Abstract
Glioblastoma (GB) is a highly heterogeneous type of incurable brain cancer with a low survival rate. Intensive ongoing research has identified several potential targets; however, GB is marred by the activation of multiple pathways, and thus common targets are highly sought. The signal regulatory scaffold IQGAP1 is an oncoprotein implicated in GB. IQGAP1 nucleates a myriad of pathways in a contextual manner and modulates many of the targets altered in GB like MAPK, NF-κB, and mTOR/PI3K/Akt1, thus positioning it as a plausible common therapeutic target. Here, we review the targets that are subjects of GB treatment clinical trials and the commonly used animal models that facilitate target identification. We propose a model in which the dysfunction of various IQGAP1 pathways can explain to a larger extent some of the GB heterogeneity and offer a platform for personalized medicine.
Keywords: Glioblastoma, Scaffold proteins, Precision medicine, IQGAP1
Background: GB is morphologically and molecularly heterogenous disease
Glioblastoma (GB) is an incurable primary brain cancer arising in the cerebral hemispheres of the brain [1]. The World Health Organization (WHO) designates the GB as a grade 4 tumor, which is the highest on the malignancy scale. Despite the intensive basic and clinical research, the 5-year survival of treated GB remains at 5% with a median survival of about 15 months [2, 3]. The disease incidence in adults is about 3.19–4.17 cases per 100,000 persons, while in pediatric patients, the incidence is about 0.85 per 100,000 persons [4] The standard-of-care for GB includes surgical tumor resection, chemotherapy, and ionizing radiation. Several factors pose challenges to effective treatment, including the diffuse nature of the tumor that limits the scope of resection, the rapid proliferative rate of the tumor cells, the activation of multiple signaling pathways, the fast development of therapy-resistant clones, and the impediment of the blood-brain barrier (BBB) to therapy [5].
Initially believed to arise from glial cells, GB has been described as invasive and undifferentiated [6, 7]. The tumors and their surrounding microenvironment exhibit a heterogeneous character due to the varying appearance of necrosis, hemorrhage, or cystic degeneration [8]. Furthermore, a strong line of evidence suggests that GB arises not only from glia but also from multiple cell types with neural stem cell-like properties that exist at variable stages of differentiation, ranging from stem cells to neurons to glia [9–11]. This tumor heterogeneity hinders the classification and treatment of GB. Consistent with this view, it has long been suggested that these heterogeneous GB cells display phenotypic variations largely defined by molecular alterations in signaling pathways rather than by differences in cell types of origin [12]. Indeed, several studies have implicated many molecules in GB pathobiology (Table 1). However, the underlying molecular mechanisms leading to the inception or maintenance of the disease remain incompletely defined, thus hindering the development of targeted therapies. Accordingly, research is currently directed at addressing the issue of heterogeneity through single-cell-based assays such as single-cell Systems Genetics Network Analysis (scSYGNAL), RNA sequencing (RNA-seq), epigenetics, Transposase Accessible Chromatin (ATAC-Seq), and gene expression Spatial Transcriptomics [13, 14]. Thus, unraveling the causes and consequences of the molecular alterations leading to GB progression represents a crucial requisite to developing new targeted and safe therapeutic approaches for GB. The aim of this review is to summarize the current developments in GB research targets, preclinical animal models, and treatment clinical trials directed at plausible molecular targets, and discuss future directions to investigate new pathways in GB management involving a scaffold signaling protein as a common target.
Table 1.
Known molecular targets in glioblastoma
| Molecule | Function | Reference(s) |
|---|---|---|
| Epidermal Growth Factor Receptor (EGFR) | Receptor with tyrosinase kinase and ligand-binding activity; induces downstream cell proliferation | [18] |
| Platelet Derived Growth Factor A (PDGFA) | Subunit of the PDGF gene umbrella (six subunits that form ligand and tyrosine kinase receptors); functions in neuroprotection, glial cell development, and hematopoiesis | [26] |
| Phosphatase and TENsin homolog on chromosome 10q23 (PTEN) | Tumor suppressor gene; regulates cell proliferation, apoptosis, and DNA repair | [25] |
| Rat Sarcoma Virus (Ras) | Collection of G-proteins; regulate intermediates with signal transduction and cell proliferation pathways | [113] |
| Retinoblastoma Protein (RB) | Tumor suppressor protein; targets G1/S cell cycle checkpoint, and negatively regulates apoptosis | [114] |
| Tumor protein p53 (p53) | Tumor suppressor protein; prevents malignant transformation of cancer cells and eliminates damaged cells | [115] |
| Janus kinase/Signal transducer and activator of transcription (JAK/STAT) | Signaling pathway that activates transcription; triggers pro-tumorigenic functions: anti-apoptosis, cancer cell proliferation, and immune suppression | [34] |
| Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) | Collection of five transcription factors; control and trigger cell proliferation, motility, and differentiation | [37, 38] |
| Sonic Hedgehog (Shh) | Signaling pathway within the hedgehog (Hh) domain; assists in organogenesis, cell homeostasis, and neural cell type specification | [116] |
Identified molecular targets in GB
A wealth of molecules has been implicated in GB, but while illustrating the heterogeneity of the disease, they fall short of providing effective treatment. Several genetic mutations and pathway alterations have been implicated in GB development, which may contribute to the inter- and intra-heterogeneity of the tumors. As depicted in Table 1, these include genetic mutations of the epidermal growth factor receptor (EGFR), over-expression of platelet-derived growth factor subunit A (PDGFA), and loss of heterozygosity of phosphatase and TENsin homolog on chromosome 10q23 (PTEN). The mutations lead to alterations in downstream effector pathways, including the small GTPase Ras, the p53 tumor suppressor known as the guardian of the genome, and the cell cycle regulator retinoblastoma (RB) protein [1]. Interestingly, a subset of human GB cells without p53 mutations were reported to over-express the proto-oncogene mouse double minute 2 (MDM2), a negative regulator of p53 [15]. Further alterations include the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor, and the Sonic Hedgehog (Shh) signaling pathway [16]. Typically, GB displays dysfunction of genes, proteins, or pathways that control cell proliferation, cell-cell adhesion, and apoptosis (Table 1); which we will discuss in more detail below.
EGFR houses a combination of transmembrane (ligand-binding) and tyrosine kinase domains that control several downstream cellular pathways [17]. Thus, mutations in EGFR alter the downstream phosphatidylinositol-3-kinase (P13K/Akt) and the mitogen-activated protein kinase (MAPK) pathways [18]. The mammalian target of rapamycin (mTOR/P13K/Akt pathway) regulates cell cycle, apoptosis, cellular stress, and cell growth. In turn, Akt1 controls mTOR, which promotes protein biosynthesis of cyclin D1 [19]. The MAPK component ERK1/2 triggers a signaling cascade of the MAPK superfamily proteins that regulate cell cycle and cell proliferation [16, 20]. Research has also shown that EGFR colocalizes with the IQ motif-containing GTPase Activating Protein 1 (IQGAP1), a scaffold oncoprotein that regulates a plethora of cellular functions, including cell-cell contacts, cell motility, cell division and proliferation, protein traffic, and apoptosis [21, 22]; however, its underlying mechanisms in these various events are just emerging as discussed later below. PTEN is a tumor suppressor that has been identified as a frequent GB target because its mutations promote uncontrolled and rapid tumor progression [23]. Like EGFR, PTEN also modulates the mTOR/PI3k/Akt signaling through the conversion of PIP3 to PIP2 where the formation of PIP3 triggers the activation of mTOR/Akt, which activates key cell proliferation pathways [24] and modulates cell proliferation, apoptosis, and DNA repair [25]. PDGFA is a subunit that forms homo-, or hetero-dimers that are involved in embryogenesis, glioma cell development, and hematopoiesis [26]. In vivo studies in mice and rats have also shown that PDGFA can trigger oligodendrocyte precursor cells [27]. Further, overexpression of PDGFA in mouse models has led to GB development [28].
The retinoblastoma (RB) protein regulates the cell cycle through the arrest of the G1/S phase [16, 29]. RB proteins, when phosphorylated, do not bind to E2F, a transcription factor that promotes cell proliferation [29]. Conversely, when RB is not phosphorylated, the protein binds to E2F, thus inhibiting cell cycle progression into the S phase [29]. The RB pathway is altered in GB in many ways, including homozygous deletion, promoter methylation, or mutation of pathway component proteins (16). The tumor suppressor p53 protein that controls cell proliferation and cell cycle progression is also altered in GB [30]. The p53 protein contributes to preventing damaged cells from propagating through the cell cycle [31]. Typically, p53 is altered in GB through deletions of the CDKN2A/ARF locus [30, 32]. Gene deletions within CDKN2B and CDKN2C, which encode tumor suppressor genes CDK4 and CDK6, promote uncontrolled cancer cell proliferation [18, 33]. The JAK/STAT signaling pathway regulates tumorigenic functions like angiogenesis and anti-apoptosis along with mediating cell responses to growth factors or cytokines [34, 35]. JAK proteins, activated by cytokine stimulation, phosphorylate STAT proteins to initiate pathway activation [35]. The STAT component includes a collection of transcription factors in the cytoplasm that are activated by phosphorylation [34]. In GB, secretion of interleukin (IL)-6, IL-8, and growth factors activate the STAT proteins and increase tumor proliferation [34, 36].
The transcription factor NF-κB controls cell proliferation, motility, and differentiation through downstream effector activation that includes EGFR, PGFR, and receptor tyrosine kinases [37, 38]. GB tumors exhibit increased NF-κB activation accompanied by tumor cell proliferation and macrophage-induced inflammation [38, 39]. The Shh is a signaling pathway that functions in embryonic development and tissue homeostasis [16]. The Shh mechanism of action includes the release of a glioma-associated oncogene homolog (GLI1) [16]. GLI1, a zinc finger protein, is stabilized in promoting tumorigenic pathways in coordination with Shh [40].
Clearly, the normal cellular functions of these variable molecules are intertwined, and when one of them becomes aberrant, their function converges to induce or sustain the GB disease state. However, key questions remain, including whether the altered protein/pathway is a cause or a consequence of the tumor progression, and how the heterogeneity in GB evolves. These questions are particularly significant because the GB heterogeneity evolves over time, and the treatment itself induces further heterogeneity in the tumor [14]. For this purpose and others, several animal models have been developed that provide advantages as well as exhibit limitations in replicating the features of human GB, and that we consider below.
Preclinical animal models in GB studies
Although several animal models are currently being used in target research, there is not a single model that captures the features and complexities of human GB, but the collective results yielded by these models offer valuable information for understanding the landscape of the disease, at least in part. Rodent models, specifically rats and mice, are the primary animal models used in GB research [41]. The four main rat-brain tumor models include the C6 glioma, 9 L/LacZ gliosarcoma, RG-2 glioma, and F98 glioma [42]. In comparison to mice models, the main advantage of the rat-brain models over the mice models is the larger physical size that allows for greater implantation and localization accuracy [41]. Each rat glioma-model corresponds to a different cell line such as C6, 9 L/LacZ, RG-2, or F98 injected into a rat [41]. The C6 glioma model was developed from Wistar-Furth adult rats and highlights histological GB characteristics including tumor necrosis, vascular alterations, and various levels of invasiveness [43, 44]. There is also significant tumor growth caused by the secretion of angiogenic factors like the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [45]. The F98 model is similar to the C6 model in that it has significant vascular alterations and invasiveness and has an infiltrative and aggressive growth pattern that mimics the dynamic of human GB growth pattern [43]. Molecularly, research has shown that the F98 model exhibits over-expression of platelet-derived growth factor subunit B (PDGFB), Ras, epidermal growth factor receptor (EGFR), and cyclin D1/D2 [46].
The 9 L/LacZ and the RG-2 both have an aggressive glioma growth pattern, along with tumor margins, and corresponding feeder vessels [43]. The 9 L model has less vascularization and smaller necrotic centers, whereas the RG2 has larger necrotic centers [43, 45]. The 9 L model is highly immunogenic and models a mutant p53 gene [42]. Molecularly, the non-immunogenic RG2 model harbors a p53 wildtype and has increased expression of PDGFB, IGF-2, Ras, Erb3/HER3, and cyclin D2 [42]. While each model has unique characteristics, their growth patterns tend to be similar within the range of two to four days [43]. A comparison of vessel growth between the models showed that 9 L rat models had a higher average length of newly observed vessels, followed by RG-2, C6, and F98 [43]. In pre-existing vessels, F98 had the greatest average length change, followed by C6, 9 L, and RG2 [43]. Disadvantages of rat models include the potential for spontaneous resection, the substantial number of rats needed for modeling, and the monetary cost and time needed for maintenance [42]. Thus, there is no single rat model that replicates the complexity of GB, and the differences among them create difficulty in effectively modeling the disease and creating clinical therapeutics.
The four main mouse-GB models include syngeneic murine, genetically engineered, cell-line xenograft, and patient-derived xenograft (PDX) [47]. Genetically engineered mouse models (GEMM) are centered around Tet-regulated (tetracycline) Cre-inducible gene (Cre/loxP) technology that create genetic alterations like mutation, activation, and inversions [48]. GEMM also allows for cells to be expressed at certain time intervals and be isolated to certain singular or multiple cells [47]. However, GEMM production can take a significant amount of development time ranging from months to years [49].
Syngeneic mouse models are those that arise from spontaneous or chemically induced gliomas. Typical chemical models include GL261, GL26, and CT-2 A, while P560 is a spontaneous model [47, 50]. Murine models allow researchers to understand molecular GB interactions, quantify immune responses, and test potential clinical therapeutics [51]. The downside of syngeneic models includes a lack of accurate tumor microenvironments as seen in human GB, susceptibility to genetic drift, and a high mutation rate [3]. The two types of xenograft mouse models are patient- or cell-line-derived. Patient-derived xenografts (PDX) preserve the GB genetic and histological features from human tissue, during mice injection [47]. Cell-line xenografts are mice injected with human GB cell lines like U87, U251, T98G, and A172 [47]. The benefits of xenografts include maintaining the high variability of the original tumor after engraftment, which helps in clinical applications [47]. However, xenografts can take several months to develop tumors [52]. Another disadvantage is that the single cell lines used to generate the model may not reflect the heterogeneity of the GB tumors [47]. While active preclinical research is ongoing, clinical trials are being applied to promising therapies directed at many signaling proteins, some of which are listed in Table 2 and discussed below.
Table 2.
Current glioblastoma treatment clinical trial
| Name (Clinical Trial Identifier # or Drug Brand Name) | Company Name | Function | Reference(s) |
|---|---|---|---|
| Berubicin (NCT04915404) | CNS Pharmaceuticals | Anthracycline agent; inhibits topoisomerase II | [55] |
|
ONC201 |
Chimerix | D2 dopamine receptor (DR) antagonist; Upregulates DR5/TRAIL (TNF-related apoptosis inducing ligand), alters MET (Mesenchymal Epithelial Transition), and inactivates Akt/ERK signaling | [58, 59] |
| WP-1122 (NCT05195723) | Moleuclin Biotech Inc. | 2-deoxy-d-glucose analog; Glycolysis inhibitor that targets hexokinase and glucose-6-phoshphate isomerase | [60] |
| VBI-1901 (NCT03382977) | VBI Vaccines Inc. | Cytomegalovirus antigen vaccine; targets gB and pp65 antigens | [61, 62] |
|
Temferon |
Geneta Science | Hematopoietic cell immuno-therapy: CD34 + hematopoietic stem/progenitor cells (HPSCs) that produce IFN-α | [33] |
| Bevacizumab (BVZ/Avastin) | Genentech, Inc. | VEGF inhibitor; prevents tumor angiogenesis | [67] |
| Erlotinib (Tarceva) | Genentech, Inc. | Used in-coordination with BVZ; anticancer agent that inhibits EGFR, causes cell cycle arrest, and initiates apoptosis | [117] |
| Docetaxel (Taxotere) | Sanofi-Aventis Inc. | Used in-coordination with BVZ; antineoplastic agent that inhibits microtubule assembly and causes G2/M cell cycle arrest | [118] |
| Trastuzumab (Herceptin) | Genentech, Inc. | Used in-coordination with BVZ; IgG1 monoclonal antibody that targets the HER2 (Human epidermal growth factor receptor 2 | [119] |
| Temozolomide (Temodar/Temodal) | Merck & Co., Inc | Alkylating agent: Imidazotetrazinone derivative that is hydrolyzed into a methyl diazonium ion | [55] |
| Carmustine Implant (Gliadel Wafers) | Arbor Pharmaceuticals | Carmustine-infused wafers that are a cell-cycle alkylating agent | [55] |
|
Rindopepimut (CDX110) |
Celldex Therapeutics | Immunotherapeutic vaccine against EGFRvIII oncogenic deletion mutant | [120] |
Current GB treatment clinical trials and approaches
As of 2023, the National Cancer Institute (NCI) lists about 382 current Phase I-III treatment clinical trials for GB on its website. Some of these are monotherapy, combination immunotherapy, or addition to standard-of-care therapy for newly diagnosed, treated, or recurrent gliomas. Immuno-therapies or small molecule inhibitors under clinical studies are directed to EGFR, FGFR, and GSK3β in addition to others listed in Table 2. As of January 2024, about 591 GB clinical trials are either recruiting or not-yet recruiting [53] with the majority being in phase I or II [54]. A selection of the therapies that received FDA-approval and have been used in GB management are listed in Table 2 and here we discuss a few additional trials. Typical therapeutic techniques in clinical trials include target therapy, immunotherapy, and cytotoxic chemotherapy [54]. Current pharmacologic clinical trials include Berubicin and ONC201. Berubicin, a doxorubicin analog that can cross the BBB, inhibits topoisomerase II (TopoII), an enzyme that alters dsDNA, and thereby induces apoptosis [55]. Phase I Berubicin trials in GB patients (n = 25) yielded an efficacy rate of 48%, including one with a complete response [56]. Berubicin has been undergoing Phase II trials and is actively recruiting patients [55]. ONC201, an orally administered drug, is a D2 dopamine receptor (DRD2) antagonist that can cross the BBB [57]. DRD2 regulates GB cell-growth by modulating receptor and ligand interactions of MET (mesenchymal-epithelial transition factor receptor) and TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), which are both involved in tumor survival [58]. Initial animal models and Phase I trial results have shown that ONCO201 led to tumor regression and is currently active in Phase II testing [59].
Recently, WP-1122, a 2-deoxy-D-glucose (2-DG) orally administered analog, received FDA orphan drug designation and has been undergoing Phase 1 trials [60]. WP-1122 functions as a glycolysis inhibitor through the manipulation of hexokinase and phosphoglucose isomerase, thus inducing apoptosis and cell cycle arrest [60]. GB vaccines are also in clinical trials; the VBI-1901 is an example of a vaccination treatment that targets immunogenic cytomegalovirus (CMV) antigens like gB and pp65 that are commonly found in GB patients [61]. Initial testing with 10 patients showed that VBI-1901 treatment led to a loss of CMV-specific CD4 cells along with a lack of patient immunological tolerance [61, 62]. VBI-1901 is currently undergoing further Phase 1 Trials and is actively recruiting patients [61, 62]. Finally, Temferon, a drug that was also recently given orphan drug designation by the FDA, is a collection of CD34 + hematopoietic stem/progenitor cells (HPSCs) that have undergone lentiviral transduction [33]. Temferon promotes the production of interferon IFN-α, an anti-tumoral cytokine that has immunomodulatory and anti-angiogenesis properties [63]. Temferon is currently undergoing Phase II testing [64]. Despite the versatility and promise of the ongoing clinical trials, the identification of more effective therapies remains a pressing goal. Treatment clinical trials continue to face many challenges, including limited patient awareness about ongoing clinical trials, the complex nature of designing and implementing experimental protocols, the restrictive eligibility criteria, and patient socio-economic disparities, such as cost, travel, and time that limit patient participation [54, 65].
Besides treatment clinical trials, some drugs have been FDA-approved. These treatments include Bevacizumab (BVZ), an intravenously administered monoclonal IgG1 antibody that was FDA-approved in 2009 and serves as a second-line treatment for GB [66]. BVZ is a therapeutic antibody that inhibits the VEGF protein to prevent tumor angiogenesis [67]. Current experiments include the use of BVZ in conjunction with various drug combinations including Erlotinib, an EGFR inhibitor; Docetaxel, an anti-microtubule agent; and Trastuzumab, an IgG1 monoclonal antibody treatment [66, 68, 69]. Another prescribed therapeutic is Temozolomide (TMZ), a chemotherapeutic agent administered orally after radiation treatment [17]. TMZ is an alkylating agent that triggers tumor cell death; however, many GB patients develop, or have a pre-existing, resistance to TMZ, thus negating the potential therapeutic effects [70]. Intravenously administered alkylating agents such as Carmustine are used in implanted biodegradable wafers known as Gliadel wafers [17, 71]. Gliadel wafers are typically inserted around the tumor areas after surgical resection [71].
Despite the available treatments, the present GB treatment bottleneck represents a lack of specific and predictive biomarkers for targeted therapy [72]. Further, each clinical therapeutic can have significant adverse side effects including diarrhea, fatigue, kidney injury, or cardiac complications, thus posing an even greater risk for patient survival [73]. Typically, pharmacological agents are used in combination with surgical resection or radiation in treating GB; however, there is still a need for effective clinical treatments due to drug-delivery challenges imposed by the blood-brain barrier (BBB) and the blood-tumor barrier (BTB) [74]. The BBB and BTB, which maintain brain homeostasis and membrane permeability, hinder the ability of drug compounds to freely diffuse into the affected brain areas [75]. Additionally, the unique GB heterogeneity requires the development of patient-specific treatment, thus layering another challenge to effective treatment. A more efficient approach would be the identification of common therapeutic targets as proposed below.
IQGAP1 as a potential common therapeutic target in GB
The role of scaffold proteins in complex human maladies is emerging as a new field of study. IQGAP1 is an oncoprotein that normally serves as a regulatory signaling scaffold (Fig. 1A) in many pathways that modulate versatile cellular functions [22]. The modular nature of the protein allows it to bind to various signaling and structural proteins, including receptors, kinases, cytoskeletal proteins, and transcriptional factors to mediate numerous cellular functions ranging from secretion, endocytosis, and cell migration to cell division and proliferation [22, 76]. As such, IQGAP1 has been implicated in many cancers [77] including GB development, invasion, and proliferation, and has been suggested as a prognostic marker in a glioma rat model [78]. Studies using U251 and U373 cell lines harvested from human GB showed that IQGAP1 levels were significantly overexpressed in GB tissue [79]. Although the role of IQGAP1 in oncogenesis has been largely attributed to protein overexpression, recent evidence suggests that subcellular mislocalization and partner dysfunction are key factors at least in certain cancers [80]. In GB, IQGAP1 localizes to podosome/invadopodia-like structures [81, 82], filopodia [83], tumor-associated microvesicles [84], and GB stem cell niches [85]. Hence, targeting IQGAP1 in GB and other cancers is now the subject of intensive research, albeit still in its infancy, and a target therapy is not yet in sight.
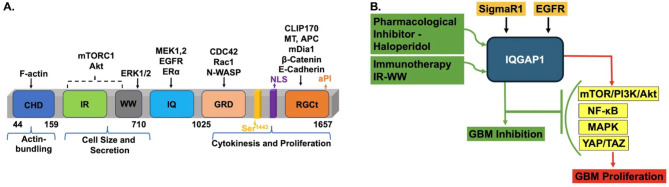
Fig. 1.
IQGAP1 Scaffold as a Common Target in glioblastoma. A. Schematic Structure of IQGAP1 and Some Glioblastoma Relevant Partners. IQGAP1 is a ubiquitous modular signaling protein that nucleates many cellular pathways. It binds a variety of receptors, including EGFR1/HER1, VEGFR, PDGFRβ, and ERα. It also binds and regulates the activities of several kinases, including the mTOR/Akt1/PI3K and the MAPK pathways. Having a nuclear localization signal (NLS) at its C-terminus, it also plays roles in the nucleus. CHD denotes the calponin homology domain; IR-WW is the IQGAP1 repeats (IR) and proline-rich (WW) region involved in protein-protein interactions; IQ denotes the isoleucine and glutamine rich region that binds calcium-calmodulin and many other proteins; GRD is the Ras-GTPase related domain that bind small GTPases like Cdc42; RGCt is the Ras GAP C-terminal domain that engages multiple protein partners some of which are indicated on the drawing. As IQGAP1 modulates many cellular functions, its dysfunction has been implicated in many human diseases, including GB. B.A Proposed Model for IQGAP1 as a Common Clinical Target in Glioblastoma. IQGAP1 serves as a scaffold of most of the molecules and pathways that have been largely implicated in GB, particularly EGFR, MAPK, PI3K/Akt1/mTOR, and YAP/TAZ. Receptor-mediated endocytosis across the BBB [88] in vivo has been demonstrated for a few peptides [89] such as insulin, insulin-like receptor, transferrin, and EGFR [90], all of which are IQGAP1-binding partners [22, 76]. Further, previous studies have shown that caffeine, antidepressants, and anti-schizophrenia drugs can cross into the BBB [91]. Accordingly, IQGAP1 pharmacological inhibitors such as Haldol [92] or inhibitory peptides such as the IR-WW fragment will have a facile route to the GB tumors, potentially making targeting IQGAP1 a more effective strategy
Because GB is defined by the activation of multiple signaling pathways as discussed above, and IQGAP1 scaffold represents a signaling hub that nucleates many of such pathways, it is intuitively appealing to propose it as a common therapeutic target in GB. Notably, many of the GB therapy targets such as EGFR, FGFR, GSK3β, and Sigma Receptor 1 (SigmaR1 or dopamine receptor), as well as many molecules listed in Table 1, are known effectors of IQGAP1 [22, 86, 87], thereby further supporting the notion that IQGAP1 scaffold can serve as an ideal upstream common target for GB marked by variable pathways.
Furthermore, IQGAP1 has been shown to modulate cell proliferation through NF-κB regulation, which leads to varied matrix metalloproteinase 2 (MMP2) protein expression [93]. MMP2, a zinc-dependent endopeptidase associated with tumor angiogenesis, has been studied in rodent models where an inverse correlation between MMP2 presence and cancer prognosis was noted [94]. Also, IQGAP1 directly binds several members of the mitogen-activated protein kinase (MAPK) cascade where it serves as a scaffold to modulate the Ras/MAPK pathway [95]. MAPK is hyperactivated in GB lesions and promotes cancer cell migration [96]. Similarly, the mTOR/PI3K/Akt1 kinase pathway has been implicated in GB and is currently a clinical therapeutic target in brain tumors [24, 97]. Several studies demonstrated that IQGAP1 directly binds and regulates the activities of the P13K/Akt1/mTOR pathway [98–100]. Interestingly, pharmacogenetic studies demonstrated that IQGAP1 exhibits a higher sensitivity to the bona fide mTOR- and PI3K-specific inhibitors like rapamycin and LY29002 [98, 101]. Again, these findings not only support the idea that IQGAP1 would be an effective clinical target in glioblastoma working upstream of key oncogenic pathways, but also that existing FDA-approved drugs can be repurposed for treatment.
Potential approaches to targeting IQGAP1 in GB
Immunotherapy and pharmacologic inhibitors with small molecules are at the forefront of precision medicine. Recently, peptides, proteins, and antibodies have become of increasing interest to the pharmaceutical industry due to their high potency, selectivity, and lack of toxicity; thus, they have been investigated as potential treatment for other brain diseases [89]. Despite the limitations imposed by the blood-brain barrier (BBB), their short duration of action, and their need for parenteral administration in the clinic, the past decade has witnessed significant advances in delivering peptides to the brain, and they now represent ~ 10% of the world’s pharmaceutical sales revenues [102]. In this regard, receptor-mediated endocytosis across the BBB has been demonstrated in vivo for a few peptides [89]. These receptors include insulin receptor, the insulin-like receptor, transferrin, and EGFR [90]; all of which bind to IQGAP1 to mediate protein traffic in a context-dependent manner [22, 76]. Therefore, it is anticipated that delivery of an IQGAP1 inhibitory peptide into brain tumors likely will be efficient and more specific. Recently, the efficacy of pharmacologic drugs like the antipsychotic drug Haloperidol (Haldol), which inhibited GB cell proliferation [92], and the inhibitory IR-WW peptide against IQGAP1 that arrested cytokinesis in cancer cells [80], have been demonstrated in cell culture and animal models (manuscript in preparation) with potential therapeutics for GB. Repurposing Haldol as anti-GB treatment will require some chemical modifications that address the known adverse side effects of Haldol such as dyskinesia. Our mechanistic cellular studies reveal effects on the cytoskeleton that could be addressed by chemical synthesis of new analogs (unpublished).
Mechanistic pharmacogenetic studies using the GB cell lines U87 and LN18 and Haldol revealed that Haldol inhibits GB cell proliferation by altering IQGAP1 signaling differentially in the two cell lines [92]. These studies uncovered previously known and unknown partners that included the Rho GTPase-activating protein 6 isoform 1 (Rho GAP). Rho GTPase is inactivated in GB leading to promoting cancer cell metastasis [103]. Interestingly, analyses of Haldol-mediated inhibition of GB cell lines identified several transcription factors in the immunoprecipitated proteins, as novel partners for IQGAP1, including myotubularin-related phosphates (MTMR), retinol dehydrogenase, and zinc finger proteins. While MTMR is known for maintaining protein catalytic activity and stability [104], it also regulates transcriptional activity by modulating the extracellular signal-regulated kinase (ERK1/2) [105]. Recently, ERK regulation of autophagic transcription via mTOR was shown to be required for GB growth that was synergistically inhibited by a combination of mTOR and ERK pharmacologic inhibitors [106]. The retinol dehydrogenase family of proteins has been shown to promote glioma cell division through upregulation of the transforming growth factor-β (TGF-β)/SMAD signaling pathway [107]. Overexpression of zinc finger proteins, a collection of transcription factors, has also been shown to promote GB cell proliferation [108]. Significantly, support for IQGAP1 scaffold as a target hub in GB transcriptional regulation is provided by the finding that the transcriptional co-activators yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ) that operate in the Hippo pathway drive the GB stem-like cell (GSC) state responsible for initiating and sustaining the GB tumors [109]. IQGAP1, via its IQ motifs, binds YAP directly and appears to inhibit its transcriptional activity [110]. It is becoming evident that IQGAP1 has a dual role in gene transcription and other cellular functions. For example, it serves as a co-activator with the estrogen receptor-α (ERα) and β-catenin while serving as an inhibitor of the nuclear factor of activated T-cell (NFAT), a family of transcription factors important in the immune response [20, 111, 112]. Thus, it is possible that IQGAP1, under physiological conditions, plays a positive or negative regulatory role in the transcription of the same gene in a context-dependent manner. This is consistent with the reports that chronic inhibition/loss or activation/expression of IQGAP1 leads to disease states like triple-negative breast cancer [80], thus justifying its designation as a molecular rheostat in cell homeostasis [22]. In our hands, Haldol inhibited cancer cells harboring activation (MDA-MB-231) or inhibition (MDA-MB-468) of IQGAP1 [80; manuscript in preparation]. The mechanism by which IQGAP1 regulates the YAP/TAZ co-activators and interplay in GB stem cell initiation and maintenance awaits further investigation. Altogether these findings, while highlighting the signaling heterogeneity of glioma cell lines, present the opportunity for harnessing the various pathways of IQGAP1 in GB to identify more personalized clinical therapeutics. Additionally, as IQGAP1 resides as a hub in the crossroads of multiple pathways, many of which are associated with GB, it presents an opportunity for developing a common marker or therapeutic target in GB.
We propose a model in which IQGAP1 serves as a regulatory scaffold at the apex of the pathways that mediate GB cell initiation and proliferation through various partners, including receptors, transcription factors, and kinases (Fig. 1B). Thus, it seems appealing to envision that targeting IQGAP1 would be a plausible therapeutic strategy in the heterogeneous nature of GB; however, much more mechanistic work is required to bring this notion to fruition.
Conclusion
In summary, Glioblastoma (GB) has proven to be difficult to classify or treat due to tumor and microenvironment heterogeneity brought by dysregulation of a variety of signaling pathways. Thus far, the majority of the implicated signaling pathways reside directly downstream of the oncoprotein IQGAP1, which normally serves as a scaffold to nucleate and regulate a variety of specialized pathways often using different combinations of the same molecules. Consequently, IQGAP1 has been associated with various cellular functions including apoptosis, cell proliferation, and cell-cell communication, and its dysfunction has been implicated in many human cancers, including GB. It is therefore fitting to propose that IQGAP1 presents an ideal target in the search for effective GB therapy. These potential therapeutics include inhibitory peptides and pharmacologic small molecule inhibitors; however, much more research is needed to realize this goal.
Animals and study approvals
All animal procedures used in this study contributing to this review were approved by the Institutional Animal Care and Use Committee (IACUC) of the University Of Toledo Health Science campus, which is AAALAC and NIH accredited and comply with or exceed the NIH regulations.
Acknowledgements
We thank the members of the Osman lab past and present for insightful discussions.
Author contributions
V. I. drafted and edited the manuscript. J. D. edited the manuscript. M. A. O conceptualized, drafted and edited the manuscript.
Funding
No external funding as of yet.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no potential conflicts of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma Multiforme: a review of its epidemiology and Pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3–9. PMID: 28239999; PMCID: PMC5563115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63. 10.1093/neuonc/nou223. PMID: 25304271; PMCID: PMC4193675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad AF, Young JS, Amara D, Berger MS, Raleigh DR, Aghi MK, et al. Mouse models of glioblastoma for the evaluation of novel therapeutic strategies. Neurooncol Adv. 2021;3(1):vdab100. 10.1093/noajnl/vdab100. PMID: 34466804; PMCID: PMC8403483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grochans S, Cybulska AM, Simińska D, Korbecki J, Kojder K, Chlubek D, et al. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers (Basel). 2022;14(10):2412. 10.3390/cancers14102412. PMID: 35626018; PMCID: PMC9139611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong ET, Lok E, Swanson KD. An evidence-based review of Alternating Electric fields Therapy for malignant gliomas. Curr Treat Options Oncol. 2015;16(8):40. 10.1007/s11864-015-0353-5. PMID: 26143265; PMCID: PMC4491358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. 10.1007/s00401-007-0243-4. Epub 2007 Jul 6. Erratum in: Acta Neuropathol. 2007;114(5):547. PMID: 17618441; PMCID: PMC1929165. [DOI] [PMC free article] [PubMed]
- 7.Jovčevska I, Kočevar N, Komel R. Glioma and glioblastoma - how much do we (not) know? Mol Clin Oncol. 2013;1(6):935–41. 10.3892/mco.2013.172. Epub 2013 Aug 26. PMID: 24649273; PMCID: PMC3916171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agnihotri S, Burrell KE, Wolf A, Jalali S, Hawkins C, Rutka JT, et al. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch Immunol Ther Exp (Warsz). 2013;61(1):25–41. 10.1007/s00005-012-0203-0. Epub 2012 Dec 7. PMID: 23224339. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. 10.1038/nature05236. Epub 2006 Oct 18. PMID: 17051156. [DOI] [PubMed] [Google Scholar]
- 10.Lan X, Jörg DJ, Cavalli FMG, Richards LM, Nguyen LV, Vanner RJ, et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature. 2017;549(7671):227–32. 10.1038/nature23666. Epub 2017 Aug 30. PMID: 28854171; PMCID: PMC5608080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puchalski RB, Shah N, Miller J, Dalley R, Nomura SR, Yoon JG, et al. An anatomic transcriptional atlas of human glioblastoma. Science. 2018;360(6389):660–3. 10.1126/science.aaf2666. PMID: 29748285; PMCID: PMC6414061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157 – 73. 10.1016/j.ccr.2006.02.019. PMID: 16530701. [DOI] [PubMed]
- 13.Park JH, Feroze AH, Emerson SN, Mihalas AB, Keene CD, Cimino PJ, et al. A single-cell based precision medicine approach using glioblastoma patient-specific models. NPJ Precis Oncol. 2022;6(1):55. 10.1038/s41698-022-00294-4. PMID: 35941215; PMCID: PMC9360428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenbarth D, Wang YA. Glioblastoma heterogeneity at single cell resolution. Oncogene. 2023;42(27):2155–2165. 10.1038/s41388-023-02738-y. Epub 2023 Jun 5. PMID: 37277603. [DOI] [PMC free article] [PubMed]
- 15.Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993;53(12):2736–9. PMID: 8504413. [PubMed] [Google Scholar]
- 16.Wong SC, Kamarudin MNA, Naidu R. Anticancer mechanism of Curcumin on Human Glioblastoma. Nutrients. 2021;13(3):950. 10.3390/nu13030950. PMID: 33809462; PMCID: PMC7998496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Wu C, Chen N, Gu H, Yen A, Cao L, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440–50. 10.18632/oncotarget.7961. PMID: 26967052; PMCID: PMC5078108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh D, Nandi S, Bhattacharjee S. Combination therapy to checkmate glioblastoma: clinical challenges and advances. Clin Transl Med. 2018;7(1):33. 10.1186/s40169-018-0211-8. PMID: 30327965; PMCID: PMC6191404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in Cancer. Front Oncol. 2014;4:64. 10.3389/fonc.2014.00064. PMID: 24782981; PMCID: PMC3995050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs MW, Li Z, Sacks DB. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J Biol Chem. 2002;277(9):7453-65. 10.1074/jbc.M104315200. Epub 2001 Dec 4. PMID: 11734550. [DOI] [PubMed]
- 21.McNulty DE, Li Z, White CD, Sacks DB, Annan RS. MAPK scaffold IQGAP1 binds the EGF receptor and modulates its activation. J Biol Chem. 2011;286(17):15010–21. 10.1074/jbc.M111.227694. Epub 2011 Feb 24. PMID: 21349850; PMCID: PMC3083173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osman MA. Cytoskeleton Dynamics in Health and Disease: role of Molecular switches and Rheostats. Cytoskeleton Health Disease. 2015;11–62. 10.1007/978-1-4939-2904-7_2.
- 23.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93(16):1246-56. 10.1093/jnci/93.16.1246. PMID: 11504770. [DOI] [PubMed]
- 24.Hashemi M, Etemad S, Rezaei S, Ziaolhagh S, Rajabi R, Rahmanian P et al. Progress in targeting PTEN/PI3K/Akt axis in glioblastoma therapy: Revisiting molecular interactions. Biomed Pharmacother. 2023;158:114204. 10.1016/j.biopha.2022.114204. Epub 2023 Jan 4. PMID: 36916430. [DOI] [PubMed]
- 25.Han F, Hu R, Yang H, Liu J, Sui J, Xiang X, et al. PTEN gene mutations correlate to poor prognosis in glioma patients: a meta-analysis. Onco Targets Ther. 2016;9:3485–92. 10.2147/OTT.S99942. PMID: 27366085; PMCID: PMC4913532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantanhede IG, de Oliveira JRM. PDGF Family expression in Glioblastoma Multiforme: Data Compilation from Ivy Glioblastoma Atlas Project Database. Sci Rep. 2017;7:15271. 10.1038/s41598-017-15045-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westermark B. Platelet-derived growth factor in glioblastoma-driver or biomarker? Ups J Med Sci. 2014;119(4):298–305. 10.3109/03009734.2014.970304. Epub 2014 Oct 24. PMID: 25342206; PMCID: PMC4248069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gai QJ, Fu Z, He J, Mao M, Yao XX, Qin Y, et al. EPHA2 mediates PDGFA activity and functions together with PDGFRA as prognostic marker and therapeutic target in glioblastoma. Signal Transduct Target Ther. 2022;7(1):33. 10.1038/s41392-021-00855-2. PMID: 35105853; PMCID: PMC8807725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahir ST, Rahmani K, Heidarymeybodi Z, Hobab H. The Status of Retinoblastoma Gene expression in brain tumors [Review of the Status of Retinoblastoma Gene expression in Brain Tumors]. World Cancer Res J. 2022. 10.32113/wcrj_20225_2318. [Google Scholar]
- 30.Zhang Y, Dube C, Gibert M Jr, Cruickshanks N, Wang B, Coughlan M, et al. The p53 pathway in Glioblastoma. Cancers (Basel). 2018;10(9):297. 10.3390/cancers10090297. PMID: 30200436; PMCID: PMC6162501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170(6):1062–78. 10.1016/j.cell.2017.08.028. PMID: 28886379; PMCID: PMC5743327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. 10.1126/scisignal.2004088. PMID: 23550210; PMCID: PMC4160307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buonfiglioli A, Hambardzumyan D. Macrophages and microglia: the cerberus of glioblastoma. Acta Neuropathol Commun. 2021;9(1):54. 10.1186/s40478-021-01156-z. PMID: 33766119; PMCID: PMC7992800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou A, Ott M, Fang D, Heimberger AB. The role and therapeutic targeting of JAK/STAT signaling in Glioblastoma. Cancers (Basel). 2021;13(3):437. 10.3390/cancers13030437. PMID: 33498872; PMCID: PMC7865703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahmideh H, Shapourian H, Moltafeti R, Tavakol C, Forghaniesfidvajani R, Zalpoor H, et al. The role of Natural products as inhibitors of JAK/STAT signaling pathways in Glioblastoma Treatment. Oxid Med Cell Longev. 2022;2022:7838583. 10.1155/2022/7838583. PMID: 36193062; PMCID: PMC9526628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao S, Wang C, Zheng Q, Qiao Y, Xu K, Jiang T, et al. STAT5 regulates glioma cell invasion by pathways dependent and independent of STAT5 DNA binding. Neurosci Lett. 2011;487(2):228–33. Epub 2010 Oct 20. PMID: 20969921. [DOI] [PubMed] [Google Scholar]
- 37.Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2(9):823–30. 10.1158/2326-6066.CIR-14-0112. PMID: 25187272; PMCID: PMC4155602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soubannier V, Stifani S. NF-κB signalling in Glioblastoma. Biomedicines. 2017;5(2):29. 10.3390/biomedicines5020029. PMID: 28598356; PMCID: PMC5489815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puliyappadamba VT, Hatanpaa KJ, Chakraborty S, Habib AA. The role of NF-κB in the pathogenesis of glioma. Mol Cell Oncol. 2014;1(3):e963478. 10.4161/23723548.2014.963478. PMID: 27308348; PMCID: PMC4905061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Lai Q, Wang D, Pei J, Tian B, Gao Y, et al. Hedgehog signaling regulates the development and treatment of glioblastoma. Oncol Lett. 2022;24(3):294. 10.3892/ol.2022.13414. PMID: 35949611; PMCID: PMC9353242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahu U, Barth RF, Otani Y, McCormack R, Kaur B. Rat and mouse brain tumor models for experimental neuro-Oncology Research. J Neuropathol Exp Neurol. 2022;81(5):312–29. 10.1093/jnen/nlac021. PMID: 35446393; PMCID: PMC9113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94(3):299–312. 10.1007/s11060-009-9875-7. Epub 2009 Apr 21. PMID: 19381449; PMCID: PMC2730996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doblas S, He T, Saunders D, Pearson J, Hoyle J, Smith N, et al. Glioma morphology and tumor-induced vascular alterations revealed in seven rodent glioma models by in vivo magnetic resonance imaging and angiography. J Magn Reson Imaging. 2010;32(2):267–75. 10.1002/jmri.22263. PMID: 20677250; PMCID: PMC2915452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giakoumettis D, Kritis A, Foroglou N. C6 cell line: the gold standard in glioma research. Hippokratia 2018 Jul-Sep;22(3):105–12. PMID: 31641331; PMCID: PMC6801124. [PMC free article] [PubMed]
- 45.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53(23):5822–7. PMID: 7694795. [PubMed] [Google Scholar]
- 46.Belloli S, Brioschi A, Politi LS, Ronchetti F, Calderoni S, Raccagni I et al. Characterization of biological features of a rat F98 model: a PET-MRI study with [18F]FAZA and [18F]FDG. Nucl Med Biol. 2013;40(6):831 – 40. 10.1016/j.nucmedbio.2013.05.004. PMID: 23915802. [DOI] [PubMed]
- 47.Kijima N, Kanemura Y. Mouse models of Glioblastoma. In: De Vleeschouwer S, editor. Glioblastoma [Internet]. Brisbane (AU): Codon; 2017. p. 29251866. Sep 27. Chapter 7. [PubMed] [Google Scholar]
- 48.Noorani I. Genetically Engineered Mouse models of Gliomas: Technological developments for translational discoveries. Cancers (Basel). 2019;11(9):1335. 10.3390/cancers11091335. PMID: 31505839; PMCID: PMC6770673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, et al. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res. 2009;69(2):431–9. 10.1158/0008-5472.CAN-08-1800. PMID: 19147555; PMCID: PMC2701484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother. 2011;60(2):153–60. 10.1007/s00262-010-0946-6. Epub 2010 Dec 1. PMID: 21120655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh T, Fakurnejad S, Sayegh ET, et al. Immunocompetent murine models for the study of glioblastoma immunotherapy. J Transl Med. 2014;12:107. 10.1186/1479-5876-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Miletic H, Sakariassen PØ, Huszthy PC, Jacobsen H, Brekkå N, et al. A reproducible brain tumour model established from human glioblastoma biopsies. BMC Cancer. 2009;9:465. 10.1186/1471-2407-9-465. PMID: 20040089; PMCID: PMC2810304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glioblastoma Multiform Clinical Trials. CTG Labs - NCBI. (n.d.). https://www.clinicaltrials.gov/search?cond=Glioblastoma+Multiforme&aggFilters=status%3Arec+not+act
- 54.Bagley SJ, Kothari S, Rahman R, Lee EQ, Dunn GP, Galanis E, et al. Glioblastoma clinical trials: current Landscape and opportunities for Improvement. Clin Cancer Res. 2022;28(4):594–602. 10.1158/1078-0432.CCR-21-2750. PMID: 34561269; PMCID: PMC9044253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pająk B. Looking for the Holy Grail-Drug candidates for Glioblastoma Multiforme Chemotherapy. Biomedicines. 2022;10(5):1001. 10.3390/biomedicines10051001. PMID: 35625738; PMCID: PMC9138518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazerooni RB, Conrad CA, Johansen M, et al. Phase I clinical pharmacokinetics of RTA 744 (berubicin(B)), a blood-brain barrier penetrating anthracycline active against high grade glioma, and evaluation of its 13-hydroxy metabolite, berubicinol (B-ol). Mol Cancer Ther. 2007;6:157. https://scholar.google.com/scholar_lookup?journal=Mol+Cancer+Ther&title=Phase+I+clinical+pharmacokinetics+of+RTA+744+(berubicin. (B)),+a+blood-brain+barrier+penetrating+anthracycline+active+against+high+grade+glioma,+and+evaluation+of+its+13-hydroxy+metabolite,+berubicinol+(B-ol)&volume=6&publication_year=2007&pages=157. [Google Scholar]
- 57.Arrillaga-Romany I, Odia Y, Prabhu VV, Tarapore RS, Merdinger K, Stogniew M, et al. Biological activity of weekly ONC201 in adult recurrent glioblastoma patients. Neuro Oncol. 2020;22(1):94–102. 10.1093/neuonc/noz164. PMID: 31702782; PMCID: PMC7080220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeon HM, Oh YT, Shin YJ, Chang N, Kim D, Woo D, et al. Dopamine receptor D2 regulates glioblastoma survival and death through MET and death receptor 4/5. Neoplasia. 2023;39:100894. 10.1016/j.neo.2023.100894. Epub 2023 Mar 25. PMID: 36972629; PMCID: PMC10066565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardner SL, Tarapore RS, Allen J, McGovern SL, Zaky W, Odia Y, et al. Phase I dose escalation and expansion trial of single agent ONC201 in pediatric diffuse midline gliomas following radiotherapy. Neurooncol Adv. 2022;4(1):vdac143. 10.1093/noajnl/vdac143. PMID: 36382108; PMCID: PMC9639395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pająk B, Siwiak-Niedbalska E, Jaśkiewicz A, Sołtyka M, Zieliński R, Domoradzki T, et al. Synergistic anticancer effect of Glycolysis and histone deacetylases inhibitors in a Glioblastoma Model. Biomedicines. 2021;9(12):1749. 10.3390/biomedicines9121749. PMID: 34944565; PMCID: PMC8698815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen PY, Reardon DA, Forst DA, Lee EQ, Haas B, Daoud T, et al. Evaluation of tumor responses and overall survival in patients with recurrent glioblastoma (GBM) from a phase IIA trial of a CMV vaccine immunotherapeutic candidate (VBI-1901). J Clin Oncol. 2022;40(16suppl):2014–2014. 10.1200/jco.2022.40.16_suppl.2014. [Google Scholar]
- 62.Wen PY, Reardon DA, Forst DA, Lee EQ, Iwamoto FM, Diaz-Mitoma F, et al. Evaluation of GM-CSF and AS01b adjuvants in a phase I/IIA trial of a therapeutic CMV vaccine (VBI-1901) against recurrent glioblastoma (GBM). J Clin Oncol. 2021;39(15suppl):2047–2047. 10.1200/jco.2021.39.15_suppl.2047. [Google Scholar]
- 63.Buckner JC, Schomberg PJ, McGinnis WL, Cascino TL, Scheithauer BW, O’Fallon JR et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma. Cancer. 2001;92(2):420 – 33. 10.1002/1097-0142(20010715)92:2%3C420::aid-cncr1338%3E3.0.co;2-3. PMID: 11466698. [DOI] [PubMed]
- 64.A Study Evaluating Temferon in Patients With Glioblastoma & Unmethylated MGMT (TEM-GBM). Clinicaltrials.gov. (2023, January 20). https://clinicaltrials.gov/study/NCT03866109
- 65.Lee EQ, Chukwueke UN, Hervey-Jumper SL, de Groot JF, Leone JP, Armstrong TS, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;21(9):1100–17. 10.1093/neuonc/noz104. PMID: 31175826; PMCID: PMC7594546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kazazi-Hyseni F, Beijnen JH, Schellens JH, Bevacizumab. Oncologist. 2010;15(8):819–25. 10.1634/theoncologist.2009-0317. Epub 2010 Aug 5. PMID: 20688807; PMCID: PMC3228024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fisher JP, Adamson DC. Current FDA-Approved therapies for high-Grade Malignant Gliomas. Biomedicines. 2021;9(3):324. 10.3390/biomedicines9030324. PMID: 33810154; PMCID: PMC8004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho MY, Mackey JR. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag Res. 2014;6:253–9. 10.2147/CMAR.S40601. PMID: 24904223; PMCID: PMC4041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boekhout AH, Beijnen JH, Schellens JH, Trastuzumab. Oncologist. 2011;16(6):800–10. 10.1634/theoncologist.2010-0035. Epub 2011 May 31. PMID: 21632460; PMCID: PMC3228213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh N, Miner A, Hennis L, Mittal S. Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review. Cancer Drug Resist. 2021;4(1):17–43. 10.20517/cdr.2020.79. Epub 2021 Mar 19. PMID: 34337348; PMCID: PMC8319838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashby LS, Smith KA, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol. 2016;14(1):225. 10.1186/s12957-016-0975-5. PMID: 27557526; PMCID: PMC4997737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a state of the science review. Neuro Oncol. 2014;16(7):896–913. 10.1093/neuonc/nou087. PMID: 24842956; PMCID: PMC4057143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magee DE, Hird AE, Klaassen Z, Sridhar SS, Nam RK, Wallis CJD et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol. 2020;31(1):50–60. 10.1016/j.annonc.2019.10.008. PMID: 31912796. [DOI] [PubMed]
- 74.Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N. Current challenges and opportunities in Treating Glioblastoma. Pharmacol Rev. 2018;70(3):412–45. 10.1124/pr.117.014944. PMID: 29669750; PMCID: PMC5907910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akhter MH, Rizwanullah M, Ahmad J, Amin S, Ahmad MZ, Minhaj MA, et al. Molecular targets and Nanoparticulate systems designed for the improved therapeutic intervention in Glioblastoma Multiforme. Drug Res (Stuttg). 2021;71(3):122–37. 10.1055/a-1296-7870. Epub 2020 Nov 9. PMID: 33167048. [DOI] [PubMed] [Google Scholar]
- 76.White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal. 2012;24(4):826–34. 10.1016/j.cellsig.2011.12.005. Epub 2011 Dec 11. PMID: 22182509; PMCID: PMC3268868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583(12):1817–24. 10.1016/j.febslet.2009.05.007. Epub 2009 May 9. PMID: 19433088; PMCID: PMC2743239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balenci L, Clarke ID, Dirks PB, Assard N, Ducray F, Jouvet A et al. IQGAP1 protein specifies amplifying cancer cells in glioblastoma multiforme. Cancer Res. 2006;66(18):9074-82. 10.1158/0008-5472.CAN-06-0761. PMID: 16982749. [DOI] [PubMed]
- 79.Diao B, Liu Y, Zhang Y, Yu J, Xie J, Xu GZ. IQGAP1–siRNA inhibits proliferation and metastasis of U251 and U373 glioma cell lines. Mol Med Rep. 2017;15(4):2074–82. 10.3892/mmr.2017.6257. Epub 2017 Feb 28. PMID: 28259970; PMCID: PMC5365011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Osman MA, Antonisamy WJ, Yakirevich E. IQGAP1 control of centrosome function defines distinct variants of triple negative breast cancer. Oncotarget. 2020;11(26):2493–511. 10.18632/oncotarget.27623. PMID: 32655836; PMCID: PMC7335670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rotoli D, Pérez-Rodríguez ND, Morales M, Maeso MD, Ávila J, Mobasheri A, et al. IQGAP1 in Podosomes/Invadosomes is involved in the progression of Glioblastoma Multiforme depending on the Tumor Status. Int J Mol Sci. 2017;18(1):150. 10.3390/ijms18010150. PMID: 28098764; PMCID: PMC5297783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rotoli D, Morales M, Maeso MD, Ávila J, Pérez-Rodríguez ND, Mobasheri A, et al. IQGAP1, AmotL2, and FKBP51 scaffoldins in the Glioblastoma Microenvironment. J Histochem Cytochem. 2019;67(7):481–94. 10.1369/0022155419833334. Epub 2019 Feb 22. PMID: 30794467; PMCID: PMC6598128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okura H, Golbourn BJ, Shahzad U, Agnihotri S, Sabha N, Krieger JR, et al. A role for activated Cdc42 in glioblastoma multiforme invasion. Oncotarget. 2016;7(35):56958–75. 10.18632/oncotarget.10925. PMID: 27486972; PMCID: PMC5302965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Zhuang X, Greene KS, Si H, Antonyak MA, Druso JE, et al. Cdc42 functions as a regulatory node for tumour-derived microvesicle biogenesis. J Extracell Vesicles. 2021;10(3):e12051. 10.1002/jev2.12051. Epub 2021 Jan 12. PMID: 33473262; PMCID: PMC7804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rotoli D, Díaz-Flores L, Gutiérrez R, Morales M, Ávila J, Martín-Vasallo P. AmotL2, IQGAP1, and FKBP51 Scaffold proteins in Glioblastoma Stem Cell niches. J Histochem Cytochem. 2022;70(1):9–16. 10.1369/00221554211025480. Epub 2021 Jun 24. PMID: 34165350; PMCID: PMC8721575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson TS, Osman MA. An emerging role for Sigma Receptor 1 in Personalized treatment of breast Cancer. Cancers (Basel). 2023;15(13):3464. 10.3390/cancers15133464. PMID: 37444574; PMCID: PMC10340381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thines L, Roushar FJ, Hedman AC, Sacks DB. The IQGAP scaffolds: critical nodes bridging receptor activation to cellular signaling. J Cell Biol. 2023;222(6):e202205062. 10.1083/jcb.202205062. Epub 2023 Apr 18. PMID: 37071417; PMCID: PMC10120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Correale J, Villa A. Cellular elements of the blood-brain barrier. Neurochem Res. 2009;34(12):2067–77. 10.1007/s11064-009-0081-y. Epub 2009 Oct 25. PMID: 19856206. [DOI] [PubMed] [Google Scholar]
- 89.Lalatsa A, Schatzlein AG, Uchegbu IF. Strategies to deliver peptide drugs to the brain. Mol Pharm. 2014;11(4):1081-93. doi: 10.1021/mp400680d. Epub 2014 Mar 21. PMID: 24601686. [DOI] [PubMed]
- 90.Frank HJ, Pardridge WM, Morris WL, Rosenfeld RG, Choi TB. Binding and internalization of insulin and insulin-like growth factors by isolated brain microvessels. Diabetes. 1986;35(6):654 – 61. 10.2337/diab.35.6.654. PMID: 3011572. [DOI] [PubMed]
- 91.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–36. 10.1007/s11481-006-9025-3. Epub 2006 Jul 6. PMID: 18040800. [DOI] [PubMed] [Google Scholar]
- 92.Iyer VJ, Osman MA. Haldol targets IQGAP1 pathway and promotes Novel Partner interactions in Glioblastoma Cell Lines. MicroPubl Biol. 2023;2023. 10.17912/micropub.biology.000822. PMID: 37228393; PMCID: PMC10203884. [DOI] [PMC free article] [PubMed]
- 93.Zhang Z, Wei Y, Li X, Zhao R, Wang X, Yang Z, et al. IQGAP1 enhances cell invasion and matrix metalloproteinase-2 expression through upregulating NF-κB activity in esophageal squamous cell carcinoma cells. Gene. 2022;824:146406. 10.1016/j.gene.2022.146406. Epub 2022 Mar 8. PMID: 35276237. [DOI] [PubMed] [Google Scholar]
- 94.Sincevičiūtė R, Vaitkienė P, Urbanavičiūtė R, Steponaitis G, Tamašauskas A, Skiriutė D. MMP2 is associated with glioma malignancy and patient outcome. Int J Clin Exp Pathol. 2018;11(6):3010–8. PMID: 31938426; PMCID: PMC6958083. [PMC free article] [PubMed] [Google Scholar]
- 95.Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25(18):7940–52. 10.1128/MCB.25.18.7940-7952.2005. PMID: 16135787; PMCID: PMC1234344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krishna KV, Dubey SK, Singhvi G, Gupta G, Kesharwani P. MAPK pathway: potential role in glioblastoma multiforme. Interdiscip Neurosurg. 2021;23. 10.1016/j.inat.2020.100901. https://pubs.acs.org/servlet/linkout?suffix=ref243/cit243&dbid=16&doi=10.1021%2Facs.jmedchem.1c01946&key=10.1016%2Fj.inat.2020.100901. http://scholar.google.com/scholar_lookup?hl=en&volume=23&publication_year=2021&pages=100901&journal=Interdiscip.+Neurosurg.&author=K.+V.+Krishna&author=S.+K.+Dubey&author=G.+Singhvi&author=G.+Gupta&author=P.+Kesharwani&title=MAPK+pathway%3A+Potential+role+in+glioblastoma+multiforme&doi=10.1016%2Fj.inat.2020.100901
- 97.Crespo S, Kind M, Arcaro A. The role of the PI3K/AKT/mTOR pathway in brain tumor metastasis. J Cancer Metastasis Treat. 2016;2:80–9. 10.20517/2394-4722.2015.72. [Google Scholar]
- 98.Tekletsadik YK, Sonn R, Osman MA. A conserved role of IQGAP1 in regulating TOR complex 1. J Cell Sci. 2012;125(Pt 8):2041–52. 10.1242/jcs.098947. Epub 2012 Feb 10. PMID: 22328503; PMCID: PMC3360921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi S, Anderson RA. And Akt-ion! IQGAP1 in control of signaling pathways. EMBO J. 2017;36(8):967–9. 10.15252/embj.201796827. Epub 2017 Mar 20. PMID: 28320738; PMCID: PMC5391135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan CW, Jin X, Zhao Y, Pan Y, Yang J, Karnes RJ, et al. AKT-phosphorylated FOXO1 suppresses ERK activation and chemoresistance by disrupting IQGAP1-MAPK interaction. EMBO J. 2017;36(8):995–1010. 10.15252/embj.201695534. Epub 2017 Mar 9. PMID: 28279977; PMCID: PMC5391142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang JB, Sonn R, Tekletsadik YK, Samorodnitsky D, Osman MA. IQGAP1 regulates cell proliferation through a novel CDC42-mTOR pathway. J Cell Sci. 2009;122(Pt 12):2024–33. 10.1242/jcs.044644. Epub 2009 May 19. PMID: 19454477; PMCID: PMC2723156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reichert JP, Tartat A, Dunn MK. Report summary: development trends for peptide therapeutics: a comprehensive quantitative analysis of peptide therapeutics in clinical development. Pept Ther Foundation. 2010.
- 103.Al-Koussa H, Atat OE, Jaafar L, Tashjian H, El-Sibai M. The role of rho GTPases in Motility and Invasion of Glioblastoma Cells. Anal Cell Pathol (Amst). 2020;2020:9274016. 10.1155/2020/9274016. PMID: 32089990; PMCID: PMC7013281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Allen EA, Amato C, Fortier TM, Velentzas P, Wood W, Baehrecke EH. A conserved myotubularin-related phosphatase regulates autophagy by maintaining autophagic flux. J Cell Biol. 2020;219(11):e201909073. 10.1083/jcb.201909073. PMID: 32915229; PMCID: PMC7594499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weidner P, Söhn M, Schroeder T, Helm L, Hauber V, Gutting T, et al. Myotubularin-related protein 7 activates peroxisome proliferator-activated receptor-gamma. Oncogenesis. 2020;9(6):59. 10.1038/s41389-020-0238-8. PMID: 32522977; PMCID: PMC7286916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang K, Luan L, Li X, Sun X, Yin J. ERK inhibition in glioblastoma is associated with autophagy activation and tumorigenesis suppression. J Neurooncol. 2022;156(1):123–37. 10.1007/s11060-021-03896-3. Epub 2021 Nov 19. PMID: 34797524. [DOI] [PubMed] [Google Scholar]
- 107.Guan F, Kang Z, Wang L, Wang K, Mao BB, Peng WC, et al. Retinol dehydrogenase 10 promotes metastasis of glioma cells via the transforming growth factor-β/SMAD signaling pathway. Chin Med J (Engl). 2019;132(20):2430–7. 10.1097/CM9.0000000000000478. PMID: 31613821; PMCID: PMC6831065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jen J, Wang YC. Zinc finger proteins in cancer progression. J Biomed Sci. 2016;23(1):53. 10.1186/s12929-016-0269-9. PMID: 27411336; PMCID: PMC4944467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Castellan M, Guarnieri A, Fujimura A, Zanconato F, Battilana G, Panciera T, et al. Single-cell analyses reveal YAP/TAZ as regulators of stemness and cell plasticity in Glioblastoma. Nat Cancer. 2021;2(2):174–88. 10.1038/s43018-020-00150-z. Epub 2020 Dec 7. PMID: 33644767; PMCID: PMC7116831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sayedyahossein S, Li Z, Hedman AC, Morgan CJ, Sacks DB. IQGAP1 binds to yes-associated protein (YAP) and modulates its transcriptional activity. J Biol Chem. 2016;291(37):19261–73. 10.1074/jbc.M116.732529. Epub 2016 Jul 20. PMID: 27440047; PMCID: PMC5016668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erdemir HH, Li Z, Sacks DB. IQGAP1 binds to estrogen receptor-α and modulates its function. J Biol Chem. 2014;289(13):9100–12. 10.1074/jbc.M114.553511. Epub 2014 Feb 18. PMID: 24550401; PMCID: PMC3979404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharma S, Findlay GM, Bandukwala HS, Oberdoerffer S, Baust B, Li Z et al. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci U S A. 2011;108(28):11381-6. 10.1073/pnas.1019711108. Epub 2011 Jun 27. Erratum in: Proc Natl Acad Sci U S A. 2011;108(41):17235. PMID: 21709260; PMCID: PMC3136327. [DOI] [PMC free article] [PubMed]
- 113.da Fonseca CO, Linden R, Futuro D, Gattass CR, Quirico-Santos T. Ras pathway activation in gliomas: a strategic target for intranasal administration of perillyl alcohol. Arch Immunol Ther Exp (Warsz). 2008 Jul-Aug;56(4):267–76. 10.1007/s00005-008-0027-0. Epub 2008 Jul 29. PMID: 18726148; PMCID: PMC2778682. [DOI] [PMC free article] [PubMed]
- 114.Biasoli D, Kahn SA, Cornélio TA, Furtado M, Campanati L, Chneiweiss H, et al. Retinoblastoma protein regulates the crosstalk between autophagy and apoptosis, and favors glioblastoma resistance to etoposide. Cell Death Dis. 2013;4(8):e767. 10.1038/cddis.2013.283. PMID: 23949216; PMCID: PMC3763445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee YJ, Seo HW, Baek JH, et al. Gene expression profiling of glioblastoma cell lines depending on TP53 status after tumor-treating fields (TTFields) treatment. Sci Rep. 2020;10:12272. 10.1038/s41598-020-68473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carballo GB, Honorato JR, de Lopes GPF, Spohr TCLSE. A highlight on sonic hedgehog pathway. Cell Commun Signal. 2018;16(1):11. 10.1186/s12964-018-0220-7. PMID: 29558958; PMCID: PMC5861627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdelgalil AA, Al-Kahtani HM, Al-Jenoobi FI, Erlotinib. Profiles Drug Subst Excip Relat Methodol. 2020;45:93–117. 10.1016/bs.podrm.2019.10.004. Epub 2019 Dec 6. PMID: 32164971. [DOI] [PubMed] [Google Scholar]
- 118.Farha NG, Kasi A, Docetaxel. 2022 Nov 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 30725927.
- 119.Garnock-Jones KP, Keating GM, Scott LJ, Trastuzumab. A review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs. 2010;70(2):215 – 39. 10.2165/11203700-000000000-00000. Erratum in: Drugs. 2011;71(12):1578. PMID: 20108993. [DOI] [PubMed]
- 120.Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H et al. ACT IV trial investigators. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. doi: 10.1016/S1470-2045(17)30517-X. Epub 2017 Aug 23. PMID: 28844499. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.