Abstract
Background
Treating children with acute severe asthma (ASA) who fail to respond to first-line inhaled bronchodilators is problematic: use of intravenous agents is inconsistent and side-effects are common. High-flow humidified oxygen (HiFlo) has shown promise in other respiratory conditions and is increasingly used in ASA, but with little evidence.
Methods
We conducted a feasibility randomised controlled trial with deferred consent to assess early HiFlo in children aged 2–11 years with ASA not responding to “burst” therapy (high-dose inhaled salbutamol ± ipratropium). Children with Paediatric Respiratory Assessment Measure (PRAM) score 5+ after “burst” were randomised to commence HiFlo or follow standard care. Candidate primary outcomes assessed were treatment failure requiring escalation, and time to meeting hospital discharge criteria.
Results
The target was met despite coronavirus disease 2019 pandemic disruption: 56 children were randomised across four sites, with deferred consent received in 50 out of 56 (89%), and mean recruitment rate 1.1 per site per month. 28 were allocated early HiFlo and 22 standard care. Data collection was complete for both candidate primary outcomes. Treatment failure requiring escalation occurred in 18 of 28 children (64%) in the HiFlo arm and in 19 of 22 (86%) in the standard care arm. Median (interquartile range) time from randomisation to meeting discharge criteria was 29.3 h (21.8–43.7 h) in the HiFlo arm and 36.8 h (24.1–46.3 h) in the standard care arm.
Conclusions
HiFlo in childhood ASA is a potentially promising intervention whose use is increasing despite lack of evidence. A definitive randomised controlled trial to assess its effectiveness is required and appears to be feasible.
Shareable abstract
High-flow humidified oxygen is a potentially promising intervention in childhood acute severe asthma, the use of which is increasing despite lack of evidence. A definitive RCT to assess its effectiveness is required and appears to be feasible. https://bit.ly/44Cn7a0
Introduction
Acute severe asthma (ASA) is a leading cause of hospital attendance in children, accounting for up to 7% of all paediatric emergency visits [1] and 8.5% of paediatric admissions from emergency departments [2], the commonest single cause, and asthma exacerbation rates are increasing [3]. Standard first-line treatment [3] for ASA in children involves high-dose inhaled bronchodilators via a spacer device or nebuliser (“burst” therapy) plus oral corticosteroids: many children improve clinically and may be discharged. Around 20% fail to respond and require more intensive, second-line treatment: commonly intravenous (i.v.) bronchodilators [4] (one or more of: aminophylline, salbutamol and magnesium sulfate). However, the evidence for efficacy is limited and inconsistent, and side-effects are common [5]. Owing to a scarcity of evidence, current guidelines [5, 6] give little steer on which second-line treatment clinicians should use. There is therefore a need to investigate other options for treating ASA to improve the effectiveness of treatment and reduce adverse effects.
High-flow humidified oxygen (HiFlo) therapy is an innovative healthcare technology that supports breathing by supplying a warm, humidified air/oxygen mixture at high flow rates via fine nasal cannulae, which has shown promising results in other acute respiratory conditions in children [7]. For example, trials in infants with acute bronchiolitis have shown improved oxygen saturation levels [8] and fewer treatment failures [9, 10], though not significantly faster weaning from oxygen [9]. In a recent large trial of children with acute respiratory failure due to a range of diagnoses, HiFlo was non-inferior to continuous positive airway pressure [11].
There have so far been no substantial randomised controlled trials (RCTs) of HiFlo in children with ASA, though its use in ASA has been rapidly increasing [12]. Retrospective observational studies of HiFlo in ASA have suggested that its use improves physiological indices and asthma severity scores [13, 14], but they have also raised concerns that its use may delay initiating other forms of respiratory support [15]. There have been two small single-centre pilot RCTs of HiFlo in acute asthma, with conflicting results [16, 17]. A recent review of HiFlo in ASA [12] concluded that “large well-designed randomised controlled trials assessing the clinical efficacy of high-flow nasal cannula oxygen for children with asthma exacerbation are urgently warranted.”
In summary, ASA in childhood is a common life-threatening emergency condition with important impacts on healthcare costs and quality of life. HiFlo is a novel therapy which may have the potential to treat ASA more effectively, but its use in this context is increasing without proof of efficacy. If HiFlo in ASA is not evaluated objectively, there is a risk that a treatment without proven benefit (but with significant costs) may drift into routine practice. There is therefore an urgent need for a well-designed, adequately powered RCT of HiFlo in ASA. A definitive RCT could be large and expensive to run, and it is unclear whether it would be feasible, how large it would need to be and what the most appropriate outcome measures would be. We conducted a multicentre feasibility RCT designed to fill this knowledge gap. The underlying hypothesis is that early use of HiFlo improves outcomes in children with ASA who fail to respond to first-line therapy. The aim of this study was to establish whether an RCT to examine this hypothesis can be conducted safely and successfully, and to generate data needed to plan the definitive RCT.
Methods
Participants
We recruited children aged 2 to 11 years who presented to the hospital emergency department (ED) with ASA and did not respond to first-line “burst” therapy (defined as high-dose inhaled salbutamol, with or without ipratropium, via inhaler and spacer or nebuliser, at least two doses over 1 h). ASA was defined as acute respiratory distress with wheeze and did not require a prior diagnosis of asthma. Failure to respond was defined as a Paediatric Respiratory Assessment Measure (PRAM) [18, 19] score of 5 or more, between 1 and 4 h after starting “burst” therapy. We excluded children with: signs of bacterial pneumonia (fever >38.5°C plus focal signs on auscultation or chest radiograph); impending respiratory failure; recognised contraindications to HiFlo therapy (air leak, decreased conscious level, recent bowel surgery or intractable vomiting); other major respiratory, cardiovascular or neurological conditions; and previous participation in the study. The study was approved by the West Midlands – Solihull Research Ethics Committee (reference: 19/WM/0219, IRAS: 261627), and is registered with ISRCTN (78297040).
Study design
The study protocol has been previously published [20]. This was a multicentre unblinded feasibility RCT with deferred consent. Eligible children were randomised online (Sealed Envelope Ltd, London, UK) 1:1 to intervention (HiFlo) or control (standard care) arms, stratified by site, age (<5 years, 5 and over) and ASA severity (PRAM score at entry: <8, 8 and over). Deferred consent [21, 22] was sought from parent or guardian once the child was stable: a child was enrolled into the study at randomisation, but only recruited after deferred consent was received.
The specific feasibility objectives were: to evaluate enrolment rates and deferred consent rates, to assess the feasibility of recording candidate primary outcome measures and estimate their variability, to assess the acceptability to families of the treatments and of the deferred consent model, and (based on all of these) to determine the design characteristics for a definitive RCT. For the first three objectives, specific targets were pre-specified (table 1).
TABLE 1.
Feasibility objectives with pre-specified thresholds for progression to a full randomised controlled trial
| Feasibility objective | Feasibility outcome measure | Threshold |
|---|---|---|
| Evaluate enrolment rates | Proportion of eligible children who were enrolled (i.e. randomised) | 50% |
| Evaluate deferred consent rates | Proportion of children with signed deferred consent amongst those enrolled into the study | 70% |
| Assess feasibility of recording candidate primary outcome measures | Proportion of data collection complete per participant, for the two candidate primary outcome measures | 80% |
Two candidate primary outcome measures were evaluated: treatment failure needing escalation of therapy; and time to meeting hospital discharge criteria (defined as maintaining oxygen saturation 92% or above without supplemental oxygen or respiratory support, plus requiring inhaled bronchodilator 4-hourly or less frequently). The following candidate secondary outcome measures were also recorded: time to hospital discharge, time to achieving PRAM score 3 or less, time to no longer requiring supplemental oxygen or respiratory support, need for and duration of i.v. bronchodilator therapy, need for noninvasive or invasive ventilation, treatment-related adverse effects, hospital readmission within 48 h, and acceptability of treatment to parents/carers and children assessed by questionnaire.
Recommendations for the number of participants to include in a feasibility study to provide precise estimates of the variability in the candidate primary outcome measures, for the purpose of estimating the sample size required for the future trial, vary between 50 [23] and 70 [24]. The larger target was initially chosen to allow for a 30% attrition to deferred consent [22] but was subsequently revised to the lower figure with agreement from the Trial Steering Committee on the basis of the observed deferred consent rate.
Equipment
HiFlo intervention was initiated using the Vapotherm Precision Flow Plus system (Vapotherm Inc., Exeter, NH, USA), chosen because this system incorporated a transfer unit with battery and cylinders to allow continuous HiFlo delivery in the ED and during transfer to the ward. Inhaled bronchodilator delivery was maintained during HiFlo using an in-line Aerogen Solo nebuliser (Aerogen Ltd, Galway, Ireland).
Study procedures
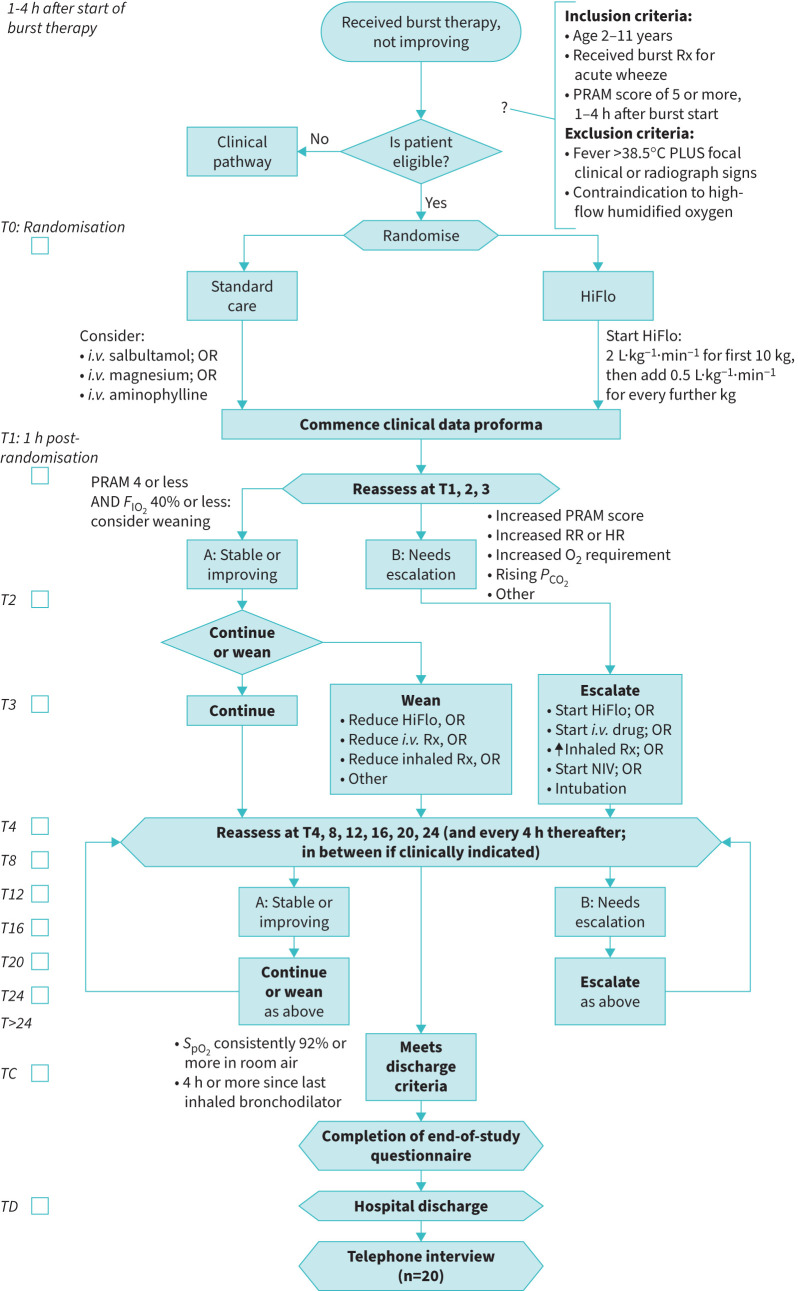
Figure 1 outlines the trial procedures. Following randomisation, in the intervention arm, HiFlo was to be commenced within 30 min of randomisation and increased to a target of 2 L·kg−1·min−1 for the first 10 kg body weight, plus 0.5 L·kg−1·min−1 for every further kilogram, with maximum 40 L·min−1. The fraction of inspired oxygen (FIO2) was adjusted to maintain oxygen saturation by pulse oximeter (SpO2) ≥92%. Other than this early commencement of HiFlo, management in both arms was pragmatic, with all treatment options available and guided by clinical choice. Progress was recorded on a clinical data proforma (CDP), and the following were pre-defined as escalations of therapy: starting an i.v. bronchodilator, starting rescue HiFlo, initiating invasive or noninvasive ventilation, or increasing the frequency of inhaled bronchodilator. Whenever an escalation was needed, the reason for this (e.g. increasing oxygen requirement, increasing PRAM score) was recorded on the CDP. As improvement occurred, weaning of therapies was also at clinical discretion according to standard principles as per figure 1. Vital signs, PRAM score, current treatment and oxygen requirement were recorded on the CDP, hourly for 4 h then 4-hourly, and time both of meeting hospital discharge criteria and of actual hospital discharge were recorded.
FIGURE 1.
Study flow chart. Rx: treatment; PRAM: Paediatric Respiratory Assessment Measure; HiFlo: high-flow humidified oxygen; i.v.: intravenous; FIO2: fraction of inspired oxygen; RR: respiratory rate; HR: heart rate; PCO2: carbon dioxide tension; NIV: noninvasive ventilation; SpO2: oxygen saturation by pulse oximeter; TC: time of meeting discharge criteria; TD: time of discharge.
Prior to discharge, an end-of-study questionnaire (ESQ), co-designed with parents of children admitted with ASA, was completed by the accompanying parent or carer. Items related to treatment effectiveness, treatment satisfaction, service satisfaction, information and consent, physical comfort, pain and communication were included. If the participating child was aged 4 years or over, they completed a shorter child-appropriate version. In addition, a purposive sample of parents/carers and healthcare professionals (HCPs) across the four sites were invited to participate in a 30-min semi-structured telephone interview with an experienced qualitative researcher to elicit their views and opinions of the therapy and the study more generally. To capture fresh impressions, parent/carer interviews were conducted within 3 weeks of discharge.
Statistical analysis
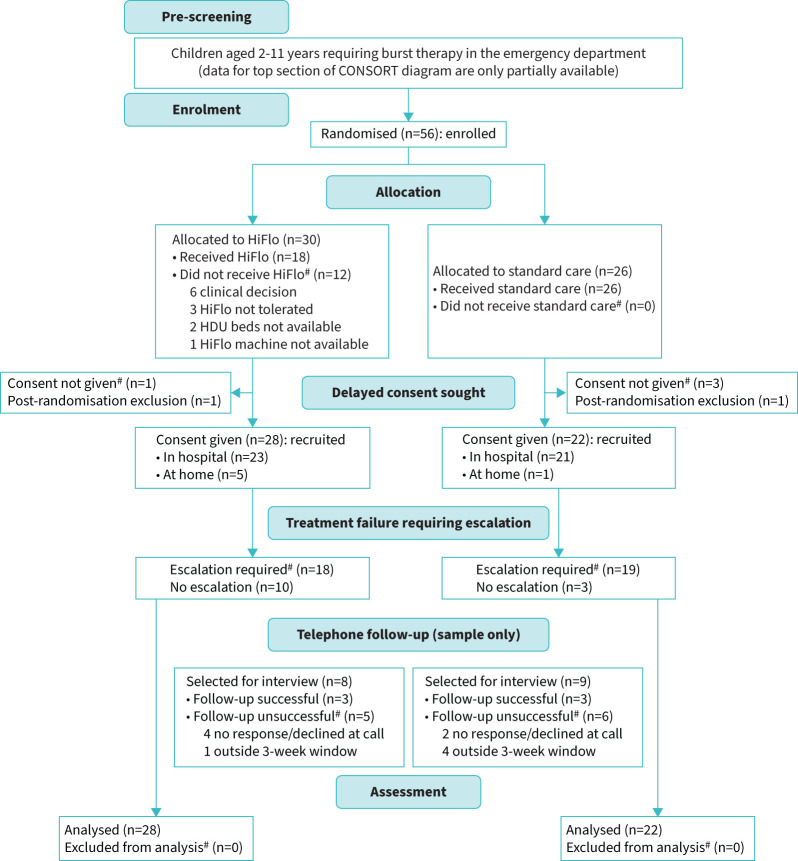
Participant flow through the trial is represented in a CONSORT flow chart (figure 2) [25]. Available cases for whom deferred consent had been obtained were analysed on an intention to treat basis. Normally distributed variables were summarised by means and standard deviations, skewed continuous variables by medians and interquartile ranges (IQR) and categorical variables by frequencies and percentages. The difference in means between trial arms for each of the candidate primary and secondary outcomes was estimated, together with bootstrapped 95% confidence intervals.
FIGURE 2.
CONSORT diagram. HiFlo: high-flow humidified oxygen; HDU: high-dependency unit.
Results
The trial opened in February 2020, but a month later was paused for 15 months during the coronavirus disease 2019 (COVID-19) pandemic due to a combination of factors including: general concerns about face-to-face research, specific concerns about HiFlo as an “aerosol generating procedure” and redeployment of research staff to clinical duties during the pandemic. The trial reopened at the lead site in July 2021 and opened at the other three sites during 2022. Recruitment closed in April 2023 when 56 children had been enrolled. The CONSORT diagram (figure 2) gives full details of numbers of children progressing through the study, and table 2 summarises participants’ baseline characteristics.
TABLE 2.
Summary of baseline characteristics by treatment group and overall
| HiFlo | Standard care | Total | |
|---|---|---|---|
| Participants | 28 | 22 | |
| Age, years | 4.8±2.3 | 5.5±3.1 | 5.1±2.7 |
| Male sex | 18 (64.3) | 14 (63.6) | 32 (64) |
| Ethnicity | |||
| Asian | 1 | 3 | 4 |
| Black | 2 | 2 | 4 |
| White | 22 | 16 | 38 |
| Mixed/other | 2 | 1 | 3 |
| Previous wheeze | |||
| No | 6 | 7 | 13 |
| Only with colds | 13 | 13 | 26 |
| With and between colds | 7 | 1 | 8 |
| Regular inhaled corticosteroid | 14 | 5 | 19 |
| Atopy (hay fever/eczema) | 15 | 8 | 23 |
| Family history of atopy | 17 | 12 | 29 |
Data are presented as n, mean±sd or n (%). HiFlo: high-flow humidified oxygen.
Feasibility outcomes
Enrolment rate amongst eligible children
Owing to disruption by the COVID-19 pandemic, it was not possible to collect data on all children presenting to all sites who met eligibility criteria; however, data were collected for sample periods at three sites. Of 254 children who required “burst” therapy, 60 met eligibility criteria and 30 (50%) were enrolled.
Deferred consent rate amongst enrolled children
Deferred consent was received on 50 of 56 children enrolled (89%): 44 families gave consent face-to-face in hospital, and a further six after telephone contact following discharge. Only one family actively declined consent; in the remaining five, consent could not be obtained in hospital and the family then could not be contacted after discharge.
The overall recruitment process (enrolment plus deferred consent) resulted in a recruitment rate of 1.1 children per site per month of being open for recruitment.
Feasibility of recording outcome measures
Table 3 shows the completeness (%) of data collection for the candidate primary and secondary outcome measures. For the two candidate primary outcome measures, data were collected on all 50 children recruited, and data collection was over 80% complete for all but one of the candidate secondary outcome measures.
TABLE 3.
Completeness of candidate primary and secondary outcome data collection
| Candidate outcome | Outcome recorded, n (%) |
|---|---|
| Primary | |
| Treatment failure needing escalation of therapy | 50 (100) |
| Time to meeting discharge criteria | 50 (100) |
| Secondary | |
| Time to actual hospital discharge | 50 (100) |
| Time to achieving PRAM score 3 or less | 41 (82) |
| Time to no longer requiring oxygen or respiratory support | 37 (74) |
| Need for and duration of i.v. therapy | 49 (98) |
| Need for noninvasive/invasive ventilation | 50 (100) |
| Treatment-related adverse effects | 50 (100) |
| Readmission within 48 h of discharge | 50 (100) |
| End-of-study questionnaire received | 42 (84) |
PRAM: Paediatric Respiratory Assessment Measure; i.v.: intravenous.
Proportion receiving allocated treatment
As expected, all 26 enrolled children allocated to standard care received this treatment; some of these children later received rescue HiFlo as an allowed escalation. However, of the 30 enrolled children allocated to the HiFlo arm, 12 did not have HiFlo initiated as allocated for a range of reasons (see figure 2). A significant factor in this was the lack of availability of high-dependency beds at sites where children on HiFlo could not be cared for on general wards, leading to the responsible clinician being reluctant to start HiFlo as allocated.
Data on candidate primary outcomes
Treatment failure requiring escalation of therapy
This outcome occurred in 18 of 28 children (64%) in the HiFlo arm and in 19 of 22 (86%) in the standard care arm. By far the commonest first escalation in both groups was commencing an i.v. bronchodilator: 16 in HiFlo and 16 in standard care, and in 90% the i.v. drug was magnesium sulfate. Three children in standard care and one in HiFlo (who had not commenced early HiFlo as allocated) had HiFlo commenced later as rescue, and one child in the HiFlo arm was escalated to noninvasive ventilation.
Time to meeting discharge criteria
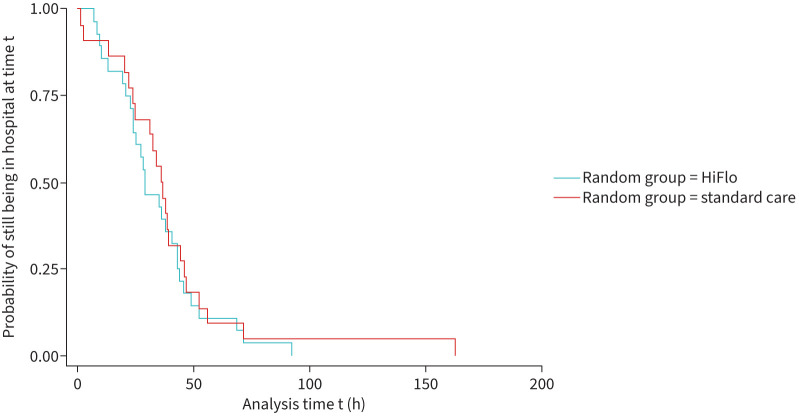
Median (IQR) time from randomisation to meeting discharge criteria was 29.3 h (21.8–43.7 h) in the HiFlo arm and 36.8 h (24.1–46.3 h) in the standard care arm. Figure 3 shows the Kaplan–Meier curves for this outcome, showing overlap with crossing points.
FIGURE 3.
Kaplan–Maier curves for time between randomisation and readiness for discharge. HiFlo: high-flow humidified oxygen.
Variance of outcomes
Table 4 shows the estimated between-arm differences in candidate primary and key secondary outcome measures, with bootstrapped 95% confidence intervals for means, for the HiFlo and standard care arms, with number of children these are based on.
TABLE 4.
Estimated mean differences (HiFlo minus standard care) with bootstrapped 95% confidence intervals for candidate outcomes
| Outcome | n | Estimated difference | 95% CI |
|---|---|---|---|
| Treatment failure needing escalation of therapy, % | 50 | −23 | −46–0 |
| Time to meeting discharge criteria, h | 50 | −5.5 | −20.3–9.2 |
| Time to actual hospital discharge, h | 50 | −1.8 | −17.4–13.9 |
| Time to achieving PRAM score 3 or less, h | 41 | 1.5 | −1.4–8.5 |
| Time to no longer requiring oxygen or respiratory support, h | 37 | 10.8 | 2.5–19.1 |
HiFlo: high-flow humidified oxygen; PRAM: Paediatric Respiratory Assessment Measure.
Table 5 summarises the results of the ESQ completed by parents/carers. The full questionnaire results from parents and children are given in the online supplementary material.
TABLE 5.
Summary results from end-of-study questionnaire (completed by 42 parents/carers)
| Statement | % responding “agree” or “strongly agree” | ||
|---|---|---|---|
| HiFlo | Standard | Total | |
| I understand that my child was in a research study during their hospital visit | 96 | 90 | 93 |
| I was aware of the study before a member of the research team spoke to us | 45 | 37 | 42 |
| I did not notice the study posters displayed in the hospital | 67 | 78 | 72 |
| I understood the treatment my child received | 100 | 100 | 100 |
| I understood the need for study randomisation to either treatment | 100 | 100 | 100 |
| I understood what deferred consent means | 100 | 100 | 100 |
| I understood why deferred consent was used | 100 | 100 | 100 |
| The treatment my child received was effective | 100 | 100 | 100 |
| The treatment my child received was unpleasant | 30 | 31 | 30.5 |
HiFlo: high-flow humidified oxygen.
In-depth telephone interviews were conducted with six parents/carers and 11 HCPs across the sites: all had given prior consent to be contacted, and the small number of parent/carer interviews reflected the difficulty in contacting parents within the pre-specified 3-week window after discharge. The detailed qualitative data report from the interviews of parents and HCPs is given in the online supplementary material.
The three core themes which emerged from the parent/carer interviews centred on: 1) the child's experiences and differences from previous admissions; 2) reassurance about treatment approaches; and 3) timeliness and clarity of information. They described their child's initial discomfort with the unfamiliar HiFlo treatment but felt that this resolved quickly. They suggested ways that younger children could be helped: “Maybe if they had a teddy bear or something they could just show, “Oh look, here's teddy with these tubes stuck on his face. We're going to do this for you”.” They emphasised the importance of reassuring parents and carers that the treatments being studied are not experimental but routinely used. In keeping with the questionnaire responses, they were generally happy with the concept and procedure of deferred consent, but most had not seen the posters displayed in the ED and flagged up the need for other approaches to pre-informing families about the study.
The HCP interviews brought out challenges that would need to be met and adaptations that might be needed for a definitive trial, focussing on 1) time and resource constraints, 2) treatment familiarity and preferences and 3) impact of unfamiliar assessment tools. HCPs expressed frustration that at times they had been unable to recruit outside office hours and during very busy winter periods due to lack of critical care beds, and flagged up the problem of maintaining training on the study in the face of high staff turnover. Unfamiliarity with using HiFlo in the ED, and with the Vapotherm system specifically, were important issues despite the extensive training conducted: greater use of videos and WhatsApp groups were suggested. Similarly, use of the unfamiliar PRAM scoring system remained an issue despite training: scoring within the required time window and lack of trust in scores were specific problems.
Discussion
This feasibility RCT of early HiFlo in ASA in children recruited to its target despite major disruption from the COVID-19 pandemic, and demonstrates that a definitive RCT is feasible. It provides important data and learning points for planning the full-scale trial. Based on our results, a trial enrolling 117 children per group, resulting in 104 children per group with deferred consent, would give 90% power at 5% significance to detect a 20% reduction in treatment escalation. This could be achieved with nine centres each recruiting 1.1 children per month over 24 months. The second candidate primary outcome, time to meeting discharge criteria, demonstrated too much variability and overlap to allow a sensible study size calculation.
Important learning points to be taken into consideration when planning the full RCT include better strategies to inform families, the choice and role of an asthma severity score, and training and logistical issues in implementing the intervention. The deferred consent model worked well and was generally acceptable to families: the deferred consent rate of 89% was comparable with other paediatric emergency studies [21, 22], and feedback was positive, but more than two-thirds of families did not recall seeing the ED poster and were therefore not aware of the study until approached later for consent. Other strategies such as using video screens and issuing an information leaflet at triage should be considered. We used the PRAM scoring system because it has been validated across the target age range [19], and employed it as an entry criterion, a documented reason for escalation and a candidate secondary outcome measure. However, PRAM was only in clinical use at one of the four sites, and despite considerable investment in training only a minority of clinical staff became comfortable with its use. This caused issues in recruitment, especially outside office hours, led to gaps in documentation and was one factor in some children not receiving allocated treatment. A recent survey [26] found that very few units in the UK use an asthma scoring system in routine clinical care, so these issues would apply to any of the published measures, but a simpler scoring system which aligns more closely with routinely collected data would improve feasibility. Finally, unfamiliarity with HiFlo and logistical issues need to be addressed in planning the definitive RCT. The high occupancy rate for paediatric critical care beds during the peak ASA seasons means that participating sites need to be able to look after children receiving HiFlo on general paediatric wards.
Provided these issues are addressed, we have demonstrated that it will be feasible to carry out a definitive RCT to determine whether early use of HiFlo improves outcomes in children with ASA who fail to respond to first-line therapy.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00168-2024.SUPPLEMENT (1.7MB, pdf)
Acknowledgements
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-1217-20024). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. We would like to acknowledge the support of Vapotherm Inc. who loaned HiFlo equipment for the study, and Aerogen Ltd who loaned nebuliser equipment. We would also like to acknowledge the skill and commitment of the clinical and research teams at all study sites in recruitment and data gathering, and specifically Kevin Donnelly, Vivien Richmond, Dan Yusef and Emma Taglivini (University Hospitals Sussex NHS Foundation Trust, Brighton), Amber Cook and Emy van der Harg (University Hospital Southampton NHS Foundation Trust, Southampton), Elsa Mathew (Leicester Royal Infirmary, Leicester) and Daisy Stroud, Emma Clarey and Nia Williams (King's College Hospital NHS Foundation Trust, London). Finally, thanks to Romanie Hannah and Becky Ramsay (University Hospitals Sussex NHS Foundation Trust, Brighton) for help in designing outcome tools, and to Chloe Bruce, Debbie Lambert and Amy Arbon (Brighton and Sussex Clinical Trials Unit, Brighton) for their support and guidance with data management and quality assurance oversight.
Provenance: Submitted article, peer reviewed.
This study is registered with ISRCTN, identifier 78297040.
Ethics statement: The main ethical considerations were: 1) the inclusion of participants (children) unable to give informed consent; 2) the inclusion of participants in an emergency care situation; and 3) the deferred consent model. All these aspects were considered in detail by the NHS Research Ethics Committee (West Midlands – Solihull Research Ethics Committee; reference: 19/WM/0219, IRAS: 261627).
Conflict of interest: A. Kapur has nothing to disclose.
Conflict of interest: H. Rojas-Anaya has nothing to disclose.
Conflict of interest: G. Roberts has nothing to disclose.
Conflict of interest: D. Roland has nothing to disclose.
Conflict of interest: A. Gupta has nothing to disclose.
Conflict of interest: M. Lazner was an advisory board member for Aerogen Ltd for non-HiFlo study-related research and received an honorarium of £952.89 for this work.
Conflict of interest: J. Bayreuther has nothing to disclose.
Conflict of interest: F. Cantle has nothing to disclose.
Conflict of interest: C. Jones has nothing to disclose.
Conflict of interest: J. Pappachan has nothing to disclose.
Conflict of interest: S. Bremner has nothing to disclose.
Conflict of interest: D. James has nothing to disclose.
Conflict of interest: S. Fitzgerald has nothing to disclose.
Conflict of interest: K. Owens has nothing to disclose.
Conflict of interest: L. Asim has nothing to disclose.
Conflict of interest: E. Khaleva has nothing to disclose.
Conflict of interest: P. Seddon, in March 2023, chaired an advisory board (unrelated to HiFlo) for Aerogen Ltd, which loaned nebuliser equipment for the study, and received an honorarium.
Support statement: This study was supported by the National Institute for Health Research, Research for Patient Benefit grant PB-PG-1217-20024. Funding information for this article has been deposited with the Crossref Funder Registry.
Data availability
Individual participant data which underlies the results reported will be made available after de-identification, beginning 3 months and ending 5 years after article publication, to researchers who provide a methodologically sound proposal to the corresponding author.
References
- 1.Chawla J, Seear M, Zhang T, et al. . Fifty years of pediatric asthma in developed countries: how reliable are the basic data sources? Pediatr Pulmonol 2012; 47: 211–219. doi: 10.1002/ppul.21537 [DOI] [PubMed] [Google Scholar]
- 2.Merrill C, Owens PL. Reasons for being admitted to the hospital through the emergency department for children and adolescents, 2004: Statistical Brief #33. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, Agency for Healthcare Research and Quality, 2007. www.ncbi.nlm.nih.gov/books/NBK61976/ [PubMed] [Google Scholar]
- 3.Kallis C, Maslova E, Morgan AD, et al. . Recent trends in asthma diagnosis, preschool wheeze diagnosis and asthma exacerbations in English children and adolescents: a SABINA Jr study. Thorax 2023; 78: 1175–1180. doi: 10.1136/thorax-2022-219757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha I, Latchem S, Amusan L, et al. National Asthma and COPD Audit Programme: Children and young people asthma clinical and organisational audit 2019/20. Combined clinical and organisational audit of children and young people asthma services in England, Scotland and Wales. London, Royal College of Physicians, 2021. www.nrap.org.uk/NRAP/welcome.nsf/0/FD67A41147AF27E4802586D80055CC2F/$file/CYPAA_combined_national_clinical_and_organisational_audit_report_May_2021.pdf [Google Scholar]
- 5.British Thoracic Society ( BTS)/Scottish Intercollegiate Guidelines Network (SIGN). SIGN 158: British Guideline on the Management of Asthma. 2019. www.sign.ac.uk/media/1773/sign158-updated.pdf
- 6.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention, 2023. Date last accessed: 1 August 2024. www.ginasthma.org
- 7.Haq I, Gopalakaje S, Fenton AC, et al. . The evidence for high flow nasal cannula devices in infants. Paediatr Respir Rev 2014; 15: 124–134. [DOI] [PubMed] [Google Scholar]
- 8.Hilliard TN, Archer N, Laura H, et al. . Pilot study of vapotherm oxygen delivery in moderately severe bronchiolitis. Arch Dis Child 2012; 97: 182–183. doi: 10.1136/archdischild-2011-301151 [DOI] [PubMed] [Google Scholar]
- 9.Kepreotes E, Whitehead B, Attia J, et al. . High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet 2017; 389: 930–939. doi: 10.1016/S0140-6736(17)30061-2 [DOI] [PubMed] [Google Scholar]
- 10.Franklin D, Babl FE, Schlapbach LJ, et al. . A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med 2018; 378: 1121–1131. doi: 10.1056/NEJMoa1714855 [DOI] [PubMed] [Google Scholar]
- 11.Ramnarayan P, Richards-Belle A, Drikite L, et al. . Effect of high-flow nasal cannula therapy vs continuous positive airway pressure therapy on liberation from respiratory support in acutely ill children admitted to pediatric critical care units: a randomized clinical trial. JAMA 2022; 328: 162–172. doi: 10.1001/jama.2022.9615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao KY, Chien YH, Mu SC. High-flow nasal cannula in children with asthma exacerbation: a review of current evidence. Paediatr Respir Rev 2021; 40: 52–57. [DOI] [PubMed] [Google Scholar]
- 13.Baudin F, Buisson A, Vanel B, et al. . Nasal high flow in management of children with status asthmaticus: a retrospective observational study. Ann Intensive Care 2017; 7: 55. doi: 10.1186/s13613-017-0278-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez Martinez F, Gonzalez Sanchez MI, Toledo Del Castillo B, et al. . Tratamiento con oxigenoterapia de alto flujo en las crisis asmáticas en la planta de hospitalización de pediatría: nuestra experiencia [Treatment with high-flow oxygen therapy in asthma exacerbations in a paediatric hospital ward: experience from 2012 to 2016]. An Pediatr (Engl Ed) 2019; 90: 72–78. doi: 10.1016/j.anpedi.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 15.Pilar J, Modesto IAV, Lopez-Fernandez YM, et al. . High-flow nasal cannula therapy versus non-invasive ventilation in children with severe acute asthma exacerbation: an observational cohort study. Med Intensiva 2017; 41: 418–424. doi: 10.1016/j.medin.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Ballestero Y, De Pedro J, Portillo N, et al. . Pilot clinical trial of high-flow oxygen therapy in children with asthma in the emergency service. J Pediatr 2018; 194: 204–210.e3. doi: 10.1016/j.jpeds.2017.10.075 [DOI] [PubMed] [Google Scholar]
- 17.Gauto Benítez R, Morilla Sanabria LP, Pavlicich V, et al. . High flow nasal cannula oxygen therapy in patients with asthmatic crisis in the pediatric emergency department. Rev Chil Pediatr 2019; 90: 642–648. [DOI] [PubMed] [Google Scholar]
- 18.Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): a responsive index of acute asthma severity. J Pediatr 2000; 137: 762–768. doi: 10.1067/mpd.2000.110121 [DOI] [PubMed] [Google Scholar]
- 19.Ducharme FM, Chalut D, Plotnick L, et al. . The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. J Pediatr 2008; 152: 476–480.e1. doi: 10.1016/j.jpeds.2007.08.034 [DOI] [PubMed] [Google Scholar]
- 20.Rojas-Anaya H, Kapur A, Roberts G, et al. . High-flow humidified oxygen as an early intervention in children with acute severe asthma: protocol for a feasibility randomized controlled trial. JMIR Res Protoc 2024; 13: e54081. doi: 10.2196/54081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harron K, Woolfall K, Dwan K, et al. . Deferred consent for randomized controlled trials in emergency care settings. Pediatrics 2015; 136: e1316–e1322. doi: 10.1542/peds.2015-0512 [DOI] [PubMed] [Google Scholar]
- 22.Lyttle MD, Rainford NEA, Gamble C, et al. . Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet 2019; 393: 2125–2134. doi: 10.1016/S0140-6736(19)30724-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012; 65: 301–308. doi: 10.1016/j.jclinepi.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 24.Teare MD, Dimairo M, Shephard N, et al. . Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014; 15: 264. doi: 10.1186/1745-6215-15-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldridge SM, Chan CL, Campbell MJ, et al. . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016; 355: i5239. doi: 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chacko J, King C, Harkness D, et al. . Pediatric acute asthma scoring systems: a systematic review and survey of UK practice. J Am Coll Emerg Physicians Open 2020; 1: 1000–1008. doi: 10.1002/emp2.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00168-2024.SUPPLEMENT (1.7MB, pdf)
Data Availability Statement
Individual participant data which underlies the results reported will be made available after de-identification, beginning 3 months and ending 5 years after article publication, to researchers who provide a methodologically sound proposal to the corresponding author.