Abstract
Vision is formed by the transmission of light stimuli to the brain through axons extending from photoreceptor cells. Damage to these axons leads to loss of vision. Despite research on neural circuit regeneration through transplantation, achieving precise axon projection remains challenging. To achieve optic nerve regeneration by transplantation, we employed the Drosophila visual system. We previously established a transplantation method for Drosophila utilizing photoreceptor precursor cells extracted from the eye disc. However, little axonal elongation of transplanted cells into the brain, the lamina, was observed. We verified axonal elongation to the lamina by modifying the selection process for transplanted cells. Moreover, we focused on N‐cadherin (Ncad), a cell adhesion factor, and Twinstar (Tsr), which has been shown to promote actin reorganization and induce axon elongation in damaged nerves. Overexpression of Ncad and tsr promoted axon elongation to the lamina, along with presynaptic structure formation in the elongating axons. Furthermore, overexpression of Neurexin‐1 (Nrx‐1), encoding a protein identified as a synaptic organizer, was found to not only promote presynapse formation but also enhance axon elongation. By introducing Ncad, tsr, and Nrx‐1, we not only successfully achieved axonal projection of transplanted cells to the brain beyond the retina, but also confirmed the projection of transplanted cells into a deeper ganglion, the medulla. The present study offers valuable insights to realize regeneration through transplantation in a more complex nervous system.
Keywords: actin dynamics, axon growth, Drosophila, transplantation, visual system
Presynapse formation in axons elongating into the lamina was observed upon overexpression of Ncad and Tsr.
1. INTRODUCTION
Vision plays a pivotal role in the acquisition of external information. Vision is initiated upon the perception of light stimuli by the photoreceptor cells within the retina. These light stimuli are converted into electrical signals within the photoreceptor cells, which are subsequently conveyed to the brain through extensive axonal extensions. Therefore, impairments to the axons of the nerve connecting the retina to the brain result in vision loss. To reacquire vision, photoreceptor cells need to reestablish the neural circuitry connecting the retina to the brain by extending novel elongated axons and forming synapses in the brain.
However, when axons are damaged, various impediments to axonal regeneration come into play, including the secretion of inhibitory molecules for axon elongation by glial cells and the formation of physical barriers due to scar tissue resulting from the injury, which collectively pose problems to nerve regeneration (Laha et al., 2017). The emergence of induced pluripotent stem (iPS) cells has provided a promising avenue for in vitro production of specific cells and tissues, attracting attention for potential regenerative transplantation approaches. In mammals, retinal ganglion cells (RGCs) play a vital role in transmitting information from the retina to the brain by extending axons. Successful transplantation of RGCs has been reported, and within these transplants, the integration of transplanted cells within the host organism and their responsiveness to light (MacLaren et al., 2006; Venugopalan et al., 2016). However, achieving precise control over the projection of transplanted cells into the brain remains challenging (Venugopalan et al., 2016). Notably, transplantation of iPS cell‐derived RGCs revealed that while these cells displayed electrophysiological functionality, their axonal projections were limited to the optic nerve head and did not reach the brain (Oswald et al., 2021; Vrathasha et al., 2022). Regenerating neural circuits through transplantation necessitates specific elements, including cell adhesion factors facilitating attachment to neighboring tissues during axon elongation, axon guidance molecules responsible for directing the course of axonal growth, cytoskeletal molecules such as actin and tubulin, and molecules crucial for inducing the formation of synapses, which are essential for transmitting information within the nerve.
Previous research has shown that the reorganization of actin filaments plays a crucial role in promoting neural regeneration. Actin filaments, major constituents of the cytoskeleton that are formed through the process of actin polymerization and depolymerization, play a key role in maintaining cell shape and enabling cell motility. The actin‐depolymerizing factor (ADF)/Cofilin is a known factor that promotes actin depolymerization. ADF/Cofilin regulates neurite outgrowth during development and is localized in the filopodia of growth cones (Flynn et al., 2012; Hylton et al., 2022). Additionally, studies have shown that overexpression of Cofilin1 in injured sciatic nerves in mice induces axon elongation, thereby facilitating nerve regeneration (Tedeschi et al., 2019).
A number of factors have been recognized for their role in inducing synapse formation, which is critical for the transmission of information within neural networks (Connor & Siddiqui, 2023). Neurexins, serving as synapse organizers and cell adhesion factors, are single transmembrane proteins predominantly localized to the presynaptic region. Their function involves inducing synapse formation through interactions with neuroligins, expressed at postsynaptic sites (Gomez et al., 2021). In mammals, neurexins are encoded by three genes, with several promoters, encoding α‐neurexin 1‐3 and β‐neurexin 1‐3.
To achieve optic nerve regeneration through transplantation, we utilized the model organism Drosophila. In the Drosophila visual system, photoreceptor cells extend axons that project directly to the optic lobe. While the structure is notably less complex than that of mammalian systems, this model presents fewer barriers to axon outgrowth and displays a precise layered organization. Studies of the Drosophila visual system have extensively analyzed axon guidance and elongation, identifying a range of associated molecules (Hakeda‐Suzuki et al., 2017; Takechi et al., 2021; Zang et al., 2021). Additionally, owing to the genetic manipulation tools available in Drosophila, it serves as an advantageous model organism for the comprehensive exploration of various genes, particularly in the context of transplantation studies. Previously, we established a method for transplanting photoreceptor precursor cells obtained from eye discs (Suzuki et al., 2018), enabling a detailed analysis of the factors pivotal in promoting optic nerve axon growth following transplantation. While cells overexpressing N‐cadherin (Ncad), encoding a cell adhesion molecule, have been transplanted, their impact has been restricted to promoting axon elongation primarily within the retina, with minimal extension beyond the retina (Suzuki et al., 2018). Therefore, we further focused on cytoskeleton‐related factors and synapse organizers.
Twinstar (Tsr), a cytoskeleton‐related gene and the Drosophila homolog of ADF/Cofilin (Gunsalus et al., 1995), has been reported to be involved in neuroblast growth, axon elongation, and retina morphogenesis (Ng & Luo, 2004; Pham et al., 2008). In Drosophila, it has been shown that actin depolymerization and cleavage lead to cellular morphological alterations. Moreover, the synapse organizer Neurexin‐1 (Nrx‐1), the Drosophila homolog of α‐neurexin, has been found to regulate synapse formation as in mammals (Li et al., 2007; Zeng et al., 2007). Nrx‐1 has also been implicated in the maturation of rhodopsin, a vital component in phototransduction, as well as in microtubule stabilization (Banerjee & Riordan, 2018; Tian et al., 2013).
We confirmed the extension of axons from the retina to the lamina by modifying the selection process of transplanted cells. Our findings demonstrated that overexpression of Ncad, encoding a cell adhesion molecule, and tsr, encoding a factor which induces actin reorganization, significantly promotes axon elongation. Furthermore, in the lamina, we observed the formation of presynaptic structures in the elongated axons 6 days posttransplantation. Additionally, our results indicated that the introduction of Nrx‐1, encoding a synapse organizer, accelerated the formation of presynaptic structures, suggesting the involvement of Nrx‐1 in axon growth. Notably, we succeeded in replicating the precise projection of Drosophila photoreceptor cells to their target tissue, the medulla. Given the rarity of stable axonal projection to the brain in previous observations, the outcomes of this study are anticipated to offer valuable insights in order to achieve regeneration through transplantation.
2. MATERIALS AND METHODS
2.1. Fly strains
Flies were kept at 25°C. The following stocks were used: UAS‐tsr (BDSC 9235), UAS‐tsr.S3A (BDSC 9237), UAS‐tsr.S3E (BDSC 9239) (Ng & Luo, 2004), UAS‐Nrx‐1‐SAM (BDSC 82741), GMR‐myr‐RFP (BDSC 7120), UAS‐myr‐RFP (II) (BDSC 7118), elav‐Gal4 (Luo et al., 1994), UAS‐FLP (BDSC 8208), GMR‐FRT‐stop‐FRT‐Gal4, and Brp‐FRT‐stop‐FRT‐GFP (Chen et al., 2014). Brp‐FRT‐stop‐FRT‐GFP (II) was generated by knock‐in of the flip‐out cassette with GFP (FRT‐stop‐FRT‐GFP) into the Brp C‐terminus.
2.2. Transplantation
The experimental procedures for transplantation were described previously (Suzuki et al., 2018). The removed eye discs were dissociated and centrifuged at rcf 775g for 5 min. The precipitated cells were resuspended by pipetting and the cells were transferred to the chamber (Figure 1a). Coverslips coated with Concanavalin A (Wako) were placed over the chamber. Cells were incubated for 1.5 h with the coverslips down and for 2.5 h with the coverslips up in a humidity‐controlled, light‐shielded case (25°C). Glass capillaries were used for transplantation (0.75 mm inner diameter, 1 mm outer diameter, NARISHIGE). Under an upright fluorescence microscope (Axio Plan2, ZEISS), cell clusters adhering to coverslips and expressing RFP were aspirated with a glass micropipette using a microinjector (NARISHIGE) filled with Mineral oil (Sigma). Parafilm was used to fix the heads of adult flies within 1 day after birth, and cell clusters were transplanted into the retina. Vaseline was also applied to the puncture site after transplantation to prevent the retina from drying out.
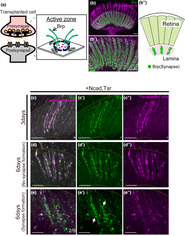
FIGURE 1.
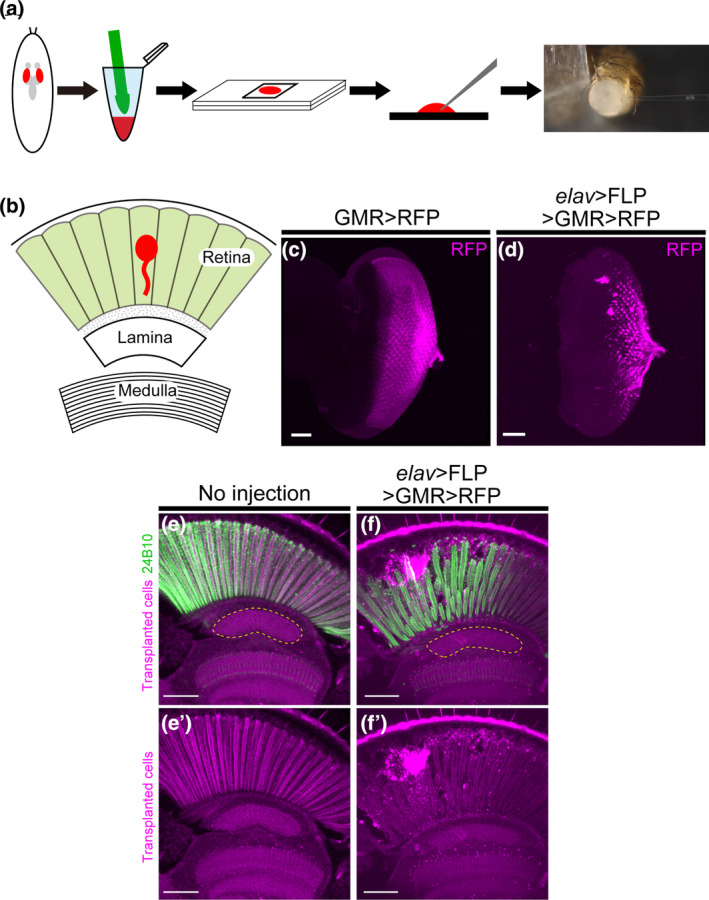
Transplantation method and its modification in Drosophila. (a) Schematic diagram of steps for transplantation. Take out the eye discs from third‐instar larvae, add dispase and collagenase, and homogenize by hand. After incubation at 25°C for 4 h, aspirate cells with a glass capillary and transplant to the adult fly. (b) Schematic diagram of the Drosophila visual system. The Drosophila visual system consists of the retina and the brain ganglia, including the lamina and the medulla. Cells are transplanted into the retina (red). (c) Third‐instar larval eye discs labeled with GMR‐myr‐RFP. Cells expressing RFP (magenta) represent developing photoreceptor cells. (d) Third‐instar larval eye discs labeled with elav‐Gal4, UAS‐FLP, GMR‐FRT‐stop‐FRT‐Gal4, and UAS‐myr‐RFP (abbreviated as elav‐FLP GMR‐FsF‐Gal4). Cells expressing RFP (magenta) represent developing neurons and photoreceptor cells. (e, f) Photoreceptor cells were stained with mAb24B10 (green). The yellow dotted line indicates the lamina region. (e) Section of an untransplanted adult head. (f) Transplantation of cells labeled with elav‐FLP GMR‐FsF‐Gal4. Observation of transplanted cells (magenta) in the Drosophila retina. Axon elongation to the lamina was observed in 18.9% of flies (n = 37). Scale bars in (c–f): 50 μm.
2.3. Observation of transplanted flies
Transplanted flies were kept at 25°C for 3 or 6 days. The experimental procedures for agarose section, fixation, and immunostaining were described previously (Hakeda‐Suzuki et al., 2011). Flies were sliced into 80 μm thick sections using a Linear slicer (Dosaka EM (D.S.K)). Samples were incubated with primary antibodies overnight at 4°C and with secondary antibodies at 25°C for 1 h. The following antibodies were used for immunohistochemistry: mAb24B10 (1:50, Developmental Studies Hybridoma Bank (DSHB)). The secondary antibodies were Alexa488‐ or Alexa633‐conjugated (1:400, Life technologies).
2.4. Observation of adult flies
The experimental procedures for adult brain dissection, fixation, and immunostaining were described previously (Hakeda‐Suzuki et al., 2017). The following antibodies were used for immunohistochemistry: mAb24B10 (1:50, DSHB). The secondary antibodies were Alexa633‐conjugated (1:400, Life technologies).
2.5. Imaging, quantification, and statistical analyses
All confocal images were obtained by a Nikon C2+ or Nikon A1 confocal microscope. The images were analyzed using NIS elements Imaris or Adobe Photoshop. Data in Figures 2, 3, and 5 were analyzed by Fisher's exact test using R Core Team (2023) (R Foundation for Statistical Computing, Vienna, Austria).
FIGURE 2.
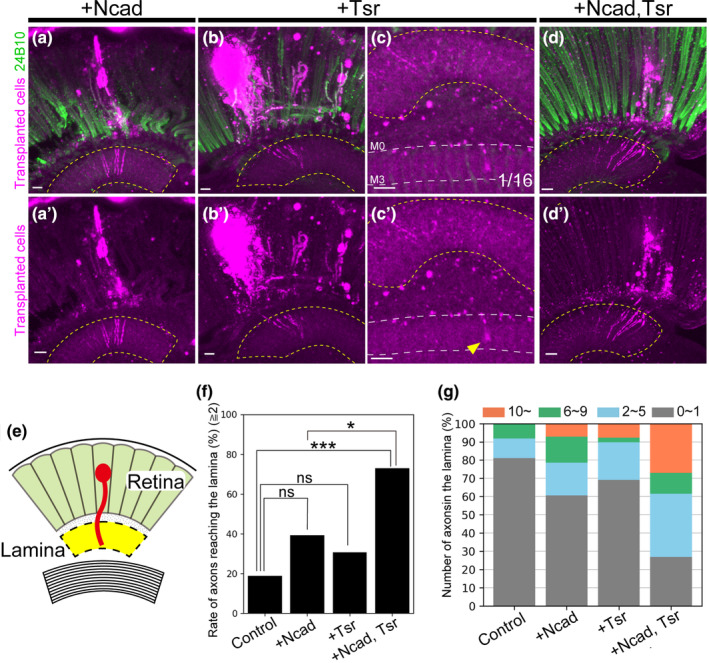
Transplantation of cells overexpressing N‐cadherin and/or Twinstar. (a–d) Photoreceptor cells were stained with mAb24B10 (green). The yellow dotted line indicates the lamina region. (a, a') Cells overexpressing N‐cadherin (Ncad) (magenta) showed axon elongation to the lamina in 39.3% of transplanted flies (n = 28). (b, b') Cells overexpressing Twinstar (tsr) (magenta) showed axon elongation to the lamina in 30.8% of transplanted flies (n = 39). (c, c') An axon elongating to the medulla was observed in one case of tsr overexpression. Yellow arrowheads indicate elongated axons, and white dotted lines indicate layers of the medulla. (d, d') Cells overexpressing Ncad and tsr showed axon elongating to the lamina in 73.1% of transplanted flies (n = 26). (e) Schematic of axon elongation to the lamina of transplanted cells. Axons reached the lamina (yellow) beyond the retina. (f) Percentage of flies with axon elongation to the lamina. Significant differences were observed between cells overexpressing Ncad and tsr and control cells and cells overexpressing Ncad alone. (g) Classification of the number of axons extending into the lamina. The number of axons was classified into four categories: ≤1 axon (gray), 2–5 axons (blue), 6–9 axons (green), and ≥10 axons (orange). In the control, no fly has more than 10 axons elongated to the lamina, while overexpression of Ncad and tsr resulted in more than five axons elongating to the lamina in about 40% of the transplanted flies. Scale bars in (a–d): 10 μm. n.s., p ≧ .05, *p < .05, ***p < .001.
FIGURE 3.
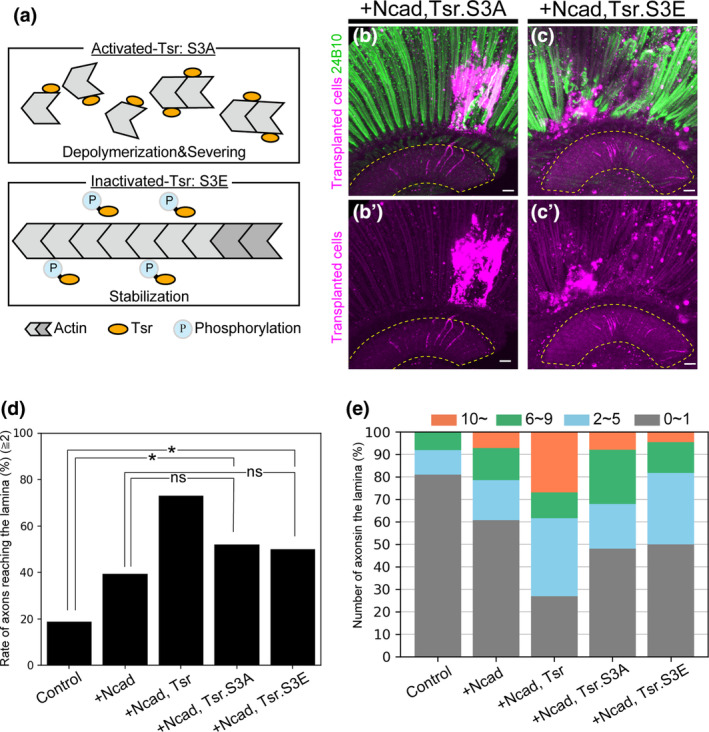
Transplantation of cells overexpressing Ncad and activated/inactivated Tsr. (a) Schematic diagram of activated/inactivated Tsr. Tsr is activated by dephosphorylation and inactivated by phosphorylation. (b, c) Photoreceptors were stained with mAb24B10 (green). The yellow dotted line indicates the lamina region. (b, b′) Cells overexpressing Ncad and tsr.S3A (magenta) elongated axons to the lamina in 52% of transplanted flies (n = 25). (c, c′) Cells overexpressing Ncad and tsr.S3E (magenta) elongated axons to the lamina in 50% of transplanted flies (n = 22). (d) Percentage of flies with axon elongation to the lamina. Significant differences were observed between cells overexpressing Ncad and tsr.S3A or tsr.S3E and control cells. No significant differences were observed compared to cells overexpressing Ncad alone. (e) Classification of the number of axons extending into the lamina. The number of axons was classified into four categories: ≤1 axon (gray), 2–5 axons (blue), 6–9 axons (green), and ≥10 axons (orange). When the activated/inactivated form of tsr was expressed, a decreasing trend in the number of axon elongations per fly was observed. Scale bar (b, c): 10 μm. n.s., p ≧ .05, *p < .05.
FIGURE 5.
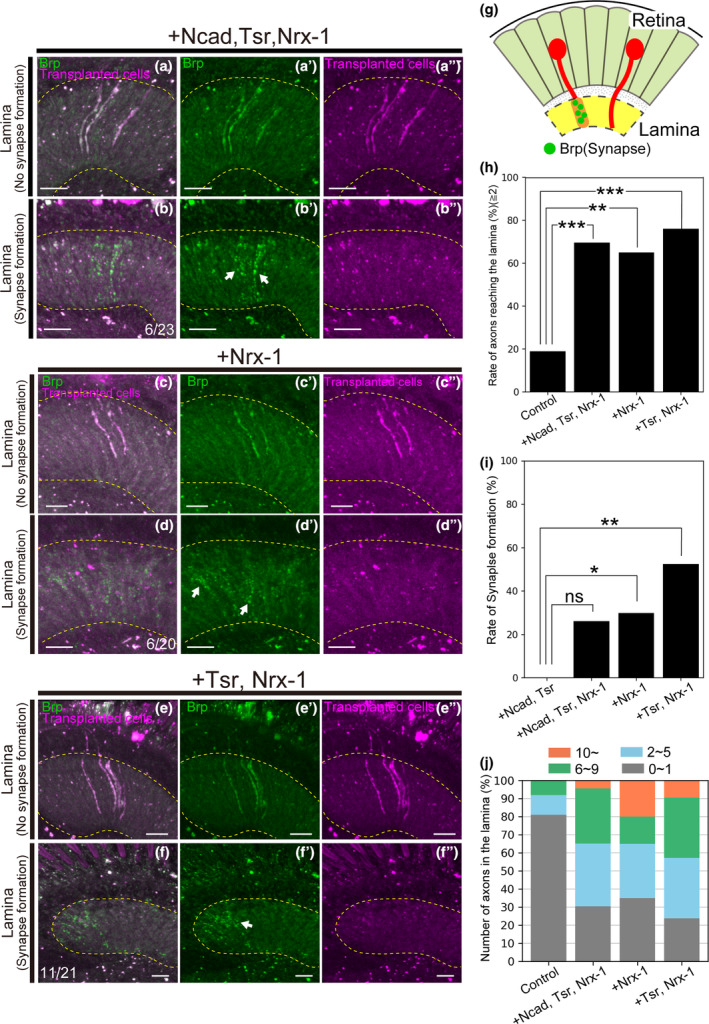
Nrx‐1 shortens the latency and promotes the formation of presynaptic structures. (a–f) Transplanted cells were labeled with RFP (magenta). Photoreceptors were stained with mAb24B10 (green). The yellow dotted line indicates the lamina region. (a‐a") Cells overexpressing Ncad, tsr, and Nrx‐1 with Brp‐GFP elongated axons to the lamina in 69.6% of flies 3 days after transplantation (n = 23). Brp signals throughout the elongated axons were observed. (b–b″) Punctate Brp signals were identified on the elongated axons in 26.1% of transplanted flies (n = 6/23). (c–c″) Cells overexpressing Nrx‐1 with Brp‐GFP elongated axons to the lamina in 65% of flies 3 days after transplantation (n = 20). Brp signals throughout the elongated axons were observed. (d–d″) Punctate Brp signals were identified on the elongated axons in 30% of transplanted flies (n = 6/20). (e–e") Cells overexpressing tsr and Nrx‐1 with Brp‐GFP elongated axons to the lamina in 76.2% of flies 3 days after transplantation (n = 21). Brp signals throughout the elongated axons were observed. (f–f″) Punctate Brp signals were identified on the elongated axons in 52.4% of transplanted flies (n = 11/21). (g) Schematic of axons that formed and did not form synapses in the lamina. (h) Percentage of flies with axon elongation to the lamina. Significant differences were observed between cells overexpressing Ncad, tsr, and Nrx‐1, overexpressing Nrx‐1, or overexpressing tsr and Nrx‐1 and controls. (i) Percentage of presynapse formation in extended axons. Significant differences were observed between cells overexpressing Nrx‐1 or overexpressing tsr and Nrx‐1 and cells overexpressing Ncad and tsr. (j) Classification of the number of axons extending into the lamina. The number of axons was classified into four categories: ≤1 axon (gray), 2–5 axons (blue), 6–9 axons (green), and ≥10 axons (orange). The percentage of flies with 10 or more elongating axons was the highest in Nrx‐1 overexpression. Scale bar (a–f):10 μm n.s., p ≧ .05, *p < .05, **p < .01, ***p < .001.
3. RESULTS
3.1. The modified transplantation method enhances axonal extension
To select cells for transplantation, cells were previously labeled using GMR‐myr‐RFP (Suzuki et al., 2018). GMR (Glass Multimer Reporter) originates from the promoter sequence of Drosophila Rh1, serving as the binding site for the transcription factor Glass (Ellis et al., 1993; Hay et al., 1994). Glass exhibits expression not only in photoreceptors, which are neuronal cells, but also in cone cells and pigment cells of the eye, which are non‐neuronal cells, suggesting that Glass plays a role in promoting the differentiation of non‐neuronal cells (Ellis et al., 1993; Morrison et al., 2018; Moses & Rubin, 1991). These findings suggest that cells labeled with GMR‐myr‐RFP included non‐neuronal cells. We hypothesized that the low percentage of photoreceptor cells in the transplanted cells prevented bundling of axons by non‐neuronal cells, which would have limited axon growth of the transplanted cells to the retina. Therefore, we attempted to reduce non‐neuronal cells by using elav‐Gal4, a neuron‐specific Gal4 driver.
In Drosophila transplantation, eye discs are initially extracted from third instar larvae, dissociated, and cultured. The cultured cells are then transplanted into adult flies (Figure 1a). In the Drosophila visual system, photoreceptor cells extend axons from the retina, a component of the compound eye, projecting these axons to the lamina and medulla—ganglia in the brain. Having transplanted cells into the adult retina, we anticipated that the transplanted cells would project axons towards the lamina or medulla (Figure 1b). The transplanted cells were labeled by combining elav‐Gal4 with UAS‐FLP, GMR‐FRT‐stop‐FRT‐Gal4, and UAS‐myr‐RFP to selectively label only the neuronal photoreceptors among the cellular components of the compound eye (abbreviated as elav‐FLP GMR‐FsF‐Gal4 hereinafter). This combined approach, involving elav‐Gal4 and GMR‐FRT‐stop‐FRT‐Gal4, not only targeted neurons exclusively but also amplified the expression of RFP in the transplanted cells, thereby aiding in the visualization of these cells.
In the eye disc, cells labeled with elav‐FLP GMR‐FsF‐Gal4 exhibited a reduction in labeled photoreceptor cells compared to those labeled with GMR‐myr‐RFP (Figure 1c,d). Previously, transplantation of cells labeled with GMR‐myr‐RFP resulted in axon elongation limited to the retina, without any observed axonal projection to the lamina (Suzuki et al., 2018). In contrast, upon transplantation of cells labeled with elav‐FLP GMR‐FsF‐Gal4, transplanted cells were observed in the retina, and axon elongation towards the lamina was observed in 18.9% of the transplanted flies (Figure 1e,f). This occurrence might be attributed to the increased presence of photoreceptor cells among the transplanted cell population.
3.2. Co‐overexpression of Ncad and tsr promotes axonal extension in transplants
To enhance the axonal elongation of cells labeled with elav‐FLP GMR‐FsF‐Gal4 towards the lamina, we transplanted cells overexpressing Ncad using the Gal4‐UAS system. Previous investigations have demonstrated that the transplantation of cells overexpressing Ncad, encoding a cell adhesion factor, promotes axon elongation in the retina (Suzuki et al., 2018). Therefore, we attempted to enhance the reachability of the lamina by overexpressing Ncad in cells labeled with elav‐FLP GMR‐FsF‐Gal4. Despite the transplantation of Ncad‐overexpressing cells resulting in axon elongation to the lamina in 39.3% of the flies, there was no significant difference compared to controls (Figure 2a,e,f).
To further promote axon elongation to the lamina, we focused on Tsr, a Drosophila homolog of ADF/Cofilin. Tsr is implicated in actin dynamics, contributing to actin depolymerization and cleavage. Prior research has demonstrated that overexpression of Cofilin1, the mouse homolog of Tsr, in damaged mouse neurons promotes nerve regeneration (Tedeschi et al., 2019). Consequently, we attempted to transplant cells overexpressing tsr, leading to axonal elongation to the lamina in 30.8% of flies. However, no significant difference was observed compared to controls (Figure 2b,e,f). In one case, overexpression of tsr resulted in axon elongation beyond the lamina, extending into the M2 and M3 layers of the medulla (Figure 2c).
We presumed that overexpression of either Ncad or tsr would promote axon elongation to the lamina. However, no significant difference was observed compared to controls (Figure 2f). We considered that a combination of Ncad, promoting adhesion to surrounding tissues, and Tsr, generating a driving force through actin depolymerization and cleavage, could potentially promote axon elongation. Subsequently, we attempted to transplant cells overexpressing both Ncad and tsr. The transplantation of cells overexpressing both Ncad and tsr led to axon elongation to the lamina in 73.1% of flies. In comparison to controls and Ncad alone, overexpression of Ncad and tsr significantly promoted axon elongation (Figure 2d–f) (control: 18.9%).
We also analyzed the number of axons extending into the lamina per fly. In controls, no more than 10 axons extended into the lamina; however, when Ncad or tsr was overexpressed, more than 10 axons extended into the lamina in some flies. Furthermore, overexpression of Ncad and tsr led to more than five axons elongating to the lamina in about 40% of the transplanted flies, indicating an increase in the number of axons elongating to the lamina (Figure 2g).
These results suggest that the combination of Ncad, a cell adhesion factor, and Tsr, which facilitates actin reorganization, promotes axon elongation beyond the retina into the lamina.
3.3. Both actin rearrangement and stabilization are crucial for axonal extension
Cofilin undergoes activation through dephosphorylation of its Ser3 residue and inactivation through phosphorylation of the same residue (Agnew et al., 1995; Moriyama et al., 1996). Activated Cofilin binds to actin, facilitating depolymerization, whereas inactivated Cofilin is unable to bind to actin, leading to the stabilization of the actin fiber (Figure 3a). Similarly, in Tsr, the Drosophila homolog of ADF/Cofilin, the unphosphorylated form of Tsr is active, while the phosphorylated form is inactive. To examine whether activated Tsr facilitates axon elongation of transplanted cells into the lamina or, conversely, whether inactivated Tsr inhibits axon elongation, we transplanted cells overexpressing activated/inactivated Tsr.
In the mushroom body, the other nervous system of the fly, the activated form of Tsr (Tsr.S3A) demonstrates a rescue of Tsr defects comparable to the wild type (Ng & Luo, 2004; Sudarsanam et al., 2020). Similarly, in mice, activated Cofilin1 has been observed to promote axon elongation to a degree equivalent to the wild type (Tedeschi et al., 2019). When we transplanted cells overexpressing tsr.S3A, mimicking the activated form, and Ncad, axon elongation to the lamina was observed in 52% of flies (Figure 3b,d). In contrast to activated Tsr, inactivated Tsr (Tsr.S3E) is known to be unable to sufficiently rescue Tsr defects in flies (Ng & Luo, 2004; Sudarsanam et al., 2020). Nonetheless, when tsr.S3E and Ncad were overexpressed, axon elongation to the lamina was observed in 50% of flies (Figure 3c,d).
The axonal elongation observed in both Ncad + activated Tsr (52%) and Ncad + inactivated Tsr (50%) does not show a significant difference compared to the overexpression of Ncad alone (Figure 3d). However, a significant difference is noted when compared to controls. This suggests that the overexpression of Ncad and tsr, whether in the activated or inactivated form, tends to promote axonal elongation. However, the most notable enhancement of axon elongation is observed when Ncad is co‐overexpressed with wild‐type tsr. Comparing the number of elongated axons, the percentage of flies with 10 or more axons reaching the lamina was higher in wild‐type tsr overexpression than in the activated/inactivated tsr overexpression (Figure 3d,e).
In transplantation, overexpression of either activated or inactivated tsr with Ncad did not promote axon elongation to the same degree as observed with overexpression of wild‐type tsr and Ncad. Additionally, notable differences were observed in the number of axons reaching the lamina. These findings suggest that Tsr plays a crucial role in promoting axon elongation by reversibly cycling between phosphorylated and dephosphorylated states.
3.4. Presynaptic structures can be formed in axons extended to the lamina
To regenerate neural circuits, synapses must be formed following axon elongation. To assess synapse formation in axons extending to the lamina from transplanted cells, we employed Brp as a presynaptic marker. Brp, a key component of the active zone in the presynapse, is visually observed as distinct dots in the lamina (Figure 4a,b). As the number of Brp dots labeled with GFP using the STaR method approximately corresponds to the number and distribution of T‐bars observed through electron microscopy, Brp serves as a marker for both synapse construction and disassembly, making it a suitable indicator for presynapse formation (Araki et al., 2020; Chen et al., 2014; Osaka et al., 2022; Sugie et al., 2015). Consequently, we examined the formation of presynaptic structures in axons elongated to the lamina 3 days after transplantation by specifically labeling transplanted cells with Brp (Brp‐GFP).
FIGURE 4.
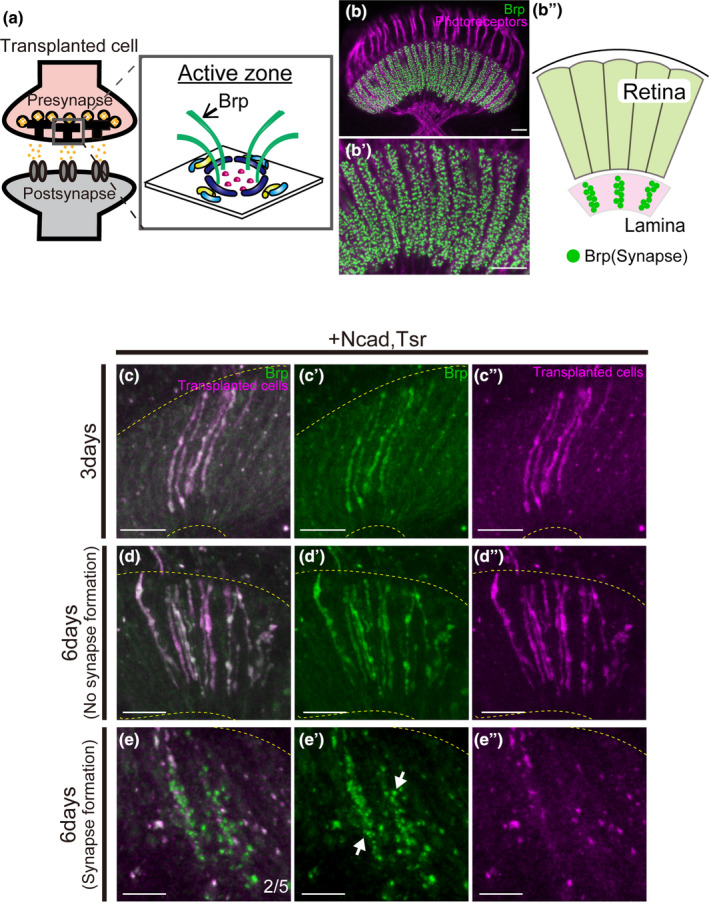
Presynapse formation in axons elongating into the lamina. (a) Schematic diagram of the synapse. The active zone is a structure present in the presynapse, and Brp is one of the active zone components. (b) Localization of endogenous Brp in the adult Lamina. Brp (green) is observed as dots in the lamina. Photoreceptor cells were stained with mAb24B10 (magenta). (c–e) Transplanted cells are labeled with RFP (magenta), Brp is labeled with GFP (green). The yellow dotted line indicates the lamina region. (c–c″) Cells overexpressing Ncad and tsr with Brp‐GFP showed axon elongation to the lamina 3 days after transplantation and a Brp signal throughout elongated axons (n = 10/16). No punctate signal was observed. (d–d″) At 6 days after transplantation, axon elongation to the lamina was observed, and the Brp signal was detected throughout axons (n = 5/7). (e–e") Punctate signals (green) were observed in the lamina in two of the cases elongating axons to the lamina (n = 2/5). In this fly 25 or 33 synapses were observed. The white arrow indicates the distinct synapse. Scale bar (b–e): 10 μm.
Transplantation of cells overexpressing Ncad and tsr with Brp‐GFP revealed the presence of Brp‐GFP signals throughout the axons extending to the lamina after 3 days. However, at this time point, the Brp‐GFP signal pattern perfectly overlapped with the axon plasma membrane (RFP), and thus no obvious punctate signal indicative of presynapse formation was observed, suggesting that presynapse induction was not sufficiently triggered in the elongated axons (Figure 4c). Considering that the short duration between transplantation and fixation may have contributed to the absence of presynapse formation, we extended this period to 6 days. After 6 days, Brp‐GFP signals perfectly overlapped with the axonal membrane in some flies as at 3 days. However, in two of the cases with axon elongation to the lamina, punctate signals stronger than the membranous RFP signal were observed at the lamina, confirming the formation of presynaptic structures (Figure 4d,e, n = 2/5).
In summary, overexpression of Ncad and tsr promotes axon elongation but necessitates a relatively extended period of 6 days for presynapse formation.
3.5. Nrx‐1 not only induces presynapse formation but also promotes axonal extension
To increase the efficiency of presynapse formation in axons extending into the lamina, we attempted to transplant cells overexpressing Nrx‐1 in addition to Ncad and tsr. Neurexins, as cell adhesion factors, are localized to the presynapse and are known to induce synapse formation (Gomez et al., 2021). Drosophila Nrx‐1 has been demonstrated to be essential for synapse formation and exhibits widespread expression from embryonic to adult stages (Li et al., 2007; Zeng et al., 2007).
We transplanted cells overexpressing Ncad, tsr, and Nrx‐1 with Brp‐GFP and observed axonal elongation after 3 days. Axonal elongation to the lamina was observed in 69.6% of the flies (Figure 5a,g,h). Notably, punctate Brp‐GFP signals were observed in the lamina, confirming the formation of presynaptic structures, in 26.1% of the transplanted flies (Figure 5b,g,i, n = 6/23). Clearly, with overexpression of Ncad, tsr, and Nrx‐1 a trend towards enhanced presynaptic formation was observed in comparison to overexpression of Ncad and tsr (Figure 5i). Although this difference was not statistically significant, we proceeded to transplant cells overexpressing Nrx‐1 exclusively. This resulted in axon elongation to the lamina in 65% of the flies, with presynapse formation confirmed in 30% of the transplanted flies (Figure 5c,d,g,h,i, n = 6/20).
As 65% of axons reached the lamina, exclusive overexpression of Nrx‐1 represented a significant difference compared to controls. This suggests that Nrx‐1 may not only enhance the efficiency of synapse formation but also play a role in axon elongation to the lamina. Therefore, we transplanted cells overexpressing tsr and Nrx‐1 to investigate whether the combination of Tsr, promoting cytoskeletal reorganization, and Nrx‐1, a cell adhesion molecule, would enhance axonal elongation to the lamina. The transplantation of cells overexpressing tsr and Nrx‐1 resulted in axon elongation to the lamina in 76.2% of the transplanted flies, the highest percentage observed (Figure 5e–h). Punctate Brp‐GFP signals were observed in 52.4% of the transplanted flies, confirming the formation of presynaptic structures (Figure 5f,i, n = 11/21). Notably, in terms of the number of elongated axons per fly, overexpression of Nrx‐1 alone yielded the highest percentage of flies with 10 or more axons (Figure 5j).
The introduction of Nrx‐1 shortened the time to presynapse formation. While the primary purpose of introducing Nrx‐1 was to induce presynapse formation, all combinations with additional Nrx‐1 seemed to promote both axon elongation and presynapse formation. Notably, axon elongation to the lamina was observed even when only Nrx‐1 was overexpressed, suggesting the involvement of Nrx‐1 in axon elongation.
3.6. Transplanted cells elongate axons to the medulla beyond the lamina
We successfully verified the axon elongation of transplanted cells to the lamina beyond the retina, as well as the furthest target tissue of the photoreceptor, the medulla. When only tsr was overexpressed, one case was observed with axon elongation reaching the M2–M4 layers of the medulla beyond the lamina (Figures 2c, 6f). When only Nrx‐1 was overexpressed, we observed axon elongation to the lamina and the formation of presynaptic structures. Additionally, in one case, axon elongation to the medulla was noted, accompanied by the formation of presynaptic structures in the elongated axon (Figure 6a,b,e,f). When tsr and Nrx‐1 were overexpressed, axon elongation to the medulla was observed in four samples with axons extending to the lamina. The most extended axon reached the M6 layer, and the formation of presynaptic structures was confirmed in half of the flies with axon elongation to the medulla (Figure 6c–f). One case of axon elongation beyond the lamina to the medulla was also observed when activated/inactivated tsr and Ncad were overexpressed or when Ncad, tsr, and Nrx‐1 were overexpressed (Figure 6f). When Ncad and tsr were overexpressed, axon elongation to the lamina was not observed (Figure 6f). To examine the cell types of transplanted cells, the results of staining with an antibody against Rh1, an R1–R6 photoreceptor‐specific rhodopsin, showed that few cells overexpressing Ncad and tsr were stained with Rh1 (data not shown). R7 and R8 photoreceptors project to the medulla, and based on the staining results, the transplanted cells with axons reaching the lamina or the medulla are likely to be R7 or R8 photoreceptors. However, rhodopsin is expressed during the late pupal stage (Earl & Britt, 2006). In the present study, cells taken from the eye discs of third instar larvae were transplanted and observed 3 days (72 h) later, potentially overlapping with the period of rhodopsin expression. Consequently, there is a possibility that the staining was conducted when rhodopsin levels were too low to be detected. Thus, we cannot exclude the possibility that the transplanted cells may have comprised R1–R6 photoreceptors.
FIGURE 6.
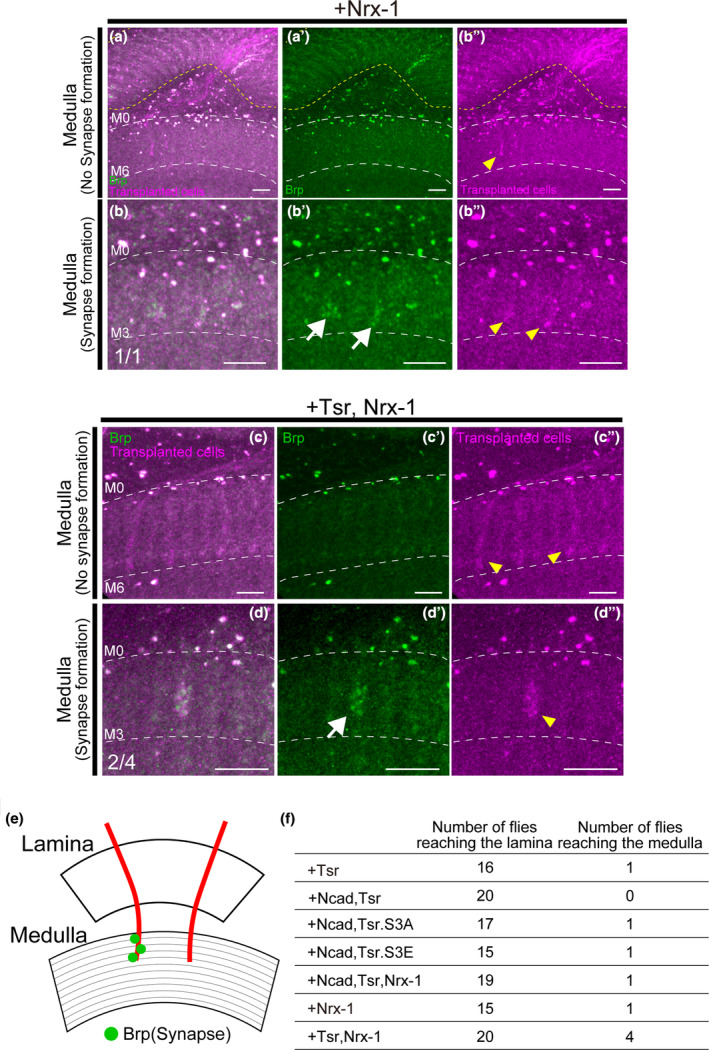
Axon elongation of transplanted cells beyond the lamina to the medulla. (a–d) Transplanted cells are labeled with RFP (magenta), Brp is labeled with GFP (green). The yellow dotted line indicates the lamina region. Yellow arrowheads indicate axons and white arrows indicate distinct synapses. (a–a") Cells overexpressing Nrx‐1 elongated axons to the M2–M5 layers of the medulla in one case among flies with axon elongation to the lamina (n = 1/15). (b–b″) Punctate Brp signals were found on the axons elongating to the medulla of cells overexpressing Nrx‐1. (c–c″) Cells overexpressing tsr and Nrx‐1 elongated axons to the medulla in four cases among flies with axon elongation to the lamina (n = 4/20). The most elongated axons reached the M6 layer of the medulla. (d–d″) Punctate Brp signals were found on the axons elongating to the medulla of cells overexpressing tsr and Nrx‐1. (e) Schematic of axons that elongated to the medulla and formed synapses and axons that did not form synapses. (f) The number of flies with axon elongation to the medulla among flies with axon elongation to the lamina. The rate of reaching the medulla was higher in transplants of cells overexpressing tsr and Nrx‐1. Scale bar (a–d): 10 μm.
Although the elongation to the medulla was limited, we successfully confirmed axonal elongation of transplanted cells to the medulla, the furthest target tissue of Drosophila photoreceptor cells, by introducing Ncad, tsr, and Nrx‐1. While no clear correlation was observed between Ncad, Tsr, and Nrx‐1, overexpression of Nrx‐1 tended to promote axon elongation to the medulla. In particular, axon elongation to the medulla was most frequently observed when cells overexpressing Nrx‐1 and tsr were transplanted. The exact mechanism underlying the induction of axon elongation to the medulla by these factors remains unclear and constitutes a subject for future investigation. These results suggest that Nrx‐1 may play a role not only in the formation of presynaptic structures but also in axon growth to the medulla.
4. DISCUSSION
In this study, we enhanced the axonal extension of transplanted cells from the retina to the lamina by modifying the selection process of transplanted cells. Furthermore, our results showed that overexpression of Ncad and tsr in cells selected through this process facilitates axon elongation. Presynapse formation in the elongated axons in the lamina was confirmed 6 days after transplantation. The introduction of Nrx‐1 led to a significant reduction in the time required for presynapse formation, shortening it to 3 days, suggesting that Nrx‐1 not only promotes presynapse formation but is also involved in axon elongation. Overexpression of Ncad, tsr, or Nrx‐1 promoted axonal projection beyond the retina, successfully replicating the accurate projection of Drosophila photoreceptor cells to their furthest target tissue, the medulla. In conclusion, these results suggest that overexpression of Ncad, tsr, and Nrx‐1 is associated with the promotion of axon elongation in transplantation. These factors emerge as effective contributors to the regeneration of neural circuits through transplantation.
4.1. Actin depolymerization and cell adhesion promote axon elongation
Axon elongation is thought to occur with the driving force of retrograde actin flow by actin turnover and Myosin II in the growth cone and adhesion to surrounding tissues, which pushes the cell membrane in the direction of elongation, thereby protruding the growth cone (Dent & Gertler, 2003; Lowery & Van Vactor, 2009; Suter & Forscher, 1998; Vitriol & Zheng, 2012). ADF/Cofilin plays a critical role in actin turnover and retrograde actin flow, contributing to neurite formation (Flynn et al., 2012; Tedeschi et al., 2019). We attempted to promote axon extension beyond the retina into the lamina by introducing tsr, which encodes a protein that induces actin reorganization. Previous research has demonstrated that Cofilin1, a protein that facilitates actin depolymerization and cleavage, promotes the elongation of injured axons in the central nervous system of mice (Tedeschi et al., 2019). Nevertheless, overexpression of tsr alone, a homolog of ADF/Cofilin, in transplanted cells did not markedly enhance axon elongation to the lamina beyond the retina, contrasting with findings from previous studies. In transplantation, cells conditioned in vitro must extend axons within the organism, having already completed their development. The expression of factors promoting actin turnover and increasing retrograde actin flow, such as Tsr, is expected to be insufficient for stabilizing axons due to their inability to adhere to the surrounding tissue. Consequently, we assumed that the adaptation to the transplanted environment is hindered, and Tsr alone does not adequately facilitate axon elongation into the lamina.
Ncad, which promotes axon elongation when co‐expressed with Tsr, plays a role in stabilizing growth cones through filopodial dynamics (Mehmet Neset Özel et al., 2015). The cytoplasmic domain of cadherin binds to β‐catenin, which associates with actin fibers via α‐catenin, leading to enhanced cell adhesion (Noordstra et al., 2023). The driving force generated by the increased retrograde actin flow due to enhanced actin turnover by overexpression of tsr, combined with stable adhesion to the surrounding environment via catenin by Ncad, may have pushed the plasma membrane in the direction of elongation and protruded the growth cone. Consequently, axon elongation beyond the retina into the lamina could be induced.
Overexpression of Ncad and the activated/inactivated form of tsr did not exhibit a significant difference compared to Ncad overexpression alone, although a notable distinction was observed compared to controls, suggesting a tendency to promote axon elongation regardless of whether Tsr is active or inactive. Previous research has demonstrated that activated Cofilin promotes axon elongation to a similar extent as the wild type (Endo et al., 2003; Tedeschi et al., 2019), reinforcing the notion that activated Tsr plays a crucial role in axon elongation. On the contrary, the influence of inactivated Tsr on axon elongation has been reported to be both promoting and non‐promoting (Ng & Luo, 2004; Sudarsanam et al., 2020). Our findings indicated that overexpression of Ncad and the inactivated form of tsr tended to facilitate axon elongation compared to controls. Two potential reasons for this promotion are considered. The first reason is actin stabilization. Since axon elongation was observed in control cells, it is presumed that the transplanted cells inherently possessed the ability to elongate axons. The introduction of the inactivated form of tsr is thought to have inhibited the depolymerization of actin fibers, leading to their stabilization. This may explain the observation of axons in the lamina without retraction. The second possibility is a shift in the ratio of inactivated to activated Tsr. Tsr (Cofilin) undergoes phosphorylation and dephosphorylation, regulated by LIM‐kinases and Slingshot (Van Troys et al., 2008). Since our experiments were conducted in the presence of endogenous Tsr, overexpression of the inactivated form of tsr might have influenced axon elongation by modulating the ratio of activated to inactivated forms of endogenous Tsr. The role of the inactivated form of Tsr in axon elongation remains controversial. While overexpression of Ncad and activated/inactivated tsr promoted axon elongation compared to controls, it reduced the number of axons reaching the lamina, implying that either activation or inactivation alone is insufficient for axon elongation. Hence, a reversible change in the phosphorylated and dephosphorylated forms of Tsr is considered crucial for the process of axon elongation.
While the introduction of Nrx‐1 was intended to induce synapse formation, its potential involvement in axon elongation is also suggested. Previous studies have identified molecules that play dual roles in both synapse formation and axon stabilization (Astigarraga et al., 2010; Hakeda‐Suzuki et al., 2017; Holbrook et al., 2012), suggesting that synapse formation causes axonal stabilization (Özel et al., 2019). One potential explanation for the dual role of Nrx‐1, promoting both synapse formation and axon elongation, could be that the synapses induced by Nrx‐1 contribute to axonal stabilization, thereby suppressing axonal retraction. Another potential mechanism contributing to the role of Nrx‐1 in axon elongation is the stabilization of microtubules. Previous research showed that the stabilization of microtubules is pivotal for axon elongation (Blanquie & Bradke, 2018). It is known that Nrx‐1 has genetic interactions with the microtubule‐associated factor Futsch and that microtubule cleavage is triggered by Nrx‐1 expression (Banerjee & Riordan, 2018). Our observations of axon elongation to the lamina in controls suggest that the transplanted cells inherently possessed the ability to elongate axons. Axon elongation to the lamina was observed even in the transplantation of cells overexpressing Nrx‐1 alone, which is presumably attributed to the non‐retraction of elongated axons resulting from microtubule stabilization induced by Nrx‐1. Moreover, the greatest axon elongation was observed after overexpression of tsr and Nrx‐1, which may be related to the actin ring. Actin is present not only in growth cones but also in axons, forming a ring structure called an actin ring (Xu et al., 2013). Previous research indicates that actin rings play a role in microtubule stabilization (Qu et al., 2017). The depolymerization and cleavage of actin induced by Tsr may have influenced the formation of actin rings, consequently stabilizing the microtubules within the axons and ultimately promoting axon elongation.
4.2. Presynapse formation in extended axons is induced by Nrx‐1
In transplantation of cells overexpressing Ncad and tsr, presynapse formation could not be confirmed 3 days after transplantation, but it could be confirmed 6 days later. While the RFP signal was weak in regions where the Brp‐GFP signal was detected, we attribute this to the thickening of the elongated axons as they matured. The presence of debris is typically associated with neurodegeneration (Osaka et al., 2022). If the observed Brp‐GFP signals were indicative of neurodegeneration, a corresponding RFP signal would be expected. However, no such RFP signal was observed. These findings suggest that the axons undergoing synaptogenesis became thicker as they matured, leading to a diminished RFP signal, with only the Brp‐GFP signal being detectable. The delayed formation of presynaptic structures by cells overexpressing Ncad and tsr may be attributed to the role of Ncad in stabilizing synapse formation rather than initiating it. Previous studies have demonstrated that Ncad plays a role in stabilizing synapses but is not indispensable for the actual formation of synapses (Suzuki & Takeichi, 2008). Moreover, other synaptic organizers have been identified (Connor & Siddiqui, 2023). Overexpression of Ncad alone might not be fully capable of initiating synapse formation.
Overexpression of Nrx‐1 promoted the formation of presynaptic structures. Previous studies have demonstrated that Nrx‐1 is a key organizer of synapse formation (Gomez et al., 2021). Consistent with this, our results showed that Nrx‐1 significantly curtails the time of presynapse formation, emphasizing its role in promoting formation of presynaptic structures. In contrast, synapse formation was observed in approximately half of the transplanted animals. To enhance the rate of synapse formation, molecules associated with synapses, such as Munc13‐1 and RIM, known to reside in the active zone and play a role in priming synaptic vesicles (Südhof, 2012), have been shown to inhibit axon elongation (Hilton et al., 2022). Considering that synapse formation may contribute to axon stabilization, as discussed earlier, the expression of synapse‐related molecules during the transplantation stage could impede axon elongation. Therefore, precise timing regulation, such as employing a light‐dependent gene expression system (de Mena & Rincon‐Limas, 2020; Qian et al., 2023), might be crucial to control the expression timing effectively.
AUTHOR CONTRIBUTIONS
R.I., S.H.‐S., and T.S. designed the experiments. R.I. and N.Y. performed the experiments. R.I. analyzed the data. R.I. and T.S. wrote the paper.
ACKNOWLEDGMENTS
We gratefully acknowledge the Kyoto Drosophila Stock Center, NIG‐Fly, Bloomington stock center, and DSHB for providing fly or antibody stocks. This work was supported by a Grants‐in‐Aid for Scientific Research (B) and a Grants‐in‐Aid for Scientific Research on Innovative Areas from MEXT (#16H06457, #21H05682, #21H02483, and #23H04220 to T.S.), a Takeda Visionary Research Grant from the Takeda Science Foundation (to T.S.), and JST SPRING (JPMJSP2106 to R.I.).
Iwanaga, R. , Yahagi, N. , Hakeda‐Suzuki, S. , & Suzuki, T. (2024). Cell adhesion and actin dynamics factors promote axonal extension and synapse formation in transplanted Drosophila photoreceptor cells. Development, Growth & Differentiation, 66(3), 205–218. 10.1111/dgd.12916
Communicating Editor: Harukazu Nakamura
REFERENCES
- Agnew, B. J. , Minamide, L. S. , & Bamburg, J. R. (1995). Reactivation of phosphorylated Actin depolymerizing factor and identification of the regulatory site. Journal of Biological Chemistry, 270(29), 17582–17587. 10.1074/jbc.270.29.17582 [DOI] [PubMed] [Google Scholar]
- Araki, T. , Osaka, J. , Kato, Y. , Shimozono, M. , Kawamura, H. , Iwanaga, R. , Hakeda‐Suzuki, S. , & Suzuki, T. (2020). Systematic identification of genes regulating synaptic remodeling in the drosophila visual system. Genes and Genetic Systems, 95(3), 101–110. 10.1266/ggs.19-00066 [DOI] [PubMed] [Google Scholar]
- Astigarraga, S. , Hofmeyer, K. , Farajian, R. , & Treisman, J. E. (2010). Three drosophila liprins interact to control synapse formation. Journal of Neuroscience, 30(46), 15358–15368. 10.1523/JNEUROSCI.1862-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, S. , & Riordan, M. (2018). Coordinated regulation of axonal microtubule organization and transport by drosophila neurexin and BMP pathway. Scientific Reports, 8, 17337. 10.1038/s41598-018-35618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquie, O. , & Bradke, F. (2018). Cytoskeleton dynamics in axon regeneration. Current Opinion in Neurobiology, 51, 60–69. 10.1016/j.conb.2018.02.024 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Akin, O. , Nern, A. , Tsui, C. Y. K. , Pecot, M. Y. , & Zipursky, S. L. (2014). Cell‐type‐specific labeling of synapses in vivo through synaptic tagging with recombination. Neuron, 81(2), 280–293. 10.1016/j.neuron.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor, S. A. , & Siddiqui, T. J. (2023). Synapse organizers as molecular codes for synaptic plasticity. Trends in Neurosciences, 46(11), 971–985. 10.1016/j.tins.2023.08.001 [DOI] [PubMed] [Google Scholar]
- de Mena, L. , & Rincon‐Limas, D. E. (2020). PhotoGal4: A versatile light‐dependent switch for spatiotemporal control of gene expression in drosophila explants. iScience, 23(7), 101308. 10.1016/j.isci.2020.101308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent, E. W. , & Gertler, F. B. (2003). Cytoskeletal dynamics and transport in growth cone motility and guidance. Neuron, 40(2), 209–227. 10.1016/S0896-6273(03)00633-0 [DOI] [PubMed] [Google Scholar]
- Earl, J. B. , & Britt, S. G. (2006). Expression of drosophila rhodopsins during photoreceptor cell differentiation: Insights into R7 and R8 cell subtype commitment. Gene Expression Patterns, 6(7), 687–694. 10.1016/j.modgep.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Ellis, M. C. , O'Neill, E. M. , & Rubin, G. M. (1993). Expression of drosophila glass protein and evidence for negative regulation of its activity in non‐neuronal cells by another DNA‐binding protein. Development, 119(3), 855–865. 10.1242/dev.119.3.855 [DOI] [PubMed] [Google Scholar]
- Endo, M. , Ohashi, K. , Sasaki, Y. , Goshima, Y. , Niwa, R. , Uemura, T. , & Mizuno, K. (2003). Control of growth cone motility and morphology by LIM kinase and slingshot via phosphorylation and dephosphorylation of cofilin. The Journal of Neuroscience, 23(7), 2527–2537. 10.1523/JNEUROSCI.23-07-02527.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, K. C. , Hellal, F. , Neukirchen, D. , Jacob, S. , Tahirovic, S. , Dupraz, S. , Stern, S. , Garvalov, B. K. , Gurniak, C. , Shaw, A. E. , Meyn, L. , Wedlich‐Söldner, R. , Bamburg, J. R. , Small, J. V. , Witke, W. , & Bradke, F. (2012). ADF/cofilin‐mediated Actin retrograde flow directs neurite formation in the developing brain. Neuron, 76(6), 1091–1107. 10.1016/j.neuron.2012.09.038 [DOI] [PubMed] [Google Scholar]
- Gomez, A. M. , Traunmüller, L. , & Scheiffele, P. (2021). Neurexins: Molecular codes for shaping neuronal synapses. Nature Reviews Neuroscience, 22, 137–151. 10.1038/s41583-020-00415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus, K. C. , Bonaccorsi, S. , Williams, E. , Verni, F. , Gatti, M. , & Goldberg, M. L. (1995). Mutations in twinstar, a drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. Journal of Cell Biology, 131(5), 1243–1259. 10.1083/jcb.131.5.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda‐Suzuki, S. , Berger‐Müller, S. , Tomasi, T. , Usui, T. , Horiuchi, S. Y. , Uemura, T. , & Suzuki, T. (2011). Golden goal collaborates with flamingo in conferring synaptic‐layer specificity in the visual system. Nature Neuroscience, 14(3), 314–323. 10.1038/nn.2756 [DOI] [PubMed] [Google Scholar]
- Hakeda‐Suzuki, S. , Takechi, H. , Kawamura, H. , & Suzuki, T. (2017). Two receptor tyrosine phosphatases dictate the depth of axonal stabilizing layer in the visual system. eLife, 6, e31812. 10.7554/eLife.31812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, B. A. , Wolff, T. , & Rubin, G. M. (1994). Expression of baculovirus P35 prevents cell death in drosophila . Development, 120(8), 2121–2129. 10.1242/dev.120.8.2121 [DOI] [PubMed] [Google Scholar]
- Hilton, B. J. , Husch, A. , Schaffran, B. , Lin, T. , Burnside, E. R. , Dupraz, S. , Schelski, M. , Kim, J. , Müller, J. A. , Schoch, S. , Imig, C. , Brose, N. , & Bradke, F. (2022). An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron, 110(1), 51–69. 10.1016/j.neuron.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook, S. , Finley, J. K. , Lyons, E. L. , & Herman, T. G. (2012). Loss of syd‐1 from R7 neurons disrupts two distinct phases of presynaptic development. Journal of Neuroscience, 32(50), 18101–18111. 10.1523/JNEUROSCI.1350-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton, R. K. , Heebner, J. E. , Grillo, M. A. , & Swulius, M. T. (2022). Cofilactin filaments regulate filopodial structure and dynamics in neuronal growth cones. Nature Communications, 13, 2439. 10.1038/s41467-022-30116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha, B. , Stafford, B. K. , & Huberman, A. D. (2017). Regenerating optic pathways from the eye to the brain. Science, 356(6342), 1031–1034. 10.1126/science.aal5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Ashley, J. , Budnik, V. , & Bhat, M. A. (2007). Crucial role of drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron, 55(5), 741–755. 10.1016/j.neuron.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery, L. A. , & Van Vactor, D. (2009). The trip of the tip: Understanding the growth cone machinery. Nature Reviews Molecular Cell Biology, 10, 332–343. 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, L. , Joyce Liao, Y. , Jan, L. Y. , & Jan, Y. N. (1994). Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes and Development, 8, 1787–1802. 10.1101/gad.8.15.1787 [DOI] [PubMed] [Google Scholar]
- MacLaren, R. E. , Pearson, R. A. , MacNeil, A. , Douglas, R. H. , Salt, T. E. , Akimoto, M. , & Ali, R. R. (2006). Retinal repair by transplantation of photoreceptor precursors. Nature, 444, 203–207. 10.1038/nature05161 [DOI] [PubMed] [Google Scholar]
- Moriyama, K. , Iida, K. , & Yahara, I. (1996). Phosphorylation of Ser‐3 of cofilin regulates its essential function on Actin. Genes to Cells, 1(1), 73–86. 10.1046/j.1365-2443.1996.05005.x [DOI] [PubMed] [Google Scholar]
- Morrison, C. A. , Chen, H. , Cook, T. , Brown, S. , & Treisman, J. E. (2018). Glass promotes the differentiation of neuronal and non‐neuronal cell types in the drosophila eye. PLoS Genetics, 14(1), e1007173. 10.1371/journal.pgen.1007173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses, K. , & Rubin, G. M. (1991). Glass encodes a site‐specific DNA‐binding protein that is regulated in response to positional signals in the developing drosophila eye. Genes and Development, 5(4), 583–593. 10.1101/gad.5.4.583 [DOI] [PubMed] [Google Scholar]
- Ng, J. , & Luo, L. (2004). Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron, 44(5), 779–793. 10.1016/j.neuron.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Noordstra, I. , Morris, R. G. , & Yap, A. S. (2023). Cadherins and the cortex: A matter of time? Current Opinion in Cell Biology, 80, 102154. 10.1016/j.ceb.2023.102154 [DOI] [PubMed] [Google Scholar]
- Osaka, J. , Yasuda, H. , Watanuki, Y. , Kato, Y. , Nitta, Y. , Sugie, A. , & Suzuki, T. (2022). Identification of genes regulating stimulus‐dependent synaptic assembly in drosophila using an automated synapse quantification system. Genes and Genetic Systems, 97(6), 297–309. 10.1266/ggs.22-00114 [DOI] [PubMed] [Google Scholar]
- Oswald, J. , Kegeles, E. , Minelli, T. , Volchkov, P. , & Baranov, P. (2021). Transplantation of miPSC/mESC‐derived retinal ganglion cells into healthy and glaucomatous retinas. Molecular Therapy ‐ Methods and Clinical Development, 21, 180–198. 10.1016/j.omtm.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özel, M. N. , Kulkarni, A. , Hasan, A. , Brummer, J. , Moldenhauer, M. , Daumann, I. M. , Wolfenberg, H. , Dercksen, V. J. , Kiral, F. R. , Weiser, M. , Prohaska, S. , von Kleist, M. , & Hiesinger, P. R. (2019). Serial synapse formation through filopodial competition for synaptic seeding factors. Developmental Cell, 50(4), 447–461. 10.1016/j.devcel.2019.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özel, M. N. , Langen, M. , Hassan, B. A. , & Hiesinger, P. R. (2015). Filopodial dynamics and growth cone stabilization in drosophila visual circuit development. eLife, 4, e10721. 10.7554/eLife.10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, H. , Yu, H. , & Laski, F. A. (2008). Cofilin/ADF is required for retinal elongation and morphogenesis of the drosophila rhabdomere. Developmental Biology, 318(1), 82–91. 10.1016/j.ydbio.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y. , Li, T. , Zhou, S. , Chen, X. , & Yang, Y. (2023). A single‐component optogenetic Gal4‐UAS system allows stringent control of gene expression in zebrafish and drosophila . ACS Synthetic Biology, 12(3), 664–671. 10.1021/acssynbio.2c00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Y. , Hahn, I. , Webb, S. E. D. , Pearce, S. P. , & Prokop, A. (2017). Periodic Actin structures in neuronal axons are required to maintain microtubules. Molecular Biology of the Cell, 28(2), 296–308. 10.1091/mbc.E16-10-0727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsanam, S. , Yaniv, S. , Meltzer, H. , & Schuldiner, O. (2020). Cofilin regulates axon growth and branching of drosophila γ‐neurons. Journal of Cell Science, 133(8), jcs232595. 10.1242/jcs.232595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof, T. C. (2012). The presynaptic active zone. Neuron, 75(1), 11–25. 10.1016/j.neuron.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugie, A. , Hakeda‐Suzuki, S. , Suzuki, E. , Silies, M. , Shimozono, M. , Möhl, C. , Suzuki, T. , & Tavosanis, G. (2015). Molecular remodeling of the presynaptic active zone of drosophila photoreceptors via activity‐dependent feedback. Neuron, 86(3), 711–725. 10.1016/j.neuron.2015.03.046 [DOI] [PubMed] [Google Scholar]
- Suter, D. M. , & Forscher, P. (1998). An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Current Opinion in Neurobiology, 8, 106–116. 10.1016/S0959-4388(98)80014-7 [DOI] [PubMed] [Google Scholar]
- Suzuki, S. C. , & Takeichi, M. (2008). Cadherins in neuronal morphogenesis and function. Development Growth and Differentiation, 50, S119–S130. 10.1111/j.1440-169X.2008.01002.x [DOI] [PubMed] [Google Scholar]
- Suzuki, T. , Oochi, K. , Hakeda‐Suzuki, S. , & Suzuki, T. (2018). Transplantation of photoreceptor precursor cells into the retina of an adult drosophila . Development Growth and Differentiation, 60(7), 442–453. 10.1111/dgd.12545 [DOI] [PubMed] [Google Scholar]
- Takechi, H. , Hakeda‐Suzuki, S. , Nitta, Y. , Ishiwata, Y. , Iwanaga, R. , Sato, M. , Sugie, A. , & Suzuki, T. (2021). Glial insulin regulates cooperative or antagonistic golden goal/flamingo interactions during photoreceptor axon guidance. eLife, 10, e66718. 10.7554/eLife.66718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, A. , Dupraz, S. , Curcio, M. , Laskowski, C. J. , Schaffran, B. , Flynn, K. C. , Santos, T. E. , Stern, S. , Hilton, B. J. , Larson, M. J. E. , Gurniak, C. B. , Witke, W. , & Bradke, F. (2019). ADF/cofilin‐mediated Actin turnover promotes axon regeneration in the adult CNS. Neuron, 103(6), 1073–1085.e6. 10.1016/j.neuron.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y. , Li, T. , Sun, M. , Wan, D. , Li, Q. , Li, P. , Zhang, Z. C. , Han, J. , & Xie, W. (2013). Neurexin regulates visual function via mediating retinoid transport to promote rhodopsin maturation. Neuron, 77(2), 311–322. 10.1016/j.neuron.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Van Troys, M. , Huyck, L. , Leyman, S. , Dhaese, S. , Vandekerkhove, J. , & Ampe, C. (2008). Ins and outs of ADF/cofilin activity and regulation. European Journal of Cell Biology, 87(8–9), 649–667. 10.1016/j.ejcb.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Venugopalan, P. , Wang, Y. , Nguyen, T. , Huang, A. , Muller, K. J. , & Goldberg, J. L. (2016). Transplanted neurons integrate into adult retinas and respond to light. Nature Communications, 7, 10472. 10.1038/ncomms10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitriol, E. A. , & Zheng, J. Q. (2012). Growth cone travel in space and time: The cellular Ensemble of Cytoskeleton, adhesion, and membrane. Neuron, 73(6), 1068–1081. 10.1016/j.neuron.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrathasha, V. , Nikonov, S. , Bell, B. A. , He, J. , Bungatavula, Y. , Uyhazi, K. E. , & Murthy Chavali, V. R. (2022). Transplanted human induced pluripotent stem cells‐ derived retinal ganglion cells embed within mouse retinas and are electrophysiologically functional. iScience, 25(11), 105308. 10.1016/j.isci.2022.105308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, K. , Zhong, G. , & Zhuang, X. (2013). Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science, 339(6118), 452–456. 10.1126/science.1232251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, Y. , Chaudhari, K. , & Bashaw, G. J. (2021). New insights into the molecular mechanisms of axon guidance receptor regulation and signaling. Current Topics in Developmental Biology, 142, 147–196. 10.1016/bs.ctdb.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, X. , Sun, M. , Liu, L. , Chen, F. , Wei, L. , & Xie, W. (2007). Neurexin‐1 is required for synapse formation and larvae associative learning in drosophila . FEBS Letters, 581(13), 2509–2516. 10.1016/j.febslet.2007.04.068 [DOI] [PubMed] [Google Scholar]


