Abstract
The low CO2-inducible NDH complex (NDH–1MS) plays a crucial role in the cyanobacterial CO2-concentrating mechanism. However, the components in this complex and the regulation mechanism are still not completely understood. Using a mutant only with NDH–1MS as active Ci sequestration system, we identified a functional gene sll1736 named as cupAR (CupA Regulator). The cupAR deletion mutant, ΔcupAR, grew faster than the WT under high CO2 (HC) condition, more evidently at low pH. The activities of O2 evolution, CO2 uptake,NDH-1, and the building up of a transthylakoid proton were stimulated in this mutant under HC conditions. The cupAR gene is cotranscribed with the NDH–1S operon (ndhF3-ndhD3-cupA) and encoded protein, which specifically suppresses the transcription level of this operon under HC conditions. Mutation of cupAR significantly upregulated the accumulation of CupA, the key protein of NDH–1MS, under HC condition. CupAR interacted with NdhD3 and NdhF3, the membrane components of NDH–1MS, while accumulation of CupAR was reduced in the ΔndhD3 mutant. Furthermore, CupAR was colocated with CupA in both NDH–1MS complex and NDH–1S subcomplex. On the other hand, deletion of ndhR, a negative regulator of the NDH–1S operon, increased the accumulation of CupAR, whereas deletion of cupAR significantly lowered NdhR. Based on these results, we concluded that CupAR is a novel subunit of NDH–1MS, negatively regulating the activities of CupA and CO2 uptake dependent on NDH–1MS by positive regulation of NdhR under enriched CO2 conditions.
Keywords: Synechocystis sp. PCC 6803, CO2-concentrating mechanism, NDH–1MS, CupA, CupAR
Cyanobacteria possess a CO2-concentrating mechanism (CCM) that functions to elevate the CO2 concentration around the active site of ribulose-1,5-bisphosphate carboxylase/oxygenase to compensate for the low affinity of ribulose-1,5-bisphosphate carboxylase/oxygenase for CO2 (1). Two CO2-uptake systems and three HCO3- transporters have been identified in Synechocystis PCC 6803 (hereafter Syn 6803) and other cyanobacterial strains (2). One of the CO2 uptake systems, NDH–1MS' complex consists of NdhD4, NdhF4, and CupB (ChyX), is a constitutive system showing low affinity for CO2; another NDH–1MS complex, consisting of NdhD3, NdhF3, and CupA (ChyY), is inducible under low CO2 (LC) conditions and has high affinity for CO2 (3, 4, 5). The expression of ndhF3-ndhD3-cupA-cupS operon was induced in Syn 6803 and Synechococcus PCC 7002 under LC conditions (6). The proteins encoded by ndhF3-ndhD3-cupA-cupS formed a small complex NDH–1S, in which CupA and a small protein CupS were identified as subunits of cyanobacterial NDH-1 (7, 8). Single-particle electron microscopy of purified NDH–1MS complex of Thermosynechoccus elongates revealed a U-shape structure (9). CupA is responsible for the U-shape by binding at the tip of the membrane-bound arm of NDH–1MS in Thermosynechoccus elongatus and Syn 6803 (10). Both CupA and CupB may have a CA-like activity (11, 12), similar to the thylakoid membrane–bound CA. EcaB regulates the conversion of CO2 to HCO3- (13). The structure of NDH–1MS has also been resolved by cryo-EM (14). CupB has been purified and identified in a 450 kDa NDH–1MS' complex (15).
CCMs are upregulated under LC conditions, whereas downregulated under HC conditions (elevated CO2 levels) at the transcriptional level (16). Several transcriptional regulators participate in this process. CmpR as a regulator activates the expression of the cmp-operon encoding the high-affinity HCO3- transporter BCT1 (17). NdhR (CcmR, encoded by sll1594) acts as a repressor of the genes involved in CCM, such as NDH–1MS and sbtA, under HC conditions (16, 18, 19). Some NdhR-regulated genes are inducible under LC conditions, suggesting that additional NdhR-independent Ci-regulatory mechanisms exist in cyanobacteria (20). Both NdhR and CmpR belong to a large family of LysR-type transcriptional regulators (21). 2-phosphoglycolate and RubP can enhance the promoter binding of CmpR (22) whereas 2-oxoglutarate and NADP+ function as corepressor for NdhR (23). Jiang et al. showed that the full-length structure of NdhR from Syn 6803 forms a complex with 2-oxoglutarate, and the NdhR regulatory domain interacts with 2-phosphoglycolate (24).
In this work, we identified a novel functional gene sll1736, named as cupAR (CupA Regulator) in Syn 6803, and found that cupAR-defective mutant, ΔcupAR, grew faster than the WT at HC conditions, especially under low pH, and showed higher activities of O2 evolution and CO2 uptake. The cupAR gene belongs to the same operon of NDH–1S, functioning as a negative regulator for NDH–1S genes that specifically suppresses the transcript levels of this operon under HC conditions. CupAR interacted with NdhD3 and NdhF3 and colocalized with CupA in both NDH–1MS complex and NDH–1S subcomplex. Deletion of ndhR increased the accumulation of CupAR, whereas deletion of cupAR significantly lowered NdhR. Our results suggest that CupAR is a novel subunit of NDH–1MS and negatively regulates the transcription of NDH–1S operon, the translation of cupA, and the activity of NDH–1MS for CO2 uptake by positive regulation of NdhR under HC conditions.
Results
Mutation of cupAR upregulates NDH–1S activity under HC conditions
To investigate the components of NDH–1MS, we isolated the complex from solubilized thylakoid membrane cupA-His6 using Ni column. MS/MS analysis of the fraction eluted by 150 mM imidazole indicated the presence of CupA and CupAR (Table S2) in addition to many potential proteins copurified with CupA (Table S1). The result suggested that the CupAR protein might be associated with CupA in NDH–1S.
To study whether CupAR participates in the function of NDH–1MS complex, we constructed cupAR-defective mutant by inserting Cm resistance cassette to cupAR gene (sll1736) in the WT strain. Complete segregation of ΔcupAR was confirmed by PCR analysis (Fig. 1C). In addition, we constructed Δ4/cupAR mutant by transforming the Δ4 mutant (ΔndhF4/ΔbicA/ΔcmpB/ΔsbtA) with genomic DNA isolated from ΔcupAR mutant. Comparison of their growth with the WT on BG11 agar plate buffered at various pHs under HC (10% CO2) or LC (air CO2) indicated that the ΔcupAR and Δ4/cupAR mutants grew faster than the WT and Δ4, respectively, especially at low pH (below 8.0) under HC conditions (Fig. 1A, left), where the expression of genes in NDH–1S operon was suppressed and also under LC conditions at pH 6.5. In contrast, the difference became insignificant at pH 8.0 under LC conditions (Fig. 1A, right). A similar trend was found in the growth curves (Fig. 1B).
Figure 1.
Growth and photosynthetic properties of the WT and mutants.A, cells were grown on agar plates at pH 8.0, pH 7.0, and pH 6.5 under HC (10% CO2 [v/v] or LC [0.03%] conditions. B, growth curves of WT and mutants under different pHs and HC (10% CO2 [v/v] or LC [0.03%] conditions. C, The transposon insertion site and PCR amplification to confirm complete knockout of cupAR (sll1736) in the mutants; photosynthetic O2 evolution rate of WT and mutants under different pHs and HC (10% CO2 [v/v]) (D) or LC (0.03%) (E) conditions. F, the initial reduction rate of P700+ after far-red light. Asterisk indicates significant differences (t test, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗∗p < 0.0001). HC, high CO2; LC, low CO2.
Knockout of cupAR did not affect photosystem II activity but enhanced cyclic electron flow
No significant differences were observed between WT and ΔcupAR in the chlorophyll (Chl) fluorescence parameters, such as QA, reflecting the redox state of electron transport carriers (Fig. S1A) and potential quantum efficiency of photosystem II (PSII), yield (II) as the indicator of the quantum yield of PSII (Fig. S1B). However, the cyclic electron flow around PSI was enhanced, as reflected by a transient increase in Chl fluorescence after termination of actinic light illumination (25) (Fig. S1C) and reflected by the initial reduction of P700+ after termination of far-red light (26) (Fig. 1F). The result indicates that CupAR negatively controls cyclic electron flow.
Knockout of cupAR stimulates the activity of photosynthetic O2 evolution and CO2 uptake under HC conditions
The activity of photosynthetic oxygen evolution of ΔcupAR mutant was higher than that of the WT under HC (10% CO2) at pH 7.0 or pH 8.0 (Fig. 1D). There was no significant difference among WT, Δ4, and Δ4/cupAR either at pH 7.0 or pH 8.0 under HC conditions (Fig. 1D) and also between WT and ΔcupAR, or between Δ4 and Δ4/cupAR at pH 7.0 or pH 8.0 under LC conditions (Fig. 1E). As shown in Fig. S1D, the rate of photosynthetic O2 evolution in ΔcupAR was higher than that in the WT by about 10 to 20% at pH 6.5 to 8.0 but was lower in the Δ4 mutant by about 60%. However, the activity was partially recovered in Δ4/cupAR by about 6% at pH 8.0 and more evidently at pH 7.0 and 6.5. On the other hand, the rate of photosynthetic CO2 uptake in ΔcupAR increased by 24% at pH 8.0 and more significantly at lower pHs, by 43% at pH 7.0 and by 40% at pH 6.5, compared with that of WT. The CO2 uptake rate in Δ4 was also decreased to about 26% of the WT at pH 8.0 and more obviously at lower pH. It was partially recovered in Δ4/cupAR by about 30% at pH 8.0, 16% at pH 7.0, and 12% at pH 6.5, respectively (Fig. S1E). The results suggest that CupAR stimulates the activity of CO2 uptake of NDH–1MS under HC conditions.
Interaction between CupAR and NdhD3, NdhF3 in vitro
To confirm whether CupAR is a component of NDH–1S, we investigated the interaction between CupAR and the components of NDH–1S (NdhD3, NdhF3, CupA, and CupS) using a yeast two-hybrid system. As shown in Figure 2A, there was strong interaction of CupAR with NdhD3 and NdhF3, respectively. No interaction was found between CupAR and CupA or CupS in vitro. The cupAR (sll1736) encodes a protein of 127 amino acids with two transmembrane regions (amino acids 15–37 and 41–63) mainly on the N-terminal region and a hydrophilic tail of 64 amino acids on the C-terminal region, suggesting that the N terminus of CupAR may insert into the thylakoid membrane and interact with NdhD3 and NdhF3. To test this possibility, we synthesized CupAR-NM containing transmembrane region of CupAR (1–63 amino acids) as AD to detect the interaction with NdhD3 and NdhF3 as BD, respectively. The data showed that the interaction can still be detected between CupAR-NM and NdhD3 or NdhF3, indicating that the CupAR-NM is essential for its interaction with the NdhD3 and NdhF3.
Figure 2.
Interaction, localization, and function of CupAR in NDH–1MS.A, yeast two-hybrid assay of CupAR interaction with NdhD3 and NdhF3. Blue precipitate represents accumulated β-galactosidase activity resulting from the activation of the lacZ reporter gene by protein–protein interaction. The induction plate was incubated at 30 °C for 28 h and then photographed. The interaction of AD with BD was assayed as a negative control. At least six independent experiments were performed, and one representative result is shown on each assay. B, Western analyses of CupA in the ΔcupAR mutant under LC and HC conditions. PetD, a subunit of cytochrome b6f complex, was used as control. C, the expression of CupAR in the cupAR-YFP and ΔndhD3 strains. PetD was used as control. D, Western analyses of NDH–1MS and NDH–1S complexes after separation by 2D-gel electrophoresis (BN-PAGE and SDS-PAGE) of thylakoid membrane proteins. The strains were probed with specific antibodies against GFP. The positions of NDH–1MS and NDH–1S complexes, respectively, in WT are indicated by red and blue arrows. E, analysis of proton gradient across the thylakoid membranes using QA (quinacridine) fluorescence quenching in WT and mutants, ΔcupAR, ΔndhD3, and M55. Intact cells of WT and mutant strains were harvested at midlogarithmic phase (optical density at 730 nm ≈ 0.5) and then suspended at a final chlorophyll concentration of 10 μg ml−1 in fresh BG11 medium with 5 μM quinacridine. The quenching of QA fluorescence was induced by illumination with actinic light (60 μmol photos m–2 s–1) after starting measurement. The QA fluorescence quenching was calculated as the ratio (ΔF/ΔFo) of the decreased fluorescence intensity (ΔF) to the background fluorescence intensity (ΔFo). Values are means ± SE of three independent measurements. Asterisk indicates significant differences (t test, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗∗p < 0.0001). HC, high CO2; LC, low CO2; NDH, NAD(P)H dehydrogenase.
Effect of CupAR protein on other subunits in NDH–1S complex
As shown in Figure 2B, the expression of CupA was hardly detected under HC condition but was induced under LC condition in WT. The expression of CupA was greatly increased in the ΔcupAR mutant under HC condition. The expression level of CupAR was similar either under HC or LC conditions but evidently reduced in ΔndhD3 mutant (Fig. 2C), indicating that NdhD3 is important for accumulation of CupAR. In contrast, CO2 concentrations had no effect on the PetD, a subunit of cytochrome b6f complex.
CupAR is localized in NDH–1MS and NDH–1S complexes
To confirm whether CupAR is colocated with CupA in NDH–1MS complex in vivo, we constructed cupAR-YFP mutant by the method as described above. The thylakoid membrane from cupA-CFP and cupAR-YFP was subjected to Blue Native-PAGE to separate the NDH–1S complex, followed by second dimension SDS-PAGE to separate the subunits of these protein complexes, which were then immunoblotted using an anti-GFP antibody. As shown in Figure 2D, CupAR is colocated with CupA in both NDH–1MS and NDH–1S complexes.
CupAR negatively controls the proton gradient across the thylakoid membranes under HC conditions
Proton gradient formation across the thylakoid membranes measured using quinacridine (QA) fluorescence quenching was faster in ΔcupAR than in WT grown under HC condition, but there was no significant difference between them when cells were grown under LC condition (Fig. 2E). In contrast, the QA fluorescence quenching was lowered in both ΔndhD3 and M55 cells, as reported in the previous study (12). The result confirms that CupAR negatively controls the function of NDH–1MS under HC conditions.
The cupAR gene is cotranscribed with the ndhF3-ndhD3-cupA operon and suppresses the expression of the other genes within this operon under HC conditions
RT–PCR experiment showed that cupS and cupAR genes are cotranscribed with cupA (Fig. 3A), indicating that sll1735-sll1736 belongs to the same operon. In HC-grown cells of WT, expression of genes in this operon except cupAR was hardly observed. The expression level of these genes was elevated after the cells were shifted to LC for 6 h. Surprisingly, in HC-grown cells of the ΔcupAR mutant, the expression levels of these genes were increased to those of LC-adapted WT cells (Fig. 3B). However, the degree of upregulation was much weaker as compared with that in the ΔndhR mutant. Furthermore, the expression of sbtA was not significantly increased in the HC-grown cells of the ΔcupAR mutant (Fig. 3B). As expected, the expression level of ndhD4 and cupB of the constitutive CO2 uptake system kept unchanged under all conditions (Fig. 3B). The results indicate that CupAR specifically suppresses the transcription of the genes in NDH–1S.
Figure 3.
Identification and analysis of the genes in ndhF3-ndhD3-cupA operon in transcriptional and translational levels.A, RT–PCR analysis of genes in ndhF3-ndhD3-cupA operon. B, expression of genes in ndhF3-ndhD3-cupA operon and cupAR under LC and HC conditions. 16S was used as control, and RTase was used as negative control. C, accumulation of NdhR and CupA in WT, ΔcupAR and ΔndhR observed by immunoblotting using antibodies against NdhR and CupA, respectively. CcmM, one of the components in carboxysome, was used as control. D, accumulation of CupAR in WT and ΔndhR. CcmM was used as control. CupAR in these strains was tagged with YFP and detected by immunoblotting using the antibody against YFP. Sample containing 20 μg thylakoid membrane proteins was loaded onto each lane. HC, high CO2; LC, low CO2.
CupAR positively regulates the expression of NdhR and negatively regulates the expression of CupA
The aforementioned results suggest that CupAR might function as a negative regulative factor for expression of genes in the ndhF3-ndhD3-cupA operon. To understand the role of CupAR and NdhR for expression of genes in the operon, the accumulation of CupA was determined in the WT, ΔcupAR, and ΔndhR strains. Immunodetective analysis showed that NdhR level in ΔcupAR was about 30% of that in the WT (Fig. 3C). In contrast, the CupA level was upregulated in ΔcupAR and much more significantly in ΔndhR (Fig. 3C), indicating CupAR positively regulates NdhR, which negatively regulates the expression of genes in the ndhF3-ndhD3-cupA operon. On the other hand, the accumulation of CupAR was remarkably increased in ΔndhR (Fig. 3D), indicating that accumulation of CupAR is negatively controlled by NdhR.
Discussion
Reverse genetics and proteomics revealed that the LC-inducible NDH–1MS is involved in CO2 uptake, which shows high affinity to CO2 (7). However, the components and activity regulation of this complex are still not completely understood. In this work, we identified a novel gene, cupAR, that negatively controlled the high affinity CO2 uptake system: the ΔcupAR mutant grew faster than the WT under HC conditions especially at pH below 8.0 (Fig. 1A). The recovery of growth of Δ4 by further deletion of cupAR (Δ4/cupAR) supports the view that CupAR acts as a negative regulator for the NDH–1MS function (Fig. 1, A and B). In contrast to the genes involved in high-affinity CO2 uptake, such as ndhD3, ndhF3, and cupA (27), cupAR was constitutively expressed under HC conditions (Figs. 2C). Knocking out of cupAR gene stimulated accumulation of CupA (Fig. 2B) and expression of genes in ndhF3-ndhD3-cupA operons (Fig. 3B), which raised the growth, photosynthetic O2 evolution (Figs. 1D and S1D), and CO2 uptake (Fig. S1E) of the mutant under HC conditions.
CupA, cupS, and cupAR are expressed as an operon, possibly together with ndhD3 and ndhF3 (Fig. 3A). Moreover, mutation of cupAR remarkably increased the transcript level of genes in NDH–1S (Fig. 3B) and the accumulation of CupA (Fig. 3C) under HC conditions. The result suggests that CupAR negatively regulates the expression of genes encoding the subunits of NDH–1S, inactivation of cupA did not change the expression of cupAR (data not shown), while the amount of CupAR was decreased obviously in ΔndhD3 mutant (Fig. 2C). The increase of cyclic electron flow (Figs. 1F and S1C) and transthylakoid membrane proton gradient (Fig. 2E) by inactivation of cupAR indicate the negative regulation of NDH-1 by CupAR. Furthermore, yeast two-hybrid analysis showed that both NdhD3 and NdhF3 interact with CupAR in vitro (Fig. 2A), indicating that NdhD3 is crucial for accumulation of CupAR and might be the binding site for CupAR. In contrast to NdhR, CupAR only functions in negative regulation of the genes in the NDH–1S operon under HC conditions, and the suppressing effect is much weaker compared with that of NdhR (Fig. 3). The results suggest that CupAR specifically regulates the genes in NDH–1S and its activity under HC conditions.
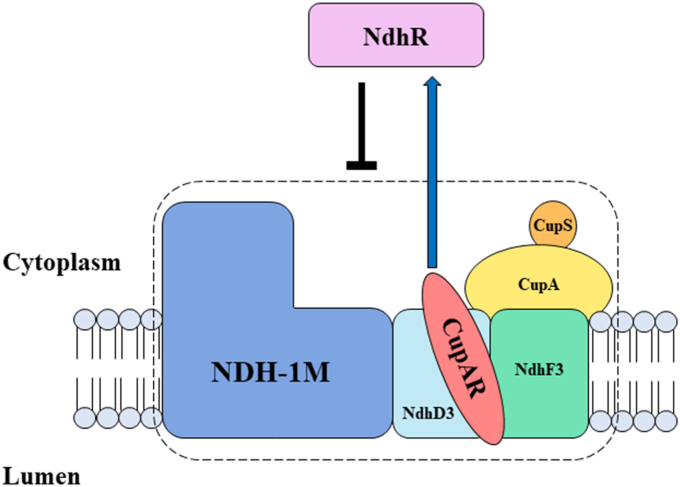
CupAR is colocated with CupA in NDH–1MS and NDH–1S (Fig. 2D), and its N-terminal region interacts with NdhD3 and NdhF3 (Fig. 2A). Based on these results, we propose a model for localization of CupAR in NDH–1MS as depicted in Figure 4. The model also indicates that CupAR enhances the expression of NdhR (Fig. 3C), which suppresses the expression of genes in NDH–1MS (Fig. 3B) and its activity (Figs. 1F, 2E, and S1C) under HC or low pH conditions.
Figure 4.
Working mode of CupAR. CupAR is located in NDH–1MS with its N terminus linked to NdhD3 and NdhF3. CupAR positively regulates NdhR, which negatively regulated NDH–1MS under HC conditions. HC, high CO2; NDH, NAD(P)H dehydrogenase.
Thus, we may conclude that CupAR is a novel subunit of NDH–1MS, negatively regulating the activities of CupA and CO2 uptake dependent on NDH–1MS by positive regulation of NdhR. Our findings provide new insights into the regulation of inorganic carbon acquisition systems in cyanobacteria (1, 2).
Experimental procedures
Culture conditions
WT and mutant cells of Syn 6803 were grown in BG-11 medium buffered with 10 mM Tris–HCl, pH 8.0, at 30 °C under 50 μmol photons m−2 s−1 in the presence of 3% CO2 in air (v/v) to obtain HC cells. LC cells were obtained by aerating HC cells with air overnight under the same light conditions. BG-11 plates were prepared by adding 1.5% agar, 10 mM Tris–HCl (pH 8.0), and 0.3% Na2S2O3. Appropriate antibiotics were added into BG-11 medium for growing the mutants and complemented strains.
Isolation and construction of mutants
The cupA-His mutant was made by adding 6-His to the C-terminal region of cupA gene. The cupAR-defective mutant, ΔcupAR, was made by replacing the sll1736 gene with chloramphenicol cassette as shown in Figure 1B. The Δ4 mutant (ΔndhF4/ΔbicA/ΔcmpB/ΔsbtA) of Syn 6803 possessing only the CupA-dependent CO2 uptake system was constructed and transformed with ΔcupAR-Cm construct to make Δ4/cupAR mutant. Transformation of Syn 6803 and cupA-CFP strain of Syn 6803 was constructed according to the method of Williams and Szalay (28). The construction and identification of other mutants, ΔcupA, cupA-CFP, and cupAR-YFP-6His, are shown in Figs. S2–S4, respectively. Primers used are listed in Table S3. The complete segregation of mutants was confirmed by PCR (Figs. S2–S4).
Chlorophyll fluorescence measurement
Chl fluorescence was measured using a portable pulse amplitude–modulated fluorometer (PAM-2000) (Walz) (29). First, 1 ml suspension of Syn 6803 cells was pipetted into a reaction cuvette with a thermostat (30 °C). One end of a multibranched fiber optic bundle was inserted into the cuvette, and while the other ends were connected to the emitter–detector unit and to different light sources. The photochemical quenching coefficients (qP) and the effective yield of PS II photochemistry ([yield (II)]) were automatically calculated by the data acquisition software DA-2000 installed in a computer connected to PAM-2000. In the example given, the intensity of red actinic light increased stepwise every 20 s. At the end of each intensity step, the fluorescence ratio parameters, including yield (II), were assessed with the application of a saturation pulse. The kinetics of the fluorescence induction curve was recorded on a computer and then exported to Excel for graphical display of the light saturation curves. The postillumination increase in Chl fluorescence was monitored using a PAM Chl fluorometer (Walz) in an emitter–detector–cuvette assembly (ED-101US) and a 101ED unit, as previously described (25, 30, 31).
Measurement of the redox state of P700
The redox change of P700 was monitored by absorbance at 810 to 830 nm, using a PAM Chl fluorometer (Walz), with an ED-P700DW-II unit for P700 absorbance changes as previously described (32). The cell suspension (1 ml) was added to a cuvette in the ED-101 US connected to far-red light with multibranched fiber optics (FR, >720 nm). Each sample was kept in the dark for 2 min to promote adaptation to dark conditions prior to measurement. Experiments were repeated three times.
Oxygen evolution and CO2 uptake
The WT and mutant cells were harvested by centrifugation and resuspended in fresh BG11 with 10 mM NaHCO3 at 5 μg Chl a ml−1. Photosynthetic oxygen evolution was measured using a Clark-type oxygen electrode (Oxylab2; Hansatech) with a saturating light (150 μmol photons m−2 s−1) at 30 °C. Data represent the means ± standard deviations of values from three cultures in parallel. CO2 uptake was measured with the LI-6400/LI-6400XT Portable Photosynthesis System (LICOR). Cells were concentrated into 100 of optical density at 730 nm and then spread with 30 μl on an agar plate as a spot into a diameter of 0.5 cm incubated in the growth chamber before measurement. Each spot was cut out into a square of 1 cm × 1 cm and put on a cover glass before measurement. Same size of agar was used as control.
Isolation of total membrane fraction
Five liters of 4-day-old cells (optical density at 730 nm = 0.6) were harvested, resuspended in 30 ml of buffer A (20 mM Hepes, pH 7.5, 5 mM sodium phosphate, 10 mM NaCl, 10 mM MgCl2, and 25% glycerol), and were broken by vortexing for 10 times at the highest speed for 20 s with 3 min interval cooling on ice. To remove the glass beads, the sample was centrifuged at 5000g for 10 min, and the membranes were subsequently collected by ultracentrifugation at 150,000g for 40 min and resuspended in buffer A.
Electrophoresis and immunoblotting
Blue Native-PAGE for thylakoid membranes was performed as described earlier (30) with slight modifications. Thylakoid membranes were washed with 330 mM sorbitol, 50 mM Bis–Tris, pH 7.0, and 0.5 mM PMSF (Sigma) and resuspended in 20% glycerol (v/v), 25 mM Bis–Tris, pH 7.0, 10 mM MgCl2, 0.1 units of RNase-free DNase RQ1 (Promega) at a Chl a concentration of 0.6 mg ml−1. The samples were incubated on ice for 10 min, and an equal volume of 3% n-dodecyl-β-maltoside was added. The solubilization was performed for 1 h on ice. Insoluble components were removed by centrifugation at 18,000g for 15 min. The collected supernatant was mixed with one-tenth volume of sample buffer (5% Serva Blue G, 100 mM Bis–Tris, pH 7.0, 30% sucrose [w/v], 500 mM ε-amino-n-caproic acid, and 10 mM EDTA). Solubilized membranes were then applied to a 0.75-mm-thick, 5 to 13.5% acrylamide gradient gel (Mighty Small mini-vertical unit; Hoefer). Samples of 3 μg Chl a were loaded on the gel. Electrophoresis was performed at 4 °C by increasing the voltage gradually from 50 up to 200 V during the 5.5-h run.
For immunoblotting, the proteins were electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) and detected by protein-specific antibodies using an ECL assay kit (Amersham Biosciences) according to the manufacturer’s protocol.
RNA extraction and RT–PCR analysis
About 200 ml of Syn 6803 strains grown in BG-11 under air condition was collected by centrifugation and quickly frozen in liquid nitrogen. Total RNA was extracted using a TRIzol Reagent Kit (Invitrogen), according to the manufacturer’s instructions, and treated with RNase-free DNase I (TAKARA) for RT–PCR.
For RT–PCR, first-strand complementary DNA was synthesized in a 25 μl reaction mixture containing 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 50 mM dNTPs, 25 U of RNasin, 200 U of M-MLV reverse transcriptase (Promega), 2 mg of total RNA, and 0.5 mg of random primers at 37 °C for 60 min. The relative concentration of complementary DNA was evaluated after serial dilutions by PCR using primers rnpB-F and rnpB-R and adjusted to the same level according to the brightness of PCR bands.
Yeast two-hybridization assay
Yeast two-hybridization assay was performed using the LexA system (Clontech). The full-length cupAR and truncated cupAR gene (cupAR-N, cupAR-M, cupAR-C, cupAR-NM, and cupAR-MC) were cloned into the PJG4-5 vector to make the prey constructs. The fragments containing ndhD3, ndhF3, cupA, and sll1735 were cloned into the PEG202 vector to make the bait constructs (primers are shown in Table S3). The bait and prey constructs together with a reporter vector pSH18-34 were cotransformed into the yeast strain EGY48 according to the manufacturer’s instructions for the Matchmaker LexA two-hybrid system (Clontech). Transformed yeast cells were diluted and dropped onto synthetic dropout plate containing X-gal and then were grown at 30 °C in darkness as described previously by Dai et al. (33).
QA fluorescence quenching analysis
Fluorescence of QA was measured at 503 nm using the PAM chlorophyll fluorometer (Maxi-version; Walz) attached to a US-370 emitter with an emission peak at 375 nm and a PM-101/D detector as described previously (34, 35). Cells were harvested at logarithmic phase and suspended in reaction mixture of fresh BG11 medium with 5 μM QA at a final Chl concentration of 10 μg ml–1. The quenching of QA fluorescence was induced by illuminating the cells with actinic light (60 μmol photons m–2 s–1) after the background fluorescence became stable after about 2 min.
Affinity chromatography
The membrane fractions of CupA-His were suspended with binding buffer at 1 mg Chl ml–1, solubilized with DM (1%) on ice for 1 h, and then centrifuged at 100,000g for 30 min. The supernatant of solubilized thylakoids was filtered through a 0.45-μm-pore-size membrane and applied on to the 1 ml Ni2+-affinity chromatography column. The column was washed with binding buffer containing 5 mM imidazole. Proteins were eluted with 3 ml of binding buffer containing 150 mM imidazole, and the eluted fractions were stored at −80 °C for further analysis.
Identification of proteins by MALDI-TOF MS and electrospray ionization tandem MS
Proteins from eluted fractions of Ni2+-affinity chromatography column were digested with trypsin. The digests were analyzed by MALDI-TOF MS and electrospray ionization MS/MS as described by Battchikova et al. (36). The concentration of protein was determined using a detergent-compatible protein assay kit Bio-Rad.
Bioinformatics tools
NCBI (National Center for Biotechnology Information), KEGG (Kyoto Encyclopedia of Genes and Genomes), and CyanoBase were used as sequence information resources. BLAST searches were performed to search for homologous sequences. To compare protein sequences or a protein sequence with an expressed sequence tag, FASTA and FASTX were applied, respectively. Domain analysis was performed by Pfam software (European Bioinformatics Institute).
Data availability
Data can be shared upon request.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This work was supported by the fund from Synthesis Biology (grant no.: 2019YFA0904602).
Author contributions
T. O. and H. M. conceptualization; M. X. and T. O. methodology; J. L. and F. Z. investigation; J. L. and F. Z. data curation; H. M. writing–original draft; T. O. writing–review & editing; T. O. and H.M. supervision; H. M. funding acquisition.
Reviewed by members of the JBC Editorial Board. Edited by Sarah E. O'Connor
Supporting information
References
- 1.Kaplan A., Reinhold L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:539–570. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa T., Kaplan A. Inorganic carbon acquisition systems in cyanobacteria. Photosynthesis Res. 2003;77:105–115. doi: 10.1023/A:1025865500026. [DOI] [PubMed] [Google Scholar]
- 3.Ohkawa H., Sonoda M., Hagino N., Shibata M., Pakrasi H.B., Ogawa T. Functionally distinct NAD(P)H dehydrogenases and their membrane localization in Synechocystis sp PCC6803. Funct. Plant Biol. 2002;29:195–200. doi: 10.1071/PP01180. [DOI] [PubMed] [Google Scholar]
- 4.Shibata M., Ohkawa H., Kaneko T., Fukuzawa H., Tabata S., Kaplan A., et al. Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11789–11794. doi: 10.1073/pnas.191258298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda S., Badger M.R., Price G.D. Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp PCC7942. Mol. Microbiol. 2002;43:425–435. doi: 10.1046/j.1365-2958.2002.02753.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa T. A gene homologous to the SUBUNIT-2 gene of nadh dehydrogenase IS essential to inorganic carbon transport of synechocystIS PCC6803. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4275–4279. doi: 10.1073/pnas.88.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang P.P., Battchikova N., Jansen T., Appel J., Ogawa T., Aro E.M. Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell. 2004;16:3326–3340. doi: 10.1105/tpc.104.026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P.P., Battchikova N., Paakkarinen V., Katoh H., Iwai M., Ikeuchi M., et al. Isolation, subunit composition and interaction of the NDH-1 complexes from Thermosynechococcus elongatus BP-1. Biochem. J. 2005;390:513–520. doi: 10.1042/BJ20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arteni A.A., Zhang P., Battchikova N., Ogawa T., Aro E.-M., Boekema E.J. Structural characterization of NDH-1 complexes of Thermosynechococcus elongatus by single particle electron microscopy. Biochim. Biophys. Acta Bioenerget. 2006;1757:1469–1475. doi: 10.1016/j.bbabio.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Folea I.M., Zhang P., Nowaczyk M.M., Ogawa T., Aro E.-M., Boekema E.J. Single particle analysis of thylakoid proteins from Thermosynechococcus elongatus and Synechocystis 6803: localization of the CupA subunit of NDH-1. FEBS Lett. 2008;582:246–251. doi: 10.1016/j.febslet.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa T., Mi H. Cyanobacterial NADPH dehydrogenase complexes. Photosynthesis Res. 2007;93:69–77. doi: 10.1007/s11120-006-9128-y. [DOI] [PubMed] [Google Scholar]
- 12.Han X., Sun N., Xu M., Mi H. Co-ordination of NDH and Cup proteins in CO2 uptake in cyanobacterium Synechocystis sp PCC 6803. J. Exp. Bot. 2017;68:3869–3877. doi: 10.1093/jxb/erx129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun N., Han X., Xu M., Kaplan A., Espie G.S., Mi H. A thylakoid-located carbonic anhydrase regulates CO2 uptake in the cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2019;222:206–217. doi: 10.1111/nph.15575. [DOI] [PubMed] [Google Scholar]
- 14.Schuller J.M., Saura P., Thiemann J., Schuller S.K., Gamiz-Hernandez A.P., Kurisu G., et al. Redox-coupled proton pumping drives carbon concentration in the photosynthetic complex I. Nat. Commun. 2020;11:494. doi: 10.1038/s41467-020-14347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu M., Ogawa T., Pakrasi H.B., Mi H. Identification and localization of the CupB protein involved in constitutive CO(2) uptake in the cyanobacterium, Synechocystis sp strain PCC 6803. Plant Cell Physiol. 2008;49:994–997. doi: 10.1093/pcp/pcn074. [DOI] [PubMed] [Google Scholar]
- 16.Wang H.L., Postier B.L., Burnap R.L. Alterations in global patterns of gene expression in Synechocystis sp PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 2004;279:5739–5751. doi: 10.1074/jbc.M311336200. [DOI] [PubMed] [Google Scholar]
- 17.Omata T., Gohta S., Takahashi Y., Harano Y., Maeda S. Involvement of a CbbR homolog in low CO2-induced activation of the bicarbonate transporter operon in cyanobacteria. J. Bacteriol. 2001;183:1891–1898. doi: 10.1128/JB.183.6.1891-1898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figge R.M., Cassier-Chauvat C., Chauvat F., Cerff R. Characterization and analysis of an NAD(P)H dehydrogenase transcriptional regulator critical for the survival of cyanobacteria facing inorganic carbon starvation and osmotic stress. Mol. Microbiol. 2001;39:455–468. doi: 10.1046/j.1365-2958.2001.02239.x. [DOI] [PubMed] [Google Scholar]
- 19.Woodger F.J., Bryant D.A., Price G.D. Transcriptional regulation of the CO2-concentrating mechanism in a euryhaline, coastal marine cyanobacterium, Synechococcus sp strain PCC 7002: role of NdhR/CcmR. J. Bacteriol. 2007;189:3335–3347. doi: 10.1128/JB.01745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaehn S., Orf I., Schwarz D., Matthiessen J.K.F., Kopka J., Hess W.R., et al. Integrated transcriptomic and metabolomic characterization of the low-carbon response using an ndhR mutant of Synechocystis sp PCC 6803. Plant Physiol. 2015;169:1540–1556. doi: 10.1104/pp.114.254045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell M.A. MOLECULAR-BIOLOGY of the lysr family of transcriptional regulators. Annu. Rev. Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura T., Takahashi Y., Yamaguchi O., Suzuki H., Maeda S.-I., Omata T. Mechanism of low CO2-induced activation of the cmp bicarbonate transporter operon by a LysR family protein in the cyanobacterium Synechococcus elongatus strain PCC 7942. Mol. Microbiol. 2008;68:98–109. doi: 10.1111/j.1365-2958.2008.06137.x. [DOI] [PubMed] [Google Scholar]
- 23.Daley S.M.E., Kappell A.D., Carrick M.J., Burnap R.L. Regulation of the cyanobacterial CO2-concentrating mechanism involves internal sensing of NADP(+) and alpha-ketogutarate levels by transcription factor CcmR. PLoS One. 2012;7:e41286. doi: 10.1371/journal.pone.0041286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y.-L., Wang X.-P., Sun H., Han S.-J., Li W.-F., Cui N., et al. Coordinating carbon and nitrogen metabolic signaling through the cyanobacterial global repressor NdhR. Proc. Natl. Acad. Sci. U. S. A. 2018;115:403–408. doi: 10.1073/pnas.1716062115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mi H.L., Endo T., Ogawa T., Asada K. Thylakoid membrane-bound, NADPH-specific pyridine-nucleotide dehydrogenase complex mediates cyclic electron-transport in the cyanobacterium synechocystis SP PCC-68038. Plant Cell Physiol. 1995;36:661–668. [Google Scholar]
- 26.Mi H.L., Endo T., Schreiber U., Ogawa T., Asada K. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine-nucleotide dehydrogenase in the cyanobacterium synechocystIS PCC-6803. Plant Cell Physiol. 1992;33:1233–1237. [Google Scholar]
- 27.Shibata M., Ohkawa H., Katoh H., Shimoyama M., Ogawa T. Two CO2 uptake systems in cyanobacteria: four systems for inorganic carbon acquisition in Synechocystis sp strain PCC6803. Funct. Plant Biol. 2002;29:123–129. doi: 10.1071/PP01188. [DOI] [PubMed] [Google Scholar]
- 28.Williams J.G., Szalay A.A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983;24:37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber U. Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynthesis Res. 1986;9:261–272. doi: 10.1007/BF00029749. [DOI] [PubMed] [Google Scholar]
- 30.Deng Y., Ye J.Y., Mi H.L. Effects of low CO2 on NAD(P)H dehydrogenase, a mediator of cyclic electron transport around photosystem I in the cyanobacterium Synechocystis PCC6803. Plant Cell Physiol. 2003;44:534–540. doi: 10.1093/pcp/pcg067. [DOI] [PubMed] [Google Scholar]
- 31.Cao B., Mijiti X., Deng L.-L., Wang Q., Yu J.-J., Anwaierjiang A., et al. Genetic characterization conferred Co-resistance to isoniazid and ethionamide in Mycobacterium tuberculosis isolates from southern xinjiang, China. Infect. Drug Resist. 2023;16:3117–3135. doi: 10.2147/IDR.S407525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mi H., Deng Y., Tanaka Y., Hibino T., Takabe T. Photo-induction of an NADPH dehydrogenase which functions as a mediator of electron transport to the intersystem chain in the cyanobacterium Synechocystis PCC6803. Photosynthesis Res. 2001;70:167–173. doi: 10.1023/A:1017946524199. [DOI] [PubMed] [Google Scholar]
- 33.Dai H., Zhang L., Zhang J., Mi H., Ogawa T., Ma W. Identification of a cyanobacterial CRR6 protein, Slr1097, required for efficient assembly of NDH-1 complexes in Synechocystis sp PCC 6803. Plant J. 2013;75:858–886. doi: 10.1111/tpj.12251. [DOI] [PubMed] [Google Scholar]
- 34.Xu M., Shi N., Li Q., Mi H. An active supercomplex of NADPH dehydrogenase mediated cyclic electron flow around Photosystem I from the panicle chloroplast of Oryza sativa. Acta Biochim. Biophys. Sin. (Shanghai) 2014;46:757–765. doi: 10.1093/abbs/gmu064. [DOI] [PubMed] [Google Scholar]
- 35.Chen X., He Z., Xu M., Peng L., Mi H. NdhV subunit regulates the activity of type-1 NAD(P)H dehydrogenase under high light conditions in cyanobacterium Synechocystis sp PCC 6803. Sci. Rep. 2016;6 doi: 10.1038/srep28361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battchikova N., Aro E.-M., Nixon P.J. Bioenergetic Processes of Cyanobacteria. Springer Publishing; New York, NY: 2011. Structure and physiological function of NDH-1 complexes in cyanobacteria; pp. 445–467. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be shared upon request.