percentage of different grades of ICANS for ssCART‐19 and cCART‐19 groups (p = 0.049; Fisher's exact test). (F) Percentage of different grades of ICANS for ssCART‐19 groups and cCART‐19 groups (p = 0.0449; Fisher's exact test).
Change in cytokine levels after CAR T‐cell therapy
Following infusion of CAR T‐cells, the peak levels of IL‐6, IL‐2, and TNF‐α within 28 days were significantly lower in the ssCART‐19 group compared to the cCART‐19 group (p < 0.05) (Figure 3A–C). However, there were no significant differences in the peak levels of IL‐4, IL‐10, IL‐17A, and IFN‐γ (p > 0.05) (Figure 3D–G). The dynamic levels of IL‐6 and TNF‐α in the ssCART‐19 group were significantly lower than those in the cCART‐19 group (p < 0.05) (Figure 3H–I). No significant differences were observed between the two groups in terms of the dynamic level of IL‐2, IL‐10, and IFN‐γ (p > 0.05) (Figure 3J–L). Although the peak and dynamic cytokine levels were not entirely consistent, the overall trend of lower cytokine release levels in the ssCART‐19 group suggested a safety benefit of ssCART‐19, which was consistent with the lower incidence of CRS observed in the ssCART‐19 group.
Figure 3.
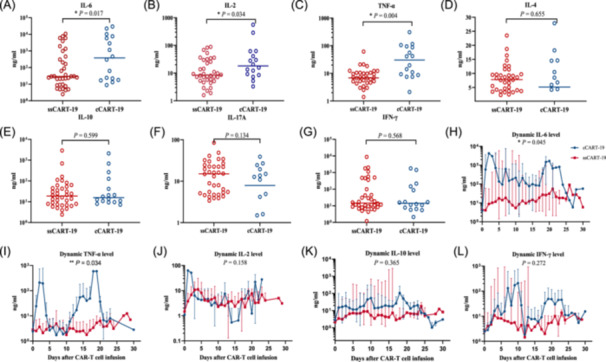
Cytokine levels in the two groups. (A–G) Scatter plots of the peak concentration of interleukin 6 (IL‐6), IL‐2, tumor necrosis factor‐α (TNF‐α), IL‐4, IL‐10, IL‐17A, and IFN‐γ. (H–L) Dynamic changes in the cytokines IL‐6, TNF‐α, IL‐2, IL‐10, and IFN‐γ within 28 days after CAR T‐cell infusion.
Treatment response and survival
On Day 28 after CAR T‐cell infusion, 91.49% (43/47) of patients achieved CR or CRi in the ssCART‐19 group, compared to 85% (34/40) in the cCART‐19 group (p = 0.999) (Figure 4). In the ssCART‐19 group, 72.34% (34/47) achieved MRD‐negative CR, while in the cCART‐19 group, 65% (26/40) achieved MRD‐negative CR. The duration of response of each patient enrolled in the study until the last visit is presented in a swimmer plot (Figure 5). After CART therapy, 20 patients (42.55%, 20/47) in the ssCART‐19 group and 18 patients (45%, 18/40) in the cCART‐19 group received allogeneic hematopoietic stem cell transplantation (allo‐HSCT) (p = 0.999) (Supporting Information S1: Figure 2). Patients in the adverse risk group who did not receive allo‐HSCT as consolidation continued to receive standard consolidation chemotherapy.
Figure 4.
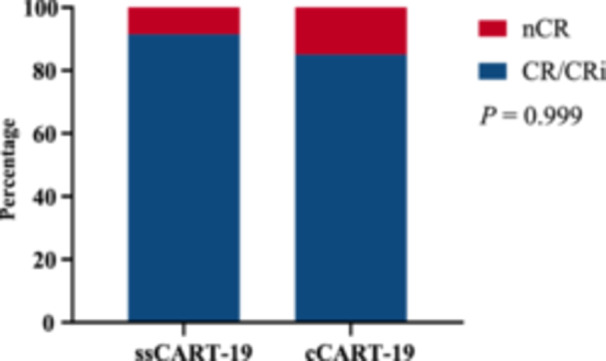
Response rates in the two groups (p = 0.999; t test).
Figure 5.
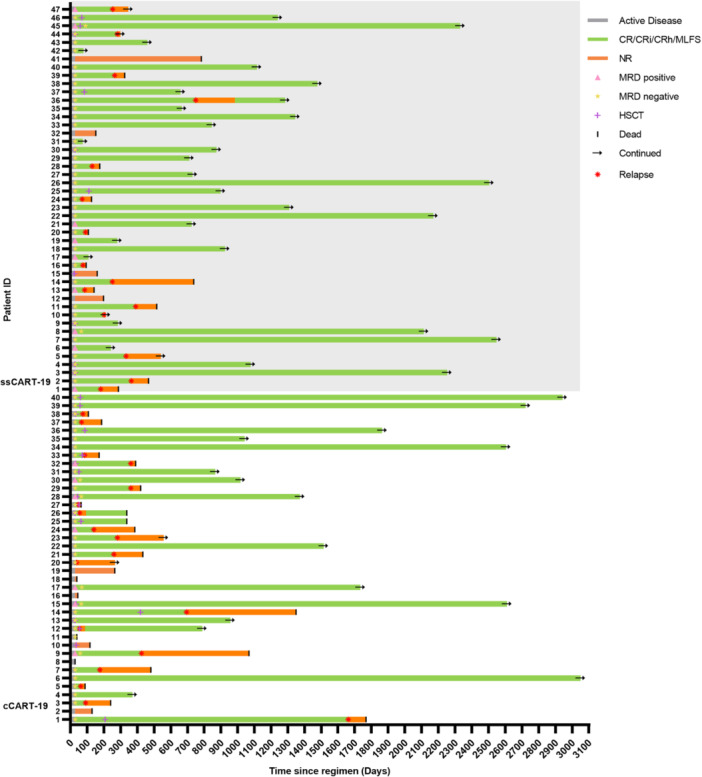
Swimmer plot of 87 patients showing treatment responses after CAR T‐cell infusion.
In the cCART‐19 group, three patients died within 28 days of CAR T‐cell infusion, including one patient who succumbed to sCRS, one patient who experienced sCRS and ICANS, and one patient who was discharged due to failure of CART therapy and subsequently died of unknown causes. In the ssCART‐19 group, one patient died of infection within 28 days.
As of the data cutoff on February 2024, the median follow‐up time was 21.9 (range: 0.33–101.7) months. The NRM was 10.4% in the ssCART‐19 group and 13.6% in the cCART‐19 group (p = 0.33) (Figure 6A). Median OS was 37.17 months for ssCART‐19 and 32.93 months for cCART‐19 (p = 0.40). The median PFS was 24.17 months for ssCART‐19 and 9.33 months for cCART‐19 (p = 0.23) (Figure 6B,C). We analyzed OS and PFS at 1, 2, and 3 years after CART therapy. The 1‐, 2‐, and 3‐year OS rates for patients in the ssCART‐19 group versus the cCART‐19 group were 67.34% versus 65.58%, 58.58% versus 55.33%, and 47.73% versus 42.43%, respectively. Similarly, the 1‐, 2‐, and 3‐year PFS rates for patients in the ssCART‐19 group versus the cCART‐19 group were 59.52% versus 45.54%, 51.04% versus 44.66%, and 44.65% versus 42.5%, respectively (Figure 6B,C; Supporting Information S1: Figure 3).
Figure 6.
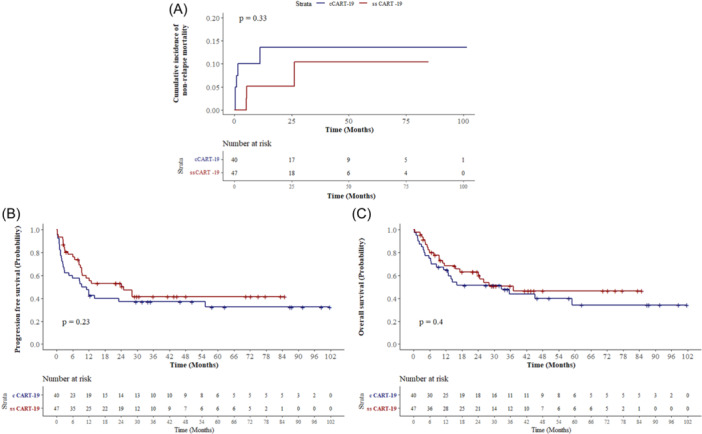
Response and survival. (A) NRM of the two groups of patients. (B, C) Kaplan–Meier curves of progression‐free survival and overall survival.
Pharmacokinetics
Following infusion of CAR T‐cells, blood levels of CAR T‐cells, measured by CAR gene copies per mg of DNA, peaked between 7 and 30 days in most patients. The ssCART‐19 and cCART‐19 groups were compared in terms of peak CAR T‐cell concentration (C max) and time to peak (T max) of CAR T‐cells expansion, and no statistically significant difference was observed between the two groups (p = 0.960; p = 0.768) (Figure 7A,B). Line graph analysis of the dynamic change process within 30 days of infusion showed that there was no significant difference in the expansion of CAR T‐cell expansion between the two groups (p = 0.438) (Figure 7C). These results suggest that the addition of IL‐6 knockdown elements does not affect the in vivo expansion of ssCART‐19 cells after infusion. The C max values were similar between the two groups, regardless of whether the patients achieved CR/CRi or NR. Specifically, for CR patients, the values were 1.16 × 105 copies (range: 8.27 × 103−1.25 × 107) versus 8.65 × 105 copies (range: 1.29 × 103−8.27 × 106), p = 0.6348. In NR patients, the values were 2.64 × 105 copies (range: 9.25 × 104−3.7 × 105) versus 4.58 × 104 copies (range: 3.05 × 104−1.8 × 106), p = 0.665 (Supporting Information S1: Figure 4A). Maximum expansion occurred on Day 7 (range: 1–24) in patients who achieved CR in the ssCART group and on Day 10 (range: 1–22) in the cCART‐19 group, with no significant difference observed between the two groups (Supporting Information S1: Figure 4B). These results indicate that the pharmacokinetics of ssCART‐19 cells were not affected by the insertion of IL‐6 knockdown elements in vivo.
Figure 7.
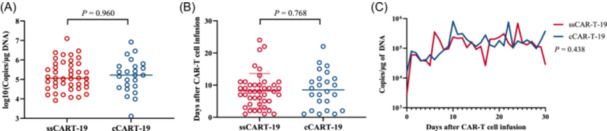
Expansion of CAR T‐cells. (A) C max in the ssCART‐19 and cCART‐19 groups (p = 0.960). (B) T max in the ssCART‐19 and cCART‐19 groups (p = 0.768). (C) Dynamic expansion of CAR T‐cells within 30 days postinfusion. The results showed no difference in the copies of CAR T‐cells in the ssCART‐19 and cCART‐19 groups (p = 0.438).
DISCUSSION
The CART therapy has shown great promise in the treatment of r/r B‐ALL. However, the occurrence of CRS and ICANS remains a major concern. Therefore, it is crucial to identify the risk factors for these toxicities and develop appropriate therapeutic and prophylactic strategies that do not compromise the efficacy of CART. In this post hoc analysis of 2 clinical trials, we compared the outcomes of patients treated with ssCART‐19 to those treated with cCART‐19.
Although there was no significant difference in NRM, median OS, and median PFS between the two groups, the 6‐month PFS was superior in the ssCART‐19 group, indicating a short‐term benefit of this treatment approach. This provides an opportunity for patients with poor prognosis to receive a treatment such as allo‐HSCT. A notable finding of our study was the significantly lower incidence of severe AEs in the ssCART‐19 group. In particular, the incidence of sCRS. In our retrospective analysis, only 17.02% of patients in the ssCART‐19 group required tocilizumab or corticosteroids, compared to 37.5% of patients in the cCART‐19 group. Corticosteroids are commonly used by clinicians to treat CRS and ICANS. 29 , 30 Prolonged use of corticosteroids can impair immune system function, promote tumor progression, and increase the risk of infection. 21 , 31 , 32 The lower incidence of CRS in the ssCART‐19 group, resulting in reduced corticosteroid use, may explain the observed 6‐month PFS benefit.
In addition, the knockdown of IL‐6 in CAR T‐cells has been shown to effectively reduce the severity of CRS. The primitive inflammatory factors produced by CAR T‐cells upon activation may be the trigger of CRS. Our previous experiments have confirmed that IL‐6, IL‐1, IFN‐γ, and IL‐2 in the supernatant of cCART‐19 activated by tumor cells can activate monocytes to secrete large amounts of IL‐6. 23 Within 30 days after CAR T‐cell infusion, serum levels of IL‐6, IL‐2, and TNF‐α levels were lower in patients in the ssCART‐19 group than in patients in the cCART‐19 group, suggesting that patients in the ssCART‐19 group had a relatively mild inflammatory response associated with CAR T‐cell infusion. These data may also partially explain the lower incidence of sCRS in the ssCART‐19 group.
Antigen stimulation from tumor cells effectively drives CAR T‐cell expansion, correlating with therapy response and CRS occurrence. Although higher CAR T‐cell expansion is associated with increased CRS severity, 33 , 34 our study shows that ssCART‐19 maintains comparable expansion and efficacy while reducing sCRS incidence compared to cCART‐19 in vivo. The integration of IL‐6 shRNA technology into ssCART‐19 did not affect T‐cell activation, proliferation, and differentiation, as confirmed by in vitro experiments and gene enrichment analysis. 23 , 35 This is consistent with findings suggesting that IL‐6 promotes CAR T‐cell proliferation and that inhibition of the IL‐6/STAT3 pathway could promote CAR T‐cell expansion. 14 , 36 Our clinical data confirm that IL‐6 knockdown does not affect the proliferation or cytotoxicity of ssCAR‐T‐19 cells.
In addition, we observed that the proportion of ICANS was lower in the ssCART‐19 group than in the cCART‐19 group. Chen et al. reported successful treatment of three patients with r/r B‐ALL involving the central nervous system using ssCART‐19, and none of them experienced ICANS. 24 By reducing the incidence of sCRS at its source, we can avoid the complexity of interventions and monitoring after the onset of ICANS. Therefore, ssCART‐19 is particularly recommended for patients at high risk of sCRS and ICANS.
In addition, the incidence of hematological toxicity, including neutropenia and severe thrombocytopenia, was reduced in the ssCART‐19 group. Notably, patients in the ssCART group had lower rates of grade ≥3 neutropenia and thrombocytopenia. Neutropenia can increase the risk of infections, particularly fungal infections, which can be life‐threatening. Juluri et al. used neutrophil and platelet recovery as indicators of hematologic toxicity, higher IL‐6 levels were associated with lower neutrophil and platelet counts. 37 Peak and dynamic levels of IL‐6 were lower after ssCART‐19 infusion, which may facilitate hematopoietic recovery.
Our clinical trials have demonstrated the safety and efficacy of ssCART‐19 in patients with r/r B‐ALL, particularly those with expected severe myelosuppression and CRS. The results support the potential of ssCART‐19 as a promising therapeutic approach for this challenging patient population. As T‐cell‐mediated aberrant macrophage activation contributes to the development of CRS, our future focus will be on disrupting the interaction between CAR T‐cells and macrophages to further improve the safety of CAR T‐cells. Relevant trial studies are currently underway.
AUTHOR CONTRIBUTIONS
Jin‐Feng Ma and Wen‐Jie Gong collected the data, performed statistical analysis, and drafted the manuscript. Lei Yu designed the CAR T‐cells. Sheng‐Li Xue and Wen‐Jie Gong treated the patients. Jia‐Wei Yan, Jing‐Wen Tan, and Hong‐Jia Zhu manufactured the CAR T‐cell and performed all experimental analyses. Xiao‐wen Tang, Hui‐Ying Qiu, Miao Miao, Yue Han, Li‐Min Li, Li‐Qing Kang, Nan Xu, Zhou Yu, Xu Jia, Mei‐Jing Liu, Zhi‐Zhi Zhang, and Miao Wang provided collected the clinical data. Chun‐Long Yan, De‐Pei Wu, and Hai‐Ping Dai revised the manuscript and supervised the study. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This work was supported by grants from the National Natural Science Foundation of China (Nos. 82470224, 82470174, 82270165, 82200249), National Key R&D Program of China (2022YFC2502703), Jiangsu Province Natural Science Foundation of China (No. BK20221235), Translational Research Grant of NCRCH (No. 2020ZKMB05), Jiangsu Province “333” Project, Social Development Project of the Science and Technology Department of Jiangsu (No. BE2021649), Gusu Key Medical Talent Program (No. GSWS2019007), Sailing Project, Scientific Research Foundation of Jining No.1 People's Hospital (2021‐QHM‐016).
Supporting information
Supporting information.
Contributor Information
Lei Yu, Email: ylyh188@163.com.
Sheng‐Li Xue, Email: slxue@suda.edu.cn.
De‐Pei Wu, Email: drwudepei@163.com.
Wen‐Jie Gong, Email: gongwenjie@suda.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361‐1365. [DOI] [PubMed] [Google Scholar]
- 2. Park JH, Rivière I, Gonen M, et al. Long‐term follow‐up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chong EA, Ruella M, Schuster SJ. Five‐year outcomes for refractory B‐cell lymphomas with CAR T‐cell therapy. N Engl J Med. 2021;384(7):673‐674. [DOI] [PubMed] [Google Scholar]
- 5. Westin JR, Kersten MJ, Salles G, et al. Efficacy and safety of CD19‐directed CAR‐T cell therapies in patients with relapsed/refractory aggressive B‐cell lymphomas: observations from the JULIET, ZUMA‐1, and TRANSCEND trials. Am J Hematol. 2021;96(10):1295‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yáñez L, Alarcón A, Sánchez‐Escamilla M, Perales MA. How I treat adverse effects of CAR‐T cell therapy. ESMO Open. 2020;4(Suppl 4):e000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T‐cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146(5):940‐948. [DOI] [PubMed] [Google Scholar]
- 8. Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25(9):1341‐1355. [DOI] [PubMed] [Google Scholar]
- 9. Shimabukuro‐Vornhagen A, Böll B, Schellongowski P, et al. Critical care management of chimeric antigen receptor T‐cell therapy recipients. CA Cancer J Clin. 2022;72(1):78‐93. [DOI] [PubMed] [Google Scholar]
- 10. Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine‐directed therapy. Blood. 2013;121(26):5154‐5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norelli M, Camisa B, Barbiera G, et al. Monocyte‐derived IL‐1 and IL‐6 are differentially required for cytokine‐release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739‐748. [DOI] [PubMed] [Google Scholar]
- 13. Xiao X, Huang S, Chen S, et al. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T‐cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res. 2021;40(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Z, Liao R, Lv J, et al. IL‐6 trans‐signaling promotes the expansion and anti‐tumor activity of CAR T cells. Leukemia. 2021;35(5):1380‐1391. [DOI] [PubMed] [Google Scholar]
- 16. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321‐3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schubert ML, Schmitt M, Wang L, et al. Side‐effect management of chimeric antigen receptor (CAR) T‐cell therapy. Ann Oncol. 2021;32(1):34‐48. [DOI] [PubMed] [Google Scholar]
- 18. Kishimoto T. IL‐6: from arthritis to CAR‐T‐cell therapy and COVID‐19. Int Immunol. 2021;33(10):515‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kauer J, Hörner S, Osburg L, et al. Tocilizumab, but not dexamethasone, prevents CRS without affecting antitumor activity of bispecific antibodies. J Immunother Cancer. 2020;8(1):e000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10(8):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bupha‐Intr O, Haeusler G, Chee L, Thursky K, Slavin M, Teh B. CAR‐T cell therapy and infection: a review. Expert Rev Anti Infect Ther. 2021;19(6):749‐758. [DOI] [PubMed] [Google Scholar]
- 22. Hill JA, Li D, Hay KA, et al. Infectious complications of CD19‐targeted chimeric antigen receptor‐modified T‐cell immunotherapy. Blood. 2018;131(1):121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang L, Tang X, Zhang J, et al. Interleukin‐6‐knockdown of chimeric antigen receptor‐modified T cells significantly reduces IL‐6 release from monocytes. Exp Hematol Oncol. 2020;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen LY, Kang LQ, Zhou HX, et al. Successful application of anti‐CD19 CAR‐T therapy with IL‐6 knocking down to patients with central nervous system B‐cell acute lymphocytic leukemia. Transl Oncol. 2020;13(11):100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gong WJ, Qiu Y, Li MH, et al. Investigation of the risk factors to predict cytokine release syndrome in relapsed or refractory B‐cell acute lymphoblastic leukemia patients receiving IL‐6 knocking down anti‐CD19 chimeric antigen receptor T‐cell therapy. Front Immunol. 2022;13:922212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625‐638. [DOI] [PubMed] [Google Scholar]
- 27. Freites‐Martinez A, Santana N, Arias‐Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE ‐ Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 2021;112(1):90‐92. [DOI] [PubMed] [Google Scholar]
- 28. Brown PA, Shah B, Advani A, et al. Acute lymphoblastic leukemia, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19(9):1079‐1109. [DOI] [PubMed] [Google Scholar]
- 29. Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T‐cell therapy. Blood. 2023;141(20):2430‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu S, Deng B, Yin Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR‐T cells for B‐cell acute lymphoblastic leukemia. Blood Cancer J. 2020;10(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006;117(3):522‐543. [DOI] [PubMed] [Google Scholar]
- 32. Banuelos J, Shin S, Cao Y, et al. BCL‐2 protects human and mouse Th17 cells from glucocorticoid‐induced apoptosis. Allergy. 2016;71(5):640‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Z, Lian Y, Ti J, Ren R, Ma L. Therapeutic efficacy and infectious complications of CD19‐targeted chimeric antigen receptor‐modified T cell immunotherapy. Anticancer Drugs. 2023;34(4):551‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang M, Munoz J, Goy A, et al. KTE‐X19 CAR T‐cell therapy in relapsed or refractory mantle‐cell lymphoma. N Engl J Med. 2020;382(14):1331‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raje N, Berdeja J, Lin Y, et al. Anti‐BCMA CAR T‐cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schluck M, Hammink R, Figdor CG, Verdoes M, Weiden J. Biomaterial‐based activation and expansion of tumor‐specific T cells. Front Immunol. 2019;10:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Juluri KR, Wu QV, Voutsinas J, et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T‐cell therapy. Blood Adv. 2022;6(7):2055‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


