Abstract
There is limited research on the clinicopathological characteristics of combined hepatocellular-cholangiocarcinoma (cHCC-CCA) currently. The aim of this study is to summerize the clinicopathological factors and prognosis of cHCC-CCA, which could help us understand this disease. 72 cases of cHCC-CCA from West China Hospital of Sichuan University were collected. Tissue components were reviewed by pathologists. Immunohistochemistry was used to detect the status of mismatch repair (MMR) and human epidermal growth factor receptor 2 (HER2) in cHCC-CCA, as well as the quantity and distribution of CD3+ T cells and CD8+ T cells. Fluorescence in situ hybridization was used to detect fibroblast growth factor receptor 2 (FGFR2) gene alteration. COX univariate and multivariate analyses were used to evaluate risk factors, and survival curves were plotted. 49 cases were classified as classic type cHCC-CCA and 23 cases as intermediate cell carcinoma. The cut-off value for diagnosing classic type was determined to be ≥ 30% for the cholangiocarcinoma component based on prognostic calculations. All tumors were MMR proficient. The rate of strong HER2 protein expression (3+) was 8.3%, and the frequency of FGFR2 gene alteration was 26.4%. CD3+ T cells and CD8+ T cells were mainly distributed at the tumor margin, and were protective factors for patients with cHCC-CCA. The overall survival of the 72 patients was 18.9 months, with a median survival of 12 months. Tumor size, TNM stage, and serum AFP level were prognostic factors for cHCC-CCA. The proportion of cholangiocarcinoma component reaching the threshold of 30%, may provide a reference for future pathology diagnosis. FGFR2 gene alteration was 26.4%, providing a clue for anti-FGFR2 therapy. However, more data is needed for further verification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12970-8.
Keywords: cHCC-CCA, Prognosis, Cut-off, HER2, FGFR2, T lymphocytes
Introduction
The incidence and mortality of primary liver cancer ranked fourth and second respectively in 2020 in China, according to the data released by WHO [1]. Combined hepatocellular cholangiocarcinoma (cHCC-CCA) is a type of primary liver cancer, accounting for approximately 2%–5% of primary liver cancer cases. cHCC-CCA has poor prognosis, a high recurrence and mortality rate [2], and currently there is insufficient research describing its clinical and biological characteristics.
cHCC-CCA is divided into classical type (areas of hepatocellular carcinoma and areas of cholangiocellular carcinoma can be seen) and intermediate cell carcinoma (the tumoural cells have a morphology intermediate between a hepatocyte and a cholangiocyte) in histopathological subtypes [2, 3]. However, there is no definitive data to support the inclusion of a minimum cut-off amount of each component in the diagnosis of classical type cHCC-CCA [2]. By contrast, the diagnostic criteria for other mixed tumors of the digestive system is clear, such as gastric adenosquamous carcinoma, whereby the squamous component accounts for≥25%of the tumor [4]. Therefore, more precise histopathological diagnostic criteria are needed for cHCC-CCA, in order to guide routine pathological diagnosis.
Novel targeted therapies for cholangiocarcinoma (CCA) have applied in the clinical in recent years. Studies have identified alterations in mismatch repair (MMR), human epidermal growth factor receptor 2 (HER2), and fibroblast growth factor receptor 2 (FGFR2) in CCA [5], and targeted drugs for these gene alterations have been applied in clinical settings as novel treatments for CCA. Therefore, it is important to understand the status of these mature biomarkers in cHCC-CCA to provide evidence to guide treatment decisions.
Microsatellite instability (MSI) refers to length changes and nucleotide substitutions in simple repeat sequences caused by defects in DNA mismatch repair (MMR) genes [6]. MSI status is a favourable prognostic biomarker and a predictor for response to immunotherapy in multiple solid malignancies [7]. Defects in MMR (dMMR) occurs in 1%-3%of CCAs [5].
HER2 is a non-ligand binding member of the EGFR family which exerts its activity through heterodimerization with other EGFR family members to promote tumor proliferation [8]. Amplification occurs in approximately 5%-26.3%of extrahepatic cholangiocarcinoma (eCCAs) and 0.9%-8%of intrahepatic cholangiocarcinoma (iCCAs) [5, 9]. HER2 has the potential to serve as a therapeutic target for biliary tract cancer [10], and is already successfully targeted against in both breast cancer [11], and gastric cancer [12].
FGFR2 aberrations have been found in 10%-45%of iCCA tumors. FGFR is composed of four transmembrane receptors (FGFR1-4) with intracellular tyrosine kinase domains. The binding of these FGFR receptors leads to angiogenesis and unregulated cell proliferation [9, 13], and related drugs have been applied in the precision treatment of biliary tract cancer [14], such as futibatinib, and pemigatinib [9, 15, 16]. Tumor infiltrating lymphocytes (TILs) are an important component of the immune microenvironment and the basis for current immunotherapeutic approaches. TILs have a variety of surface-specific antigens, of which CD3 and CD8 are generally associated with T lymphocytes [17]. Some studies have shown that the quantity and distribution of CD3+and CD8+T cells in bile duct tumors and hepatocellular carcinoma (HCC) tumors [18, 19]. However, the role of MMR, HER2, FGFR2, CD3+and CD8+T cells in cHCC-CCA is yet to be elucidated.
Thus, in this study, we retrospectively collected a series of 72 cHCC-CCA patient samples, analyzed the clinicopathological features, MMR, HER2, and FGFR2 gene status,the number and position of CD3+and CD8+T cells, in addition to prognostic factors, providing evidence for understanding this tumor entity.
Materials and methods
Patient selection
We retrospectively retrieved the medical records of patients diagnosed with cHCC-CCA at West China Hospital between 2015 and 2019. A total of 1,024 cases of cholangiocellular carcinoma, 9,872 cases of hepatocellular carcinoma, and potential 123 cases of cHCC-CCA, were retrieved.
Data were filtered according to defined inclusion and exclusion criteria, resulting in eighty-two cHCC-CCA cases. Inclusion criteria included: (1) patients diagnosed with primary cHCC-CCA; (2) no history of radiotherapy or chemotherapy; (3) no preoperative hepatic arterial chemoembolization (TACE) or other tumor-related treatments performed; (4) absence of other primary or secondary tumors; and (5) availability of complete medical records and follow-up data. Exclusion criteria were: (1) biopsy tissue sample only; (2) Non-tumor-related death.
The diagnosis of cHCC-CCA was determined in accordance with the 2019th WHO criteria. Immunohistochemistry (IHC) also aided in the diagnosis. Positive immunostaining for AFP, HepPar-1, GPC-3, and ARG1 may suggest derivation from hepatocytes, while positive staining for CK7 and CK19 may indicate origin from bile duct cells. The dual expression of hepatocytic and cholangiocytic markers in the tumour cells supports the intermediate cell carcinoma nature. Nevertheless, the diagnosis of cHCC-CCA primarily relies on the histological features of the tumor, with IHC serving as a supplementary tool rather than a definitive diagnostic criterion.
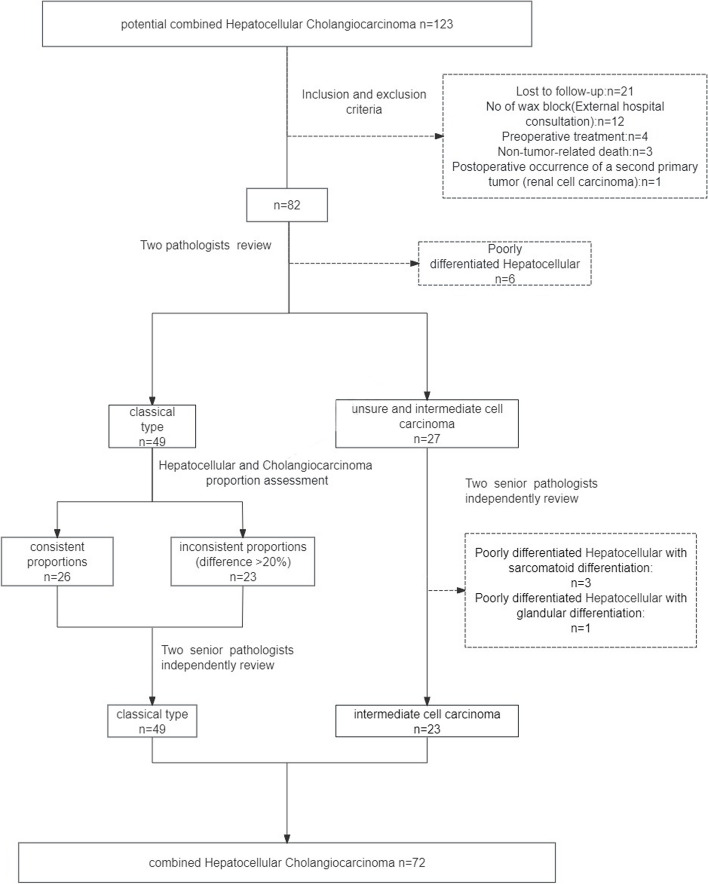
The pathologist reviewed independently according to this standard, and the specific process is as follows. Tissue slides of the 82 cases were then independently reviewed and classified by two pathologists (ZJ Lu and XF Gu). Two senior pathologists (D Jiang and D He) independently reviewed and conducted a separate analysis of intermediate cell carcinoma and those cases with inconsistent diagnosis. The four pathologists all come from gastroenterology subspecialties. Discrepancies were resolved through a detailed examination of histological morphology and immunohistochemical findings. A total of 72 patients were ultimately included in the study. Figure1illustrates the patient selection process, which ultimately included 72 patients.
Fig. 1.
Patients selection flow diagram of this study
The follow-up deadline was September 18, 2022, and follow-up methods included outpatient clinic visits, telephone communication. Overall survival (OS) was defined as the time from the day of surgery to the date of death, with the last follow-up time for surviving patients. Disease-free survival (DFS) was calculated from the date of surgery until the date of disease recurrence, death, or the last follow-up visit without evidence of recurrence.
Tissue microarray (TMA) preparation
After completing the histological evaluation as mentioned above, representative regions were selected and tissue microarrays were produced. Three tissue cores were extracted for each case. For classical type, one core was taken from the area of CCA, one core from the area of hepatocellular carcinoma (HCC), and one core from the tumor margin. For intermediate cell carcinoma, two cores were taken from the tumor area, and one core from the tumor margin. Each core had a diameter of 2 mm. A total of 6×8 cores were collected from each paraffin block. A total of 216 points were analyzed.
Immunohistochemistry (IHC)
Tissue sections (4μm) obtained from formalin-fixed paraffin-embedded (FFPE) TMA were subjected to immunostaining using the Benchmark Ultra-Advanced Staining System (Roche, Ventana Medical Systems) with positive and negative controls.
The following antibodies were used: AFP (OTI5D2, Zhongshan JinQiao, ZM-0009), HepPar-1 (OCH1E5, Zhongshan JinQiao, ZM-0131), GPC-3 (1G12, Zhong shan JinQiao, ZM-0146), ARG1 (EP261, Zhongshan JinQiao, ZA-0615), CK7 (EP16, Zhongshan JinQiao, ZA-0573), CK19 (UMAB2, Zhongshan JinQiao, ZM-0074), HER2 (4B5, ROCHE, 790–4493), MLH1 (M1, Roche Ventana MedicalSystems, Inc), MSH2 (G29-1129, Roche Ventana Medical Systems, Inc), PMS2 (A16-4, Roche Ventana Medical Systems, Inc), MSH6 (SP93, Roche Ventana Medical Systems, Inc), CD3 (LN10, Zhongshan JinQiao, ZM-0417), CD8 (SP16, Zhongshan JinQiao, ZA-0508).
IHC interpretation criteria
Two pathologists (Y Li and D Jiang) independently performed a double-blind assessment of the IHC markers.
AFP, HepPar-1 and GPC-3: Positivity for AFP, HepPar-1 and GPC-3 was determined by brownish-yellow granules presenting in the cytoplasm or cytomembrane. AGR1: Positivity for ARG1 was determined by brownish-yellow granules presenting in the cytoplasm or nuclear. CK7: Positivity for CK7 was determined by brownish-yellow granules presenting in the cytomembrane. CK19: Positivity for CK19 was determined by brownish-yellow granules presenting in the cytomembrane.
HER2: Negative (0): no staining or≤10%of invasive cancer cells showing incomplete faint membrane staining; 1+ : > 10%of invasive cancer cells showing incomplete faint membrane staining; 2+ : > 10%of invasive cancer cells showing weak to moderate intensity complete membrane staining or 10%of invasive cancer cells showing strong and complete membrane staining; 3+ : > 10%of invasive cancer cells showing strong and uniform membrane staining.
MMR: Nuclear staining of MLH1, MSH2, MSH6, and PMS2 in tumor cells was regarded as positive for MMR. When tumor nuclei were negative for each marker, the positive expression of normal glandular epithelium, infiltrating lymphocytes, vascular endothelial cells or fibroblasts were observed and served as internal positive controls. Cases were defined as proficient MMR tumors (pMMR) if positive tumor nuclear expression of all four MMR proteins were verified, and dMMR if the complete loss of tumor cell immunoreactivity for at least one of the MMR proteins.
CD3 and CD8: Positivity for CD3 and CD8 was determined by brownish-yellow granules presenting in the cytomembrane. Calculate the average number of positive cells in 4 randomly selected high power fields from each tissue core.
Fluorescence in situ hybridization (FISH)
FISH for FGFR2 was performed on tissue sections (4μm) from FFPE TMA according to an established laboratory protocol, as previously described [20]. A commercially available locus specific FGFR2 probe (Anbiping, F.01197–01) that contained 377-KB Spectrum Green fluorescent DNA in the FGFR2 3' direct marker and 446-KB Spectrum Red fluorescent DNA in the FGFR2 5' direct marker was used for hybridization. Two pathologists (Y Li and D Jiang) evaluated the FISH slides independently after screening the entire section, 100 non-overlapping nuclei, which were clearly recognized and had unequivocal signals, were counted for each case. If 20%of cells showed 1 Red 1 Green 1 Fusion (1Red 1Green distance > two signal dots), it was classified as breakage; if > 20%of cells had > 5 color signals (dots), it was classified as amplification.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software, andP < 0.05 was considered statistically significant. Wilcoxon rank-sum test was performed to assess the non-normal distribution data and categorical variables. Spearman’s rank correlation analysis was employed to investigate the association between the distribution of CD3+T cells, CD8+T cells and overall survival. Kaplan–Meier analysis was used to assess the survival rate associated with each prognostic factor and to plot the survival curve. Independent prognostic factors were determined by COX multiple regression analysis. An ROC curve was constructed to calculate the cut-off value of tissue components for cHCC-CCA.
Results
Patient demographics and clinical characteristics
The main clinical features of this study group are presented in Table1. The median age at diagnosis was 50.5 years (mean: 51.4±9.1ys; range: 27–71ys), and demonstrated a gender disparity, with 87.5%of the patients identified as male. Infection with the hepatitis virus was present in 88.9%of the patients. The levels of serum AFP and CA19-9, biomarkers for HCC and CCA, were elevated in 35/72 (≥200ng/ml) and 25/72 (≥37U/ml) of the patients, respectively. And 40/72 of patients had a tumor size greater than 5 cm. Lymph node metastasis was observed in 12/19 of the patients, and the data on lymph node involvement were available for only 19 patients. Among the patients, 13/72 were graded as stage II, 45/72 stage III, and 14/72 were stage IV at the time of diagnosis. Data on recurrence was available for only 32 cases, whilst data on metastasis was available for 29 cases. The rate of recurrence, and rate of metastasis was estimated to be 12/32 and 12/29 respectively. However, due to the comparatively short survival time of patients, accurate information regarding recurrence or metastasis could not be determined.
Table 1.
Clinicopathological features of the combined hepatocellular cholangiocarcinoma (cHCC-CCA)
| Variable | cHCC-CCA n (%) | Subtypes | ||||
|---|---|---|---|---|---|---|
| Classical type n (%) | Intermediate cell carcinoma n (%) | Z△ | P△ | |||
| Age | < 60 years | 60 (83.3) | 44 (89.8) | 16 (69.6) | -1.613 | 0.107 |
| ≥60 years | 12 (16.7) | 5 (10.2) | 7 (30.4) | |||
| Gender | Male | 63 (87.5) | 45 (91.8) | 18 (78.2) | -1.858 | 0.063 |
| Female | 9 (12.5) | 4 (8.2) | 5 (21.8) | |||
| Hepatitis B/C infection | Present | 64 (88.9) | 44 (89.8) | 20 (87.0) | -0.051 | 0.959 |
| Absent | 8 (11.1) | 5 (10.2) | 3 (13.0) | |||
| Serum AFP level | ≥200 ng/ml | 35 (48.6) | 24 (49.0) | 11 (47.8) | -0.433 | 0.665 |
| Serum CA199 level | ≥37 U/ml | 25 (34.7) | 17 (36.7) | 8 (34.8) | -0.120 | 0.905 |
| Tumor size | < 5 cm | 32 (44.4) | 22 (44.9) | 10 (43.5) | -0.112 | 0.911 |
| ≥5 cm | 40 (55.6) | 27 (55.1) | 13 (56.5) | |||
| Lymph node involvement | Present | 12 (63.2) | 7 (58.3) | 5 (71.4) | -1.220 | 0.223 |
| Absent | 7 (36.8) | 5 (41.7) | 2 (28.6) | |||
| TNM stage | I | 0 (0) | 0 (0) | 0 (0) | -1.506 | 0.132 |
| II | 13 (18.1) | 11 (22.4) | 2 (8.7) | 0.302 | 1 | |
| III | 45 (62.5) | 30 (61.2) | 15 (65.2) | 0.084 | 1 | |
| IV | 14 (19.4) | 8 (16.3) | 6 (26.1) | 0.467 | 0.981 | |
| Local recurrence | Present | 12 (37.5) | 8 (36.3) | 4 (40) | 0 | 1 |
| Absent | 20 (62.5) | 14 (63.7) | 6 (60) | |||
| Metastasis | Present | 12 (41.4) | 7 (43.8) | 5 (38.5) | 0.267 | 1 |
| Absent | 17 (58.6) | 9 (56.3) | 8 (61.5) | |||
| Histology differentiation | Well | 0 (0) | 0 (0) | 0 (0) | - | - |
| Moderate | 21 (29.2) | 21 (42.9) | 0 (0) | |||
| Poor | 51 (70.8) | 28 (57.1) | 23 (100) | |||
| MMR status | dMMR | 0 (0) | 0 (0) | 0 (0) | 0 | 1 |
| pMMR | 72(100%) | 49(100%) | 23(100%) | |||
| HER2 protein expression | 0 | 55 (76.4) | 39 (79.6) | 16 (69.6) | 0.720 | 0.678 |
| 1+ | 8 (11.1) | 4 (8.2) | 4 (17.4) | 0.365 | 0.999 | |
| 2+ | 3 (4.2) | 2 (4.1) | 1 (4.3) | 0.011 | 1 | |
| 3+ | 6 (8.3) | 4 (8.2) | 2 (8.7) | -0.193 | 1 | |
| FGFR2 gene aberration | Breakage | 12 (16.7) | 11 (22.4) | 1 (4.3) | 0.028 | 1 |
| Amplification | 7 (9.7) | 4 (8.2) | 3 (13.0) | 0.193 | 1 | |
|
CD3+T cell Median (IQR), number/HPF |
Tumor center | 11.3 (8.6–20) | 11 (3–80) | 11.5 (2–80) | 0.379 | 0.999 |
| Tumor margin | 80 (30–157.5) | 70 (5–300) | 100 (10–400) | 0.944 | 0.334 | |
|
CD8+T cell Median (IQR), number/HPF |
Tumor center | 10 (5–18.3) | 9 (2–35) | 11.5 (1.5–65) | 0.821 | 0.510 |
| Tumor margin | 50 (20–116) | 50 (5–220) | 80 (5–180) | 0.692 | 0.725 | |
Abbreviations AFP Alpha-fetoprotein, CA199 Carbohydrate antigen 19–9, TNM Stage Tumor Node Metastasis stage, MMR Mismatch repair proteins, dMMR defect in mismatch repair proteins, pMMR proficient in mismatch repair proteins, HER2 human epidermal growth factor receptor 2, FGFR2 fibroblast growth factor receptor 2, IQR interquartile range, HPF high-power field
“-” cannot be calculated. Z△: Wilcoxon rank-sum test of comparison of classical type to intermediate cell carcinoma. P△ thePvalue of comparison of classical type to intermediate cell carcinoma
In this study, there were 49 cases of classical type and 23 cases of intermediate cell carcinoma. Classification of both types was unrelated to patient gender, age, HBV/HCV viral infection, tumor size, histological grade, tumor TNM stage, presence of lymph node metastasis, or elevation of AFP and CA19-9 levels.
Histopathology
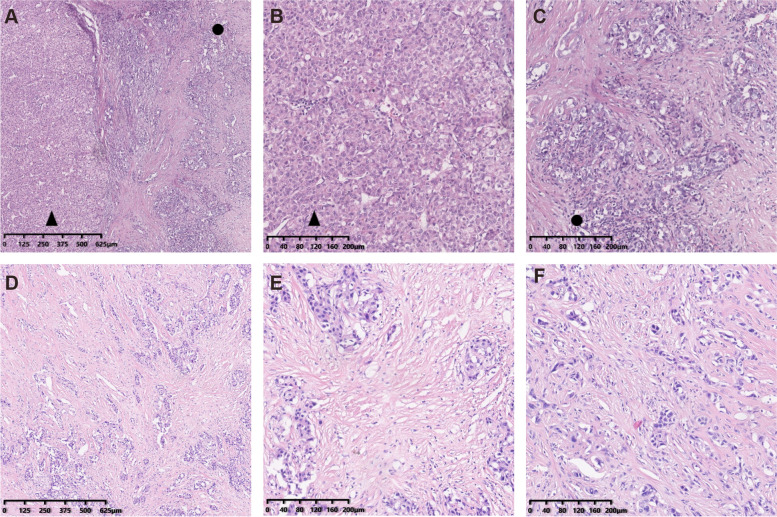
Forty-nine cases of cHCC-CCA were classified as classic type, characterized by the simultaneous presence of HCC and CCA components within the same liver tumor, with either a poorly defined or sharp transition between the two components (Fig.2A-C).
Fig. 2.
Hematoxylin and Eosin (H&E) images of combined hepatocellular cholangiocarcinoma (cHCC-CCA). A Classical type with hepatocellular carcinoma region (black solid triangle) and cholangiocarcinoma region (black solid cycle); B Hepatocellular carcinoma region; C Cholangiocarcinoma region; D-F Intermediate type of cHCC-CCA
Twenty-three cases were classified as intermediate type, with morphological features intermediatory between hepatocytes and cholangiocytes at the cellular level. These tumors appear homogeneous at lower magnification, while at higher magnification, small tumor cells with scant cytoplasm are arranged in cords, strands, trabeculae, and occasional gland-like structures within an abundant fibrous stroma (Fig.2D-F).
Immunohistochemical characteristics
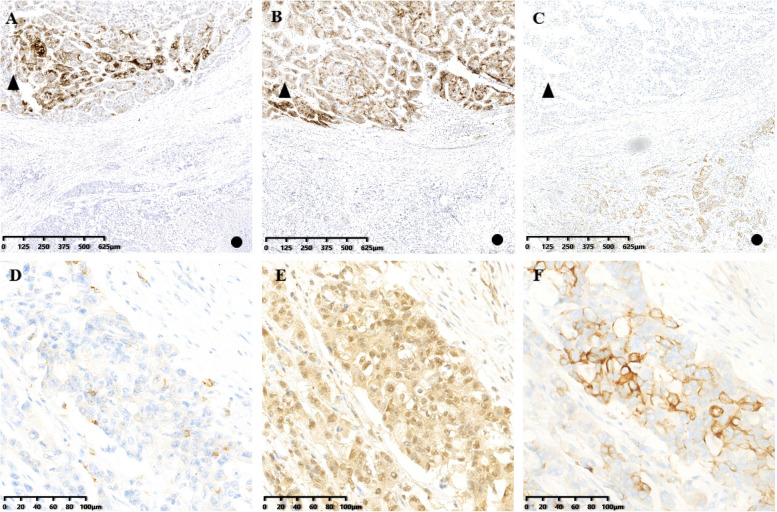
Immunohistochemical characteristics of the classical type cHCC-CCA, HCC area commonly exhibits AFP (Fig.3A), HepPar-1, ARG1 and GPC-3 (Fig.3B) positivity, while CCA area often displays CK7 and CK19 (Fig.3C) positivity. Intermediate cell carcinoma frequently exhibits both hepatocellular and bile duct cell markers (Fig.3D-F). Representative images of two types of cHCC-CCA immunohistochemistry are shown in Fig.3.
Fig. 3.
Representative images of signature antibody in two types of cHCC-CCA immunohistochemistry (IHC). AExpression of AFP in classical type, AFP was positive in hepatocellular carcinoma region (black solid triangle) and negative in cholangiocarcinoma region (black solid cycle); B Expression of GPC-3 in in classical type, GPC-3 was positive in hepatocellular carcinoma region (black solid triangle) and negative in cholangiocarcinoma region (black solid cycle); C Expression of CK19 in classical type, CK19 was positive in cholangiocarcinoma region (black solid cycle) and negative in hepatocellular carcinoma region (black solid triangle); D Expression of HepPar-1 in intermediate cell carcinoma; E Expression of GPC-3 in intermediate cell carcinoma; F Expression of CK19 in intermediate cell carcinoma
Biomarkers alterations
All 72 tumors were classified as pMMR.
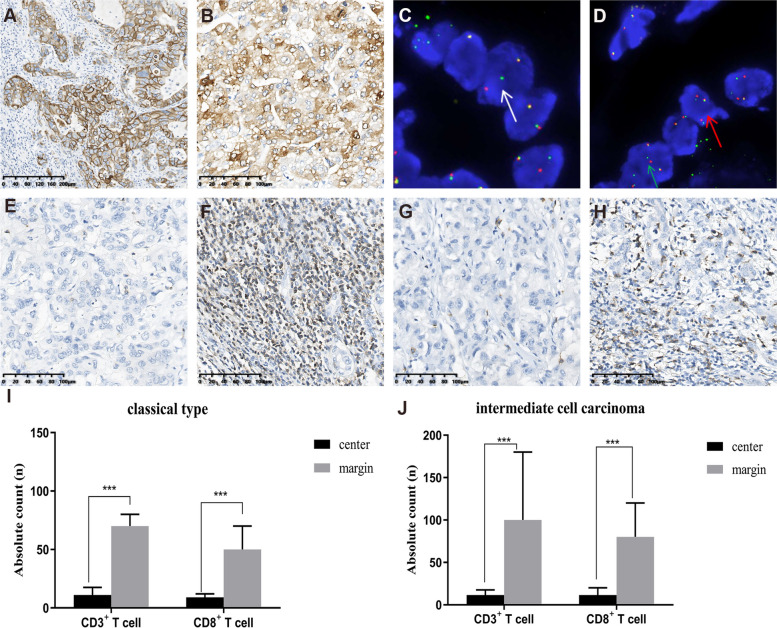
Among the 72 cases, a total of 6 (8.3%) tumors showed strong expression (3+) of the HER2 protein. Of these, 4 (5.5%) cases were classic type and expressed exclusively in the cholangiocarcinoma area (Fig.4A), whilst 2 (2.8%) were intermediate cell carcinoma (Fig.4B). No significant differences in HER2 status between the classical type and intermediate type were identified (Table1).
Fig. 4.
Representative images of HER2 immunohistochemistry (IHC), FGFR fluorescence in situ hybridization (FISH), and CD3+ T and CD8+ T cells of cHCC-CCA.AExpression of HER2 in classical type; BExpression of HER2 in intermediate cell carcinoma; CFGFR2 gene breakage, 1 fused signal, 1 green signal and 1 red signal (1F1G1R, white arrow); DFGFR2 gene amplification, 3 fused signals (3F, red arrow), 2 fused signals and 1 red signal 1 green signal (2F1R1G, green arrow); ECD3+T cells in the tumor center; FCD3+T cells at the tumor margin; GCD8+T cells in the tumor center; HCD8+T cells at the tumor margin; I-Jbar charts showing the quantification of CD3+ T and CD8+ T cells in the tumor center and margin of classical type and intermediate cell carcinomas (Median±interquartile range); ***P < 0.001
Among the total 72 cases, FGFR2 gene breakage were observed in 12 (16.7%) cases, and FGFR2 amplification in 7 (9.7%) cases. Within the 49 combined type cHCC-CCA cases, 11 (15.3%) cases showed FGFR2 breakage, of which 9 (12.5%) cases showed breakage only in the iCCA component, 2 (2.8%) cases showed breakage in both components; and 4 (5.5%) cases showed FGFR2 amplification in both components. Among the 23 intermediate type cHCC-CCA cases, 1 (1.4%) showed FGFR2 breakage, and 3 (4.2%) cases showed FGFR2 amplification (Fig.4C-D). The color signal pattern of FGFR2 amplification was predominantly 2–3 Fusion (F) and 1 green (G) 1 red (R), followed by 3-4F with occasional accompanying 1G or 1R. No significant differences in FGFR2 alteration between the classical type and intermediate type were identified (Table1).
TILs
The results demonstrated that in 72 cases, the number of CD3+T cells in the center region of the tumor was 11.3 (interquartile range, IQR 8.6–20)/high-power field (HPF), and at the tumor margin, it was 80 (IQR 30–157.5)/HPF. In classical type cases, the number CD3+T cells in the center region of the tumor was 11 (IQR 3–80)/HPF, and at the tumor margin, it was 70 (IQR 5–300)/HPF. In Intermediate cell carcinoma cases, the number of CD3+T cells in the center region of the tumor was 11.5 (IQR 2–80)/HPF, and at the tumor margin, it was 100 (IQR 10–400)/HPF. In 72 cases, the number of CD8+T cells in the center region of the tumor was 10 (IQR 5–18.3)/HPF, and it was 50 (IQR 20–116)/HPF at the tumor margin. In classical type cases, the number CD8+T cells in the center region of the tumor was 9 (IQR 2–35)/HPF, and it was 50 (IQR 5–220)/HPF at the tumor margin. In Intermediate cell carcinoma cases, the number of CD8+T cells in the center region of the tumor was 11.5 (IQR 1.5–65)/HPF, and it was 80 (IQR 5–180)/HPF at the tumor margin (Table1). CD3+and CD8+T cells primarily localised at the tumoral margin in both types of cHCC-CCA tumors and were positively correlated with OS (Fig.4E-J, Table2). In the classical type, no statistically significant difference was observed in the number of CD3+ (Z = -0.218, P = 0.828) and CD8+ (Z = -0.165, P = 0.869) T cells between the CCA component and the HCC component (Supplementary Table 1).
Table 2.
Correlation between the quantity of CD3 and CD8 (centerv.s.margin) positive cells, and overall survival in classical type and intermediate cell carcinoma
| Overall survival time | ||
|---|---|---|
| Classical type (n =49) | Intermediate cell carcinoma (n =23) | |
| Tumor center, CD3+T cell | ρ =0.365** | ρ =0.135 |
| Tumor margin,CD3+T cell | ρ =0.705** | ρ =0.641** |
| Tumor center, CD8+T cell | ρ =0.084 | ρ =0.366 |
| Tumor margin,CD8+T cell | ρ =0.700** | ρ =0.617** |
ρ: spearman’ s correlation coefficient
*P < 0.05
**P < 0.01
***P < 0.001
Survival
The OS of the 72 patients with cHCC-CCA was 18.9 months, with a median survival of 12 months. The 1-, 2-, and 3-year postoperative OS rates were 51.4%, 26.4%, and 16.7%, respectively. DFS was 13.7 months, with a median relapse duration of 5 months. Postoperative pathology revealed lymph node metastases in 12/19 patients, local recurrence in 12/32 patients, and distant metastasis in 12/29 patients.
The mean OS of the 49 patients with classical type cHCC-CCA was 16.5 months, and the median survival was 7 months. The 1-, 2-, and 3-year postoperative OS rates for classical type patients were 46.9%, 28.6%, and 14.3%, respectively. The mean OS of the 23 patients with intermediate cell carcinoma was 22.3 months, and the median survival was 7 months. The median follow-up time was 12 months (interquartile range 3–24 months).
Prognostic impact factors
Survival curve analysis revealed that a tumor maximum diameter of 5 cm or more, TNM stage, and elevated serum AFP levels were significantly associated with OS in the cohort of 72 patients with cHCC-CCA (Fig.5A-G). However, age, gender, HER2 expression and FGFR2 alteration did not show any significant correlation with OS.
Fig. 5.
Prognostic factors of Kaplan–Meier curve analysis in combined hepatocellular cholangiocarcinoma (cHCC-CCA), and cut off value analysis of ROC curve. A-C The tumor size, TNM stage and serum AFP level in 72 cHCC-CCA cases; D-F The tumor size, TNM stage, and serum AFP level in the classical type tumors; G serum AFP level in the intermediate cell carcinoma; H cut off value analysis of ROC curve
COX univariate and multivariate analysis revealed that TNM stage, and AFP levels were independent prognostic factors in the 72 cases of cHCC-CCA. In 49 cases of classical types cHCC-CCA, tumor size, TNM stage, and AFP levels were independent prognostic factors (Table3). Since only AFP levels (HR=0.217; 95%CI=0.066–0.712; P =0.012) were found to be associated with prognosis in the univariate analysis (Supplementary Table 2), no multivariate analysis was performed for variable intermediate cell carcinoma.
Table 3.
COX univariate and multivariate analysis of prognostic factors
| Variable | cHCC-CCA (n=72) | cHCC-CCA-classical type (n =49) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Age | Ref | 0.471 | Ref | 0.951 | ||||
| < 60 years | 0.792 (0.420–1.492) | 1.028 (0.432–2.445) | ||||||
| ≥60 years | ||||||||
| Gender | 0.912 | 0.919 | ||||||
| Female | Ref | Ref | ||||||
| Male | 0.961 (0.472–1.957) | 1.055 (0.374–2.978) | ||||||
| Hepatitis B/C infection | 0.488 | 0.068 | ||||||
| Present | Ref | Ref | ||||||
| Absent | 0.722 (0.288–1.813) | 0.366 (0.124–1.079) | ||||||
| Serum AFP level | < 0.001 | < 0.001 | 0.023 | 0.017 | ||||
| < 200 ng/ml | Ref | Ref | Ref | Ref | ||||
| ≥200 ng/ml | 0.298 (0.173–0.514) | 0.343 (0.193–0.610) | 0.456 (0.232–0.897) | 0.440 (0.225–0.861) | ||||
| Serum CA199 level | 0.987 | 0.452 | ||||||
| < 37 U/ml | Ref | Ref | ||||||
| ≥37 U/ml | 1.004 (0.599–1.684) | 1.271 (0.681–2.373) | ||||||
| Tumor size | 0.041 | 1.510 (0.893–2.554) | 0.124 | 0.014 | 2.345 (1.168–4.710) | 0.017 | ||
| < 5 cm | Ref | Ref | ||||||
| ≥5 cm | 1.695 (1.022–2.812) | 2.193 (1.169–4.113) | ||||||
| Lymph node involvement | 0.401 | 0.173 | ||||||
| Present | Ref | Ref | ||||||
| Absent | 0. 669 (0.262–1.709) | 0.383 (0.097–1.522) | ||||||
| TNM stage | 0.018 | 0.039 | 0.024 | 0.032 | ||||
| II | Ref | Ref | Ref | Ref | ||||
| III | 2.847 (1.138–6.148) | 2.683 (1.223–5.885) | 2.911 (1.228–6.905) | 3.257 (1.323–8.020) | ||||
| IV | 3.284 (1.335–8.080) | 2.884 (1.146–7.263) | 3.983(1.386–11.450) | 3.297 (1.105–9.840) | ||||
| Local recurrence | 0.122 | 0.355 | ||||||
| Present | Ref | Ref | ||||||
| Absent | 0.571 (0.281–1.162) | 0.659 (0.273–1.594) | ||||||
| Metastasis | 0.169 | 0.617 | ||||||
| Present | Ref | Ref | ||||||
| Absent | 0.603 (0.293–1.240) | 0.799 (0.331–1.926) | ||||||
| Histology differentiation | 0.454 | |||||||
| Moderate | Ref | |||||||
| Poor | 1.217 (0.728–2.035) | |||||||
| HER2 | 0.174 | 0.252 | ||||||
| 0~2+ | Ref | Ref | ||||||
| 3+ | 1.818 (0.770–4.263) | 1.866(0.642–5.418) | ||||||
| FGFR2 gene aberration | 0.782 | 0.959 | ||||||
| Present | Ref | Ref | ||||||
| Absent | 0.923 (0.522–1.630) | 1.017(0.530–1.954) | ||||||
Abbreviations HR Hazard risk, CI Confidence interval, Ref Reference, AFP Alpha-fetoprotein, CA199 Carbohydrate antigen 19–9, TNM Stage Tumor Node Metastasis stage
Tissue components Cut-off Value
In the classic type cHCC-CCA, using a 10% composition of cholangiocarcinoma as the threshold, an ROC curve was constructed based on survival time, and it was ultimately determined that when the cholangiocarcinoma component was ≥ 30%, the difference in survival time between the two groups was most significant (P =0.009, AUC= 0.717). Therefore, this study will consider a cholangiocarcinoma component of ≥30% as the diagnostic cut-off value for the classic type cHCC-CCA, which presents a poor OS than those with a cholangiocarcinoma component of less than 30% (Fig.5H).
Discussion
In this study, we collected 72 cases of cHCC-CCA for the analysis of clinical characteristics and prognosis. Histological features were summarized, and the expression of MMR, HER2 proteins, alteration of FGFR2 gene, and the quantity and distribution of CD3+or CD8+T cell were observed to provide evidence for the application of targeted therapy for cHCC-CCA.
The demographic characteristics and risk factors of cHCC-CCA are similar to those of both HCC and iCCA [2]. The average age for patients in this study was 50±10.9 years, which is consistent with the average age of incidence for iCCA. Among them, 63/72 were male, similar to the higher incidence rate in males for HCC and iCCA. Hepatitis virus infection has been identified as a risk factor for HCC and iCCA, and 64/72 of the patients in this study showed a history of hepatitis virus infection. Although alpha-fetoprotein (AFP) and carbohydrate antigen 19–9 (CA19-9) are biomarkers for HCC and CCA, few previous studies have detected an increase in both markers [21]. In this study, the AFP elevation rate was 35/72 and the CA19-9 elevation rate was 25/72, which is in line with previous research. Previous studies have demonstratedthat lymph node metastasis occurs in approximately 8.3%-60.0%of cHCC-CCA cases [22], and the present study showed a similar lymph node metastasis rate of 63.2% (12/19). The majority of patients were diagnosed at advanced stages, indicating the need for continued efforts in early diagnosis of cHCC-CCA.
After re-evaluation by four pathologists, we classified the 72 cases of cHCC-CCA into 49 cases of classical type and 23 cases of intermediate cell carcinoma. The ratio of classic to intermediate in this series was 2:1, and there is currently rare literature reporting the ratio of these two types.
In the current diagnositic criteria for classic type cHCC-CCA, no cut-off value for the proportion of HCC and CCA components exists. We found that tumours with iCCA component ≥30% have poor overall survival, and this cut-off value can be used as a diagnostic criterion for cHCC-CCA. A similar result was presented in a recent published study, showing that the tumours with HCC component≥65%have a better survival [23]. It is hoped that this will provide support for the diagnosis and subsequent treatment of cHCC-CCA. During pathological diagnosis, we recommend that when a region of HCC contains a large amount of fibrous tissue or glandular differentiation, carefully evaluation for the presence of cholangiocarcinoma is essential. Diagnosing the intermediate cell carcinoma is difficult, and can easily be misdiagnosed as CCA. The tumor cells of the intermediate type are a single morphological feature between hepatocytes and bile duct cells, with small and uniform tumors arranged in cords, chains, and trabeculae occasionally forming gland-like structures within a rich fibrous interstitium. The dual expression of hepatocytic and cholangiocytic markers in the tumour cells supports the intermediate hepatobiliary cell nature. It is important to note that IHC alone is not sufficient for a diagnosis of cHCC-CCA without supportive histomorphology. Due to the different postoperative treatment methods for HCC, CCA, and cHCC-CCA, caution must be exercised during the diagnosis process to ensure accurate diagnosis, and hence the most appropriate treatment decision pathway [2].
The preferred treatment for cHCC-CCA remains surgical intervention, and effective targeted therapies for cHCC-CCA are still under exploration [24].This study involved assessing the status of four MMR proteins and HER2 proteins in cHCC-CCA, as well as examining the aberrant status of FGFR2 genes and the quantity and localization of CD3+T cells and CD8+T cells. A published study shows the common gene mutations of cHCC-CCA and provides more research directions for targeted therapy [25]. Compared with this, to address the clinical demand for targeted therapies, our research focuses on whether some commonly altered biomarkers in CCA were also altered in cHCC-CCA, in the hopes of providing more choices for the targeted treatment of cHCC-CCA using established CCA treatment protocols. Our results show that all 72 cases were pMMR tumors, possibly due to the small sample size of the study, and hence the collection and further analysis of additional cases should performed in order to validate this finding. Strong (3+) HER2 protein expression presented in 8.2%of the classical type and 8.7%of the intermediate cell carcinoma. Previous studies have shown that amplification of HER2 occurs in approximately 5%-26.3%of eCCAs and 0.9%-8%of iCCAs [5, 9], which is consistent with our results. We also observed that HER2 expression in the CCA region of classical type cHCC-CCA was similar to that of CCA, while expression in the intermediate cell carcinoma was generally weak and diffuse.
FGFR2 gene breakage were detected in 22.4%of the classical type, and 4.3%of the intermediate cell carcinoma cases respectively, whilst FGFR2 amplification was detected in 8.1%of the classical type and 13.0%of the intermediate cell carcinomas. During the interpretation process, we observed that in FGFR2 amplified cases, most cells demonstrated paired red and green signals, with only a few cases showing an isolated increase in either red or green signal. Previous research has shown that FGFR2 aberrations have been found in 10%-45%of iCCA tumors [9], which is within the range of our study. Currently, the testing for and targeted drugs against both HER2 and FGFR2 have been applied in CCA [26], and the results highlighted here demonstrate that it is imperative to incorporate testing for HER2 and FGFR2 into routine assessment for cHCC-CCA in clinical practice.
Broad analysis of immune microenvironment-related cells and gene expression profiles of cHCC-CCA were reported [27, 28], however, our study specifically observe a spatial distribution of CD3+T cells and CD8+T cells to provide a clue of microenvironment from a histology perspective. In this study, we demonstrated that CD3+and CD8+T cells primarily accumulate in tumor margin, and were positively correlated with prognosis in cHCC-CCA. No statistical significance was observed between CCA and HCC components regarding the quantity of CD3+and CD8+T cells. One previously published study investigated the density and distribution of CD3+and CD8+T cells in HCC and CCA, revealing a lower total number of CD8+T cells in CCA, compared to HCC. CD3+and CD8+T cells mainly cluster at the margin of CCA, and patients with higher concentrations of CD3+and CD8+T cells have better prognosis [18]. The prognostic impact of the accumulation and localisation of predominantly CD3+T cells in HCC is currently debated. The density of CD8+T cells is lower in the center of the tumor, as opposed to the margin, and CD8+T lymphocytes in HCC have been indentified as a protective factor. Higher expression of CD8+TILs in the infiltrative margin, intratumoral, and perivascular areas is associated with improved survival in HCC [18, 19].
In this study, the OS of the 72 patients with cHCC-CCA was 18.9 months, with a median survival of 12 months. The DFS of the 72 patients with cHCC-CCA was 13.7 months, with a median relapse time of 5 months. This is consistent with the previous findings on OS and DFS in cHCC-CCA [3].
The results showed that tumor size 5 cm, TNM stage, and elevated AFP level were prognostic factors for cHCC-CCA. Previous studies have identified that the incidence of lymph node metastasis in cHCC-CCA is about 8.3%-60.0% [22], which was racpitulated in this study, with an incidence of lymph node metastasis of 63.2%. Despite this, lymph node metastasis was not identified an independent risk factor for overall survival in cHCC-CCA patients, potentially due to the small number of lymph node metastases obtained. However, because the incidence of lymph node metastasis in cHCC-CCA is relatively high, it is necessary to observe the distribution of lymph node lesions and metastasis in patients undergoing surgical resection for cHCC-CCA, and to perform lymph node dissection as required. Tumor recurrence and metastasis are also common factors affecting patient prognosis, however, due to limited information, recurrence and metastasis were not considered as factors affecting patient prognosis in this study. It is hoped that future studies can increase the sample size to obtain a more complete picture of the prognostic impact of cHCC-CCA metastatic dissemination.
The objective of this study is to refine pathological diagnostic criteria, improve the accuracy of the prognosis of patients, and explore the potential targeted biomarkers in cHCC-CCA by using the progress in the field of precision therapy for CCA. These efforts will advance clinical decision-making and optimize treatment strategies for specific molecular features of cCC-CCA, ultimately improving patient prognosis and quality of care. Recently, several articles have reported clinicopathological characteristics and genetic changes of cHCC-CCA [29–31], which have some similarity with our study. But our finding provides a diagnostic cut-off values for the different tissue components for the disease, from a pathological diagnosis view. We hope our study can add some clue for the understanding of cHCC-CCA.
There were some limitations of this study, including the relatively small sample size, however it represents the current limit of data collection for cHCC-CCA due to the low incidence rate and lack of early clinical information. Further follow-up will be conducted to collect more cases of cHCC-CCA for future studies. The usage of TMA for TILs observation is another drawback, but we sample the tissue from different tumor regions, including sample from different tissue components and sample from tumor center or margin, in an attempt to counteract the bias of sampling. Additionally, gene-level validation for HER2 was not performed in this study, and as such we plan to include FISH testing for HER2 in our future studies.
In summary, cHCC-CCA has a high mortality rate, exhibiting characteristics of both hepatocellular carcinoma and cholangiocarcinoma. The optimal cutoff value of ≧30%for the CCA component is expected to aid classical type cHCC-CCA diagnosis. Alterations in HER2 and FGFR2 was detected in cHCC-CCA, and corresponding indicators should be considered for detection in clinical diagnosis, to ensure optimal patient treatment strategies. Tumor size 5 cm, TNM staging, and serum elevated AFP levels (≥200ng/ml) affect the prognosis of patients with cHCC-CCA, and personalized treatment can be applied based on these factors.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- cHCC-CCA
Combined hepatocellular cholangiocarcinoma
- CCA
Cholangiocarcinoma
- MMR
Mismatch repair
- HER2
Human epidermal growth factor receptor 2
- FGFR2
Fibroblast growth factor receptor 2
- MSI
Microsatellite instability
- dMMR
Defects in MMR
- eCCAs
Extrahepatic cholangiocarcinoma
- iCCAs
Intrahepatic cholangiocarcinoma
- FGFR1-4
Fibroblast growth factor receptors 1-4
- TILs
Tumor infiltrating lymphocytes
- HCC
Hepatocellular carcinoma
- TACE
Hepatic arterial chemoembolization
- OS
Overall survival
- DFS
Disease-free survival
- TMA
Tissue microarray
- FFPE
Formalin-fixed paraffin-embedded
- pMMR
Proficient MMR
- AFP
Alpha-fetoprotein
- CA19-9
Carbohydrate antigen 19–9
Authors’ contributions
Yue Li and Dan Jiang designed the study. Yue Li, Du He, Zi-Jian Lu, Xia-Fei Gu, and Dan Jiang reviewed the tissue slides; Yue Li, Xiao-Yu Liu, Min Chen, Yu Zhou, and Yin-Xia Tu conducted the experiments. Yue Li and Xian Zhang analyzed data. Yue Li and Gemma Owen wrote the manuscript; Dan Jiang revised the manuscript. Gemma Owen modified and embellished the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Resident Physician Research Fund of West China Hospital, Sichuan University [Project No. 20220220200723] from Yue Li.
Availability of data and materials
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of West China Hospital. The Ethics Committee of West China Hospital agrees to waive informed consent because this retrospective observational study uses medical records/biological specimens from previous clinical diagnosis, the risk of which was not greater than the minimum risk.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer (IARC). IARC Global Cancer Observatory. Globocan 2020,March, 2021.https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf. Cited 15 July 2023.
- 2.World Health Organization. WHO Classification of tumors. 5th ed. Digestive System. Lyon: International Agency for Research on Cancer; 2019.260–264.
- 3.Aurélie Beaufrère J C, Valérie Paradis .Combined hepatocellular-cholangiocarcinoma: An update Beaufrère J Hepatol. 74:5:1212-1224.10.1016/j.jhep.2021.01.035 [DOI] [PubMed]
- 4.World Health Organization. WHO Classification of tumors. 5th ed. Digestive System. Lyon: International Agency for Research on Cancer; 2019.98.
- 5.Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J Hepatol. 2020;73(1):170–85 10.1016/j.jhep.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Hacking S, Chou C, Baykara Y, Wang Y, Uzun A, Gamsiz Uzun ED. MMR Deficiency Defines Distinct Molecular Subtype of Breast Cancer with Histone Proteomic Networks. Int J Mol Sci. 2023;24(6):5327 10.3390/ijms24065327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtney J. Riedinger ,Paulina J. Haight and Floor J. Backes et al.Epigenetic MMR defect identifies a risk group not accounted for through traditional risk stratification algorithms in endometrial cancer. Front. Oncol. 13:1147657.10.3389/fonc.2023.1147657 [DOI] [PMC free article] [PubMed]
- 8.Bhavya Yarlagadda, Vaishnavi Kamatham, Ashton Ritter, Faisal Shahjehan, Pashtoon M. Kasi.Trastuzumab and pertuzumab in circulating tumor DNA ERBB2-amplified HER2-positive refractory cholangiocarcinoma.NPJ Precis Oncol. ,2019, 2019 Aug 19;3:19.10.1038/s41698-019-0091-4. [DOI] [PMC free article] [PubMed]
- 9.Yang W, Sun Y. Promising Molecular Targets for the Targeted Therapy of Biliary Tract Cancers: An Overview. Onco Targets Ther. 2021Feb;25(14):1341–66.10.2147/OTT.S297643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuzillet C, Artru P, Assenat E, et al. Optimizing Patient Pathways in Advanced Biliary Tract Cancers: Recent Advances and a French Perspective. Targ Oncol. 2023;18:51–76.10.1007/s11523-022-00942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhamar M, Alkamachi B, Mehrotra H, et al. Clinical significance of quantitative categorization of HER2 fluorescent in situ hybridization results in invasive breast cancer patients treated with HER2-targeted agents. Mod Pathol. 2021;34:720–34.10.1038/s41379-020-00728-z. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Li X, Chen P, Gao Y. From Anti-HER-2 to Anti-HER-2-CAR-T Cells: An Evolutionary Immunotherapy Approach for Gastric Cancer. J Inflamm Res. 2022Jul;17(15):4061–85.10.2147/JIR.S368138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13..Miguel Zugman, Gehan Botrus ,Roberto Carmagnani Pestana and Pedro Luiz Serrano Uson Junior .Precision Medicine Targeting FGFR2 Genomic Alterations in Advanced Cholangiocarcinoma: Current State and Future Perspectives.Front Oncol. ,2022, 2022 Apr 4;12:860453.10.3389/fonc.2022.860453 [DOI] [PMC free article] [PubMed]
- 14.Raevskaya O, Appelman H, Razumilava N. A Contemporary Approach to Diagnosis and Treatment of Combined Hepatocellular-Cholangiocarcinoma. Curr Hepatol Rep. 2020;19(4):478–8510.1007/s119-020-00556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzo A. Targeted Targeted Therapies in Advanced Cholangiocarcinoma: A Focus on FGFR Inhibitors. Medicina. 2021;57:458.10.3390/medicina57050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuanyuan Zheng, Yan Li and Jiao Feng et al. Cellular based immunotherapy for primary liver cancer. J Exp Clin Cancer Res 40, 250 (2021).10.1186/s13046-021-02030-5 [DOI] [PMC free article] [PubMed]
- 17.Liu D, Heij LR, Czigany Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41(1):127.10.1186/s13046-022-02340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Jin W, Wang S, Ding H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Front Immunol. 2021Sep;3(12): 729705.10.3389/fimmu.2021.729705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu TH, Chen X, Zhang XH, Zhang EC, Sun CX. Clinicopathological characteristics and prognostic factors for intrahepatic cholangiocarcinoma: a population-based study. Sci Rep. 2021;11(1):3990.10.1038/s41598-021-83149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang D, Peng R, Yan X, et al. Synovial sarcoma showing loss of a green signal in SS18 fluorescence in situ hybridization: a clinicopathological and molecular study of 12 cases. Virchows Arch. 2017;471:799–807.10.1007/s00428-017-2211-2. [DOI] [PubMed] [Google Scholar]
- 21.Li R, Yang D, Tang CL, et al. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016Feb;25(16):158.10.1186/s12885-016-2156-x.PMID:26917546;PMCID:PMC4768404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HS, Bae JS, Jang KY, et al. Clinicopathologic study on combined hepatocellular carcinoma and cholangiocarcinoma: with emphasis on the intermediate cell morphology. J Korean Med Sci. 2011Aug;26(8):1023–30.10.3346/jkms.2011.26.8.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Y, Zheng X, Zhou C, et al. Combined hepatocellular carcinoma-cholangiocarcinoma with a predominant HCC component: better survival and MRI-based prediction. Eur Radiol. 2023;33:1412–21.10.1007/s00330-022-09131-5. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Wang L, Teng F, Zhang T, Zhao Y, Chen Z. The clinical characteristics and prognostic factors of combined Hepatocellular Carcinoma and Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma after Surgical Resection: A propensity score matching analysis. Int J Med Sci. 2021Jan 1;18(1):187–98.10.7150/ijms.50883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue R, et al. Genomic and Transcriptomic Profiling of Combined Hepatocellular and Intrahepatic Cholangiocarcinoma Reveals Distinct Molecular Subtypes. Cancer Cell. 2019Jun 10;35(6):932-947.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proskuriakova E. K. Current Targeted Therapy Options in the Treatment of Cholangiocarcinoma: a literature review. Cureus. 2022;14(6):e26233.10.7759/cureus.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen CT, et al. Immune Profiling of Combined Hepatocellular-Cholangiocarcinoma Reveals Distinct Subtypes and Activation of Gene Signatures Predictive of Response to Immunotherapy. Clin Cancer Res. 2022Feb 1;28(3):540–51. [DOI] [PubMed] [Google Scholar]
- 28.Yagi N, et al. Component with abundant immune-related cells in combined hepatocellular cholangiocarcinoma identified by cluster analysis. Cancer Sci. 2022May;113(5):1564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito T, Ishii T, Takeda H, Sumiyoshi S, Tomofuji K, Wakama S, et al. Comprehensive analyses of the clinicopathological features and genomic mutations of combined hepatocellular-cholangiocarcinoma. Hepatol Res. 2024;54(1):103–15.10.1111/hepr.13965. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki M, Sato Y, Nakanuma Y. Expression of fibroblast growth factor receptor 2 (FGFR2) in combined hepatocellular-cholangiocarcinoma and intrahepatic cholangiocarcinoma: clinicopathological study. Virchows Arch. 2024;484:915–23.10.1007/s00428-024-03792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vij M, Veerankutty FH, Rammohan A, Rela M. Combined hepatocellular cholangiocarcinoma: A clinicopathological update. World J Hepatol. 2024;16(5):766–75 10.4254/wjh.v16.i5.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.