Abstract
Background
Cold is an important environmental limiting factor affecting plant yield and quality. Capsicum (chili pepper), a tropical and subtropical vegetable crop, is extremely sensitive to cold. Although H2S is an important signaling regulator in the responses of plant growth and development to abiotic stress, few studies have examined its effects on cold-sensitive capsicum varieties. Through biotechnology methods to enhance the cold resistance of peppers, to provide some reference for pepper breeding, investigated molecular regulation by H2S of responses to cold stress in cold-sensitive capsicum plants, via physiological and transcriptomic analyses.
Results
In capsicum seedlings, exogenous H2S enhanced relative electrical conductivity (REC) and levels of malondialdehyde (MDA) under cold stress, maintained membrane integrity, increased the activity of enzymatic and non-enzymatic antioxidants, balanced reactive oxygen species levels (O2·− and H2O2), and improved photosynthesis, mitigating the damage caused by cold. In addition, 416 differentially expressed genes (DEGs) were involved in the response to cold stress after H2S treatment. These DEGs were mainly enriched in the ascorbate–glutathione and starch–sucrose metabolic pathways and plant hormone signal-transduction pathways. Exogenous H2S altered the expression of key enzyme-encoding genes such as GST, APX, and MDHAR in the ascorbate–glutathione metabolism pathway, as well as that of regulatory genes for stimulatory hormones (auxin, cytokinins, and gibberellins) and inhibitory hormones (including jasmonate and salicylic acid) in the plant hormone signal-transduction pathway, helping to maintain the energy supply and intracellular metabolic stability under cold stress.
Conclusions
These findings reveal that exogenous H2S improves cold tolerance in cold-sensitive capsicum plants, elucidating the molecular mechanisms underlying its responses to cold stress. This study provides a theoretical basis for exploring and improving cold tolerance in capsicum plants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05635-y.
Keywords: Cold stress, H2S, Enzymatic antioxidant, Non-enzymatic antioxidant, Photosynthesis, Transcriptome
Introduction
Temperature is a major environmental factor affecting plant growth and development. Excessively high or low environmental temperatures can affect plants. Extensive research has shown that cold stress significantly affects the quality and yield of grain, oil, and horticultural crops [1]. the vegetable Capsicum annuum L. (capsicum) contains multiple vitamins, with vitamin C being the most abundant. It can be used fresh or as a spice during the green and ripe stages. Dried capsicum and capsicum powder are rich in vitamins and capsaicin, making them important export products in China [2]. capsicum is are highly sensitive to temperature; seedlings exposed to temperatures below 5–10 °C can suffer from cold damage, preventing them from safely overwintering or withstanding frost in early spring [3]. Plants subjected to cold stress rapidly accumulate large amounts of reactive oxygen species (ROS), primarily the superoxide anion (O2·−), hydrogen peroxide (H2O2), hydroxyl radicals, and singlet oxygen, which are highly oxidative. Excessive ROS levels can disrupt the dynamic balance of the cellular environment and impair metabolic functions [4, 5]. When plants are subjected to abiotic stress, their enzymatic and non-enzymatic systems are rapidly activated. NADPH oxidase catalyzes electron transfer from the cytoplasm to O2, leading to the formation of O2·− [6]. Dismutation then occurs spontaneously or via the action of SOD [7], generating H2O2. APX then converts H2O2 to H2O with high affinity, thereby alleviating excessive ROS levels and reducing damage to the plants [8]. Studies have found that cold stress can damage the photosynthetic organs of plants, affecting the opening and closing of leaf stomata, impairing CO₂ absorption, damaging the thylakoid membranes of chloroplasts, affecting the reaction centers of photosystems, and inhibiting the activity of key enzymes in the Calvin cycle [9]. Photosynthesis-related metabolism typically involves the synthesis of starch, which helps maintain respiration in the dark, enabling symbionts to supply sugars during both the day and night [10]. Sucrose, the primary organic carbon source, is transported from photosynthetic leaves to non-photosynthetic tissue via the phloem. In the phloem, sucrose is hydrolyzed into glucose and fructose by invertase (INV) after phloem unloading, or is degraded into UDP glucose and fructose by sucrose synthase (SUS). Sucrose can be degraded into hexoses for various purposes [11]. In addition to supporting nighttime metabolic functions, starch rapidly degrades under light conditions, aiding the production of organic acids and sugars, increasing the turgor pressure of guard cells and promoting stomatal opening [12]. The key starch-degrading enzyme involved in this process is β-amylase 1 (BAM1), which works in conjunction with α-amylase 3 (AMY3) to degrade starch. Arabidopsis mutants lacking AMY3 show a substantial reduction in osmotic-stress-induced starch degradation, resulting in reduced accumulation of sugars and proline (Pro) and reduced water-uptake capacity [13, 14].
H2S, a signaling molecule in plants, participates in various activities such as flowering, fruit setting, senescence, photosynthesis, and sucrose and starch synthesis and degradation [15, 16]. Exogenous H2S can increase endogenous H2S levels and the activity of key enzymes, and alters the responses of multiple signaling pathways to abiotic stress. For example, following salt stress, H2S works synergistically with Ca2+ to increase the concentration of sodium and potassium ions on both sides of the membrane, maintaining membrane lipid integrity and redox homeostasis [17]. Exogenous H2S can enhance the resistance of grapes to stress by inducing superoxide dismutase (SOD) activity and ICE1 and CBF3 expression [18]. Spraying bananas with H2S during storage can increase their osmotic substance levels and oxidase activity, helping to maintain their quality and extending their shelf life by enhancing stress resistance and delaying ripening [19]. H2S participates in regulating the upstream and downstream signaling of various plant hormones. In cucumber explants, IAA (indole-3-acetic acid) depletion inhibits adventitious root formation, whereas administration of H2S alleviates this inhibition [20]. In maize seedlings, H2S acts as a downstream signaling molecule in salicylic acid (SA)-induced heat tolerance. In Arabidopsis, H2S induces stomatal movement via an abscisic acid (ABA)-acid-dependent pathway [21].
In recent years, with the increasing severity of cold stress, capsicums, whether used as vegetables or spices, hold a significant market share. However, due to their preference for warm conditions, they cannot safely overwinter during the winter months. To ensure a steady supply of peppers during winter, developing cold-tolerant varieties has become one of the primary goals for breeders. However, research on H₂S in cold-sensitive chili capsicum seedlings is relatively limited. This study investigates the effects of H₂S pretreatment followed by short-term cold stress on chili capsicum seedlings, focusing on major physiological, biochemical, and transcriptomic changes. It analyzes the molecular mechanisms of differential gene expression, enzymatic systems, and starch-sucrose metabolism pathways in response to H₂S after low-temperature exposure, providing a reference for future research.
Materials and methods
Plant materials
The experiment was carried out in the vegetable Laboratory of Horticulture College of Sichuan Agricultural University in January 2022. Under cold stress, an artificial climate box (Model: RGX300EF, Tianjin Test Instrument Co., LTD., Tianjin, China.) was used to set the temperature at 5 ℃ for 48 h. The experimental material was a cold-sensitive pepper (Dongxing capsicum) screened in our laboratory, and the seeds were stored at the Vegetable Laboratory of Horticulture College, Sichuan Agricultural University. NaHS, an H2S donor, was purchased from McLean. Hypohtrine (HT), an H2S scavenger, was purchased from Sigma Aldrich (St Louis, MO, USA).
Experimental design
Full and intact capsicum seeds were selected and sown in a 12 × 6 well seedling tray in growth medium (nutrient soil: vermiculite, 5:5 v/v). Seedlings were grown at 25/18°C (day/night) with a 12 h /12 h light / dark photoperiod (light intensity, 300 µmol m− 2 s− 1; relative humidity, 75%). Once the seedlings had 3– 4 true leaves, they were transplanted into 10 × 10 × 15 cm pots. Once they had 7–8 true leaves, the exogenous treatment was applied continuously for 3 d to prepare them for cold stress. These temperature, humidity, and light conditions were maintained throughout the seedling stage. The cold stress temperature was set to 5 ℃ (± 1 °C).
The experimental design involved four treatments: Normal temperature control (with 200 mL water), at 25 °C (‘Control’); three cold treatments were used, namely cold stress alone (with 200 mL water; ‘Cold’), or cold stress with 200 mL of NaHS pretreatment at 0.5 mmol·L−¹ (‘H2S’) or 200 mL of HT pretreatment at 0.2 mmol·L⁻¹ (‘HT’). Each treatment was 15 plants and repeated 3 times. The exogenous substances were applied continuously for 3 d. The following day, the seedlings were placed in a growth chamber to induce cold stress. Leaf samples were collected for physiological and transcriptomic analyses at different time points (0, 6, 12, 24, and 48 h). Each sample was bioreplicated three times.
Determination of physiological and biochemical indices
Fresh leaves (0.1 g) were placed in a test tube containing 30 ml of deionized water and allowed to stand for 6 h. The conductivity of deionized water (R0) was measured using a conductivity meter. The conductivity of the sample solution was measured before and after boiling, recorded as R1 and R2, respectively. The relative electrolyte leakage (REC) was calculated using the formula: REC (%) = (R1 – R0) ×100/ (R2 - R0). Malondialdehyde (MDA) content was determined using the thiobarbituric acid method, and proline (Pro) content was measured using the sulfosalicylic acid method [22]. Superoxide anion (O₂·⁻) content was assessed using the p-aminobenzoic acid method [23], and hydrogen peroxide (H₂O₂) content was measured using the potassium iodide spectrophotometric method [24]. The activities of peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) were determined using guaiacol, nitroblue tetrazolium (NBT), and UV absorbance methods, respectively [25]. The activities of Monodehydroascorbate reductase (MDAHR) at 340 nm and Dehydroascorbate reductase (DHAR) at 265 nm were also measured [26]. Ascorbic acid (AsA), dehydroascorbic acid (DHA), glutathione (GSH), and glutathione disulfide (GSSG) contents were determined following the methods described by Noctor and Foyer [27].
The photosynthetic parameters were determined using a Li-Cor 6400XT (Gene Company Limited, Hong Kong, China) portable photosynthesis measurement system. Photosynthetic indices were measured using fully unfolded leaves. With a red/blue LED as the light source, the flow rate was 500 mL min− 1, the CO2 concentration was 400 µmol− 1, and the PAR was 1000 µmol m− 2 s− 1. The photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) of the best functional leaves (3rd to 4th true leaves) of pepper seedlings in each treatment were determined, and each treatment was repeated three times. The enzyme activity changes of RubisCO, FBA, and SBP were determined using an ELISA kit (Shanghai Fwei Biotechnology Co., Ltd., Fengxian District, Shanghai, China).
The determination of endogenous H2S followed Sekiya et al.‘s methylene blue method [28]. 0.1 g fresh chilli leaves were ground into homogenization on ice with 0.9 ml 20 mM pre-cooled Tris-HCl buffer (pH 8.0), and then centrifuged at 4℃ and 12,000×g for 20 min, and the supernatant was taken for the determination solution. A zinc acetate absorption well was placed in the small test tube to be installed, and then 100µL 30 mM FeCl3 (dissolved in 1.2 MHCl) and 100µL 20 mM N, N-dimethyl14 - p-phenylenediamine (dissolved in 7.2 M HCl) were added to the test liquid. The sealing film of the test tube will react quickly at 37℃ for 30 min. At the wavelength of 670 nm, the absorbance was determined to find the corresponding H2S concentration and calculate the content according to the prepared standard curve.
RNA extraction, library construction, and sequencing
RNA extraction from the 12 samples was performed by Biomarker Technologies Co., Ltd. (http://www.biomarker.com.cn/, Beijing, China). Total RNA purity and concentration were measured using a Nano-Drop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and RNA integrity was assessed using an Agilent 2100 Bioanalyzer/Lab Chip GX (Agilent, Santa Clara, CA, USA). After confirming total RNA quality, cDNA library construction and sequencing were performed. Sequencing was performed using the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) in PE150 mode to obtain raw data. The raw data were then filtered to remove reads containing adapters and low-quality sequences (Include reads with a removal ratio of N greater than 10% and reads where the number of bases with quality value Q ≤ 10 constitutes more than 50% of the entire read.), resulting in high-quality clean reads. These clean reads were aligned to the reference genome of Capsicum annuum (Capsicum_annuum GCF_000710875.1 genome. fa) using HISAT2 for subsequent analysis [29]. Genes were identified as differentially expressed using thresholds of Fold Change ≥ 1.5 and Pvalue < 0.01. Differential expression analysis and functional annotation analysis of differentially expressed genes (DEGs) were performed based on DEG expression.
Validation of transcriptome data by qRT-PCR
To verify the reliability of the data, this study selected 7 antioxidant genes and H2S synthesizing genes for qRT-PCR to verify the transcriptomic data according to the trend of antioxidant enzyme activity. RNA samples were provided by Biomarker Technologies Co., Ltd., and cDNA was synthesized from total RNA using a HiScript II Q Select RT Super Mix qPCR kit (Vazyme, Nanjing, China). Primers (Additional File 1, Supplementary Table 1) were designed by National Center for Biotechnology Information (NCBI: https://www.ncbi.nlm.nih.gov/). qRT–PCR was performed on the Light-Cycler 480II (Roche, Basel, Switzerland), using AceQqPCR SYBR Green Master Mix (Vazyme, China). The PCR reaction conditions were as follows: denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. To determine relative fold differences for each sample, the threshold constant (Ct) value was normalized to the Ct value of UBI-3, and set relative to control samples (0 h) according to the formula 2(−ΔCt) [30]. Three independent technical replicates were analyzed for each sample.
Statistical analysis
All data were sorted using Excel 2016 software and analyzed using one-way analysis of variance (ANOVA) with SPSS 26.0 (IBM Corporation, Armonk, NY, USA). Duncan’s new complex range method was applied at a 5% level of significance (P < 0.05). Data are presented as the mean ± Standard Error (SE, n = 3). Graphs were created using Origin 21 and Adobe Illustrator.
Results
Effects of H2S on membrane lipid peroxidation and ROS under cold stress
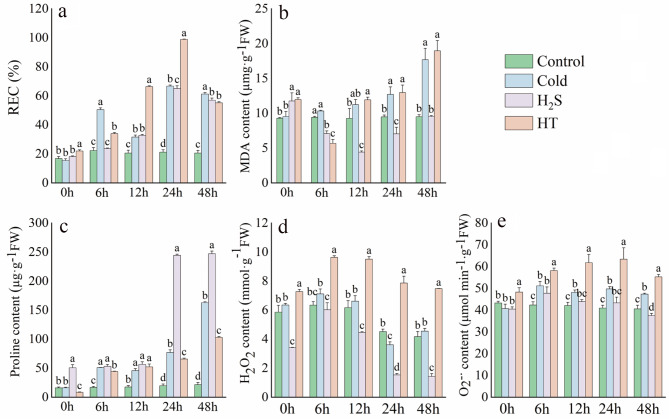
Under cold stress alone, REC initially increased then decreased over time, whereas MDA and Pro levels gradually increased over time. Relative to the Cold treatment, the H2S treatment exhibited significantly lower REC, by 52.94%, 24.59%, and 16.36%, at 6, 24, and 48 h, respectively, however, there was no significant increase at 12 h (Fig. 1a), as well as significantly lower MDA content, by 31.54%, 60.63%, 44.50%, and 46.12%, at 6, 12, 24 and 48 h, respectively (Fig. 1b). Relative to the Cold treatment, the H2S treatment exhibited significantly higher Pro content, by 19.07%, 68.46%, and 34.06%, at 12 h and 24 h (Fig. 1c). However, relative to the Control and Cold treatments, the HT group exhibited higher REC, MDA levels and Pro content.
Fig. 1.
Effects of H2S on membrane lipids and ROS in capsicum plants under cold stress. a: Relative electrical conductivity (REC); b: Malondialdehyde (MDA); c: Proline content; d: H2O2; e: O2−
ROS are key indicators that respond directly to cold stress, with O2·− and H2O2 being the predominant forms. Under cold stress, O2·− and H2O2 increased initially, then decreased. Relative to the Control and Cold treatments, the H2S treatment exhibited significantly lower O2·− content, by 7.62% and 20.85%, at 48 h (Fig. 1e). Relative to the Cold treatment, the H2S treatment achieved significantly lower H2O2 content at 24 h and 48 h, by 57.51% and 68.48%, respectively (Fig. 1d). However, under HT treatment, O2·− and H2O2 levels were significantly elevated, exacerbating the damage caused by ROS. These findings reveal that H2S can alleviate cold stress by reducing REC and MDA levels, increasing Pro content to maintain osmotic stability, and reducing ROS accumulation.
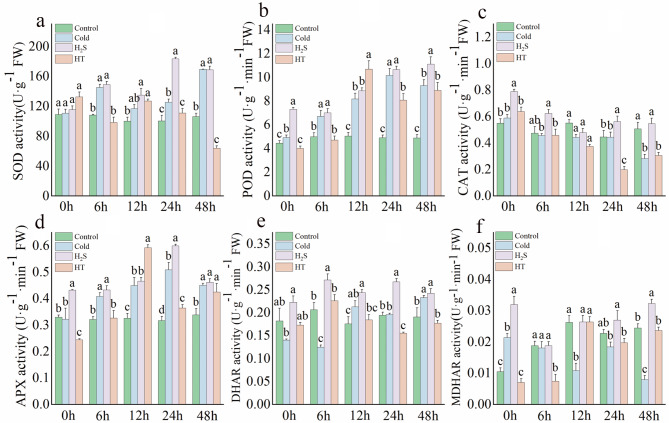
Effects of H2S on antioxidant enzyme activity in capsicum seedlings under cold stress
Under cold stress, SOD activity initially increased and then decreased, POD activity gradually increased, CAT activity gradually decreased, and APX activity initially increased and then decreased (Fig. 2a–d). There were no distinct trends in DHAR and MDHAR activity, although their activity was significantly higher under cold stress than in the normal control. H2S treatment significantly increased the activity of the other enzymes. After 48 h of cold stress, relative to the Control, SOD was elevated in the HT and H2S treatments, by 37.19% and 37.03%, respectively, and POD activity was elevated by 44.39% and 55.95%, respectively (Fig. 2a, b). CAT activity was lower under the H2S treatment than in the control, but only at 12 h of cold stress, and was significantly higher than in the Control and Cold groups at the other time points (Fig. 2c). After 24 h of cold stress, APX and DHAR enzyme activity peaked in the H2S group, exhibiting values that were 47.07% and 15.01% higher than in the Control and 27.21% and 26.50% higher than in the cold stress group, respectively (Fig. 2de). Although MDHAR activity was also elevated, the difference was not significant (Fig. 2f). In contrast, the HT group exhibited the opposite trend.
Fig. 2.
Effects of H2S on antioxidant enzyme activity in capsicum seedlings under cold stress. a: SOD; b: POD; c: CAT; d: APX; e: DHAR; f: MDHAR
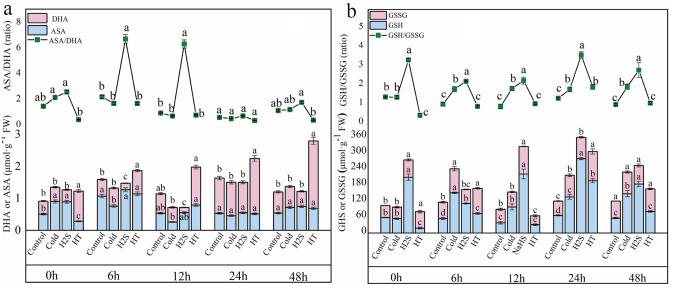
Effects of H2S on the AsA–GSH system in capsicum seedlings under cold stress
Cold stress reduced the levels of AsA (a component of the AsA–GSH system), relative to the Control. H2S treatment increased AsA content, but the increase was significant only after 6 h of cold stress. Under cold stress, H2S treatment did not alter DHA content, whereas it increased the AsA/DHA ratio significantly, by 361.72% and 491.90%, at 6 and 12 h, respectively (Fig. 3a). Relative to the Control and Cold groups, the H2S group exhibited significantly higher GSH and GSSG content: after 12 h of cold stress, GSH content was higher following H2S treatment, by 610.50% and 126.43%, respectively, than in the Control and Cold groups; similarly, after 24 h of cold stress, GSSG content was higher following H2S treatment, by 81.59% and 35.26%, respectively (Fig. 3b). Treatment with H2S significantly increased the GSH/GSSG ratio under cold stress. This indicates that H2S alleviates the effects of cold stress on capsicum by regulating AsA and GSH content, thereby altering the AsA/DHA and GSH/GSSG ratios.
Fig. 3.
Effects of H2S on the AsA–GSH system in capsicum seedlings under cold stress. a: AsA and DHA content and AsA/DHA ratio; b: GSH and GSSG content and GSH/GSSG ratio
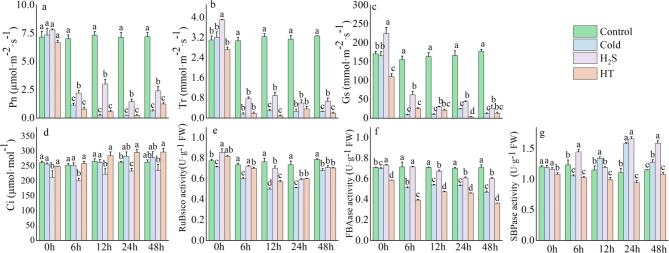
Effects of H2S on photosynthesis-related enzyme activity and photosynthetic parameters in capsicum seedlings under cold stress
Under cold-stress, H2S improved photosynthetic parameters and Calvin-cycle enzyme activity. Under cold stress, the net photosynthetic rate (Pn), stomatal conductance (Gs), and transpiration rate (Tr) decreased significantly over time, whereas intercellular CO2 concentration (Ci) increased (Fig. 4a–d). Relative to the Cold group, H2S pretreatment achieved significantly higher Pn, Gs, and Tr, and lower Ci, following 6–48 h of cold stress. After 24 h of cold stress, the H2S group exhibited higher Pn, Gs, and Tr, by 82.60% (Fig. 4a), 44.21%, and 55.37% (Fig. 4b and c), respectively, and a reduction in Ci of 21.47% (Fig. 4d). In contrast, the HT group exhibited the opposite trends in the gas-exchange parameters.
Fig. 4.
Effects of cold stress on photosynthetic parameters and Calvin-cycle enzyme activity in capsicum seedlings. a: Net photosynthetic rate (Pn); b: Transpiration rate (Tr); c: Stomatal conductance (Gs); d: CO2 concentration (Ci); e: RuBisCO activity; f: Fructose-1,6-diphosphate aldolase (FBAase) activity; g: Sedoheptulose1,7-diphosphate (SBPase) activity
Under cold stress, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) activity initially declined then increased, while Fructose-1,6-diphosphate aldolase (FBAase) activity decreased continuously and Sedoheptulose1,7-diphosphate (SBPase) activity increased continuously (Fig. 4e–g). Relative to the Cold group, the H2S group exhibited higher RuBisCO activity (by 13.34%; Fig. 4e), FBAase activity (by 12.35%; Fig. 4f), and SBPase activity (by 4.63%; Fig. 4g) after 24 h of stress. However, relative to the Control and Cold groups, HT treatment achieved significantly lower activity of all three enzymes. These results indicate that exogenous H2S can mitigate the effects of cold stress on photosynthesis in capsicum seedlings.
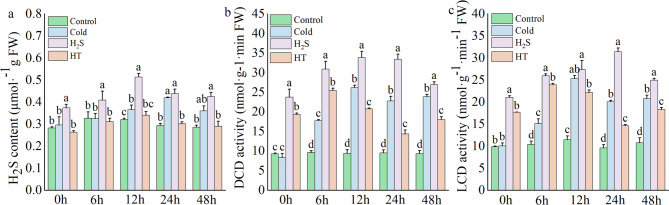
Effects of H2S on endogenous H2S in capsicum seedlings under cold stress
Relative to the Control group, the Cold group exhibited significantly higher endogenous H2S content after 0–12 h of stress, whereas the H2S group exhibited elevated endogenous H2S content at all time points. In contrast, relative to the Control group, HT treatment either reduced or maintained endogenous H2S levels (Fig. 5a). LCD and DCD are key enzymes involved in the synthesis of endogenous H2S in plants. Under cold stress, LCD and DCD activity first increased and then decreased in the in H2S group, peaking at 24 h, with increases of 69.41% and 36.01%, respectively, relative to the Control group, and 71.39%, and 31.71%, respectively, relative to the Cold group (Fig. 5b, c). This indicates that exogenous H2S not only promotes the accumulation of endogenous H2S during cold stress but also increases endogenous H2S levels under normal conditions, while simultaneously enhancing the activity of synthesis enzymes (LCD and DCD).
Fig. 5.
Effects of H2S on endogenous H2S content in capsicum under cold stress. a: Endogenous H2S content; b: LCD; C: DCD
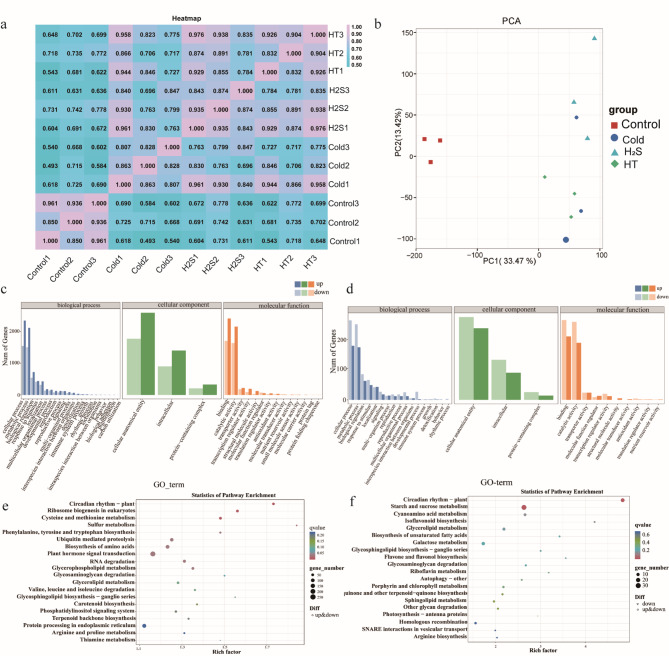
Transcriptome library construction, quality assessment, and GO and KEGG analysis
RNA-seq analysis using the eukaryotic reference transcriptome for the 12 samples yielded 76.23 Gb of clean data, with each sample generating at least 5.81 Gb of clean data. At least 92.33% of the bases were Q30 bases. The clean reads from each sample were aligned to the designated reference genome, with alignment efficiencies of 90.84–95.36% (Additional File 1, Supplementary Table 2). Correlation analysis revealed that the correlation coefficient of the biological replicates, comparing the different treatments, reached 0.95 (Fig. 6a), demonstrating the strong reproducibility of the data. In the Principal Component Analysis (PCA), PC1 accounted for 33.47% and PC2 for 13.42% of the variance (Fig. 6b), indicating similarity in gene expression among the biological replicates. These results confirm the high reproducibility of the samples and highlight the variation in gene expression between the different samples.
Fig. 6.
Transcriptome sample correlation analysis, principal component analysis, and GO and KEGG enrichment analyses. a: Correlations between samples; b: Principal Component Analysis (PCA); c: GO enrichment analysis (Control vs. Cold treatments); d: GO enrichment analysis (Cold vs. H2S treatments); e: KEGG enrichment analysis (Control vs. Cold treatments); f: KEGG enrichment analysis (Cold vs. H2S treatments)
Using the Gene Ontology (GO) database, GO enrichment analysis was conducted, considering the Biological Process (BP), Molecular Function (MF), and Cellular Component (CC) terms and comparing the Control and Cold treatments and the Cold and H2S treatments (Fig. 6c, d). The most enriched BP categories were reproduction, immune system processes, and behavior. The most enriched CC categories were intracellular, protein-containing complex, and cellular anatomical entity, and the most enriched MF categories were catalytic activity, structural molecule activity, and transporter activity. We further mapped the DEGs to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to analyze the metabolic pathways. In the Control vs. Cold treatment comparison, 3707 DEGs were enriched in 134 metabolic pathways (Additional File 1, Supplementary Table 3), with the top five most significantly enriched pathways being plant-circadian rhythm, ribosome biogenesis in eukaryotes, cysteine and methionine metabolism, sulfur metabolism, and phenylalanine, tyrosine, and tryptophan biosynthesis. In the Cold vs. H2S treatment comparison, 416 DEGs were enriched in 105 metabolic pathways (Additional File 1, Supplementary Table 4), with the top five most significantly enriched pathways being plant circadian rhythm, starch and sucrose metabolism, cyano-amino acid metabolism, isoflavonoid biosynthesis, and glycerolipid metabolism (Fig. 6e, f).
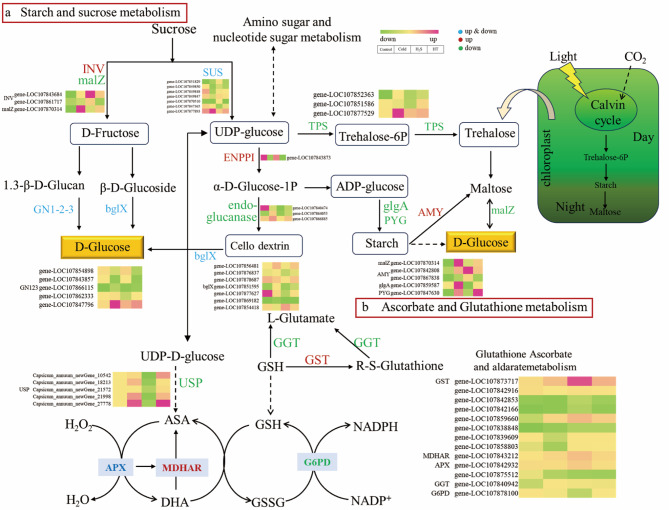
Effects of H2S on starch sucrose metabolism and the ascorbate–glutathione pathway in capsicum seedlings under cold stress
Transcriptome data analysis of cold-sensitive capsicum seedlings treated with H2S under cold stress identified 33 DEGs related to the cold stress response and sugar metabolism. Seven of these DEGs encode sucrose synthase (SUS), which promotes the synthesis of UDP-glucose (five upregulated and two downregulated). Following H2S treatment, ectonucleotide pyrophosphatase, which promotes the synthesis of the starch and cellulose glycosyl donors ADP-glucose and GDP-glucose, was upregulated; two genes encoding beta-fructofuranosidase (INV), which promotes the formation of D-fructose and D-glucose, were upregulated; one alpha-glucosidase gene, which prevents the hydrolysis of glucosidic bonds downregulation of three endoglucanase genes, was downregulated; and seven beta-glucosidase (bglX) genes were differentially regulated (one upregulated and six downregulated). Following H2S treatment, five differentially regulated glucan endo-1,3-beta-glucosidase 1/2/3 (GN1_2_3) genes were identified (two upregulated and three downregulated); these inhibit cellulose decomposition and affect sucrose breakdown into D-glucose (Fig. 7, a); and trehalose 6-phosphate synthase (TPS) was downregulated. Although by NaHS treatment promoted ADP-glucose formation, it affected the efficiency of the conversion of photosynthetically produced sucrose into starch by downregulating starch synthase (glgA) and glycogen phosphorylase (PYG) genes, while upregulating the alpha-amylase (AMY) gene, thus accelerating the conversion of starch into maltose. H2S induced downregulation of the alpha-glucosidase (malZ) gene, thus hindering the breakdown of maltose into D-glucose.
Fig. 7.
Analysis of differentially expressed genes (DEGs) involved in the metabolism of starch, sucrose, ascorbate, and glutathione. a: Starch and sucrose metabolism; b: Ascorbate and glutathione metabolism. Blue: Upregulated or downregulated; Red: Upregulated; Green: Downregulated
Metabolic pathway analysis following H2S treatment identified 18 common DEGs related to GSH and ascorbic acid (Fig. 7b). Among these, two DEGs encoding gamma-glutamyl-transpeptidase 3 (GGT) were downregulated, whereas eight encoding glutathione S-transferase (GST) were upregulated, promoting the synthesis of R-S-glutathione; and two DEGs encoding L-ascorbate peroxidase (APX), regulating the conversion of H2O2 to H2O and the formation of DAH from AsA, were identified, with one upregulated and one downregulated. One of the identified DEGs, encoding glucose-6-phosphate 1-dehydrogenase (G6PD), participates in promoting the conversion of GSSG back to GSH.
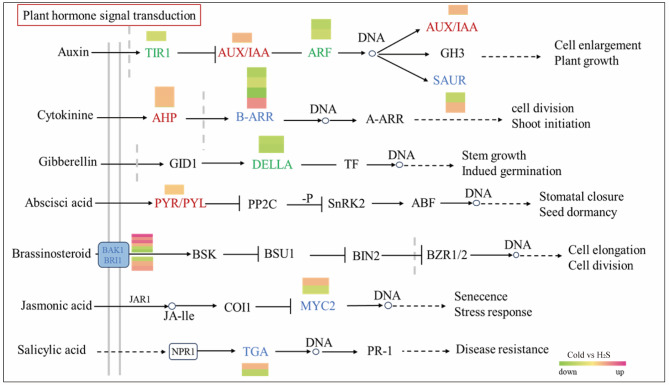
Effects of H2S on plant hormone signal transduction in capsicum seedlings cold stress
Transcriptome analysis of capsicum seedlings treated with H2S identified DEGs related to hormone signal transduction in response to cold stress, mainly involving stimulators (such as Aux, GA, cytokinin [CTK], and MEL) and inhibitors (such as ETH, ABA, SA, and JA). Following H2S treatment, in the Aux signal-transduction pathway, one DEG encoding a growth protein (Aux/IAA) with inhibitory function and one DEG related to the SAUR family protein were upregulated; one gene encoding the TIR1 receptor protein was downregulated; and two genes encoding ARF family proteins were downregulated. In terms of CTK synthesis, two genes encoding histidine-containing phosphotransfer proteins (AHP) were upregulated by H2S treatment, and four genes in the two-component response regulator ARR-B family were differentially regulated (one upregulated and three downregulated), collectively modulating CTK under cold stress. In terms of GA synthesis, two DEGs encoding DELLA proteins were downregulated by H2S treatment. In terns of ABA synthesis, H2S treatment upregulated one DEG (PYR/PYL); when phosphorylated by ABA-receptor kinases, PYR/PYL enhances PP2C inhibition and leads to the activation of the ABA signal. BR binds to the extracellular domain of BRI1 and activates its intracellular domain. Activated BRI1 then phosphorylates its negative regulator, BKI1, allowing it to bind to its coreceptor BAK1. BRI1 and BAK1 phosphorylate each other to fully activate BR signaling. Following NaHS treatment, two DEGs encoding BRI1 were upregulated and one downregulated, while four encoding BAK1 were upregulated and two downregulated. Two DEGs encoding bHLH zip transcription factor (MYC2) regulate two branches of the JA signaling pathway. MYC2 (gene-LOC107850747), which is negatively self-regulated, is essential for the expression of pathogen defense genes, whereas EGL1 (gene-LOC107865400), which is positively regulated by MYC2, participates in the response of plants to cold stress. SA is an essential hormone related to disease resistance; within the transcription factor TGA family, TGA7 (upregulated following H2S treatment) and TGA1 (downregulated) interact to activate PR gene expression.
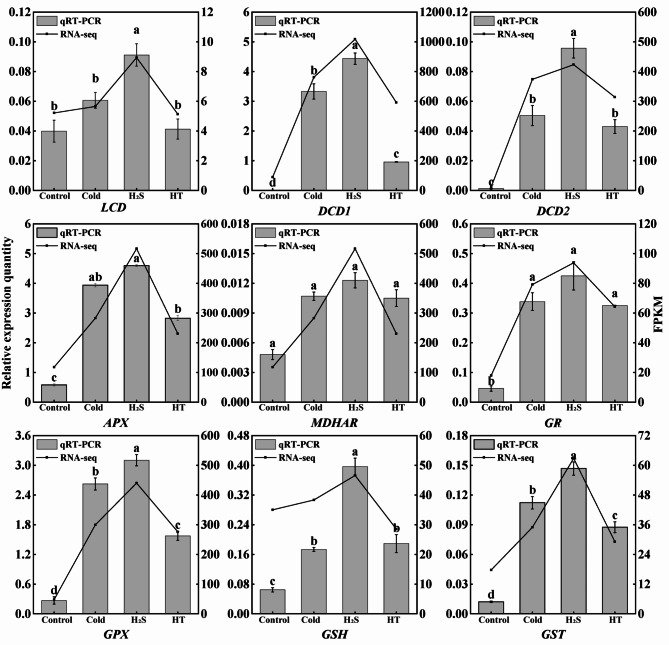
Quantitative real-time PCR (q-PCR) validation
To verify the reliability of the data, this study selected 9 antioxidant genes and H2S synthesizing genes for qRT-PCR according to the trend of antioxidant enzyme activity. Relative to the Control and Cold groups, the H2S group exhibited significantly higher FPKM values and RNA-seq-based expression of LCD, CDD1, DCD2, APX, MDHAR, GR, GPX, GSH, and GST (Fig. 8), and these levels exhibited the same trends, indicating that the samples generated reliable RNA-Seq data.
Fig. 8.
Quantitative real-time PCR (q-PCR) validation
Discussion
Capsicum, a warm-loving vegetable crop, is highly sensitive to temperature. Cold stress can have different detrimental effects on capsicum plants depending on the growth stage. Cold damages membrane lipids, increasing membrane permeability and leading to cellular metabolic disorders. REC and MDA are commonly used indicators of membrane lipid damage, whereas Pro acts as an osmoprotectant [31]. In studies on H2S alleviating aluminum stress in rice, it was found that H2S can enhance root elongation, reduce aluminum content in root tips, decrease MDA and H2O2 levels, and increase the activity of antioxidant enzymes [32]. Here, as shown in Fig. 1, H2S application reduced REC and MDA levels following cold stress. Additionally, it increased Pro content, which is consistent with the results observed in cucumbers under low-temperature stress [33]. This suggests that H2S helps to maintain membrane lipid integrity in capsicum seedlings and increases the active osmotic capacity of Pro, thereby enhancing its tolerance to cold injury. When ROS accumulation leads to oxidative stress, the elevated H2S levels can be reduced via both enzymatic and non-enzymatic pathways [34]. exogenous H2S can reduce the accumulation of H2O2 and O2·– in barley roots [35] and also enhance antioxidant enzyme activity in wheat [36]. Here, in capsicum seedlings, cold injury resulted in excessive production of ROS-related O2·− and H2O2. H2S application alleviated these effects by activating antioxidant enzymes (namely SOD, POD, CAT, APX, DHAR, and MDHAR) to continuously reduce or eliminate ROS levels and to maintain ROS homeostasis physiologically stable levels. SOD primarily converts O2·− into H2O2, which is then reduced to H2O by electrons provided by APX (as shown in Fig. 2). Studies on aluminum stress in soybeans and cadmium stress in Bermuda grass have generated similar conclusions on the role of H2S [35, 37].
The non-enzymatic antioxidant system, also known as the AsA–GSH cycle, is one of the main systems of plant defense against stress. GSH is a small redox-active molecule that occurs primarily in two stable forms, GSH and GSSG. DHA is a reversibly oxidized form of AsA. GSH participates in the AsA–GSH cycle [38]. DHA can maintain α-tocopherol and zeaxanthin in their reduced states and protect proteins from denaturation via oxidation of thiol groups, thereby preserving cell membrane integrity [35]. Here, H2S regulated the non-enzymatic antioxidants AsA, DHA, GSSG, and GSH in the AsA–GSH cycle in capsicum seedlings (as shown in Fig. 3). Similar conclusions were drawn by Kaya et al. in their study on capsicum under high zinc stress [39]. H2S treatment upregulated GST, MDHAR, and APX and downregulated GGT, USP, and G6PD (as shown in Fig. 7b), promoting the conversion of H2O2 to H2O. This indicates that both enzymatic and non-enzymatic systems are involved in protecting the membrane integrity of pepper seedlings. In rice leaves, H2S improves photosynthetic capacity by increasing the stomatal aperture and density. In Arabidopsis, in contrast, NaHS inhibits NO production and induces stomatal closure [40], and H2S reduces stomatal diameter and alleviates cold damage [41]. The role of H2S in stomatal dynamics therefore remains controversial. Here, cold treatment rapidly reduced Pn, Gs, Tr, and Ci levels, whereas H2S application significantly increased Pn, Gs, and Tr levels, indicating that H2S can promote stomatal opening and enhance the photosynthetic capacity of capsicum seedlings under cold stress. Non-stomatal factors are the primary factors causing the long-term reduction in photosynthetic capacity at low temperatures [42]. H2S can promote CO2 transport, upregulate photosynthesis-related enzymes, and mediate thiol group redox reactions to improve photosynthesis [43]. Following cold stress, RuBisCO, FBA, and SBP activity was reduced, while H2S increased this activity, promoting CO2 fixation via the dark reaction. In maize leaves under iron deficiency, application of H2S upregulated the protein and gene expression of RuBisCO and phenylpyruvate decarboxylase [44].
Starch plays a significant role in regulating stress tolerance, by releasing energy, sugars, and derivative metabolites via its degradation [45]. When sucrose enters the vacuole, it is converted into glucose and fructose by invertase (INV) and sucrose synthase (SUS) [11]. In moss, ABA-induced starch degradation improves cold tolerance [46], and drought-resistant beans can degrade more starch than drought-sensitive beans [21]. The activity of AMY, an endohydrolase that cleaves α-1,4-glycosidic linkages in starch, is associated with starch degradation [47]. Here, H2S upregulated INV and SuS in the sucrose-metabolism pathway, thus regulating endoglucanase enzyme-encoding genes such as GN123, bglX, ENPPI, and genes encoding cellulose synthesis-related endoglucanase, as shown in Fig. 7, thus promoting monosaccharide accumulation in capsicum seedlings. H2S downregulated genes encoding starch-related enzymes, including glgA and PYG, the seaweed sugar-related gene TPS, and malZ, but upregulated AMY. This indicates that, in capsicum seedlings, H2S primarily regulates starch breakdown by regulating AMY to facilitate the conversion of starch into sugar, thereby enhancing osmotic stability and cold tolerance. This indicates that the use of H2S can regulate the breakdown of accumulated starch and sucrose in pepper seedlings into monosaccharides for absorption, thereby maintaining the energy supply of pepper seedlings after cold stress and enhancing their cold tolerance.
Crosstalk between H2S and hormones comprises one of the most important signaling mechanisms in plants [48, 49]. Plant hormones include stimulators such as Aux, GA, CTK, and MEL, and inhibitors such as ETH, ABA, SA, and JA (Fig. 9) [50]. Here, NaHS activated downstream stimulators, including the auxin-responsive protein IAA and SAUR family proteins, promoting Aux synthesis; it upregulated CTK synthesis-related AHP and ARR-B and enhanced their DNA transcription. NaHS downregulated DELLA proteins, in the GA synthesis pathway. In the presence of inhibitors, NaHS increases the inhibition of PP2Cs by activating ABA signaling (by upregulating PYR/PYL expression). For Arabidopsis, Aroca et al. reached a similar conclusion [51], finding that H2S regulates guard-cell stomatal closure in response to cold stress via ABA signaling [20]. H2S affects JA and SA synthesis by upregulating MYC2 and TGA. Treating tomato seedlings with NAA upregulated DES1 expression and DES activity, leading to the accumulation of endogenous H2S, which stimulates lateral-root growth [52]. In tomatoes, H2S and GA mutually promoted each other to effectively alleviate boron stress in tomatoes, whereas HT eliminated this effect [53]. Aroca et al. [51] observed reduced persulfidation of the ABA receptors PYR1and PYL1 in Arabidopsis DES1-knockout mutants. In the epidermis of the faba bean, Arabidopsis, and Impatiens, H2S promotes stomatal closure by regulating the ABA-dependent pathway and ABC transporters located in guard cells [46]. In maize seedlings under heat stress, SA activates endogenous H2S and its synthesizing-enzyme LCD, whereas H2S does not significantly affect PAL and BA2H (the key enzymes in SA biosynthesis) or endogenous SA levels; H2S thus acts as a downstream signal of SA to regulate plant physiological functions [54]. Therefore, in capsicum seedlings, H2S alleviates cold stress by activating the ABA-dependent signaling pathway and promoting endogenous H2S accumulation. H2S pretreatment upregulates downstream transcription-factors expression under cold stress by promoting interactions between BR and the extracellular domain of BRI1 and its co-receptor, BAK1 [55]. BRI1 and BAK1 participate in the pathway regulating sugar responses via G protein subunits [56]. After being subjected to low-temperature stress, pepper seedlings exhibit changes in the levels of various plant hormones as part of their stress response mechanisms. Exogenous H2S induces structural genes such as IAA and CTK, which are involved in regulating defense functions, thereby enhancing the cold tolerance of pepper seedlings.
Fig. 9.
Effects of H2S on plant hormone signal transduction in capsicum seedlings under cold stress
Here, H2S was applied to alleviate the effects of cold damage in capsicum seedlings. Based on these findings, H2S regulates various plant hormones and carbon compounds such as starch and sucrose. This work provides some reference for using biotechnological methods to help vegetables or other plants resist cold stress. Future research will focus on in-depth studies of H2S signaling molecules in balancing ROS mechanisms.
Conclusions
These findings elucidate the mechanisms whereby H2S pretreatment regulates the alleviation of cold stress in cold-sensitive capsicum seedlings. The findings indicate that H2S can alleviate damage to capsicum seedlings caused by cold stress by regulating the accumulation of physiologically active substances, maintaining membrane integrity, enhancing enzymatic and non-enzymatic antioxidant activity, maintaining ROS homeostasis, and improving photosynthesis. In capsicum seedlings under cold stress, H2S transcriptionally upregulates key genes involved in sucrose and starch metabolism, ascorbic acid and GSH metabolism, and hormone-signaling pathways (including GST, APX, MDHAR, and AMY), while downregulating genes such as GGT, G6PD, USP, glgA, PYG, and malZ. H2S thereby maintains sugar accumulation and osmotic stability, thus enhancing the cold tolerance of cold-sensitive capsicum seedlings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
A: Conceptualization, X S. and B C.; B: methodology, Z X., L L. and J X.; C : software, W T., and M X.; D: validation, D W. and B C.; E: formal analysis, L Z. and Z H.; F: investigation, Y L. and B S.; G: resources, X S. and Z X.; H: data curation, X S., Z X and L Z.; I: writing—original draft preparation, X S.; J: writing—review and editing, X S. and B C.; K: visualization, B C., Z X. and Y T.; L: supervision, Y L., B S. and H L.; M: project administration, H L.; N: funding acquisition, H L.;
Funding
This work was supported by breeding research in vegetables (2021YFYZ0022).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue Ping Song, Bi Yan Cao and Ze Ping Xu, equally contributed to this work and are co-first authors.
References
- 1.Awasthi R, Bhandari K, Nayyar H. Temperature stress and redox homeostasis in agricultural crops. Front Environ Sci. 2015;3:11. [Google Scholar]
- 2.González-Aguilar GA, Cruz GL. Polyamines induced by hot water treatments reduce chilling injury and decay in pepper fruit [J]. Postharvest Biol Technol. 2000;18(1):19–26. [Google Scholar]
- 3.Gonzálezaguilar GA, Gayosso L, Cruz R, Fortiz J, Báez R, Wang CY. Polyamines induced by hot water treatments reduce chilling injury and decay in pepper fruit. Postharvest Biol Technol. 2000;18:19–26. [Google Scholar]
- 4.Cui X, Wang Y, Wu J, Han X, Gu X, Lu T, Zhang Z. The RNA editing factor DUA 1 is crucial to chloroplast development at low temperature in rice. New Phytol. 2019;221:834–49. [DOI] [PubMed] [Google Scholar]
- 5.Munro D, Tre berg JR. A radical shift in perspective: Mitochondria as regulators of reactive oxygen species. J Exp Biol. 2017;220(7):1170–80. [DOI] [PubMed] [Google Scholar]
- 6.Kaundal A, Rojas CM, Mysore KS. Measurement of NADPH oxidase activity in plants. Bio-Protocol. 2012. http://www.bio-protocol.org/e278
- 7.Kaur N, Dhawan M, Sharma I, et al. Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol. 2016;16:131. 10.1186/s12870-016-0824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyagi S, Verma PC, Singh K, Upadhyay SK. Molecular characterization of ascorbate peroxidase (APX) and APX–related (APX–R) genes in Triticum aestivum L. Genomics. 2020;112(6):4208–23. [DOI] [PubMed] [Google Scholar]
- 9.Fang P, Yan M, Chi C, Wang M, Zhou Y, Zhou J, Shi K, Xia X, Foyer CH, Yu J. Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 2019;180:2061–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas D, Sharkey. The end game(s) of photosynthetic carbon metabolism, Plant Physiology, Volume 195, Issue 1, May 2024, Pages 67–78. [DOI] [PMC free article] [PubMed]
- 11.Mathan J, Singh A, Ranjan A. Sucrose transport and metabolism control carbon partitioning between stem and grain in rice. J Exp Bot. 2021;72(12):4355–72. [DOI] [PubMed] [Google Scholar]
- 12.Valerio C, Costa A, Marri L, Issakidis-Bourguet E, Pupillo P, Trost P, Sparla F. Thioredoxin-regulated b-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J Exp Bot. 2011;62:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horrer D, Fl€utsch S, Pazmino D, Matthews JSA, Thalmann M, Nigro A, Leonhardt N, Lawson T, Santelia D. Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr Biol. 2016;26:362–70. [DOI] [PubMed] [Google Scholar]
- 14.Martina Zanella GL, Borghi C, Pirone et al. β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J Exp Bot, 2016, 67 – 6, 1819–26. [DOI] [PubMed]
- 15.Mei Y, Chen H, Shen W, et al. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 2017;17:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Cai W, Ji T, Ye L, Lu Y, Yuan T. WRKY13 enhances cadmium tolerance by promoting D-cysteine desulfhydrase and hydrogen sulfide production. Plant Physiol. 2020;183:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan M, Siddiqui M, Mukherjee S. Calcium-hydrogen sulfide crosstalk during K+-deficient NaCl stress operates through regulation of Na+/H + antiport and antioxidative defense system in mung bean roots. Plant Physiol Biochem. 2021;159:211–25. [DOI] [PubMed] [Google Scholar]
- 18.Fu P, Wang W, Hou L, Liu X. Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc Bot Pol. 2013;56:126–9. [Google Scholar]
- 19.Luo ZS, Li DD, Du RX, Mou WS. Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci Hortic. 2015;183:144–51. [Google Scholar]
- 20.Lin YT, Li MY, Cui WT, Lu W, Shen WB. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J Plant Growth Regul. 2012;31:519–28. [Google Scholar]
- 21.García-Mata C, Lamattina L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010;188:977–84. [DOI] [PubMed] [Google Scholar]
- 22.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–7. [Google Scholar]
- 23.Ke D, Sun G, Wang Z. Effects of superoxide radicals on ACC synthase activity in chilling-stressed etiolated mung bean seedlings. Plant Growth Regul. 2007;51:83–91. [Google Scholar]
- 24.Chakrabarty D, Datta SK. Micropropagation of gerbera: lipid peroxidation and antioxidant enzyme activities during Acclimati-Zation process. Acta Physiol Plant. 2008;30:325–31. [Google Scholar]
- 25.De Azevedo Neto AD, Prisco JT, Enéas-Filho J, Medeiros JV, Gomes-Filho E. Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol. 2005;162:1114–22. [DOI] [PubMed] [Google Scholar]
- 26.Nokano Y, Asada K. Hydrogen peroxide scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–80. [Google Scholar]
- 27.Noctor G, Foyer CH. Intracellular redox compartmentation and ROS-Related communication in regulation and signaling. Plant Physiol. 2016;171:1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekiya J, Schmidt A, Wilson LG, Filner P. Emission of hydrogen sulfide by leaf tissue in response to l-cysteine. Plant Physiol. 1982;70:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements[J]. Nat Methods. 2015;12(4):357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol. 2006;15(1):56–61. 10.1097/00019606-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Rosisca JR, de Oliveira CMG, Sartori AVD, Stolf-Moreira R, Silva MADE, Morais H. Electrical conductivity as an indicator of damage due to low temperatures in beans leaves. Semin Cienc Agrar. 2019;40:1011–22. [Google Scholar]
- 32.Zhu CQ, Zhang JH, Sun LM, Zhu LF, Abliz B, Hu WJ, et al. Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast Al contents in rice. Front Plant Sci. 2018;9:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Zhang. X, Cai. B, Pan. D et al. Physiological response and transcription profiling analysis reveal the role of glutathione in H2S-induced chilling stress tolerance of cucumber seedlings. Plant Science, 2020, 291 (2020) 110363. [DOI] [PubMed]
- 34.Liu H, Wang J, Liu J, et al. Hydrogen sulfide (H2S) signaling in plant development and stress responses. aBIOTECH. 2021;2:32–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H, Ye T, Chan Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers). Plant Physiol Biochem. 2014;74:99–107. [DOI] [PubMed] [Google Scholar]
- 36.Fu MM, Dawood M, Wang NH, Wu F. Exogenous hydrogen sulfide reduces cadmium uptake and alleviates cadmium toxicity in barley. Plant Growth Regul. 2019;89:227–37. [Google Scholar]
- 37.Wang H, Ji F, Zhang Y, Hou J, Liu W, Huang J, et al. Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminium toxicity. Plant Cell Environ. 2019;42(8):2340–56. [DOI] [PubMed] [Google Scholar]
- 38.Aghdam MS, Mahmoudi R, Razavi F, Rabiei V, Soleimani A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H2S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci Hortic. 2018;238:264–71. [Google Scholar]
- 39.Kaya C, Ashraf M, Akram NA. Hydrogen sulfide regulates the levels of key metabolites and antioxidant defense system to counteract oxidative stress in pepper (Capsicum annuum L.) plants exposed to high zinc regime. Environ Sci Pollut Res. 2018;25:12612–8. [DOI] [PubMed] [Google Scholar]
- 40.Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, et al. A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem. 2010;48(12):931–5. [DOI] [PubMed] [Google Scholar]
- 41.Jin ZP, Shen JJ, Qiao ZJ, Yang GD, Wang R, Pei TX. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun. 2011;414(3):481–6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang HH, Xu N, Teng ZY, Wang JR, Ma S, Wu X, Sun GY. 2-Cys prx plays a critical role in scavenging H2O2 and protecting photosynthetic function in leaves of tobacco seedlings under drought stress. J Plant Interact. 2018;14(1):119–28. [Google Scholar]
- 43.Fengjiao Liu X, Fu G, Wu Y, Feng F, Li H, Bi X, Ai. Hydrogen peroxide is involved in hydrogen sulfide-induced carbon assimilation and photoprotection in cucumber seedlings. Environ Exp Bot, 2020, 175–0098.
- 44.Chen J, Wu FH, Wang WH, Wang WH, Hu WJ, Simon M, et al. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot. 2011;62(13):4481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu S, Wang Y, Huang W, et al. Enhanced Accumulation of Carbohydrate and Starch in Chlorella Zofingiensis Induced by Nitrogen Starvation. Appl Biochem Biotechnol. 2014;174:2435–45. [DOI] [PubMed] [Google Scholar]
- 46.Nagao M, Minami A, Arakawa K, Fujikawa S, Takezawa D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J Plant Physiol. 2005;162:169–80. [DOI] [PubMed] [Google Scholar]
- 47.Hiteshi K, Gupta R. Thermal adaptation of alpha-amylases: a review. Extremophiles. 2014;18:937–44. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee A, Roychoudhury A. Strigolactones: multi-level regulation of biosynthesis and diverse responses in plant abiotic stresses. Acta Physiol Plant. 2018a;40:86. [Google Scholar]
- 49.Banerjee A, Roychoudhury A. Interactions of brassinosteroids with major phytohormones: antagonistic effects. J Plant Growth Regul. 2018b;37:1025–32. [Google Scholar]
- 50.Li ZG, Xiang RH, Wang JQ. Hydrogen sulfide–Phytohormone Interaction in plants under physiological and stress conditions. J Plant Growth Regul. 2021;40:2476–84. [Google Scholar]
- 51.Aroca A, Benito JM, Gotor C, Romero LC. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J Exp Bot. 2017;68:4915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang T, Cao Z, Li J, Shen W, Huang L. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem. 2014;76:44–51. [DOI] [PubMed] [Google Scholar]
- 53.Kaya C, Sarıoğlu A, Ashraf M, Alyemeni NS, Ahmad P. Gib-berellic acid-induced generation of hydrogen sulfide alleviates boron toxicity in tomato (Solanum lycopersicum L.) plants. Plant Physiol Biochem. 2020;153:53–63. [DOI] [PubMed] [Google Scholar]
- 54.Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ. Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013;36:1564–72. [DOI] [PubMed] [Google Scholar]
- 55.Gozde S, Demirer., Donald J, Gibson. et al. Xiaoyan Yue.,. Phosphate deprivation-induced changes in tomato are mediated by an interaction between brassinosteroid signaling and zinc. New Phytologist, 2023, 239: 1368–1383. [DOI] [PubMed]
- 56.Peng Y, Chen L, Li S, et al. BRI1 and BAK1 interact with G proteins and regulate sugar-responsive growth and development in Arabidopsis. Nat Commun. 2018;9:1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.