Abstract
Epithelioid inflammatory myofibroblastic sarcoma, a variant of the inflammatory myofibroblastic tumor, is a rare tumor that is not well described in the radiologic literature. We present a case of a 14-year-old male adolescent who presented with fever, fatigue, and weight loss symptoms and was found to have an abdominal mass on contrast enhanced CT. Initial differentials included lymphoma, pheochromocytoma, desmoid, and sarcoma, and pathological evaluation revealed an epithelioid inflammatory myofibroblastic sarcoma. The mass was separate from the surrounding structures of the left upper abdomen with unique radiologic features not previously described in the literature. Prior literature examples of epithelioid inflammatory myofibroblastic sarcoma described a heterogenous, enhancing lobulated mass, and our case was a lobulated, avidly enhancing homogenous mass on CT with surrounding inflammation and avid uptake on PET/CT. In addition to the imaging features, we describe the surgical findings, the pathologic features of the tumor, and the oncologic treatment of this patient. This case highlights the importance of including rare tumors such as epithelioid inflammatory myofibroblastic sarcoma as a potential differential consideration of an avidly enhancing homogenous abdominal mass in an adolescent.
Keywords: Epithelioid inflammatory myofibroblastic sarcoma, Inflammatory myofibroblastic tumor, Pediatrics, Anaplastic lymphoma kinase, Crizotinib
Background
Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is a rare, aggressive variant of an inflammatory myofibroblastic tumor [1]. Inflammatory myofibroblastic tumor (IMT) is a mesenchymal based neoplasm with borderline malignant potential, usually benign and made up of myofibroblastic spindle cells [2]. It mostly arises from the abdomen, including the mesentery and omentum; however, it is also associated with pulmonary, mediastinal, and head/neck origins. More locally aggressive forms tend to display expression of anaplastic lymphoma kinase (ALK) [3]. One review reported a mean age of onset of 13.2 years old with range of 3 weeks to 74 years old, and a mean tumor size at the time of presentation of 7.8 cm in diameter [3]. Compared to cases of IMT, the EIMS variant is rare, often intra-abdominal, and has different clinical and pathological features. EIMS can have a more aggressive course, with a higher rate of local recurrence, but is generally not associated with metastatic disease [1]. ALK expression in a nuclear membrane or perinuclear pattern is characteristic of EIMS [4]. Histopathologically, it is composed of round to epithelioid or histiocytoid tumor cells with a minor spindle cell component [1,4]. Although the imaging feature of inflammatory myofibroblastic tumors have been described in the literature, the imaging features of epithelioid inflammatory myofibroblastic sarcomas are not well described [5,6]. We describe the imaging and pathologic features of this tumor, the surgical and oncological treatment, and the follow up of this pediatric patient.
Case presentation
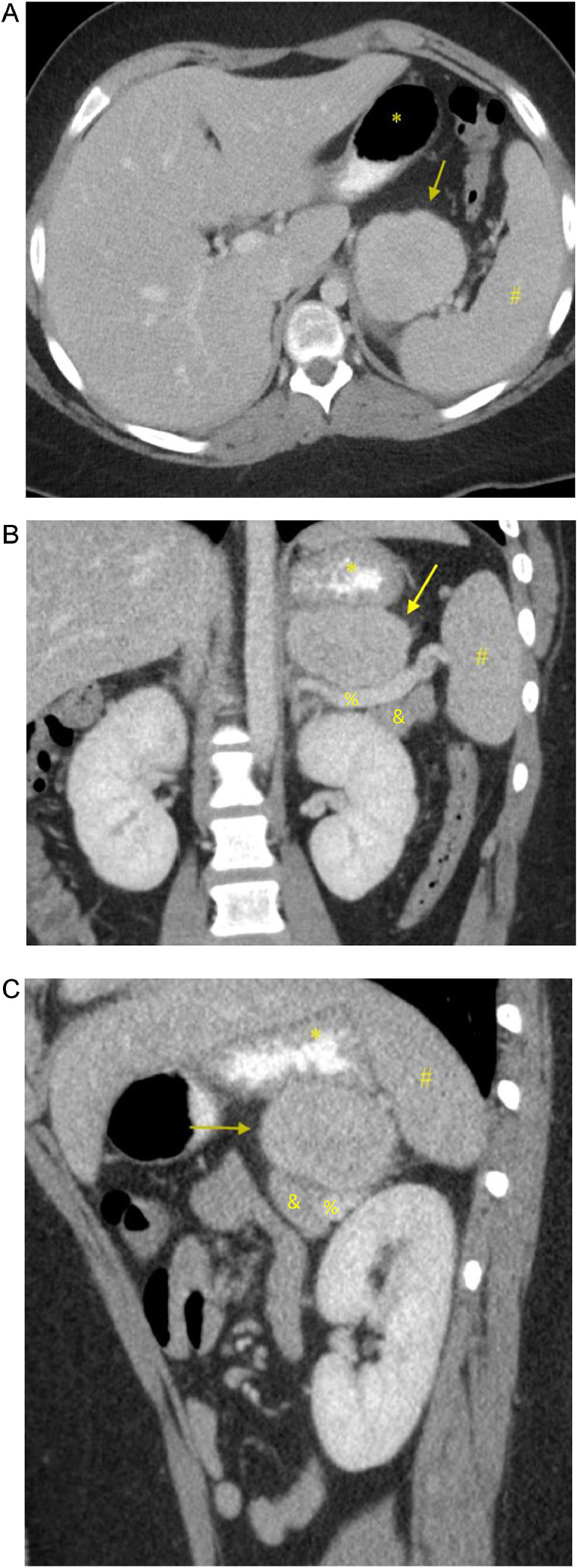
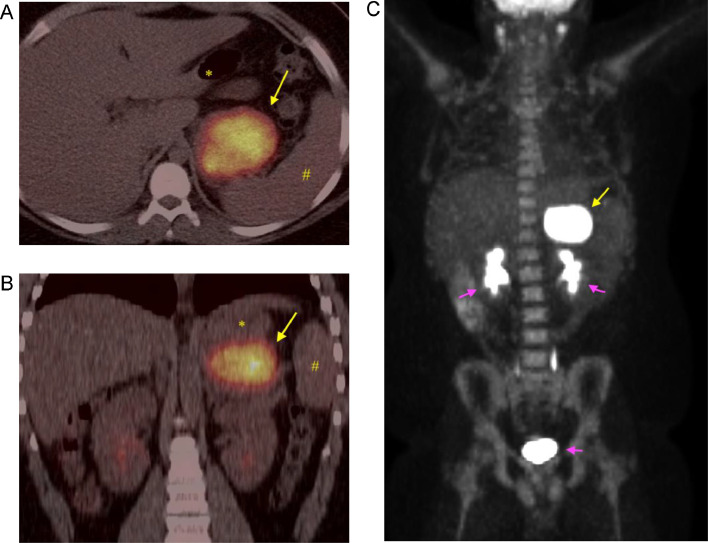
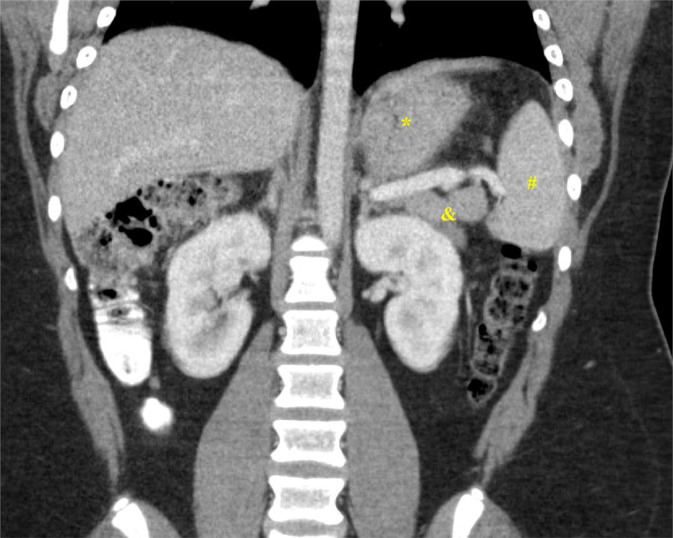
Our patient is a 14-year-old boy who presented to Pediatric Oncology with a 6-week history of headache, fatigue, fever, and weight loss. Early in the disease course, he had been evaluated by primary care for these symptoms, with associated cough and congestion. He was initially treated for allergies and sinus infection; however, he had persistent headache, fatigue, and fevers of increasing degree. Due to continued symptoms and weight loss, he had lab work done that showed a mildly elevated white count with neutrophil predominance, elevated platelet count, elevated inflammatory markers, and anemia (consistent with anemia of inflammation). To evaluate his headaches, a CT head without contrast had been performed, which was negative. There was no lymphadenopathy and no palpable organomegaly or mass on physical exam. CT neck, chest, and abdomen/pelvis was ordered due to concern for malignancy. The CT abdomen/pelvis revealed a 6.2 × 5.7 × 3.8 cm, enhancing, lobulated marginated soft tissue mass within the left upper quadrant abdomen with adjacent fat stranding (Fig. 1). There was homogenous enhancement without signs of necrosis and there was no metastatic disease in the chest. The mass was in proximity to the stomach, left adrenal gland, left kidney, pancreas, and spleen, and the mass did not definitively invade into these organs. Differential diagnosis at the time included lymphoma, pheochromocytoma, sarcoma, and desmoid tumor. PET/CT (10.6 mCi FDG) was performed to evaluate for metastatic disease, which demonstrated uniform intense FDG uptake of the left upper quadrant mass with max SVU of 45 (Fig. 2). No FDG-avid abdominopelvic lymphadenopathy or additional distant abnormal metabolic activity was identified.
Fig. 1.
Enhancing soft tissue mass (yellow arrow) in the left upper quadrant. (A) Axial portal venous phase CT imaging with oral contrast of the abdomen shows an enhancing, irregular mass with adjacent fat stranding and distinct margin separation from the stomach (*) and spleen (#). (B and C) Coronal and Sagittal CT imaging of the upper abdominal mass without invasion of the splenic vein (%), stomach (*),pancreas (&), and spleen (#).
Fig. 2.
FDG PET/CT imaging. (A and B) Axial and coronal PET/CT imaging shows avid and homogenous FDG uptake in the left upper quadrant abdominal mass (yellow arrow). Stomach (*) and spleen (#) are separate from the mass. (C) PET MIP coronal imaging of the avidly hypermetabolic left upper quadrant mass (yellow arrow). No hypermetabolic uptake was identified to suggest metastases. Normal physiologic excretion of radiotracer from the kidneys and bladder (purple arrows).
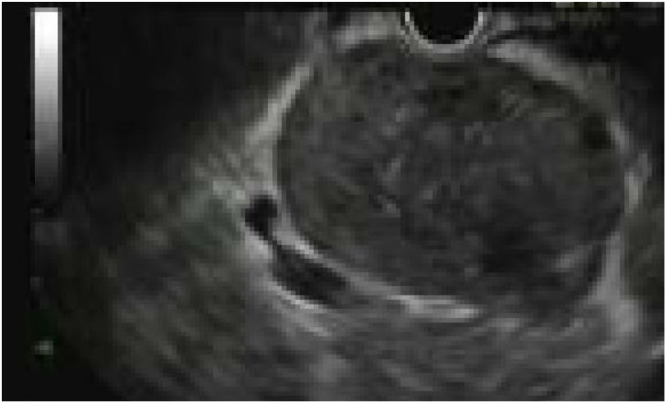
Given the proximity to the stomach, Gastroenterology was consulted and performed an endoscopic guided ultrasound fine needle aspiration biopsy through the stomach. On the endoscopic ultrasound (EUS) exam, the mass abutted, but did not appear to arise from or invade either the stomach or the pancreas (Fig. 3). Cytology of the aspirated cells demonstrated a spindle cell neoplasm with ALK expression. Some of the cells were epithelioid with varying degrees of cytologic atypia. Flow cytometry, mycobacterial cultures, and acid-fast bacteria (AFB) stains were all negative.
Fig. 3.
Endoscopic Ultrasound. Greyscale image shows a homogenous mass abutting the stomach. No necrosis identified.
Pediatric general surgery resected the mass via a laparoscopic approach. The mass was identified inferior to the greater curvature of the stomach. The lesion was well-capsulated with vessels covering the surface of the tumor. The tumor was densely adherent to the stomach, splenic artery and the anterior aspect of the pancreas, but it was not invading these structures. The tumor was dissected off the pancreas, splenic hilum, and splenic artery and removed intact without disrupting the integrity of either structures or tumor capsule.
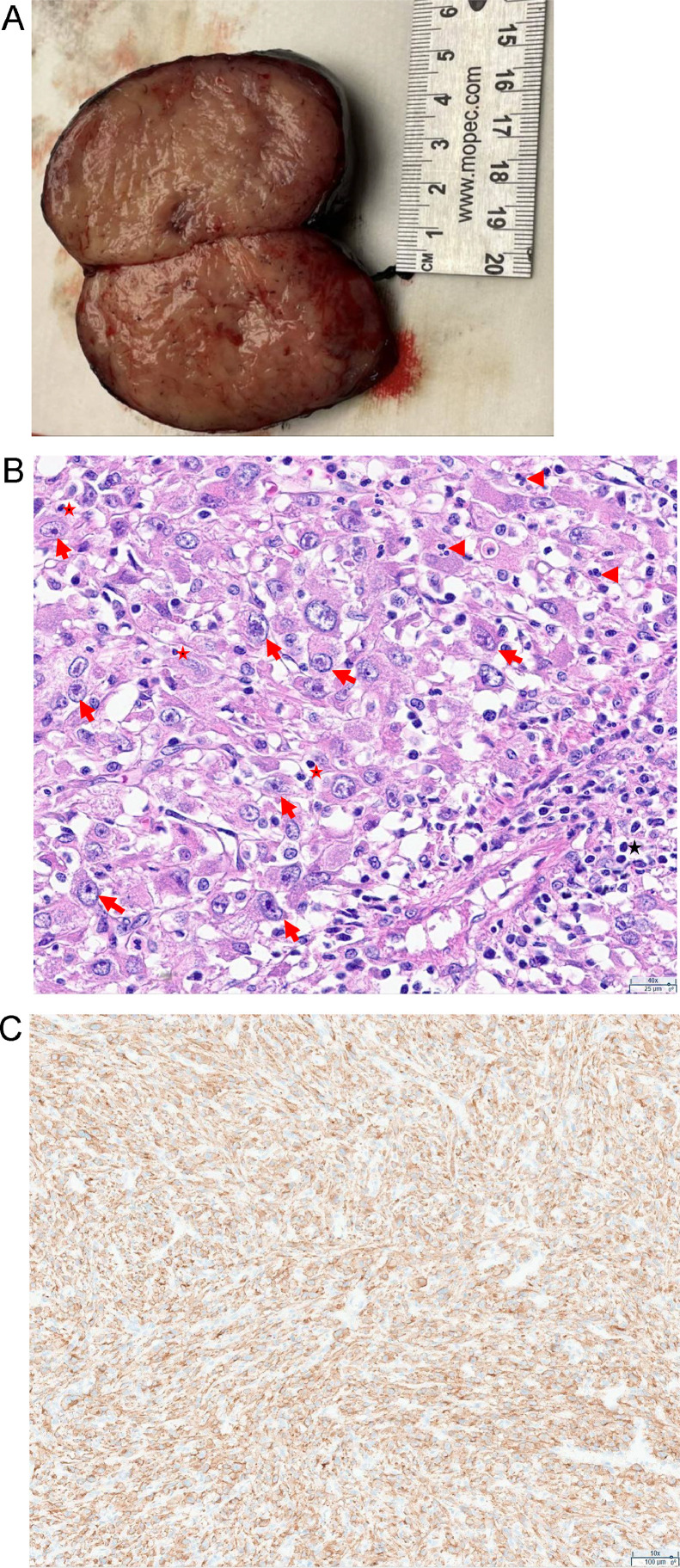
Pathology of the mass revealed a soft tissue mass with a mitotic rate of 1 per 10 HPF, no tumor necrosis, no lymphovascular invasion, and no perineural invasion (Fig. 4). The resection margin was 0.1 mm, but negative for tumor. Cytoarchitectural features were very typical of an epithelioid inflammatory myofibroblastic sarcoma. The specimen had sheets of large epithelioid or polygonal cells with eosinophilic cytoplasm and atypical vesicular nuclei with variably prominent nucleoli. There was a prominent mixed inflammatory infiltrate consisting of lymphocytes and neutrophils. Immunostains showed positivity for ALK with a cytoplasmic distribution, while SMA, desmin, MYOD1 and CD30 were negative. NGS mutation panel detected the CLTC-ALK (C31;A20) gene fusion.
Fig. 4.
Pathology images of the tumor. (A) Gross appearance of the well-circumscribed firm mass with tan cut surface and no necrosis. (B) H&E staining view showing sheets of large epithelioid or polygonal tumor cells with eosinophilic cytoplasm and atypical vesicular nuclei with variably prominent nucleoli (arrows). The tumor cells are intimately admixed with inflammatory infiltrate consisting of lymphocytes (star) and neutrophils (arrowhead). (C) ALK protein immunohistochemistry reveals cytoplasmic staining.
Following surgery, the patient's fevers, headaches, and fatigue rapidly resolved, and his white blood cells, hemoglobin, and platelets normalized. He was started on oral crizotinib, a tyrosine kinase inhibitor, which reduces the cellular proliferation and survival mediated by ALK fusion proteins. He is receiving 500 mg of crizotinib twice per day, due to his BSA of greater than 1.7 m [2], with a plan to continue this treatment for at least 1 year. He returned for follow up CT imaging 3, 6, and 9 months later with no evidence for local disease recurrence or metastatic disease (Fig. 5).
Fig. 5.
Postoperative coronal CT image of the upper abdomen 3 months after resection. No evidence of recurrence of the EIMS tumor. CT scans at 6 and 9 months after resection also showed no recurrence. There was normal appearance to the stomach (*), spleen (#), and pancreas (&).
Discussion
EIMS is a variant of the IMT, which occurs primarily in children, adolescents, and has a male predominance [1]. It is most commonly intra-abdominal, but may also have pulmonary and head/neck locations. It was first named EIMS in 2011 as a more aggressive subtype of IMT, characterized by epithelioid to round cell morphology, inflammatory infiltrate and ALK positivity on immunohistochemistry [4]. It is a rare tumor, with 43 cases previously discussed in the literature as of 2021 [1]. There are few publications describing this tumor's imaging characteristics. In a 2016 study with 5 cases of EIMS, CT imaging findings in a 55-year-old man included a solid, lobulated, heterogenous mass with mild adjacent stranding in the pelvic cavity and a 22-year-old man's CT scan revealed a solid, heterogeneous mass of the right abdominal cavity measuring 20 × 15 cm [7]. Our case differed from these examples by way of homogenous avid enhancement with more circumscribed lobulated margins. However, similar to prior reports, the tumor in our case exhibited adjacent mesenteric fat stranding, in keeping with the inflammatory state of this tumor. The mass was homogenous on EUS and it was FDG avid on PET/CT imaging. Published patients with EIMS commonly presented with symptoms of abdominal pain, mass and/or sometimes with ascites [1,8]. Our patient presented with fever, weight loss, and fatigue, suggestive of the B symptoms of lymphoma, prompting the CT scan which identified the tumor. Approximately 20% of patients with IMT present with these symptoms [8], but it is unknown how often these symptoms are present in EIMS. The EIMS variant is not known to be associated with metastases; however, it is known to have a high rate of local recurrence.
Primary neoplasms originating in the soft tissue of the retroperitoneum are exceedingly rare, accounting for 0.16% of a 25,647 tumor registry in 1 study [9]. The EIMS tumor needs to be considered in this rare subset of tumors. Differential diagnoses for retroperitoneal (RP) region neoplasms include liposarcomas of varying differentiation, leiomyomas, leiomyosarcomas, solitary fibrous tumors, desmoid tumors, extra-adrenal pheochromocytomas, cystic-based neoplasms, and neurogenic tumors like neurofibromas, schwannomas, ganglioneuromas, rhabdomyosarcomas [9]. One initial step in assessment involves classifying the mass as predominantly solid or cystic [10]. Although imaging and patient demographics can aid in RP mass classification, tissue sampling is necessary for definitive histologic diagnosis due to considerable overlap in imaging features [10].
In our case, cystic-based masses such as lymphatic malformations, tailgut cysts, and cystic teratomas can be ruled out due to the solid features of our patient's mass. Lipomatous neoplasms can also be excluded because of the absence of intralesional fat and minimal heterogeneity. Distinguishing solitary fibrous tumor from EIMS tumor can be challenging given their solitary and highly vascular nature [9]. While solitary fibrous tumors can occur at any age, they are most common in the fifth to seventh decades of life, and rarely in the pediatric population [11]. Neurogenic tumors like extra-adrenal paragangliomas, ganglioneuroblastomas, and ganglioneuromas can arise along the sympathetic chain within the retroperitoneum, exhibiting variable radiologic findings such as contrast enhancement heterogeneity, calcifications, possible hemorrhage, and/or central necrosis [12,13].
Lymphoma is less likely in our patient due to the solitary nature of the mass without nodal involvement. Desmoid tumors share similar characteristics with retroperitoneal EIMS, including being well-circumscribed, solid, enhancing, and hyperdense masses. Desmoid tumors are associated with familial adenomatous polyposis (FAP), most commonly affecting the abdominal wall (50%), mesentery (41%), and retroperitoneum (9%) in a AJR study [14]. Additionally, pheochromocytoma was considered in the differential due to its well-circumscribed, solid, avidly enhancing mass; however, pheochromocytoma are typically associated with the adrenal medulla, and less commonly found extra-adrenally.
The mainstay of treatment is of myofibroblastic tumors is surgical resection, with targeted molecular therapy via RTK inhibition being applied in certain clinical scenarios with variable effectiveness [15]. In a recent study, 2 patients treated with crizotinib therapy after biopsy showed a decrease in radiographic size after 1 year with no evidence of disease recurrence after 1.5 years [15]. Another case series included 4 pediatric patients with unresected EIMS, all of whom demonstrated response to crizotinib, though 2 did have disease recurrence on therapy [16]. In our patient, surgical resection was completed up-front and crizotinib was started to prevent recurrence. CT scans performed 3, 6, and 9 months after surgery demonstrated complete resection of the left upper quadrant abdominal mass without evidence of tumor recurrence. The patient was being treated with crizotinib at the time of follow-up CT scan evaluations with plan to continue with treatment for 12 months.
Conclusion
Few case reports detail the imaging findings of epithelioid inflammatory myofibroblastic sarcoma. We describe a case of a 14-year-old boy with an intra-abdominal enhancing soft tissue mass with mild surrounding soft tissue stranding, lobulated margins and no necrosis or nearby organ invasion. It was homogenous on EUS and avidly hypermetabolic on PET/CT imaging. There was no metastatic disease at time of presentation and following complete surgical resection and on treatment with an ALK inhibitor crizotinib, the patient remains disease free. This case highlights the importance of including such rare tumors in the differential diagnosis when appropriate.
Patient consent
We have obtained written and informed consent for publication of this article from the legal guardian of the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Batool S, Ahuja A, Chauhan DS, Bhardwaj M, Meena AK. Epithelioid inflammatory myofibroblastic sarcoma: the youngest case reported. Autops Case Rep. 2021;11 doi: 10.4322/acr.2021.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inflammatory myofibroblastic tumor. https://rarediseases.info.nih.gov/diseases/7146/inflammatory-myofibroblastic-tumor [accessed 23.08.24].
- 3.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31(4):509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 4.Marino-Enriquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35(1):135–144. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim WS, Cheon JE, Shin SM, Youn BJ, Kim IO, et al. Inflammatory myofibroblastic tumors of the abdomen as mimickers of malignancy: imaging features in nine children. AJR Am J Roentgenol. 2009;193(5):1419–1424. doi: 10.2214/AJR.09.2433. [DOI] [PubMed] [Google Scholar]
- 6.Cantera JE, Alfaro MP, Rafart DC, Zalazar R, Muruzabal MM, Barquin PG, et al. Inflammatory myofibroblastic tumours: a pictorial review. Insights Imaging. 2015;6(1):85–96. doi: 10.1007/s13244-014-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu L, Liu J, Lao IW, Luo Z, Wang J. Epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological, immunohistochemical and molecular cytogenetic analysis of five additional cases and review of the literature. Diagn Pathol. 2016;11(1):67. doi: 10.1186/s13000-016-0517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gros L, Dei Tos AP, Jones RL, Digklia A. Inflammatory myofibroblastic tumour: state of the art. Cancers (Basel) 2022;14(15) doi: 10.3390/cancers14153662. :3662–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Dasuqi K, Irshaid L, Mathur M. Radiologic-pathologic correlation of primary retroperitoneal neoplasms. Radiographics. 2020;40(6):1631–1657. doi: 10.1148/rg.2020200015. [DOI] [PubMed] [Google Scholar]
- 10.Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Jr., Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011;31(4):949–976. doi: 10.1148/rg.314095132. [DOI] [PubMed] [Google Scholar]
- 11.Penel N, Amela EY, Decanter G, Robin YM, Marec-Berard P. Solitary fibrous tumors and so-called hemangiopericytoma. Sarcoma. 2012;2012 doi: 10.1155/2012/690251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goenka AH, Shah SN, Remer EM. Imaging of the retroperitoneum. Radiol Clin North Am. 2012;50(2):333–355. doi: 10.1016/j.rcl.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics. 2003;23(1):29–43. doi: 10.1148/rg.231025050. [DOI] [PubMed] [Google Scholar]
- 14.Einstein DM, Tagliabue JR, Desai RK. Abdominal desmoids: CT findings in 25 patients. AJR Am J Roentgenol. 1991;157(2):275–279. doi: 10.2214/ajr.157.2.1853806. [DOI] [PubMed] [Google Scholar]
- 15.Kerr DA, Thompson LDR, Tafe LJ, Jo VY, Neyaz A, Divakar P, et al. Clinicopathologic and genomic characterization of inflammatory myofibroblastic tumors of the head and neck: highlighting a novel fusion and potential diagnostic pitfall. Am J Surg Pathol. 2021;45(12):1707–1719. doi: 10.1097/PAS.0000000000001735. [DOI] [PubMed] [Google Scholar]
- 16.Trahair T, Gifford AJ, Fordham A, Mayoh C, Fadia M, Lukeis R, et al. Crizotinib and surgery for long-term disease control in children and adolescents with ALK-positive inflammatory myofibroblastic tumors. JCO Precis Oncol. 2019:3. doi: 10.1200/PO.18.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]