Abstract
Subnormal T-cell production of interleukin-2 (IL-2) in human immunodeficiency virus (HIV) disease has been described; however, it is not clear whether failure to synthesize IL-2 represents a selective or global defect in T-cell cytokine production. We evaluated the intracellular production of gamma interferon (IFN-γ) and IL-2 in CD4+ cells that were stimulated with staphylococcal enterotoxin B or cytomegalovirus antigen. Strikingly, IFN-γ and IL-2 are differentially regulated in T cells of HIV-infected patients such that the numbers of CD69+ cells or IFN-γ-positive cells that make IL-2 are proportionally decreased in CD4+ T cells from HIV-infected patients. These findings demonstrate a selective defect in IL-2 production and suggest that enumeration of IFN-γ-producing cells in response to T-cell receptor stimulation, while providing some estimate of antigen-reactive cell frequency, may not reflect or predict “normal” T-cell function in HIV-infected patients.
CD4+ T-cell function in human immunodeficiency virus (HIV) disease is markedly impaired. CD4+ T cells from HIV-infected individuals fail to proliferate appropriately following stimulation with antigen or mitogen (2, 6, 7, 9, 10, 12) and display enhanced susceptibility to apoptosis (5, 8). These defects are accompanied by reduced production of the immunoregulatory cytokine interleukin-2 (IL-2) (2, 7). IL-2 production may be critical in HIV disease, since this cytokine acts as an important T-cell growth factor. Moreover, the addition of exogenous IL-2 to patient T cells enhances T-cell proliferation (12) and protects from apoptosis (1) in vitro. Thus, production of IL-2 by patient T cells may be especially important for proper immune function.
Recent progress in intracellular cytokine staining has allowed for the evaluation of cytokine production by defined T-cell populations on a single-cell basis. This technique has been used to evaluate immune responsiveness in HIV-infected patients (3, 4, 11). Commonly, gamma interferon (IFN-γ) production by patient T cells is used to measure immune responses to recall antigen, HIV antigen, or mitogens in vitro. The relative ease of detecting IFN-γ makes it a useful and sensitive tool for identifying antigen-reactive cells.
Despite the practical advantages of detecting IFN-γ production in T-cell populations, measuring production of this cytokine may not provide a complete determination of functionality of cells from HIV-infected patients. T cells from HIV-infected patients with advanced disease have poor proliferation responses to HIV antigens and yet may respond by producing IFN-γ (11). We have described poor proliferation in CD4+ T cells from HIV-infected patients even though these cells express CD25 and CD69 activation markers following T-cell receptor stimulation (13). Thus, some functional responses, such as IFN-γ production or expression of CD25 and CD69, may be maintained in HIV disease whereas others, such as proliferation, are lost.
Contrary to this suggestion, it has been argued that proliferation failure may be an artifact of prolonged cell culture, during which cells may be predisposed to an early apoptotic death (11). The detection of intracellular cytokine avoids these complications, since the assay can be performed in 5 to 6 h ex vivo. Therefore, it could be argued that cytokine responses better reflect the true nature of immune recognition and function in HIV disease.
To determine whether selective defects in cytokine production might be present in HIV disease, we tested the capacity of patient CD4+ T cells to produce IL-2 and IFN-γ in response to staphylococcal enterotoxin B (SEB), a superantigen with potent cytokine-inducing potential. Whole blood from HIV-infected patients or healthy controls was stimulated with SEB and subsequently treated with brefeldin A to prevent the release of cytokine from intracellular stores. IFN-γ and IL-2 production was measured in CD4+ CD69+ cells by three-color flow cytometry. CD69 is a marker of T-cell activation and is commonly used to enrich for antigen-reactive cells in intracellular cytokine assays. In comparison to healthy donor cells, patient CD69+ cells tended to have reduced IL-2 responses, but relatively normal IFN-γ responses, on a per cell basis (Fig. 1 and Table 1). The addition of anti-CD28 antibody at 1 μg/ml to the stimulation had little effect on cytokine production in response to SEB (data not shown). Thus, with CD69 as a denominator of activated cells, it was clear that most patient responses were suboptimal in IL-2 production but normal in IFN-γ production.
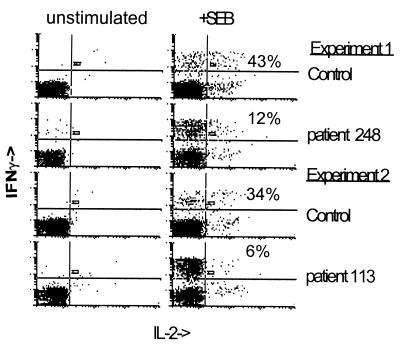
FIG. 1.
Differential production of IL-2 and IFN-γ in CD69+ cells. Whole blood was incubated with SEB (Sigma) (5 μg/ml) for 2 h before Golgi-stop reagent (BD Pharmingen) containing brefeldin A was added. Cells were incubated for an additional 3 h, at which time the cells were treated with Facs lyse solution (Becton Dickinson) and frozen at −86°C overnight. Frozen cells were thawed and treated with Facs permeabilizing solution (Becton Dickinson) and then stained with anti-CD69 phycoerythrin-conjugated antibody, anti-CD4 peridinin chlorophyll protein-conjugated antibody, and either anti-IL-2 or anti-IFN-γ fluorescein isothiocyanate-conjugated antibody (Becton Dickinson). Cells stained with isotype control antibodies were used to establish quadrants. Unstimulated cells in this case were exposed to anti-CD28 antibody (1 μg/ml) to control for additional experiments in which cells were stimulated with SEB plus anti-CD28 antibody (data not shown). The numbers shown indicate the percentages of CD69+ cells that were IL-2 positive following SEB stimulation.
TABLE 1.
Expression of IL-2 and IFN-γ following SEB stimulationa
| Patient or control | HIV RNA in plasma (no. of copies/ml) | No. of CD4 cells/μl | CD69+ % IL-2+ | CD69+ % IFN-γ+ | IFN-γ+ % IL-2+ |
|---|---|---|---|---|---|
| Patients | |||||
| 536 | <400 | 486 | 15.1 | 25.5 | ND |
| 248 | 15,000 | 202 | 11.5 | 32.1 | 12.3 |
| 138 | 21,000 | 309 | 11.8 | 26.5 | 12.4 |
| 121 | 25,000 | 460 | 6.4 | 36.1 | 5.9 |
| 261 | 32,000 | 680 | 9.0 | ND | 21 |
| 113 | 65,000 | 250 | 21.7 | 52.6 | 5.7 |
| 091 | 97,000 | 190 | 9 | 22 | |
| 107 | 520,000 | 112 | 11.7 | 12.3 | 24 |
| Mean | 12.0∗ | 29.6 | 13.6† | ||
| Median | 28,500 | 275.9 | 11.6 | 26.5 | 12.35 |
| Controls | |||||
| 1 | 16.7 | 17.1 | 34.4 | ||
| 2 | 11.2 | 24.8 | 32 | ||
| 3 | 19.4 | 25.4 | 43.2 | ||
| 4 | 9.7 | 48 | 39 | ||
| 5 | 22 | 29 | ND | ||
| 6 | 18.3 | 25.5 | 29 | ||
| 7 | 25 | ND | ND | ||
| 8 | 21 | ND | 20.0 | ||
| Mean | 17.9 | 28.3 | 33.0 | ||
| Median | 18.3 | 25.5 | 33.2 |
P values from two-tailed t tests of comparisons between the average responses of patients and healthy donors are as follows: ∗, P < 0.03; †, P < 0.001. ND, not done.
The differential production of IFN-γ and IL-2 in HIV disease was even more dramatically illustrated by using IFN-γ-producing cells as the denominator for the enumeration of IL-2-producing cells. In HIV-infected patients, the proportion of IFN-γ-producing cells that could simultaneously make IL-2 was markedly reduced compared to cells from healthy donors (Fig. 2 and Table 1). Again, addition of anti-CD28 antibody made little difference (data not shown). These observations suggest that CD4+ T cells activated through T-cell receptor stimulation to produce IFN-γ have a decreased capacity to make IL-2 in HIV disease.
FIG. 2.
Reduced proportions of IFN-γ-producing cells make IL-2 in CD4+ T cells from HIV-infected patients. Whole blood was stimulated and processed as described in the legend to Fig. 1 except that cells were stained with anti-CD4 peridinin chlorophyll protein-conjugated antibody, anti-IFN-γ phycoerythrin-conjugated antibody, and anti-IL-2 fluorescein isothiocyanate-conjugated antibody. The numbers shown indicate the percentages of IFN-γ-producing cells that were IL-2 positive following SEB stimulation.
Cytokine production following superantigen stimulation may differ from that induced by antigen stimulation, since superantigen does not require processing by professional antigen-presenting cells and can elicit responses from either naïve or memory cell populations with the appropriate T-cell receptor variable chain. Therefore, to determine whether differential production of IL-2 and IFN-γ might also be observed in response to antigen, whole blood was exposed to cytomegalovirus (CMV) antigen and assessed for cytokine production. Following CMV antigen stimulation, patient cells demonstrated a proportional reduction in IL-2 production both in the CD69+ T-cell population and in IFN-γ producing cells; however, the IFN-γ responses of CD69+ cells in patients and in controls were similar (Fig. 3 and Table 2). These results are consistent with what was found with SEB stimulation and indicate that the differential expression of IFN-γ and IL-2 is also seen in memory CD4+ T cells responding to antigen in HIV-infected patients.
FIG. 3.
Reduced IL-2 production in CMV-stimulated cells. Whole blood was stimulated with CMV antigen (3 μg/ml) with the addition of anti-CD28 and anti-CD49d antibodies (BD Pharmingen) at 3 μg/ml and analyzed by three-color flow cytometry. Unstimulated cells were exposed to costimulatory antibodies alone. Cells were gated on CD4+ cells, and histograms were generated based on the stains shown.
TABLE 2.
Expression of IL-2 and IFN-γ following CMV stimulation
| Patient or control | HIV RNA in plasma (no. of copies/ml) | No. of CD4 cells/μl | CD69+ % IL-2+ | CD69+ % IFN-γ+ | IFN-γ+ % IL-2+ |
|---|---|---|---|---|---|
| Patients | |||||
| 141 | 23,000 | 598 | 20.5 | 90.2 | 24 |
| 150 | 135,000 | 344 | 10 | 85 | 9 |
| 266 | 91,000 | 784 | 5 | 70 | 8 |
| 265 | 196,000 | 63 | 13.5 | 53 | 22 |
| 164 | 93,000 | 104 | 11.0 | 45 | NDa |
| 195 | 70,000 | 464 | ND | ND | 33.6 |
| Mean | 101,000 | 393 | 12.0 | 68.6 | 19.3 |
| Controls | |||||
| 1 | 57 | 62 | 41 | ||
| 2 | 24 | 53 | 26 | ||
| 3 | 19.4 | 50 | ND | ||
| 4 | ND | ND | 62 | ||
| Mean | 33.5 | 55 | 43 |
ND, not done.
While diminished IL-2 expression has been noted in HIV disease (2, 7), this study clearly demonstrates a selective impairment in IL-2 expression by CD4+ T cells in HIV disease on an individual-cell basis following T-cell receptor activation. Importantly, these analyses were performed under conditions in which cells were incubated for a short time and maintained in whole blood. Therefore, differences in production of IL-2 and IFN-γ cannot be explained by culture-induced artifacts, as might occur in proliferation assays of long duration.
Important inferences can be drawn from the results of this study, which shows that CD4+ T cells from HIV-infected patients maintain a selective defect in IL-2 production while IFN-γ expression is preserved. As IL-2 is a critical mediator of immune competence in health and disease, this selective defect in IL-2 expression after T-cell receptor stimulation may be a key determinant of immune dysfunction in HIV infection. Selective impairment of IL-2 production in memory cells also might limit appropriate expansion of antigen-reactive cells following in vivo challenge. This may in part explain the reduced vaccine responses that have been observed in HIV-infected patients (14). Furthermore, while T-cell production of IFN-γ may provide an indication that T cells with defined receptor specificity are present, enumeration of these cells may not provide an appropriate demonstration of functional immune competence in HIV disease.
Acknowledgments
We thank Robert Asaad for assistance in acquiring blood samples.
Scott Sieg is supported by National Research Service grant AI07024-21, from the National Institutes of Health. These studies were also supported by the Case Western Reserve University Center for AIDS Research (grant AI36219).
REFERENCES
- 1.Adachi Y, Oyaizu N, Than S, McCloskey T W, Pahwa S. IL-2 rescues in vitro lymphocyte apoptosis in patients with HIV infection. J Immunol. 1996;157:4184–4193. [PubMed] [Google Scholar]
- 2.Clerici M, Stocks N I, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. J Clin Investig. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gea-Banacloche J C, Migueles S A, Martino L, Shupert W L, McNeil A C, Sabbaghian M S, Ehler L, Prussin C, Stevens R, Lambert L, Altman J, Hallahan C W, Bernaldo de Quiros J C L, Conners M. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 4.Goulder P J R, Altfeld M A, Rosenberg E S, Nguyen T, Tang Y, Eldridge R L, Addo M M, He S, Muckerjee J S, Phillips M N, Bunce M, Kalams S A, Sekely R P, Walker B D, Brander C. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J Exp Med. 2001;193:181–193. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J C. Activation-induced death by apoptosis in CD4+ T cells from HIV-1 infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurley R J, Ikeuchi K, Byrn R A, Anderson K, Groopman J E. CD4+ lymphocyte function with early human immunodeficiency virus infection. Proc Natl Acad Sci USA. 1989;86:1993–1997. doi: 10.1073/pnas.86.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane C L, Depper J M, Greene W C, Whalen G, Waldmann T A, Fauci A S. Qualitative analysis of immune function in patients with acquired immune deficiency syndrome. N Engl J Med. 1985;313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 8.Meyaard L, Otto S A, Jonker R R, Janneke Mijnster M, Keet R P M, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 9.Miedema F, Chantal Petit A J, Terpstra F G, Eeftinck Schattenkerk J K M, de Wolf F, Al B J M, Roos M, Lange J M A, Danner S A, Goudsmit J, Schellekens P T A. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men: HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Investig. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musey L K, Krieger J N, Hughes J P, Schacker T W, Corey L, McElrath M J. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J Infect Dis. 1999;180:278–284. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- 11.Pitcher C J, Quittner C, Peterson D M, Conners M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1998;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 12.Seder R A, Grabstein K H, Berzofsky J A, McDyer J F. Cytokine interactions in human immunodeficiency virus-infected individuals: roles of interleukin (IL)-2, IL-12, and IL-15. J Exp Med. 1995;182:1067–1078. doi: 10.1084/jem.182.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieg, S. F., C. V. Harding, and M. M. Lederman. HIV-1 infection impairs cell cycle progression of CD4+ T cells without affecting early activation responses. J. Clin. Investig., in press. [DOI] [PMC free article] [PubMed]
- 14.Valdez H, Smith K Y, Landay A, Connick E, Kuritzkes D R, Kessler H, Fox L, Spritzler J, Roe J, Lederman M B, Lederman H M, Evans T G, Heath-Chiozzi M, Lederman M M. Response to immunization with recall and neoantigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. ACTG 375 team. AIDS Clinical Trials Group. AIDS. 2000;14:11–21. doi: 10.1097/00002030-200001070-00002. [DOI] [PubMed] [Google Scholar]