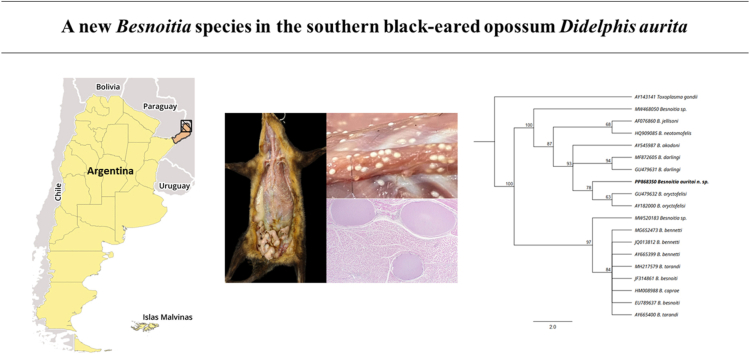
Abstract
Besnoitia spp. are cyst-forming coccidian parasites with a broad host range, infecting various wild and domestic animal species. Northamerican opossums (Didelphis virginiana) are severely affected by the infection with B. darlingi. This study presents a case of infection with Besnoitia in a road-killed female southern black-eared opossum (Didelphis aurita) in Misiones, Argentina. Many 0.5–1 mm cysts were observed in several muscles and visceral organs and were microscopically identified in skeletal muscles, tongue, and heart. Histological analysis disclosed multiple spherical cysts with a myriad of bradyzoites like-cells and a well-defined cyst wall. A small number of degenerate and ruptured cysts, surrounded by mild to moderate inflammation were observed. Genomic DNA from an individual cyst and muscle was extracted and ITS1 marker and 18S rRNA gene fragments from sarcocystid protozoa were successfully amplified by PCR and sequenced. The 18S sequence exhibited 100% identity with sequences of B. darlingi and B. oryctofelisi. Comparison of the complete ITS1 sequence (259 bp) revealed an identity of 99.2% with B. oryctofelisi and 97.7% with B. darlingi. This result together with the phylogeny positioning, suggest that the Besnoitia sp. in the present case differ from B. darlingi, being closely related with B. oryctofelisi.
Keywords: Besnoitia sp., Didelphis aurita, Argentina, Infection, PCR, Histopathology
Graphical abstract
Highlights
-
•
Numerous 0.5–1 mm Besnoitia tissue cysts found in a Didelphis aurita from Argentina.
-
•
The obtained ITS1 sequence differed from B. darlingi from Northamerican opossums.
-
•
Besnoitia sp. recorded in this work could be a new species.
-
•
Besnoitia spp. from the Americas seems to have a common ancestor.
1. Introduction
The southern black-eared opossum, Didelphis aurita, inhabits the Atlantic Forest in South America. It is distributed in southeastern Brazil, from Bahia to Rio Grande do Sul, eastern Paraguay and northeastern Argentina, in northern and central Misiones province (Gardner, 2007). This species is frequent in continuous and remnant native forests associated with streams (Massoia et al., 2012; Chemisquy et al., 2019). It is less frequent in anthropic modified environments than its sympatric close related White-eared opposum (Didelphis albiventris) (Cruz et al., 2019). Roadkill poses a substantial threat to different species within protected natural areas of the Atlantic Forest, both in Brazil and Argentina. The southern black-eared opossum is among the most frequently affected mammals by roadkill in northern Misiones (Chemisquy et al., 2019). Despite its association with remnant native forests and frequent roadkill incidents, there is no evidence suggesting that southern black-eared opossum is at risk of conservation concern (Chemisquy et al., 2019; Cruz et al., 2019). It is currently listed as "Least Concern" in the International Union for Conservation of Nature's Red List of Threatened Species (Astúa et al., 2021).
Related to the parasites and pathogens occurring in southern black-eared opossum, numerous potentially pathogenic agents have been identified in this host in Brazil, such as Mycoplasma sp. (Oliveira et al., 2023), Salmonella enterica (Casagrande et al., 2011), Ehrlichia sp. (Guimarães et al., 2008), among others. Moreover, several protozoans and helminths have been detected in the southern black-eared opossum, including Sarcocystis spp., Toxoplasma gondii, Schistosoma mansoni, and Cruzia tentaculata (Casagrande et al., 2009; Costa Neto et al., 2019; Bezerra-Santos et al., 2021). In Argentina, Hartmann (Hartmann, 2023) mentioned the presence of seven helminths, including the nematodes Capillaria sp., C. tentaculata, Cyclophyllidea, the trematode Rhopalias sp. and two acanthocephalans. These findings underline the importance of understanding the role of these marsupials in wild cycles of pathogens and assessing their role as potential reservoirs and transmitters of parasites in the region. Besnoitia is a genus of cosmopolitan apicomplexan protozoans included in the family Sarcocystidae. Different Besnoitia species parasitize a broad intermediate host range, such as cattle, equids, rodents, opossums, among others, developing tissue cysts (Dubey and Yabsley, 2010). Felids are definitive hosts for some Besnoitia species, in which the parasites undergo a sexual multiplication in the intestine, leading to the production and shedding of oocysts (Dubey et al., 2003a; Dubey and Yabsley, 2010; Smith and Frenkel, 1977, 1984; Verma et al., 2017; Wallace and Frenkel, 1975). Currently, at least 10 species of Besnoitia are described; however, only in a few of them, the complete life cycles are known (Basso 2018). In North and South America, different intermediate hosts have been documented, such as the virginian opossum (Didelphis virginiana) for B. darlingi, domestic rabbits (Oryctolagus cuniculus) for B. oryctofelisi, and different rodent species for B. akodoni, B. jellisoni and B. neotomofelis (Dubey et al., 2003a, 2003b; Dubey and Yabsley, 2010; Venturini et al., 2002). Domestic cats (Felis catus) can serve as definitive hosts for B. neotomofelis, B. darlingi, B. oryctofelisi and B. wallacei (Olias et al., 2011). In the case of B. darlingi, bobcats (Lynx rufus) were shown to be natural definitive hosts (Verma et al., 2017). Besnoitia oocysts are morphologically similar to T. gondii oocysts and can be easily misidentified (Dubey and Yabsley, 2010). Given that the different Besnoitia species cannot be differentiated based on cyst morphology, molecular methods are necessary for its identification (Venturini et al., 2002; Basso, 2018; Schares et al., 2020). The ITS1 region is highly conserved within each Besnoitia species and is being used as the main target for molecular identification techniques (Schares et al., 2020).
In Argentina, Besnoitia-like cysts have been previously documented in various hosts, including those from B. oryctofelisi in domestic rabbits (Venturini et al., 2002), and from undescribed Besnoitia species in pichis (Zaedyus pichiy) (Superina et al., 2009) and in vizcachas (Lagostomus maximus) (Cwirenbaum et al., 2021).
In wild mammals, besnoitiosis typically manifests with cysts in the skin, skeletal muscle, and visceral organs, accompanied by acute and chronic inflammatory processes (Jack et al., 1989; Elsheikha et al., 2003; Juan-Salles et al., 2004; Shaw et al., 2009; Superina et al., 2009). Several studies suggest that factors such as young age, immunological naivety, immunosuppression, and stress may predispose individuals to clinical disease (Glover et al., 1990; Ellis et al., 2012). This study documents a putative new species of Besnoitia in southern black-eared opossum, incorporating histopathological, and molecular aspects.
2. Materials and methods
2.1. Study area
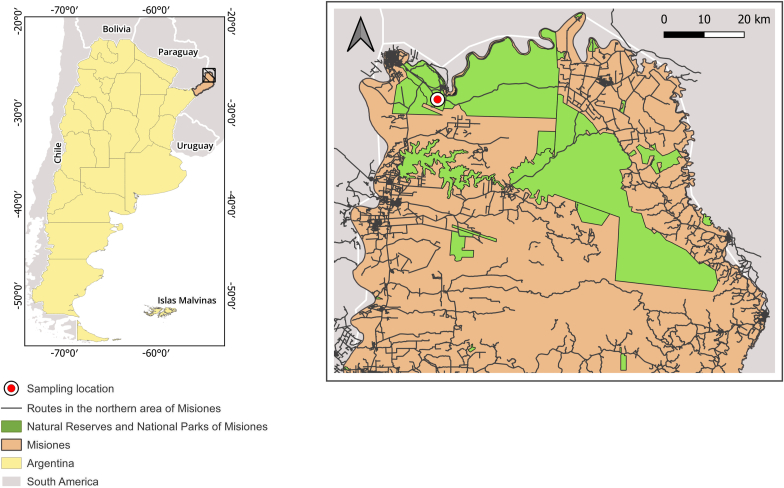
The sampling was carried out in the Iguazú National Park (PNI), located in the department of Iguazú, northwest of the province of Misiones, Argentina (Fig. 1). The area is bordered by Brazil to the north and Paraguay to the west. In addition, the area is known to receive more than one million tourists from all over the world who visit the PNI every year. The PNI is included in the Upper Paraná Atlantic Forest ecoregion and is part of the largest continuous remnant of Atlantic Forest in the world (25° 55′ 52.32″ S; 54° 15 '30.60″ W). Despite its highly fragmented state, the Misiones rainforest remains one of the most biologically diverse ecosystems in the world (Cullen et al., 2001) and hosts the most varied mammalian community in Argentina (De Angelo et al., 2008). Within this diverse community, several carnivorous species may prey on didelphids, including the cougar (Puma concolor), jaguar (Panthera onca), ocelot (Leopardus pardalis), jaguarundi (Herpailurus yagouaroundi), margay (Leopardus wiedii), southern tiger cat (Leopardus guttulus), crab-eating fox (Cerdocyon thous), and greyheaded tayra (Eira barbara) (Presley, 2000; Wang, 2002; Facure et al., 2003; Moreno et al., 2006; Tófoli et al., 2009; Perilli et al., 2016).
Fig. 1.
Map of Argentina showing Misiones province (left). Detailed area of Northwest Misiones (right); red dot shows the point where the road-killed southern black-eared opossum (Didelphis aurita) was found in the National Route 101 traversing the National Park Iguazú (green). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The vegetation is a subtropical semi-deciduous forest composed of different plant communities, such as gallery forests, bamboo forests, forests dominated by palmettos (Euterpe edulis), araucaria forests (Araucaria angustifolia), among others (Di Bitetti et al., 2003; Galindo-Leal and de Gusmão Câmara, 2003). This area is characterized by an altitude of 220 m and has a subtropical climate, with annual rainfall ranging between 1700 and 2100 mm and an average annual temperature of 20 °C (Ligier, 2000).
2.2. Animal samples
A female wild southern black-eared opossum was retrieved deceased following a vehicular collision on National Route 101 (Misiones, Argentina), a roadway traversing the PNI, on August 25th, 2023 (Fig. 1). The carcass was handled and sampled in accordance with protocols sanctioned by the technical office of the National Parks Administration (IF-2023-34961534-APN-DRNEA#APNAC), adhering to the guidelines proposed by the WOAH (World Organisation for Animal Health) for wildlife disease surveillance (https://www.woah.org/app/uploads/2021/09/a-wildlifehealth-conceptnote.pdf). A comprehensive dataset detailing parameters such as sex, reproductive status, age category, weight, and body condition score was recorded. Subsequently, a complete necropsy was conducted, scrutinizing each body cavity for anomalies, and systematically documenting the macroscopic characteristics of each organ.
Tissue samples for histopathology, including skeletal muscle, heart, tongue, lung, liver, spleen, kidney, intestines, diaphragm, lymph nodes, fetuses, brain, and cerebellum, were collected and preserved in 10% formalin. Preservation included both a segment of healthy tissue and, when present, a segment of any identified lesions. A pooled sample comprising muscular tissues, was stored at −80 °C and subsequently prepared for DNA extraction.
2.3. Histopathological studies
Formalin-fixed tissue samples were processed by standard histologic techniques using a Leica TP1020 tissue processor. Briefly: tissues were embedded in paraffin, sections of 4-μm-thick were prepared using a Leica RM2245 microtome, which were stained with hematoxylin and eosin. Slides were examined by optical microscopy and lesions were characterized. Photographs were obtained with a Leica DM750 microscope and a Leica ICC50W camera.
2.4. Molecular identification and phylogenetic analysis
DNA was extracted using a commercially available kit, according to the manufacturer's instructions (ADN PuriPrep-T kit; Inbio-Highway, Argentina) from pooled skeletal muscles containing several cysts and one individual cyst retrieved from skeletal muscle. For 18S rRNA gene, a fragment of around 650 bp was amplified by conventional PCR, using SarcoF and SarcoR primers (Moré et al., 2011). Another conventional PCR, targeting the ITS1 and flanking regions was performed using primers SU1F and 5.8SR2 (Gjerde, 2014). Both PCRs were carried out as previously described by Bentancourt Rossoli et al. (2023). Each PCR routine included a negative control (DNA extraction process control sample), a no template control (NTC, ultrapure water), and a positive control (S. miescheriana DNA for 18S rRNA and S. rileyi DNA for ITS1 PCR). A GeneAmp PCR System 9700 cycler (Applied Biosystems) was used to perform all PCR assays. Amplification products were examined after electrophoresis in a 1.5% agarose gel stained with ethidium bromide and photographed with a UV light image system (E-Box, Vilber, France). Obtained amplicons were excised from the gels and purified using a commercial kit according to the manufacturer's instructions (Zymoclean™ Gel DNA recovery Kit, Zymo Research, USA) and submitted for Sanger sequencing to Microsynth, Balgach, Switzerland (https://srvweb.microsynth.ch), along with both primers used for each PCR. Sequences obtained were analyzed and aligned using the Geneious Prime software (https://www.geneious.com). The consensus sequences with trimmed primers were compared with the GenBank database by BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The obtained ITS1 sequence was aligned with sequences of Besnoitia spp. and a distance tree was constructed using Bayesian inference with gamma rate variation and HKY85 substitution model (MrBayes plugin, Geneious Prime software). A ITS1 sequence from Toxoplasma gondii (AY143141) was used as an outgroup. All the sequences used for the phylogenetic tree are listed in Table 1. The obtained 18S rRNA fragment sequence was also aligned with other Besnoitia spp. sequences (which have at least 90% coverage with our sequence) and a phylogenetic tree was constructed using the same model and software mentioned before. A 18S rRNA sequence from Toxoplasma gondii (OR805035) was used as an outgroup to root the tree.
Table 1.
Besnoitia spp. ITS1 sequences from different hosts used for constructing the phylogenetic tree. The Toxoplasma gondii sequence was used as outgroup. ∗obtained from cell culture derived parasites.
| Coccidian species | Host | GenBank Accession | Locality | Author |
|---|---|---|---|---|
| Toxoplasma gondii | not indicated | AY143141 | USA | Su et al. (2003) |
| Besnoitia sp. | Acinonyx jubatus | MW468050 | Namibia | Schares et al. (2021) |
| B. jellisoni | not indicated∗ | AF076860 | Australia | Ellis et al. (2000) |
| B. neotomofelis | Neotoma micropus | HQ909085 | Texas, USA | Charles et al., 2012 |
| B. akodoni | Akodon montensis | AY545987 | Brazil | Dubey et al. 2003a,b |
| B. darlingi | Lynx rufus | MF872605 | Mississippi, USA | Verma et al. (2017) |
| B. darlingi | Didelphis virginiana | GU479631 | Mississippi, USA | Dubey et al. 2003a,b |
| Besnoitia sp. | Didelphis aurita | PP868350 | Misiones, Argentina | This study |
| B. oryctofelisi | Oryctolagus cuniculus | GU479632 | Argentina | Rosenthal et al. (2016) |
| B. oryctofelisi | Oryctolagus cuniculus | AY182000 | Argentina | Dubey et al. 2003a,b |
| Besnoitia sp. | Equus asinus | MW520183 | Italy | Villa et al. (2021) |
| B. bennetti | Equus asinus | MG652473 | Belgium | Liénard et al. (2018) |
| B. bennetti | Equus asinus | JQ013812 | Pennsylvania, USA | Ness et al. (2012) |
| B. bennetti | Equus asinus | AY665399 | Michigan, USA | Elsheikha et al., 2005 |
| B. tarandi | Rangifer tarandus caribou | MH217579 | Quebec, Canada | Schares et al. (2019) |
| B. besnoiti | Bos taurus | JF314861 | Bologna, Italy | Gentile et al. (2012) |
| B. caprae | Capra aegagrus hircus | HM008988 | Iran | Namazi et al. (2011) |
| B. besnoiti | Bos taurus | EU789637 | Spain | Fernández-García et al., 2009 |
| B. tarandi | Rangifer tarandus | AY665400 | Finland | Dubey et al. (2004) |
3. Results
3.1. Macroscopic examination
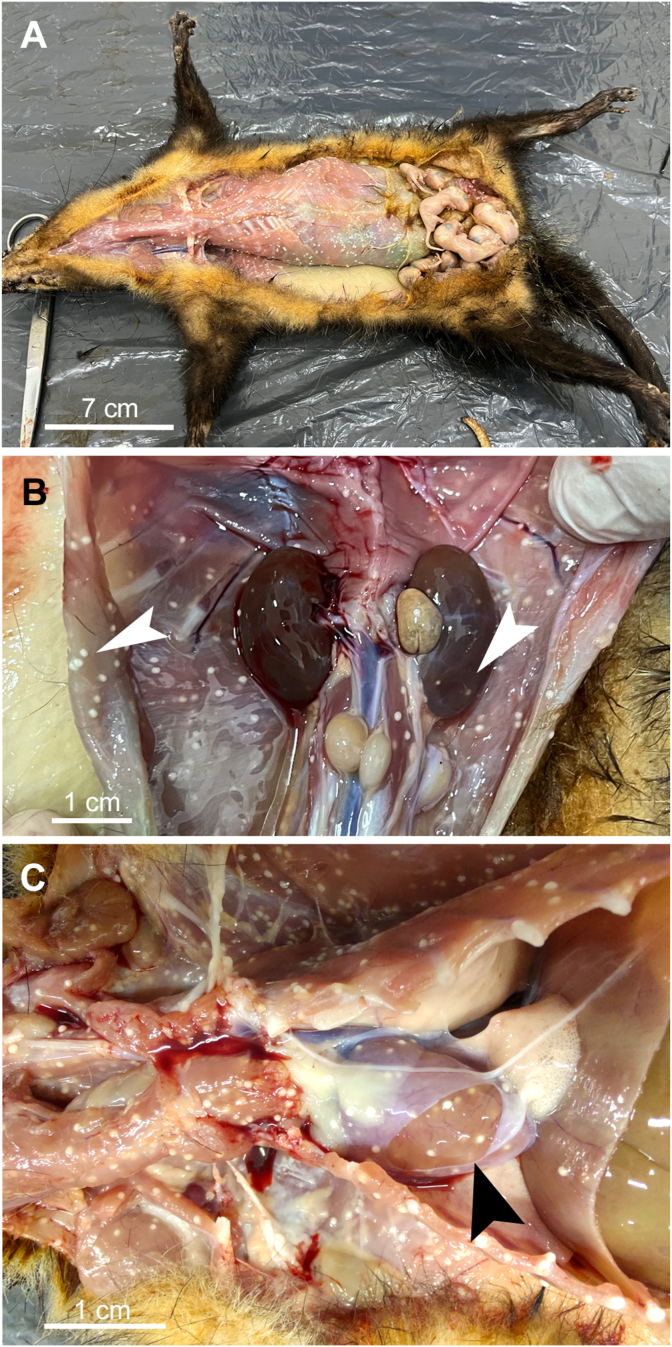
The specimen collected was an adult female of southern black-eared opossum, weighing 790 g, with eight embryos in the marsupium (Fig. 2a). Macroscopic examination revealed the presence of a huge number of macroscopic whitish cysts in various organs of the adult female, including subcutaneous tissue, teats, fasciae, skeletal muscles, heart, liver, kidneys, and spleen; however, skeletal muscles and heart were the most affected organs (Fig. 2). The cysts measured between 0.5 and 1 mm in diameter and were predominantly located superficially, although some were detected deep within the tissues, particularly in skeletal muscle and heart (Fig. 2b and c). Based on fat reserves, muscle mass, visible bony prominences, and organ shape, the body condition of the animal was considered good.
Fig. 2.
Dissection of a female southern black-eared opossum (Didelphis aurita). Multiple whitish cysts in skeletal muscles (A), in diaphragm and kidney (white arrowheads) (B), and heart (black arrowhead) (C).
3.2. Histopathological findings
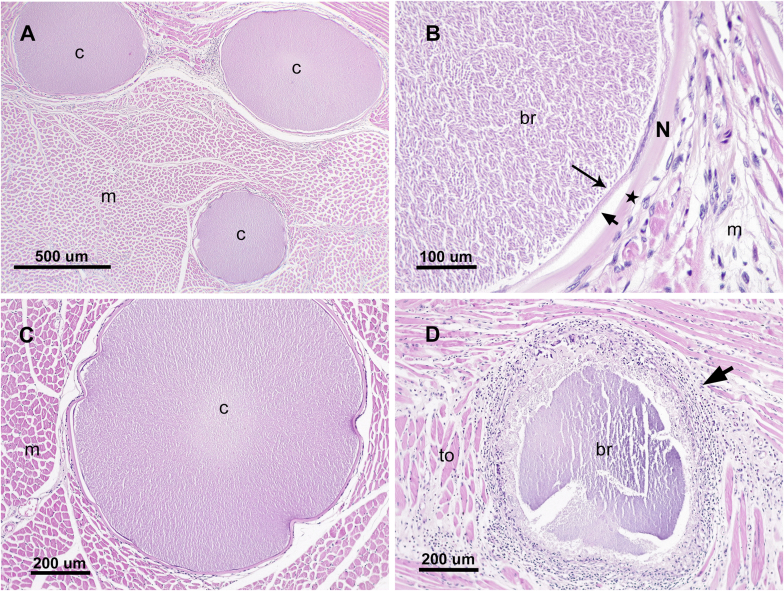
Histopathological analysis revealed multiple cysts, located mostly in skeletal muscle, tongue, and heart of the adult female. No cysts were found in the fetuses. The cysts were spherical, measured up to 1000 μm, and had a 10–20 μm thick-three-layered wall. The outermost layer was an eosinophilic and hyalinized capsule of collagen fibers. The next visible layer was the cytoplasm of the host cell; sometimes compressed host cell nuclei could be seen in it. The inner layer was a thin parasitophorous vacuole, which contained a myriad of approximately 2 × 5 μm basophilic bradyzoites-like cells (Fig. 3a and b). Most of the cysts had no tissue reaction (Fig. 3c). Some cysts were surrounded by a mild number of lymphocytes, macrophages, plasma cells, and eosinophils. There were a small number of degenerated and ruptured cysts, surrounded by mild to moderate inflammation, composed by macrophages, lymphoid cells, eosinophils, and giant cells (Fig. 3d). Other findings were multifocal bronchointerstitial pneumonia, with intralesional nematodes; diffuse pleocelular esophagitis with intralesional nematodes; and reactive lymphoid tissue in spleen and lymph nodes. No other lesions were found in the rest of the organs examined. Three slides with histopathological sections from muscle, tongue and heart containing Besnoitia sp. cysts were stored at the Helminthological Collection Museo de La Plata, La Plata, Buenos Aires, Argentina (code MLP-Pr 105).
Fig. 3.
Histopathological sections of Besnoitia sp. cysts in muscles stained with hematoxylin and eosin. Cysts with bradyzoites (c) in skeletal muscles (m) (A). Detail of a cyst wall in the heart muscle (m), composed of three layers: capsule (star), host cell cytoplasm (short arrow), and parasitophorous vacuole (long arrow) with a myriad of bradyzoites (br). Note the compressed nucleus of the host cell (N) (B). Higher magnification of a Besnoitia sp. cyst (c) in skeletal muscle (m) showing absence of tissue reaction (C). Degenerated Besnoitia sp. cyst on the tongue (to) surrounded by severe inflammation (short arrow) (D).
3.3. Molecular characterization and phylogenetic positioning of a putative new Besnoitia sp.
The two samples processed were positive by the 18S rRNA PCR and the amplicons were suitable for subsequent sequencing. The obtained sequences (625bp, primers trimmed) from muscles and one individual cyst, were identical between them, and exhibited 100% identity and coverage with previously described sequences of B. darlingi (MF872605) and B. oryctofelisi (GU479632).
By ITS1 PCR the sample from the individual cyst showed a more concentrated product and was sequenced, resulting in a consensus sequence (with trimmed primers) of 484bp (including complete ITS1, and 18S rRNA and 5.8S flanking regions). The identity was 98.56% (100% coverage) with Besnoitia darlingi (MF872605) and 99.54% (89% coverage) with Besnoitia oryctofelisi (GU479632). When only the complete ITS1 sequence (259bp) was considered, the percentage of identity was 99.23% (two single nucleotide polymorphisms-SNPs) with B. oryctofelisi sequences (GU479632 and AY182000) and 97.7% (4 SNPs and 2 gaps) with B. darlingi sequences (GU479631, HQ163919, AF489696), all with 100% coverage.
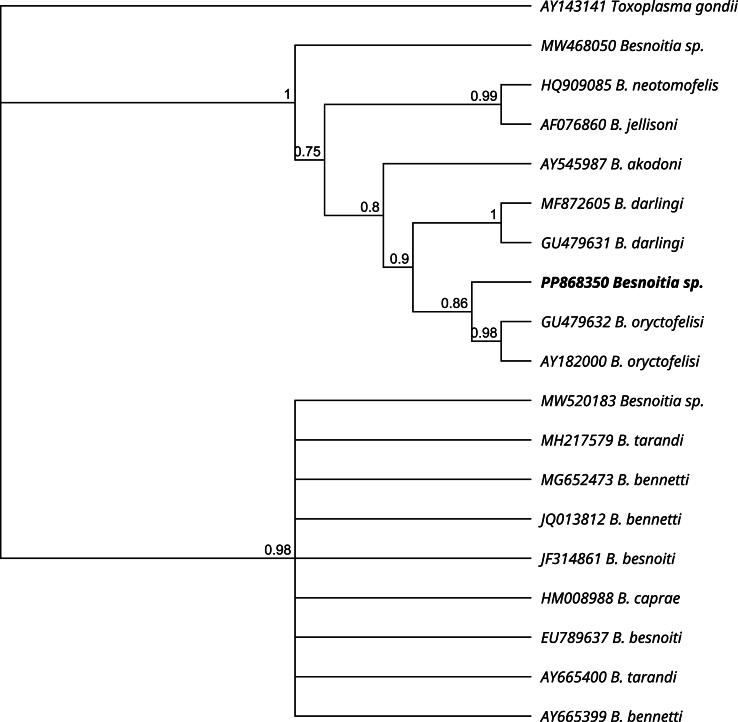
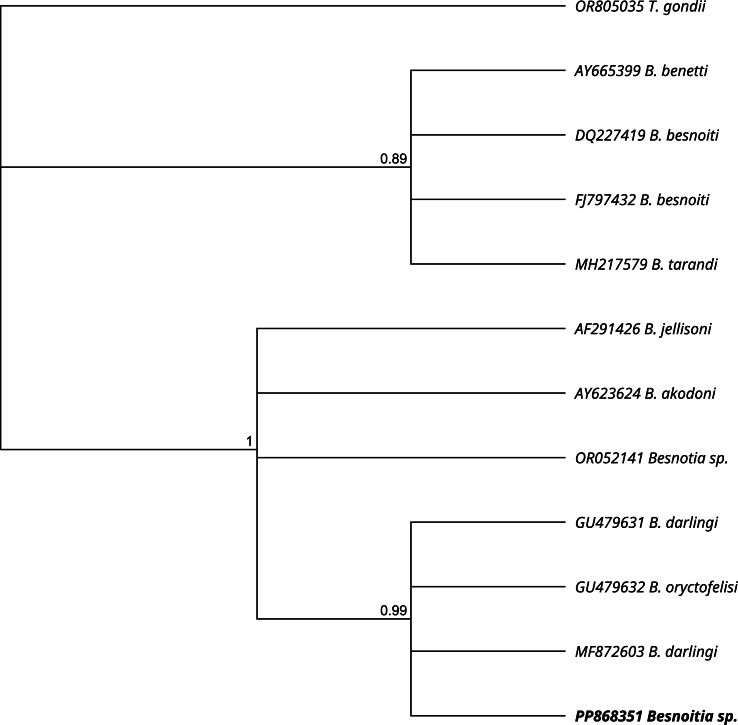
The obtained sequences of complete ITS1 containing 18S and 5.8S rRNA gene flanking regions (Accession number PP868350) and 18S rRNA gene fragment (Accession number PP868351) were registered in the GenBank. In the phylogenetic tree (Fig. 4) our sequence is positioned on a branch closely related to B. oryctofelisi, and as close relatives in a sister group appear B. darlingi and B. akodoni sequences. The phylogeny using 18S rRNA sequences positioned the sequence of Besnoitia sp. from southern black-eared opossum together with sequences of B. darlingi and B. oryctofelisi and closely related to B. akodoni, B. jellisoni and a Besnoitia sp. from rodents (Fig. 5).
Fig. 4.
Phylogenetic distance tree using Bayesian inference with gamma rate variation and HKY85 substitution model (MrBayes plugin, Geneious Prime software) of ITS1 sequences from Besnoitia spp. A ITS1 sequence from Toxoplasma gondii (AY143141) was used as an outgroup. Branches are labelled with the posterior probability. The sequence obtained in the present study appears in bold.
Fig. 5.
Phylogenetic distance tree using Bayesian inference with gamma rate variation and HKY85 substitution model of 18S rRNA fragment sequences from Besnoitia spp. A 18S rRNA sequence from Toxoplasma gondii (OR805035) was used as an outgroup. Branches are labelled with the posterior probability. The sequence obtained in the present study appears in bold.
4. Discussion
This study documented a putative new Besnoitia species from southern black-eared opossum in Misiones, Argentina, which was characterized by molecular analyses. In Argentina, Besnoitia parasites had so far only been recorded in three mammal species: domestic rabbits (Venturini et al., 2002), Patagonian pichis (Superina et al., 2009) and wild vizcachas (Cwirenbaum et al., 2021). In this sense, southern black-eared opossum is the third native wild mammal documented as a host of Besnoitia in Argentina.
In the present study, Besnoitia sp. showed preference for muscular and cardiac tissues in the hosts, similar to previous reports from other Besnoitia spp. (Juan-Salles et al., 2004; Superina et al., 2009; Ellis et al., 2012). Virginia opossum severely infected with B. darlingi showed a general deteriorating condition with multiple round, firm, white cysts in the ear pinnae, lips, tongue, retina and iridal tissue, skeletal muscle, as well as in the myocardium, liver, kidneys, lungs, spleen and other organs (Ellis et al., 2012; Gardner, 2019). In this study, the most largely affected tissues were skeletal muscle, heart and tongue and the animal presented a good body condition. The observation of a high number of large cysts suggests an advanced stage of chronic infection. Histopathological analysis confirmed the presence of cysts exhibiting the typical characteristics of Besnoitia (Venturini et al., 2002; Shaw et al., 2009). The majority of the cysts showed minimal tissue reaction, suggesting a modulation of the host immune response, a phenomenon commonly observed in chronic infections by some sarcocystid parasites (Frenkel, 1989). This is also consistent with observations done in rabbits and vizcachas infected with Besnoitia sp. in Argentina, where no severe cellular damage or inflammation was recorded in the tissues surrounding the cysts (Venturini et al., 2002; Cwirenbaum et al., 2021). The inflammation surrounding degenerated and ruptured cysts may indicate acute episodes of localized immune response, possibly induced by the release of parasitic antigens (Superina et al., 2009). In pichis and maras, the infection with Besnoitia sp. has been associated with acute and chronic pulmonary inflammations (Juan-Salles et al., 2004; Superina et al., 2009); however, the broncho-interstitial pneumonia observed in our case appears to be related to a nematode infection, as no protozoan cysts were found in the lungs. Similarly, pulmonary oedema and congestion with intralesional nematodes have been previously reported in Virginia opossum co-infected with B. darlingi (Ellis et al., 2012).
So far, besnoitiosis in opossums was assumed to be caused by B. darlingi. However, accurate species identification in intermediate hosts can only be reliably achieved through molecular methods (Schares et al., 2020). In our study, the obtained 18S rRNA sequences showed 100% identity with both B. darlingi and B. oryctofelisi sequences. However, when using ITS1, a target with a higher discriminative power (Schares et al., 2020), it was revealed that our sequence had a higher identity with B. oryctofelisi (but with 2 SNPs) and differed extensively from B. darlingi (4 SNPs and 2 gaps). Moreover, in the cladogram performed with Bayesian inference using ITS1 sequences, Besnoitia sp. is placed as a sister group from B. oryctofelisi found in rabbits from Argentina, and both Besnoitia species are closely associated with B. akodoni from the sigmodontine rodent Akodon montensis from Brazil, and from B. darlingi isolated from Virginia opossum and a bobcat in USA. In addition, B. neotomofelis from the Southern Plains woodrat (Neotoma micropus), B. jellisoni from the white-footed deer mouse (Peromyscus maniculatus) from the USA, and Besnoitia sp. oocysts shed by a cheetah in Namibia are phylogenetically closer related to this group as to other Besnoitia species affecting ruminants and equids (Fig. 4). These genetic similarities and phylogenetic placement suggest that all Besnoitia species detected in rodents, marsupials, rabbits as intermediate hosts or felids as definitive hosts could have a common ancestor, as previously suggested by Oyarzún-Ruiz et al. (2023). Interestingly, almost all these species have been described in the Americas.
Besnoitiosis in opossums has been increasingly linked to severe debilitation and mortality, with factors such as youth, immunosuppression, and stress possibly linked to enhanced susceptibility to development of clinical manifestations (Ellis et al., 2012). The extensive presence of cysts within vital organs or ocular tissues can predispose them to predation and compromised foraging abilities (Gardner, 2019), as might have happened to the specimen under scrutiny in this study. However, the specimen was in apparent good body condition, with no external injuries.
The study of road-killed specimens offers valuable access to internal anatomical insights, shedding light on dietary habits, parasitic fauna, and pathologies, being an important resource for opportunistic surveillance. Previous investigations in the same region as the present study, have provided essential insights into the zoonotic and ecological significance of parasites, revealing novel parasite-host cycles in Argentina (Arrabal et al., 2017, 2020, 2023; Maldonado et al., 2019). In this study, a putative new species of Besnoitia was identified, which prompts further investigation into the natural transmission cycle in the area. Evidence suggests that the domestic cat may not serve as the optimal definitive host for certain Besnoitia species, with low intensity of oocyst excretion noted in B. neotomofelis, B. darlingi, and B. oryctofelisi (Olias et al., 2011). Notably, bobcats have been identified as natural definitive hosts for B. darlingi, underlining the potential role of wild felids in the life cycle of Besnoitia parasites (Verma et al., 2017; Schares et al., 2020). The Atlantic Forest harbors six species of wild felids, with opossums serving as significant dietary complements, depending on the felid species (Crawshaw Jr, 1995; Tófoli et al., 2009; Bianchi et al., 2011; dos Santos et al., 2022). It is possible to assume that some of these felids could be definitive hosts for the Besnoitia sp. Identified here. Nevertheless, further studies are needed to identify the hosts and the identity of this potentially new Besnoitia species.
Funding
The molecular studies were made possible by internal funding from the Institute of Parasitology of the University of Bern, Switzerland.
CRediT authorship contribution statement
Juan Pablo Arrabal: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Conceptualization. Gastón Moré: Writing – review & editing, Writing – original draft, Resources, Methodology, Formal analysis. María Marcela Orozco: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Conceptualization. Elisa Helman: Writing – original draft, Visualization, Methodology. Juliana Notarnicola: Writing – review & editing, Visualization. Walter Basso: Writing – review & editing, Resources, Formal analysis. Bárbara Betina Hartmann: Writing – original draft. Andrea Schapira: Methodology. Leonardo Minatel: Writing – review & editing, Visualization, Methodology, Investigation, Conceptualization.
Declaration of competing interest
We, Arrabal Juan Pablo, Moré Gastón, Orozco María Marcela, Helman Elisa, Notarnicola Juliana, Basso, Walter, Hartmann Bárbara Betina, Schapira Andrea, and Minatel Leonardo, authors from the manuscript intitled “A new Besnoitia species in the southern black-eared opossum Didelphis aurita” report no conflict of interest.
Acknowledgments
We extend our sincere gratitude to Florencia Trubbo, from Garrahan Hospital (Buenos Aires, Argentina) for her excellent technical assistance; to Eduardo Lestani for his willingness to collect roadkill opossums; to Patricio Ramírez Llorens for his active participation in the detection of roadkill; to Lucila Garcia Macchi for her assistance during the necropsy; and to Francisco Brusa from Museo de La Plata for his assistance with the slides deposited at the collection. Special thanks to the Ministry of Ecology and Natural Resources of Misiones and to the NEA regional delegation of the Iguazú National Park. Additionally, JPA, MMO, BH, GM, and JN are members of CONICET Researcher's Career.
References
- Arrabal J.P., Avila H.G., Rivero M.R., Camicia F., Salas M.M., Costa S.A., Nocera C.G., Rosenzvit M.C., Kamenetzky L. Echinococcus oligarthrus in the subtropical region of Argentina: first integration of morphological and molecular analyses determines two distinct populations. Vet. Parasitol. 2017;240:60–67. doi: 10.1016/j.vetpar.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Arrabal J.P., Pérez M.G., Arce L.F., Kamenetzky L. First identification and molecular phylogeny of Sparganum proliferum from endangered felid (Panthera onca) and other wild definitive hosts in one of the regions with highest worldwide biodiversity. Int. J. Parasitol. Parasites. Wildl. 2020;13:142–149. doi: 10.1016/j.ijppaw.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrabal J.P., Arce L.F., Macchiaroli N., Kamenetzky L. Ecological and molecular associations between neotropical wild felids and Taenia (Cestoda: taeniidae) in the Atlantic Forest: a new report for Taenia omissa. Parasitol. Res. 2023;122:2999–3012. doi: 10.1007/s00436-023-07989-y. [DOI] [PubMed] [Google Scholar]
- Astúa D., de la Sancha N., Costa L. The IUCN Red List of Threatened Species; 2021. Didelphis aurita (Amended Version of 2015 Assessment) 2021: e.T40500A197310366. [DOI] [Google Scholar]
- Basso W. In: Infectious Diseases of Livestock, Part I. Anipedia, South Africa (2018) Coetzer J.A.W., Thomson G.R., Maclachlan N.J., Penrith M.L., editors. 2018. Besnoitiosis.https://www.anipedia.org/resources/besnoitiosis/1116 [Google Scholar]
- Bentancourt Rossoli J.V., Moré G., Soto-Cabrera A., Moore D.P., Morrell E.L., Pedrana J., Scioli M.V., Campero L.M., Basso W., Hecker Y.P., Scioscia N.P. Identification of Sarcocystis spp. in synanthropic (Muridae) and wild (Cricetidae) rodents from Argentina. Parasitol. Res. 2023;123:31. doi: 10.1007/s00436-023-08036-6. PMID: 38085379. [DOI] [PubMed] [Google Scholar]
- Bezerra-Santos M.A., Ramos R.A.N., Campos A.K., Dantas-Torres F., Otranto D. Didelphis spp. opossums and their parasites in the Americas: a One Health perspective. Parasitol. Res. 2021;120:4091–4111. doi: 10.1007/s00436-021-07072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande R.A., César M.O., Pena H.F.J., Zwarg T., Teixeira R.H.F., Nunes A.L.V., Neves D. do V.D. de A., Gomes M., Quagglia Neto F., Milanello L., Fontenelle J.H., Matushima E.R. Ocorrência de Sarcocystis spp. en gambas (Didelphis aurita e Didelphis albiventris) en regiones del Estado de São Paulo, Brasil. Braz. J. Vet. Res. Anim. Sci. 2009;46:101–106. [Google Scholar]
- Casagrande R.A., Laranjeira Lopes L.F., Moura dos Reis E., dos Prazeres Rodrigues D., Matushima E.R. Isolamento de Salmonella enterica em gambas (Didelphis aurita e Didelphis albiventris) do Estado de Sao Paulo, Brasil. Cien. Rural. 2011;41:492–496. [Google Scholar]
- Charles R.A., Kjos S., Ellis A.E., Dubey J.P., Shock B.C., Yabsley M.J. Parasites and vector-borne pathogens of southern plains woodrats (Neotoma micropus) from southern Texas. Parasitol. Res. 2012;110:1855–1862. doi: 10.1007/s00436-011-2710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemisquy M.A., Varela D., Martin D.M. In: Categorización 2019 de los mamíferos de Argentina según su riesgo de extinción. Lista Roja de los mamíferos de Argentina. SAyDS–SAREM, editor. 2019. Didelphis aurita.http://cma.sarem.org.ar Online version: [Google Scholar]
- Costa-Neto S.F., Cardoso T.S., Boullosa R.G., Maldonado A., Gentile R. Metacommunity structure of the helminths of the black-eared opossum Didelphis aurita in peri-urban, sylvatic and rural environments in south-eastern Brazil. J. Helminthol. 2019;93:720–731. doi: 10.1017/S0022149X18000780. [DOI] [PubMed] [Google Scholar]
- Crawshaw Jr P.G. University of Florida; 1995. Comparative Ecology of Ocelot (Felis pardalis) and Jaguar (Panthera onca) in a Protected Subtropical Forest in Brazil and Argentina. [Google Scholar]
- Cruz P., Iezzi M.E., De Angelo C., Varela D., Di Bitetti M.S. Landscape use by two opossums is shaped by habitat preferences rather than by competitive interactions. J. Mammal. 2019;100:1966–1978. [Google Scholar]
- Cullen L., Bodmer E.R., Valladares-Pádua C. Ecological consequences of hunting in Atlantic forest patches, São Paulo, Brazil. Oryx. 2001;35:137–144. [Google Scholar]
- Cwirenbaum R., Schmidt A.R., Cortasa S.A., Corso M.C., Vitullo A.D., Dorfman V.B., Halperin J. First record of an infection by tissue cyst-forming coccidia in wild vizcachas (Lagostomus maximus, Rodentia) of Argentina. Int. J. Parasitol. Parasites Wildl. 2021;16:52–58. doi: 10.1016/j.ijppaw.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bitetti M.S., Placci G., Dietz L.A. Una Visión de Biodiversidad para la Ecorregión del Bosque Atlántico del Alto Paraná: Diseño de un Paisaje para la Conservación de la Biodiversidad y prioridades para las acciones de conservación. World Wildlife Fund. 2003:154. [Google Scholar]
- De Angelo C., Paviolo A., Di Blanco A., Di Bitetti M. Guía de huellas de los mamíferos de Misiones y otras áreas del subtrópico de Argentina. Ediciones del Subtrópico. 2008:17–21. [Google Scholar]
- Dubey J.P., Sreekumar C., Lindsay D.S., Hill D., Rosenthal B.M., Venturini L., Venturini M.C., Greiner E.C. Besnoitia oryctofelisi n. sp. (Protozoa: Apicomplexa) from domestic rabbits. Parasitology. 2003;126:521–539. [PubMed] [Google Scholar]
- Dubey J.P., Sreekumar C., Rosenthal B.M., Lindsay D.S., Grisard E.C., Vitor R.W. Biological and molecular characterization of Besnoitia akodoni n. sp. (Protozoa: Apicomplexa) from the rodent Akodon montensis in Brazil. Parassitologia. 2003;45:61–70. [PubMed] [Google Scholar]
- Dubey J.P., Sreekumar C., Rosenthal B.M., Vianna M.C.B., Nylund M., Nikander S., Oksanen A. Redescription of Besnoitia tarandi (Protozoa: Apicomplexa) from the reindeer (Rangifer tarandus) Int. J. Parasitol. 2004;34:1273–1287. doi: 10.1016/j.ijpara.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Yabsley M.J. Besnoitia neotomofelis n. sp. (Protozoa: Apicomplexa) from the southern plains woodrat (Neotoma micropus) Parasitology. 2010;137:1731–1747. doi: 10.1017/S0031182010000636. [DOI] [PubMed] [Google Scholar]
- Dos Santos J.G., Entringer Júnior H., Srbek Araujo A.C. Food habits of the ocelot (Leopardus pardalis) in a lowland atlantic forest of southeastern Brazil. Mastozool. Neotrop. 2022;29(2) [Google Scholar]
- Ellis J.T., Holmdahl O.J.M., Ryce C., Njenga J.M., Harper P.A., Morrison D.A. Molecular phylogeny of Besnoitia and the genetic relationships among Besnoitia of cattle, wildebeest and goats. Protist. 2000;151:329–336. doi: 10.1078/S1434-4610(04)70031-0. [DOI] [PubMed] [Google Scholar]
- Ellis A.E., Mackey E., Moore P.A., Divers S.J., Hensel P., Carmichael K.P., Accola P., Brown J., Gottdenker N., Keel M.K., Shock B.C., Yabsley M.J. Debilitation and mortality associated with besnoitiosis in four Virginia opossums (Didelphis virginiana). J. Zoo Wildl. Med. 2012;43:367–374. doi: 10.1638/2011-0181.1. [DOI] [PubMed] [Google Scholar]
- Facure K.G., Giaretta A.A., Monteiro-Filho E.L.A. Food habits of the crab-eating-fox, Cerdocyon thous, in an altitudinal forest of the Mantiqueira Range, southeastern Brazil. Mammalia. 2003;67:503–512. [Google Scholar]
- Fernández-García A., Risco-Castillo V., Pedraza-Díaz S., Aguado-Martínez A., Álvarez-García G., Gómez-Bautista M., Collantes-Fernández E., Ortega-Mora L.M. First isolation of Besnoitia besnoiti from a chronically infected cow in Spain. J. Parasitol. 2009;95:474–476. doi: 10.1645/GE-1772.1. [DOI] [PubMed] [Google Scholar]
- Frenkel J.K. Tissue-dwelling intracellular parasites: infection and immune responses in the mammalian host to Toxoplasma, Sarcocystis and Trichinella. Amer. Zool. 1989;29:455–467. [Google Scholar]
- Galindo-Leal C., de Gusmão Câmara I. In: Atlantic Forest of South America: Biodiversity Status, Threats, and Outlook. Galindo-Leal C., de Gusmão Câmara I., editors. Island Press; Washington: 2003. Atlantic Forest hotspot status: an overview; pp. 3–11. [Google Scholar]
- Gardner A.L. University of Chicago Press; 2007. Mammals of South America Volume 1: Marsupials, Xenarthrans, Shrews and Bats. [Google Scholar]
- Gardner A. Medical Management of Wildlife Species: a Guide for Practitioners. 2019. Natural history and medical management of opossums; pp. 297–311. [Google Scholar]
- Gentile A., Militerno G., Schares G., Nanni A., Testoni S., Bassi P., Gollnick N.S. Evidence for bovine besnoitiosis being endemic in Italy—first in vitro isolation of Besnoitia besnoiti from cattle born in Italy. Vet. Parasitol. 2012;184:108–115. doi: 10.1016/j.vetpar.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Molecular characterisation of Sarcocystis rileyi from a common eider (Somateria mollissima) in Norway. Parasitol. Res. 2014;113:3501–3509. doi: 10.1007/s00436-014-4062-y. [DOI] [PubMed] [Google Scholar]
- Glover G., Swendrowski M., Cawthorn R. An epizootic of besnoitiosis in captive caribou (Rangifer tarandus caribou), reindeer (Rangifer tarandus tarandus) and mule deer (Odocoileus hemionus hemionus) J. Wildl. Dis. 1990;26:186–195. doi: 10.7589/0090-3558-26.2.186. [DOI] [PubMed] [Google Scholar]
- Guimarães A., Macedo Raimundo J., Silva A.T.D., Carpintero F.M., Pires J.R., Benevenute J.L., Machado R.Z., André M.R., Divan Baldani C. Detection of a putative novel genotype of Ehrlichia sp. from opossums (Didelphis aurita) from Brazil. Rev. Bras. Parasitol. Vet. 2008;28:140–144. doi: 10.1590/S1984-296120180068. [DOI] [PubMed] [Google Scholar]
- Hartmann B.B. Didelphis albiventris y Didelphis aurita (Mammalia, Didelphimorphia) en ambientes selváticos y antrópicos en el departamento Iguazú, Misiones. Trabajo Integrador Final de Especialización. Universidad Nacional de Misiones. 2023;53pp https://rid.unam.edu.ar/handle/20.500.12219/5424 [Google Scholar]
- Jack S.W., Van Alstine W.G., Swackhamer J. Besnoitiasis in Indiana opossums. J. Vet. Diagn. Invest. 1989;1:189–191. doi: 10.1177/104063878900100223. [DOI] [PubMed] [Google Scholar]
- Juan-Salles C., Rico-Hernandez G., Garner M.M., Barr B.C. Pulmonary besnoitiasis in captive maras (Dolichotis patagonum) associated with interstitial pneumonia. Vet. Pathol. 2004;41:408–411. doi: 10.1354/vp.41-4-408. [DOI] [PubMed] [Google Scholar]
- Liénard E., Nabuco A., Vandenabeele S., Losson B., Tosi I., Bouhsira É., Prévot F., Sharif S., Franc M., Vanvinckenroye C., Caron Y. First evidence of Besnoitia bennetti infection (Protozoa: Apicomplexa) in donkeys (Equus asinus) in Belgium. Parasit. Vectors. 2018;11:1–7. doi: 10.1186/s13071-018-2993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligier H.D. Informe para la fundación vida silvestre argentina. Instituto Nacional de Tecnología Agropecuaria (INTA); Corrientes: 2000. Caracterización Geomorfológica y Edáfica de la Provincia de Misiones. [Google Scholar]
- Maldonado L.L., Arrabal J.P., Rosenzvit M.C., Oliveira G.C.D., Kamenetzky L. Revisiting the phylogenetic history of helminths through genomics, the case of the new Echinococcus oligarthrus genome. Front. Genet. 2019;10:708. doi: 10.3389/fgene.2019.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoia E., Chebez J.C., Bosso A. Fundación de Historia Natural Félix de Azara; Buenos Aires: 2012. Los mamíferos silvestres de la provincia de Misiones, Argentina. [Google Scholar]
- Moré G., Abrahamovich P., Jurado S., Bacigalupe D., Marin J.C., Rambeaud M., Venturini L., Venturini M.C. Prevalence of Sarcocystis spp. in Argentinean cattle. Vet. Parasitol. 2011;177:162–165. doi: 10.1016/j.vetpar.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Moreno R.S., Kays R.W., Samudio R. Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. J. Mammal. 2006;87:808–816. [Google Scholar]
- Namazi F., Oryan A., Sharifiyazdi H. Genetic characterization of the causative agent of besnoitiosis in goats in Iran on the basis of internal transcribed spacer rDNA and its comparison with Besnoitia species of other hosts. Parasitol. Res. 2011;108:633–638. doi: 10.1007/s00436-010-2107-4. [DOI] [PubMed] [Google Scholar]
- Ness S.L., Peters-Kennedy J., Schares G., Dubey J.P., Mittel L.D., Mohammed H.O., Bowman D.D., Felippe M.J., Wade S.E., Shultz N., Divers T.J. Investigation of an outbreak of besnoitiosis in donkeys in northeastern Pennsylvania. J. Am. Vet. Med. Assoc. 2012;240:1329–1337. doi: 10.2460/javma.240.11.1329. [DOI] [PubMed] [Google Scholar]
- Olias P., Schade B., Mehlhorn H. Molecular pathology, taxonomy and epidemiology of Besnoitia species (Protozoa: Sarcocystidae) Infect. Genet. Evol. 2011;11:1564–1576. doi: 10.1016/j.meegid.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Oliveira Á.F.X., Calchi A.C., Mongruel A.C.B., Stocco A.V., Stocco N.V., Costa A.C., Mureb E.N., Pires J.R., Guimarães A., Balthazar D.A., Machado R.Z., André M.R., Divan Baldani C. Molecular detection of hemoplasmas in rescued black-eared opossums (Didelphis aurita Wied-Neuwied, 1826) from southeastern Brazil, with evidence of a novel genotype infecting marsupials. Rev. Bras. Parasitol. Vet. 2023;32 doi: 10.1590/S1984-29612023015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún-Ruiz P., Thomas R.S., Santodomingo A.M., Uribe J.E., Ardila M.M., Echeverry D.M., Muñoz-Leal S., Silva-de la Fuente M.C., Loyola M., Palma C.J., Landaeta-Aqueveque C., Henríquez A. Survey and molecular characterization of Sarcocystidae protozoa in wild cricetid rodents from Central and Southern Chile. Animals. 2023;13:2100. doi: 10.3390/ani13132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perilli M.L., Lima F., Rodrigues F.H., Cavalcanti S.M. Can scat analysis describe the feeding habits of big cats? A case study with jaguars (Panthera onca) in Southern Pantanal, Brazil. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley S.J. Eira barbara. Mamm. Species. 2000;636:1–6. 2000. [Google Scholar]
- Rosenthal B.M., Dunams-Morel D., Ostoros G., Molnár K. Coccidian parasites of fish encompass profound phylogenetic diversity and gave rise to each of the major parasitic groups in terrestrial vertebrates. Infect. Genet. Evol. 2016;40:219–227. doi: 10.1016/j.meegid.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Schares G., Jutras C., Bärwald A., Basso W., Maksimov A., Schares S., Tuschy M., Conraths F.J., Brodeur V. Besnoitia tarandi in Canadian woodland caribou–isolation, characterization and suitability for serological tests. Int. J. Parasitol. Parasites Wildl. 2019;8:1–9. doi: 10.1016/j.ijppaw.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G., Dubey J.P., Rosenthal B., Tuschy M., Bärwald A., Conraths F.J. Sensitive, quantitative detection of Besnoitia darlingi and related parasites in intermediate hosts and to assess felids as definitive hosts for known and as-yet undescribed related parasite species. Int. J. Parasitol. Parasites Wildl. 2020;11:114–119. doi: 10.1016/j.ijppaw.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G., Joeres M., Rachel F., Tuschy M., Czirják G.Á., Maksimov P., Conraths F.J., Wachter B. Molecular analysis suggests that Namibian cheetahs (Acinonyx jubatus) are definitive hosts of a so far undescribed Besnoitia species. Parasit. Vectors. 2021;14:1–10. doi: 10.1186/s13071-021-04697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Grasperge B., Nevarez J., Reed S., Long L., Rademacher N., Sánchez-Migallón Guzmán D. Besnoitia darlingi infection in a Virginia opossum (Didelphis virginiana). J. Zoo. Wildl. Med. 2009;40:220–223. doi: 10.1638/2008-0165.1. PMID: 19368269. [DOI] [PubMed] [Google Scholar]
- Smith D.D., Frenkel J.K. Besnoitia darlingi (Protozoa: Toxoplasmatinae): cyclic transmission by cats. J. Parasitol. 1977;63:1066–1071. [PubMed] [Google Scholar]
- Smith D.D., Frenkel J.K. Besnoitia darlingi (Apicomplexa, Sarcocystidae, Toxoplasmatinae): transmission between opossums and cats. J. Protozool. 1984;31:584–587. doi: 10.1111/j.1550-7408.1984.tb05510.x. [DOI] [PubMed] [Google Scholar]
- Su C., Evans D., Cole R.H., Kissinger J.C., Ajioka J.W., Sibley L.D. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- Superina M., Garner M.M., Aguilar R.F. Health evaluation of free-ranging and captive pichis (Zaedyus pichiy; Mammalia, Dasypodidae), in Mendoza province, Argentina. J. Wildl. Dis. 2009;45:174–183. doi: 10.7589/0090-3558-45.1.174. [DOI] [PubMed] [Google Scholar]
- Tófoli C.F., Rohe F., Setz E.Z.F. Jaguarundi (Puma yagouaroundi) (Geoffroy, 1803) (Carnivora, Felidae) food habits in a mosaic of Atlantic Rainforest and eucalypt plantations of southeastern Brazil. Braz. J. Biol. 2009;69:871–877. doi: 10.1590/s1519-69842009000400015. [DOI] [PubMed] [Google Scholar]
- Villa L., Gazzonis A.L., Diezma-Diaz C., Perlotti C., Zanzani S.A., Ferrucci F., Álvarez-García G., Manfredi M.T. Besnoitiosis in donkeys: an emerging parasitic disease of equids in Italy. Parasitol. Res. 2021;120:1811–1819. doi: 10.1007/s00436-021-07089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini L., Petruccelli M., Piscopo M., Unzaga J.M., Venturini M.C., Bacigalupe D., Basso W., Dubey J.P. Natural Besnoitia sp. infection in domestic rabbits from Argentina. Vet. Parasitol. 2002;107:273–278. doi: 10.1016/s0304-4017(02)00156-5. [DOI] [PubMed] [Google Scholar]
- Verma S.K., Cerqueira-Cezar C.K., Murata F.H.A., Lovallo M.J., Rosenthal B.M., Dubey J.P. Bobcats (Lynx rufus) are natural definitive host of Besnoitia darlingi. Vet. Parasitol. 2017;248:84–89. doi: 10.1016/j.vetpar.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Wallace G.D., Frenkel J.K. Besnoitia species (Protozoa, Sporozoa, Toxoplasmatidae): recognition of cyclic transmission by cats. Science. 1975;188:369–371. doi: 10.1126/science.804183. [DOI] [PubMed] [Google Scholar]
- Wang E. Diets of ocelots (Leopardus pardalis), margays (L. wiedii), and oncillas (L. tigrinus) in the Atlantic rainforest in southeast Brazil. Stud. Neotrop. Fauna E. 2002;37:207–212. [Google Scholar]