Abstract
The brain in the genus Homo expanded rapidly during evolution, accelerated by a reciprocated interaction between neural, cognitive, and ecological niches (triadic niche construction, or TNC). This biologically costly expansion incubated latent cognitive capabilities that, with a quick and inexpensive rewiring of brain areas in a second phase of TNC, provided the basis for Homo sapiens specific abilities. The neural demands for perception of the human body in interaction with tools and the environment required highly integrated sensorimotor domains, inducing the parietal lobe expansion seen in humans. These newly expanded brain areas allowed connecting the sensations felt in the body to the actions in the world through the cognitive function of “projection”. In this opinion article, we suggest that as a relationship of equivalence between body parts, tools and their external effects was established, mental mechanisms of self‐objectification might have emerged as described previously, grounding notions of spatial organization, idealized objects, and their transformations, as well as socio‐emotional states in the sensing agent through a self‐in‐the‐world map. Therefore, human intelligence and its features such as symbolic thought, language, mentalizing, and complex technical and social behaviors could have stemmed from the explicit awareness of the causal relationship between the self and intentional modifications to the environment.
Keywords: brain expansion, hominid evolution, niche construction, parietal cortex
1. NICHE CONSTRUCTION AND THE RISE OF HUMAN COGNITION
The theory of triadic niche construction (TNC) posits that human evolution involved a specific process that accelerated the emergence of novel abilities, such as symbolic thought, language, mentalizing, and complex technical and social behaviors, which served as the basis to the rise of modern technological civilization (Iriki & Taoka, 2012). A key concept in TNC is niche construction, which is a set of events by which organisms select and modify components of their environments in which they and their descendants live, affecting the ecological processes and environmental factors that impact their subsequent evolution (Bateson & Gluckman, 2011; Laland et al., 2017). More specifically, TNC posits that human evolution was driven by the mutual interaction between three domains: ecological, neural, and cognitive. The ecological niche being the habitat and environment of a species and the neural niche being the biological substrate that supports specific cognitive capacities. Meanwhile, the cognitive niche encompasses these newly acquired cognitive capacities that allow exploiting the environment through reasoning and knowledge (Iriki & Taoka, 2012).
TNC argues that these three domains are mutually co‐constructive, meaning that they influence each other in a reciprocal manner. For example, the ecological niche can shape the evolution of the neural niche by providing selective pressures that favor the development of certain cognitive capacities. In turn, the neural niche can influence the ecological niche by allowing individuals to exploit the environment in new and innovative ways. That means that humans (including archaic humans) influenced their own evolutionary trajectory by first modifying the environment and then adapting to this new environment. The cognitive niche is particularly important in TNC, as it is the domain that allows humans to actively shape their own environment in a goal‐oriented fashion. This is done through the use of tools, language, and other cultural artifacts. By modifying their environment, humans can create new opportunities for learning and development, which in turn can lead to further cognitive evolution. Archeological, neurobiological, and psychological studies suggest that the process of TNC was subtended by two different, successive mechanisms that have been designated as two phases of TNC: TNC‐1 and TNC‐2 (Iriki et al., 2021).
TNC‐1 is assumed to have begun with the rise of stone toolmaking in early Homo. The rate of expansion in cranial capacity during this period occurred much faster in humans than it had in their Australopithecine ancestors (Iriki et al., 2021), which indicates that humans experienced an expansion of neural tissue dedicated to novel cognitive functions (Krubitzer & Dooley, 2013). Stone toolmaking constitutes an important milestone because it enabled the development of different types of tools for different and specialized purposes. Integrating such tools into a habitat modifies how individuals interact with and exploit their habitat, thereby creating a novel ecological niche that can evolve alongside tool complexification. This combination of hereditary (genetic and extra‐genetic) and environmental changes dominated as the main evolutionary force during that period (Iriki & Taoka, 2012). Tools, therefore, are not only manifestations of the human biological–cognitive niche but also an active and essential part of human development (Bruner, 2021).
Given the numerous species that use tools, it is necessary to ask, “Why did this pattern occur only in humans?” New Caledonian crows spontaneously remove leaves from twigs and use them to retrieve food from crevices (Kenward et al., 2005). Chimpanzees can manufacture tools, such as termite fishing probes, and teach their techniques to younger individuals (Musgrave et al., 2020). However, it is important to make a distinction between tools and “objects”, as defined by Bruner and Gleeson (2019). A tool, as opposed to an object, must fit three criteria: be represented within the body schema of the brain, be part of a productive chain in which a sequence of behaviors involving the tool or tools must be necessary to achieve a target, and be integrated with and necessary to a cultural niche (Bruner, 2021; Bruner & Gleeson, 2019). Presumably, human lineages possessed distinctive traits, perhaps pertaining to a set of features favorable to TNC that allow tool use within these criteria. Humans possessed large brains, which correlate with behavioral innovation (Reader & Laland, 2002) and the capacity to cope with novel environments (Bateson & Gluckman, 2011). Furthermore, brain expansion occurred through the addition of a diversity of anatomical and functional brain regions, which may have functioned as a form of preadaptation to develop new functions (Anderson, 2010; Krubitzer, 2009).
2. THE EVOLUTION OF THE BODY AND THE BRAIN ARE CLOSELY LINKED
The physical form of the body itself is shaped by the environment through evolution, which, through niche construction, shapes the environment. This process also applies to the brain, as seen in the example of tool usage and concurrent brain expansion. Therefore, it is reasonable to believe that the cognitive dimension would develop under similar pressures and hence produce similar effects.
Foraging and navigation can also be seen as examples of complex behaviors that involve multiple cognitive processes, such as learning, pathfinding, and pattern recognition. Despite this, insects, which possess much simpler nervous systems than vertebrates, are able to successfully navigate novel environments, find efficient routes, and return to known food locations and their nests (Collett et al., 2013). These behaviors are possible because of a close interdependence between the limitations of their bodies and their nervous systems (Wystrach, 2021). Wystrach illustrates how ants can navigate without complex visuospatial representations or allocentric spatial representations by relying on their own bodily constraints. An ant's immobile eyes, low‐resolution vision, and tendency to walk mostly forward simplify the navigation problem: scene recognition becomes less demanding, memory load is reduced, and body rotation substitutes mental rotation. Different modes and sensitivity in sensory perception can also generate new cognitive strategies. Bees are able to perceive light polarization, facilitating light‐guided navigation (Kraft et al., 2011); flies' bilateral sensitivity to odors allows them to perceive odor gradients with high accuracy, reducing their dependence on other senses (Raman et al., 2008).
These examples from the insect world demonstrate how body, perception, and behavior are closely integrated. Accordingly, more complex bodies, defined here as morphological and sensorimotor complexity, require more accurate representations of the self‐body, which interacts with the environment. In hominids, the verticalization of the body axis, which culminated in constant terrestrial bipedalism in humans, emphasizes the distinction between the hands and feet. These two features evolved for dexterous object manipulation and locomotion, respectively. Verticality also freed the hands from supporting the body, allowing primates to sit and manipulate objects in front of their eyes, further developing hand–eye coordination (Tia et al., 2023). Bipedalism also enabled primates to move while manipulating objects. Although these features are present in other animals, their combination in hominids, including adaptations to efficiently manipulate and modify objects in their environment such as including binocular vision and neuromuscular control of fine digit movements, is unique.
Verticalization also constrained the implementation of neuronal resources to facilitate the transition between coordinate systems derived from the dissociation of body, eye/head, and hand axes (Iriki & Taoka, 2012). Indeed, object locations needed to be represented not only with the body/trunk as a reference but also with the head/eyes and hands as references moving relative to the body. For such complex organisms, negotiating the environment required elaborate strategies relying on internal maps of one's surroundings grounded through visual and vestibular systems, as well as egocentric and allocentric spatial information processing (Fitzpatrick et al., 2006; Grön et al., 2000). The combination of these maps with the human morphology, with wide reach limbs able to maintain manipulation dexterity further away from the body, allowed for a large interpersonal space encoded separately from other maps, but which must still be integrated with the representations of the external space (Tia et al., 2023).
The new physical features derived from this verticalization demanded a close integration between the visual and sensorimotor domains to enable their efficient utilization. That is, because primates manipulate complex objects while observing the object and its transformations in response to the movement of their own limbs, visual and somato‐motor information require a common spatial reference frame (Cohen & Andersen, 2002). One of the main regions responsible for this integration, the parietal cortex, grew disproportionately large in humans compared with other mammals and even other primates (Goldring & Krubitzer, 2017; Kaas & Stepniewska, 2016). Even a comparison to Neanderthals, which show similar cranial capacity to modern humans, reveals morphological differences, with the latter having more elongated parietal regions (Pereira‐Pedro et al., 2020). This area has important roles in object recognition, such as during visual rotation and active object manipulation (Hsiao, 2008; Sakata et al., 1986), as well as during movement planning on a cognitive level (Andersen & Cui, 2009). It is also important to language and social functions (Blakemore et al., 2005; Simon et al., 2002). Object manipulation, tool use, and constructive skills are cognitive visuomotor functions that are essential for human cognition. Together with language and mathematical reasoning, these abilities have helped make the human brain unique, allowing humans to develop complex technologies and societies (Bruner et al., 2023).
These changes were a result of the hereditary and environmental feedback loop caused by increasingly swift modifications in the environment and the adaptations required as a result (Iriki & Taoka, 2012). This progressively led to the second phase of TNC, which is thought to have occurred only in Homo sapiens. Considering the almost identical or larger cranial capacity of late Homo, early H. sapiens, and modern humans, as well as the speed at which TNC‐2 emerged, its occurrence cannot be attributed solely to brain expansion. Instead, TNC‐2 more likely involved a rewiring of existing neural tissue. This process—which consisted of reusing existing neural resources of basic human abilities for novel, abstract, and advanced cognitive functions—was considerably faster and less costly than TNC‐1 (Figure 1). Similarly to how only a few changes in regulatory genes seem to be sufficient for humans to develop limbs different from those of other primates (Prabhakar et al., 2008), changes in gene expression levels appear to be largely responsible for producing the uniqueness of the human brain by modifying its organization and activity (Cáceres et al., 2003; Preuss, 2011; Shi et al., 2006). Heterochrony is another factor that could have taken part in this rewiring process. It is a change in the timing or rate of events during embryonic development, leading to changes in size, shape, and proportion of organs, such as parts of the brain (Klingenberg, 1998; Koyabu et al., 2014), with small modifications in development possibly having significant behavior implications (Wobber et al., 2010).
FIGURE 1.
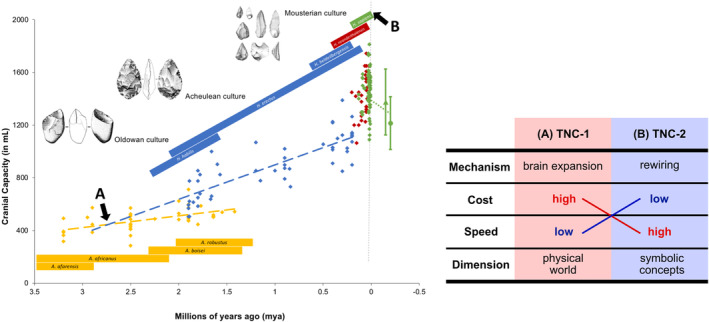
The rise of stone toolmaking in the genus Homo was marked by a steep increase in cranial capacity (graph, modified from Matzke, 2006), coinciding with the start of TNC‐1 (A arrow). Such a process was comparatively slow and biologically costly, acquiring novel cognitive functions that operate on the dimension of the physical world, such as the tool manufacturing itself and their interaction with the immediate environment. TNC‐1 prepared the neural substrate for TNC‐2. Starting at the peak of human cranial capacity (B arrow) and involving a rewiring of the existing neural tissue, a relatively fast and inexpensive process, TNC‐2 allowed humans to operate in a symbolic dimension of abstract concepts, language, and other functions that support complex technical and social behavior. TNC, triadic niche construction.
The changes that led to TNC‐2 did not occur in isolation, but in conjunction with the ongoing process of TNC‐1. Both TNC‐1 and TNC‐2 involved modifications of the existing neural tissue, but they differed in their speed, direction, and magnitude. TNC‐1 was a slower and more gradual process that radically increased the size and complexity of the brain, while TNC‐2 was a faster and more subtle process from the point of view of morphological changes, with alterations in the organization and function of the brain. Both processes were influenced by different evolutionary pressures, such as environmental changes, social interactions, and cultural innovations. Therefore, TNC‐1 and TNC‐2 should not be seen as separate or sequential events, but as simultaneous and interactive aspects of human brain evolution.
When analyzed from a contemporary perspective (post‐TNC‐2), TNC‐2 appears to require an unlikely confluence of specific factors to develop. Regardless, it is important to clarify that the relationship between these niches is not one of linear causality but rather of parallel development, as it is generally understood in the literature on regular niche construction. TNC happened continuously, albeit fast, with evidence indicating a gradual process of development, such as in the gradual advancement of tools, language, cooperation, art, and abstract thought.
However, TNC‐2 must have been accelerated in H. sapiens by a specific context or event. A viable candidate for this is the function of projection. Projection is a mechanism connecting internal representations with real external physical objects. When an object is seen, touched, or moved, perception happens within the self; however, it must be projected onto the external world, where it actually happens (Bretas et al., 2020; Pylyshyn, 2011). It relies on the integration of multiple sources of sensory information about objects and beings perceived around the body to construct a map of the “world‐around‐the‐body.” This projected reality combines external information derived from the physical world with self‐related information—that is, bodily sensations derived from sensory input. In this way, this projected reality is grounded in the body that is sensing. That is the same mechanism that allows a blind man to locate a barrier at the tip of his walking stick instead of at his hand, or a person to feel touch in a rubber hand as if it was part of their own body (rubber hand illusion), projecting an internal model of the perceived world onto the actual, physical environment (Bretas et al., 2020). This mechanism may derive from the neuronal reorganization associated with tool manufacture and use, as tools are used consciously, with multiple steps required to achieve a planned goal. Locating the external source of a sensation felt in the body through a tool and producing the appropriate motor response requires linking the feeling produced at the surface of the body to actions observed in the environment at the end of the tool. As the tool modifies this process by interposing between the individual and the environment, it spatially disconnects the sensation in the body from the place where the action occurs and from the motor responses performed to move the tool. However, tool users are capable of correctly accounting for a tool by incorporating their body into an internally constructed representation of the world that includes social and sensorimotor space (Bretas et al., 2020). The neuronal substrates involved in sensorimotor and functional properties of tool usage did not develop alone but rather in parallel with other cognitive functions to handle the intended use of tools, the processes of toolmaking, and the behavioral context in which a tool is used (Kastner et al., 2017). Parietal areas may be involved in that process by transforming the visual input of objects and tools into intrinsic coordinates referencing their position in relation to each other and the subject's own body. Complex manufacture depends on seeing and understanding a real or imagined example and on the sequence of actions that link the eye and the hand (Bruner et al., 2023). The complexity of tool usage and toolmaking can be approached through the concept of action hierarchies (Stout, 2011). According to this view, superordinate levels, which represent abstract or spatially and temporally distant goals, are divided into simpler subgoals and actions. This process allows for task flexibility and context‐specific adaptation and could represent a basis by which early modern humans developed complex technologies.
3. FROM THE SELF‐BODY TO THE SELF‐IN‐THE‐WORLD MAP
Manipulating tools induces a rapid updating of the body schema in the anterior intraparietal cortex, enabling tools to be incorporated and represented as extensions of the body (Maravita & Iriki, 2004). At the neuronal level, tool use increases intracortical connections between the intraparietal sulcus and the temporoparietal junction, which participates in building a subjective sense of self (Iriki, 2006). Interactions between the anterior intraparietal area and the temporoparietal junction during tool use may result from a recalibration of the body schema in the anterior intraparietal area driven by the intention to incorporate the tool into the internal body self‐representation. By definition, tool usage is a goal‐oriented activity, as tools are used with a purpose, bringing explicit awareness to the self‐body that is connected with the subject's own intentions (Iriki, 2006). This causal relationship, established between the agent's body and the tools, allows the agent to project the internal representation of the self onto an object—that is, the tool—while also self‐objectifying its own body, establishing a spatial and causal connection between the self‐body and the environment. Furthermore, tools have permanence and exist independently of the agent using them. When the same object, which is sometimes incorporated into one's own body image, is manipulated by other agents, it can produce results that are identical to when manipulated by the self. As visual stimuli can be perceived equally in the self‐body as well as in the other, whereas the tool can only be felt when manipulated by the self (through somatosensation), this process becomes an intersection between the self and the other, allowing the subject to regard itself as an individual within a social world (Iriki, 2006; Ishida et al., 2010). Simultaneously, one's own mental states can be projected onto the other, perhaps as a basis for mentalizing.
The fact that a tool that was originally an external object works as a part of one's body would conversely indicate that one's body can also be regarded as an external object. By gradually expanding the self‐objectification, the entire body of oneself comes into play in the representational world. Because one's body is closely related to the notion of one's self, it may be considered that the self is included as an object of one's cognitive map. The fact that the self and the body are constituents of the representation suggests that they can be projected to the world. In other words, the self becomes a player in the projected reality. This type of projection is called the secondary projection, a cognitive component of TNC‐2. Therefore, the internal representation of the body, and by extension, the self (Serino et al., 2013), can be combined with that of the surrounding world to create a self‐in‐the‐world map where the self becomes an object that can be manipulated in the representation based on the intention and desire of the original self. By the construction of a self‐in‐the‐world map, human activities are released from now and here. In this new representation, the projected self can be manipulated in the same manner as an object to fulfill new cognitive functions, such as retrospection (episodic memory), prospection (planning) and symbolic thought (Iriki et al., 2021). Namely, cognitive functions established in TNC‐1 were modified and combined during TNC‐2, giving rise to more advanced, abstract functions. One possible neuronal substrate to support the function of projection is the secondary somatosensory cortex (S2).
To construct a comprehensive representation of the self‐in‐the‐world, one must combine a representation of the self that is built on the body schema and integrates multiple sensory inputs with additional information relative to the surrounding world. The S2 comprises multiple body maps and receives information from visual, auditory, and vestibular sources that relate to the self and environment (Bretas et al., 2020). Moreover, this area contains neurons with complex multimodal and attention‐modulated responses with large receptive fields, which are sometimes bilateral or include multiple body segments (Robinson & Burton, 1980; Taoka et al., 2013, 2016). A study in humans found that the S2 can be activated both when subjects are touched and when they observe others being touched (Keysers et al., 2004). This property could intervene in self–other interactions, social understanding, and empathy (Keysers et al., 2004; Seger et al., 2004), as projection evolved to also include self‐representation (Bretas et al., 2020; Iriki et al., 2021). Indeed, social cognition observed in apes seems to support more individualistic and competitive contexts, whereas the human hunter–gatherer niche promoted collaborative aspects of social cognition (Whiten & Erdal, 2012). Within newly developed social conditions, a stable communication system might have evolved from repeated pairwise interactions, giving rise to language (Pinker, 2013). Nevertheless, one must consider how relatively fast and early an infant can acquire language and learn to manipulate objects with precise movements of their fingers, highlighting the importance of the neural niche in these developments.
The new functions afforded by TNC‐2 could include understanding and abstract manipulation of the self and the world, enabling humans to locate the self in particular times and places and plan activities with temporally and spatially distant goals (e.g. agriculture, migration). These new functions could lead to more complex societies arising from new abilities related to self‐consciousness and mentalizing, as well as the need for cooperation when performing technologically advanced activities. This advancement of technological strategies is reflected by the intensification and diversification of stone tools in the late Homo genus. This milestone presumably marks the onset of obligatory tool use, which means that tools not only assisted individual needs but were also essential to survival in specific ecological and cultural niches (Bruner & Gleeson, 2019; Shea, 2017). In these new niches, the subject must understand not only the environment but also its position and role within it. By connecting a body representation that can include tools and nearby objects into it and mapping both causal and spatial relationships between the agent's own body and the world, the S2 represents the relationship of the self as a social agent with environmental structures under abstract spatial dimensions (Corradi‐Dell'Acqua et al., 2009).
4. CONCLUSION
The brain expansion of TNC‐1 did not continue into the late stages of human evolution. However, the elongation of the parietal outline in H. sapiens compared with Homo neanderthalensis, which occurred alongside an overall decrease in cranial capacity and is associated with changes in the anatomo‐functional organization of the S2 and associative parietal regions (Bruner & Iriki, 2016), indicates that these brain areas likely had an important role in TNC‐2. The complexification of the associative cortex, which comprises the S2 and is involved in combining different modes of sensation and perception of the self‐body, as well as the diversification of its functions, may form the basis of the cognitive revolution which took place in H. sapiens. Nevertheless, the question of the uniqueness of human S2 in comparison with other primates requires further investigation (Bretas et al., 2020).
Although brain rewiring in TNC‐2 is a fast and efficient process that is considered to have produced a wealth of new abilities in H. sapiens, it required some preconditions to occur. Human lineages possessed large brains with specialized regions, which endowed them with a set of cognitive and sensorimotor skills. Beginning with Homo habilis, the complexification of stone‐tool manufacture and use indicates the existence of a range of skills necessary to plan and produce tools and transmit tool‐related knowledge. From this point on, cognitive notions of spatial organization, idealized objects and their transformations, as well as socio‐emotional states, were mapped onto the external world through the mechanism of projection, bringing explicit awareness to the causal relationship between the self and intentional modifications to the environment. This process was embedded in the neural rewiring that circumscribed TNC‐2, allowing the manipulation of this world (in both physical and symbolic dimensions) in a “positive feedback loop.”
Bretas, R. , Tia, B. , & Iriki, A. (2024). The self‐in‐the‐world map emerged in the primate brain as a basis for Homo sapiens abilities. Development, Growth & Differentiation, 66(6), 342–348. 10.1111/dgd.12939
Communicating Editor: Shuichi Onami
This article is part of the special issue “Emergence in Biological Systems: Challenges to Bridging Hierarchies.”
REFERENCES
- Andersen, R. A. , & Cui, H. (2009). Intention, action planning, and decision making in parietal‐frontal circuits. Neuron, 63(5), 568–583. 10.1016/j.neuron.2009.08.028 [DOI] [PubMed] [Google Scholar]
- Anderson, M. L. (2010). Neural reuse: A fundamental organizational principle of the brain. Behavioral and Brain Sciences, 33(4), 245–266. 10.1017/S0140525X10000853 [DOI] [PubMed] [Google Scholar]
- Bateson, P. P. G. , & Gluckman, P. D. (2011). Plasticity, robustness, development and evolution. Cambridge University Press. [Google Scholar]
- Blakemore, S.‐J. , Bristow, D. , Bird, G. , Frith, C. , & Ward, J. (2005). Somatosensory activations during the observation of touch and a case of vision–touch synaesthesia. Brain, 128(7), 1571–1583. 10.1093/brain/awh500 [DOI] [PubMed] [Google Scholar]
- Bretas, R. , Taoka, M. , Suzuki, H. , & Iriki, A. (2020). Secondary somatosensory cortex of primates: Beyond body maps, toward conscious self‐in‐the‐world maps. Experimental Brain Research, 238(2), 259–272. 10.1007/s00221-020-05727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner, E. (2021). Evolving human brains: Paleoneurology and the fate of middle Pleistocene. Journal of Archaeological Method and Theory, 28(1), 76–94. 10.1007/s10816-020-09500-8 [DOI] [Google Scholar]
- Bruner, E. , Battaglia‐Mayer, A. , & Caminiti, R. (2023). The parietal lobe evolution and the emergence of material culture in the human genus. Brain Structure and Function, 228(1), 145–167. 10.1007/s00429-022-02487-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner, E. , & Gleeson, B. T. (2019). Body cognition and self‐domestication in human evolution. Frontiers in Psychology, 10, 1111. 10.3389/fpsyg.2019.01111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner, E. , & Iriki, A. (2016). Extending mind, visuospatial integration, and the evolution of the parietal lobes in the human genus. Quaternary International, 405, 98–110. 10.1016/j.quaint.2015.05.019 [DOI] [Google Scholar]
- Cáceres, M. , Lachuer, J. , Zapala, M. A. , Redmond, J. C. , Kudo, L. , Geschwind, D. H. , Lockhart, D. J. , Preuss, T. M. , & Barlow, C. (2003). Elevated gene expression levels distinguish human from non‐human primate brains. Proceedings of the National Academy of Sciences, 100(22), 13030–13035. 10.1073/pnas.2135499100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, Y. E. , & Andersen, R. A. (2002). A common reference frame for movement plans in the posterior parietal cortex. Nature Reviews Neuroscience, 3(7), 553–562. 10.1038/nrn873 [DOI] [PubMed] [Google Scholar]
- Collett, M. , Chittka, L. , & Collett, T. S. (2013). Spatial memory in insect navigation. Current Biology, 23(17), R789–R800. 10.1016/j.cub.2013.07.020 [DOI] [PubMed] [Google Scholar]
- Corradi‐Dell'Acqua, C. , Tomasino, B. , & Fink, G. R. (2009). What is the position of an arm relative to the body? Neural correlates of body schema and body structural description. Journal of Neuroscience, 29(13), 4162–4171. 10.1523/JNEUROSCI.4861-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, R. C. , Butler, J. E. , & Day, B. L. (2006). Resolving head rotation for human bipedalism. Current Biology, 16(15), 1509–1514. 10.1016/j.cub.2006.05.063 [DOI] [PubMed] [Google Scholar]
- Goldring, A. B. , & Krubitzer, L. A. (2017). Evolution of parietal cortex in mammals: From manipulation to tool use. In Evolution of nervous systems (pp. 259–286). Elsevier. 10.1016/B978-0-12-804042-3.00086-5 [DOI] [Google Scholar]
- Grön, G. , Wunderlich, A. P. , Spitzer, M. , Tomczak, R. , & Riepe, M. W. (2000). Brain activation during human navigation: Gender‐different neural networks as substrate of performance. Nature Neuroscience, 3(4), 404–408. 10.1038/73980 [DOI] [PubMed] [Google Scholar]
- Hsiao, S. (2008). Central mechanisms of tactile shape perception. Current Opinion in Neurobiology, 18(4), 418–424. 10.1016/j.conb.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Iriki, A. (2006). The neural origins and implications of imitation, mirror neurons and tool use. Current Opinion in Neurobiology, 16(6), 660–667. 10.1016/j.conb.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Iriki, A. , Suzuki, H. , Tanaka, S. , Bretas Vieira, R. , & Yamazaki, Y. (2021). The sapient paradox and the great journey: Insights from cognitive psychology, neurobiology, and phenomenology. Psychologia, 63(2), 151–173. 10.2117/psysoc.2021-B017 [DOI] [Google Scholar]
- Iriki, A. , & Taoka, M. (2012). Triadic (ecological, neural, cognitive) niche construction: A scenario of human brain evolution extrapolating tool use and language from the control of reaching actions. Philosophical Transactions of the Royal Society B, 367(1585), 10–23. 10.1098/rstb.2011.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, H. , Nakajima, K. , Inase, M. , & Murata, A. (2010). Shared mapping of own and Others' bodies in Visuotactile bimodal area of monkey parietal cortex. Journal of Cognitive Neuroscience, 22(1), 83–96. 10.1162/jocn.2009.21185 [DOI] [PubMed] [Google Scholar]
- Kaas, J. H. , & Stepniewska, I. (2016). Evolution of posterior parietal cortex and parietal‐frontal networks for specific actions in primates. Journal of Comparative Neurology, 524(3), 595–608. 10.1002/cne.23838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner, S. , Chen, Q. , Jeong, S. K. , & Mruczek, R. E. B. (2017). A brief comparative review of primate posterior parietal cortex: A novel hypothesis on the human toolmaker. Neuropsychologia, 105, 123–134. 10.1016/j.neuropsychologia.2017.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward, B. , Weir, A. A. S. , Rutz, C. , & Kacelnik, A. (2005). Behavioural ecology: Tool manufacture by naive juvenile crows. Nature, 433(7022), 121. 10.1038/433121a [DOI] [PubMed] [Google Scholar]
- Keysers, C. , Wicker, B. , Gazzola, V. , Anton, J.‐L. , Fogassi, L. , & Gallese, V. (2004). A touching sight: SII/PV activation during the observation and experience of touch. Neuron, 42(2), 335–346. 10.1016/S0896-6273(04)00156-4 [DOI] [PubMed] [Google Scholar]
- Klingenberg, C. P. (1998). Heterochrony and allometry: The analysis of evolutionary change in ontogeny. Biological Reviews, 73(1), 79–123. 10.1017/S000632319800512X [DOI] [PubMed] [Google Scholar]
- Koyabu, D. , Werneburg, I. , Morimoto, N. , Zollikofer, C. P. E. , Forasiepi, A. M. , Endo, H. , Kimura, J. , Ohdachi, S. D. , Truong Son, N. , & Sánchez‐Villagra, M. R. (2014). Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nature Communications, 5(1), 3625. 10.1038/ncomms4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft, P. , Evangelista, C. , Dacke, M. , Labhart, T. , & Srinivasan, M. V. (2011). Honeybee navigation: Following routes using polarized‐light cues. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1565), 703–708. 10.1098/rstb.2010.0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer, L. (2009). In search of a unifying theory of complex brain evolution. Annals of the new York Academy of Sciences, 1156(1), 44–67. 10.1111/j.1749-6632.2009.04421.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer, L. , & Dooley, J. (2013). Cortical plasticity within and across lifetimes: How can development inform us about phenotypic transformations? Frontiers in Human Neuroscience, 7, 620. 10.3389/fnhum.2013.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland, K. , Odling‐Smee, J. , & Endler, J. (2017). Niche construction, sources of selection and trait coevolution. Interface Focus, 7(5), 20160147. 10.1098/rsfs.2016.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita, A. , & Iriki, A. (2004). Tools for the body (schema). Trends in Cognitive Sciences, 8(2), 79–86. 10.1016/j.tics.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Matzke, N. (2006). Fun with hominin cranial capacity datasets (and excel), part 2. The Panda's Thumb. https://pandasthumb.org/archives/2006/09/fun-with-homini-1.html
- Musgrave, S. , Lonsdorf, E. , Morgan, D. , Prestipino, M. , Bernstein‐Kurtycz, L. , Mundry, R. , & Sanz, C. (2020). Teaching varies with task complexity in wild chimpanzees. Proceedings of the National Academy of Sciences, 117(2), 969–976. 10.1073/pnas.1907476116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira‐Pedro, A. S. , Bruner, E. , Gunz, P. , & Neubauer, S. (2020). A morphometric comparison of the parietal lobe in modern humans and Neanderthals. Journal of Human Evolution, 142, 102770. 10.1016/j.jhevol.2020.102770 [DOI] [PubMed] [Google Scholar]
- Pinker, S. (2013). Language, cognition, and human nature. Oxford University Press. 10.1093/acprof:oso/9780199328741.001.0001 [DOI] [Google Scholar]
- Prabhakar, S. , Visel, A. , Akiyama, J. A. , Shoukry, M. , Lewis, K. D. , Holt, A. , Plajzer‐Frick, I. , Morrison, H. , FitzPatrick, D. R. , Afzal, V. , Pennacchio, L. A. , Rubin, E. M. , & Noonan, J. P. (2008). Human‐specific gain of function in a developmental enhancer. Science, 321(5894), 1346–1350. 10.1126/science.1159974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss, T. M. (2011). The human brain: Rewired and running hot: The human brain: Rewired and running hot. Annals of the new York Academy of Sciences, 1225(S1), E182–E191. 10.1111/j.1749-6632.2011.06001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylyshyn, Z. W. (2011). Things and places: How the mind connects with the world. The MIT Press. [Google Scholar]
- Raman, B. , Ito, I. , & Stopfer, M. (2008). Bilateral olfaction: Two is better than one for navigation. Genome Biology, 9(3), 212. 10.1186/gb-2008-9-3-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader, S. M. , & Laland, K. N. (2002). Social intelligence, innovation, and enhanced brain size in primates. Proceedings of the National Academy of Sciences, 99(7), 4436–4441. 10.1073/pnas.062041299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, C. J. , & Burton, H. (1980). Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. Fascicularis. Journal of Comparative Neurology, 192(1), 69–92. 10.1002/cne.901920105 [DOI] [PubMed] [Google Scholar]
- Sakata, H. , Shibutani, H. , Ito, Y. , & Tsurugai, K. (1986). Parietal cortical neurons responding to rotary movement of visual stimulus in space. Experimental Brain Research, 61(3), 658–663. 10.1007/BF00237594 [DOI] [PubMed] [Google Scholar]
- Seger, C. A. , Stone, M. , & Keenan, J. P. (2004). Cortical activations during judgments about the self and an other person. Neuropsychologia, 42(9), 1168–1177. 10.1016/j.neuropsychologia.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Serino, A. , Alsmith, A. , Costantini, M. , Mandrigin, A. , Tajadura‐Jimenez, A. , & Lopez, C. (2013). Bodily ownership and self‐location: Components of bodily self‐consciousness. Consciousness and Cognition, 22(4), 1239–1252. 10.1016/j.concog.2013.08.013 [DOI] [PubMed] [Google Scholar]
- Shea, J. J. (2017). Occasional, obligatory, and habitual stone tool use in hominin evolution. Evolutionary Anthropology: Issues, News, and Reviews, 26(5), 200–217. 10.1002/evan.21547 [DOI] [PubMed] [Google Scholar]
- Shi, P. , Bakewell, M. , & Zhang, J. (2006). Did brain‐specific genes evolve faster in humans than in chimpanzees? Trends in Genetics, 22(11), 608–613. 10.1016/j.tig.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Simon, O. , Mangin, J.‐F. , Cohen, L. , Le Bihan, D. , & Dehaene, S. (2002). Topographical layout of hand, eye, calculation, and language‐related areas in the human parietal lobe. Neuron, 33(3), 475–487. 10.1016/S0896-6273(02)00575-5 [DOI] [PubMed] [Google Scholar]
- Stout, D. (2011). Stone toolmaking and the evolution of human culture and cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1567), 1050–1059. 10.1098/rstb.2010.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka, M. , Tanaka, M. , Hihara, S. , Ojima, H. , & Iriki, A. (2013). Neural response to movement of the hand and mouth in the secondary somatosensory cortex of Japanese monkeys during a simple feeding task. Somatosensory & Motor Research, 30(3), 140–152. 10.3109/08990220.2013.779246 [DOI] [PubMed] [Google Scholar]
- Taoka, M. , Toda, T. , Hihara, S. , Tanaka, M. , Iriki, A. , & Iwamura, Y. (2016). A systematic analysis of neurons with large somatosensory receptive fields covering multiple body regions in the secondary somatosensory area of macaque monkeys. Journal of Neurophysiology, 116(5), 2152–2162. 10.1152/jn.00241.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia, B. , Bretas, R. , Yamazaki, Y. , & Iriki, A. (2023). The body in the world: Tools and somato‐centric maps in the primate brain. In Cognitive archaeology, body cognition, and the evolution of visuospatial perception (pp. 85–107). Elsevier. 10.1016/B978-0-323-99193-3.00011-8 [DOI] [Google Scholar]
- Whiten, A. , & Erdal, D. (2012). The human socio‐cognitive niche and its evolutionary origins. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1599), 2119–2129. 10.1098/rstb.2012.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber, V. , Wrangham, R. , & Hare, B. (2010). Application of the heterochrony framework to the study of behavior and cognition. Communicative & Integrative Biology, 3(4), 337–339. 10.4161/cib.3.4.11762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wystrach, A. (2021). Movements, embodiment and the emergence of decisions. Insights from insect navigation. Biochemical and Biophysical Research Communications, 564, 70–77. 10.1016/j.bbrc.2021.04.114 [DOI] [PubMed] [Google Scholar]


