Abstract
Clinical and experimental evidence indicates that the hepatitis C virus (HCV) E2 glycoprotein (HCV/E2) is the most promising candidate for the development of an effective anti-HCV vaccine. Identification of the human epitopes that are conserved among isolates and are able to elicit protective antibodies would constitute a significant step forward. This work describes the mapping of the B-cell epitopes present on the surface of HCV/E2, as recognized by the immune system during infection, by the analysis of the reciprocal interactions of a panel of human recombinant Fabs derived from an HCV-infected patient. Three unrelated epitopes recognized by antibodies with no neutralization-of-binding (NOB) activity were identified; a fourth, major epitope was defined as a clustering of minor epitopes recognized by Fabs endowed with strong NOB activity.
Hepatitis C Virus (HCV) is the major causative agent of blood-borne non-A, non-B hepatitis (12, 19). The tendency of HCV infection toward chronicity (9), with persistent and continuous viral replication (4), suggests that in the majority of cases the host immune response is unable to tackle and eradicate the infection. The commonest way of controlling viral diseases is by developing vaccines able to prevent viral spreading typically by eliciting neutralizing antibodies. In the case of HCV, although specific humoral immunity can be readily detected and the demonstration of anti-HCV antibodies establishes a serologic diagnosis of infection (3), it is controversial whether the humoral response affords any protection (5, 13, 14, 20, 26, 29). However, recent reports describing the dynamics of intrahost evolution in an HCV-positive population during primary infection have shown that a crucial phase for disease outcome lies at a time point corresponding to the production of antibodies by the infected host (15, 23). These data suggest an important role for antibodies in the evolution of HCV infection.
An important viral structure studied as an antibody response target is the HCV E2 envelope glycoprotein (HCV/E2). Successful protection of chimpanzees by immunization with glycoproteins E1 and E2 has been ascribed to the induction of specific anti-E2 antibodies (11) that seem to be able to neutralize the binding of E2 to susceptible cells. These molecules are commonly referred to as antibodies with neutralization-of-binding (NOB) activity (28). Although the assessment of the efficacy of this class of antibodies in inhibiting HCV infection and replication has been hampered by the poor growth efficiency of HCV in cell culture, high titers of NOB antibodies have been seen to correlate with the natural resolution of chronic HCV infection (18).
These considerations show that the study of the antibody response against HCV/E2 can greatly contribute to the development of an effective vaccine. This goal is usually pursued by using panels of mouse monoclonal antibodies. Since in the case of this viral pathogen the murine model is not consistent with the human antibody response (1), the generation from an infected patient of human monoclonal antibodies representing discrete parts of the immune response is more suitable to the study of this aspect of virus-host interplay (10).
Cloning of the immune repertoire of an HCV-infected patient on phage display combinatorial vectors and generation of recombinant monoclonal Fab fragments (7, 27) have demonstrated that inhibition of binding of HCV/E2 to cells varies widely from one antibody clone to another.
The failure of traditional approaches such as peptide scanning (16) to identify the epitopes recognized by these molecules is probably connected with the fact that, when assayed by the phage display technology, the most important part of the in vivo antiviral response is usually directed against conformational and heavily glycosylated regions (17), a finding confirmed by the recent work of Allander et al. (2). An alternative approach consists of analyzing the reciprocal interactions of recombinant Fab pairs assuming that Fabs inhibiting each other's binding are directed against overlapping parts of the E2 molecules and that Fab pairs that do not interact define two discrete B-cell epitopes. Two Fabs with identical inhibition patterns would thus be likely to define the same B epitope.
The human B epitopes present on HCV/E2 and recognized by our panel of Fabs were thus analyzed by a competitive enzyme-linked immunosorbent assay (ELISA) using FLAG-labeled Fabs against unlabeled Fabs. For production of the above-mentioned FLAG-labeled Fabs (FLAG-Fabs), Fab genes were inserted in the pComb3/FLAG vector (R. Burioni, unpublished data), adding an epitope (FLAG) to the carboxy-terminal end of the heavy-chain fragment recognized specifically by a mouse anti-FLAG monoclonal antibody (Sigma, Saint Louis, Mo.).
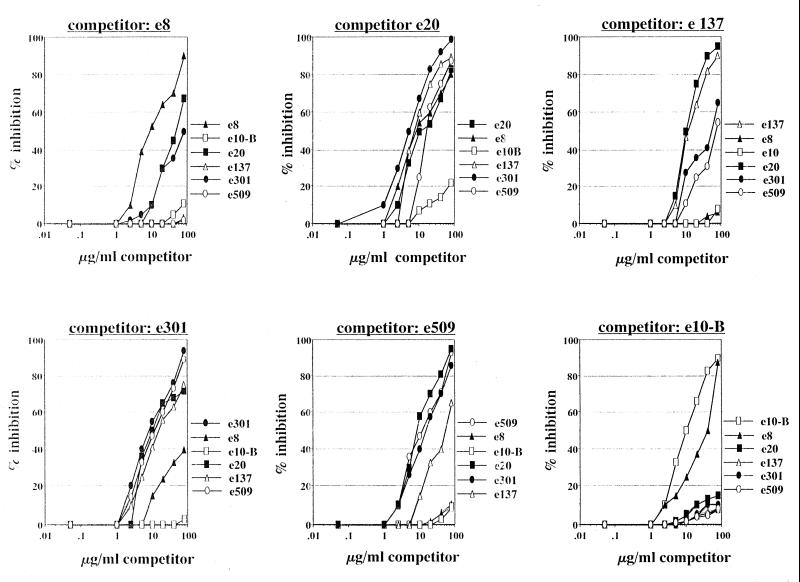
For competition assays, ELISA plates (Costar, Corning, N.Y.) were then coated with recombinant HCV/E2 (genotype 1a, strain H) (7, 22, 24) and blocked with phosphate-buffered saline (PBS)–1% bovine serum albumin for 1 h at 37°C; subsequently, 50 μl of a purified preparation of a competing Fab at known concentrations (Fig. 1) was added to the wells and the mixture was incubated for 2 h at 37°C. After this step, an appropriate amount of FLAG-Fabs was added directly to the wells to obtain a final concentration giving approximately 60% of the maximum optical density at 450 nm (OD450) in the ELISA (Table 1) and the mixture was incubated for an additional 30 min. Plates were then washed 10 times with PBS–0.05% Tween, and binding of the FLAG-Fab probe to the antigen was revealed with anti-FLAG M2 mouse monoclonal antibody (Sigma; 10 μg/ml in PBS) and demonstrated, after another wash, by addition of peroxidase-conjugated anti-mouse immunoglobulin serum (Sigma; 1:700 in PBS). After a final wash, 100 μl of substrate (Sigma) was added and the OD450s of the plates were read after 30 min at room temperature in the dark. A negative control sample containing an excess of a purified control human Fab directed against herpes simplex virus glycoprotein D (8) and corresponding to 0% inhibition was included. Wells with no labeled Fab were always included to rule out nonspecific reactivity of the FLAG-Fab detection system.
FIG. 1.
Inhibition of binding of probe FLAG-Fab antigen by previous binding of different concentrations of purified unlabeled Fabs used as competitors.
TABLE 1.
Human recombinant Fabs used in this studya
| Human Fab | HCV/E2 50% NOB titer (μg/ml) | Concn as probe in competition ELISA (μg/ml) | NOB activity |
|---|---|---|---|
| e8 | >40 | 10 | None |
| e20 | 3 | 5 | High |
| e137 | 40 | 20 | None |
| e301 | 3 | 5 | High |
| e509 | 0.3 | 20 | Highest |
| e10-B | >40 | 0.05 | None |
The generation, purification, and characterization of these anti-HCV/E2 Fabs have been described elsewhere (7, 27). Fab e10-B is a novel anti-HCV/E2 Fab with no NOB activity selected by using antibody-coated HCV/E2 (6). Fifty percent NOB concentration (28) and concentrations used in competition experiments (FLAG-Fabs) are indicated for each antibody fragment. Underlined Fabs are endowed with NOB activity.
Final results were determined as percent inhibition with the following formula: percent inhibition = 100 × [(OD450 of probe FLAG-Fab alone − OD450 of probe FLAG-Fab with competitor Fab)/OD450 of probe FLAG-Fab alone] (Fig. 1).
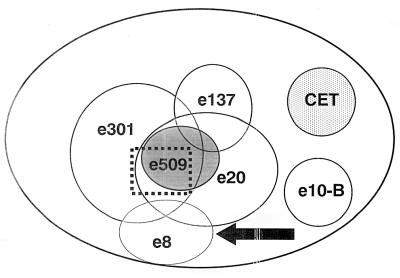
At least six different epitopes were characterized. As they did not inhibit each other, Fabs e137 and e8 were defined as recognizing two distinct epitopes on HCV/E2 and since they had no NOB activity, these epitopes were defined as nonneutralizing. A third, different epitope was recognized by e10-B, as expected since this Fab had been selected by using antibody-coated HCV/E2 (6). In brief, after binding of recombinant HCV/E2 to ELISA plates and blocking of the wells with PBS–3% bovine serum albumin 70 μl of a mixture containing purified human monoclonal recombinant anti-HCV/E2 Fabs e8, e20, e137, e301, and e509 (10 μg of each clone per ml) was added to the wells and incubated for 1 h at 37°C. Antibody-coated HCV/E2 was then used to select Fab e10-B. Competition binding experiments demonstrated that, as expected, the binding of Fab e10B to HCV/E2 is not inhibited by any other Fab. By contrast, previous binding of Fab e10B inhibited the binding of Fab e8 to HCV/E2. This effect was asymmetric, as e10B inhibited e8 but was not itself inhibited (6). This may be due to a modification of the structure of the glycoprotein subsequent to the binding of e10-B. The epitopes defined by these families of Fabs (e8, e137, and e10-B) were thus completely distinct. The other three Fabs (e20, e301, and e509) recognized three overlapping but different epitopes, which are probably clustered to form a major epitope on the surface of HCV/E2. These three Fabs belong to families endowed with strong NOB activity. The analysis of inhibition data allowed to observe the reciprocal reactivity of all Fabs in the matrix shown in Table 2. It is important to stress that Fab e509, which had the highest NOB titer (Table 1), seems to define the minimal region that needs to be recognized for neutralization of binding. By using these data, a two-dimensional surface map of the human epitopes on HCV/E2 was also drawn (Fig. 2).
TABLE 2.
Reaction pattern matrix showing reciprocal interactions of pairs of monoclonal antibodies to HCV/E2 proteina
| Monoclonal antibody | Inhibition of probe monoclonal antibody
|
|||||
|---|---|---|---|---|---|---|
| e8 | e20 | e137 | e301 | e509 | e10-B | |
| e8 | ++ | ++ | − | + | − | − |
| e20 | ++ | ++ | ++ | ++ | ++ | − |
| e137 | − | ++ | ++ | ++ | + | − |
| e301 | + | ++ | ++ | ++ | ++ | − |
| e509 | − | ++ | + | ++ | ++ | − |
| e10-B | ++ | − | − | − | − | ++ |
| CET | − | − | − | − | − | − |
++, >60% inhibition at a competing Fab concentration of 80 μg/ml; +, 40 to 60% inhibition; − <30% inhibition. Underlined Fabs are endowed with NOB activity. Binding of each FLAG-Fab was inhibited by the corresponding unlabeled Fab, bearing out the correctness of the experimental procedure. All inhibitions were symmetric, with the exception of Fab e10-B, which probably induced a conformational change that prevented the further recognition of HCV/E2 by Fab e8 (6). CET represents six monoclonal antibodies directed against a known HCV/E2 epitope (21). Two of them (CET3 and CET5) did not bind to the recombinant E2 used in our study and were not tested in the competition ELISA. The other four were used as competitors at known concentrations (100 and 5 μg/ml) against each human unlabeled Fab. Binding of human Fabs was revealed by peroxidase-conjugated anti-human Fab serum (Sigma).
FIG. 2.
Two-dimensional surface-like map of the human B-cell epitopes present on the surface of HCV/E2 as recognized by the monoclonal antibodies used in this study. Overlapping circles indicate reciprocal inhibition. Fabs endowed with NOB activity are underlined. The putative region mediating the interaction of HCV/E2 with the cellular target is indicated by the dotted line. Due to modifications that can be induced by antigen-antibody interactions, this diagram does not correspond to the actual physical map.
The first conclusion that can be derived from the data presented in this paper is that all NOB antibodies recognized a specific region of HCV/E2, which may be the one involved in binding of the virus to the cellular target. Although other explanations are possible, such as an antibody-induced modification of the protein conformation preventing further interaction of HCV/E2 with the cellular target, the antibodies directed against this epitope are useful reagents in view of a better definition of this key step in virus-host interaction. A second aspect is that only a cluster of epitopes was recognized by antibodies with the NOB effect. Immunoglobulins directed against the e137 and e8 epitopes not only lack this activity but can also displace from the virus other antibodies (like e301 and e20) that can inhibit important viral functions. In addition, antibodies binding to the e10-B epitope can reduce the NOB activity of other immunoglobulins even without displacing them (6). The eventual NOB effect is the sum of the interactions among all antibody clones, and the absence of NOB activity in HCV-positive sera can be due to a prevalence of the effect of NOB-inactive clones. Studies are in progress to evaluate these synergies.
To identify the physical location of the epitopes on the surface of HCV/E2, competition experiments were performed with a panel of mouse monoclonal Fabs directed against a specific mouse B-cell epitope present in the HCV/E2 region and spanning amino acid residues 527 to 560 (21). In no experiment was binding of human Fabs inhibited by previous binding of an excess of mouse monoclonal antibody to HCV/E2 (data not shown). This indicates that the human B-cell epitopes defined in this study did not correspond to the murine epitope recognized by the CET monoclonal antibodies (Fig. 2).
Our Fabs were also tested by ELISA, as previously described (7), for the ability to bind a synthetic peptide corresponding to HVR-1 derived from a sequence of HCV/E2 with the 1a genotype (H isolate, amino acids 388 to 409) (16) used to produce the recombinant HCV/E2 employed in this study. As expected, no binding was evidenced since library selection was performed with strategies designed to favor antibodies directed against epitopes conserved among different genotypes. Although these data are not conclusive, as they require testing of reactivity against HCV/E2 with HVR-1 deleted, as well as against different HVRs expressed in the context of a whole E2 glycoprotein, they leave open the possibility that NOB antibodies directed against regions in which HVR-1 is not directly involved as an epitope might be present in the repertoire of infected patients.
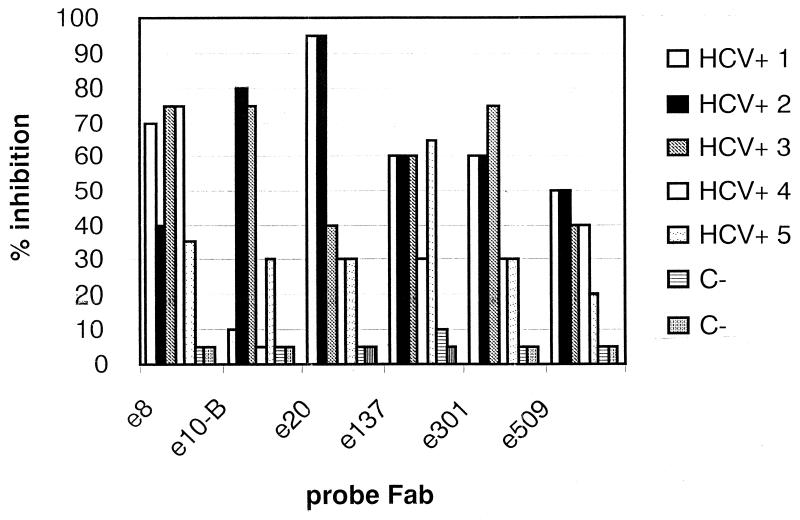
Finally, to exclude the artifactual nature of our Fabs, we evaluated by competitive ELISA the abilities of HCV-positive and HCV-negative sera to inhibit Fab binding to the antigen. As shown in Fig. 3, the results indicate that all of the positive patients, albeit to different extents, had antibodies able to bind to the epitopes recognized by our Fabs. Remarkably, in some cases, inhibition of FLAG-Fab binding was noted in serum dilutions of up to 1:500 (data not shown). Previous binding to HCV/E2 of a mixture of the six Fabs (10 μg of each clone per ml) described in this work was able to inhibit the binding to HCV/E2 of sera obtained from HCV-positive patients (1:200 dilution) up to 75% in an ELISA format (R. Burioni, unpublished data). An assay able to measure the amount of serum antibodies directed against the different epitopes is being developed. No binding to HCV/E2 and no inhibition were demonstrated in the HCV-negative sera, even at the highest concentrations (data not shown).
FIG. 3.
Inhibition of binding of human recombinant Fabs by previous binding of a 1:20 dilution of sera obtained from HCV-infected patients and from two HCV-negative controls. All HCV-positive sera were demonstrated to contain HCV RNA of genotype 1b and to have an ELISA titer against HCV/E2 of >1:500.
Although additional studies are needed for the fine characterization of human B-cell epitopes, this paper provides useful data for a better understanding of virus-host interplay in this infection. The absence of an in vitro neutralization model requires further investigations aimed at correlating NOB and true neutralization activity. If this is proved, antibodies recognizing neutralizing and conserved viral epitopes similar to the ones described in this study will have the potential for therapeutic use. The availability of a panel of human recombinant Fabs and of detailed information on the epitopes recognized by them would allow easy in vitro evaluation of antigens (25), which would be a considerable advance for an infection lacking an animal model. Molecules demonstrated in vitro to be able to stimulate selectively the production of neutralizing antibodies in immunized patients will be the best candidates for further in vivo studies.
Acknowledgments
We thank Silvio Bighi, Mario Perotti, and Cristiano Scalpelli for valuable assistance. We are deeply grateful to Giovanni Gasbarrini for critical help and continuous and enthusiastic support.
This work was supported by grants from the Istituto Superiore di Sanità to R.B. and M.C.
REFERENCES
- 1.Ahmed M, Shikata T, Esumi M. Murine humoral immune response against recombinant structural proteins of hepatitis C virus distinct from those of patients. Microbiol Immunol. 1996;40:169–176. doi: 10.1111/j.1348-0421.1996.tb03321.x. [DOI] [PubMed] [Google Scholar]
- 2.Allander T, Drakenberg K, Beyene A, Rosa D, Abrignani S, Houghton M, Widell A, Grillner L, Persson M A. Recombinant human monoclonal antibodies against different conformational epitopes of the E2 envelope glycoprotein of hepatitis C virus that inhibit its interaction with CD81. J Gen Virol. 2000;81:2451–2459. doi: 10.1099/0022-1317-81-10-2451. [DOI] [PubMed] [Google Scholar]
- 3.Alter H J, Purcell R H, Shih J W, Melpolder J C, Houghton M, Choo Q L, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 4.Alter M J, Margolis H S, Krawczynski K, Judson F N, Mares A, Alexander W J, Hu P Y, Miller J K, Gerber M A, Sampliner R E, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 5.Bjoro K, Froland S S, Yun Z, Samdal H H, Haaland T. Hepatitis C infection in patients with primary hypogammaglobulinemia after treatment with contaminated immune globulin. N Engl J Med. 1994;331:1607–1611. doi: 10.1056/NEJM199412153312402. [DOI] [PubMed] [Google Scholar]
- 6.Burioni, R., F. Bugli, N. Mancini, D. Rosa, C. DiCampli, G. Moroncini, A. Manzin, S. Abrignani, P. E. Varaldo, M. Clementi, and G. Fadda. Non-neutralizing human antibody fragments against hepatitis C virus E2 glycoprotein modulate neutralization of binding activity of human recombinant Fabs. Virology, in press. [DOI] [PubMed]
- 7.Burioni R, Plaisant P, Manzin A, Rosa D, Delli Carri V, Bugli F, Solforosi L, Abrignani S, Varaldo P E, Fadda G, Clementi M. Dissection of human humoral immune response against hepatitis C virus E2 glycoprotein by repertoire cloning and generation of recombinant Fab fragments. Hepatology. 1998;28:810–814. doi: 10.1002/hep.510280331. [DOI] [PubMed] [Google Scholar]
- 8.Burioni R, Williamson R A, Sanna P P, Bloom F E, Burton D R. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc Natl Acad Sci USA. 1994;91:355–359. doi: 10.1073/pnas.91.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerny A, Chisari F V. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- 10.Chanock R M, Crowe J E, Jr, Murphy B R, Burton D R. Human monoclonal antibody Fab fragments cloned from combinatorial libraries: potential usefulness in prevention and/or treatment of major human viral diseases. Infections Agents Dis. 1993;2:118–131. [PubMed] [Google Scholar]
- 11.Choo Q L, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 13.Farci P, Alter H J, Govindarajan S, Wong D C, Engle R, Lesniewski R R, Mushahwar I K, Desai S M, Miller R H, Ogata N, et al. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258:135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 14.Farci P, Alter H J, Wong D C, Miller R H, Govindarajan S, Engle R, Shapiro M, Purcell R H. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA. 1994;91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder J C, Strazzera A, Chien D Y, Munoz S J, Balestrieri A, Purcell R H, Alter H J. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 16.Geysen H M, Meloen R H, Barteling S J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci USA. 1984;81:3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadlock K G, Lanford R E, Perkins S, Rowe J, Yang Q, Levy S, Pileri P, Abrignani S, Foung S K. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J Virol. 2000;74:10407–10416. doi: 10.1128/jvi.74.22.10407-10416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii K, Rosa D, Watanabe Y, Katayama T, Harada H, Wyatt C, Kiyosawa K, Aizaki H, Matsuura Y, Houghton M, Abrignani S, Miyamura T. High titers of antibodies inhibiting the binding of envelope to human cells correlate with natural resolution of chronic hepatitis C. Hepatology. 1998;28:1117–1120. doi: 10.1002/hep.510280429. [DOI] [PubMed] [Google Scholar]
- 19.Kuo G, Choo Q L, Alter H J, Gitnick G L, Redeker A G, Purcell R H, Miyamura T, Dienstag J L, Alter M J, Stevens C E, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 20.Lai M E, Mazzoleni A P, Argiolu F, De Virgilis S, Balestrieri A, Purcell R H, Cao A, Farci P. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet. 1994;343:388–390. doi: 10.1016/s0140-6736(94)91224-6. [DOI] [PubMed] [Google Scholar]
- 21.Lee J W, Kim K, Jung S H, Lee K J, Choi E C, Sung Y C, Kang C Y. Identification of a domain containing B-cell epitopes in hepatitis C virus E2 glycoprotein by using mouse monoclonal antibodies. J Virol. 1999;73:11–18. doi: 10.1128/jvi.73.1.11-18.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesniewski R, Okasinski G, Carrick R, Van Sant C, Desai S, Johnson R, Scheffel J, Moore B, Mushahwar I. Antibody to hepatitis C virus second envelope (HCV-E2) glycoprotein: a new marker of HCV infection closely associated with viremia. J Med Virol. 1995;45:415–422. doi: 10.1002/jmv.1890450411. [DOI] [PubMed] [Google Scholar]
- 23.Manzin A, Solforosi L, Debiaggi M, Zara F, Tanzi E, Romano L, Zanetti A R, Clementi M. Dominant role of host selective pressure in driving hepatitis C virus evolution in perinatal infection. J Virol. 2000;74:4327–4334. doi: 10.1128/jvi.74.9.4327-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogata N, Alter H J, Miller R H, Purcell R H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parren P W, Fisicaro P, Labrijn A F, Binley J M, Yang W P, Ditzel H J, Barbas C F, Burton D R. In vitro antigen challenge of human antibody libraries for vaccine evaluation: the human immunodeficiency virus type 1 envelope. J Virol. 1996;70:9046–9050. doi: 10.1128/jvi.70.12.9046-9050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piazza M, Sagliocca L, Tosone G, Guadagnino V, Stazi M A, Orlando R, Borgia G, Rosa D, Abrignani S, Palumbo F, Manzin A, Clementi M. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537–1544. [PubMed] [Google Scholar]
- 27.Plaisant P, Burioni R, Manzin A, Solforosi L, Candela M, Gabrielli A, Fadda G, Clementi M. Human monoclonal recombinant Fabs specific for HCV antigens obtained by repertoire cloning in phage display combinatorial vectors. Res Virol. 1997;148:165–169. doi: 10.1016/s0923-2516(97)89904-9. [DOI] [PubMed] [Google Scholar]
- 28.Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner A J, Lau J Y, Choo Q L, Chien D, Pileri P, Houghton M, Abrignani S. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759–1763. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zibert A, Schreier E, Roggendorf M. Antibodies in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology. 1995;208:653–661. doi: 10.1006/viro.1995.1196. [DOI] [PubMed] [Google Scholar]