Abstract
Purpose
Skin pigmentation influences peripheral oxygen saturation (SpO2) compared to arterial saturation of oxygen (SaO2). Occult hypoxemia (SaO2 ≤ 88% with SpO2 ≥ 92%) is associated with increased in-hospital mortality in venovenous-extracorporeal membrane oxygenation (VV-ECMO) patients. We hypothesized VV-ECMO cannulation, in addition to race/ethnicity, accentuates the SpO2-SaO2 discrepancy due to significant hemolysis.
Methods
Adults (≥ 18 years) supported with VV-ECMO with concurrently measured SpO2 and SaO2 measurements from over 500 centers in the Extracorporeal Life Support Organization Registry (1/2018–5/2023) were included. Multivariable logistic regressions were performed to examine whether race/ethnicity was associated with occult hypoxemia in pre-ECMO and on-ECMO SpO2-SaO2 calculations.
Results
Of 13,171 VV-ECMO patients, there were 7772 (59%) White, 2114 (16%) Hispanic, 1777 (14%) Black, and 1508 (11%) Asian patients. The frequency of on-ECMO occult hypoxemia was 2.0% (N = 233). Occult hypoxemia was more common in Black and Hispanic patients versus White patients (3.1% versus 1.7%, P < 0.001 and 2.5% versus 1.7%, P = 0.025, respectively). In multivariable logistic regression, Black patients were at higher risk of pre-ECMO occult hypoxemia versus White patients (adjusted odds ratio [aOR] = 1.55, 95% confidence interval [CI] = 1.18–2.02, P = 0.001). For on-ECMO occult hypoxemia, Black patients (aOR = 1.79, 95% CI = 1.16–2.75, P = 0.008) and Hispanic patients (aOR = 1.71, 95% CI = 1.15–2.55, P = 0.008) had higher risk versus White patients. Higher pump flow rates (aOR = 1.29, 95% CI = 1.08–1.55, P = 0.005) and on-ECMO 24-h lactate (aOR = 1.06, 95% CI = 1.03–1.10, P < 0.001) significantly increased the risk of on-ECMO occult hypoxemia.
Conclusion
SaO2 should be carefully monitored if using SpO2 during ECMO support for Black and Hispanic patients especially for those with high pump flow and lactate values at risk for occult hypoxemia.
Keywords: Pulse oximetry, Arterial blood gas, Venovenous extracorporeal membrane oxygenation, Racial/ethnical disparities, Hypoxemia
Introduction
Arterial blood gas (ABG) analysis is the gold-standard method to measure arterial oxygen saturation (SaO2) as it directly measures the partial pressure of oxygen in arterial blood [1]. Pulse oximetry is a standard non-invasive continuous method of monitoring peripheral oxygen saturation (SpO2) via measurement of hemoglobin saturation through spectroscopy. Darker skin pigmentation may worsen occult hypoxemia (previously defined as SaO2 ≤ 88% despite SpO2 ≥ 92%) [2] as was seen in a cohort of 10,001 intensive care unit (ICU) patients where Black patients experienced occult hypoxemia threefold more than White patients [3]. In a study of respiratory failure patients 6 h before extracorporeal membrane oxygenation (ECMO) cannulation, the frequency of pre-ECMO occult hypoxemia in Black patients was higher than in White patients [2]. However, this study analyzed only pre-ECMO oxygen saturation values and did not account for clinically relevant covariates, such as hemolysis or vasopressor usage.
In addition to skin pigmentation bias, SpO2 is also unable to distinguish between oxygen bound to hemoglobin (oxyhemoglobin), carbon monoxide bound to hemoglobin (carboxyhemoglobin), or oxidized hemoglobin (methemoglobin or sulfhemoglobinemia), which can further cause inaccurate pulse oximetry readings [4, 5]. This is particularly important in ECMO patients, where accurate oxygenation measurements are critical and their complex physiology [6] can further influence the accuracy of these measurements. Specifically, high ECMO pump flow rate and larger cannula size [7] can cause hemolysis due to mechanical shearing of red blood cells [8–10], leading to formation of carboxyhemoglobin and thus causing inaccurate SpO2 measurements. This phenomenon was observed in a cohort of 40 venovenous (VV)-ECMO patients; however, this study is limited by small sample size and did not account for ECMO-relevant covariates or race/ethnicity in their analyses. [11]
We sought to address these previous limitations using concurrently measured SpO2 and SaO2 data points within a multicenter, international cohort of ECMO patients, to examine for discrepancy by race/ethnicity. In addition, we hypothesized that ECMO-induced hemolysis would worsen occult hypoxemia in VV-ECMO patients. Finally, as the single-lumen cannula may require greater resistance of perfusion and overall pump flow to maintain adequate perfusion, we also hypothesized that single versus double-lumen cannulation strategies may impact the SpO2-SaO2 discrepancy.
Methods
Study Design and Population
This study was approved by the Johns Hopkins Hospital Institutional Review Board (IRB00216321) on 10/22/2019. The study title is “Retrospective Analysis of Outcomes of Patients on Extracorporeal Membrane Oxygenation”. All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975. The Extracorporeal Life Support Organization (ELSO) Registry is an international multicenter registry from over 500 ECMO centers [12]. The Registry collects demographic information, pre-ECMO comorbidities, pre-ECMO and on-ECMO laboratory and hemodynamic information, on-ECMO complications, and outcomes. Comorbidity information was recorded using International Classification of Diseases, 10th Revision (ICD-10) codes.
We included patients who were (1) 18 years of age or older; (2) supported with VV-ECMO; and (3) had data on race/ethnicity. We excluded repeat ECMO runs within individual patients, patients without data on hypoxemia, and patients with extreme outlier values for the difference between SpO2 and SaO2.
Definitions
SpO2 was defined as the non-invasive pulse oximeter measured oxyhemoglobin saturation, while SaO2 was the percent arterial blood oxyhemoglobin saturation from ABG. Pre-ECMO ventilator-type settings included conventional ventilation, high-frequency oscillatory ventilation, other high-frequency ventilation (high-frequency jet ventilation or percussive ventilation), other non-specified ventilations, and no ventilation. Pre-ECMO mechanical circulatory support included intra-aortic balloon pump, Impella®, and left ventricular assist device. Pre-ECMO vasopressor infusions included dopamine, epinephrine, norepinephrine, phenylephrine, and vasopressin. Pre-ECMO inotrope infusions included dobutamine, enoximone, levosimendan, milrinone, nicardipine, nitroglycerin, nitroprusside, and tolazoline. Infusions were treated as a binary covariate (i.e., the presence or absence of the infusion). Pre-ECMO vasopressor and inotrope infusions were employed for at least 6 h within 24 h of the start of ECMO cannulation.
Single-lumen cannulation involves placement in two vascular access sites and double-lumen cannulation involves placement of a cannula in single vascular access site. Definitions for on-ECMO complications are provided in the eMethods.
Race/ethnicity was coded per patient as one of Asian, Black, Hispanic, Middle Eastern or North African, Native American, Native Pacific Islander, Multiple, Other, Unknown, and White. We restricted our primary analysis to Asian, Black, Hispanic, and White patients. White patients were chosen as the reference comparator based on previous literature showing the first pulse oximeters were calibrated to this race/ethnicity. [13]
Outcomes
Occult hypoxemia was defined as SaO2 ≤ 88% with a time-matched SpO2 ≥ 92% [2]. The primary outcomes were the occult hypoxemia (binary variable) and SpO2-SaO2 discrepancy (continuous variable), which were compared between different races/ethnicities. We also assessed the accuracy and precision for SpO2 to predict SaO2.
Statistical Analysis
Continuous variables were assessed for normality with the Kolmogorov–Smirnov test, and all were determined to be not normally distributed. Therefore, these variables are denoted as median with interquartile range (IQR). Categorical variables are represented as frequency with percentages. The Kruskal–Wallis, Wilcoxon rank-sum, and Pearson chi-square tests were utilized to compare continuous and categorical variables, respectively. SpO2–SaO2 differences were compared with Kruskal–Wallis and Wilcoxon rank-sum tests. A P value < 0.05 was considered statistically significant (eMethods contain more details).
Bland–Altman analyses were used to assess agreement between SpO2 and the gold standard, SaO2, by calculating the difference between SpO2 and SaO2, the mean of SpO2 and SaO2, the estimated bias (median difference), and limits of agreement (median and 95% limits of agreement at 2.5th and 97.5th percentiles) using a nonparametric method to estimate the limits given the non-normality of the differences between SpO2 and SaO2. The one-sample Wilcoxon signed-rank test was used to compare the median difference between SpO2 and SaO2 (estimated bias) for Asian, Black, and Hispanic patient groups as compared to the median value for White patients (hypothetical value set at 0). Boxplots were performed to visually assess the SpO2–SaO2 discrepancy, while scatterplots were used to visually assess the correlation between SpO2 and SaO2. Spearman correlations were conducted for pre-ECMO and on-ECMO SpO2 and SaO2 by race/ethnicity.
Multivariable logistic regressions were performed to examine whether race/ethnicity was associated with occult hypoxemia in pre-ECMO and on-ECMO measurements. One pre-ECMO SpO2–SaO2 pair and a corresponding on-ECMO SpO2–SaO2 pair were measured from the same patient for adequate comparison. We selected covariates a priori that were hypothesized to be associated with the SpO2–SaO2 discrepancy based on clinical judgment and prior data. Covariates in both the pre-ECMO and on-ECMO models included age, sex, presence of pre-ECMO temporary mechanical circulatory support (tMCS), and presence of pre-ECMO vasopressor and inotrope infusions. The on-ECMO model also included hemolysis, hyperbilirubinemia, cannulation strategy (single- versus double lumen), ECMO pump flow rate, and on-ECMO serum lactate value. Adjusted odds ratios (OR) are presented with 95% confidence intervals (CIs).
Receiver-operating characteristic curve (ROC) analyses were performed to determine the accuracy of on-ECMO SpO2 in predicting SaO2. Based on previous literature [14–17], the following on-ECMO SaO2 thresholds were tested: 88%, 92%, and 95%. All SpO2 values were tested to detect the specific SaO2 threshold at or below its value. Area under the receiver-operating characteristic curve (AUC), sensitivity, and specificity were calculated. In addition, the AUC for each SaO2 threshold was compared by race/ethnicity groups. All statistical analyses were performed using R Studio (R 4.1.2, www.r-project.org) or IBM SPSS Statistics for Windows, Version 28.0 (IBM Corp, Armonk, NY).
Results
Study Population
Of 30,407 VV-ECMO patients, we included 13,171 patients for our study after applying the inclusion and exclusion criteria (Fig. 1). Of 13,171 VV-ECMO patients (median age = 48.6 years, 66% male), 7772 (59%) were White, 2114 (16%) were Hispanic, 1777 (14%) were Black, and 1,508 (11%) were Asian (Table 1). Of 9851 patients with valid COVID-19 data, 59% (N = 5830) had a diagnosis of SARS CoV-2 and COVID-19. The median ECMO duration was 11.4 days (IQR = 5.7–22.9). Of patients with complete cannulation information (N = 12,966), 9,833 (76%) received single-lumen VV-ECMO versus 3133 (24%) received double-lumen VV-ECMO. Black patients were less likely to have single-lumen cannulation (N = 1206, 69%) compared to other races/ethnicities.
Fig. 1.
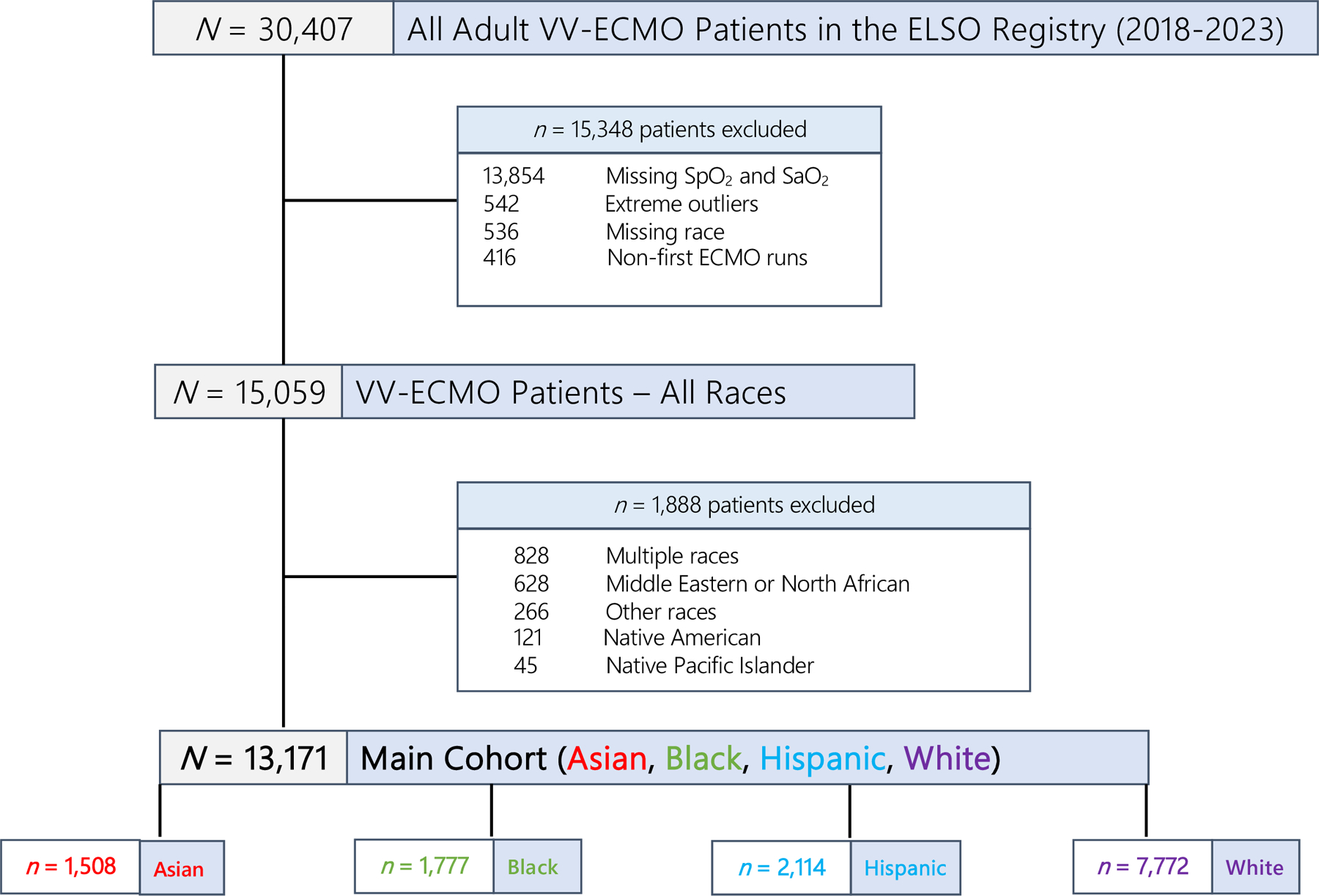
Creation of study cohort from the ELSO Registry (1/2018–5/2023)
Table 1.
Baseline characteristics and clinical variables of VV-ECMO patients stratified by race/ethnicity
| Total (N = 13,171) | Asian (n = 1508, 11%) | Black (n = 1777, 14%) | Hispanic (n = 2114, 16%) | White (n = 7772, 59%) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 48.6 (37.0–58.3) | 49.8 (38.7–60.7) | 44.3 (32.4–55.0) | 44.9 (35.4–54.5) | 50.2 (38.3–59.5) |
| Male | 8675 (66) | 1029 (68) | 1021 (58) | 1519 (72) | 5106 (66) |
| Body mass index, kg/m2 | 30.7 (26.0–36.4) | 25.6 (22.9–29.7) | 32.5 (26.5–38.7) | 31.1 (27.1–36.2) | 31.1 (26.5–36.9) |
| Year ECLS | |||||
| 2018 | 1626 (12) | 217 (14) | 197 (11) | 120 (6) | 1092 (14) |
| 2019 | 2096 (16) | 296 (20) | 250 (14) | 198 (9) | 1352 (17) |
| 2020 | 3756 (29) | 472 (31) | 537 (30) | 881 (42) | 1866 (24) |
| 2021 | 3909 (30) | 377 (25) | 514 (29) | 683 (32) | 2335 (30) |
| 2022 | 1576 (12) | 137 (9) | 250 (14) | 206 (10) | 983 (13) |
| 2023 | 208 (2) | 9 (1) | 29 (2) | 26 (1) | 144 (2) |
| Past medical history | |||||
| Diabetes | 1372 (10) | 96 (6) | 242 (14) | 316 (15) | 718 (9) |
| Hypertension | 1873 (14) | 112 (7) | 331 (19) | 285 (13) | 1145 (15) |
| Atrial fibrillation | 845 (6) | 36 (2) | 108 (6) | 94 (4) | 607 (8) |
| Cardiomyopathy | 177 (1) | 10 (1) | 40 (2) | 27 (1%) | 100 (1) |
| COPD | 478 (4) | 20 (1) | 46 (3) | 21 (1) | 391 (5) |
| COVID-19 status (N = 9851) | 5830 (59) | 621 (59) | 722 (53) | 1433 (79) | 3054 (54) |
| Pre-ECMO support | |||||
| tMCS | 310 (2) | 23 (2) | 71 (4) | 17 (1) | 199 (3) |
| Vasopressor infusions | 8119 (62) | 992 (66) | 1059 (60) | 1194 (57) | 4874 (63) |
| Inotrope infusions | 870 (7) | 82 (5) | 144 (8) | 113 (5) | 531 (7) |
| Pre-ECMO mean blood pressure (mm Hg) | 77 (69–88) | 78 (69–90) | 78 (69–90) | 80 (71–89) | 76 (68–87) |
| Pre-ECMO ABG | |||||
| pH | 7.28 (7.19–7.36) | 7.28 (7.19–7.36) | 7.27 (7.17–7.35) | 7.29 (7.20–7.37) | 7.28 (7.18–7.36) |
| HCO3-(mEq/L) | 26.6 (22.1–31.4) | 25.0 (21.0–29.6) | 26 (22.1–30.2) | 28.0 (24.0–34.0) | 26.6 (22.0–31.2) |
| PaO2 | 67 (56–84) | 65 (56–80) | 68 (53–87) | 68 (57–84) | 68 (56–84) |
| PaCO2 | 59 (48–74) | 58 (46–74) | 59 (47–74) | 60 (49–75) | 59 (48–73) |
| Lactate (mmol/L) | 1.8 (1.2–3.0) | 1.9 (1.2–3.2) | 1.8 (1.2–3.4) | 1.6 (1.2–2.4) | 1.7 (1.2–3.0) |
| SpO2 | 91 (85–95) | 90 (84–94) | 91 (84–96) | 91 (86–95) | 91 (86–95) |
| SaO2 | 91 (85–95) | 90 (84–94) | 90 (83–95) | 91 (85–95) | 91 (85–95) |
| On-ECMO mean blood pressure (mm Hg) | 76 (70–85) | 79 (71–88) | 77 (71–86) | 77 (70–85) | 75 (69–83) |
| On-ECMO pulse pressure (mm Hg) | 55 (46–66) | 52 (43–63) | 55 (45–68) | 56 (47–67) | 56 (46–67) |
| On-ECMO ABG | |||||
| pH | 7.40 (7.36–7.44) | 7.40 (7.36–7.46) | 7.40 (7.36–7.44) | 7.41 (7.37–7.45) | 7.40 (7.36–7.44) |
| HCO3-(mEq/L) | 27 (24–31) | 26 (23–29) | 26 (23–29) | 28 (24–32) | 27 (24–31) |
| PaO2 | 78 (66–100) | 83 (68–107) | 80 (67–109) | 75 (63–94) | 78 (66–98) |
| PaCO2 | 44 (38–50) | 42 (36–48) | 43 (38–48) | 44 (39–51) | 44 (39–50) |
| Lactate (mmol/L) | 1.5 (1.1–2.2) | 1.6 (1.2–2.4) | 1.5 (1.1–2.2) | 1.4 (1.1–2.0) | 1.5 (1.0–2.2) |
| SpO2 | 96 (93–98) | 97 (94–99) | 97 (93–99) | 95 (92–98) | 96 (93–98) |
| SaO2 | 95 (92–98) | 96 (93–98) | 96 (93–98) | 95 (91–97) | 95 (93–97) |
| Pump flow (4 h) | 4.10 (3.60–4.65) | 3.95 (3.40–4.36) | 4.15 (3.65–4.70) | 4.13 (3.68–4.70) | 4.15 (3.60–4.70) |
| Pump flow (24 h) | 4.14 (3.60–4.72) | 3.95 (3.40–4.42) | 4.12 (3.60–4.72) | 4.20 (3.70–4.79) | 4.20 (3.65–4.80) |
| Cannulation strategy (N = 12,966) | |||||
| Single lumen (two sites) | 9,833 (76) | 1,279 (88) | 1,206 (69) | 1,572 (75) | 5,776 (75) |
| Double lumen (one site) | 3,133 (24) | 169 (12) | 545 (31) | 525 (25) | 1,894 (25) |
| Days on ECMO support | 11.4 (5.7–22.9) | 11.3 (5.8–23.2) | 10.1 (5.3–21.2) | 15.8 (7.6–30.2) | 10.7 (5.3–21.0) |
| ECMO complications | |||||
| ECMO circuit mechanical failure | 1224 (9) | 138 (9) | 156 (9) | 237 (11) | 693 (9) |
| Renal replacement therapy | 3419 (26) | 368 (24) | 483 (27) | 490 (23) | 2078 (27) |
| Hemolysis | 659 (5) | 44 (3) | 80 (5) | 155 (7) | 380 (5) |
| Hyperbilirubinemia | 534 (4) | 56 (4) | 69 (4) | 114 (5) | 295 (4) |
| Cardiac arrhythmia | 1202 (9) | 93 (6) | 156 (9) | 165 (8) | 788 (10) |
| Gastrointestinal hemorrhage | 790 (6) | 92 (6) | 112 (6) | 156 (7) | 430 (6) |
ECMO extracorporeal oxygenation membrane, tMCS temporary mechanical circulatory support, VV venovenous
Occult Hypoxemia
Overall, on-ECMO occult hypoxemia was observed in 2.0% of patients (233 of 11,709). On-ECMO occult hypoxemia was more common in Black (3.1% vs 1.7%, P < 0.001) and Hispanic (2.5% vs 1.7%, P = 0.025) patients versus White patients. The proportion of on-ECMO occult hypoxemia was similar between Asian and White patients (1.6%% vs 1.7%, P = 0.787).
In a multivariable logistic regression, Black patients were at higher risk of pre-ECMO occult hypoxemia versus White patients (aOR = 1.55, 95% CI = 1.18–2.02, P = 0.001; Fig. 2A, eTable 1). No other race/ethnicity group differences were found.
Fig. 2.
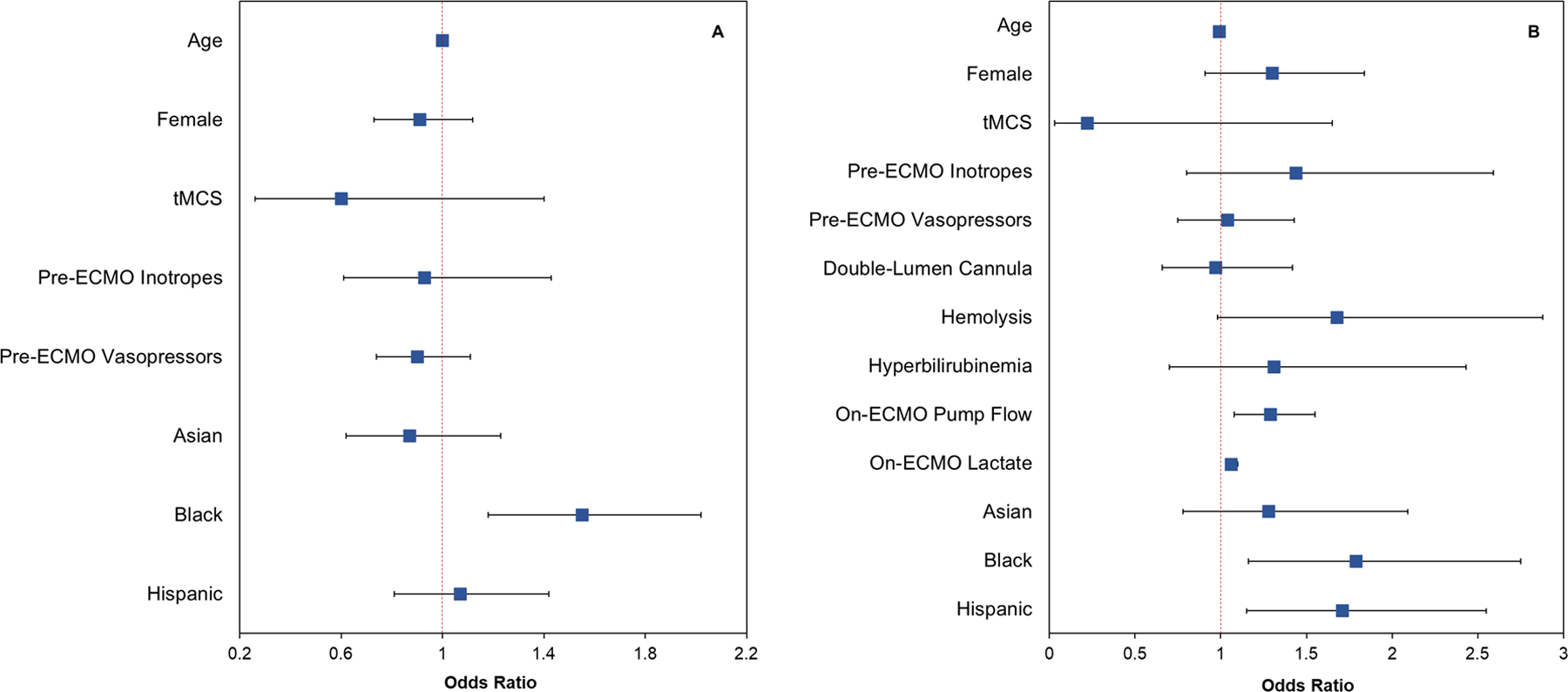
Forest plots of odds ratios and 95% confidence intervals from the multivariable logistic regression for occult hypoxemia in A pre-ECMO SpO2-SaO2 and B on-ECMO SpO2-SaO2 pairs. Pre-ECMO and on-ECMO SpO2-SaO2 measurements were extracted from the same patient
For on-ECMO occult hypoxemia, multivariable logistic regression analysis found that Black patients (aOR = 1.79, P = 0.008) and Hispanic patients (aOR = 1.71, P = 0.008) had greater risk versus White patients (Table 2, Fig. 2B). Other significant risk factors included pump flow rate at 24 h on ECMO (aOR = 1.29, P = 0.005) and on-ECMO lactate (aOR = 1.06, P < 0.001; Table 2).
Table 2.
Factors associated with on-ECMO occult hypoxemia frequencies in multivariable logistic regression analysis
| OR | 95% CI | P value | |
|---|---|---|---|
| Age | 0.99 | 0.98–1.00 | 0.069 |
| Female sex | 1.30 | 0.91–1.84 | 0.147 |
| Race/ethnicity | |||
| White | Reference | ||
| Black | 1.79 | 1.16–2.75 | 0.008 |
| Asian | 1.28 | 0.78–2.09 | 0.332 |
| Hispanic | 1.71 | 1.15–2.55 | 0.008 |
| Pre-ECMO support | |||
| Pre-ECMO tMCS | 0.22 | 0.03–1.65 | 0.142 |
| Pre-ECMO inotrope infusions | 1.44 | 0.80–2.59 | 0.228 |
| Pre-ECMO vasopressor infusions | 1.04 | 0.75–1.43 | 0.830 |
| ECMO physiology | |||
| Double-lumen cannula (one-site) | 0.97 | 0.66–1.42 | 0.874 |
| Hemolysis | 1.68 | 0.98–2.88 | 0.060 |
| Hyperbilirubinemia | 1.31 | 0.70–2.43 | 0.402 |
| Pump flow at 24 h on-ECMO | 1.29 | 1.08–1.55 | 0.005 |
| On-ECMO lactate | 1.06 | 1.03–1.10 | < 0.001 |
CI confidence interval, ECMO extracorporeal membrane oxygenation, OR odds ratio, tMCS temporary mechanical circulatory support
SpO2–SaO2 Discrepancy
Figure 3 depicts boxplots of the on-ECMO SpO2–SaO2 difference for each race/ethnicity. The overall on-ECMO estimated bias for the entire cohort was 1.0 (median difference between SpO2 and SaO2) with 95% limits of agreement at −6.0 and 7.0 (2.5th and 97.5th percentiles). In Bland–Altman plots, the limits of agreement for Asian, Black, and Hispanic patients were wider as compared to White patients (eFig. 1). In addition, the median difference of the SpO2-SaO2 difference for Asian, Black, and Hispanic patients was significantly higher than the hypothetical value set at the median for White patients. The overall Spearman correlation coefficient comparing on-ECMO SpO2 with SaO2 was moderate (rs = 0.69, P < 0.001). The Spearman correlation coefficients comparing on-ECMO SpO2 with SaO2 were relatively comparable in magnitude across the race/ethnicity groups, and all were significant (P < 0.001): Asian patients (rs = 0.69), Black patients (rs = 0.67), Hispanic patients (rs = 0.72), and White patients (rs = 0.68), but Fisher r-to-z transformation did find a significant difference in rs between White and Hispanic patients (z = − 2.84, P = 0.005). Each race/ethnicity group was found to have greater on-ECMO discrepancies as a continuous variable versus White patients after adjustment (eTable 2). Supplementary analysis of other races/ethnicities (Middle Eastern or North African, Multiple, Native American, Native Pacific Islander, and Other) was also conducted. The overall on-ECMO estimated bias for additional races/ethnicities was 1.1%. The on-ECMO estimated biases for Middle Eastern or North African, Multiple, Native American, Native Pacific Islander, and Other was 3.0%, 1.0%, 0.64%, − 1.1%, 0.47%, and 0.63%, respectively.
Fig. 3.
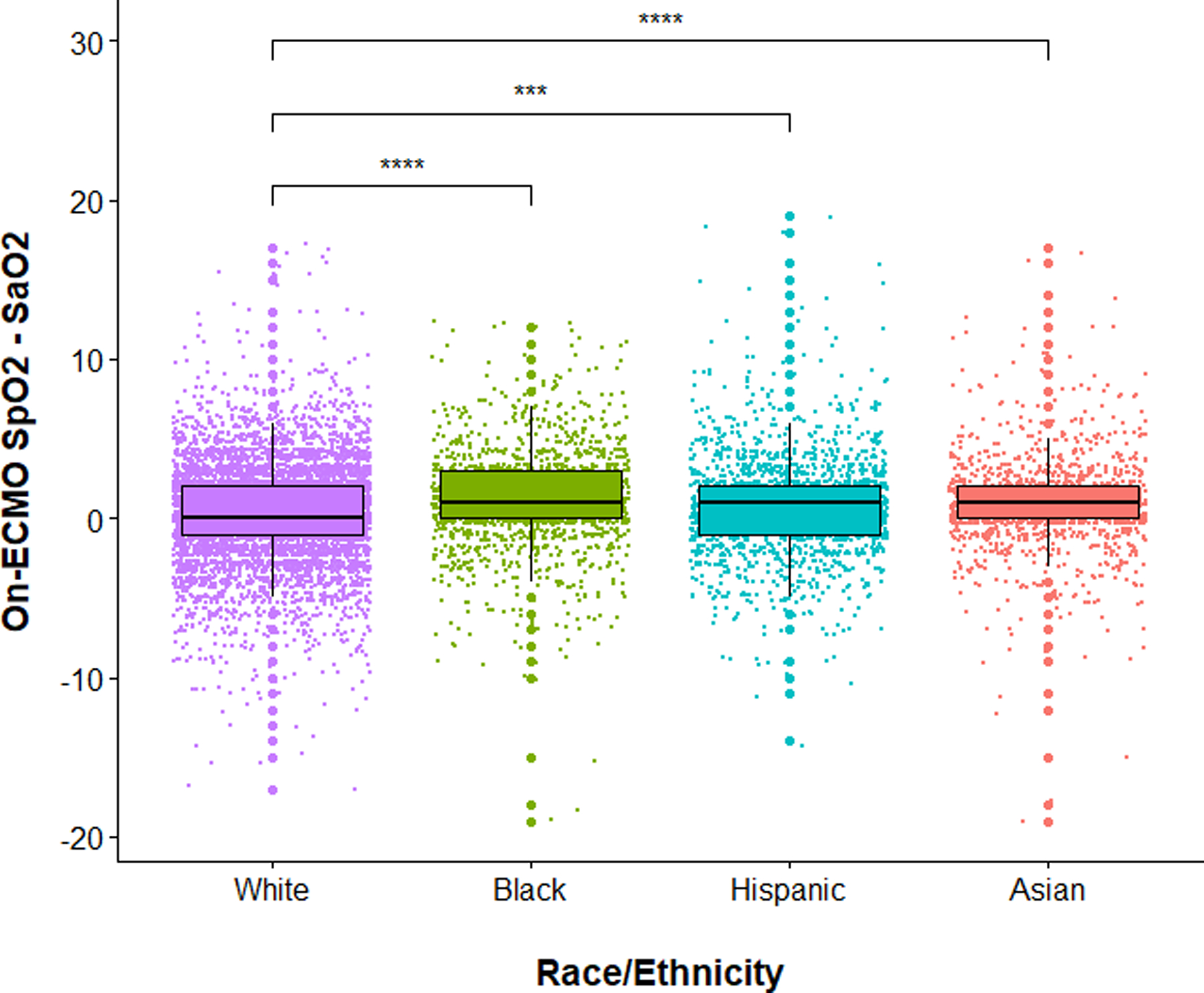
Boxplots showing pulse oximetry (SpO2) overestimates arterial blood gas (SaO2) in Black, Asian, and Hispanic venovenous (VV)-extracorporeal membrane oxygenation (ECMO) patients, compared to White VV-ECMO patients. Purple color = White patients. Green color = Black patients. Turquoise color = Hispanic patients. Red color = Asian patients. Solid black line represents the median value while the upper and lower limits of the boxes represent the 75% and 25% quartiles, respectively. White patients were used as the reference comparator. **** and *** represent P values < 0.0001 and < 0.001, respectively
On-ECMO Receiver-Operating Characteristic Curve Analyses
At an 88% SaO2 threshold, SpO2 predicted hypoxemia with an AUC of 0.89 (95% CI = 0.88–0.90), at a 92% threshold, SpO2 predicted hypoxemia with an AUC of 0.87 (95% CI = 0.86–0.88), and at a 95% threshold, SpO2 predicted hypoxemia with an AUC of 0.83 (95% CI = 0.83–0.84; eFig. 2, eResults), sensitivity of 0%, and specificity of 100%.
Exploratory Analysis: Single Lumen Versus Double Lumen
There was no difference in the incidence of on-ECMO occult hypoxemia for single-lumen versus dual-lumen cannulation (2% vs 2%, P = 0.527). However, the correlation between on-ECMO SpO2 and SaO2 was weaker for single lumen (rs = 0.68, P < 0.001) versus double lumen (rs = 0.72, P < 0.001) using Fisher r-to-z transformation to compare rs values (z = − 3.45, P < 0.001). In patients with confirmed hemolysis, there was no difference in the incidence of on-ECMO occult hypoxemia for single-lumen versus double-lumen cannulation (6% vs 4%, P = 0.463). The correlation between on-ECMO SpO2 and SaO2 was weaker for single lumen (rs = 0.67, P < 0.001) versus double lumen (rs = 0.83, P < 0.001) using Fisher r-to-z transformation to compare rs values (z = − 4.28, P < 0.001).
Exploratory Analysis: COVID-19 vs Non-COVID-19
In those with COVID-19 (N = 5,830, 59%), the incidence of pre-ECMO occult hypoxemia did not differ for patients with and without COVID-19 (4% vs 4% P = 0.993), but the incidence of on-ECMO occult hypoxemia was significantly greater for patients with COVID-19 (2.4% vs 1.5%, P = 0.003).
Sensitivity Analyses
To reduce heterogeneity, subgroup analysis was performed between different primary indications for VV-ECMO. The frequency of occult hypoxemia in the subset of VV-ECMO patients with a primary indication of ARDS was similar to that of COPD or asthma patients (2.8% vs. 0%, P = 0.31; 2.7% vs. 2.1%, P = 0.53). The frequency of hemolysis in ARDS patients was similar to that of COPD patients (5.4% vs. 2.8%, P = 0.49) but greater than that of asthma patients (5.4% vs. 1%, P = 0.008).
In sensitivity analysis of only SpO2 < 90% datapoints, the overall on-ECMO estimated bias was − 2.68%. The on-ECMO estimated bias within this subgroup was − 2.92%, − 2.96%, − 3.65%, and − 1.39% for White, Black, Asian, and Hispanic VV-ECMO patients, respectively. The frequency of occult hypoxemia was higher in older patients (≥ 48.6 years, median age) versus younger patients (< 48.6 years, 2.7% vs. 1.8%, P < 0.001). The frequency of occult hypoxemia was greater in patients with higher 4-h pump flows (≥ 4.100 L/min, median pump flow rate) versus patients with lower 4-h pump flows (< 4.100 L/min, 2.8% vs. 1.8%, P < 0.001). The frequency of occult hypoxemia was greater in patients with higher 24-h pump flows (≥ 4.15 L/min, median pump flow rate) versus patients with lower 24-h pump flows (< 4.15 L/min, 2.8% vs. 2.1%, P = 0.016).
Comment
In this retrospective observational analysis of the international multicenter ELSO Registry, we found that the frequency of unadjusted and adjusted occult hypoxemia in VV-ECMO patients was higher in Black and Hispanic patients versus White patients. After adjusting for clinically relevant risk factors, we found that ECMO support exacerbated occult hypoxemia (compared to pre-ECMO). This finding suggests that, in addition to race/ethnicity, being supported by VV-ECMO may lead to more inaccurate pulse oximetry measurements likely mediated by greater ECMO pump flow and corresponding associated hemolysis.
Pre-ECMO vs On-ECMO Occult Hypoxemia
While a previous ELSO Registry study using solely pre-ECMO SpO2 and SaO2 measurements found a similar frequency of occult hypoxemia in Hispanic patients compared to White patients [2], we found a higher frequency of occult hypoxemia in Hispanic versus White patients in “on-ECMO” SpO2 and SaO2 values. This finding suggests the SpO2-SaO2 discrepancy is worse during the time that patients were supported with ECMO. Besides one single-center study analyzing this discrepancy in 57 VV-ECMO patients [17], which found the unadjusted frequency of occult hypoxemia to be greater in Black versus White patients but not in Hispanic or Asian versus White patients, no other literature exists pertaining to this discrepancy directly for patients supported on ECMO.
Undetected hypoxemia during cannulation is important as ECMO patients are already at high risk for many complications, such as acute brain injury from inadequate oxygen delivery [18], which increases mortality [19, 20]. Occult hypoxemia may increase in-hospital mortality due to increased risk of organ dysfunction [21, 22], lung injury [23], and worse neurocognition [24]. Accordingly, monitoring for occult hypoxemia in patients with such risk factors (Black and Hispanic race/ethnicity, high pump flow, and lactate) in ECMO is extremely important.
Additionally, compared to this pre-ECMO ELSO study, our study has several strengths including (1) having a larger sample size (N = 13,171 versus N = 1562), (2) the use of methodically rigorous statistical methods such as the Kolmogorov–Smirnov test to assess for non-normality and nonparametric analyses when comparing the SpO2–SaO2 discrepancy, and (3) the adjustment of more clinically relevant and ECMO-specific covariates versus only adjusting for measured SpO2 and sex as was done in this prior study.
Cannulation Strategy, ECMO Pump Flow Rate, and Hemolysis
In contrast to previous data [6], we found no differences in the SpO2–SaO2 discrepancy between patients with single-lumen versus double-lumen, perhaps because the rates of hemolysis in our population were similar (5.5% versus 5.4%) which may be the primary driver of increased SpO2–SaO2 discrepancy via carbon monoxide production increasing carboxyhemoglobin. However, we still observed the correlation between SpO2 versus SaO2 was weaker in single lumen versus double lumen, with and without confirmed hemolysis. However, relative to single lumen, the double-lumen cannula enhanced the SpO2–SaO2 discrepancy in our multivariable linear regression analysis. Overall, further research is warranted to investigate the association between cannulation strategy and the SpO2–SaO2 discrepancy.
Although “ELSO-defined” hemolysis during ECMO was not independently associated with occult hypoxemia, higher ECMO pump flow rates were associated with higher occurrence of occult hypoxemia. This finding may indicate varying degree of hemolysis is occurring with high ECMO pump flow rates [6, 7, 25]. Due to shear mechanical stress that is produced by high pump flow rates in combination with large cannula sizes [7] throughout the ECMO circuit and oxygenator, ECMO-associated hemolysis can occur [26]. Furthermore, intravascular hemolysis, which has been reported to occur in 18% of ECMO-supported patients, can increase plasma hemoglobin [27]. This hemoglobin can then scavenge endothelial-derived nitric oxide, which causes oxidative stress and triggers inflammatory signaling pathways that can potentially worsen the discrepancy as well. [25, 28]
Limitations
In line with previous studies [2, 3, 29, 30], we duly note that race/ethnicity was used as a substitute for skin tone as clinicians do not obtain this information and this data was not available in the ELSO Registry. However, future studies examining the relationship between skin tone and the SpO2–SaO2 discrepancy are needed to confirm our results, ideally using dermatology scales, such as the Fitzpatrick skin type or Monk Skin Tone Scale. The ELSO Registry is an international consortium of over 500 ECMO centers; therefore, heterogeneity in data collection and the patient cohort inherently exists [31]. However, this diversity in sampling may be offset by the large sample size of our study. Frequency of occult hypoxemia was relatively low in our cohort; however, it was measured at a single timepoint during ECMO cannulation and may not reflect the SpO2–SaO2 discrepancy throughout the entire ECMO run, as observed in previous studies [6, 17]. Furthermore, center volume may be an important explanatory variable in our analysis but this variable was not available in this study. Additionally, there are several other limitations with the ELSO Registry including lack of granular data on skin perfusion (e.g., perfusion index, mottling score, or recapillarization time), Vasopressor Dose Equivalence scores which have previously confounded the SpO2–SaO2 discrepancy in ECMO patients [6], timestamps for when SpO2 and SaO2 data were collected, or specific markers for hemolysis, such as haptoglobin, lactate dehydrogenase, or free hemoglobin. Information regarding bridge to transplantation as an indication for VV-ECMO was also not available in this study, which could confound our results as these patients may be able to better tolerate hypoxemia. Outcome data such as mortality was also not available, but is likely important for future studies to determine if occult hypoxemia in ECMO patients is associated with worse clinical outcomes. Finally, prospective observational studies are needed to assess causation effects and appropriately suggest a correction factor by race/ethnicity for pulse oximeters.
Conclusion
Black and Hispanic VV-ECMO patients are at higher risk for occult hypoxemia and overestimated true oxygen saturation measurements compared to White patients. Patients who were supported with ECMO had an increased risk of the SpO2–SaO2 discrepancy compared to the pre-ECMO SpO2–SaO2 discrepancy, which was greater in non-White patients. As occult hypoxemia and SpO2–SaO2 discrepancy may worsen in patients with ECMO support, clinicians should carefully monitor ABGs during ECMO support for those with such risk factors.
Supplementary Material
Funding
SPK is supported by NHLBI (5K08HL14332). SMC is supported by NHLBI (1K23HL157610).
Declarations
Conflict of Interest Dr. Tonna is supported by a Career Development Award from the National Institutes of Health/National Heart, Lung, And Blood Institute (K23HL141596). Dr. Tonna is the Chair of the Registry Committee of the Extracorporeal Life Support Organization (ELSO). Dr. Brodie receives research support from and consults for LivaNova. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira, and Cellenkos. He is the President-elect of the Extracorporeal Life Support Organization (ELSO) and the Chair of the Executive Committee of the International ECMO Network (ECMONet) and he writes for UpToDate. Dr. Lorusso is a consultant for Medtronic, LivaNova, Getinge, and ASbiomed and Member of the Medical Advisory Board of Eurosets and Xenios. He is the ELSO Research Committee Chair, and Honorary Secretary of EuroELSO. The authors do not have any additional conflicts of interest to declare.
Abbreviations
- ABG
Arterial blood gas
- aOR
Adjusted odds ratio
- AUC
Area under the receiver-operating characteristic curve
- CI
Confidence interval
- COVID-19
Coronavirus Disease 19
- ECMO
Extracorporeal membrane oxygenation
- ELSO
Extracorporeal Life Support Organization
- IQR
Interquartile range
- PaO2
Partial pressure of oxygen
- ROC
Receiver-operating characteristic
- SaO2
Oxygen saturation measured by arterial blood gas
- SpO2
Oxygen saturation measured by pulse oximetry
- SD
Standard deviation
- tMCS
Temporary mechanical circulatory support
- VV-ECMO
Venovenous ECMO
Footnotes
Extended author information available on the last page of the article
Publication History: This manuscript was uploaded to the preprint server “Research Square” with the following DOI: https://doi.org/10.21203/rs.3.rs-3617237/v1.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00408-024-00711-4.
IRB Approval This study was approved by the Johns Hopkins Hospital Institutional Review Board (IRB00216321) on 10/22/2019. The study title is “Retrospective Analysis of Outcomes of Patients on Extracorporeal Membrane Oxygenation.” All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975.
References
- 1.Castro D, Patil SM, Keenaghan M (2022) Arterial blood gas. Stat-Pearls. StatPearls Publishing, Treasure Island: (Copyright © 2022, StatPearls Publishing LLC) [PubMed] [Google Scholar]
- 2.Valbuena VSM, Barbaro RP, Claar D et al. (2022) Racial bias in pulse oximetry measurement among patients about to undergo extracorporeal membrane oxygenation in 2019–2020. Chest 161:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS (2020) Racial bias in pulse oximetry measurement. N Engl J Med 383:2477–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jubran A (1999) Pulse oximetry. Crit Care 3:R11–r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley RG, Aks SE, Eshom JL, Rydman R, Schaider J, Shayne P (1994) The pulse oximetry gap in carbon monoxide intoxication. Ann Emerg Med 24:252–255 [DOI] [PubMed] [Google Scholar]
- 6.Kalra A, Shou BL, Zhao D et al. (2024) ECMO physiological factors influence pulse oximetry and arterial oxygen saturation discrepancies. Ann Thorac Surg 117:1221–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauri T, Spinelli E, Ibrahim Q et al. (2023) Impact of drainage cannula size and blood flow rate on the outcome of patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: an ELSO registry analysis. Am J Respir Crit Care Med 208:105–107 [DOI] [PubMed] [Google Scholar]
- 8.Tripathi RS, Papadimos TJ (2011) ECMO and endogenous carboxyhemoglobin formation. Int J Crit Illn Inj Sci 1:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appelt H, Philipp A, Mueller T et al. (2020) Factors associated with hemolysis during extracorporeal membrane oxygenation (ECMO)-Comparison of VA- versus VV ECMO. PLoS ONE 15:e0227793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BG, Carlin JB, Tibballs J, Mead H, Hochmann M, Osborne A (1998) Accuracy of two pulse oximeters at low arterial hemoglobin-oxygen saturation. Crit Care Med 26:1128–1133 [DOI] [PubMed] [Google Scholar]
- 11.Nisar S, Gibson CD, Sokolovic M, Shah NS (2020) Pulse oximetry is unreliable in patients on veno-venous extracorporeal membrane oxygenation caused by unrecognized carboxyhemoglobinemia. ASAIO J 66:1105–1109 [DOI] [PubMed] [Google Scholar]
- 12.Lorusso R, Alexander P, Rycus P, Barbaro R (2019) The extracorporeal life support organization registry: update and perspectives. Ann Cardiothorac Surg 8:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jubran A (2015) Pulse oximetry. Crit Care 19:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louw A, Cracco C, Cerf C et al. (2001) Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med 27:1606–1613 [DOI] [PubMed] [Google Scholar]
- 15.Kollef MH, Schuster DP (1995) The acute respiratory distress syndrome. N Engl J Med 332:27–37 [DOI] [PubMed] [Google Scholar]
- 16.Brochard L, Roudot-Thoraval F, Roupie E et al. (1998) Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The multicenter trail group on tidal volume reduction in ARDS. Am J Respir Crit Care Med 158:1831–1838 [DOI] [PubMed] [Google Scholar]
- 17.Kalra A, Shou BL, Zhao D et al. (2023) Racial and ethnical discrepancy in hypoxemia detection in patients on extracorporeal membrane oxygenation. JTCVS Open. 14:145–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalra A, Bachina P, Shou BL, et al. (2024) Acute brain injury risk prediction models in venoarterial extracorporeal membrane oxygenation patients with Tree-based machine learning: An eLSO registry analysis. JTCVS Open. 10.1016/j.xjon.2024.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho SM, Canner J, Caturegli G et al. (2021) Risk factors of ischemic and hemorrhagic strokes during venovenous extracorporeal membrane oxygenation: analysis of data from the extracorporeal life support organization registry. Crit Care Med 49:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho SM, Canner J, Chiarini G et al. (2020) Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med 48:e897–e905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong AI, Charpignon M, Kim H et al. (2021) Analysis of discrepancies between pulse oximetry and arterial oxygen saturation measurements by race and ethnicity and association with organ dysfunction and mortality. JAMA Netw Open 4:e2131674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry NR, Hanson AC, Schulte PJ et al. (2022) Disparities in hypoxemia detection by pulse oximetry across self-identified racial groups and associations with clinical outcomes. Crit Care Med 50:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Covassin N, Fan Z et al. (2020) Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc 95:1138–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikkelsen ME, Christie JD, Lanken PN et al. (2012) The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 185:1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Materne LA, Hunsicker O, Menk M, Graw JA (2021) Hemolysis in patients with extracorporeal membrane oxygenation therapy for severe acute respiratory distress syndrome—a systematic review of the literature. Int J Med Sci 18:1730–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams DC, Turi JL, Hornik CP et al. (2015) Circuit oxygenator contributes to extracorporeal membrane oxygenation-induced hemolysis. ASAIO J 61:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zangrillo A, Landoni G, Biondi-Zoccai G et al. (2013) A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 15:172–178 [PubMed] [Google Scholar]
- 28.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ (2007) Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood 110:2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fawzy A, Wu TD, Wang K et al. (2022) Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med 182:730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valbuena VSM, Seelye S, Sjoding MW et al. (2022) Racial bias and reproducibility in pulse oximetry among medical and surgical inpatients in general care in the Veterans Health Administration 2013–2019: multicenter, retrospective cohort study. BMJ 378:e069775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enumah ZO, Etchill EW, Kim BS et al. (2024) Racial disparities among patients on venovenous extracorporeal membrane oxygenation in the pre-coronavirus disease 2019 and coronavirus disease 2019 eras: a retrospective registry review. JTCVS Open 17:162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


