Abstract
Background
This study aimed to investigate what treatment are selected for malignant brain tumors, particularly glioblastoma (GBM) and primary central nervous system lymphoma (PCNSL), in real-world Japan and the costs involved.
Methods
We conducted a questionnaire survey regarding treatment selections for newly diagnosed GBM and PCNSL treated between July 2021 and June 2022 among 47 institutions in the Japan Clinical Oncology Group-Brain Tumor Study Group. We calculated the total cost and cost per month of the initial therapy for newly diagnosed GBM or PCNSL.
Results
The most used regimen (46.8%) for GBM in patients aged ≤74 years was ‘Surgery + radiotherapy concomitant with temozolomide’. This regimen’s total cost was 7.50 million JPY (Japanese yen). Adding carmustine wafer implantation (used in 15.0%), TTFields (used in 14.1%), and bevacizumab (BEV) (used in 14.5%) to the standard treatment of GBM increased the cost by 1.24 million JPY for initial treatment, and 1.44 and 0.22 million JPY per month, respectively. Regarding PCNSL, ‘Surgery (biopsy) + rituximab, methotrexate, procarbazine, and vincristine (R-MPV) therapy’ was the most used regimen (42.5%) for patients of all ages. This regimen incurred 1.07 million JPY per month. The three PCNSL regimens based on R-MPV therapy were in ultra-high-cost medical care (exceeding 1 million JPY per month).
Conclusions
Treatment of malignant brain tumors is generally expensive, and cost-ineffective treatments such as BEV are frequently used. We believe that the results of this study can be used to design future economic health studies examining the cost-effectiveness of malignant brain tumors.
Keywords: glioblastoma, primary central nervous system lymphoma, treatment regimen, high-cost medical care, cost
As regimens used in GBM or PCNSL are costly, we believe that it will be necessary in the future to take cost-effectiveness into consideration in selecting a treatment.
Introduction
Glioblastoma (GBM) is one of the most malignant primary brain tumors and diffusely infiltrates the central nervous system (1,2). In Japan, GBM is a rare cancer, accounting for 1.68 cases of 100 000 people per year (3). Postoperative concomitant chemoradiotherapy with temozolomide (TMZ) and adjuvant TMZ are the standard treatments for GBM worldwide (4), with a median overall survival (OS) period of 14.6 months (5).
As GBM has the poor prognosis, treatment development is currently underway to determine what to add to TMZ to prolong survival for GBM. Firstly, carmustine wafer implantation (Gliadel) is an intracavity sustained-release formulation containing carmustine, a nitrosourea alkylating antineoplastic agent, implanted on the resection surface during the resection of malignant gliomas (6). Secondly, bevacizumab (BEV) is another drug approved for GBM treatment. Although there are two randomized controlled trials (AVAglio and RTOG0805) on BEV in combination with TMZ plus chemoradiotherapy for newly diagnosed GBM, it is not considered the standard of care for newly diagnosed GBM, mainly because two large placebo-controlled phase III trials showed no significant differences in OS, and this has not been approved for use in newly diagnosed GBM in any country other than Japan. Thirdly, the NovoTTF-100A system is a portable device that generates a low-intensity, intermediate-frequency alternating electric field called a tumor-treating field (TTF), which is believed to kill cancer cells by inhibiting their replication (7). However, the actual treatment selections for GBM in the real world as well as their associated costs have not been fully investigated.
According to the Report of the Brain Tumor Registry of Japan (2005–2008) (5), primary central nervous system lymphoma (PCNSL) accounts for 4.9% (814 per 4 years) of all primary brain tumors, and the incidence of PCNSL has been increasing in recent years. Currently, the standard treatment for PCNSL is HD-MTX-based remission induction therapy and consolidation therapy with high-dose cytarabine (AraC) or WBRT. The 2-year survival rate of patients treated with rituximab + MTX + procarbazine + vincristine (R-MPV), which is a combination of HD-MTX-based multiple agent remission induction therapy and HD-AraC, consolidation pharmacotherapy, and 23.4 Gy of reduced dose whole brain radiation, was 90% (8).
In addition to the standard treatment for these malignant brain tumors, further therapeutic development should be conducted to enable prolonged survival and may achieve a cure in the future. However, the high development costs of these new drugs render them costly. Japan has a universal health insurance system that significantly reduces patients’ out-of-pocket expenses, even when medical costs are high (9). The reduced costs come from insurance premiums and taxes paid by Japanese citizens. As the cost of medical care continues to increase, the burden on the public is approaching its limit. In the future, it will be necessary to consider drug costs and the effects of treatment choices on patient outcomes and the effects on healthcare costs and the use of limited healthcare resources from a broad perspective. Particularly, the burden of cancer continues to grow, and the disease is becoming a major economic burden for all industrialized countries (10,11). Therefore, the Japan Clinical Oncology Group (JCOG) Health Economic Committee considered that it was necessary to discuss sustainable medical care for the next generation of patients.
In the present study, we conducted a survey at a hospital in the JCOG-BTSG to reveal the treatment options available for GBM and PCNSL among malignant brain tumors and the medical costs for each regimen. This study aimed to investigate what treatment are available for malignant brain tumors, particularly GBM and PCNSL, in real-world Japan and the costs involved. It was led by the JCOG Health Economic Committee.
Materials and methods
A questionnaire survey
A questionnaire survey was conducted at 47 JCOG-BTSG-registered centers to determine the initial treatment regimens used for malignant brain tumors that are not curable: (1) newly diagnosed GBM and (2) PCNSL. The survey was conducted using Google Form, which included the name of the facility and the name of the researchers. The lists of initial treatment regimens established in the questionnaire survey items are shown in Tables 1 (newly diagnosed GBM) and Table 2 (PCNSL). There were 11 treatment regimens for GBM and eight treatment regimens for PCNSL (Tables 1 and 2). The content of each treatment regimen was extracted from regimens used mainly in Japan. In the survey, the total number of patients receiving each treatment was collected, but individual patient data were not collected. The number of patients who were treated with each regimen was divided by ‘age ≤ 74 years/≥ 75 years (at the start of treatment)’ in the survey. The study period covered cases of newly diagnosed GBM or PCNSL from July 2021 to June 2022 at each institution.
Table 1.
Initial treatment regimens for newly diagnosed GBM surveyed in the questionnaire.
| Treatment regimens | 74 years old or younger | 75 years old or older | ||
|---|---|---|---|---|
| No. of patients (n = 530) | (%) | No. of patients (n = 203) | (%) | |
| Surgery + RT (60Gy/30fr) | 5 | 1.0 | 14 | 6.9 |
| Surgery + RT (60Gy/30fr) + TMZ | 248 | 46.8 | 16 | 7.9 |
| Surgery + RT (60Gy/30fr) + TMZ + Carmustine wafer implantation | 57 | 10.8 | 6 | 3.0 |
| Surgery + RT (60Gy/30fr) + TMZ + TTFields | 56 | 10.6 | 3 | 1.5 |
| Surgery + RT (60Gy/30fr) + TMZ + Carmustine wafer implantation + TTFields | 29 | 5.5 | 0 | 0 |
| Surgery + RT (40Gy/15fr) + TMZ | 40 | 7.6 | 117 | 57.7 |
| Surgery + RT (25Gy/5fr) + TMZ | 14 | 2.7 | 22 | 10.9 |
| Surgery + RT (60Gy/30fr) + TMZ + BEV | 54 | 10.2 | 24 | 11.9 |
| Surgery + RT (60Gy/30fr) + TMZ + BEV + Carmustine wafer implantation | 12 | 2.3 | 1 | 0.5 |
| Surgery + RT (60Gy/30fr) + TMZ + BEV + TTFields | 10 | 1.9 | 0 | 0 |
| Surgery + RT (60Gy/30fr) + TMZ + BEV + Carmustine wafer implantation + TTFields | 5 | 1.0 | 0 | 0 |
| Number of facilities that responded to the survey: 42 of the 47 JCOG-BTSG-registered centers (89.4%) | ||||
Abbreviation: RT, radiation therapy; TMZ, temozolomide, TTFields, tumor-treating fields; BEV, bevacizumab.
Table 2.
Initial treatment regimens for newly diagnosed PCNSL surveyed in the questionnaire.
| Treatment regimens | 74 years old or younger | 75 years old or older | ||
|---|---|---|---|---|
| No. of patients (n = 172) | (%) | No. of patients (n = 86) | (%) | |
| Surgery (biopsy) + HD-MTX therapy | 11 | 6.4 | 12 | 14.0 |
| Surgery (biopsy) + HD-MTX therapy + Tirabrutinib | 2 | 1.2 | 3 | 3.5 |
| Surgery (biopsy) + HD-MTX therapy + ASCT/HDC | 3 | 1.8 | 0 | 0 |
| Surgery (biopsy) + HD-MTX therapy + WBRT | 7 | 4.1 | 5 | 5.9 |
| Surgery (biopsy) + R-MPV therapy | 73 | 42.5 | 46 | 53.5 |
| Surgery (biopsy) + R-MPV therapy + Tirabrutinib | 12 | 7.0 | 8 | 9.4 |
| Surgery (biopsy) + R-MPV therapy + ASCT/HDC | 19 | 11.1 | 0 | 0 |
| Surgery (biopsy) + R-MPV therapy + WBRT | 45 | 26.2 | 12 | 14.0 |
| Number of facilities that responded to the survey: 39 of the 47 JCOG-BTSG-registered centers (83.0%) | ||||
Abbreviation: HD-MTX, high-dose methotrexate; WBRT, whole brain radiotherapy; R-MPV, rituximab, methotrexate, procarbazine, and vincristine; ASCT/HDC, autologous stem cell transplantation/high-dose chemotherapy.
Calculation of the cost of each treatment for malignant brain tumors
This study calculated the total cost of the initial therapy for newly diagnosed GBM and PCNSL (not including the cost of maintenance therapy). Medical costs were calculated based on Japanese receipt scores. Monthly cost was calculated based on the treatment duration for each treatment regimen. Among the treatments, we defined ‘high-cost medical care’ as treatments of ≥0.5 million JPY per month and ‘ultra-high-cost medical care’ as treatments of ≥1 million JPY per month, as defined by the JCOG Health Economics Committee.
Results
General information
Questionnaires were collected from patients with newly diagnosed GBM or PCNSL treated between April 2022 and March 2023 from 47 JCOG-BTSG registries. The questionnaires collected from patients with newly diagnosed GBM received responses from 42 of all 47 JCOG-BTSG-registered centers (89.4%). In contrast, the survey for PCNSL received responses from 39 of the 47 JCOG-BTSG-registered centers (83.0%). Among these centers, the total numbers of patients surveyed for GBM and PCNSL were 733 and 258, respectively. In Japan, the Center for Cancer Genomics and Advanced Therapeutics offers a next-generation sequencing-based comprehensive genomic profiling test for patients with malignant brain tumors. This test targets patients with GBM or PCNSL. The cost is 0.56 million JPY (12).
Results of a survey of treatment regimens used for newly diagnosed GBM
Overall, 733 GBM cases were reported, of which 530 GBM cases were reported for those aged ≤74 years and 203 GBM cases were reported for those aged ≥75 years. The proportions of elderly and non-elderly patients receiving each treatment regimen for newly diagnosed GBM are shown in Fig. 1 and Table 1. The most used regimen for GBM in patients aged ≤74 years was ‘Surgery + radiotherapy (RT) (60 Gy/30 fr) + TMZ,’ (248/530 cases, 46.8%), which is the standard treatment for non-elderly patients with GBM. The next most used regimen was ‘Surgery + RT (60 Gy/30 fr) + TMZ’ plus (1) carmustine wafer implantation or (2) TTFields, or (3) BEV, which were almost equally used. In contrast, the most used regimen for GBM in patients aged ≥75 years was ‘Surgery + RT (40 Gy/15 fr) + TMZ’ (117/203 cases, 57.7%), which is the standard treatment for elderly patients with GBM. The next most used regimens were ‘Surgery + RT (60 Gy/30 fr) + TMZ plus BEV’ (24/203 cases, 11.9%), and ‘Surgery + RT (25 Gy/5 fr) + TMZ’ (22/203 cases, 10.9%), which were performed as clinical trials. ‘Carmustine wafer implantation’ and ‘TTFields’ tended not to be used in elderly patients with GBM. Overall, the percentages of carmustine wafer implantation, TTFields, and BEV used were 110/733; 15.0%, 103/733; 14.1%, and 106/733; 14.5%, respectively.
Fig. 1.
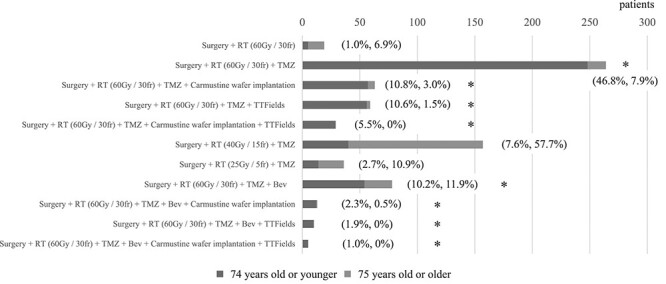
The proportion of each regimen for newly diagnosed GBM, selected by JCOG-participating physicians, according to the age group. Percentages show the proportion of patients receiving the regimen in each age groups (<75 years or > 75 years). The bold star highlights the regimens with high- or ultra-high- cost.
Comparison of the cost of each treatment regimen used for newly diagnosed GBM
The cost of treatment for eight of the 11 regimens for GBM used in the survey was investigated (Table 3). Among all 733 GBM patients, 521 patients (71.1%) were in the ‘high-cost medical care’ group, while 116 (15.8%) were in the ‘ultra-high-cost medical care’ group. As divided to age groups, 471/530 (88.9%) patients ≤74 years belonged to the ‘high-cost medical care’ group, while 112/530 (21.1%) were in the ‘ultra-high-cost medical care’ group. Among patients ≥75 years, 50/203 (24.6%) were in the ‘high-cost medical care’ group, compared to 4/203 (2.0%) in the ‘ultra-high-cost medical care’ group with a low percentage.
Table 3.
Total cost of each treatment regimen and monthly cost for newly diagnosed GBM.
| Treatment | Surgery cost (million JPY) | Cost for the first 6 months of treatment (including 3 courses of TMZ maintenance therapy) (million JPY) | Cost for the first 1 year of treatment (including 9 courses of TMZ maintenance therapy) (million JPY) | Cost for initial treatment without recurrence +12 courses of TMZ maintenance therapy (million JPY) | Cost per month (million JPY) | high-cost medical care | ultra-high-cost medical care |
|---|---|---|---|---|---|---|---|
| Surgery + RT (60Gy/30fr) + TMZ (Standard of care) (JCOG0911) | 1.32 | 7.95 | 8.85 | 9.3 | 0.74 | ○ | |
| Surgery + RT (60Gy/30fr) + TMZ + Carmustine wafer implantation (JCOG1703) | 1.32 | 9.19 | 10.09 | 10.54 | 0.84 | ○ | |
| Surgery + RT (60Gy/30fr) + TMZ + TTFields | 1.32 | 12.27 | 21.81 | 26.58 | 1.82 | ○ | ○ |
| Surgery + RT (60Gy/30fr) + TMZ + Carmustine wafer implantation + TTFields | 1.32 | 13.51 | 23.05 | 27.82 | 1.92 | ○ | ○ |
| Surgery + RT (60Gy/30fr) + TMZ + BEV | 1.32 | 8.61 | 10.83 | 12.31 | 0.9 | ○ | |
| Surgery + RT (60Gy/30fr) + TMZ + BEV + Carmustine wafer implantation | 1.32 | 9.85 | 12.07 | 13.55 | 1.01 | ○ | ○ |
| Surgery + RT (60Gy/30fr) + TMZ + BEV + TTFields | 1.32 | 12.93 | 23.79 | 29.59 | 1.98 | ○ | ○ |
| Surgery + RT (60Gy/30fr) + TMZ + BEV + Carmustine wafer implantation + TTFields | 1.32 | 14.17 | 25.03 | 30.83 | 2.09 | ○ | ○ |
Abbreviation: RT, radiation therapy; TMZ, temozolomide; TTFields, tumor-treating fields; BEV, bevacizumab; JCOG, Japan Clinical Oncology Group.
The total cost of ‘Surgery + RT (60 Gy/30 fr) + TMZ’, the standard treatment for GBM in non-elderly patients as initial treatment, was 7.50 million JPY, including 1.32 million JPY for surgery and 1.00 million JPY for radiation therapy. One course of maintenance therapy cost 0.15 million JPY per month for TMZ maintenance therapy. The cost of ‘Surgery + RT (60 Gy/30 fr) + TMZ (standard of care)’ for the first 6 months, 1 year, and up to 12 courses of TMZ maintenance therapy is shown in Table 3. Adding carmustine wafer implantation to the standard treatment of GBM in non-elderly patients increased the cost by 1.24 million JPY for initial treatment, adding TTFields increased the cost by 1.44 million JPY per month, and adding BEV increased the cost by 0.22 million JPY per a month. ‘Surgery + RT (60 Gy/30 fr) + TMZ’ (standard of care), adding ‘TTFields regimen,’ ‘carmustine wafer implantation + TTFields regimen,’ ‘BEV + carmustine wafer implantation + TTFields regimen,’ ‘BEV + carmustine wafer implantation + TTFields regimen,’ and ‘BEV + carmustine wafer implantation + TTFields regimen’ were regimens for ultra-high-cost medical care.
Results of a survey of treatment regimens used for PCNSL
A total of 258 PCNSL cases were reported, of which 172 were reported in patients aged ≤74 years and 86 were reported in patients aged ≥75 years. The proportions of elderly and non-elderly patients in each treatment regimen for PCNSL are shown in Fig. 2 and Table 2. ‘Surgery (biopsy) + R-MPV therapy’ was the commonly used regimen for patients with PCNSL of all ages (119/258 cases, 46.1%). As the European Association of Neuro-Oncology guidelines state that RT should be avoided in elderly patients with PCNSL (13), ‘Surgery (biopsy) + R -MPV therapy + WBRT’ was the second most common treatment regimen. Autologous stem cell transplantation high-dose chemotherapy was not performed in patient with PCNSL aged >75 years.
Fig. 2.
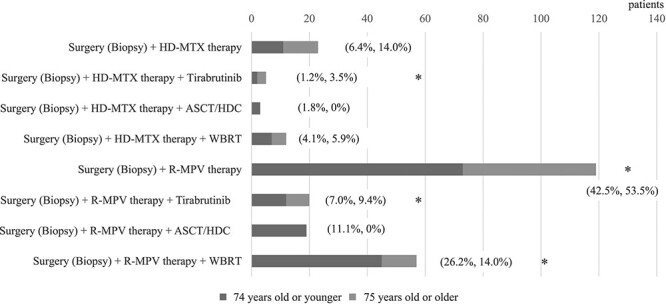
The proportion of each regimen for newly diagnosed PCNSL, selected by JCOG-participating physicians, according to the age group. Percentages show the proportion of patients receiving the regimen in each age groups (<75 years or > 75 years). The bold star highlights the regimens with high- or ultra-high-cost.
Comparison of the cost of each treatment regimen used for PCNSL
The total cost of six of the eight regimens for PCNSL used in the survey was investigated (Table 4). Among the 258 patients with PCNSL, 201 (77.9%) were in the ‘high-cost medical care’ group, while 196 (76.0%) were in the ‘ultra-high-cost medical care’ group. Among patients ≤74 years, 132/172 (76.7%) were in the ‘high-cost medical care’ group, compared to 130/172 (75.6%) in the ‘ultra-high-cost medical care’ group. In contrast, among patients ≥75 years, 69/86 (80.2%) were in the ‘high-cost medical care’ group, compared to 66/86 (76.7%) in the ‘ultra-high-cost medical care’ group.
Table 4.
Total cost of each treatment regimen and monthly cost for newly diagnosed PCNSL.
| Treatment | Surgery (biopsy) cost (million JPY) | Cost for the first 6 months of treatment (Cost for initial treatment without recurrence) (million JPY) | Cost per month (million JPY) | high-cost medical care | ultra-high-cost medical care |
|---|---|---|---|---|---|
| Surgery (biopsy) + HD-MTX therapy (Previous standard of care, JCOG1114) | 0.2 | 2 | 0.33 | ||
| Surgery (biopsy) + HD-MTX therapy + Tirabrutinib | 0.2 | 4.73 | 0.79 | ○ | |
| Surgery (biopsy) + HD-MTX therapy + WBRT | 0.2 | 2.9 | 0.48 | ||
| Surgery (biopsy) + R-MPV therapy | 0.2 | 6.4 | 1.07 | ○ | ○ |
| Surgery (biopsy) + R-MPV therapy + Tirabrutinib | 0.2 | 8.22 | 1.37 | ○ | ○ |
| Surgery (biopsy) + R-MPV therapy + WBRT (Standard of care) | 0.2 | 7.9 | 1.32 | ○ | ○ |
Abbreviation: HD-MTX, high-dose methotrexate; WBRT, whole brain radiotherapy; R-MPV, rituximab, methotrexate, procarbazine, and vincristine; JCOG, Japan Clinical Oncology Group.
The most frequently used regimen at the JCOG-BTSG-registered centers was ‘Surgery (biopsy) + R-MPV therapy,’ with a total cost of 6.4 million JPY and 1.07 million JPY per month. The cost of the stereotactic brain tumor biopsy surgery was 0.2 million JPY. Regarding chemotherapy, MTX therapy (two courses) cost 0.7 million JPY per month, R-MPV therapy (two courses) cost 1.4 million JPY per month, and tirabrutinib cost 0.91 million JPY per month. The three regimens based on R-MPV therapy involved ultra-high-cost medical care, with a monthly cost exceeding 1 million JPY. In contrast, adding tirabrutinib to HD-MTX or R-MPV therapy resulted in an increase in 0.91 million JPY per month.
Discussion
Both GBM and PCNSL are rare brain tumors with poor prognoses. Although these tumors are treated with what is considered the standard care, little consideration has been given to the cost of the standard treatment itself, the cost of new drugs added to the standard treatment, and the cost-benefit ratio of these new drugs (14,15). Furthermore, clinical trials for the development of new therapies in Japan have not been conducted in a cost-benefit manner. In this study, we investigated the treatment regimens used for GBM and PCNSL at JCOG-BTSG-registered centers with the most experience in treating brain tumors in Japan and the cost of these regimens.
The standard treatment for GBM is ‘Surgery + RT (60 Gy/30 fr) + 6–12 cycles of TMZ’ (4,16). In our series, this regimen was also the commonly used regimen for GBM patients with 264 of 733 cases (36.0%). In the current study, 15.0% (110/733) of the patients with GBM were treated with carmustine wafer implantation. However, there are not many prospective randomized clinical trials that compare these treatment regimen with groups of patients treated with other agents. Although there are some reports from retrospective studies [17–19), there is still no evidence that carmustine wafer implantation in GBM leads to a prolonged prognosis. We are currently awaiting the results of a randomized phase III study, JCOG1703 (16), for newly diagnosed maximally resected GBM comparing carmustine wafer implantation followed by chemoradiotherapy with TMZ with chemoradiotherapy alone (16). While it remains to be seen how carmustine wafer implantation during surgery much improves the prognosis for of patients with GBM will improve with carmustine wafer implantation during surgery, we should firmly consider the 1.24 million JPY increase over standard therapy. Therefore, this drug is not generally used for GBM in routine practice before the results of the phase III trial, making it less available than it should be.
For GBM, the following two treatments (TTFields and BEV) are representative of prospective randomized clinical trials. A randomized phase III trial was conducted to evaluate the efficacy of the TTFields in newly diagnosed GBM (20). A total of 695 patients with GBM were randomized to receive TMZ maintenance with TTFields or maintenance with TMZ alone after completion of the initial treatment with the Stupp regimen. In patients with newly diagnosed GBM, the median OS period was significantly prolonged by 4.9 months in the TTFields group compared with TMZ alone (20.9 months vs. 16.0 months). A prolonged OS benefit of 4.9 months for TTFields must be considered for cost-effectiveness, considering that the cost per month for TTFields is 1.44 million JPY. A French research group performed a cost-effectiveness analysis of TTFields (21). The analysis using the Markov model showed that the addition of TTFields to the standard treatment with TMZ increased the life expectancy by 4.08 months (0.34 life-years gained (LYG)) and the cost per patient by €185 476. The incremental cost-effectiveness ratio (ICER) was €549 909/LYG. Therefore, this study emphasizes that the current cost of TTFields has an ICER that is significantly high to be cost-effective. However, other research groups tested different models and concluded that TTF remains a less cost-effective intervention, significantly hindering its dissemination to potentially eligible patients (22). Thus, given that TTFields are costly, there is a difference in opinion as to whether they are cost-effective. In the present study, 14.1% (103/733) of patients with GBM were treated with TTF. In Japan, the use of TTF increases the monthly amount by 1 440 000 JPY, with the ICER estimated at 17280000JPY/LYG (incremental cost: 5875200JPY; incremental effectiveness: 0.34 LYG). Therefore, it is considered to be a less cost-effective treatment since it far exceeds the willingness-to-pay (WTP) threshold in Japan (7.5 million yen/QALY).
In the AVAglio study, compared chemoradiotherapy with TMZ plus BEV (23) and chemoradiotherapy with TMZ plus placebo in patients with newly diagnosed GBM, the median OS period was not significantly different at 16.8 and 16.7 months, respectively. Another randomized controlled trial of BEV in combination with chemoradiotherapy with TMZ for newly diagnosed GBM is the RTOG0805 trial (24) showed no difference in OS between BEV-treated (median survival, 15.7 months) and placebo-treated patients (median survival, 16.1 months). Therefore, BEV is not considered the standard of care for newly diagnosed GBM worldwide, partly because of the lack of significant OS differences in two large placebo-controlled phase III trials. However, the use of BEV for newly diagnosed GBM has been inconsistently approved by insurance in Japan. Even in the JCOG-BTSG registry, 106/733 (14.5%) patients with newly diagnosed GBM were treated with BEV despite a lack of OS prolongation (Fig. 1). One of the reasons why BEV is often used in Japan is that 49.2% of patients newly diagnosed with GBM in Japan have a Karnofsky performance status (KPS) of ≤70 and BEV is used to improve performance status of patients. Some patients with GBM with low KPS may benefit from additional BEV treatment with RT + TMZ, but considering that BEV costs 0.22 million JPY per month, the use of BEV for newly diagnosed GBM should be discouraged, at least for patients with high performance status in Japan.
Three to five cycles of HD-MTX have been the standard care for PCNSL in Japan for a long time (25), and the JCOG-BTSG conducted the JCOG1114 study comparing HD-MTX + WBRT versus HD-MTX + TMZ + WBRT plus adjuvant MTX (26). Based on the results of several clinical trials for PCNSL (8,27,28), R-MPV is considered the standard of care for PCNSL in the JCOG-BTSG, and some clinical trials are ongoing. ‘Surgery (biopsy) + R-MPV therapy’ was the most used regimen at the JCOG-BTSG centers in patients with PCNSL aged ≤74 years (73/172 cases, 42.5%) and in patients with PCNSL aged ≥75 years (46/86 cases, 53.5%). R-MPV therapy, the standard treatment for PCNSL, belongs to the ‘ultra-high-cost medical care’ group, indicating that the cost of the standard treatment itself is high. If another therapy is added to this standard therapy, the cost will naturally be even higher. Because there are no results of clinical trials comparing it with R-MPV therapy, it is difficult to discuss the cost-effectiveness of PCNSL treatment regimens; however, there are some reports of the cost-effectiveness of PCNSL treatment regimens (29,30). A retrospective study on the cost-effectiveness of rituximab plus methotrexate with AraC (R-MA regimen) has been reported (30). Thirty-seven patients who received the R-M regimen showed good OS at low costs. The International Extranodal Lymphoma Study Group-32 randomized patients with PCNSL into three groups: methotrexate-AraC, methotrexate-AraC-rituximab, and methotrexate-AraC-thiotepa-rituximab (MATRix) as induction therapy. The MATRix regimen significantly improved complete remission (29). The MATRix regimen had a 3.05 quality-adjusted life year (QALY) gain at an additional cost of $75 513, with an ICER of $24 758/QALY gain (29). Thus, the MATRix regimen appears to be the optimal induction therapy for PCNSL patients, both clinically and economically.
In the treatment of newly-diagnosed GBM, TMZ plus BEV had no significant difference in median OS period compared to TMZ alone. Thus, in the present study, if the 76 patients treated with ‘Surgery + RT (60 Gy/30 fr) + TMZ + BEV Surgery + RT (60 Gy/30 fr + TMZ + BEV)’, assuming those patients survived 12 months, the cost of BEV could be reduced by 200.64 million JPY, considering an increase of 0.22 million JPY per month in the cost of BEV. Since Japan’s estimated medical cost in 2022 is 46 trillion JPY, this represents a 0.004% reduction in medical costs.
One limitation is that this survey did not cover all brain tumor treatment centers in Japan, only those registered with the JCOG-BTSG. The treatment selections for malignant brain tumors at non-JCOG participating centers may differ from those at JCOG participating centers that have experts in malignant brain tumors. Therefore, the results may differ if the disease population increases. In addition, because the survey period was limited to 1 year, the possibility of bias cannot be denied. Additionally, individual patient data for GBM and PCNSL were not collected. Thus, this study did not consider information, such as the actual duration of administration, drug discontinuation, or dose reduction in individual cases. Therefore, we may not have been able to accurately assess the cost-benefit ratio of each regimen for each disease. Therefore, future prospective clinical trials on GBM and PCNSL should evaluate treatment regimens and cost-benefits. There are still no ongoing surveys or studies for cost containment for malignant brain tumors. Considering the increasing healthcare costs in Japan, healthcare professionals should have a perspective for cost-effectiveness optimization for malignant brain tumors.
Conclusions
In the present study, we investigated the types of treatment regimens used for GBM or PCNSL and the proportion of elderly/non-elderly patients in each treatment at JCOG-BTSG-registered centers in Japan and provided information on the cost per month of each treatment regimen and whether it was high-cost or ultra-high-cost medical care. Treatment of malignant brain tumors is generally expensive, and substantial number of patients are treated by high-cost drugs with unproven or denied benefit. Although this study analyzed only survival time, it is important to discuss the maintenance of Performance Status or Quality of life of patients in the future. We believe that the results of this study can be used to design future health economic studies examining the cost-effectiveness of malignant brain tumors, particularly GBM and PCNSL.
Contributor Information
Kazuya Motomura, Department of Neurosurgery, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Japan.
Keita Sasaki, JCOG Data Center/Operations Office, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, Japan.
Narushi Sugii, Department of Neurosurgery, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Japan.
Shigeru Yamaguchi, Department of Neurosurgery, Faculty of Medicine, Hokkaido University, North 15, West 7, Kita-ku, Sapporo, Japan.
Hirotaka Inoue, Department of Neurosurgery, Graduate School of Medical Sciences, 1-1-1, Honjo, Chuo-ku, Kumamoto University, Kumamoto, Japan.
Akito Oshima, Department of Neurosurgery, Yokohama City University, 3-9, Fukuura, Kanazawa, Yokohama, Japan.
Kazuhiro Tanaka, Department of Neurosurgery, Kobe University Hospital, 7-5-1 Kusunoki-cho, Chuo-ku, Kobe, Japan.
Yoshihiro Otani, Department of Neurological Surgery, Okayama University Faculty of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, kita-ku, Okayama, Japan.
Mitsuaki Shirahata, Department of Neurosurgery/Neuro-oncology, Saitama Medical University International Medical Center, 1397-1 Yamane, Hidaka, Saitama, Japan.
Ichiyo Shibahara, Department of Neurosurgery, Kitasato University School of Medicine, 1-15-1, Kitasato, Minami-ku, Sagamihara, Japan.
Motoo Nagane, Department of Neurosurgery, Kyorin University Faculty of Medicine, 6-20-2 Shinkawa, Mitaka, Tokyo, Japan.
Shunsuke Tsuzuki, Department of Neurosurgery, Tokyo Women's Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo, Japan.
Tomoo Matsutani, Department of Neurological Surgery, Chiba University Graduate School of Medicine, 1-8-1 Inohana, Chuou-ku, Chiba, Japan.
Yoshihiro Tsukamoto, Department of Neurosurgery, Brain Research Institute, Niigata University, 1-757 Asahimachi-dori, Chuo-ku, Niigata, Japan.
Noriyuki Kijima, Department of Neurosurgery, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka, Japan.
Kenichiro Asano, Department of Neurosurgery, Hirosaki University Graduate School of Medicine, Zaifu-cho 5, Hirosaki, Japan.
Makoto Ohno, Department of Neurosurgery and Neuro-Oncology, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo, Japan.
Akihiro Inoue, Department of Neurosurgery, Ehime university school of medicine, 454, Shitsukawa, Toon, Ehime, Japan.
Yohei Mineharu, Department of Neurosurgery, Kyoto University Graduate School of Medicine, 54 Kawahara-cho Shogoin Sakyo-ku, Kyoto, Japan.
Keisuke Miyake, Department of Neurological Surgery, Kagawa University Faculty of Medicine, 1750-1 Ikenobe, Miki-cho, Kita-gun, Kagawa, Japan.
Yuta Mitobe, Department of Neurosurgery, Yamagata University Hospital, 2-2-2, Iida-Nishi, Yamagata, Japan.
Mitsuto Hanihara, Department of Neurosurgery, University of Yamanashi, 1110 Shimokato, Chuo, Yamanashi, Japan.
Yu Kawanishi, Department of Neurosurgery, Kochi Medical School, Kochi University, Kohasu, Oko-cho, Nankoku, Kochi, Japan.
Shoichi Deguchi, Department of Neurosurgery, Shizuoka Cancer Center, Shimonagakubo 1007, Nagaizumi, Sunto-gun, Shizuoka, Japan.
Masato Saito, Department of Neurosurgery, Asahikawa Medical University, 2-1-1-1 Midorigaoka Higashi, Asahikawa, Japan.
Ryosuke Matsuda, Department of Neurosurgery, Nara Medical University, Shijo-cho 840, Kashihara, Nara, Japan.
Kenta Ujifuku, Department of Neurosurgery, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki, Japan.
Hideyuki Arita, Department of Neurosurgery, Osaka International Cancer Institute, 3-1-69, Otemae, Chuo-ku, Osaka, Japan.
Yuichi Sato, Department of Neurosurgery, Iwate Medical University School of Medicine, 2-1-1 Idai-dori, Yahaba, Shiwa, Iwate, Japan.
Shinji Yamashita, Department of Neurosurgery, Miyazaki University, 5200 Kihara Kiyotake, Miyazaki, Japan.
Ushio Yonezawa, Department of Neurosurgery, Hiroshima University Hospital, 1-2-3 Minamiku Kasumi, Hiroshima, Japan.
Junya Yamaguchi, Department of Neurosurgery, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Japan.
Yasutomo Momii, Department of Neurosurgery, Oita University Faculty of Medicine, 1-1 Idaigaoka Hasama-machi Yufu, Oita, Japan.
Takahiro Ogawa, Department of Neurosurgery, Kyoto Prefectural University of Medicine, 465 Kajii-cho, Kawaramachi-Hirokoji, Kamigyo-ku, Kyoto, Japan.
Atsushi Kambe, Department of Brain and Neurosciences, Division of Neurosurgery, Faculty of Medicine, Tottori University, 36-1 Nishi-cho, Yonago, Tottori, Japan.
Shigeo Ohba, Department of Neurosurgery, Fujita Health University, 1-98Dengakugakubo, Kutsukake-cho, Toyoake, Aichi, Japan.
Junya Fukai, Department of Neurological Surgery, Wakayama Medical University School of Medicine, 811-1 Kimiidera, Wakayama, Japan.
Norihiko Saito, Department of Neurosurgery, Toho University Ohashi Medical Center, 2-22-36, Ohashi, Meguro, Tokyo, Japan.
Masashi Kinoshita, Department of Neurosurgery, Kanazawa University, 13-1 Takara-machi, Kanazawa, Japan.
Koichiro Sumi, Department of Neurological Surgery, Nihon University School of Medicine, 30-1 Oyaguchikamicho, Itabashi-ku, Tokyo, Japan.
Ryohei Otani, Department of Neurosurgery, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo, Japan.
Takeo Uzuka, Department of Neurosurgery, Dokkyo Medical University Hospital, 880 Kitakobayashi, Mibu-machi, Shimotsuga-gun, Tochigi, Japan.
Noriyoshi Takebe, Department of Neurosurgery, Medical Research Institute Kitano Hospital, PIIF Tazuke-kofukai, 2-4-20 Ohgimachi, Kita-ku, Osaka, Japan.
Shinichiro Koizumi, Department of Neurosurgery, Hamamatsu University School of Medicine, 1-20-1, Handayama, Higashi-ku, Hamamatsu, Shizuoka, Japan.
Ryuta Saito, Department of Neurosurgery, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Japan.
Yoshiki Arakawa, Department of Neurosurgery, Kyoto University Graduate School of Medicine, 54 Kawahara-cho Shogoin Sakyo-ku, Kyoto, Japan.
Yoshitaka Narita, Department of Neurosurgery and Neuro-Oncology, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo, Japan.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Funding
This work was supported in part by the Research Fund of National Federation of Health Insurance Societies (Kenpo-ren) and the National Cancer Center Research and Development Fund (2023-J-03).
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Broekman ML, Maas SLN, Abels ER, Mempel TR, Krichevsky AM, Breakefield XO. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol 2018;14:482–95. 10.1038/s41582-018-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motomura K, Natsume A, Kishida Y, et al. Benefits of interferon-β and temozolomide combination therapy for newly diagnosed primary glioblastoma with the unmethylated MGMT promoter: a multicenter study. Cancer 2011;117:1721–30. 10.1002/cncr.25637. [DOI] [PubMed] [Google Scholar]
- 3. Narita Y. Incidence of brain tumors in Japan. Nippon Rinsho 2023;81:7–11. [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5. Report of Brain Tumor Registry of Japan . Brain Tumor Registry of Japan (2005-2008) 14th edition. Neurol Med Chir (Tokyo) 57:9–102. 10.2176/nmc.sup.2017-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brem H, Mahaley MS Jr, Vick NA, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg 1991;74:441–6. 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 7. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 2012;48:2192–202. 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 8. Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013;31:3971–9. 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito M. Health insurance systems in Japan: a neurosurgeon's view. Neurol Med Chir (Tokyo) 2004;44:617–28. 10.2176/nmc.44.617. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol 2011;12:933–80. 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 11. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med 2011;364:2060–5. 10.1056/NEJMsb1013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Omura T, Takahashi M, Ohno M, et al. Clinical application of comprehensive genomic profiling tests for diffuse gliomas. Cancers (Basel) 2022;14:2454. 10.3390/cancers14102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoang-Xuan K, Deckert M, Ferreri AJM, et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro Oncol 2023;25:37–53. 10.1093/neuonc/noac196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Messali A, Hay JW, Villacorta R. The cost-effectiveness of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma in the United States. Neuro Oncol 2013;15:1532–42. 10.1093/neuonc/not096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prica A, Chan K, Cheung M. Combined modality therapy versus chemotherapy alone as an induction regimen for primary central nervous system lymphoma: a cost-effectiveness analysis. Neuro Oncol 2014;16:1384–91. 10.1093/neuonc/nou057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kadota T, Saito R, Kumabe T, et al. A multicenter randomized phase III study for newly diagnosed maximally resected glioblastoma comparing carmustine wafer implantation followed by chemoradiotherapy with temozolomide with chemoradiotherapy alone; Japan clinical oncology group study JCOG1703 (MACS study). Jpn J Clin Oncol 2019;49:1172–5. 10.1093/jjco/hyz169. [DOI] [PubMed] [Google Scholar]
- 17. Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 2003;5:79–88. 10.1093/neuonc/5.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet 1995;345:1008–12. 10.1016/S0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 19. Bock HC, Puchner MJ, Lohmann F, et al. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev 2010;33:441–9. 10.1007/s10143-010-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance Temozolomide vs maintenance Temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–16. 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol 2016;18:1129–36. 10.1093/neuonc/now102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Connock M, Auguste P, Dussart C, Guyotat J, Armoiry X. Cost-effectiveness of tumor-treating fields added to maintenance temozolomide in patients with glioblastoma: an updated evaluation using a partitioned survival model. J Neurooncol 2019;143:605–11. 10.1007/s11060-019-03197-w. [DOI] [PubMed] [Google Scholar]
- 23. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–22. 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 24. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hiraga S, Arita N, Ohnishi T, et al. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J Neurosurg 1999;91:221–30. 10.3171/jns.1999.91.2.0221. [DOI] [PubMed] [Google Scholar]
- 26. Mishima K, Nishikawa R, Narita Y, et al. Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro Oncol 2023;25:687–98. 10.1093/neuonc/noac246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015;125:1403–10. 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 2007;25:4730–5. 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 29. Beca JM, Raza K, Mow E, Keech J, Kouroukis CT. Cost-effectiveness analysis of rituximab with methotrexate, cytarabine and thiotepa for the treatment of patients with primary central nervous system lymphoma. Leuk Lymphoma 2020;61:1097–107. 10.1080/10428194.2020.1711902. [DOI] [PubMed] [Google Scholar]
- 30. Yuan X, Yu T, Huang Y, et al. Rituximab with high-dose methotrexate is effective and cost-effective in newly diagnosed primary central nervous system lymphoma. Sci Rep 2022;12:21541. 10.1038/s41598-022-24922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


