Abstract
Background
Wheat is one of major sources of human cadmium (Cd) intake. Reducing the grain Cd concentrations in wheat is urgently required to ensure food security and human health. In this study, we performed a field experiment at Wenjiang experimental field of Sichuan Agricultural University (Chengdu, China) to reveal the effects of FeCl3 and Fe2(SO4)3 on reducing grain Cd concentrations in dwarf Polish wheat (Triticum polonicum L., 2n = 4x = 28, AABB).
Results
Soil application of FeCl3 and Fe2(SO4)3 (0.04 M Fe3+/m2) significantly reduced grain Cd concentration in DPW at maturity by 19.04% and 33.33%, respectively. They did not reduce Cd uptake or root-to-shoot Cd translocation, but increased Cd distribution in lower leaves, lower internodes, and glumes. Meanwhile, application of FeCl3 and Fe2(SO4)3 up-regulated the expression of TpNRAMP5, TpNRAMP2 and TpYSL15 in roots, and TpYSL15 and TpZIP3 in shoots; they also downregulated the expression of TpZIP1 and TpZIP3 in roots, and TpIRT1 and TpNRAMP5 in shoots.
Conclusions
The reduction in grain Cd concentration caused by application of FeCl3 and Fe2(SO4)3 was resulted from changes in shoot Cd distribution via regulating the expression of some metal transporter genes. Overall, this study reports the physiological pathways of soil applied Fe fertilizer on grain Cd concentration in wheat, suggests a strategy for reducing grain Cd concentration by altering shoot Cd distribution.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05652-x.
Keywords: Wheat, Heavy metal, Cd stress, Fe fertilizer, Shoot Cd distribution
Introduction
Cadmium (Cd) is one of the most toxic metals and the primary heavy metal pollutant in arable soils [1]. Moreover, Cd is readily mobile and absorbed by plant roots and inhibits plant growth and development by impairing nutrient uptake, transpiration, and photosynthesis [2–4]. In crops, absorbed Cd is subsequently translocated into shoots and accumulates in edible parts [5, 6]. In China, each year, the Cd level in approximately 1.46 million tons of agricultural products exceeds the national standard (0.10 mg/kg, GB2762-2022), posing a potential risk to human health [7].
Wheat is a staple food for more than 50% of the world’s population. The incidence of arable soils contaminated by Cd in wheat-producing areas (GB/T 41685 − 2022) has been increasing [8, 9]. Meanwhile, wheat has a strong tendency to accumulate Cd in grains. Increasing of wheat grains accumulate Cd exceeding the maximum level of 0.1 mg/kg in China [10, 11], and even 0.20 mg/kg in European countries and the United States [12, 13]. Thus, wheat is a major source of human Cd uptake in many countries and regions [14]. Reducing Cd concentration in wheat grains is urgently required to ensure food security and human health.
Grain Cd concentration in wheat is mainly mediated by Cd uptake in roots, root-to-shoot translocation, and shoot-to-grain remobilization [8, 11, 15–19]. These processes are depending on wheat genotype and controlled by genetic factors [11]. Cd uptake and transport in plants depend on essential metal transporters, such as the IRT (iron-regulated transporter), NRAMP (natural resistance-associated macrophage protein), ZIP (zinc transporter), HMA (P1B-ATPase-Heavy metal associated protein), and YSL (yellow stripe-like transporters) families [20–28]. The expression levels of genes encoding metal transporters are easily regulated by environmental factors, such as essential nutrients, light and temperature [11, 14]. Cd uptake in roots is also influenced by the rhizosphere soil’s properties, such as pH and Cd bioavailability [29]. Thus, the grain Cd accumulation in wheat is mediated by environmental factors [11]; and agronomic practices, such as the application of iron (Fe) fertilizer, could be used to reduce grain Cd accumulation in wheat.
Fe (II) fertilizers such as FeSO4 and EDTA-Na2Fe have been used to mediate grain Cd accumulation in rice [30]. However, the synergistic/antagonistic relationship between Fe fertilizers and Cd developed on the application strategy and forms of Fe fertilizers. For example, soil application of EDTA-Na2Fe reduced grain Cd concentrations in rice; while, foliar application of EDTA-Na2Fe, FeSO4, or soil application of FeSO4 increased grain Cd concentrations [30]. Fe(III) fertilizers FeCl3 and Fe2(SO4)3 can enhance the mobility of Cd in contaminated soils by extracting Cd from soil in the form of Cd2+ and Cd-Cl+complex [31]. Our previous hydroponic experiment found that FeCl3 and Fe2(SO4)3 differentially reduced root Cd uptake and promoted root-to-shoot Cd translocation in dwarf Polish wheat (DPW; Triticum polonicum L., 2n = 4x = 28, AABB) seedlings [32]. Notably, Cd concentration in wheat grains is positively correlated with Cd uptake and root-to-shoot translocation [33]. Thus, we hypothesized that soil application of FeCl3 or Fe2(SO4)3 may influence Cd uptake and transport in wheat, finally affecting grain Cd concentration.
Our previous hydroponic experiment also showed that FeCl3 and Fe2(SO4)3 differentially regulated the expression levels of metal transporter genes, such as TpIRT1 and TpYSL15 [32]. However, whether soil application of FeCl3 or Fe2(SO4)3 can regulate the expression levels of metal transporter genes (e.g., TpIRT1 and TpYSL15) to alter Cd uptake and grain Cd concentration in DPW remains unclear.
The aim of this study was to reveal the effects of soil application of FeCl3 or Fe2(SO4)3 on grain Cd concentration in DPW in the field. To achieve this objective, we evaluated the plant growth, photosynthetic parameters, and metal concentrations in tissues; calculated the parameters of Cd uptake, root-to-shoot translocation, and distribution in shoots; analyzed the expression levels of metal transporter genes, such as TpIRT1 and TpYSL15. The findings may facilitate the reduction of grain Cd accumulation in wheat production by presenting a reasonable and effective agronomic strategy.
Materials and methods
Plant materials
Dwarf Polish wheat (DPW, Triticum polonicum L., 2n = 4x = 28, AABB) is a landrace of Turpan, Xinjiang Province, China, with a seed rate of approximately 75%. It shows high uptake and tolerance of Cd and carries other valuable genes, such as the long kernel gene KL-PW and dwarfing gene Rht-B1b [29, 34–36].
Field growth and treatments
During the wheat-growing season at 2018–2019, DPW seeds were sown in the Wenjiang experimental field of Sichuan Agricultural University (30°43′N; 103°52′E), Chengdu, China. The soil (0–15 cm depth) characteristics are presented in Supplemental Table S1. The total Cd concentration in the soil exceeded the safety threshold values (0.30 mg/kg, pH < 6.5). Before sowing, N-P-K compound fertilizer (N/P2O5/K2O = 15:15:15, GB/T 15063 − 2009) was applied one-time with 600 kg/ha. The experiment was conducted in a randomized complete block design with three planted plots for each treatment; and all experiments were conducted at three different pieces of paddy field. Each plot contained 30 rows with twenty seeds planted in each row at 2 m intervals.
At the tillering stage, the 30 rows of each plot were randomly reclassified into three groups and treated with Fe(III) fertilizers. For group 1, the control (CK), Fe(III) fertilizer was not applied. For groups 2 and 3, FeCl3 (0.04 M Fe3+/m2) and Fe2(SO4)3 (0.04 M Fe3+/m2) were applied, respectively. Specifically, Fe(III) fertilizer was dissolved in 5 L distilled water and uniformly applied to the soil of each subgroup, and the CK soil was irrigated with 5 L distilled water.
At the grain filling (flowering after 15 d) stage and maturity, 30 plants were randomly sampled from each treatment of each plot (three plants each row) and then divided into grains, glumes, rachises, node 1, internode 1, flag leaves, lower nodes, lower internodes, lower leaves, and roots. All samples were dried at 105 °C for 1 h and at 80 °C for 3 d and weighed.
Investigation of agronomic traits and photosynthetic parameters
Plant height, spike length, tiller number and dry weight of tissues were investigated at grain filling stage (on the 185th day after sowing) and maturity (on the 203th day after sowing). At the grain filling stage, photosynthetic parameters, including the assimilation rate, transportation rate, internal CO2, stomatal conductance, vapor pressure deficit, and water use efficiency, were measured using a portable photosynthesis system (CIRAS-3, PP systems, Amesbury, USA). Monthly temperature, maximum temperature, minimum temperature, precipitation, wet days, and vapour pressure were obtained from the Climatic Research Unit (CRU TS version 4.08, https://crudata.uea.ac.uk/cru/data/hrg/).
Determination of metal concentrations
Concentrations of Cd and Fe in each sample were measured using the method described by Yao et al. (2023) [32]. In brief, each dried sample was ground to powder, and 0.20 g powder was digested using a mixture of acids (HNO3/HClO4; v/v, 4:1) at 280 °C. The digested solution was loaded onto an inductively coupled plasma mass spectrometer (ICP-MS; NexION, 2000; PerkinElmer, USA) to determine the concentrations of Cd and Fe. Reference standard solutions of Cd and Fe were purchased from the Guobiao Testing and Certification Company (Beijing, China).
Calculation of cd uptake, translocation and distribution
Cd uptake, root-to-shoot translocation and shoot distribution were calculated using the methods described by Shi et al. (2019) [37] and Cheng et al. (2021) [11].
(1) Tissue Cd content = Tissue Cd concentration × Tissue dry weight;
(2) Whole plant Cd content = ∑Cd content in each tissue;
(3) Cd uptake = Whole plant Cd content ÷ Whole plant dry weight;
(4) Shoot Cd content = Whole plant Cd content – Root Cd content;
(5) Cd translocation factor (TF) = Shoot Cd content ÷ whole plant Cd content;
(6) Cd distribution = Tissue Cd content ÷ whole plant Cd content;
(7) Continuous Cd uptake = Whole plant Cd content at maturity stage − Whole plant Cd content at early grain filling stage.
Total RNA extraction and RT-qPCR
At the grain filling stage, roots and shoots were sampled from each subgroup and frozen in liquid nitrogen for RNA isolation. Total RNA was isolated using the Total RNA Kit II (Omega, United States). cDNA was synthesized from 1 µg total RNA by using the M-MLV First Strand cDNA Synthesis Kit (Omega, United States). The expression levels of the metal transporter genes (TpIRT1, TpYSL15, TpHMA2, TpNRAMP2, TpNRAMP5, TpZIP1, TpZIP3, and TpZIP5) were normalized using the method Chai et al. (2022) described [36]. The specific primers we used are listed in Supplemental Table S2. TpACTIN and TpGAPDH were used as reference genes [38]. The software CFX Manager 3.1 (Bio-Rad, United States) and the 2ΔΔCt method were used to calculate the relative expression level.
Statistical analysis
All data are reported as the mean values of three replicates with standard deviations. Statistical analyses were performed using a one-way analysis of variance (ANOVA) and Turkey’s test using SPSS 20 (IBM Corporation, USA). Graphs were plotted using SigmaPlot (version 14.0; Systat Software Inc., USA).
Results
Wheat growth at grain filling and maturity
During the entire growth stage, all plants were very healthy. At the grain filling stage, soil application of FeCl3 and Fe2(SO4)3 significantly enhanced photosynthetic parameters, including assimilation rate, transportation rate, and stomatal conductance, but did not change internal CO2 concentration and water use efficiency when compared with CK (Table 1). Moreover, they did not change the plant height, spike length, and tiller numbers of DPW (Supplemental Figure S1), but differentially changed the dry weight of several tissues per plant. For example, FeCl3 application did not changed the dry weight of all tissues of DPW at the grain filling stage, but significantly increased dry weight of grain by 16.80% and dry weight of lower nodes by 0.10% at maturity. Fe2(SO4)3 application significantly increased dry weight of grain by 51.70% and decreased dry weight of root by 28.69% at the grain filling stage, and increased dry weight of grain by 15.47% and dry weights of glumes, lower nodes, lower internodes, and whole plants at maturity when compared with those of the CK (Table 2).
Table 1.
Photosynthetic parameters of DPW under Fe fertilizers treatments at grain filling stage
| Treatments | Assimilation rate | Transpiration rate | Internal CO2 | Stomatal conductance | Vapour pressure deficit | Water use efficiency |
|---|---|---|---|---|---|---|
| (μ mol m-2 s-1) | (m mol m-2 s-1) | (ppm) | (m mol m-2 s-1) | (mb) | (%) | |
| CK | 17.20 ± 0.17 b | 5.73 ± 0.35 b | 293.33 ± 3.33 a | 303.33 ± 2.03 b | 2.03 ± 0.03 a | 3.17 ± 0.13 a |
| FeCl3 | 21.27 ± 0.50 a | 7.33 ± 0.33 a | 295.67 ± 1.20 a | 453.67 ± 12.41 a | 1.90 ± 0.06 a | 2.90 ± 0.06 a |
| Fe2(SO4)3 | 20.05 ± 1.25 a | 7.25 ± 0.15 a | 305.00 ± 7.00 a | 435.00 ± 12.00 a | 1.90 ± 0.01 a | 2.80 ± 0.20 a |
Values are presented as means ± standard deviation (n = 3); different lowercase indicated significant differences in photosynthetic parameters between treatments at the grain filing stage (P ≤ 0.05).
Table 2.
Dry weight of DPW under Fe fertilizers treatments at grain filling and maturity stages
| Tissues | Grain filling stage | Maturity stage | |||||
|---|---|---|---|---|---|---|---|
| CK | FeCl3 | Fe2(SO4)3 | CK | FeCl3 | Fe2(SO4)3 | ||
| Grains | 1.47 ± 0.07 b | 1.46 ± 0.11 b | 2.23 ± 0.30 a | 3.75 ± 0.14 B | 4.38 ± 0.12 A | 4.33 ± 0.24 A | |
| Rachises | 1.25 ± 0.26 a | 1.39 ± 0.17 a | 1.32 ± 0.05 a | 2.02 ± 0.03 A | 1.88 ± 0.18 A | 2.55 ± 0.34 A | |
| Glumes | 6.69 ± 0.72 a | 7.56 ± 0.23 a | 7.28 ± 0.57 a | 6.25 ± 0.30 B | 5.91 ± 0.59 B | 8.52 ± 0.46 A | |
| Node 1 | 0.29 ± 0.07 a | 0.32 ± 0.03 a | 0.29 ± 0.06 a | 0.59 ± 0.02 A | 0.59 ± 0.03 A | 0.58 ± 0.02 A | |
| Internode 1 | 4.38 ± 0.76 a | 3.99 ± 0.68 a | 4.26 ± 1.17 a | 5.16 ± 0.28 A | 4.64 ± 0.18 A | 4.96 ± 0.21 A | |
| Flag leaves | 5.53 ± 0.58 a | 6.91 ± 1.10 a | 6.27 ± 0.62 a | 5.45 ± 0.24 AB | 4.72 ± 0.87 B | 6.37 ± 0.44 A | |
| Lower nodes | 1.22 ± 0.25 a | 1.15 ± 0.08 a | 0.93 ± 0.08 a | 1.72 ± 0.09 C | 1.90 ± 0.04 B | 2.14 ± 0.07 A | |
| Lower internodes | 5.87 ± 0.44 a | 6.33 ± 0.46 a | 5.70 ± 1.10 a | 6.19 ± 0.71 B | 6.93 ± 0.24 AB | 7.44 ± 0.24 A | |
| Lower leaves | 9.56 ± 1.03 a | 10.80 ± 0.33 a | 10.40 ± 0.81 a | 10.37 ± 0.54 AB | 8.66 ± 0.80 B | 10.60 ± 0.83 A | |
| Roots | 2.37 ± 0.44 a | 2.00 ± 0.04 ab | 1.69 ± 0.02 b | 1.93 ± 0.04 A | 1.91 ± 0.04 A | 1.31 ± 0.08 B | |
| Whole plant | 38.63 ± 1.77 a | 41.90 ± 2.36 a | 40.38 ± 3.10 a | 43.44 ± 1.07 B | 41.53 ± 1.90 B | 48.49 ± 1.50 A | |
Values are presented as means ± standard deviation (n = 3); different lowercase and uppercase letters indicate the significant differences in tissue dry weights among treatments at grain filling and maturity stages, respectively (P ≤ 0.05).
Tissue Cd and Fe concentrations at the grain filling and maturity stages
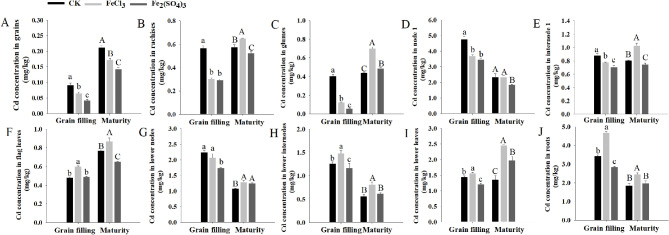
For the CK, the grain Cd concentrations of DPW were 0.09 mg/kg and 0.21 mg/kg at the grain filling and mature stages, respectively. Soil application of FeCl3 and Fe2(SO4)3 dramatically reduced the grain Cd concentrations to 0.07 mg/kg and 0.04 mg/kg at the grain filling stage, and 0.17 mg/kg (reduced ratio of 19.04%) and 0.14 mg/kg (reduced ratio of 33.33%) at maturity, respectively (Fig. 1A). To reveal the underlying mechanisms, we investigated Cd concentrations in other tissues. At the grain filling stage, Fe2(SO4)3 application significantly reduced the Cd concentrations of all tissues (except flag leaves) with the decreased ranges from 7.86% (lower internodes) to 85.78% (glumes) when compared with the CK; while, FeCl3 application only reduced the Cd concentrations of rachis (46.12%), glumes (70.01%), node 1 (22.20%), and internodes 1 (11.95%), but increased the Cd concentrations of flag leaves (24.89%), lower internodes (17.11%), lower leaves (8.29%), and roots (36.35%) (Fig. 1B and J). At maturity, different results were observed. Compared with the CK, the application of Fe2(SO4)3 decreased the Cd concentrations of rachis (8.81%), node 1 (21.35%), internode 1 (7.41%) and flag leaves (15.45%) but increased the Cd concentrations of glumes (11.12%), lower nodes (15.91%), and lower leaves (23.08%). The application of FeCl3 increased the Cd concentrations of all tissues (except node 1) with the increased range from 12.65% (flag leaves) to 67.67% (lower leaves) (Fig. 1B and J).
Fig. 1.
Tissue Cd concentration of DPW at grain filling and maturity stages
A-J: Grains, rachises, glumes, node 1, internode 1, flag leaves, lower nodes, lower internodes, lower leaves and roots, respectively. Values are presented as means ± standard deviation (n = 3); lowercase and uppercase letters indicate the significant differences in Cd concentrations among treatments at grain filling and maturity stages, respectively (P ≤ 0.05)
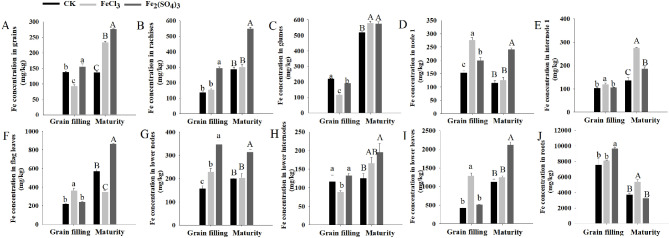
The grain Fe concentrations of DPW were similar at approximately of 137.83 mg/kg at both stages for the CK (Fig. 2A). Soil application of FeCl3 significantly reduced grain Fe concentration to 92.79 mg/kg at the grain filling stage and increased it to 234.26 mg/kg at maturity; application of Fe2(SO4)3 increased the grain Fe concentration to 156.22 mg/kg at the grain filling stage and to 276.48 mg/kg at maturity (Fig. 2A). Soil application of FeCl3 and Fe2(SO4)3 also influenced the Fe concentrations of other tissues (Fig. 2B and J). Compared with the CK, application of FeCl3 significantly reduced the Fe concentrations of glumes and lower internodes but increased those of node 1, internode 1, flag leaves, lower nodes, and lower leaves at the grain filling stage and increased the Fe concentrations of glumes, internode 1 and roots but reduced those of flag leaves at maturity (Fig. 2B and J). Application of Fe2(SO4)3 increased the Fe concentrations of rachises, node 1, lower nodes, and roots but reduced those of glumes at the grain filling stage and increased the Fe concentrations of all tissues (except roots) at maturity (Fig. 2B and J).
Fig. 2.
Tissue Fe concentration of DPW at grain filling and maturity stages
A-J: Grains, rachises, glumes, node 1, internode 1, flag leaves, lower nodes, lower internodes, lower leaves and roots, respectively. Values are presented as ± standard deviation (n = 3); lowercase and uppercase letters indicate the significant differences in Fe concentrations among treatments at grain filling and maturity stages, respectively (P ≤ 0.05)
Uptake and root-to-shoot translocation of Cd and Fe
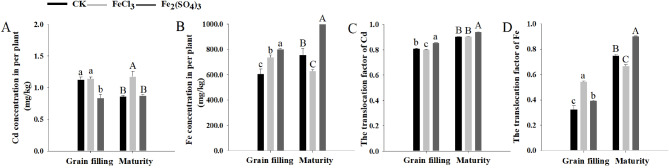
For the CK, Cd concentration of the whole plant was 1.13 mg/kg at the grain filling stage and 0.86 mg/kg at maturity. Soil application of FeCl3 did not alter the Cd concentration of the whole plant at the grain filling stage but increased it to 1.18 mg/kg at maturity, and the application of Fe2(SO4)3 reduced the Cd concentration of the whole plant to 0.84 mg/kg at the grain filling stage but did not change that at maturity (Fig. 3A). For the CK, Fe concentration in the whole plant was 607.68 mg/kg at the grain filling stage and 756.05 mg/kg at maturity. Application of FeCl3 significantly increased the Fe concentration of the whole plant to 739.12 mg/kg at the grain filling stage, but that decreased to 628.54 mg/kg at maturity; the application of Fe2(SO4)3 increased the Fe concentration of the whole plant to 802.24 mg/kg at the grain filling stage and 1007.39 mg/kg at maturity (Fig. 3B).
Fig. 3.
Per plant concentration and TF of Cd and Fe in DPW
A: Cd concentration; B: Fe concentration; C: Cd TF; D: Fe TF. Values are presented as ± standard deviation (n = 3); lowercase and uppercase letters indicate significant differences in Cd and Fe concentration in per plant and TF among treatments at grain filling and maturity stages, respectively (P ≤ 0.05)
For the CK, root-to-shoot Cd translocation was 0.81 at the grain filling stage and 0.90 at maturity; root-to-shoot Fe translocation was 0.32 at the grain filling stage and 0.75 at maturity. Compared with the CK, the application of FeCl3 significantly inhibited the root-to-shoot Cd translocation at the grain filling stage but did not influence that at maturity (Fig. 3C). It increased the root-to-shoot Fe translocation at the grain filling stage but reduced that at maturity (Fig. 3D). By contrast, the application of Fe2(SO4)3 dramatically increased the root-to-shoot translocation of Cd and Fe at the grain filling stage or maturity (Fig. 3C and D).
Continuous absorption or efflux of Cd during grain filling
The change in the Cd content of the whole plant during grain filling represents the continuous absorption or efflux of Cd. During grain filling, Cd content of the whole plant was significantly reduced in the CK but increased with soil application of Fe2(SO4)3 and did not change with soil application of FeCl3. These results indicate that DPW showed a continuous Cd efflux under CK; however, soil application of Fe2(SO4)3 promoted continuous Cd absorption (Supplemental Figure S2).
Distribution and shoot remobilization of Cd during grain filling
At the grain filling stage, Cd was mainly distributed in lower leaves (31.66%), followed by lower internodes (17.02%) and roots (18.73%), subsequently in internodes 1 (8.88%), lower nodes (6.29%), glumes (6.25%), flag leaves (6.10%), node 1 (3.13%), rachises (1.62%), and was the lowest in the grains for the CK (Table 3). Compared with the CK, soil application of Fe2(SO4)3 and FeCl3 significantly increased Cd distribution in the lower leaves, lower internodes, and flag leaves but decreased Cd distribution in the grains, rachis, glumes, and lower nodes (Table 3). At maturity, Cd was also mainly distributed in lower leaves (37.55%) and subsequently in flag leaves (11.24%), internodes 1 (11.15%), roots (9.57%), lower internodes (9.23%), glumes (7.37%), lower nodes (4.94%), node 1 (3.72%), rachises (3.11%) and grains (2.13%) for the CK (Table 3). Compared with the CK, soil application of Fe2(SO4)3 and FeCl3 also significantly increased Cd distribution in lower leaves, lower internodes, and glumes but reduced it in grains, internode 1, flag leaves, and node 1 (Table 3). Moreover, the Cd distribution ratios of the lower leaves, lower nodes, flag leaves, and glumes were significantly higher in the soil application of Fe2(SO4)3 than FeCl3.
Table 3.
Proportion of cd content in DPW at grain filling and maturity stages
| Tissues | Grain filling | Maturity | Change value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CK | FeCl3 | Fe2(SO4)3 | CK | FeCl3 | Fe2(SO4)3 | CK | FeCl3 | Fe2(SO4)3 | |
| Grains | 0.31% ± 0.01 a | 0.20% ± 0.02 c | 0.28% ±0.01 b | 2.13% ± 0.12 A | 1.56% ± 0.21 B | 1.46% ± 0.17 B | 1.82% | 1.36% | 1.18% |
| Rachises | 1.62% ± 0.12 a | 0.89% ± 0.15 b | 1.15% ± 0.11 b | 3.11% ± 0.26 A | 2.51% ± 0.14 B | 2.78% ± 0.19 A | 1.48% | 1.62% | 1.63% |
| Glumes | 6.25% ± 0.54 a | 1.93% ± 0.32 b | 1.25% ± 0.25 c | 7.37% ± 0.23 C | 8.47% ± 0.19 B | 9.82% ± 0.32 A | 1.11% | 6.54% | 8.57% |
| Node 1 | 3.13% ± 0.17 a | 2.45% ± 0.22 b | 2.94% ± 0.12 a | 3.72% ± 0.23 A | 2.81% ± 0.43 B | 2.53% ± 0.31 B | 0.58% | 0.36 | -0.41% |
| Internode 1 | 8.88% ± 0.22 a | 6.47% ± 0.32 b | 8.95% ± 0.17 a | 11.15% ± 0.16 A | 9.78% ± 0.25 B | 8.74% ± 0.31 C | 2.27% | 3.31% | -0.20% |
| Flag leaves | 6.10% ± 0.12 b | 8.67% ± 0.43 a | 9.12% ± 0.37 a | 11.24% ± 0.24 A | 8.39% ± 0.35 C | 9.79% ± 0.42 B | 5.14 | -0.28% | 0.66% |
| Lower nodes | 6.29% ± 0.31 a | 4.97% ± 0.24 b | 4.82% ± 0.18 b | 4.94% ± 0.13 B | 5.03% ± 0.11 B | 6.28% ± 0.32 A | -1.35% | 0.05% | 1.46% |
| Lower internodes | 17.02% ± 0.11 b | 19.58% ± 0.32 a | 19.81% ± 0.26 a | 9.23% ± 0.17 B | 11.46% ± 0.32 A | 10.78% ± 0.43 A | -7.79 | -8.12% | -9.03% |
| Lower leaves | 31.66% ± 0.54 b | 35.25% ± 0.39 a | 37.36% ± 0.27 a | 37.55% ± 0.65 B | 40.35% ± 0.47 B | 41.74% ± 0.28 A | 5.89% | 5.10% | 4.38% |
| Roots | 18.73% ± 0.76 a | 19.59% ± 0.34 a | 14.31% ± 0.46 b | 9.57% ± 0.13 A | 9.64% ± 0.22 A | 6.07% ± 0.43 B | -9.16 | -9.95% | -8.24% |
Values are presented as means ± standard deviation (n = 3); different lowercase and uppercase letters indicate the significant differences in tissue proportion of Cd content
From the grain filling stage to maturity, Cd distribution in the lower internodes and roots was significantly reduced in all treatments (Table 3). Cd distribution in lower nodes was also reduced in the CK. The reduction in Cd distribution indicates that these tissues were sources of Cd remobilization during grain filling. Cd distribution in the lower leaves, glumes, rachises, and grains was enhanced in all treatments (Table 3). Cd distributions in lower nodes in Fe2(SO4)3 application, flag leaves and internode 1 in the CK, and internode 1 in FeCl3 application also increased. The increase in Cd distribution indicates that these tissues were sinks for Cd remobilization.
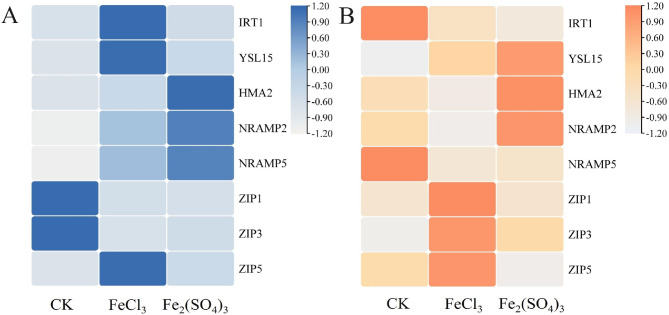
Relative expression of metal transporter genes
Soil application of FeCl3 and Fe2(SO4)3 also differentially regulated the expression of metal transporter genes, including TpIRT1, TpYSL15, TpHMA2, TpNRAMP2, TpNRAMP5, TpZIP1, TpZIP3 and TpZIP5, in DPW roots and shoots at the filling stage (Fig. 4). TpIRT1, TpNRAMP5, TpZIP3, and TpZIP5 were mainly expressed in the roots; TpHMA2, TpYSL15, and TpZIP3 were mainly expressed in the roots and shoots; and TpNRAMP2 was mainly expressed in the shoots.
Fig. 4.
Heatmap of metal transporter genes in root and shoot of DPW
A: Root; B: shoot. Values are presented as ± standard deviation (n = 3). Different expressions are performed in gradient ramp; the deeper the color, the higher gene expression.
In the roots, compared with the CK, soil application of FeCl3 and Fe2(SO4)3 significantly upregulated the expression levels of TpYSL15 and TpNRAMP5 but downregulated the expression levels of TpZIP1 and TpZIP3 (Fig. 4A). The expression of TpIRT1 and TpHMA2 was upregulated by FeCl3 and Fe2(SO4)3 application (Fig. 4A). However, the expression level of TpZIP5 was unchanged.
In shoots, compared with the CK, soil application of FeCl3 and Fe2(SO4)3 significantly upregulated the expression levels of TpYSL15 and TpZIP3 but downregulated the expression levels of TpNRAMP5 and TpIRT1 (Fig. 4B). The expression levels of TpHMA2 and TpNRAMP2 were dramatically upregulated by Fe2(SO4)3 application but downregulated by FeCl3 application (Fig. 4B).
Discussion
In this study, the soil application of FeCl3 and Fe2(SO4)3 reduced the grain Cd concentration in DPW at maturity (Fig. 1A). This reduction did not result from plant growth or development because there was no change in plant height, spike length, or tiller number. However, we did not exclude the dilution effect of grain dry weight on the reduction in grain Cd concentration, although the increased ratios of grain dry weight per plant (16.80% and 15.47%) were significantly lower than the decreased ratios of grain Cd concentration (19.04% and 33.33%) (Fig. 1A). Grain weight is jointly regulated by pre-anthesis nutrient accumulation and photosynthesis during grain filling [39, 40]. Thus, the increase in grain dry weight might have resulted from the enhanced assimilation rate, transportation rate, and stomatal conductance at the grain filling stage caused by the soil application of FeCl3 and Fe2(SO4)3 (Tables 1 and 2). The enhances may promote aboveground biomass accumulation and transport to grains, because they are closely and positively correlated [41].
Our previous hydroponic experiment showed that application of FeCl3 and Fe2(SO4)3 significantly reduced Cd uptake but promoted root-to-shoot Cd translocation in DPW seedlings [32]. In this study, soil application of FeCl3 significantly enhanced Cd uptake but did not change root-to-shoot Cd translocation, and soil application of Fe2(SO4)3 did not change Cd uptake but promoted root-to-shoot Cd translocation in DPW at maturity (Fig. 3A and C). These differences may be due to different growth conditions (hydroponic and field), external Cd concentration (80 µM and 0.30 mg/kg) and growth period (seedling and mature). The enhanced Cd uptake by FeCl3 application probably resulted from the soil application of FeCl3 (1) promoting Cd bioavailability [31], and (2) upregulating the expression level of a Cd absorber gene TpIRT1 when compared with the CK (Fig. 4A; [27]). The potential reasons for the unchanged Cd uptake and enhanced root-to-shoot Cd translocation by Fe2(SO4)3 were that soil application of Fe2(SO4)3 (1) did not change Cd bioavailability [31], or (2) did not change the expression level of Cd absorber gene TpIRT1 when compared with those of the CK (Fig. 4A), but (3) significantly upregulated the expression level of TpHMA2, whose homologous gene OsHMA2 is responsible for root-to-shoot Cd translocation in rice [42]. Soil application of Fe2(SO4)3 upregulated the expression level of Cd absorber gene TpNRAMP5, which potentially promoted the continuous absorption of Cd during grain filling (Table 3; [22]). This may also explain why Fe2(SO4)3 application reduced Cd uptake at the grain filling stage but did not change that at maturity (Fig. 3A). TpZIP1, whose homologous gene OsZIP1 encodes a Cd efflux transporter that limits excess Cd accumulation in rice [43], was highly expressed in the roots under CK (Fig. 4A), supporting that it was responsible for Cd efflux during grain filling (Table 3). Cd uptake, root-to-shoot Cd translocation, and Cd continuous absorption positively determine the grain Cd concentration in wheat [11, 15, 17–19]. Thus, the reduction in grain Cd concentration in the DPW caused by soil application of FeCl3 and Fe2(SO4)3 was independent of Cd uptake, root-to-shoot Cd translocation, and continuous Cd absorption. These results differed from those of the soil application of EDTA-Na2Fe, which reduced the grain Cd concentration in rice by reducing Cd uptake and root-to-shoot Cd translocation [30].
Intrinsically, soil application of FeCl3 and Fe2(SO4)3 reduced the grain Cd concentration by altering shoot Cd distribution or redistribution (Table 3). During grain filling in wheat, Cd remobilization from internodes and leaves mainly contributes to grain Cd concentration [11, 17, 18]. In this study, Cd was remobilized from lower internodes to grains and glumes in DPW, which was why soil application of FeCl3 and Fe2(SO4)3 increased Cd distribution in glumes during grain filling (Table 3). Internodes store pre-anthesis reserves in their phloem parenchyma and play a critical role in grain yield formation [44, 45]. However, in this study, lower nodes played a distinct role in reducing the gain in Cd concentration. This node is a core site for the xylem-to-phloem transfer of Cd [28, 46], and the transfer of Cd to grains via the phloem is restricted to lower nodes of wheat [17]. Soil application of FeCl3 and Fe2(SO4)3 completely inhibited Cd remobilization from lower nodes and altered the sinks (Table 3). Thus, they limited the xylem-to-phloem transfer of Cd in lower nodes and then increased Cd distributions in lower internodes and lower leaves via the xylem when compared with the CK (Table 3; [15, 47], but decreased Cd distributions in grains, rachises, node 1, internode 1 and flag leaves (Table 3). Additionally, Cd distributions in the lower leaves, lower nodes, and glumes were significantly higher in the Fe2(SO4)3 treatment than in the FeCl3 treatment (Table 3), resulting in a lower grain Cd concentration in the former than in the latter (Fig. 1A). The changes in Cd distribution in these tissues did not correspond to the changes in dry weight (Tables 2 and 3). Thus, tissue senescence did not catalyze Cd distribution from vegetative tissues to grains during grain filling, similar to previous study in durum wheat [18].
Another reason for the distribution of Cd is the regulation of metal transporter genes.
In rice, knockout of OsNRAMP2 and OsHMA2 significantly limits Cd distribution to upper nodes and developing tissues [47, 48]. In this study, expression levels of TpHMA2 and TpNRAMP2 in DPW shoots were upregulated by Fe2(SO4)3 application and downregulated by FeCl3 application (Fig. 4B), which did not illustrate changes in Cd distribution. Thus, TpHMA2 and TpNRAMP2 were not responsible for the change in Cd distribution due to the soil application of Fe2(SO4)3 and FeCl3. We also found that soil application of Fe2(SO4)3 and FeCl3 regulated the expression of TpIRT1 and TpNRAMP5, to affect Cd distribution in DPW shoots and grain Cd concentration. The reasons for this are as follows: (1) soil application of Fe2(SO4)3 and FeCl3 significantly downregulated the expression levels of TpIRT1 and TpRNRAMP5 in DPW shoots when compared with those of the CK (Fig. 4B), consistent with the increases in Cd distributions of lower leaves, lower internodes, and glumes (Table 3), and the decrease in Cd concentration of grains; (2) TpIRT1 and TpRNRAMP5 individually encode a Cd influx transporter because the expression of TpIRT1 and TpRNRAMP5 increased the Cd concentration in yeast (Peng et al., 2018; Jiang et al., 2021); (3) knockout of OsNRAMP5, the homologous genes of TpNRAMP5 increased the metal accumulation in the lower leaves but reduced that in the shoot elongating zone and nodes [49] (Huang et al., 2024). Since OsZIP3 is responsible for the preferential distribution of Zn to developing tissue in rice [50] (Sasaki et al., 2015); thus, soil application of Fe2(SO4)3 and FeCl3 via upregulating the expression of its homologous gene TpZIP3 to increase grain Zn concentration in DPW.
Conclusion
In conclusion, the present study showed that soil application of Fe2(SO4)3 and FeCl3 (0.04 M Fe3+/m2) significantly reduced grain Cd concentration and increased grain yield per plant in DPW. Thus, applying appropriate concentrations of Fe fertilizer in agricultural production can promote the yield and protect the grain quality. The reductions in grain Cd concentration were independent of wheat plant growth and development, Cd uptake, root-to-shoot translocation, and root continuous absorption, and resulted from the increase in Cd distribution in the lower leaves, lower internodes, and glumes. Soil application of Fe2(SO4)3 and FeCl3 increased Cd distribution in these tissues by upregulating the expression levels of TpYSL15 and TpZIP3, and downregulating the expression levels of TpIRT1, TpNRAMP5 in shoots.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- Cd
cadmium
- CK
the control
- DPW
dwarf Polish wheat
- HMA
p1B-ATPase-Heavy metal associated protein
- ICP-MS
inductively coupled plasma mass spectrometry
- IRT
iron regulated transporter
- K
potassium
- N
nitrogen
- NRAMP
natural resistance-associated macrophage protein
- P
phosphorus
- RT-qPCR
quantitative reverse transcription polymerase chain reaction
- TF
translocation factor
- YSL
yellow stripe 1-like proteins
- ZIP
Zinc regulated transporter/IRT-like protein
Author contributions
QY, YC and YW wrote the main manuscript text. QY, YY, JC and XL prepared Figs. 1, 2, 3 and 4. QY, MH and DL prepared Tables 1, 2 and 3. YC, YW, YZ, HK, JZ, DW, XF, LS and HZ discussed the results and commented on the paper. All authors reviewed the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (32272032 and 32301752), the China Postdoctoral Science Foundation (2022M712291), and the Cultivation Project of Sichuan Province Science and Technology Innovation Seedling Project (MZGC20230112).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Wang, Email: wangyi@sicau.edu.cn.
Yiran Cheng, Email: chengyiran@sicau.edu.cn.
References
- 1.Rai PK, Lee SS, Zhang M, Tsang YF, Ki-Hyun K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ Int. 2019;125:365–85. [DOI] [PubMed] [Google Scholar]
- 2.Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Chen W, Liu F, Wan Y. Physiological responses of peanut seedlings to exposure to low or high cadmium concentration and the alleviating effect of exogenous nitric oxide to high cadmium concentration stress. Plant Biosystems-An Int J Dealing all Aspects Plant Biology. 2020;154:405–12. [Google Scholar]
- 4.Zhang L, Zhang C, Du B, Lu B, Zhou D, Zhou J, Zhou J. Effects of node restriction on cadmium accumulation in eight Chinese wheat (Triticum turgidum) cultivars. Sci Total Environ. 2020;725:138358. [DOI] [PubMed] [Google Scholar]
- 5.Sarker MJ, Polash AU, Islam MA, Rima NN, Farhana T. Heavy metals concentration in native edible fish at upper Meghna River and its associated tributaries in Bangladesh: a prospective human health concern. SN Appl Sci. 2020;2:1–13. [Google Scholar]
- 6.Shi T, Wang Y. Heavy metals in indoor dust: spatial distribution, influencing factors, and potential health risks. Sci Total Environ. 2021;755:142367. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Xiong J, Tao L, Cao Z, Tang W, Zhang J, Yu X, Fu G, Zhang X, Lu Y. Regulatory mechanisms of nitrogen (N) on cadmium (cd) uptake and accumulation in plants: a review. Sci Total Environ. 2020;708:135186. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Luo N, Li Y, Cai Q, Li H, Mo C, Wong M. Cadmium in rice: transport mechanisms, influencing factors, and minimizing measures. Environ Pollut. 2017;224:622–30. [DOI] [PubMed] [Google Scholar]
- 9.Shang E, Xu E, Zhang H, Huang C. Temporal-spatial trends in potentially toxic trace element pollution in farmland soil in the major grain-producing regions of China. Sci Rep. 2019;9:19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Zhang P, Adeel M, Guo Z, Chetwynd AJ, Ma C, Bai T, Hao Y, Rui Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as fe fertilizers. Environ Pollut. 2021;269:116134. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Yang T, Xiang W, Li S, Fan X, Kang H, Wu D, Zhang H, Zeng J, Zhou Y, Wang Y. Ammonium-nitrogen addition at the seedling stage does not reduce grain cadmium concentration in two common wheat (Triticum aestivum L.) cultivars. Environ Pollut. 2021;286:117575. [DOI] [PubMed] [Google Scholar]
- 12.Guttieri MJ, Baenziger PS, Frels K, Carver B, Arnall B, Wang SC, Akhunov E, Waters BM. Prospects for selecting wheat with increased zinc and decreased cadmium concentration in grain. Crop Sci. 2015;55:1712–28. [Google Scholar]
- 13.Codex Alimentarius Commission. General standard for contaminants and toxins in food and feed. CODEX Stand. 2019; 193–1995.
- 14.Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel M, Ok YS. Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf. 2016;130:43–53. [DOI] [PubMed] [Google Scholar]
- 15.Clemens S, Aarts MGM, Thomine S, Verbruggen N. Plant science: they key to preventing slow cadmium poisoning. Trends Plant Sci. 2013;18:1360–85. [DOI] [PubMed] [Google Scholar]
- 16.Uraguchi S, Fujiwara T. Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice. 2012;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubo K, Kobayashi H, Fujita M, Ota T, Minamiyama Y, Watanabe T, Nakajima T, Shinano T. Varietal differences in the absorption and partitioning of cadmium in common wheat (Triticum aestivum L). Environ Exp Bot. 2016;124:79–88. [Google Scholar]
- 18.Yan B, Nguyen C, Pokrovsky OS, Candaudap F, Coriou C, Bussiere S, Robert T, Cornu JY. Contribution of remobilization to the loading of cadmium in durum wheat grains: impact of post-anthesis nitrogen supply. Plant Soil. 2018;424:591–606. [Google Scholar]
- 19.Yan B, Nguyen C, Pokrovsky OS, Candaudap F, Coriou C, Bussi`ere S, Robert T, Cornu JY. Cadmium allocation to grains in durum wheat exposed to low cd concentrations in hydroponics. Ecotoxicol Environ Saf. 2019;184:109592. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki A, Yamaji N, Ma JF. Overexpression of OsHMA3 enhances cd tolerance and expression of zn transporter genes in rice. J Exp Bot. 2014;65:6013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pottier M, Oomen R, Picco C, Giraudat J, Scholz-Starke J, Richaud P, Carpaneto A, Thomine S. Identification of mutations allowing natural resistance associated macrophage proteins (NRAMP) to discriminate against cadmium. Plant J. 2015;83:625–37. [DOI] [PubMed] [Google Scholar]
- 22.Peng F, Wang C, Zhu J, Zeng J, Kang H, Fan X, Sha L, Zhang H, Zhou Y, Wang Y. Expression of TpNRAMP5, a metal transporter from Polish wheat (Triticum polonicum L.), enhances the accumulation of Cd, Co and Mn in transgenic Arabidopsis plants. Planta. 2018;247:1395–406. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Tang Y, Zhou C, Xie S, Xiao S, Baker AJ, Qiu R. Mechanisms of Fe biofortification and mitigation of cd accumulation in rice (Oryza sativa L.) grown hydroponically with Fe chelate fertilization. Chemosphere. 2017;175:275–85. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Wang C, Liu Y, Yu K, Zhou Y. GmHMA3 sequesters cd to the root endoplasmic reticulum to limit translocation to the stems in soybean. Plant Sci. 2018;207:27–9. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Chen X, Yao Q, Long D, Fan X, Kang H, Zeng J, Sha L, Zhang H, Zhou Y, Wang Y. Overexpression of TtNRAMP6 enhances the accumulation of cd in Arabidopsis. Gene. 2019;696:225–32. [DOI] [PubMed] [Google Scholar]
- 26.Moore RET, Ullah I, Oliveira VH, Hammond SJ, Strekopytov S, Tibbett M, Dunwell JM, Rehkmper M. Cadmium isotope fractionation reveals genetic variation in cd uptake and translocation by Theobroma cacao and role of natural resistance associated macrophage protein 5 and heavy metal ATPase-family transporters. Hortic Res. 2020;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Chen X, Chai S, Sheng H, Sha L, Fan X, Zeng J, Kang H, Zhang H, Xiao X, Zhou Y, Vatamaniuk OK, Wang Y. TpIRT1 from Polish wheat (Triticum polonicum L.) enhances the accumulation of Fe, Mn, Co, and Cd in Arabidopsis. Plant Science. 2021; 312: 111508. [DOI] [PubMed]
- 28.Zhang Q, Huang D, Xu C, Zhu H, Feng R, Zhu Q. Fe fortification limits rice cd accumulation by promoting root cell wall chelation and reducing the mobility of cd in xylem. Ecotoxicol Environ Saf. 2022;240:113700. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Y, Wang C, Chai S, Shuai W, Sha L, Zhang H, Kang H, Fan X, Zhou Y, Wang Y. Ammonium N influences the uptakes, translocations, subcellular distributions and chemical forms of cd and zn to mediate the Cd/Zn interactions in dwarf Polish wheat (Triticum polonicum L.) seedlings. Chemosphere. 2018;193:1164–71. [DOI] [PubMed] [Google Scholar]
- 30.Shao G, Chen M, Wang D, Xu C, Mou R, Cao Z, Zhang X. Using iron fertilizer to control cd accumulation in rice plants: a new promising technology. Sci China C. 2008;51:245–53. [DOI] [PubMed] [Google Scholar]
- 31.Makino T, Takano H, Kamiya T, Itou T, Sekiya N, Inahara M, Sakurai Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: cd extraction mechanism and bench-scale verification. Chemosphere. 2008;70:1035–43. [DOI] [PubMed] [Google Scholar]
- 32.Yao Q, Li W, Liu Y, Cheng Y, Xiao X, Long D, Zeng J, Wu D, Sha L, Fan X, Kang H, Zhang H, Zhou Y, Wang Y. FeCl3 and Fe2(SO4)3 differentially reduce cadmium accumulation in Polish wheat (Triticum polonicum L.) seedlings by exporting cd from roots. Environ Pollut. 2023;317:120762. [DOI] [PubMed] [Google Scholar]
- 33.Kubo K, Watanabe Y, Oyanagi A, Kaneko S, Chono M, Matsunaka H, Seki M, Fujita M. Cadmium concentration in grains of Japanese wheat cultivars: genotypic difference and relationship with agronomic characteristics. Plant Prod Sci. 2008;11:243–9. [Google Scholar]
- 34.Cheng Y, Bao Y, Chen X, Yao Q, Wang C, Chai S, Zeng J, Fan X, Kang H, Sha L, Zhang H, Zhou Y, Wang Y. Different nitrogen forms differentially affect cd uptake and accumulation in dwarf Polish wheat (Triticum polonicum L.) seedlings. J Hazard Mater. 2020;400:123209. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y, Liu R, Yang T, Yang S, Chen J, Huang Y, Long D, Zeng J, Wu D, Kang H, Fan X, Sha L, Zhang H, Zhou Y, Wang Y. Genetic factors of grain cadmium concentration in Polish wheat (Triticum polonicum L). Plant Physiol. 2024. 10.1093/plphys/kiae353. [DOI] [PubMed] [Google Scholar]
- 36.Chai S, Yao Q, Liu R, Xiang W, Xiao X, Fan X, Zeng J, Sha L, Kang H, Zhang H, Long D, Wu D, Zhou Y, Wang Y. Identification and validation of a major gene for kernel length at the P1 locus in Triticum polonicum. Crop J. 2022;10:387–96. [Google Scholar]
- 37.Shi G, Li D, Wang Y, Liu C, Hu Z, Lou L, Rengel Zed, Cai Q. Accumulation and distribution of arsenic and cadmium in winter wheat (Triticum aestivum L.) at different developmental stages. Sci Total Environ. 2019;667:532–9. [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Deng X, Huang Y, Fang X, Zhang J, Wan H, Yang C. Comparison of subcellular distribution and chemical forms of cadmium among four soybean cultivars at young seedlings. Environ Sci Pollut Res. 2015;22:19584–95. [DOI] [PubMed] [Google Scholar]
- 39.Serrago RA, Alzueta I, Savin R, Slafer GA. Understanding grain yield responses to source-sink ratios during grain filling in wheat and barley under contrasting environments. Field Crops Res. 2013;150:42–51. [Google Scholar]
- 40.Cui H, Luo Y, Li C, Chang Y, Jin M, Li Y, Wang Z. Effects of nitrogen forms on nitrogen utilization, yield, and quality of two wheat varieties with different gluten characteristics. Eur J Agron. 2023;149:126919. [Google Scholar]
- 41.Zheng T, Zhang X, Yin G, Wang L, Han Y, Chen L, Huang F, Tang J, Xia X, He Z. Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1982 and 2008. Field Crops Res. 2011;122:225–33. [Google Scholar]
- 42.Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa N, Nakanishi H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012;35:1948–57. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Feng S, Zhang B, Wang M, Cao H, Rono JK, Chen X, Yang Z. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019;19:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Bragado R, Vicente R, Molero G, Serret MD, Maydup ML, Araus JL. New avenues for increasing yield and stability in C3 cereals: exploring ear photosynthesis. Curr Opin Plant Biol. 2020;13:1–12. [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Wang Z, Xiao F, Yang L, Li G, Ding Y, Paul MJ, Li W, Liu Z. Dynamics of dry matter accumulation in internodes indicates source and sink relations during grain-filling stage of japonica rice. Field Crops Res. 2021;263:108009. [Google Scholar]
- 46.Guo J, Zhang X, Ye D, Huang H, Wang Y, Zheng Z, Li T, Yu H. Crucial roles of cadmium retention in node II for restraining cadmium transport from straw to ear at reproductive period in a grain low-cadmium rice line (Oryza sativa L). Ecotoxicol Environ Saf. 2020;205:111323. [DOI] [PubMed] [Google Scholar]
- 47.Yamaji N, Sasaki A, Xia J, Yokosho K, Ma JF. A node-based switch for preferential distribution of manganese in rice. Nat Commun. 2013;4:2442. [DOI] [PubMed] [Google Scholar]
- 48.Chang J, Xie Y, Zhang H, Zhang S, Zhao F. The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germination and affects cd distribution to rice grain. Plant Soil. 2022;476:79–95. [Google Scholar]
- 49.Huang S, Konishi N, Yamaji N, Ma JF. Local distribution of manganese to leaf sheath is mediated by OsNramp5 in rice. New Phytol. 2024;241:1708–19. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki A, Yamaji N, Mitani-Ueno N, Kashino M, Ma JF. A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant J. 2015;84:374–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.