Abstract
Wnt is a family of secreted signaling proteins involved in the regulation of cellular processes, including maintenance of stem cells, carcinogenesis, and cell differentiation. In the context of early vertebrate embryogenesis, graded distribution of Wnt proteins has been thought to regulate positional information along the antero‐posterior axis. However, understanding of the molecular basis for Wnt spatial distribution remains poor. Modified states of heparan sulfate (HS) proteoglycans are essential for Wnt8 localization, because depletion of N‐deacetylase/N‐sulfotransferase 1 (NDST1), a modification enzyme of HS chains, decreases Wnt8 levels and NDST1 overexpression increases Wnt8 levels on the cell surface. Since overexpression of NDST1 increases both deacetylation and N‐sulfation of HS chains, it is not clear which function of NDST1 is actually involved in Wnt8 localization. In the present study, we generated an NDST1 mutant that specifically increases deacetylation, but not N‐sulfation, of HS chains in Xenopus embryos. Unlike wild‐type NDST1, this mutant did not increase Wnt8 accumulation on the cell surface, but it reduced canonical Wnt signaling, as determined with the TOP‐Flash reporter assay. These results suggest that N‐sulfation of HS chains is responsible for localization of Wnt8 and Wnt8 signaling, whereas deacetylation has an inhibitory effect on canonical Wnt signaling. Consistently, overexpression of wild‐type NDST1, but not the mutant, resulted in small eyes in Xenopus embryos. Thus, our NDST1 mutant enables us to dissect the regulation of Wnt8 localization and signaling by HS proteoglycans by specifically manipulating the enzymatic activities of NDST1.
Keywords: HSPGs, morphogen, NDST, Wnt, Xenopus
Heparan sulfate (HS) chains are important modulators of Wnt proteins in the extracellular space. The bifunctional HS modification enzyme N‐deacetylase/N‐sulfotransferase 1 (NDST1) has a positive effect on Wnt signaling; however, which activity is actually involved in Wnt signaling remains unclear. In the present work, we dissected these activities by introducing mutations into NDST1.

1. INTRODUCTION
The Wnt family of secreted signaling proteins contributes to carcinogenesis, stem cell maintenance, and animal development (Albrecht et al., 2021; Clevers et al., 2014; Nusse & Clevers, 2017). In the context of early vertebrate development, β‐catenin‐dependent (canonical) Wnt signaling conveys positional information along the antero‐posterior (AP) axis (Kiecker & Niehrs, 2001; Niehrs, 2022), contributing to AP patterning. Wnt has been considered a morphogen that gives positional information to cells or tissues through its concentration gradient; therefore, its spatial distribution, or gradient formation, in embryonic tissue has been intensively studied (Farin et al., 2016; Mii et al., 2017; Pani & Goldstein, 2018; Strigini & Cohen, 2000; Zecca et al., 1996). To date, several models have been proposed to explain extracellular Wnt transport (Mehta et al., 2021; Routledge & Scholpp, 2019). For instance, Wnt carriers, such as secreted Frizzled‐related proteins (sFRPs) and Wnt inhibitory factor 1 (WIF1) (de Almeida Magalhaes et al., 2023; Mii & Taira, 2009; Mii & Taira, 2011), extracellular vesicles (Gross et al., 2012), and cell protrusions, including cytonemes (Stanganello et al., 2015; Zhang et al., 2023), mediate Wnt transport. In addition to these mediators, several lines of evidence show that association with cell surface molecules also affects Wnt transport.
Heparan sulfate proteoglycans (HSPGs) are heavily involved in the transport and spatial distribution of Wnt (Matsuo & Kimura‐Yoshida, 2014; Mii, 2020; Mii & Takada, 2020; Yan & Lin, 2009). In fact, HSPGs are involved in the spatial distribution of many morphogens, including Decapentaplegic (Dpp), Hedgehog (Hh), and Wingless (Wg) in Drosophila (Franch‐Marro et al., 2005; Han et al., 2004; Tabata & Takei, 2004; Takei et al., 2004; Yan & Lin, 2009) and fibroblast growth factors (FGFs) in vertebrates (Matsuo & Kimura‐Yoshida, 2014; Shimokawa et al., 2011). An HSPG molecule consists of a core protein, such as Glypican or Syndecan, and several heparan sulfate (HS) chains, which are initially synthesized as linear co‐polymers of glucuronic acid (GluA) and N‐acetyl glucosamine (GlcNAc), thus forming [GlcA‐GlcNAc]n (Esko & Selleck, 2002; Yan & Lin, 2009). Then, HS chains are modified in multiple steps, including N‐ and O‐sulfation and epimerization, initiated with N‐sulfation by N‐deacetylase/N‐sulfotransferase (NDST), using 3′‐phosphoadenosine 5′‐phosphosulfate (PAPS) as the sulfonate donor (Esko & Selleck, 2002). These HS chain modifications are crucial for functions of HSPGs, such as specific binding of secreted proteins and their downstream signaling (Ai et al., 2003; Baeg et al., 2004; Han et al., 2005).
Based on immunostaining with monoclonal antibodies that recognize specific modifications of HS chains, including NAH46, which recognizes [GlcA‐GlcNAc]n (Suzuki et al., 2008), and HepSS‐1, which recognizes [GlcA‐GlcNS]n (Kure & Yoshie, 1986; van den Born et al., 2005), localization of modified HS chains in embryonic tissues has been reported (Mii et al., 2017; Takatoh & Hanaoka, 2010). Interestingly, HS chains recognized by these antibodies are not distributed uniformly on the cell surface. Instead, they exhibit punctate patterns. Furthermore, since puncta stained by each antibody rarely overlap, [GlcA‐GlcNAc]‐rich and [GlcA‐GlcNS]‐rich HSPGs appear to form distinct clusters on the cell surface, which we refer to as N‐acetyl‐rich and N‐sulfo‐rich HS clusters. Comparisons of the localization of these puncta with that of endogenous Wnt8 in Xenopus embryos revealed that endogenous Wnt8 strongly co‐localizes with N‐sulfo‐rich HS clusters. Consistently, knockdown of NDST1 reduces cell surface Wnt8 localization as well as canonical Wnt signaling, and vice versa. Thus, N‐sulfation of HS chains appears crucial for Wnt8 binding and signaling (Mii et al., 2017).
However, because of the N‐deacetylase activity of NDST1, overexpression of NDST1 also increases the levels of deacetyl (also known as N‐unsubstituted) HS [GlcA‐GlcNH3 +], and the monoclonal antibody JM403 specifically recognizes [GlcA‐GlcNH3 +]n structures in HS chains, demonstrating the presence of deacetyl states in tissues in vivo (Takatoh & Hanaoka, 2010; van den Born et al., 1995; van den Born et al., 2003; van den Born et al., 2005), although the biological functions of deacetyl modifications are poorly understood. Thus, whether either deacetylation or N‐sulfation of HS chains actually contributes to the distribution of Wnt8 and Wnt8 signaling remains unknown. In the present study, we created mutant constructs of the bifunctional enzyme NDST1, intending to suppress either N‐deacetylase or N‐sulfotransferase activity, in order to study modification states of HS chains involved in Wnt8 localization and downstream canonical Wnt signaling. We found that a mutant with amino acid substitutions at PAPS‐binding sites (PBSs) in the N‐sulfotransferase domain of NDST1 specifically exhibits increased deacetylation, but not N‐sulfation, of HS chains. Using this mutant form of NDST1, we examined which modification state is actually involved in the distribution of Wnt8 and Wnt8 signaling in Xenopus embryos.
2. MATERIALS AND METHODS
2.1. Xenopus embryo manipulation and microinjection
All experiments involving Xenopus laevis were approved by the Institutional Animal Care and Use Committee, National Institutes of Natural Sciences. Manipulation of X. laevis and microinjection experiments were performed according to standard methods (Sive et al., 2000). Briefly, unfertilized Xenopus eggs were obtained by injection of gonadotropin (ASKA Pharmaceutical), and eggs were fertilized with testis homogenates. After fertilization, eggs were de‐jellied in 4% cysteine solution (pH 7.8) and incubated in 1/10× Steinberg's solution at 14–20°C. Embryos were staged according to Nieuwkoop and Faber (1967). For mRNA microinjection, mRNAs were synthesized from plasmid DNA with the mMessage mMachine SP6 kit (Invitrogen) and purified with the RNeasy Micro kit (QIAGEN) or by phenol–chloroform extraction. Microinjections were performed in four‐cell stage embryos. Amounts of injected mRNA are described in figure legends. For cell tracing, mRNA encoding mRuby2‐KRas or mRFP1 was used.
2.2. Plasmids used for mRNA synthesis
We used the following plasmids as templates of mRNA synthesis: pCSf107‐Xndst1, pCSf107‐sp‐mVenus‐wnt8 (Mii et al., 2017), pCSf107‐mRuby2‐kras, pCSf107‐mRFP1, and pCS2 + memRFP. For constructing pCSf107‐Xndst1‐MdAc and pCSf107‐Xndst1‐MNS, the coding sequence of Xndst1 was mutated by PCR to substitute C482 and K610 with tryptophan and alanine, respectively, according to a previous report for mouse NDST1 (Figure S1a) (Bengtsson et al., 2003). To construct pCSf107‐Xndst1ΔST, the coding sequence of the N‐sulfotransferase domain (F600‐R878) was removed by PCR (Figure S2a). To construct pCSf107‐Xndst1‐mPBS, a synthetic coding sequence was obtained from Integrated DNA Technologies, in which all amino acids in PBS1 and PBS2 are substituted with alanine (Figure 1a).
FIGURE 1.

A PAPS‐binding site mutant (mPBS) of NDST1 exhibited increased deacetylation, but not N‐sulfation, in Xenopus embryos. (a) Domain structure of NDST1 and amino acid sequences of PAPS‐binding sites (PBSs). Sequences of wild‐type (WT) NDST1 and mPBS are shown. (b) Sequence conservation of PBSs among species. Numbering of the residues is based on Xenopus Ndst1.L. (c) Overexpression of WT NDST1 in the animal cap region increased both JM403 and HepSS‐1 staining and decreased NAH46 staining. WT NDST1‐overexpressing cells exhibited a non‐cell‐autonomous increase in JM403 staining (arrows). (d) Overexpression of NDST1‐mPBS in the animal cap region increased JM403 staining, but not HepSS‐1 staining, and decreased NAH46 staining. mRNAs were injected into the animal region of a ventral blastomere at the four‐cell stage. Embryos were fixed at Stage 11.5. Amounts of mRNAs: ndst1 (WT or mPBS), 1000 pg/embryo; mRuby‐kras, 100 pg/embryo. Scale bar: 20 μm.
2.3. Fluorescence image acquisition
Fluorescence images were acquired mainly with a laser‐scanning confocal microscope (Leica TSC SP8 system, objective: HC PL APO2 ×40/NA1.10 W CORR water immersion).
2.4. Live imaging
For live imaging, embryos were mounted on 35‐mm glass‐based dishes (Iwaki, 3910‐035) using a silicone chamber made in‐house with holes with a diameter of 1.7–1.8 mm.
2.5. Immunostaining
Xenopus embryos were fixed with MEMFA (0.1 M MOPS, pH 7.4, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde) and dehydrated with EtOH to improve HS chain staining. Fixed embryos were rehydrated with TBT (1× TBS, 0.2% BSA, 0.1% Triton X‐100). Blocking was performed with TBTS (TBS with 10% heat‐immobilized FBS) at 70°C for 40 min. Primary antibodies and their dilutions were as follows: NAH46 (recognizing [GlcA‐GlcNAc]n, purchased from Seikagaku, or prepared in‐house, mouse monoclonal IgM, 1:50), HepSS‐1 (recognizing [GlcA‐GlcNS]n, purchased from Seikagaku, or prepared in‐house, mouse monoclonal IgM, 1:400), and JM403 (recognizing [GlcA‐GlcNH3 +]n, purchased from Seikagaku or Amsbio [370730‐1], mouse monoclonal IgM, 1:200). Primary antibodies were diluted with TBTS. Secondary antibodies (goat anti‐mouse IgM mu chain Alexa Fluor 488, A21042, Invitrogen, 1:500; goat polyclonal anti‐mouse IgM mu chain Alexa Fluor 647, ab150123, Abcam, 1:500) were diluted with TBTS. Embryos were incubated in antibody solution overnight at 4°C and washed with TBT six times for 30 min after each antibody incubation step.
2.6. Luciferase reporter assays
Luciferase reporter assays were carried out as previously described (Mii & Taira, 2009). Multiple comparisons were carried out with pairwise t‐tests in which significance levels (p‐values) were adjusted by the Holm method after performing the Shapiro–Wilk test to assess normality of datasets, using R.
2.7. Image analyses
For quantification of JM403 staining in MdAc‐overexpressing embryos (Figure S1d), regions of interest (ROIs) at cell boundaries were selected with a width of five pixels, and signal intensities were measured by ImageJ. For measurement of eye size (Figure 4b), the area of the eye was measured with ImageJ using brush selection to obtain the ROI. Multiple comparisons were carried out with pairwise Wilcoxon rank‐sum tests in which significance levels (p‐values) were adjusted by the Holm method, using R.
FIGURE 4.
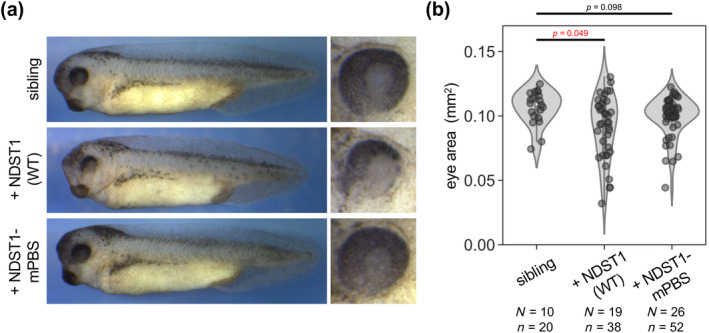
Overexpression of WT NDST1 resulted in smaller eyes, whereas overexpression of NDST1‐mPBS did not. (a) Morphological phenotypes of injected embryos at Stage 35–36. Overexpression of WT NDST1 caused smaller eyes compared to siblings, whereas overexpression of NDST1‐mPBS did not. mRNAs were injected into the animal region of both dorsal blastomeres at the four‐cell stage, targeting the anterior neuroectoderm. (b) Quantification of eye size in embryos. Statistical analysis was performed with the Wilcoxon rank‐sum test. Numbers of embryos (N) and numbers of eyes (n) are indicated. Amounts of mRNAs: ndst1 (WT or mPBS), 2000 pg/embryo.
3. RESULTS AND DISCUSSION
NDST1 is a bifunctional enzyme that has both N‐deacetylase and N‐sulfotransferase domains (Figure 1a) (Bengtsson et al., 2003; Dou et al., 2015; van den Born et al., 2003). To manipulate either deacetylation or N‐sulfation of HS chains, we constructed several mutants of NDST1 (Figures 1a, S1, and S2). Deacetylation and N‐sulfation of HS chains can be examined by immunostaining with monoclonal antibodies recognizing [GlcA‐GlcNH3 +]n (JM403) and [GlcA‐GlcNS]n (HepSS‐1) (van den Born et al., 2003; van den Born et al., 2005). Indeed, overexpression of wild‐type (WT) NDST1 increased JM403 staining, as previously described in cultured cells (van den Born et al., 2005), and also increased HepSS‐1 staining, whereas it reduced NAH46 staining, which is dependent upon N‐acetyl HS [GlcA‐GlcNAc]n, as we previously reported (Figure 1c) (Mii et al., 2017). Unexpectedly, we found that overexpression of WT NDST1 caused a non‐cell‐autonomous increase in JM403 staining, but not HepSS‐1 staining (Figure 1c). Although the underlying mechanisms are unknown, we speculate that overexpression of NDST1 may cause mis‐localization of NDST1 to the extracellular space, where deacetylation possibly occurs, but N‐sulfation does not, due to a lack of PAPS.
Another study found that point mutants of NDST1 lacked these activities (MdAc lacks deacetylase activity and MNS lacks N‐sulfotransferase activity) in an in vitro assay using cell lysates (Bengtsson et al., 2003; Sueyoshi et al., 1998; Wei & Swiedler, 1999). However, in our experiments, MdAc increased JM403 staining although the deacetylase activity of MdAc appeared lower than that of WT, and MNS increased HepSS‐1 staining when overexpressed in Xenopus embryos (Figure S1). Thus, these mutants were not useful for our purposes. Overexpression of NDST1ΔST, which lacks the entire N‐sulfotransferase domain, increased not only JM403, but also HepSS‐1 staining (Figure S2). Similar observations have also been reported with overexpression of the MNS mutant in cultured cells and with in vitro assays using isolated N‐deacetylase and N‐sulfotransferase domains, suggesting that deacetylation is the rate‐limiting step for subsequent N‐sulfation and that HS deacetylated by mutant NDST1 can be a substrate for subsequent N‐sulfation by endogenous NDST1 (Bengtsson et al., 2003; Dou et al., 2015). Thus, simple loss of N‐sulfotransferase activity cannot be used to specifically increase deacetylation in Xenopus embryos. Another approach was required for our purpose.
PAPS is a donor of sulfonate groups for N‐sulfation, and PBSs in NDST1 have been identified through structural and biochemical analyses (Gorokhov et al., 2000; Kakuta et al., 1998; Kakuta et al., 1999). Among the NDST1 mutants that we examined, we found that a mutant that has amino acid substitutions in the PBSs (NDST1‐mPBS, Figure 1a,b) increases JM403 staining, but not HepSS‐1 staining (Figure 1d). At a low frequency, NDST1‐mPBS appears to reduce HepSS‐1 staining, implying a dominant‐negative effect on N‐sulfation (Figure S3). Although the mechanisms underlying the differences in N‐sulfation between cells overexpressing mPBS, MNS, and ΔST are unknown, we will discuss this point later. In addition, NDST1‐mPBS reduced NAH46 staining, similarly to WT NDST1 (Figure 1d). Overall, NDST1‐mPBS increases deacetylation of HS, but does not increase N‐sulfation. Thus, we utilized this mutant to examine the effects of HS modifications on Wnt8 regulation.
Previously, we showed that accumulation of Wnt8 on the cell surface is increased by overexpression of NDST1 (Mii et al., 2017). However, whether deacetylation or N‐sulfation of HS chains is actually involved in the distribution of Wnt8 remains unknown. Therefore, we examined whether NDST1‐mPBS‐overexpressing cells accumulate Wnt8, using monomeric Venus‐tagged Wnt8 (mVenus‐Wnt8). In contrast to WT NDST1, overexpression of NDST1‐mPBS did not increase mVenus‐Wnt8 accumulation on the cell surface (Figure 2). Thus, N‐sulfation, but not deacetylation, of HS chains likely causes Wnt8 accumulation.
FIGURE 2.

Upon overexpression of NDST1‐mPBS, mVenus‐tagged Wnt8 (mV‐Wnt8) does not accumulate on the cell surface. (a) Schematic illustration of microinjection of mRNAs. Microinjection was performed at the four‐cell stage. (b) Upon overexpression of WT NDST1, mV‐Wnt8 (closed arrowheads) accumulated on the cell surface, while it did not upon overexpression of NDST1‐mPBS (open arrowheads). Embryos were observed at Stage 11.5–12 by live imaging. Amounts of mRNAs: ndst1 (WT and mPBS), 1000 pg/embryo; mRFP1, 100 pg/embryo. Scale bar: 20 μm.
We showed that N‐sulfo‐rich HS is involved in canonical Wnt signaling activated by Wnt8, because N‐sulfo‐rich HS clusters co‐localize with signaling components such as Fzd8 and phosphorylated LRP6, as well as Wnt8 (Mii et al., 2017). Consistently, knockdown of NDST1 reduces canonical Wnt signaling, whereas overexpression of NDST1 enhances it (Mii et al., 2017; Yamamoto et al., 2023). However, whether deacetylation or N‐sulfation of HS chains is actually required for canonical Wnt signaling remains unknown. Therefore, we examined whether NDST1‐mPBS enhances canonical Wnt signaling activated by Wnt8. Overexpression of WT NDST1 enhanced the activity of the TOP‐Flash reporter, which has three TCF‐binding sites in its promoter region and is frequently used to evaluate canonical Wnt signaling (van de Wetering et al., 1997); however, overexpression of NDST1‐mPBS reduced reporter activity (Figure 3). This result suggests that N‐sulfation, but not deacetylation, of HS chains is involved in canonical Wnt signaling activated by Wnt8.
FIGURE 3.

Canonical Wnt signaling required N‐sulfation‐rich HS chains rather than N‐deacetylation‐rich HS chains. TOP‐Flash reporter assay. Numbers of measured pools (n, each pool contained three embryos at Stage 11.5) are indicated. Multiple comparisons were carried out with pairwise t‐tests in which significance levels (p‐values) were adjusted by the Holm method, after performing Shapiro–Wilk tests to assess normality of datasets. mRNAs and TOP‐Flash reporter DNA were injected into the animal region of a ventral blastomere at the four‐cell stage. Amounts of mRNAs: wnt8, 100 pg/embryo; ndst1 (WT or mPBS), 500 pg/embryo. Amount of TOP‐Flash reporter DNA: 100 pg/embryo.
We also examined whether these differences in activity resulted in morphological phenotypes of Xenopus embryos when overexpressed. mRNA of either WT ndst1 or ndst1‐mPBS was injected into dorsal blastomeres of four‐cell stage embryos, targeting the anterior neuroectoderm. Embryos injected with WT ndst1 exhibited significantly smaller eyes compared with uninjected siblings (Figure 4a,b). However, eye size of embryos injected with ndst1‐mPBS did not differ from that of uninjected siblings. Thus, overexpression of WT NDST1, but not NDST1‐mPBS, causes smaller eyes, a hallmark of activated canonical Wnt signaling (Cavodeassi et al., 2005).
N‐Sulfation of HS chains, which is catalyzed by NDST, is considered the initial step for further HS modifications, such as O‐sulfation and epimerization (Esko & Selleck, 2002). Overexpression of NDST1 causes cell surface accumulation of Wnt8 and enhances canonical Wnt signaling (Mii et al., 2017; Yamamoto et al., 2023). On the other hand, little is known about the biological significance of N‐deacetylation, which is also catalyzed by NDST, although N‐deacetylation of HS chains is detectable in several tissues and cultured cells (Nadanaka et al., 2014; Takatoh & Hanaoka, 2010; van den Born et al., 1995). One of the ways to understand this significance is dissection of N‐deacetylase and N‐sulfotransferase activities using in vivo models. In this study, we constructed a mutant form of NDST1 that exhibits increased deacetylation, but not N‐sulfation, of HS chains (Figure 1). Using this mutant form, we demonstrated that accumulation of Wnt8 and enhancement of canonical Wnt signaling by Wnt8 largely depend on N‐sulfation (Figures 2 and 3). Interestingly, overexpression of NDST1‐mPBS resulted in reduced Wnt8 activity, when examined with the TOP‐Flash reporter assay (Figure 3); however, when overexpressed in the anterior neuroectoderm, it did not affect eye size (Figure 4). In our TOP‐Flash reporter assay, signaling by overexpressed Wnt8 was specifically examined. However, in the anterior neuroectoderm, other Wnt ligands and other signaling molecules are involved in eye field patterning (Fujimura, 2016; Kiecker & Niehrs, 2001). We speculate that these different contexts may have different effects on NDST1‐mPBS in our TOP‐Flash reporter assay and on embryo eye size.
Overexpression of NDST1‐mPBS stably reduced canonical Wnt signaling activated by Wnt8 (Figure 3), but not HepSS‐1 staining (Figures 1 and S3). Overexpression of NDST1‐mPBS may not significantly change the overall amounts/density of N‐sulfation on HS chains, because HepSS‐1 staining is not much affected. At the same time, it could inhibit Wnt8 binding to HS chains by enhancing deacetylation to destroy binding motifs on HS chains for Wnt8. Consistently, a Wnt3a‐binding motif of HS chains, which was identified from a pool of synthetic oligosaccharides, contains four repeats that can be modified by N‐sulfation (Gao et al., 2016). Thus, excess deacetylation possibly inhibits binding of Wnt ligands to functional binding motifs by interrupting continuous N‐sulfation. Alternatively, excess deacetylation may not inhibit binding of Wnt8, but may inhibit Wnt signaling, as reported with 6‐O‐sulfation of HS (Ai et al., 2003). We think that these possibilities could explain the observed inconsistency between Wnt signaling and HepSS‐1 staining.
Mutations that simply impair N‐sulfotransferase activity increase both deacetylation and N‐sulfation when overexpressed in cultured cells (Bengtsson et al., 2003). We observed similar outcomes when NDST1ΔST, which lacks the entire N‐sulfotransferase domain, was overexpressed in Xenopus embryos. These results can be explained if we assume that N‐deacetylation is the rate‐limiting step for N‐sulfation and that HS chains deacetylated by N‐sulfotransferase‐defective NDST1 mutants can subsequently be rapidly N‐sulfated by endogenous NDST1 (Bengtsson et al., 2003). In contrast, we found that overexpression of NDST1‐mPBS increased deacetylation, as visualized by JM403 staining, but did not increase or even decreased N‐sulfation, as visualized with HepSS‐1 staining (Figure 1). This result suggests that HS chains deacetylated by NDST1‐mPBS cannot be further N‐sulfated by endogenous NDST1. We speculate that NDST1‐mPBS may remain on HS chains after deacetylation, thereby preventing their N‐sulfation by endogenous NDST1, and possibly acting as a dominant‐negative form. Indeed, we observed that NDST1‐mPBS reduced HepSS‐1 staining at a low frequency (Figure S3), possibly reflecting low expression of endogenous ndst1. Consistently, HepSS‐1 staining intensity in intact embryos appears to differ among cells (Mii et al., 2017), implying divergent expression of endogenous ndst1. However, considering the high dosage of NDST1‐mPBS (1000 pg/embryo) in our experiments, its dominant‐negative activity should be weak, if any. Based on structural analysis with cryo‐electron microscopy, both the deacetylase and sulfotransferase domains of NDST1 have “binding clefts” for HS chains (Mycroft‐West et al., 2023). Thus, mPBS, MNS, and ΔST are all expected to interact with HS chains. However, their binding to HS chains may differ in several regards, including affinity, kinetics, and positions. Furthermore, binding of PAPS to mPBS and MNS could also differ because MNS has only one substitution in the PBS1 region, whereas PBS1 and PBS2 are fully substituted in mPBS (Figures 1 and S1). Analysis of the binding kinetics of these mutants and HS chains or PAPS may improve our understanding of their different effects on N‐sulfation. Nevertheless, NDST1‐mPBS could serve as a tool to dissect the roles of deacetylation and N‐sulfation in the regulation of HSPGs by HS chains.
AUTHOR CONTRIBUTIONS
This project was conceived by MS and YM with input from ST. MS and YM designed and performed experiments. MS and YM wrote the manuscript, and ST, MS and YM edited the manuscript. YM supervised the project together with ST.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Supporting information
FIGURE S1. Activity of point mutants of NDST1 in Xenopus embryos. (a) Schematic illustration of point mutants of NDST1 (MdAc and MNS). Arrowheads indicate the position of mutated amino acids. Sequences around the mutated sites are highly conserved among species. (b) Immunostaining with monoclonal antibody JM403 in embryos overexpressing WT or point mutants of NDST1. Overexpression of a point mutant lacking N‐deacetylase activity (MdAc) caused a detectable increase in JM403 staining (arrowheads), although the level of JM403 staining appears weaker than that with overexpression of WT NDST1. See also quantification in (d). Overexpression of a point mutant lacking N‐sulfotransferase activity (MNS) caused increased JM403 staining, similarly to WT NDST1. WT NDST1‐ or MNS mutant‐overexpressing cells exhibited a non‐cell‐autonomous increase in JM403 staining (arrows). (c) Immunostaining with monoclonal antibody HepSS‐1 in embryos overexpressing WT or point mutants of NDST1. Overexpression of the MdAc mutant caused a detectable increase in HepSS‐1 staining, although the level of HepSS‐1 staining appears weaker than that with overexpression of WT NDST1. Overexpression of the MNS mutant caused an increase in HepSS‐1 staining, similarly to WT NDST1. (d) Quantification of JM403 staining in MdAc mutant‐overexpressing embryos. JM403 staining on cell boundaries in tracer‐negative and ‐positive areas is quantified, showing MdAc significantly increased JM403 staining. Numbers of embryos, 3; numbers of cell boundaries, 15 (5 boundaries from each embryo) for tracer‐negative and ‐positive areas. mRNAs were injected into the animal region of a ventral blastomere at the four‐cell stage. Embryos were fixed at Stage 11.5. Amounts of mRNAs: ndst1 (WT, MdAc, or MNS), 500 pg/embryo; mRFP1 (tracer), 500 pg/embryo. Scale bar: 20 μm.
FIGURE S2. Activity of NDST1ΔST in Xenopus embryos. (a) Schematic illustration of a deletion construct that lacks the entire sulfotransferase domain (NDST1ΔST). (b) Overexpression of NDST1ΔST caused an increase in JM403 staining (arrowheads), as expected because the intact deacetylase domain is retained. (c) Overexpression of NDST1ΔST caused an increase in HepSS‐1 staining. Because NDST1ΔST is likely to have lost its N‐sulfotransferase activity, the observed increase in HepSS‐1 staining is probably due to endogenous NDST1, after an increase in HS deacetylation, affording the substrate for N‐sulfation. mRNAs were injected into the animal region of a ventral blastomere at the four‐cell stage. Embryos were fixed at Stage 11.5. Amounts of mRNAs: ndst1 (WT, NDST1ΔST), 500 pg/embryo; memRFP (tracer), 100 pg/embryo.
FIGURE S3. NDST1‐mPBS reduced HepSS‐1 staining at a low frequency in Xenopus embryos. Overexpression of NDST1‐mPBS in the animal cap region appears to reduce HepSS‐1 staining at a low frequency (arrowheads). mRNAs were injected into the animal region of a ventral blastomere at the four‐cell stage. Embryos were fixed at Stage 11.5. Amounts of mRNAs: ndst1‐mPBS, 1000 pg/embryo; mRuby‐kras, 100 pg/embryo. Scale bar: 20 μm.
ACKNOWLEDGMENTS
We thank Seikagaku Corp. for the NAH46 antibody, Dr. Asako Shindo for pCS2 + memRFP, and Dr. Steven D. Aird for editing the manuscript. This work was supported in part by the following programs: KAKENHI (22H02637 to YM; 23H04930 to YM and ST; 21H02498 and 22H05642 to ST; and 22J12262 to MS) and JST‐PRESTO (JPMJPR194B to YM). MS is a JSPS Research Fellow.
Suzuki, M. , Takada, S. , & Mii, Y. (2024). Dissection of N‐deacetylase and N‐sulfotransferase activities of NDST1 and their effects on Wnt8 distribution and signaling in Xenopus embryos. Development, Growth & Differentiation, 66(3), 248–255. 10.1111/dgd.12915
Communicating Editor: NAOTO UENO
REFERENCES
- Ai, X. , Do, A. T. , Lozynska, O. , Kusche‐Gullberg, M. , Lindahl, U. , & Emerson, C. P., Jr. (2003). QSulf1 remodels the 6‐O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. The Journal of Cell Biology, 162, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht, L. V. , Tejeda‐Munoz, N. , & De Robertis, E. M. (2021). Cell biology of canonical Wnt signaling. Annual Review of Cell and Developmental Biology, 37, 369–389. [DOI] [PubMed] [Google Scholar]
- Baeg, G. H. , Selva, E. M. , Goodman, R. M. , Dasgupta, R. , & Perrimon, N. (2004). The wingless morphogen gradient is established by the cooperative action of frizzled and Heparan sulfate proteoglycan receptors. Developmental Biology, 276, 89–100. [DOI] [PubMed] [Google Scholar]
- Bengtsson, J. , Eriksson, I. , & Kjellen, L. (2003). Distinct effects on heparan sulfate structure by different active site mutations in NDST‐1. Biochemistry, 42, 2110–2115. [DOI] [PubMed] [Google Scholar]
- Cavodeassi, F. , Carreira‐Barbosa, F. , Young, R. M. , Concha, M. L. , Allende, M. L. , Houart, C. , Tada, M. , & Wilson, S. W. (2005). Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta‐catenin pathway. Neuron, 47, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers, H. , Loh, K. M. , & Nusse, R. (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science, 346, 1248012. [DOI] [PubMed] [Google Scholar]
- De Almeida Magalhaes, T. , Liu, J. , Chan, C. , et al. (2023). Extracellular carriers control lipid‐dependent secretion, delivery, and activity of WNT morphogens. Developmental Cell, 59, 244–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, W. , Xu, Y. , Pagadala, V. , Pedersen, L. C. , & Liu, J. (2015). Role of deacetylase activity of N‐deacetylase/N‐sulfotransferase 1 in forming N‐sulfated domain in Heparan sulfate. The Journal of Biological Chemistry, 290, 20427–20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko, J. D. , & Selleck, S. B. (2002). Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annual Review of Biochemistry, 71, 435–471. [DOI] [PubMed] [Google Scholar]
- Farin, H. F. , Jordens, I. , Mosa, M. H. , Basak, O. , Korving, J. , Tauriello, D. V. F. , de Punder, K. , Angers, S. , Peters, P. J. , Maurice, M. M. , & Clevers, H. (2016). Visualization of a short‐range Wnt gradient in the intestinal stem‐cell niche. Nature, 530, 340–343. [DOI] [PubMed] [Google Scholar]
- Franch‐Marro, X. , Marchand, O. , Piddini, E. , Ricardo, S. , Alexandre, C. , & Vincent, J. P. (2005). Glypicans shunt the wingless signal between local signalling and further transport. Development, 132, 659–666. [DOI] [PubMed] [Google Scholar]
- Fujimura, N. (2016). WNT/beta‐catenin signaling in vertebrate eye development. Frontiers in Cell and Development Biology, 4, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. , Xu, Y. , Liu, J. , & Ho, M. (2016). Epitope mapping by a Wnt‐blocking antibody: Evidence of the Wnt binding domain in heparan sulfate. Scientific Reports, 6, 26245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorokhov, A. , Perera, L. , Darden, T. A. , Negishi, M. , Pedersen, L. C. , & Pedersen, L. G. (2000). Heparan sulfate biosynthesis: A theoretical study of the initial sulfation step by N‐deacetylase/N‐sulfotransferase. Biophysical Journal, 79, 2909–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. C. , Chaudhary, V. , Bartscherer, K. , & Boutros, M. (2012). Active Wnt proteins are secreted on exosomes. Nature Cell Biology, 14, 1036–1045. [DOI] [PubMed] [Google Scholar]
- Han, C. , Belenkaya, T. Y. , Khodoun, M. , Tauchi, M. , Lin, X. , & Lin, X. (2004). Distinct and collaborative roles of drosophila EXT family proteins in morphogen signalling and gradient formation. Development, 131, 1563–1575. [DOI] [PubMed] [Google Scholar]
- Han, C. , Yan, D. , Belenkaya, T. Y. , & Lin, X. (2005). Drosophila glypicans dally and dally‐like shape the extracellular wingless morphogen gradient in the wing disc. Development, 132, 667–679. [DOI] [PubMed] [Google Scholar]
- Kakuta, Y. , Pedersen, L. G. , Pedersen, L. C. , & Negishi, M. (1998). Conserved structural motifs in the sulfotransferase family. Trends in Biochemical Sciences, 23, 129–130. [DOI] [PubMed] [Google Scholar]
- Kakuta, Y. , Sueyoshi, T. , Negishi, M. , & Pedersen, L. C. (1999). Crystal structure of the sulfotransferase domain of human heparan sulfate N‐deacetylase/ N‐sulfotransferase 1. The Journal of Biological Chemistry, 274, 10673–10676. [DOI] [PubMed] [Google Scholar]
- Kiecker, C. , & Niehrs, C. (2001). A morphogen gradient of Wnt/beta‐catenin signalling regulates anteroposterior neural patterning in Xenopus. Development, 128, 4189–4201. [DOI] [PubMed] [Google Scholar]
- Kure, S. , & Yoshie, O. (1986). A syngeneic monoclonal antibody to murine meth‐a sarcoma (HepSS‐1) recognizes heparan sulfate glycosaminoglycan (HS‐GAG): Cell density and transformation dependent alteration in cell surface HS‐GAG defined by HepSS‐1. Journal of Immunology, 137, 3900–3908. [PubMed] [Google Scholar]
- Matsuo, I. , & Kimura‐Yoshida, C. (2014). Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369, 20130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, S. , Hingole, S. , & Chaudhary, V. (2021). The emerging mechanisms of Wnt secretion and signaling in development. Frontiers in Cell and Development Biology, 9, 714746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mii, Y. (2020). Heparan sulfate clusters regulate distribution and signaling of Wnt morphogens. Trends in Glycoscience and Glycotechnology, 32, E205–E211. [Google Scholar]
- Mii, Y. , & Taira, M. (2009). Secreted frizzled‐related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development, 136, 4083–4088. [DOI] [PubMed] [Google Scholar]
- Mii, Y. , & Taira, M. (2011). Secreted Wnt "inhibitors" are not just inhibitors: Regulation of extracellular Wnt by secreted frizzled‐related proteins. Development, Growth & Differentiation, 53, 911–923. [DOI] [PubMed] [Google Scholar]
- Mii, Y. , & Takada, S. (2020). Heparan sulfate proteoglycan clustering in Wnt signaling and dispersal. Frontiers in Cell and Development Biology, 8, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mii, Y. , Yamamoto, T. , Takada, R. , Mizumoto, S. , Matsuyama, M. , Yamada, S. , Takada, S. , & Taira, M. (2017). Roles of two types of heparan sulfate clusters in Wnt distribution and signaling in Xenopus. Nature Communications, 8, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycroft‐West, C. J. , Abdelkarim, S. , Duyvesteyn, H. M. E. , Gandhi, N. S. , Skidmore, M. A. , Owens, R. J. , & Wu, L. (2023). Structural and mechanistic characterization of bifunctional heparan sulfate N‐deacetylase‐N‐sulfotransferase 1. BioRxiv. 10.1101/2023.08.30.555497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka, S. , Purunomo, E. , Takeda, N. , Tamura, J. , & Kitagawa, H. (2014). Heparan sulfate containing unsubstituted glucosamine residues: Biosynthesis and heparanase‐inhibitory activity. The Journal of Biological Chemistry, 289, 15231–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs, C. (2022). The role of Xenopus developmental biology in unraveling Wnt signalling and antero‐posterior axis formation. Developmental Biology, 482, 1–6. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop, P. D. , & Faber, J. (1967). Normal table of Xenopus laevis (Daudin). North Holland. [Google Scholar]
- Nusse, R. , & Clevers, H. (2017). Wnt/beta‐catenin signaling, disease, and emerging therapeutic modalities. Cell, 169, 985–999. [DOI] [PubMed] [Google Scholar]
- Pani, A. M. , & Goldstein, B. (2018). Direct visualization of a native Wnt in vivo reveals that a long‐range Wnt gradient forms by extracellular dispersal. eLife, 7, e38325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge, D. , & Scholpp, S. (2019). Mechanisms of intercellular Wnt transport. Development, 146, dev176073. [DOI] [PubMed] [Google Scholar]
- Shimokawa, K. , Kimura‐Yoshida, C. , Nagai, N. , Mukai, K. , Matsubara, K. , Watanabe, H. , Matsuda, Y. , Mochida, K. , & Matsuo, I. (2011). Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Developmental Cell, 21, 257–272. [DOI] [PubMed] [Google Scholar]
- Sive, H. L. , Grainger, R. M. , & Harland, R. M. (2000). Early development of Xenopus laevis. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Stanganello, E. , Hagemann, A. I. , Mattes, B. , et al. (2015). Filopodia‐based Wnt transport during vertebrate tissue patterning. Nature Communications, 6, 5846. [DOI] [PubMed] [Google Scholar]
- Strigini, M. , & Cohen, S. M. (2000). Wingless gradient formation in the drosophila wing. Current Biology, 10, 293–300. [DOI] [PubMed] [Google Scholar]
- Sueyoshi, T. , Kakuta, Y. , Pedersen, L. C. , Wall, F. E. , Pedersen, L. G. , & Negishi, M. (1998). A role of Lys614 in the sulfotransferase activity of human heparan sulfate N‐deacetylase/N‐sulfotransferase. FEBS Letters, 433, 211–214. [DOI] [PubMed] [Google Scholar]
- Suzuki, K. , Yamamoto, K. , Kariya, Y. , Maeda, H. , Ishimaru, T. , Miyaura, S. , Fujii, M. , Yusa, A. , Joo, E. J. , Kimata, K. , Kannagi, R. , Kim, Y. S. , & Kyogashima, M. (2008). Generation and characterization of a series of monoclonal antibodies that specifically recognize [HexA(+/−2S)‐GlcNAc]n epitopes in heparan sulfate. Glycoconjugate Journal, 25, 703–712. [DOI] [PubMed] [Google Scholar]
- Tabata, T. , & Takei, Y. (2004). Morphogens, their identification and regulation. Development, 131, 703–712. [DOI] [PubMed] [Google Scholar]
- Takatoh, J. , & Hanaoka, K. (2010). Spatially and temporally regulated expression of specific heparan sulfate epitopes in the developing mouse olfactory system. Development, Growth & Differentiation, 52, 169–180. [DOI] [PubMed] [Google Scholar]
- Takei, Y. , Ozawa, Y. , Sato, M. , Watanabe, A. , & Tabata, T. (2004). Three drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development, 131, 73–82. [DOI] [PubMed] [Google Scholar]
- Van De Wetering, M. , Cavallo, R. , Dooijes, D. , et al. (1997). Armadillo coactivates transcription driven by the product of the drosophila segment polarity gene dTCF. Cell, 88, 789–799. [DOI] [PubMed] [Google Scholar]
- Van Den Born, J. , Gunnarsson, K. , Bakker, M. A. , et al. (1995). Presence of N‐unsubstituted glucosamine units in native heparan sulfate revealed by a monoclonal antibody. The Journal of Biological Chemistry, 270, 31303–31309. [DOI] [PubMed] [Google Scholar]
- Van Den Born, J. , Pikas, D. S. , Pisa, B. J. , Eriksson, I. , Kjellen, L. , & Berden, J. H. (2003). Antibody‐based assay for N‐deacetylase activity of heparan sulfate/heparin N‐deacetylase/N‐sulfotransferase (NDST): Novel characteristics of NDST‐1 and ‐2. Glycobiology, 13, 1–10. [DOI] [PubMed] [Google Scholar]
- Van Den Born, J. , Salmivirta, K. , Henttinen, T. , et al. (2005). Novel heparan sulfate structures revealed by monoclonal antibodies. The Journal of Biological Chemistry, 280, 20516–20523. [DOI] [PubMed] [Google Scholar]
- Wei, Z. , & Swiedler, S. J. (1999). Functional analysis of conserved Cysteines in Heparan SulfateN‐deacetylase‐N‐sulfotransferases. The Journal of Biological Chemistry, 274, 1966–1970. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T. , Kambayashi, Y. , Tsukano, K. , & Michiue, T. (2023). Ndst1, a heparan sulfate modification enzyme, regulates neuroectodermal patterning by enhancing Wnt signaling in Xenopus. Development, Growth & Differentiation, 65, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, D. , & Lin, X. (2009). Shaping morphogen gradients by proteoglycans. Cold Spring Harbor Perspectives in Biology, 1, a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca, M. , Basler, K. , & Struhl, G. (1996). Direct and long‐range action of a wingless morphogen gradient. Cell, 87, 833–844. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Brunt, L. , Ono, Y. , Rogers, S. , & Scholpp, S. (2023). Cytoneme‐mediated transport of active Wnt5b–Ror2 complexes in zebrafish. Nature, 625, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Activity of point mutants of NDST1 in Xenopus embryos. (a) Schematic illustration of point mutants of NDST1 (MdAc and MNS). Arrowheads indicate the position of mutated amino acids. Sequences around the mutated sites are highly conserved among species. (b) Immunostaining with monoclonal antibody JM403 in embryos overexpressing WT or point mutants of NDST1. Overexpression of a point mutant lacking N‐deacetylase activity (MdAc) caused a detectable increase in JM403 staining (arrowheads), although the level of JM403 staining appears weaker than that with overexpression of WT NDST1. See also quantification in (d). Overexpression of a point mutant lacking N‐sulfotransferase activity (MNS) caused increased JM403 staining, similarly to WT NDST1. WT NDST1‐ or MNS mutant‐overexpressing cells exhibited a non‐cell‐autonomous increase in JM403 staining (arrows). (c) Immunostaining with monoclonal antibody HepSS‐1 in embryos overexpressing WT or point mutants of NDST1. Overexpression of the MdAc mutant caused a detectable increase in HepSS‐1 staining, although the level of HepSS‐1 staining appears weaker than that with overexpression of WT NDST1. Overexpression of the MNS mutant caused an increase in HepSS‐1 staining, similarly to WT NDST1. (d) Quantification of JM403 staining in MdAc mutant‐overexpressing embryos. JM403 staining on cell boundaries in tracer‐negative and ‐positive areas is quantified, showing MdAc significantly increased JM403 staining. Numbers of embryos, 3; numbers of cell boundaries, 15 (5 boundaries from each embryo) for tracer‐negative and ‐positive areas. mRNAs were injected into the animal region of a ventral blastomere at the four‐cell stage. Embryos were fixed at Stage 11.5. Amounts of mRNAs: ndst1 (WT, MdAc, or MNS), 500 pg/embryo; mRFP1 (tracer), 500 pg/embryo. Scale bar: 20 μm.
FIGURE S2. Activity of NDST1ΔST in Xenopus embryos. (a) Schematic illustration of a deletion construct that lacks the entire sulfotransferase domain (NDST1ΔST). (b) Overexpression of NDST1ΔST caused an increase in JM403 staining (arrowheads), as expected because the intact deacetylase domain is retained. (c) Overexpression of NDST1ΔST caused an increase in HepSS‐1 staining. Because NDST1ΔST is likely to have lost its N‐sulfotransferase activity, the observed increase in HepSS‐1 staining is probably due to endogenous NDST1, after an increase in HS deacetylation, affording the substrate for N‐sulfation. mRNAs were injected into the animal region of a ventral blastomere at the four‐cell stage. Embryos were fixed at Stage 11.5. Amounts of mRNAs: ndst1 (WT, NDST1ΔST), 500 pg/embryo; memRFP (tracer), 100 pg/embryo.
FIGURE S3. NDST1‐mPBS reduced HepSS‐1 staining at a low frequency in Xenopus embryos. Overexpression of NDST1‐mPBS in the animal cap region appears to reduce HepSS‐1 staining at a low frequency (arrowheads). mRNAs were injected into the animal region of a ventral blastomere at the four‐cell stage. Embryos were fixed at Stage 11.5. Amounts of mRNAs: ndst1‐mPBS, 1000 pg/embryo; mRuby‐kras, 100 pg/embryo. Scale bar: 20 μm.


